Abstract
Patient: Male, 72-year-old
Final Diagnosis: Infective endocarditis
Symptoms: Falls • weakness
Medication: —
Clinical Procedure: Removal of pacemaker
Specialty: Cardiology
Objective:
Rare disease
Background:
Following transvenous lead extraction (TLE) for infective endocarditis, a fibrinous remnant, or “ghost”, that previously encapsulated the lead may remain. The main aim of this case report was to highlight the importance of identification of ghosts, their negative implications, and the importance of close monitoring.
Case Report:
A 72-year-old male with a history of heart failure with non-ischemic cardiomyopathy and remote cardiac resynchronization therapy defibrillator (CRT-D) placement as well as atrioventricular node ablation for atrial fibrillation presented following a mechanical fall. An initial evaluation revealed methicillin-resistant Staphylococcus aureus bacteremia; the suspected nidus was an indwelling chemotherapy port for non-Hodgkin’s lymphoma. Echocardiography demonstrated vegetations on the aortic and mitral valves, and the right atrial device lead concerning for infective endocarditis. After TLE, a temporary transvenous wire was placed. Definitive pacing was then achieved by a Micra leadless pacemaker (LP). We opted with LP technology via the Micra device with plan for subcutaneous implantable cardioverter defibrillator (SICD) implantation to mitigate the risk of infection recurrence. After completion of 6 weeks of antibiotics, a pre-SICD transesophageal echocardiogram identified a 1.3 cm mobile echo-dense “ghost” in the right atrium. SICD was implanted as planned. Following expert consensus, no specific therapy was implemented when the ghost was identified. At 3 months, echocardiography revealed the absence of the ghost. At 1-year follow-up, no infection recurrence was noted.
Conclusions:
The presence of ghosts after transvenous lead extraction is associated with poor outcome and infection recurrence thus requiring diligent monitoring and serial echocardiography as optimal management is yet to be defined.
MeSH Keywords: Defibrillators, Implantable; Endocarditis, Bacterial; Pacemaker, Artificial
Background
Infective endocarditis is associated with substantial morbidity and mortality and is a class 1 indication for removal of all intra-cardiac hardware [1]. Following a transvenous lead extraction (TLE), there may be a persistent remnant or sheath that encapsulated the previous lead referred to as a “ghost”. A ghost is defined as a mobile mass left behind after TLE that often follows the lead’s intracardiac route into the right cardiac cavities [2]. While initially considered to represent fibrous sheaths, they may also be infectious vegetations and such distinction in clinical practice poses challenges. To this end, positron emission tomography/computed tomography (PET-CT) and single photon emission computed tomography with conventional tomography (SPECT-CT) with radioisotope-labeled leukocytes have been shown to reliably confirm or exclude infection in lead-dependent infective endocarditis and thus could be extrapolated to evaluate ghosts [2,3]. Ghosts are observed in 8% to 14% of patients that undergo TLE and remain a novel entity lacking any specific guidelines for detection or management [4]. We aim to alert the clinician about this novel complication and its important clinical significance as it portends poor outcomes.
Case Report
A 72-year-old Caucasian male presented from his nursing home to another facility following a mechanical fall. Patient had 2 recent Emergency Department evaluations for generalized weakness and cough without an identified etiology. On arrival, he was hemodynamically stable (blood pressure 105/70 mmHg) and exhibited perianal and genital excoriations with associated suprapubic swelling and generalized weakness on physical examination. The patient’s medical history included chronic non-ischemic heart failure with reduced ejection fraction (NYHA class III, AHA class C), ventricular tachycardia, remote cardiac resynchronization therapy defibrillator (CRT-D), atrioventricular node ablation for drug-refractory atrial fibrillation on Xarelto, chronic kidney disease (stage IIIa), and remote embolic stroke. His left ventricular ejection fraction (LVEF) was reported to improve after CRT-D implantation to 55% to 60% but was reduced to 25% 1-year prior to presentation. The patient also had non-Hodgkin’s lymphoma in remission and was previously treated via a right sided tunneled catheter that placed 4 years ago. An initial evaluation of non-specific generalized weakness revealed methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. Transesophageal echocardiography demonstrated multiple vegetations on the aortic and mitral valves, and the right atrial device lead. After he was diagnosed with definite infective endocarditis per the modified Duke criteria (2 major criteria), he was transferred to our facility for TLE.
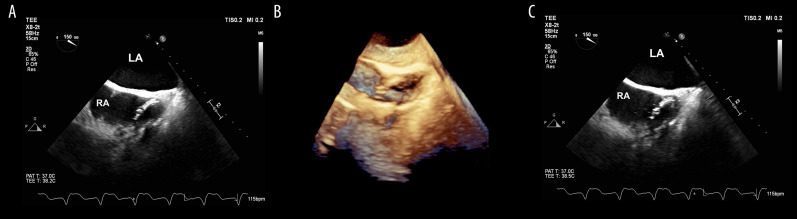
He initially completed removal of his indwelling right subclavian port, the suspected infectious nidus, and subsequently completed a full cardiac device system extraction. The patient was dependent upon pacing following his atrioventricular node ablation and was bridged with a temporary transvenous wire until blood cultures demonstrated no residual bacteremia. A Micra LP (Medtronic Inc., Minneapolis, MN, USA) was then implanted into the apical septal region of the right ventricle (QRS duration 200 ms) and the temporary wire was removed (Figure 1). A peripherally inserted central catheter was placed for long-term intravenous antibiotics (vancomycin 1.5 g intravenous daily for 6 weeks) and he was subsequently discharged. Seven weeks later, he underwent a planned subcutaneous implantable cardioverter defibrillator (SICD) implantation (Defib Sub-Q A219 Emblem MRI Guidant CR). Prior to SICD implantation, a transesophageal echocardiogram was performed to re-evaluate the vegetations and a 1.3 cm tubular and mobile echo-dense ghost was seen in the right atrium (Figure 2, Video 1). He was subsequently discharged without any change in management or further intervention. Three months after SICD placement, the patient presented with inappropriate shock due to lead displacement and underwent lead revision. During that admission, a transthoracic echocardiogram did not show any remnant of the ghost or valvular vegetations. One year after ghost identification, the patient has had no recurrence of infection.
Figure 1.
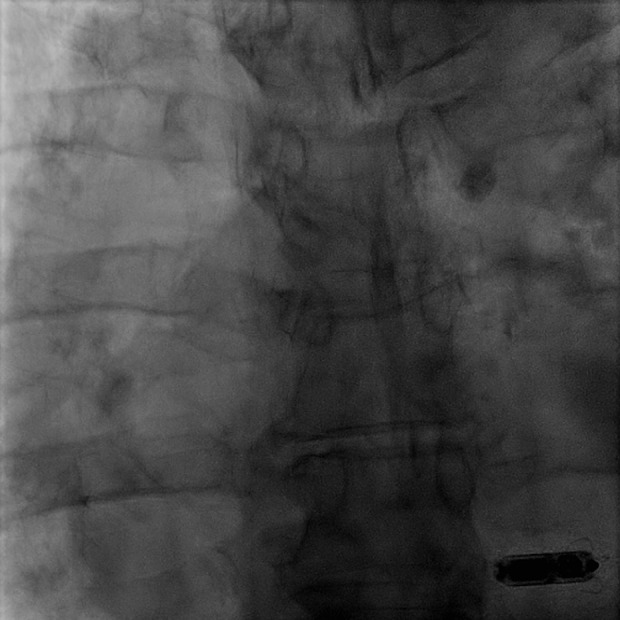
Leadless pacemaker. Micra leadless pacemaker (Medtronic Inc., Minneapolis, MN, USA) positioned in the right ventricular apex.
Figure 2.
Transesophageal echocardiogram reveals ghost. (A, C) Transesophageal echocardiography shows a 1.3 cm tubular mobile echo-dense remnant in the right atrium. (B) 3-dimensional transesophageal echocardiography image of the right atrium with 2-dimensional biplane revealing the “ghost.” RA – right atrium; LA – left atrium.
Discussion
First reported in 2008 by Rizello et al., a ghost remains a novel entity posing challenging clinical questions. Ghost pathogenesis stems from our understanding of the intrinsic encapsulation process that cardiac leads undergo. The 2 main sequential mechanisms are thrombosis with subsequent collagenous fibrosis [5] and endothelialization of the fibrous capsule surrounding the lead [6]. Such encapsulation prevents lead migration and subsequent thrombus formation. When indicated for lead malfunction, wire fractures, insulation damage, and lead migration, TLE rarely results in residual ghost. However, ghosts are readily identified after TLE when indicated for infection [4,7]. Infected leads are found to be more easily extracted than noninfected leads [8]; interestingly, the infectious process is suspected to break the seal between collagenous adhesions, endocardial surfaces, and the lead itself. Histologically, ghosts consist of infected fibrous sheaths mixed with vegetation [7]. Thus, infection has emerged as a major driver of ghost pathogenesis, yet the presence of ghosts is not a criterion for the diagnosis of infective endocarditis. Further studies are warranted to evaluate the need for interval monitoring with blood cultures and/or echocardiography after encountering a ghost. Insight into ghost pathogenesis has made significant strides while questions remain regarding therapeutic and prognostic implications.
In our case, the ghost was found after a 6-week course of antibiotic therapy for infective endocarditis. It remains unclear if that implies active infectivity obliging the prolongation of the antibiotic course. According to expert consensus, “no specific therapy is indicated for patients with [ghosts]” [9]. However, emerging evidence suggests that ghosts are harbingers of poor outcomes. Narducci et al., in an 11-month-long prospective study, reported that patients with ghosts have a 3.4-fold higher all-cause mortality compared to patients without ghosts [4]. It was postulated that the independent association of ghosts with death reflected the fact that they represent a marker of a constellation of high-risk features (older age, endocarditis, co-morbidities) [4]. A prospective evaluation by Diemberger et al. demonstrated that ghosts are not only associated with death but are also associated with cardiac device-related infective endocarditis (CDRIE) relapse/recurrence (hazard ratio [HR] 4.594; P=0.046) [10]. Nonetheless, the presence of ghosts appears to identify a sicker population who may be at higher risk of death; for such patients, careful monitoring, closer follow-up, and prompt tailored therapies are warranted to prevent worsening or complications.
Traditionally, transesophageal echocardiography has been the cornerstone in the diagnosis of infective endocarditis. Intracardiac echocardiography is a relatively recent addition and has been shown to increase diagnostic accuracy when transesophageal echocardiography is inconclusive for infective endocarditis. A prospective study evaluating patients with suspected cardiac device infection found that intracardiac echocardiography performed better than transesophageal echocardiography in identifying intracardiac vegetations, specifically identifying vegetations not seen by transesophageal echocardiography in 15% of patients with possible infective endocarditis [11]. With its superior ability to evaluate right-sided cardiac structures, intracardiac echocardiography is already proven useful in ghost detection intra-procedurally during TLE and may play an important role in constructing guidelines for ghost management [4]. Whilst specific diagnostic guidelines remain unknown, the utility of advanced imaging such as PET-C and SPET-C shows promise in patients with CDRIE. PET-C, and more so, SPET-C have shown high sensitivities (65% and 73.7%, respectively) and specificities (88% and 81%, respectively) for the diagnosis CDRIE [2,3]. This can be extrapolated to the diagnostic workup of ghosts when determining whether infection is present.
Current therapeutics dictate that, with infective endocarditis and pacemaker dependence, the use of temporary transvenous pacing, leadless pacing, or epicardial pacing are required. In our case, we elected to place an LP device for permanent pacing (Micra; Medtronic Inc., Minneapolis, MN, USA). This puts in question the safety and feasibility of placing an LP in the setting of infective endocarditis and/or a potentially infective residual ghost. In one study following post-CDRIE LP implantation, no recurrence of device infections was reported during a mean follow-up of 16 months with a maximum of 45 months [12]. Early LP implantation thus looks to be a viable non-surgical therapy independent of ongoing infection. Another feasible solution is epicardial pacemaker implantation utilizing a minimally invasive subxiphoid approach [13]. With its single-procedure approach, epicardial pacemaker implantation has been shown to be associated with shorter hospital stay without an increased risk of late infection or difference in mortality or morbidity in comparison to transvenous pacing [14]. Prospective randomized data on LP therapy and epicardial pacemakers in CDRIE and uniquely, as in this case, the subset of patients with ghosts are required to further validate these approaches.
Conclusions
While single-center prospective studies eluded to ghosts’ association with poor outcomes, large prospective multi-center studies are warranted to establish prognosis and the best management options when encountering a ghost. Pertinent questions yet to be answered concerning predisposing risk factors (diabetes, rheumatologic diseases, or autoimmune disorders) for ghost development, the role of serial imaging, and the role appropriate duration of antibiotic therapy. Further description of the role of ghosts toward clinical outcomes may avert their residual risk as a large body of evidence confirms that ghosts are to be feared rather than ignored.
Video 1.
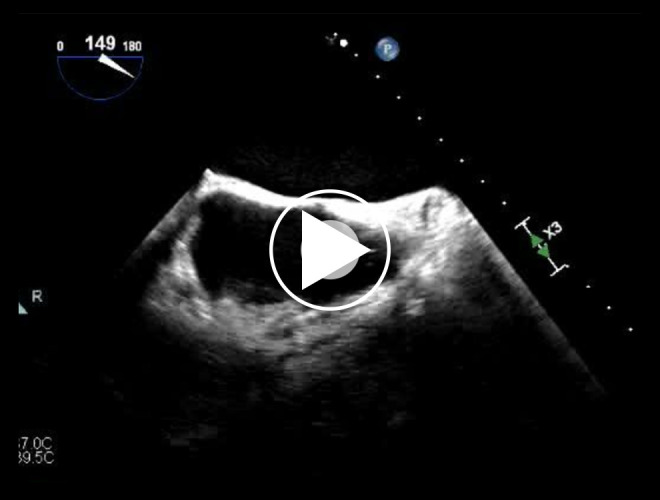
Transesophageal echocardiogram reveals the “ghost” as a mobile echo-dense mass in the right atrium.
Acknowledgments
Special thanks to Sreedhar Billakanty MD, Nagesh Chopra MD, Eugene Fu MD, Steve Nelson MD, Allan Nicholas MD, Greg Kidwell MD, James Kleman MD, Dennis A. Calnon MD, Karanvir S. Grewal MD, Todd G. Matros MD, Jay T. Mukherjee MD, David R. Richards DO, and Akira Wada MD.
Footnotes
Conflicts of interest
None.
References:
- 1.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: A scientific statement from the American Heart Association. Circulation. 2010;121(3):458–77. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 2.Małecka BA, Ząbek A, Dębski M, et al. The usefulness of SPECT-CT with radio-isotope-labeled leukocytes in diagnosing lead-dependent infective endocarditis. Adv Clin Exp Med. 2019;28(1):113–19. doi: 10.17219/acem/92315. [DOI] [PubMed] [Google Scholar]
- 3.Juneau D, Golfam M, Hazra S, et al. Positron emission tomography and single-photon emission computed tomography imaging in the diagnosis of cardiac implantable electronic device infection: A systematic review and meta-analysis. Circ Cardiovasc Imaging. 2017;10(4):e005772. doi: 10.1161/CIRCIMAGING.116.005772. [DOI] [PubMed] [Google Scholar]
- 4.Narducci ML, Di Monaco A, Pelargonio G, et al. Presence of ‘ghosts’ and mortality after transvenous lead extraction. Europace. 2017;19(3):432–40. doi: 10.1093/europace/euw045. [DOI] [PubMed] [Google Scholar]
- 5.Stokes K, Anderson J, McVenes R, McClay C. The encapsulation of polyurethane-insulated transvenous cardiac pacemaker leads. Cardiovasc Pathol. 1995;4(3):163–71. doi: 10.1016/1054-8807(95)00023-x. [DOI] [PubMed] [Google Scholar]
- 6.Esposito M, Kennergren C, Holmstrom N, et al. Morphologic and immunohistochemical observations of tissues surrounding retrieved transvenous pacemaker leads. J Biomed Mater Res. 2002;63(5):548–58. doi: 10.1002/jbm.10306. [DOI] [PubMed] [Google Scholar]
- 7.Le Dolley Y, Thuny F, Mancini J, et al. Diagnosis of cardiac device-related infective endocarditis after device removal. JACC Cardiovasc Imaging. 2010;3(7):673–81. doi: 10.1016/j.jcmg.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Byrd CL, Wilkoff BL, Love CJ, et al. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994–1996. U.S. Extraction Database, MED Institute. Pacing Clin Electrophysiol. 1999;22(9):1348–57. doi: 10.1111/j.1540-8159.1999.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 9.Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503–e51. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Diemberger I, Biffi M, Lorenzetti S, et al. Predictors of long-term survival free from relapses after extraction of infected CIED. Europace. 2018;20(6):1018–27. doi: 10.1093/europace/eux121. [DOI] [PubMed] [Google Scholar]
- 11.Narducci ML, Pelargonio G, Russo E, et al. Usefulness of intracardiac echo-cardiography for the diagnosis of cardiovascular implantable electronic device-related endocarditis. J Am Coll Cardiol. 2013;61(13):1398–405. doi: 10.1016/j.jacc.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Beurskens NEG, Tjong FVY, Dasselaar KJ, et al. Leadless pacemaker implantation after explantation of infected conventional pacemaker systems: A viable solution? Heart Rhythm. 2019;16(1):66–71. doi: 10.1016/j.hrthm.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Kempa M, Laskawski G, Budrejko S, et al. Implantation of a dual-chamber pacemaker with epicardial leads in adults using a minimally invasive subxyphoid approach. Pacing Clin Electrophysiol. 2019;42(5):537–41. doi: 10.1111/pace.13651. [DOI] [PubMed] [Google Scholar]
- 14.Amraoui S, Sohal M, Li A, et al. Comparison of delayed transvenous reim-plantation and immediate surgical epicardial approach in pacing-dependent patients undergoing extraction of infected permanent pacemakers. Heart Rhythm. 2015;12(6):1209–15. doi: 10.1016/j.hrthm.2015.02.023. [DOI] [PubMed] [Google Scholar]