Abstract
Introduction:
The use and availability of oral and inhalable products containing cannabidiol (CBD) as the principal constituent has increased with expanded cannabis/hemp legalization. However, few controlled clinical laboratory studies have evaluated the pharmacodynamic effects of oral or vaporized CBD or CBD-dominant cannabis.
Methods:
Eighteen healthy adults (9 men; 9 women) completed four, double-blind, double-dummy, drug administration sessions. Sessions were separated by ≥1 week and included self-administration of 100 mg oral CBD, 100 mg vaporized CBD, vaporized CBD-dominant cannabis (100 mg CBD; 3.7 mg THC), and placebo. Study outcomes included: subjective drug effects, vital signs, cognitive/psychomotor performance, and whole blood THC and CBD concentrations.
Results:
Vaporized CBD and CBD-dominant cannabis increased ratings on several subjective items (e.g., Like Drug Effect) relative to placebo. Subjective effects did not differ between oral CBD and placebo and were generally higher for CBD-dominant cannabis compared to vaporized CBD. CBD did not increase ratings for several items typically associated with acute cannabis/THC exposure (e.g., Paranoid). Women reported qualitatively higher ratings for Pleasant Drug Effect than men after vaporized CBD and CBD-dominant cannabis use. CBD-dominant cannabis increased heart rate compared to placebo. Cognitive/psychomotor impairment was not observed in any drug condition.
Conclusions:
Vaporized CBD and CBD-dominant cannabis produced discriminable subjective drug effects, which were sometimes stronger in women, but did not produce cognitive/psychomotor impairment. Subjective effects of oral CBD did not differ from placebo. Future research should further elucidate the subjective effects of various types of CBD products (e.g., inhaled, oral, topical), which appear to be distinct from THC-dominant products.
Keywords: Cannabidiol, Cannabis, THC, Pharmacodynamics, Vaporizer
1. Introduction
In the U.S., cannabis is currently legal for medicinal purposes in 33 states and the District of Columbia (D.C) and is legal for non-medicinal (i.e., recreational) purposes in 11 states. Many countries aside from the U.S. also now permit medicinal (e.g., Australia) and/or non-medicinal cannabis use (e.g., Canada). In addition to cannabis, the U.S. Agriculture Improvement Act of 2018 (aka, The Farm Bill) recently removed hemp and its derivative products from the controlled substances list in the U.S.; this act defined hemp as cannabis containing < 0.3 % Δ-9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis (Bridges and Hanson, 2017). Coincident with these legislative changes, an expansive retail market of cannabinoid-containing products has emerged.
Cannabidiol (CBD) is a principal constituent of many cannabis/hemp products. Presently, there is a rapidly-growing industry of CBD products (Corroon and Kight, 2018). This industry has emerged due to the purported health benefits of CBD for a variety of conditions such as anxiety, pain, and inflammation (Blessing et al., 2015; Corroon and Phillips, 2018; Devinsky et al., 2017; Maroon and Bost, 2018; Poleg et al., 2019; Walsh et al., 2017). However, phase 3 clinical trials to support the use of CBD for health indications other than rare seizure disorders is lacking (Bonn-Miller et al., 2019). Retail CBD products vary with respect to product formulation as well as intended route of administration. CBD is most commonly administered orally in a liquid solution (e.g., tinctures, elixirs) or CBD-infused food/beverage product, but CBD products intended for pulmonary administration are also prevalent. For example, there are many cannabis chemotypes for which the raw plant product has a high concentration of CBD and a low concentration of THC (i.e. CBD-dominant). These CBD-dominant cannabis chemotypes can be inhaled as dried flower or in the form of concentrated cannabis- or hemp-derived CBD oils/extracts (Corroon and Kight, 2018; Corroon and Phillips, 2018; Spindle et al., 2019a). Despite their current widespread availability, it remains unclear whether many CBD products will remain on the market, as products using CBD as a food additive and CBD products marketed for their supposed health benefits without approval by the U.S. Food and Drug Administration (FDA) are in violation of the U.S. Federal Food, Drug, and Cosmetic Act according to the FDA (FDA, 2020).
The acute pharmacodynamic effects (e.g., subjective, cognitive, and physiological drug-induced effects) of CBD have been characterized in controlled research, but prior studies have mostly been limited to oral CBD administration. In most published studies, oral CBD has produced negligible pharmacodynamic drug effects. For example, oral CBD doses of 600 mg (Martin-Santos et al., 2012), 800 mg (Babalonis et al., 2017; Haney et al., 2016), and 900 mg (Arndt and de Wit, 2017) did not alter subjective drug effects, cognitive performance, and/or physiological measures (e.g., heart rate, blood pressure) compared with placebo. In one study, however, oral CBD doses of 1500 mg and 4500 mg produced discriminable subjective drug effects, increasing ratings of drug liking compared to placebo (Schoedel et al., 2018). Only two published laboratory studies have examined the acute effects of vaporized CBD, despite the growing popularity of this method of administration. In the first study (Hindocha et al., 2015), 16 mg of vaporized CBD improved emotional facial recognition relative to placebo, but did not produce discriminable subjective drug effects. In the second study (Solowij et al., 2019), participants inhaled 400 mg vaporized CBD alone and in combination with 8 mg THC on separate days, and 4 mg CBD in combination with 8 mg THC. After inhaling the 400 mg CBD dose alone, participants reported higher subjective ratings of intoxication (e.g., Feel Stoned) compared with placebo, but these ratings were lower than those observed when participants inhaled 8 mg of THC either alone or in combination with either dose of CBD. The 400 mg CBD dose had no influence on heart rate (HR) or blood pressure (BP) and the 4 mg CBD dose did not alter subjective effects.
Additional research on the acute effects of oral and inhalable CBD products is needed for several reasons. First, prior studies have only examined one route of administration in isolation, which has not allowed for direct pharmacodynamic comparisons between oral and inhaled CBD at the same dose. Second, men have generally been oversampled in prior controlled research on CBD. Given that women exhibit greater sensitivity to some effects of THC relative to men (Cooper and Haney, 2014; Fogel et al., 2017), there is a need to explore whether the acute effects of CBD also differ between men and women. Lastly, additional research is needed to characterize the effects of CBD products that also contain low concentrations of THC because high CB D/low THC products make up a growing segment of the cannabis/hemp markets, especially where cannabis or hemp has been legalized. Indeed, hemp-derived CBD products can legally contain up to a threshold amount of THC in many jurisdictions (e.g., 0.3 % in the U.S.). Moreover, recent research has shown that CBD products sold under the guise of meeting the legal definition of hemp may contain THC concentrations that exceed the legal limit, including those advertised as THC free (Bonn-Miller et al., 2017; Poklis et al., 2019).
In this study, we sought to extend prior controlled research on CBD and address some of the limitations of previous studies noted above. We characterized, and compared, the acute pharmacodynamic effects (i.e., subjective drug effects, cognitive/psychomotor performance, vital signs) as well as pharmacokinetics (i.e., whole blood concentrations of CBD and THC) of oral CBD, vaporized CBD, vaporized CBD-dominant cannabis that contained a low concentration of THC, and placebo, in nine male and nine female infrequent cannabis users.
2. Method
All study procedures were completed at the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU). The Johns Hopkins University School of Medicine Institutional Review Board (IRB) approved the study and the study was conducted in compliance with the ethics of the Declaration of Helsinki.
2.1. Participants
Study volunteers were recruited with print and online media advertisements and word-of-mouth communication. Interested potential participants first completed a brief telephone interview and those who appeared eligible were invited for a laboratory screening visit. At this screening visit, written informed consent was obtained and procedures were completed to determine study eligibility.
In order to be eligible, participants had to be 18–45 years of age, have a body mass index (BMI) between 19 and 36 kg/m2, self-report prior experience with cannabis but no cannabis use for ≥ 30 days prior to their first experimental session, and be in good health as determined via a medical history interview, a 12-lead electrocardiogram (ERG), blood chemistry, hematology, and serology analysis, and a physical examination. Additionally, at the screening visit and prior to each drug exposure session, participants had to test negative for recent use of cannabis/other illicit drugs (via urinalysis), alcohol (via an alcohol breathalyzer), and pregnancy (via urine test; females only). Individuals were excluded from study participation if they currently used prescription or over the-counter medications or other products (e.g., supplements) that could impact their safety. Females who were breastfeeding, had a positive pregnancy test, or who were planning to become pregnant were excluded from participation.
2.2. Study design and procedure
This study utilized a within-subjects crossover design and was double-blind (study staff and participants were blinded to drug dose). A double-dummy blinding procedure was also used to control for expectancy effects whereby study participants administered both an oral dose (either placebo or active) and an inhaled dose (either placebo or active) in all drug administration sessions. Participants completed a total of 4 dosing conditions in separate experimental sessions, separated by ≥ 1 week. The 4 conditions were: (1) 100 mg oral CBD and vaporized placebo cannabis; (2) oral placebo and 100 mg vaporized CBD; (3) oral placebo and vaporized CBD-dominant cannabis containing 100 mg CBD and 3.7 mg THC; and (4) oral placebo and vaporized placebo cannabis (placebo condition). Sessions were completed in a randomized order.
On the morning of each drug administration session, participants arrived to the BPRU at approximately 7:30 am. They first provided a urine sample which was tested for recent drug use and pregnancy (if female) and took an alcohol breathalyzer. Negative results were required on all of these tests to proceed with the session. Next, recent use of cannabis, alcohol, and tobacco since the last laboratory visit was ascertained with the Timeline Follow-Back (TLFB) questionnaire (Sobell and Sobell, 1992); at screening, a 90-day TLFB was administered. Participants then completed baseline pharmacodynamic assessments (see section 2.4), provided baseline biological specimens, and consumed a standard low-fat breakfast of toast and jam. At the onset of each session, an intravenous catheter was inserted into a forearm vein of the participant’s arm to allow for repeated blood sampling.
After completing the baseline procedures, participants orally administered either 100 mg of CBD or a comparable placebo (see Study Drug section). Exactly 1 h after oral ingestion, participants used the Volcano Medic® vaporizer (Storz and Bickel, Tuttelingen, Germany) to inhale either 100 mg vaporized CBD, vaporized CBD-dominant cannabis containing 100 mg CBD and 3.7 mg THC, or placebo vapor. Study doses were heated at 204 °C (400 °F) and the resulting aerosol was captured in a balloon with a one-way valve for inhalation; participants inhaled the contents of 3 balloons in an ad libitum manner within 10 min. New balloons were used in each drug administration session and the device was thoroughly cleaned between sessions to avoid contamination from prior doses. Because we had previously observed differences in aerosol visibility between vaporized drugs (e.g., placebo and active cannabis), the balloons were covered with an opaque bag to help maintain the study blind. After drug administration, participants completed pharmacodynamic assessments for 8 h and biological specimens were obtained for 58 h; upon discharge, they were compensated for their time. Data for the first 8 h only are presented in this report because the pharmacokinetic results obtained after this time are beyond the scope of this paper and are described elsewhere (Spindle et al., 2019b).
2.3. Study drug
All study drugs were prepared and dispensed by the Johns Hopkins BPRU Pharmacy. CBD-dominant cannabis and placebo cannabis were acquired from the National Institute on Drug Abuse (NIDA) Drug Supply Program. The CBD-dominant cannabis contained 10.5 % CBD, 0.39 % THC, 0.02 % Δ8THC, and 0.05 % Cannabinol (CBN). The placebo cannabis contained 0.001 % THC, 0.003 % CBD, and 0.005 % CBN; Δ8THC was not detected. The same quantity of plant material (953 mg) was used in active and placebo sessions; this amount was selected to yield a CBD dose of 100 mg. The CBD-dominant cannabis used in this study was selected because it was representative of typical CBD-dominant cannabis products available for retail purchase in terms of the percent CBD (approximately 10 %) as well as the ratio of CBD:THC (approximately 25:1), (Hazekamp et al., 2016; Jikomes and Zoorob, 2018). For the vaporized pure CBD condition, participants inhaled synthetic crystalline CBD that was obtained from Albany Molecular Research Inc. (Rensselaer, NY, USA); this product was confirmed to not contain THC or other contaminants by independent testing. The Volcano Medic® was used to heat and aerosolize both the cannabis and CBD to enable user inhalation of study drugs.
All study participants orally ingested 100 mg CBD in 1 of their 4 experimental sessions. However, three different drug formulations were used for oral CBD dosing during the study (this was done to compare CBD pharmacokinetics across different formulations). The first 6 study completers ingested CBD in a size 0 gelcap filled with cellulose, the next 6 completers ingested 1 mL of Epidiolex (an oral CBD product produced by GW Pharmaceuticals, Greenwich, England) mixed with 9 mL pharmacy-grade cherry-flavored syrup, and the final 6 completers ingested CBD suspended in 10 mL pharmacy-grade cherry-flavored syrup. Oral placebo doses for the first 6 participants consisted of gelcaps filled with inert cellulose, and placebo oral doses for the final 12 participants was 10 mL cherry-flavored syrup. We selected 100 mg CBD as the target dose in this study to match the single unit dose of Epidiolex (i.e., 1 mL contains 100 mg CBD). For the purposes of data presentation and analyses in this manuscript, we collapsed all oral CBD dose data together because there were no effects of oral dose formulation on any pharmacodynamic outcomes.
2.4. Outcome measures
All study outcomes were measured at baseline and 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 8 h after oral dosing in each session. The 1 -h time point occurred immediately after the vapor dose administration period was completed.
2.4.1. Subjective measures
Subjective drug effects were assessed with the Drug Effect Questionnaire (DEQ) (Spindle et al., 2018; Vandrey et al., 2017). DEQ items were presented on a visual analog scale anchored from 0 (not at all) to 100 (extremely); participants recorded their responses by clicking the mouse cursor at any point on a 100 mm horizontal line. The 21 items from the DEQ can be found in Table 1.
Table 1.
Mean (SD) Peak Values for Pharmacodynamic Measures Across CBD Dosing Conditions.
| Placebo | 100 mg Oral CBD | 100 mg Vaporized CBD | CBD-dominant Cannabis (100mg CBD/3.7mg THC) | |
|---|---|---|---|---|
| Subjective Measures | ||||
| Drug Effect Questionnaire | ||||
| Drug Effect | 8.0 (17.8) | 4.7 (6.4) | 20.2 (22.2)+ | 52.7 (36.8)*# |
| Unpleasant | 2.9 (11.8) | 0.3 (1.2) | 4.7 (13.0) | 7.1 (12.7) |
| Pleasant | 9.2 (21.0) | 7.3 (10.7) | 31.4 (35.2)*+ | 48.4 (35.2)*# |
| Like Drug | 12.9 (29.2) | 17.0 (29.2) | 38.6 (38.1)*+ | 55.4 (34.8)* |
| Sick | 2.3 (9.5) | 0.4 (1.1) | 4.4 (12.5) | 7.4 (17.8) |
| Heart Racing | 2.8 (9.3) | 3.0 (8.4) | 4.4 (8.8) | 15.2 (23.6)*# |
| Anxious/Nervous | 3.2 (9.3) | 4.6 (11.7) | 5.3 (13.6) | 5.0 (6.9) |
| Relaxed | 58.8 (31.9) | 60.9 (30.0) | 69.1 (27.7) | 75.8 (22.8)* |
| Paranoid | 0.2 (0.4) | 1.2 (4.3) | 0.6 (1.5) | 0.8 (2.4) |
| Sleepy | 37.2 (29.1) | 36.4 (26.1) | 34.8 (32.0) | 50.9 (31.7)# |
| Alert | 47.7 (31.1) | 50.1 (35.3) | 52.7 (31.3) | 56.5 (31.0) |
| Irritable | 4.7 (13.7) | 1.6 (4.8) | 4.9 (17.7) | 5.4 (17.9) |
| Vigorous/ motivated | 29.8 (26.2) | 34.3 (31.3) | 35.3 (30.02 | 31.2 (30.5) |
| Restless | 6.9 (12.2) | 6.7 (13.2) | 6.8 (15.2) | 10.4 (18.1) |
| Hungry/Have Munchies | 30.8 (27.4) | 31.5 (27.3) | 45.8 (31.7)+ | 48.2 (33.5) |
| Craving | 1.2 (3.5) | 1.4 (5.2) | 5.6 (15.1) | 6.4 (19.0) |
| Dry Mouth | 4.1 (7.8) | 6.6 (14.6) | 16.1 (21.1)*+ | 35.9 (33.6)*# |
| Dry/red eyes | 2.2 (6.4) | 1.2 (2.8) | 2.9 (9.4) | 11.0 (20.1)* |
| Trouble with Memory | 1.0 (4.0) | 0.9 (2.9) | 1.9 (5.6) | 6.4 (18.1) |
| Throat Irritated | 2.7 (6.7) | 2.0 (4.2) | 27.0 (26.2)*+ | 59.9 (32.1)*# |
| Difficulty Performing Routine Tasks | 2.4 (5.8) | 1.1 (3.4) | 1.6 (4.7) | 7.8 (15.6)*# |
| Cognitive Measures | ||||
| Digit Symbol Substitution Task (DSST) | ||||
| Total Attempted | 53.6 (10.2) | 54.4 (20.2) | 53.7 (9.0) | 54.9 (6.3) |
| Total Correct | 52.6 (11.0) | 49.4 (15.4) | 52.1 (10.6) | 54.1 (6.3) |
| % Correct | 0.99 (0.02) | 0.94 (0.02) | 0.99 (0.02) | 0.99 (0.01) |
| Divided Attention Task | ||||
| Mean Distance from Stimulus (in pixels) | 27.3 (19.7) | 23.4 (16.8) | 29.7 (21.3) | 33.8 (31.7) |
| Mean average RT | 1575.6 (400.9) | 1366.0 (552.2) | 1442.1 (362.2) | 1472.0 (412.9) |
| Total Correct (peripheral numbers) | 23.6 (0.6) | 22.2 (5.5) | 23.3 (1.4) | 23.3 (1.0) |
| Paced Serial Addition Task (PASAT) | ||||
| Total Correct | 83.2 (12.4) | 79.9 (22.0) | 82.9 (10.6) | 86.3 (4.1) |
| Mean RT Correct | 1400.0 (190.2) | 1338.7 (372.7) | 1428.7 (186.1) | 1427.1 (168.2) |
| Mean RT Incorrect | 1410.0 (401.2) | 1356.8 (466.7) | 1402.7 (383.6) | 1536.8 (425.4) |
| Vital Signs | ||||
| Heart Rate | 70.0 (11.2) | 71.0 (11.2) | 68.7 (10.6) | 76.2 (10.2)*# |
| Systolic BP | 127.4 (8.7) | 125.8 (9.2) | 126.0 (11.5) | 128.8 (11.3) |
| Diastolic BP | 79.9 (10.0) | 78.9 (9.7) | 77.0 (9.5) | 81.6 (12.1)# |
Note: Asterisks (*) = significant difference from placebo condition; plus (+) = significant difference between oral and vaporized CBD conditions; pound sign (#) = significant differences between vaporized CBD and vaporized CBD-dominant cannabis (all ps < .05); RT = reaction time (in milliseconds); BP=blood pressure. Items for the DEQ were assessed using a visual analog scale with scores ranging from 0 (“not at all”) to 100 (“extremely”).
2.4.2. Cognitive/psychomotor tasks
A battery of three computerized cognitive and psychomotor performance tasks were administered, which have previously been shown to be sensitive to acute intoxication from oral and vaporized THC-dominant cannabis (Spindle et al., 2018; Vandrey et al., 2017). These tasks are also generalizable to functioning in the workplace and operating a motor vehicle. During the screening visit, participants were trained on all performance tasks until they reached a stable baseline in order to minimize practice effects during experimental sessions.
The Digit Symbol Substitution Task (DSST) is a measure of psychomotor ability and requires participants to replicate geometric patterns presented on the computer screen using the keyboard for 90 s (the main outcomes of the DSST are total attempted, total correct, and the percentage correct). In addition to being sensitive to acute cannabis intoxication (Spindle et al., 2018; Vandrey et al., 2017), the DSST has been shown to be a valid and reliable predictor of general cognitive impairment caused by brain injury and a variety of psychiatric conditions (Jaeger, 2018). The computerized Paced Serial Addition Task (PASAT), a measure of working memory, required participants to view a series of single digit integers in rapid succession. Participants add each presented number with the number that was most recently presented and then must select the sum of these two numbers from choices presented on their screen (the main outcomes of the PASAT are the total correct trials out of 90, and reaction time for correct and incorrect responses). The PASAT has also been shown to be a valid and reliable tool for detecting working memory deficits due to cannabis exposure (Spindle et al., 2018; Vandrey et al., 2017) or other reasons (e.g., aging, brain damage) and has high convergent validity with other working memory tasks and high test-retest reliability (Nikravesh et al., 2017). Finally, on the Divided Attention Task (DAT), participants track a central stimulus with a mouse cursor while also simultaneously monitoring and responding to peripheral stimuli that appear in the corners of the screen. The DAT primary outcomes include: the number of correct peripheral numbers identified, reaction time on correct responses to peripheral stimuli, and mean distance (in computer pixels) of their cursor from the central stimulus. The DAT is reliably sensitive to acute cannabis intoxication (Spindle et al., 2018; Vandrey et al., 2017) and other factors that cause impairment (e.g., alcohol intoxication, sleep deprivation), and performance on this measure is highly correlated with driving performance in controlled research (Jongen et al., 2015, 2014; Jongen et al., 2016).
2.4.3. Physiological measures
Blood pressure (BP), both systolic and diastolic (mmHg), and heart rate (HR; beats per minute, or bpm) were measured with an automated monitor while participants were in a seated position.
2.4.4. Blood specimens
Blood specimens were collected with 10 mL gray-top vacutainer tubes. Whole blood CBD and THC concentrations were determined using liquid chromatography–tandem mass spectrometry (LC–MS/MS); these analyses were performed by Immunalysis Corporation (Pomona, CA). The limit of quantitation (LOQ) was 0.5 ng/mL while the upper limit of linearity (ULOL) was 100 ng/mL.
2.5. Data presentation and analysis
Participant demographic characteristics are presented using descriptive statistics (i.e., means and standard deviations; SDs). Data for all pharmacodynamic outcomes were analyzed using repeated measures regressions (i.e., linear mixed models), each of which used a first order autoregressive (AR1) covariance structure. Separate regressions were conducted on each individual outcome and each model included three factors: dosing condition (4 levels), time (10 levels), and sex (2 levels). Planned contrasts were conducted to compare peak scores (i.e., the single highest value observed across all time points) across conditions for each outcome. Specifically, peak scores in all active dosing conditions were compared to placebo, 100 mg oral CBD was compared to 100 mg vaporized CBD, and 100 mg vaporized CBD was compared to CBD-dominant cannabis (100 mg CBD/3.7 mg THC). Peak scores for men and women were compared within each dosing condition as an exploratory analysis of potential sex differences. When calculating peak effects, values occurring > 3 h after drug administration were excluded, as data beyond this period would likely be influenced by participant fatigue, boredom, or other factors; prior controlled research has also consistently found peak effects from cannabinoids (when inhaled or orally ingested) occur within this timeframe (Newmeyer et al., 2017; Schwope et al., 2012; Spindle et al., 2018; Vandrey et al., 2017). Analyses were conducted in SPSS Version 25 using the MIXED procedure (SPSS, 2005) and figures were created using GraphPad Prism (Version 8). Note that the MIXED procedure assumes data is sampled from a normal distribution.
3. Results
Supplementary Table 1 depicts results from repeated measures regression statistical analyses (i.e., F and p values for main effects and interactions). Table 1 displays peak effects for each pharmacodynamic outcome and includes the results of the various planned contrasts. Figs. 1,2, and 3 show the time course of several subjective effects (Pleasant Drug Effect and Like Drug Effect), whole blood THC and CBD concentrations, and HR, respectively. Each figure displays these results in the full sample and also stratified by sex.
Fig. 1.
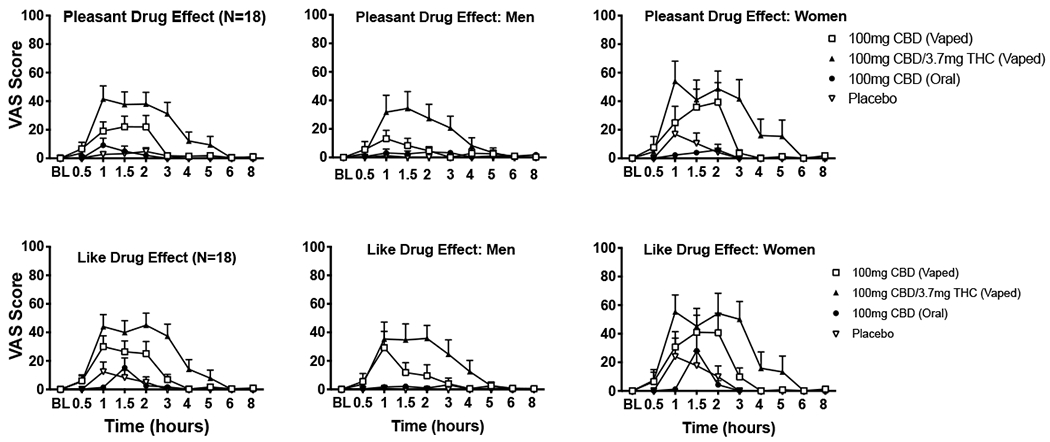
Mean ratings (+ SEM) for visual analog scale (VAS) items Pleasant Drug Effect (top panels) and Like Drug Effect (bottom panels) from the Drug Effect Questionnaire (DEQ) displayed over time for the four dosing conditions. These results are depicted for the entire sample (N = 18; far left panels), men only (N = 9; middle panels), and women only (N = 9; far right panels). Scores ranged from 0 (not at all) to 100 (extremely). Oral doses were administered at baseline (BL) time point, inhaled doses were administered at hour 1.
Fig. 2.
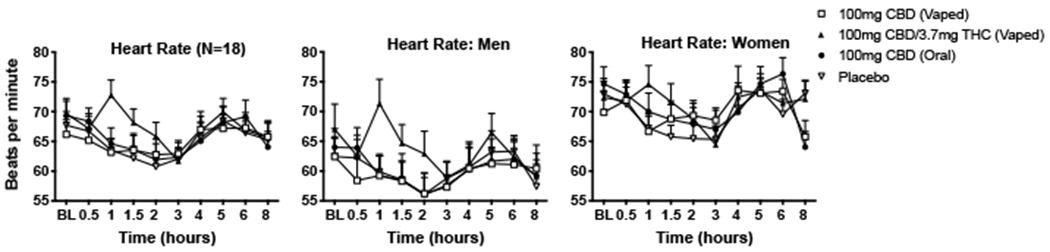
Mean (+ SEM) values for heart rate (in beats per minute; bpm) displayed over time for the four dosing conditions. These results are depicted for the entire sample (N = 18; far left panels), men only (N = 9; middle panels), and women only (N = 9; far right panels). Oral doses were administered at baseline (BL) time point, inhaled doses were administered at hour 1.
Fig. 3.
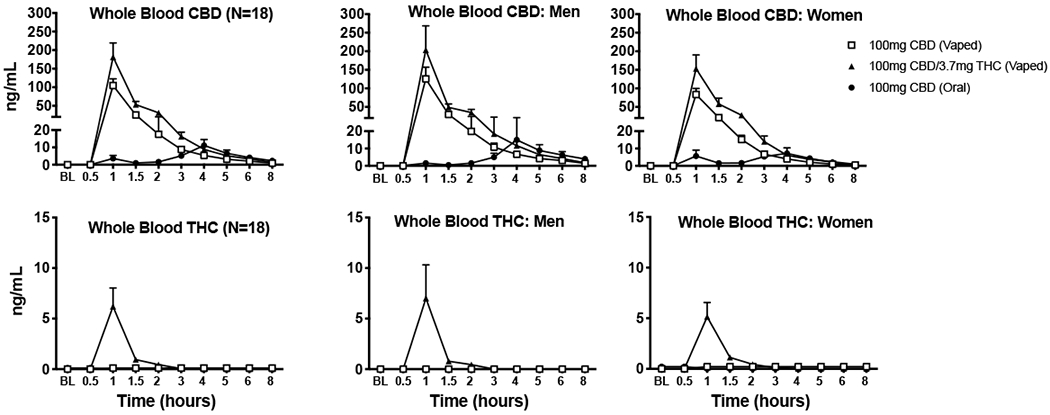
Mean (+ SEM) whole blood concentrations (in ng/mL) for CBD (top panels) and THC (bottom panels) displayed over time for the three active dosing conditions (i.e., 100 mg vaporized CBD; 100 mg oral CBD; CBD-dominant cannabis with 100 mg CBD and 3.7 mg THC). These results are depicted for the entire sample (N = 18; far left panels), men only (N = 9; middle panels), and women only (N = 9; far right panels). Oral doses were administered at baseline (BL) time point, inhaled doses were administered at hour 1.
3.1. Participants
Eighteen healthy adults (9 men and 9 women) completed the study. Seven other individuals provided informed consent but did not meet inclusion/exclusion criteria. Another individual was enrolled in the study but tested positive for cannabis (via urinalysis) before his first session and was removed from the study. On average, the 18 study completers had not used cannabis for 148 days (SD = 250 days) prior to their first experimental session. Of note, 69 % of participants reported never using CBD prior to the study (this information was not collected for two completers); the remaining participants reported between 1–3 lifetime uses of CBD. These individuals were 31 years old on average (SD = 6 years) and were mostly White/non-hispanic (67 %). Supplementary Table 2 details additional demographic characteristics of study completers.
3.2. Subjective drug effects
Main effects of condition were observed for Drug Effect, Pleasant Drug Effect, Like Drug Effect, Heart Racing, Dry Mouth, Throat Irritated, and Difficulty with Routine Tasks; condition by time interactions were also observed for each of these items with the exception of Difficulty with Routine Tasks. As shown in Table 1, planned contrasts revealed that vaporized CBD and CBD-dominant cannabis produced higher ratings of Pleasant Drug Effect, Like Drug Effect, Dry Mouth, and Throat Irritated compared to placebo (all ps < .05). Vaporized CBD-dominant cannabis also produced higher ratings for Drug Effect, Heart Racing, Relaxed, Dry/Red Eyes, and Difficulty with Routine Tasks (all ps < .05).
Additional planned comparisons revealed higher ratings for Drug Effect, Pleasant Drug Effect, Like Drug Effect, Hungry/Have Munchies, Dry Mouth, and Throat Irritated following administration of 100 mg vaporized CBD compared with 100 mg oral CBD (Table 1; all ps < .05). Lastly, subjective ratings were higher for CBD-dominant cannabis relative to vaporized CBD for Drug Effect, Pleasant Drug Effect, Heart Racing, Sleepy, Dry Mouth, Throat Irritated and Difficulty with Routine Tasks (all ps < .05).
Main effects of sex and/or sex by condition interactions were observed for several subjective items (see Supplementary Table 1). Exploratory planned comparisons revealed sex differences on peak ratings for several of these items. In the CBD-dominant cannabis condition, women reported significantly higher mean (SD) ratings than men for Pleasant Drug Effect (women: 61.2 [32.2]; men: 35.6 [35.0]), Dry Mouth (women: 55.1 [35.2]; men: 16.6 [18.2]), Dry/Red Eyes (women: 16.7 [27.1]; men: 5.3 [7.3]), Trouble with Memory (women: 12.6 [24.7]; men: 0.2 [0.7]), Throat Irritated (women: 82.8 [17.6]; men: 37.1 [26.5]), and Difficulty with Routine Tasks (women: 15.4 [19.7]; men: 0.2 [0.7]; all ps < .05). In the vaporized CBD condition, females also reported significantly higher mean (SD) ratings than men for Dry Mouth (women: 28.3 [23.3]; men: 3.7 [8.3]) and Throat Irritated (women: 42.7 [28.4]; men: 11.3 [10.0]; all ps < .05). In the vaporized CBD condition, women also reported qualitatively higher ratings than men for some items such as Pleasant Drug Effect (women: 42.9 [43.8]; men: 20.0 [20.6]) and Like Drug Effect (women: 46.1 [40.7]; men: 31.0 [36.2]); see Fig. 1. However, sex differences on these items did not reach statistical significance for vaporized CBD.
3.3. Cognitive performance
Overall, performance on the DSST, PASAT, and DAT was similar across dosing conditions, suggesting cognitive/psychomotor ability was not adversely impacted by the doses of CBD/THC administered in this study. Main effects of sex were observed for several cognitive task outcomes: overall, compared to women, men performed worse on cognitive tasks. However, these differences were ostensibly not driven by drug effects, as no sex by condition interactions were detected for any cognitive outcome.
3.4. Physiological outcomes
Main effects of time were observed for systolic BP, diastolic BP, and HR. There was also a time by condition interaction for HR. Planned contrasts demonstrated that the mean peak HR (i.e., beats-per-minute; bpm) observed after inhalation of CBD-dominant cannabis was higher than those observed in the placebo and vaporized CBD conditions (see Fig. 2). Main effects of sex were observed for systolic BP and HR (overall, compared to women, men had a lower HR, and had a higher systolic BP), but the lack of sex by condition interactions for these outcomes suggests these differences did not result from THC/CBD exposure.
3.5. Pharmacokinetics
Fig. 3 displays mean whole blood CBD and THC concentrations over time for each dosing condition (for the overall sample and for men and women only). In the CBD-dominant cannabis condition, mean (SD) peak concentration for THC in whole blood was 6.2 (7.8) ng/mL (men: 7.0 ng/mL [10.0] and women: 5.1 ng/mL [4.3]). Mean (SD) peak whole blood CBD concentrations were 181.4 (160.8) ng/mL in the vaporized CBD-dominant cannabis condition (men: 203.4 ng/mL [194.9] and women: 153.0 ng/mL [111.3]), 104.6 (76.5) ng/mL in the vaporized CBD condition (men: 125.4 ng/mL [95.2] and women: 83.7 ng/mL [48.8]), and 11.1 (14.7) ng/mL in the oral CBD condition (men: 15.0 ng/mL [18.30] and women: 7.2 ng/mL [9.5]).
3.6. Adverse events
No unanticipated or serious adverse events occurred in this study.
4. Discussion
As cannabis/hemp legalization has expanded in recent years, products with the cannabinoid CBD as the principal constituent have become increasingly popular. In particular, CBD-dominant products intended for either oral (e.g., tinctures) or pulmonary administration (e.g., vape pens), which often contain low concentrations of the psychoactive cannabis constituent THC, are now widely available. Despite their ubiquity in licit and illicit cannabis markets, however, controlled research to understand the acute effects of CBD products is severely limited. Such research is critical to elucidating the abuse liability and safety profile of different CBD products, determining the potential for CBD products to produce impairment in the workplace or while driving, informing dosing recommendations for use of cannabis/CBD for medical purposes, and evaluating whether these products can impact drug testing designed to detect illicit cannabis use. Ultimately, answering these types of questions with systematic research will be critical to informing public polices surrounding cannabis products.
In the present study, 100 mg of vaporized pure CBD produced discriminable subjective drug effects (e.g., increases on ratings of Pleasant Drug Effect, Like Drug Effect) that were greater than placebo and the same dose of oral CBD. In a previous study (Solowij et al., 2019), 400 mg vaporized pure CBD also increased subjective feelings of intoxication (i.e., ratings of Stoned), but 16 mg of vaporized pure CBD had no impact on subjective effects in another study (Hindocha et al., 2015). Thus, high doses of vaporized CBD appear to be capable of producing positive subjective drug effects. The observed differences in subjective effects between vaporized and oral CBD in this study may be the result of higher bioavailability of CBD when inhaled as opposed to orally ingested (as evidenced by higher blood CBD concentrations), or differences with respect to pharmacokinetic time course (inhalation results in absorption of a large bolus dose with immediate peak effect). Interestingly, the effects observed from vaporized CBD were dissimilar from those commonly observed following the use of THC-dominant products. For example, pure CBD did not increase ratings of Anxious/Nervous, Hungry/Have Munchies, or Trouble with Memory compared with placebo, and had no impact on cognitive performance or physiological outcomes (e.g., HR); THC administration reliably influences all of these outcomes, particularly in infrequent cannabis users (Arkell et al., 2019; Spindle et al., 2018; Vandrey et al., 2017). Participants in this study often reported that the drug effect they experienced was difficult to describe. For example, one participant described that they felt “different” after drug administration but that the specific effects were not familiar or easy to articulate. Thus, future research should attempt to further elucidate the pharmacodynamic effects of various CBD products, perhaps by interviewing experienced CBD users using qualitative methods, in order to inform the development of CBD-specific subjective effect questionnaires.
CBD-dominant cannabis produced significantly stronger subjective drug effects (e.g., Drug Effect, Sleepy, Dry Mouth, Heart Racing) and increased HR to a greater extent than oral and vaporized pure CBD. CBD-dominant cannabis likely had a stronger impact on pharmacodynamic outcomes because it contained a low concentration of THC (3.7 mg). Notably, the concentration of THC in the cannabis product used in this study is comparable to many CBD-dominant cannabis and hemp products that are widely available in the U.S. For example, hemp products can legally contain up to 0.3 % THC (Bridges and Hanson, 2017), which is only 0.09 % lower than the THC concentration of the cannabis used in this study. Overall, findings from the present study demonstrate that even very low amounts of THC can be physiologically active (e.g., acutely increase HR) and produce moderate to high subjective drug effects which is important considering that many commercial CBD products advertised as THC free contain low concentrations of THC (Bonn-Miller et al., 2017; Poklis et al., 2019). Consumers of CBD products should be aware that in the current unregulated market, they may unknowingly purchase a product with THC that is capable of producing stronger effects than CBD by itself.
Women reported stronger subjective drug effects on several items compared to men following inhalation of vaporized CBD and CBD-dominant cannabis. Interestingly, several of these effects were positive in nature (Pleasant Drug Effect) while others were negative in nature (Throat Irritation). In previous studies of THC-dominant cannabis and oral THC (Cooper and Haney, 2014; Fogel et al., 2017), women have also reported more pronounced subjective drug effects compared to men, but the present study is the first to suggest that women may also be more sensitive to some of the acute effects of inhaled CBD. Due to the limited power of detecting sex differences in this study, additional research, with larger sample sizes, is needed to replicate these findings, to explore possible mechanisms for this difference, and to understand whether heightened acute sensitivity to CBD in women influences use patterns of CBD products (e.g., frequency, product type or dose used).
This study had several limitations worth noting. First, this study only used one type of CBD-dominant cannabis, one vaporizer, one dose of THC and CBD, and only studied two routes of CBD administration (oral and pulmonary). Given the diversity of available CBD-dominant products and other possible routes of CBD administration (e.g., transdermal/topical, sublingual), much more research is needed in this area. Second, participants were infrequent cannabis users who were often using CBD for the first time during the study. Thus, it is unclear whether our findings are generalizable to experienced cannabis/CBD users. Third, this study was conducted in a laboratory setting which may have impacted subjective responses and other outcomes. Lastly, this study may have been underpowered to detect sex differences on some pharmacodynamic outcomes. That said, the sex differences that were observed highlight the need for further, and more exhaustive, exploration of sex differences, ideally at a wide range of CBD doses and using various routes of administration.
5. Conclusion
This study characterized the pharmacodynamic effects of CBD (both oral and vaporized), as well as CBD-dominant cannabis that contained a high CBD, but low THC concentration. Overall, CBD-dominant cannabis produced the strongest pharmacodynamic outcomes, increasing cardiovascular effects and ratings for several subjective items to a greater extent than placebo and pure CBD. However, vaporized pure CBD also produced significantly greater subjective drug effects compared with placebo and oral CBD, though these acute effects were unlike those observed in prior controlled studies of THC-dominant cannabis. Given the ongoing expansion of legal cannabis and the recent legalization of hemp in the U.S. (cannabis plants with ≤0.3 % THC), additional research is needed to determine the acute pharmacodynamics and pharmacokinetics of the numerous novel CBD-dominant products that are readily available to consumers.
Supplementary Material
Acknowledgments
Acknowledgments
We thank the support staff of the Johns Hopkins University Behavioral Pharmacology Research Unit for outstanding contributions to the implementation of this study. We also thank the many individuals involved with the NIDA Drug Supply Program and AMRI Global for providing study drugs.
Funding
This research was supported by the Substance Abuse and Mental Health Services Administration (SAMHSA) and the National Institute on Drug Abuse (NIDA; T32DA07209).
Footnotes
Declaration of Competing Interest
Dr. Vandrey has served as a consultant or received honoraria from Zynerba Pharmaceuticals, FSD Pharma, and Canopy Health Innovations Inc.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2020.107937.
References
- Arkell TR, Lintzeris N, Kevin RC, Ramaekers JG, Vandrey R, Irwin G, Haber PS, McGregor IS, 2019. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology (Berl.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt DL, de Wit H, 2017. Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis Cannabinoid Res. 2 (1), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, Walsh SL, 2017. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depen 172, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR, 2015. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12 (4), 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R, 2017. Labeling accuracy of cannabidiol extracts sold online. JAMA 318 (17), 1708–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Pollack CV Jr., Casarett D, Dart R, ElSohly M, Good L, Guzman M, Hanus L, Hill KP, Huestis MA, Marsh E, Sisley S, Skinner N, Spahr J, Vandrey R, Viscusi E, Ware MA, Abrams D, 2019. Priority considerations for medicinal cannabis-related research. Cannabis Cannabinoid Res. 4 (3), 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges M, Hanson K, 2017. Regulating hemp and cannabis-based products. NCSL Legisbrief 25 (37), 1–2. [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2014. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 136, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, Kight R, 2018. Regulatory status of cannabidiol in the United States: a perspective. Cannabis Cannabinoid Res. 3 (1), 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, Phillips JA, 2018. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 3 (1), 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Wright S, 2017. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N. Engl. J. Med 377 (7), 699–700. [DOI] [PubMed] [Google Scholar]
- FDA, 2020. FDA Regulation of Cannabis and Cannabis-Derived Products. Including Cannabidiol (CBD). https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd.
- Fogel JS, Kelly TH, Westgate PM, Lile JA, 2017. Sex differences in the subjective effects of oral Delta(9)-THC in cannabis users. Pharmacol. Biochem. Behav 152, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, Walsh SL, 2016. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked Cannabis. Neuropsycho pharmacology 41 (8), 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazekamp A, Tejkalová K, Papadimitriou S, 2016. Cannabis: from cultivar to chemovar II—a metabolomics approach to Cannabis classification. Cannabis Cannabinoid Res. 1 (1), 202–215. [Google Scholar]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV, 2015. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur. Neuropsychopharmacol 25 (3), 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, 2018. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol 38 (5), 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jikomes N, Zoorob M, 2018. The cannabinoid content of legal Cannabis in Washington state varies systematically across testing facilities and popular consumer products. Sci. Rep 8 (1), 4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen S, Vuurman E, Ramaekers J, Vermeeren A, 2014. Alcohol calibration of tests measuring skills related to car driving. Psychopharmacology 231 (12), 2435–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen S, Perrier J, Vuurman EF, Ramaekers JG, Vermeeren A, 2015. Sensitivity and validity of psychometric tests for assessing driving impairment: effects of sleep deprivation. PLoS One 10 (2), e0117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen S, Vuurman E, Ramaekers J, Vermeeren A, 2016. The sensitivity of laboratory tests assessing driving related skills to dose-related impairment of alcohol: a literature review. Accid. Anal. Prev 89, 31–48. [DOI] [PubMed] [Google Scholar]
- Maroon J, Bost J, 2018. Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int 9, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farre M, Zuardi AW, McGuire PK, 2012. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des 18 (32), 4966–4979. [DOI] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Abulseoud OA, Huestis MA, 2017. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. 175, 67–76. [DOI] [PubMed] [Google Scholar]
- Nikravesh M, Jafari Z, Mehrpour M, Kazemi R, Amiri Shavaki Y, Hossienifar S, Azizi MP, 2017. The paced auditory serial addition test for working memory assessment: psychometric properties. Med. J. Islam. Repub. Iran 31, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Mulder HA, Peace MR, 2019. The unexpected identification of the cannabimimetic, 5F-ADB, and dextromethorphan in commercially available cannabidiol e-liquids. Forensic Sci. Int. 294, 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg S, Golubchik P, Offen D, Weizman A, 2019. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 89, 90–96. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, Etges T, Sommerville K, 2018. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: A randomized, double-blind, controlled trial. Epilepsy Behav. 88, 162–171. [DOI] [PubMed] [Google Scholar]
- Schwope DM, Bosker WM, Ramaekers JG, Gorelick DA, Huestis MA, 2012. Psychomotor performance, subjective and physiological effects and whole blood Delta(9)-tetrahydrocannabinol concentrations in heavy, chronic cannabis smokers following acute smoked cannabis. J. Anal. Toxicol 36 (6), 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back: a technique for asses- sing self-reported alcohol consumption In: Allen JP, Litten RZ (Eds.), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Human Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- Solowij N, Broyd S, Greenwood LM, van Hell H, Martelozzo D, Rueb K, Todd J, Liu Z, Galettis P, Martin J, Murray R, Jones A, Michie PT, Croft R, 2019. A randomised controlled trial of vaporised Delta(9)-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. Eur. Arch. Psychiatry Clin. Neurosci. [DOI] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R, 2018. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Netw Open 1 (7), e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Bonn-Miller MO, Vandrey R, 2019a. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr. Opin. Psychol 30, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Kuntz D, Mitchell JM, Bigelow GE, Flegel R, Vandrey R, 2019b. Urinary pharmacokinetic profile of cannabinoids following administration of vaporized and oral cannabidiol and vaporized CBD-dominant cannabis. J. Anal. Toxicol [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS I, 2005. Linear Mixed-effects Modeling in SPSS: An Introduction to the MIXED Procedure.
- Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, Cone EJ, 2017. Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J. Anal. Toxicol 41 (2), 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh Z, Gonzalez R, Crosby K, M ST, Carroll C, Bonn-Miller MO, 2017. Medical cannabis and mental health: a guided systematic review. Clin. Psychol. Rev 51, 15–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


