Abstract
People living with HIV (PLH) may be at increased risk of experiencing both chronic pain and opioid dependence. Physical therapy (PT) has been shown to be effective as a nonpharmacological strategy for mitigating chronic pain in the general population, however, there is gap in research investigating PT to reduce chronic pain and opioid use among PLH. This case series describes the feasibility of an innovative PT intervention to decrease chronic pain and opioid use at a multidisciplinary human immunodeficiency virus (HIV) clinic. Participants (n = 4) were evaluated and given an individualized PT “package” consisting of manual therapy, exercise prescription, Transcutaneous Electrical Nerve Stimulation, and pain coping strategies. Pre- and postintervention outcomes were measured for pain reports, opioid use, and quality-of-life measures. After the intervention, all participants reported decrease or total elimination of both pain measured on the 0–10 numerical rating scale and opioid use measured in morphine milligram equivalents (MME). A paired t-test showed a significant difference (<.05) in the preintervention and postintervention pain scores and MME values. Results of this case series suggest in this sample that the described PT intervention is a feasible approach to mitigating chronic pain and opioid use among PLH and should be implemented on a larger scale for maximal effect.
Keywords: chronic pain, HIV/AIDS, opioids
Introduction
Chronic pain, defined as pain lasting over 3 months, impacts an estimated 10%–40% of U.S. adults resulting in $635 billions of direct and indirect costs.1,2 Historically, the standard of care for chronic pain treatment in the United States has emphasized pharmacological management.3 The amount of opioids prescribed and sold in the United States has quadrupled since 1999, but the overall amount of pain reported by Americans has remained static.3 Currently, as many as 25% of patients receiving long-term opioid prescriptions from a primary care provider struggle with opioid use disorder.4 The increased use of opioid analgesics to manage chronic pain over the last several decades combined with amplified prevalence of opioid use disorder, addiction and overdose deaths highlights the urgency for alternative, safe, and effective treatment for chronic pain.5,6 The Directors of the National Institutes of Health (NIH) and the National Institute on Drug Abuse (NIDA) issued a statement in 2017 seeking an “all hands on deck” approach to combat the current opioid epidemic, emphasizing the importance of a multidisciplinary approach to this complex crisis.5
Human immunodeficiency virus (HIV) has been shown to increase the risk for chronic pain, affecting an estimated 39%–85% of people living with HIV (PLH), compared with ∼11% of the general population.2,7–9 Chronic pain etiology among PLH is multifactorial, resulting from HIV disease progression, chronic inflammation, nerve damage, and side effects from antiretroviral therapy (ART).7–10 Chronic pain has emerged as a treatment priority for PLH and is associated with psychological and functional morbidity, decreased use of ART, and reduced retention in HIV primary care.11
The HIV Medical Association has recommended a biopsychosocial strategy approach to treating chronic pain among PLH, emphasizing that nonopioid drugs as well as nonpharmacological methods should be first-line approaches.2 Despite the increased attention to and call for nonopioid pain interventions, more research is needed to establish an effective nonpharmacological approach that reduces chronic pain in PLH.
Opioid use may be of special risk to PLH; when opioids are used concurrently with antiretroviral medications, adverse pharmacokinetic and pharmacodynamic interactions may occur.12 Prescription opioid misuse appears to be higher among PLH than the general population due to multifactorial reasons, including increased incidence of pain among PLH and adverse psychological histories, such as trauma, depression, and anxiety.12–14 PLH with a history of drug use are more likely to present clinically with pain and—even when prescribed opioids—continue to experience higher levels of pain.12,13,15 In addition, an association has been found between problematic use of opioids, a current history of psychiatric disorders, history of substance abuse, and poor adherence to ART in PLH.12 These factors may contribute to the increased opioid use seen in PLH and emphasize the need for a nonopioid solution to the chronic pain experienced by PLH.
Physical therapy (PT) utilizes a multidimensional, patient-centered approach, including therapeutic exercise, manual therapy, and patient education and is widely regarded as a cost-effective, low-risk treatment for acute and chronic pain.16–19 In addition to positively influencing physical, social, and health domains, PT has been associated with patients requiring fewer prescription analgesics, visits to physicians, advanced imaging, and surgical procedures.19
Advances in life expectancy for PLH have led to systemic and age-related comorbidities, including chronic pain.2,19 A preliminary body of research suggests that when chronic pain in this patient population is managed through skilled PT, PLH have reported decrease in pain levels and use of analgesics as well as increased functional independence and quality of life.17,18,20,21 Despite these promising studies, there remains a gap in research investigating PT to reduce chronic pain and opioid use among PLH.
This case series study describes an innovative PT intervention to decrease chronic pain and opioid use at the Ponce de Leon Center, a multidisciplinary HIV clinic in Atlanta, GA. The Ponce Center is one of the largest and most comprehensive HIV clinics in the United States serving the most vulnerable and at-risk populations in Atlanta, where the burden of HIV disease is high: Atlanta currently ranks fifth in the nation for total number of adults and adolescents living with HIV.17 The clinic has over 6,200 enrolled HIV-positive patients, with ∼90% of these patients identifying with underrepresented minority groups. Over 70% of enrolled patients live below the federal poverty level; 42% are uninsured, and 26% receive Medicaid. More than 70% of PLH who live in Atlanta reside within 2 miles of the Ponce Center, in an area recognized as a spatial clustering of the Atlanta HIV epidemic.18 Weekly PT has been available onsite at the clinic since 2014 and is one component of the multidisciplinary Palliative Care Program.
Materials and Methods
Approval for this study was granted by the Institutional Review Board of the home institution. Informed consent was obtained from each subject. Inclusion criteria included: (1) HIV-positive adults age ≥18 years enrolled at the Ponce de Leon Center with a noncancer, chronic pain diagnosis (>3 months); (2) undetectable HIV viral load (HIV-1 RNA <50 copies/mL), which was tested before enrollment; (3) currently on chronic opioid therapy (>3 months). Exclusion criteria for this study included: (1) Inability to safely participate in PT due to comorbidities, including but not limited to unstable cardiac disease, psychiatric instability, or other pathology; (2) inability to communicate symptoms or response to treatment with investigators.
Eligible participants were referred by their physicians and further screened for eligibility through electronic chart review and introductory phone call to participants. Pre- and postintervention data were collected through the following three instruments and their corresponding scoring criteria: (1) The Brief Pain Inventory (BPI)22; (2) the 36-Item Short Form Survey (SF-36)23 for quality-of-life measurements; (3) the HIV, Opioid and Pain Survey (HOPS)©, an original questionnaire generated by the investigators to gather data on demographic and social information, pain experience, and opioid usage. The numerical rating scale (0–10) was recorded pre- and postintervention and at each visit.24 Change in pain was assessed through the minimal clinically important difference (MCID) for chronic musculoskeletal pain, which is one point or 15.0% change from baseline.25 Morphine milligram equivalents (MME) are used to equate different opioid doses into one standardized value to allow for risk evaluation and comparison, which was recorded pre- and postintervention.26
The PT-based interventions were performed and documented during each session and included manual therapy, personalized exercise prescription, various pain coping strategies, and Transcutaneous Electrical Nerve Stimulation (TENS). TENS is a noninvasive, nonpharmacological treatment for acute and chronic pain, delivered to the patient through surface electrodes placed on the pain site. The resulting deep tissue stimulation relieves pain by both peripheral and central mechanisms.27 Each participant also received education on pain management and opioid weaning. Opioid dose reduction was done under the supervision of the prescribing provider. Study criteria included PLH with chronic pain currently prescribed opioid analgesics, however, there were no explicit specifications regarding timeline of opioid weaning in the criteria. Although all clinic providers at the study site adhere to national opioid prescribing guidelines, not all patients are necessarily being actively weaned from opioids. Decisions on opioid weaning were on a case-by-case basis, with input by the referring physician, physical therapist, and patient. The opioid weaning was embedded within the intervention aimed primarily to mitigate chronic pain, which would decrease patients’ need for opioid analgesics to manage pain.
The planned number of PT visits was 8 ± 2 sessions lasting 45 min in duration. Participants were seen primarily for in-person clinic visits, which included one phone session. Data were deidentified and recorded in a secure digital, password-protected database.
Pain coping skills were tailored to the individual participant's pain site and etiology, but focused on three self-management techniques to be utilized at the initial onset of pain, before taking an opioid analgesic: (1) Perform their individualized home PT stretching/exercise routine; (2) put the TENS unit on the painful area for 20 min (clinically recommended time); (3) diaphragmatic/paced breathing with the following instructions: “Take a deep breath in slowly through your nose, letting your chest and lower belly expand. Breathe out slowly through your mouth with slightly pursed lips. Focus on the sound of your breath and the feeling of your chest, belly and body filling with air, then letting go.”
Results
After physician-referred participants were screened for inclusion criteria, 14 participants met all criteria for the study. Three participants were interested in the study, but were unable to attend a clinic appointment for an initial evaluation due to transportation limitations. Seven participants completed their initial preintervention interview, but were unable to continue in this cohort due to extenuating life circumstances. The final sample size for this study cohort was n = 4. Participants’ ages ranged from 31 to 65, with a mean age of 49.4 years. All participants identified as African American. Two participants were female and two were male. All participants had multiple sites of pain, including lower back, upper back, neck, shoulder, hip, and knee. Participants’ duration of pain varied from 4 months to 40 years.
Although the planned number of PT visits were 8 ± 2, the number of visits varied per participant ranging from 5 to 10 visits based on participant needs, and response to treatment. Detailed PT interventions for each participant are described in Table 1.
Table 1.
Physical Therapy Intervention By Participant
| Participant | Pain location | TENS location | Therapeutic exercise/HEP | Manual therapy | Pain coping strategies |
|---|---|---|---|---|---|
| 1 | Thoracic spine, low back | Mid back and low back | Upper back and bilateral upper trapezius stretch; calf stretches; lower trunk rotation | Soft tissue mobilization to bilateral quadratus lumborum | Patient education on performing HEP and using TENS unit before taking morning opioid dose. |
| 2 | Low back, bilateral shoulders, cervical spine | Shoulders and low back | Upper trapezius, levator scapulae, and standing stretches; lower trunk rotations; walking program | Soft tissue mobilization to upper trapezius, levator scapula, rhomboids, piriformis, quadratus lumborum, paraspinals | Patient education on performing HEP and using TENS before taking opioids. Also educated on using walking as pain distraction. |
| 3 | Low back, L knee, B calves | Patient used patch on lower extremity instead of back due to postsurgical hardware precautions | hamstring stretches; lower trunk rotation; walking 15 to 20 min 3–4 × /week, calf stretches | hamstring, calf and quadriceps stretches; soft tissue mobilization to gastrocnemius, fibularis longus | Patient education on not switching fentanyl patch automatically at 3 days and delaying putting new patch on after removing old one. |
| 4 | Bilateral knees, low back pain | Low back | Hamstring curls, LAQ, tree huggers, hamstring stretch, prone press-ups, proper bed mobility technique, and squat biomechanics | Soft tissue mobilizations to bilateral QL and erector spinae | Patient education on first using TENS unit and performing HEP before taking opioids. |
HEP, Home Exercise Program; TENS, Transcutaneous Electrical Nerve Stimulation.
Pain data were recorded using the Numeric Rating Scale (NRS), which is widely recognized as a valid, reliable, and easily administered subjective pain tool.24 The average preintervention pain report using the NRS for participants was 7.5/10. After the intervention, two participants had complete resolution (0/10) of pain (100% reduction), one participant had a final pain report of 1/10 (87.5% reduction), and the final participant had a final pain rating of 3/10 (62.5% reduction) (Fig. 1).
FIG. 1.
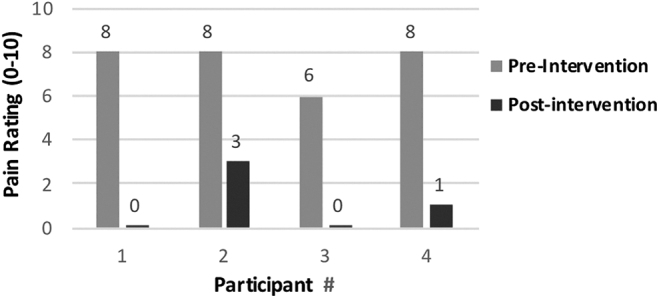
Pre- and postintervention change in pain.
Results of the study show that all participants reported a change in pain over five times the MCID, reporting a reduction in pain of at least five points. A paired t-test showed a significant difference (<.05) in the preintervention and postintervention pain scores; t = −15.381807, p = <.00001.
All participants showed a decrease in opioid use, measured by MME. Participants’ MME change, including years taking opioids, are described in Table 2. There was also a significant difference (<.05) in pre- and postintervention MME; t = −3.270308, p = .01367.
Table 2.
Pre- and Postintervention Change in Morphine Milliequivalents
| Participant | Years on opioids | MME initial | MME final | % Decrease |
|---|---|---|---|---|
| 1 | 7 | 4 | 0 | 100 |
| 2 | 1 | 45 | 1.07 | 98 |
| 3 | 6 | 60 | 25.7 | 57 |
| 4 | 4 | 5 | 0 | 100 |
MME, morphine milligram equivalents.
Pre- and postintervention SF-36 scores were recorded for all four participants. Upon completing the postintervention SF-36, all participants reported improvement in the categories of: general health, pain, emotional wellbeing, energy/fatigue, and role imitations due to physical health. One participant reported a decrease in social functioning, and one in role limitations due to emotional problems. Of note, these two participants had significant life stressors (homicides of family members), which occurred near the end of the intervention.
The BPI is a self-reported measurement tool used to clinically evaluate pain, including severity, interference with activities of daily life, and percent relief afforded by pain treatments and/or medications.22 On pre- and post-BPI questionnaires, all participants reported decreased pain severity (mean 46% decrease in severity), decreased pain interference with life activities (mean 53% decrease in interference), and increased pain relief (mean 50% increased relief) after the intervention. A paired t-test did not reveal a significant difference in pre- and postintervention pain severity (t = −1.8946, p = .1546), however, significant difference was found for both pre- and postintervention pain interference (t = 2.9357, p = .0261), and pre- and postintervention pain relief (t = 3.0000, p = .0240). Figure 2 details changes in participant scores in each BPI domain at pre- and postintervention.
FIG. 2.
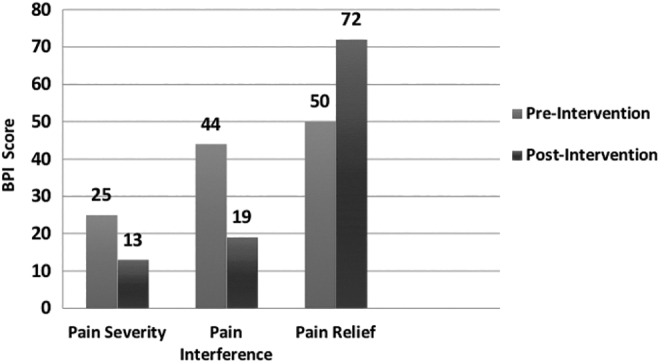
Brief Pain Inventory: mean scores pre- and postintervention.
Discussion
Chronic pain has emerged as a treatment priority among PLH. However, there is a paucity of research describing nonpharmacological means of managing chronic pain with concurrent opioid weaning within this patient population. Even on a regimen of prescribed opioids, many PLH still express high levels of pain without improvements in physical function or quality of life. This case report describes the results of a pilot cohort of PLH receiving PT treatment as a means for chronic pain reduction and opioid weaning in a multidisciplinary HIV clinic.
The results of the study show a decrease or elimination in both pain levels and opioid use after the PT intervention. The Centers for Disease Control and Prevention (CDC) recommends a decrease of 10% of original dose per month as a safe starting point for opioid weaning for individuals with long-term opioid use.28 All participants in this case series exceeded CDC long-term opioid use tapering guidelines with a range in weekly decrease from 8% to 25%. All participants reported improvements in quality of life related to pain, physical function, and general health in the postintervention SF-36 assessment.
Due to the case series type of study, a major limitation of generalizability includes the small sample size. That said, the small sample allowed us to focus on individual subjects’ experiences during the intervention, and what factors limited success in decreasing opioid use. In this patient population of financially underserved, health-disparate PLH, various social determinants of health must be taken into account when planning interventions to maximize benefit to the participants. Due to extenuating life stressors such as financial and housing insecurity and transportation limitations, some eligible participants were unable to attend their appointments and/or dropped out of the study. Future studies within this patient population will provide transportation vouchers to participants and text alerts to remind participants of their appointments to increase study adherence. In addition, low numbers of in-clinic provider referrals to PT was a limiting factor to participant recruitment. Future study recruitment will include reaching out to referring providers to advertise the study and its potential to benefit patients experiencing chronic pain and opioid use.
This patient population is commonly affected by additional psychosocial factors that may adversely influence their pain levels and quality of life, such as illicit drug use, reliance on nonprescribed opioids, depression, and other mental health issues, financial restrictions, and social isolation.29,30 For example, the two participants who experienced traumatic events during the study's SF-36 scores were impacted despite the fact that their pain was eliminated. Although these extenuating factors were discussed and documented during each session, there was a lack of control for these factors when analyzing MME, BPI, and SF-36 scores. In addition, not measuring the potential use of nonprescribed opioids or illicit drug use by the participants may impact the conclusion that PT is solely responsible for reducing pain levels and improving quality of life. Due to the high incidence of life stressors among this patient population, all participants were given information regarding mental health support services offered at the Ponce Center.
As the opioid epidemic continues to present a public health crisis in the United States, finding a nonpharmacological treatment method for chronic pain management is crucial. Chronic pain is well understood to be connected to deterioration in the quality of life, physical function, and psychological function among PLH.29,30 Given the multifaceted etiology of chronic pain and opioid addiction, a multifaceted approach to pain and opioid mitigation must be utilized. An emphasis on both psychosocial and physical domains is a holistic approach to better address opioid usage, pain management, and improve the quality of life for those living with HIV. While our results should not be overstated or generalized due to the small sample size, results are promising when considering this study within a larger sample. In conclusion, in a nonrandomized sample of HIV-positive adults at one HIV clinic, the proposed PT intervention appears to be a feasible, effective, nonpharmacological method to decrease chronic pain and analgesic use in a selected cohort of persons living with HIV.
Acknowledgments
The author wishes to acknowledge the collaboration of Dr. Kimbi Hagen, EdD; Dr. Melody Palmore, MD; Linda Gates, RN; Dr. Anne-Marie McKenzie-Brown, MD; and the patients and staff at the Grady Ponce de Leon Center.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Funding for this project was provided by:
-
1.
The National Institutes of Health, National Institute of Drug Abuse, Loan Repayment Program. Physical Therapy for Nonpharmaceutical Chronic Pain Mitigation and Opioid Use Reduction Among People Living with HIV (L30 DA046878).
-
2.
National Institutes of Health, Center for AIDS Research at Emory University. Opportunity Award Grant. Physical Therapy for Nonpharmaceutical Chronic Pain Mitigation and Opioid Use Reduction Among People Living with HIV (P30AI050409).
References
- 1. Dowell D, Haegerich TM, Chou R: CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65:1–49 [DOI] [PubMed] [Google Scholar]
- 2. Gaskin DJ, Richard P: The economic costs of pain in the United States. J Pain 2012;13:715. [DOI] [PubMed] [Google Scholar]
- 3. Bruce DR: Merlin J, Lum PJ, Ahmed E, Alexander C, Corbett AH, Foley K, Leonard K, Treisman GJ, Selwyn P. 2017 HIVMA of IDSA clinical practice guideline for the management of chronic pain in patients living With HIV. Clin Infect Dis 2017;65:e1–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reuben DB, Alvanzo AA, Ashikaga T, et al. : National Institutes of Health pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med 2015;162:295–300 [DOI] [PubMed] [Google Scholar]
- 5. Volkow ND, Collins FS: The role of science in addressing the opioid crisis. N Engl J Med 2017;377:391–394 [DOI] [PubMed] [Google Scholar]
- 6. Nicol AL, Hurley AW, Benzon HT: Alternatives to opioids in the pharmacologic management of chronic pain syndromes: A narrative review of randomized, controlled, and blinded clinical trials. Anesth Analg 2017;125:1682–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paulozzi LJ, Mack KA, Hockenberry JM: Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines—United States, 2012. MMWR Morb Mortal Wkly Rep 2014;63:563–568 [PMC free article] [PubMed] [Google Scholar]
- 8. Scott W: The psychosocial context of chronic pain on people living with HIV. Pain Rep 2019;4:e721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pullen S, Pak J, Hunt K, et al. : Physical therapy as an adjunct treatment for people living with HIV/AIDS: Provider needs assessment–Phase II. J Allied Health 2014;43:e45–e52 [PubMed] [Google Scholar]
- 10. Merlin JS, Bulls HW, Vucovich LA, Edelman EJ, Starrels JL: Pharmacologic and non-pharmacologic treatments for chronic pain in individuals with HIV: A systematic review. AIDS Care 2016;28:1506–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newshan G, Bennett J, Holman S: Pain and other symptoms in ambulatory HIV patients in the age of highly active antiretroviral therapy. J Assoc Nurses AIDS Care 2002;13:78–83 [DOI] [PubMed] [Google Scholar]
- 12. Bruce RD, Moody DE, Altice FL, et al. : A review of pharmacological interactions between HIV or hepatitis C virus medications and opioid agonist therapy: Implications and management for clinical practice. Expert Rev Clin Pharmacol 2013;6:249–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krashin DL, Merrill JO, Trescot AM: Opioids in the management of HIV-related pain. Pain Physician 2012;15(3 Suppl):ES 157–168 [PubMed] [Google Scholar]
- 14. Uebelacker LA, Weisberg RB, Herman DS, et al. : Chronic pain in HIV-infected patients: Relationship to depression, substance use, and mental health and pain treatment. Pain Med 2015;16:1870–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robinson-Papp J, Elliott K, Simpson DM, et al. : Problematic prescription opioid use in an HIV-infected cohort: The importance of universal toxicology testing. J Acquir Immune Defic Syndr 2012;61:187–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsao J, Stein J, Dobalian A: Pain, problem drug use history, and aberrant analgesic use behaviors in persons living with HIV. Pain 2007;133:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mgbemena O, Westfall AO, Ritchie CS, et al. : Preliminary outcomes of a pilot physical therapy program for HIV-infected patients with chronic pain. AIDS Care 2015;27:244–247 [DOI] [PubMed] [Google Scholar]
- 18. deBoer H, Andrews M, Cudd S, et al. : Where and how does physical therapy fit? Integrating physical therapy into interprofessional HIV care. Disabil Rehabil 2019;41:1768–1777 [DOI] [PubMed] [Google Scholar]
- 19. Hanney WJ, Masaracchio M, Liu X, Kolber MJ: The influence of physical therapy guideline adherence on healthcare utilization and costs among patients with low back pain: A systematic review of the literature. PLoS One 2016;11:e0156799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parker R, Stein DJ, Jelsma J: Pain in people living with HIV/AIDS: A systematic review. J Int AIDS Soc 2014;17:18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pullen S, del Rio C, Brandon D, et al. : Associations between chronic pain, analgesic use and physical therapy among adults living with HIV in Atlanta, GA: A retrospective cohort study. AIDS Care 2020;32:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cleeland CS, Ryan KM: Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–138 [PubMed] [Google Scholar]
- 23. RAND Corporation: 36-Item Short Form Survey (SF-36) Scoring Instructions. Available at https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html, accessed April26, 2019
- 24. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP: Validity of four pain intensity rating scales. Pain 2011;152:2399–2404 [DOI] [PubMed] [Google Scholar]
- 25. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W: Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283–291 [DOI] [PubMed] [Google Scholar]
- 26. Gilson AM, Maurer MA, Ryan KM, Cleary JF, Rathouz PJ: Using a morphine equivalence metric to quantify opioid consumption: Examining the capacity to provide effective treatment of debilitating pain at the global, regional, and country levels. J Pain Symptom Manage 2013;45:681–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vance CG, Dailey DL, Rakel BA, Sluka KA: Using TENS for pain control: The state of the evidence. Pain Manag 2014;4:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guideline Resources: CDC Opioid Guideline Mobile App | Drug Overdose | CDC Injury Center. Available at https://www.cdc.gov/drugoverdose/prescribing/app.html (Published April 10, 2019), accessed April26, 2019
- 29. Isenberg SR, Maragh-Bass AC, Ridgeway K, Beach MC, Knowlton AR: A qualitative exploration of chronic pain and opioid treatment among HIV patients with drug use disorders. J Opioid Manag 2017;13:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerbi GB, Habtemariam T, Robnett V, Nganwa D, Tameru B: Psychosocial factors as predictors of HIV/AIDS risky behaviors among people living with HIV/AIDS. J AIDS HIV Res 2012;4:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]


