Abstract
Cataract is the primary cause of visual impairment and can be corrected by cataract surgery. We investigated the impact of cataract on the risk of depression along with the benefits of cataract surgery. Patients newly diagnosed with cataract by ophthalmologists between 2001 and 2015 were identified from the National Health Insurance Research Database (NHIRD) in Taiwan. Non-cataract individuals were recruited by 1:1 matching for age, sex and index year. After propensity score matching, 233,258 patients in total were included in our study: 116,629 in each of the cataract and non-cataract cohorts. The primary outcome was the new diagnosis of depression by psychiatrists. In a mean follow-up period of 7.8 years, cataract was significantly associated with increased risk of developing depression (adjusted hazard ratio [aHR] = 1.78, 95% confidence interval [CI] 1.70–1.87, p < 0.001). We further divided the cataract cohort into surgery and non-surgery groups. Notably, cataract surgery group was associated with a decreased risk of depression compared with non-surgery patients (aHR = 0.75, 95% CI 0.71–0.79, p < 0.001). Our results emphasise the importance of regular screening for depression among cataract patients and the beneficial effect of cataract surgery in reducing the risk of depression.
Subject terms: Risk factors, Depression, Lens diseases, Vision disorders
Introduction
According to the Global Burden of Disease Study, depressive disorders are the third non-fatal leading contributor to the global disease burden in 20171. Depression is associated with functional impairment, risk of dementia, and increased mortality2. With insufficient knowledge and stigmatizing attitudes toward depression among Taiwanese people3, the situation regarding undiagnosed cases may be worse than that in other countries. In addition, approximately 50% of patients with depression drop out of the treatment before receiving the antidepressant therapy of 6–9 months that is recommended in Taiwan4, which will increase the risk of recurrence. Identifying risk factors that can be addressed may help in controlling the burden of depression, both in Taiwan and globally.
In clinical practice, depressive symptoms are common among patients with eye disease, with a pooled prevalence of 25% (range 5.4–57%)5,6. However, depression is often left unrecognised or untreated in the ophthalmological clinic, which can negatively affect therapy outcomes and quality of life5,7. Previous studies have suggested the correlation between depression and visual impairment8,9. A recent nationwide cohort study indicated a longitudinal association between visual impairment and depressive symptoms10. Although the leading cause of visual impairment and blindness in the elderly is cataract11, studies comparing the risk of depression between cataract patients and healthy individuals are still limited and are either mixed anxiety and depression together12, or have selection bias13.
Visual impairment caused by cataract is curable with cataract surgery, one of the most common operative procedures performed worldwide because of its high efficacy and minimal complications14. However, the impact of cataract surgery on depressive symptoms remains controversial. Some studies have demonstrated the beneficial effects of cataract surgery on depression15–20, whereas others have suggested no remarkable impact13,21,22.
Depression in cataract patients is an important issue that lacks sufficient evidence. A majority of previous studies are restricted on account of having a cross-sectional design12,22, follow-up period less than 3 months15,18–21, sample size less than 10013,15,18,21,22, and single-institutional bias15,18–20,22. In the present study, we performed a nationwide population-based cohort study to examine (1) the long-term association between cataracts and the risk of developing depression and (2) the impact of cataract surgery on the risk of depression.
Results
Patient characteristics
Initially, 280,970 participants were included in our study, with 140,485 participants in each of the cataract and non-cataract cohorts following 1:1 matching based on age (in 5-year increments), sex and index year. The overall mean follow-up time was 7.8 years, and the overall mean age was 62.6 years (range 20–101 years). The number of comorbidities was higher in the cataract cohort after age/sex/index year matching. Following 1:1 propensity score matching, there were 116,629 participants in each of the cataract and non-cataract cohorts. All baseline characteristics were well balanced following propensity score matching, with all standardised differences less than 0.1 (Table 1).
Table 1.
Baseline characteristics of patients with and without cataract.
| Age/sex/index year matching | Propensity score matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cataract (n = 140,485) | No cataract (n = 140,485) | SMD | Cataract (n = 116,629) | No cataract (n = 116,629) | SMD | |||||
| n | % | n | % | n | % | n | % | |||
| Age (years) | 62.7 ± 10.1 | 62.5 ± 10.3 | 0.020 | 62.9 ± 10.4 | 62.6 ± 10.5 | 0.033 | ||||
| < 65 | 82,315 | 58.6 | 83,441 | 59.4 | 0.016 | 66,908 | 57.4 | 68,456 | 58.7 | 0.027 |
| ≥ 65 | 58,170 | 41.4 | 57,044 | 40.6 | 0.016 | 49,721 | 42.6 | 48,173 | 41.3 | 0.027 |
| Sex | ||||||||||
| Male | 70,556 | 50.2 | 70,556 | 50.2 | 0.000 | 57,668 | 49.5 | 57,236 | 49.1 | 0.007 |
| Female | 69,929 | 49.8 | 69,929 | 49.8 | 0.000 | 58,961 | 50.6 | 59,393 | 50.9 | 0.007 |
| Income (NTD) | ||||||||||
| Dependent | 31,673 | 22.6 | 32,983 | 23.5 | 0.022 | 27,205 | 23.3 | 24,075 | 20.6 | 0.065 |
| 15,840–29,999 | 70,049 | 49.9 | 71,094 | 50.6 | 0.015 | 56,864 | 48.8 | 57,569 | 49.4 | 0.012 |
| 30,000–44,999 | 23,851 | 17.0 | 22,571 | 16.1 | 0.025 | 19,465 | 16.7 | 21,248 | 18.2 | 0.040 |
| 45,000 or more | 14,912 | 10.6 | 13,837 | 9.9 | 0.025 | 13,095 | 11.2 | 13,737 | 11.8 | 0.017 |
| Comorbidities | ||||||||||
| CCI | 1.5 ± 1.9 | 1.0 ± 1.8 | 0.291 | 1.3 ± 1.8 | 1.2 ± 1.9 | 0.055 | ||||
| HTN | 56,151 | 40.0 | 38,951 | 27.7 | 0.261 | 40,589 | 34.8 | 38,680 | 33.2 | 0.035 |
| DM | 35,082 | 25.0 | 14,925 | 10.6 | 0.382 | 15,428 | 13.2 | 14,925 | 12.8 | 0.013 |
| CVA | 10,240 | 7.3 | 9,150 | 6.5 | 0.031 | 8,705 | 7.5 | 8,572 | 7.4 | 0.004 |
| Heart failure | 3,749 | 2.7 | 3,043 | 2.2 | 0.033 | 3,091 | 2.7 | 2,870 | 2.5 | 0.012 |
| CAD | 16,869 | 12.0 | 10,296 | 7.3 | 0.159 | 12,460 | 10.7 | 10,267 | 8.8 | 0.063 |
| Asthma | 5,366 | 3.8 | 3,692 | 2.6 | 0.067 | 4,358 | 3.7 | 3,672 | 3.2 | 0.032 |
| COPD | 10,111 | 7.2 | 7,555 | 5.4 | 0.075 | 8,553 | 7.3 | 7,443 | 6.4 | 0.038 |
| CKD | 3,743 | 2.7 | 2,142 | 1.5 | 0.080 | 2,603 | 2.2 | 2,132 | 1.8 | 0.028 |
| Cirrhosis | 1,716 | 1.2 | 1,517 | 1.1 | 0.013 | 1,457 | 1.3 | 1,406 | 1.2 | 0.004 |
| Arthritis | 19,076 | 13.6 | 11,391 | 8.1 | 0.177 | 13,076 | 11.2 | 11,390 | 9.8 | 0.047 |
| Malignancy | 6,803 | 4.8 | 6,641 | 4.7 | 0.005 | 6,057 | 5.2 | 6,232 | 5.3 | 0.007 |
Continuous data are expressed as mean ± standard deviation and categorical data are expressed as number and percentage.
CAD coronary artery disease, CCI Charlson Comorbidity Index, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, NTD New Taiwan Dollar, SMD standardised mean difference.
Within the cataract cohort, 60,454 patients who underwent cataract surgery were classified as the surgery group, and 80,031 patients who did not undergo cataract surgery were classified as the non-surgery group. The median time from first cataract diagnosis to surgery was 398 days. Following 1:1 propensity score matching, there were 58,699 participants in each of the surgery and non-surgery groups, with a balanced distribution of baseline characteristics (Supplementary Table S1).
Risk of depression in the cataract and non-cataract cohorts
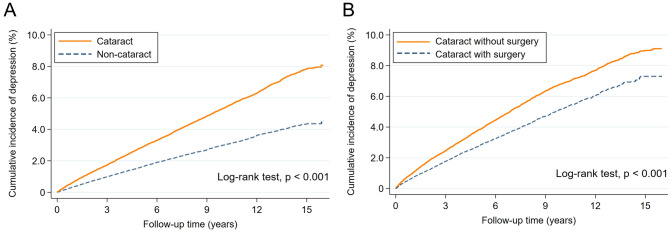
During the mean follow-up period of 7.8 years, Kaplan–Meier analysis revealed that the cumulative incidence of developing depression was consistently higher in the cataract cohort compared with the non-cataract cohort during the entire follow-up period (age/sex/index year matching: 5.43 vs. 2.84 per 1,000 person-years; propensity score matching: 5.37 vs. 3.03; log-rank test, p < 0.001) (Fig. 1A). Diagnosis of cataract was associated with a significantly higher risk of developing depression in univariable (age/sex/index year matching: crude hazard ratio [HR] = 1.92, 95% CI 1.84–2.01, p < 0.001; propensity score matching: crude HR = 1.78, 95% CI 1.70–1.87, p < 0.001) and multivariable (age/sex/index year matching: adjusted HR [aHR] = 1.81, 95% CI 1.73–1.89, p < 0.001; propensity score matching: aHR = 1.72, 95% CI 1.64–1.80, p < 0.001) Cox proportional hazard regression models (Table 2).
Figure 1.
Cumulative incidence of depression in (a) cataract and non-cataract cohorts and (b) cataract surgery and non-surgery groups in the cataract cohort.
Table 2.
Risk of developing depression in patients with and without cataract.
| Age/sex/index year matching | Propensity score matching | |||
|---|---|---|---|---|
| Cataract | No cataract | Cataract | No cataract | |
| N | 140,485 | 140,485 | 116,629 | 116,629 |
| Events | 3,015 | 6,141 | 2,620 | 5,084 |
| Person-years | 1,062,304 | 1,129,970 | 865,395 | 945,965 |
| Incidence ratea | 2.84 | 5.43 | 3.03 | 5.37 |
| Univariable model | ||||
| HR (95% CI) | 1.92 (1.84–2.01) | 1 (ref.) | 1.78 (1.70–1.87) | 1 (ref.) |
| p value | < 0.001 | < 0.001 | ||
| Multivariable modelb | ||||
| aHR (95% CI) | 1.81 (1.73–1.89) | 1 (ref.) | 1.72 (1.64–1.80) | 1 (ref.) |
| p value | < 0.001 | < 0.001 | ||
CI confidence interval, HR hazard ratio, aHR adjusted hazard ratio, ref. reference.
aPer 1,000 person-years.
bMultivariable Cox proportional hazards regression model with adjustments for baseline characteristics shown in Table 1.
Cox regression models revealed a higher risk of developing depression in both the cataract surgery (age/sex/index year matching: aHR = 1.72, 95% CI 1.62–1.82, p < 0.001; propensity score matching: aHR = 1.65, 95% CI 1.54–1.76, p < 0.001) and non-surgery (age/sex/index year matching: aHR = 2.30, 95% CI 2.19–2.41, p < 0.001; propensity score matching: aHR = 2.14, 95% CI 2.03–2.27, p < 0.001) groups compared with the risk of developing depression in the non-cataract cohort (Table 3).
Table 3.
Risk of developing depression in cataract patients with and without surgery compared with that in non-cataract patients.
| N | Events | Person-years | Incidence ratea | Univariable model | Multivariable modelb | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | aHR (95% CI) | p value | |||||
| Age/sex/index year matching | ||||||||
| No cataract | 140,485 | 3,015 | 1,062,304 | 2.84 | 1 (ref.) | 1 (ref.) | ||
| Cataract without surgery | 80,031 | 4,141 | 588,539 | 7.04 | 2.47 (2.35–2.59) | < 0.001 | 2.30 (2.19–2.41) | < 0.001 |
| No cataract | 140,485 | 3,015 | 1,062,304 | 2.84 | 1 (ref.) | 1 (ref.) | ||
| Cataract with surgery | 60,454 | 2,000 | 381,548 | 5.24 | 1.82 (1.72–1.93) | < 0.001 | 1.72 (1.62–1.82) | < 0.001 |
| Propensity score matching | ||||||||
| No cataract | 75,381 | 1,794 | 550,956 | 3.26 | 1 (ref.) | 1 (ref.) | ||
| Cataract without surgery | 75,381 | 3,906 | 555,873 | 7.03 | 2.16 (2.04–2.28) | < 0.001 | 2.14 (2.03–2.27) | < 0.001 |
| No cataract | 60,399 | 1,363 | 435,722 | 3.13 | 1 (ref.) | 1 (ref.) | ||
| Cataract with surgery | 60,399 | 1,996 | 381,237 | 5.24 | 1.65 (1.54–1.77) | < 0.001 | 1.65 (1.54–1.76) | < 0.001 |
CI confidence interval, HR hazard ratio, aHR adjusted hazard ratio, ref. reference.
aPer 1,000 person-years.
bMultivariable Cox proportional hazards regression model with adjustments for baseline characteristics shown in Table 1.
Analyses stratified by age and sex
Stratified analyses by age or sex also revealed similar results. A higher risk of depression was found in the cataract cohort compared with the non-cataract cohort in young (< 65 years) and old (≥ 65 years) age groups as well as males and females. Cataract patients with and without surgery had a higher risk of depression compared with the non-cataract controls in all the different sex or age strata. Detailed statistical results were shown in Table 4.
Table 4.
Age- and sex-stratified analyses for the risk of developing depression according to cataract status and cataract surgery history after propensity score matching.
| Univariable model | Multivariable modela | |||
|---|---|---|---|---|
| HR (95% CI) | p value | aHR (95% CI) | p value | |
| Age < 65 years | ||||
| No cataract | 1 (ref.) | 1 (ref.) | ||
| Cataract overall | 1.92 (1.81–2.04) | < 0.001 | 1.82 (1.71–1.94) | < 0.001 |
| Cataract without surgery | 2.20 (2.05–2.36) | < 0.001 | 2.15 (2.01–2.31) | < 0.001 |
| Cataract with surgery | 1.80 (1.63–1.99) | < 0.001 | 1.79 (1.62–1.98) | < 0.001 |
| Age ≥ 65 years | ||||
| No cataract | 1 (ref.) | 1 (ref.) | ||
| Cataract overall | 1.60 (1.49–1.72) | < 0.001 | 1.55 (1.44–1.67) | < 0.001 |
| Cataract without surgery | 2.09 (1.91–2.29) | < 0.001 | 2.10 (1.92–2.30) | < 0.001 |
| Cataract with surgery | 1.51 (1.37–1.66) | < 0.001 | 1.50 (1.36–1.65) | < 0.001 |
| Male | ||||
| No cataract | 1 (ref.) | 1 (ref.) | ||
| Cataract overall | 1.81 (1.68–1.95) | < 0.001 | 1.69 (1.56–1.82) | < 0.001 |
| Cataract without surgery | 2.18 (1.99–2.39) | < 0.001 | 2.11 (1.93–2.31) | < 0.001 |
| Cataract with surgery | 1.64 (1.48–1.82) | < 0.001 | 1.61 (1.45–1.79) | < 0.001 |
| Female | ||||
| No cataract | 1 (ref.) | 1 (ref.) | ||
| Cataract overall | 1.78 (1.67–1.89) | < 0.001 | 1.74 (1.63–1.85) | < 0.001 |
| Cataract without surgery | 2.17 (2.02–2.33) | < 0.001 | 2.16 (2.01–2.32) | < 0.001 |
| Cataract with surgery | 1.67 (1.52–1.83) | < 0.001 | 1.67 (1.52–1.83) | < 0.001 |
The non-cataract cohort was used as the reference group when calculating HR.
CI confidence interval, HR hazard ratio, aHR adjusted hazard ratio, ref. reference.
aMultivariable Cox proportional hazards regression model with adjustments for baseline characteristics shown in Table 1.
Comparison between surgery and non-surgery groups in the cataract cohort
Cumulative incidence curves indicated that the cumulative incidence of developing depression was higher in cataract patients who did not undergo surgery compared with those who did undergo surgery (Fig. 1B). Cox regression models also suggested that cataract patients receiving cataract surgery had a significantly lower risk of depression (age/sex/index year matching: aHR = 0.74, 95% CI 0.70–0.78, p < 0.001; propensity score matching: aHR = 0.75, 95% CI 0.71–0.79, p < 0.001) compared with the risk of depression in those who did not receive surgery (Table 5). Furthermore, the age- and sex-stratified analyses revealed similar findings (Supplementary Table S2).
Table 5.
Risk of developing depression among cataract patients with and without surgery.
| Age/sex/index year matching | Propensity score matching | |||
|---|---|---|---|---|
| Cataract surgery | No surgery | Cataract surgery | No surgery | |
| N | 60,454 | 80,031 | 58,669 | 58,669 |
| Events | 2,000 | 4,141 | 1,960 | 2,996 |
| Person-years | 381,548 | 588,539 | 369,599 | 436,290 |
| Incidence ratea | 5.24 | 7.04 | 5.30 | 6.87 |
| Univariable model | ||||
| HR (95% CI) | 0.73 (0.69–0.77) | 1 (ref.) | 0.75 (0.71–0.80) | 1 (ref.) |
| p value | < 0.001 | < 0.001 | ||
| Multivariable modelb | ||||
| aHR (95% CI) | 0.74 (0.70–0.78) | 1 (ref.) | 0.75 (0.71–0.79) | 1 (ref.) |
| p value | < 0.001 | < 0.001 | ||
CI confidence interval, HR hazard ratio, aHR adjusted hazard ratio, ref. reference.
aPer 1,000 person-years.
bMultivariable Cox proportional hazards regression model with adjustments for baseline characteristics shown in Table 1.
Discussion
In our nationwide cohort study, we analysed the long-term association between cataract and the risk of depression. Compared with the non-cataract control, the cataract cohort had a higher risk of developing depression (aHR = 1.72) despite adjustment for possible confounders. Furthermore, the aHR increased to 2.14 for cataract patients who did not undergo surgery compared with non-cataract individuals. On the other hand, the risk of depression significantly decreased in cataract patients who underwent surgery (aHR = 0.75) compared with those who did not. These results were consistent regardless of sex and age subgroups.
To date, limited studies have evaluated the differences in the risk of depression between cataract and non-cataract patients12,13. McGwin et al. in 2003 found a marginally higher depressive score in cataract patients (7.6 and 5.3 in cataract and non-cataract patients, respectively); this was measured using the Center for Epidemiological Studies-Depression Scale, ranging from 0 to 6013. Wang et al.12 conducted another community-based survey and found slightly higher odds of mental health contacts for depression or anxiety in cataract patients (OR 1.33). Although these two studies reported a significant but weak association, both had some limitations. The former study enrolled only half of the eligible subjects and had different distributions of age and sex between the cataract and non-cataract patients. The latter study had a cross-sectional design, which cannot represent the longitudinal correlation, and combined depression and anxiety in their outcomes12. Therefore, this population-based longitudinal study with a strict diagnosis criterion for depression indicated that cataract is an evident long-term risk factor for depression.
Although some previous studies have evaluated the possible benefit of cataract surgery on depression13,15–23, the results are controversial as some of these studies indicated that the depressive symptoms did not improve after cataract surgery13,21,22. Moreover, most of these studies had follow-up periods of < 1 year13,15–23, and only one was a population based study16. Therefore, we conducted this 16-year nationwide cohort study to enhance the reliability and indicated that the risk of depression was reduced by 25% in the cataract surgery group compared with that in the non-surgery group. The result is consistent with that reported by another population-based study that found that the number of mental health contacts for depression or anxiety declined by 18.8% 1 year after cataract surgery16.
It is crucial to determine whether further preventive management is required for depression in post-surgical patients. We only found one previous study that included healthy individuals as a control to evaluate the impact of cataract surgery on depressive symptoms13. However, that study found an insignificant trend of improvement of depressive symptoms in post-surgical patients compared with non-surgery cataract patients and healthy individuals. It may be due to a relatively small sample size and short follow-up period (342 individuals with up to 1-year follow-up)13. The present study also included non-cataract individuals as control. With a larger sample size and long-term follow-up, this study was able to successfully demonstrate a decreased but still higher risk of depression in post-surgical patients compared with healthy individuals. Collectively, these results suggest that while cataract surgery has a beneficial effect in reducing the risk of depression, further attention and preventive management for depression is still required in post-surgical individuals.
Most previous studies have been restricted to older patients with an average age of around 70 years12,13,16–19,21 and did not examine whether the correlation between cataract and depression differed between different age sex groups13,16,19,21. Therefore, we attempted to include all patients regardless of age and stratified the analyses by age and sex. Increased risk of developing depression was observed in both younger and older groups as well as in males and females. The decreased risk of depression in the surgery group was also noticed in all strata. These results indicate the increased risk of depression in cataract patients and the beneficial effect of cataract surgery in reducing the risk of depression regardless of age and sex.
The exact underlying mechanisms involved in the observed association between cataract and depression remain unclear, but some contributing factors have been hypothesised. Studies have found that visually impaired patients have difficulties in daily activities, especially instrumental activities of daily life and leisure activities24,25. Visually impaired individuals rely more on their cognitive function and are consequently more vulnerable to age-related cognitive decline24. They also have a higher institutionalisation rate26 and visual hallucination risk, which is known as Charles Bonnet syndrome27,28. Studies have shown improved quality of life, mental health, and economic state in patients following cataract surgery15,29. Therefore, future studies are required to develop a multifactor strategy for the prevention and management of depression in patients with cataract or visual impairment.
From a public health perspective, the present study identified a higher risk of depression in cataract patients. Previous studies have also revealed an increased risk of depression associated with glaucoma30 and other eye diseases. The prevalence of depressive symptoms can be as high as 25% for patients with any eye disease6. By taking into account all these findings, we suggest that ophthalmologists be vigilant about hidden depression and cooperate with psychiatrists. In addition, as we know that cataract may contribute to the development of depression and that cataract surgery may effectively correct this risk, psychiatrists could ask their patients about their visual acuity condition and refer them to an ophthalmologist if needed.
The strengths of the present study include the nationwide population-based longitudinal design, the strict criteria for cataract and depression that requires diagnosis by specialists, and the direct comparison of cataract patients with non-cataract individuals. However, there are still some limitations that need to be acknowledged here. First, the study design was retrospective and used claims-based data; therefore, we could not obtain granular data on some clinical characteristics such as lifestyle, smoking status and alcohol history. Bias related to unmeasured or unknown confounders was a potential issue given the nature of the study. Second, we used the diagnosis of cataract without obtaining the detailed visual acuity of individuals. This means that the cataract cohort would include more mild cases, which would diminish the impact of cataract on developing depression and steer the results toward the null hypothesis. We were also unable to identify whether patients had cataract in one or both eyes and whether surgery had been performed for both affected eyes. This means that the cataract surgery group included patients who still had an unoperated eye, which may have reduced the observed beneficial effect of cataract surgery. As a result, the association may be stronger than we were able to show. Third, while the strict criteria of depression enhance the diagnostic accuracy, it may result in the underestimated incidence of depression in both the cataract and non-cataract cohorts. Last, the present study was performed in a Taiwanese population; thus, the generalisation of the study findings to populations of other countries, cultures or races remains undetermined, and further studies are required to examine external generalizability.
In summary, the present study is the first nationwide longitudinal study evaluating the impact of cataract and cataract surgery by directly comparing cataract patients with non-cataract individuals. It contributes to the literature by indicating a long-term association of cataract and increased depression risk, demonstrating the beneficial effect of cataract surgery in reducing the risk of depression and clarifying the presence of these correlations regardless of age and sex. The results emphasise the importance of regular screening for depression among cataract patients and the beneficial effect of cataract surgery in preventing depression. Future studies are required to elucidate the underlying mechanisms and develop prevention and management strategies for depression in cataract patients.
Methods
Data sources
The present study used data from the Longitudinal Health Insurance Database (LHID) in Taiwan from 2000 to 2016. The LHID contains data from two million enrollees who were randomly sampled from the National Health Insurance Research Database (NHIRD), which includes 99% of the total population in Taiwan. The NHIRD was derived from Taiwan’s National Health Insurance (NHI) programme, which is a single-payer mandatory enrolment healthcare system with universal coverage for outpatient, inpatient, and emergency services. This comprehensive cohort enables the association between cataract and risks of depression to be examined. To protect the privacy and data security, the Health and Welfare Data Science Center encrypted personal identifiers in the LHID. Further details of NHIRD can be found in the previous article31. The Research Ethics Committee of Buddhist Tzu Chi General Hospital approved this study protocol (REC No: IRB107-217-B), and the requirement for informed consent was waived due to the use of anonymised data. Our research was performed in accordance with relevant guidelines/regulations.
Study population
We included a cataract cohort as the exposure group and a non-cataract cohort as the non-exposure (comparison) group. The cataract cohort comprised adult patients diagnosed with cataract between 2001 and 2015 in the LHID. Diagnosis of cataract was defined by at least one inpatient or two outpatient diagnoses by an ophthalmologist, using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM diagnosis code 366). The index date was defined as the date of the first diagnosis of cataract. To enhance the likelihood of distinguishing newly diagnosed cataract patients, we excluded patients diagnosed with cataract in 2000. Patients diagnosed with depression (the primary outcome) before cataract diagnosis were also excluded.
The non-cataract cohort was also retrieved from the registry of beneficiaries in the LHID. We applied 1:1 matching based on age (in 5-year increments), sex and index year to identify the non-cataract (comparison) cohort. The index date for paired non-cataract patients was assigned as the same date as that of the matched cataract case. In line with the cataract cohort, patients previously diagnosed with depression before the index date were excluded.
Patients in the cataract cohort were further divided into surgery and non-surgery groups to evaluate the impact of cataract surgery on the risk of depression. Cataract patients who underwent cataract surgery during the follow-up period were identified by order code in the NHIRD and classed as the surgery group. Those who did not undergo surgery were classed as the non-surgery group.
Outcome measures
The primary outcome was new diagnosis of depression, which required diagnosis by a psychiatrist either once in the inpatient service or twice in the outpatient clinic. The NHIRD used ICD-9-CM codes as diagnostic codes before 2016 then started to follow the ICD-10-CM codes in 2016; therefore, diagnosis of depression was identified by the ICD-9-CM codes from 2000 to 2015 (ICD-9-CM codes 296.2, 296.3, 300.4 and 311) and ICD-10-CM codes in 2016 (ICD-10-CM F32, F33 and F34.1). The follow-up period for each individual was from the index date to the development of depression, death, or December 31, 2016 (the last day in our database). The date of death was identified by linking the LHID to the National Register of Deaths in Taiwan.
Covariates
We used baseline characteristics from both the outpatient and inpatient reimbursement claims in the LHID as potential confounders. Diagnosis of pre-existing comorbidity was confirmed by at least two clinic diagnoses or one inpatient diagnosis preceding the index date. The Charlson comorbidity index score was calculated to quantify the degree of comorbidities32,33. Information on income level was retrieved as a representation of socioeconomic status. The monthly income level was divided into four groups (New Taiwan dollars ≥ 45,000; 30,000–44,999; 15,840–29,999; and financially dependent) based on the income-related NHI premiums as described in detail previously34.
Propensity score matching
To minimise possible selection bias caused by differences in baseline characteristics, we further performed propensity score matching for each direct comparison, including (1) cataract vs. non-cataract cohorts, (2) cataract surgery group vs. non-cataract cohort, (3) cataract non-surgery group vs. non-cataract cohort and (4) surgery vs. non-surgery groups among the cataract cohort. We used a logistic regression model including all baseline covariates listed in Table 1 to calculate a propensity score, which assessed the propensity for belonging to a particular group. Participants in different groups were matched 1:1 based on the nearest-neighbour matching method without replacement, using a calliper width equal to 0.2-times the standard deviation of the logit of the propensity score35,36. To ensure that the two different matching methods did not influence our results, all analyses were conducted using both the initial age, sex and index year matching and 1:1 propensity score matching.
Statistical analyses
We used a t test for continuous variables and the χ2 test for categorical variables for comparison of baseline characteristics. In the survival analysis, we used Kaplan–Meier methods to estimate the cumulative incidences with log-rank tests for comparison between cumulative incidence curves. Univariable and multivariable Cox proportional hazard regression models were performed to calculate HRs and 95% confidence intervals (CIs) for the risk of developing depression. All baseline characteristics listed in Table 1 (including age, sex, financial state and comorbidities) were considered as covariates and adjusted by multivariable Cox proportional hazard regression models to reduce possible confounding effects. To diminish any potential bias caused by immortal time before surgery in the cataract surgery group, we redefined the index date as the date of surgery instead of the date of diagnosis of cataract when performing comparisons related to the surgery group. We further performed stratified analyses according to age and sex subgroups. Standardised difference was used to assess any differences in baseline characteristics between groups, and a value of < 0.1 was considered negligible37. A two-sided probability value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA, RRID: SCR_004635) and Stata version 15 (Stata Corporation, College Station, TX, USA, RRID: SCR_012763).
Supplementary information
Author contributions
Study conception and design: H.H. and C.L. Acquisition of data: H.H., P.L. and C.L. Analysis and interpretation of data: P.C., P.L., S.L., H.H. and C.L. Preparation of the manuscript: P.C., H.H., and C.L. Critical revision: P.C., P.L., S.L., J.W., H.H., and C.L. All authors approved the final version of the manuscript for publication.
Data availability
The dataset used in this study is managed by the Taiwan Ministry of Health and Welfare, and, thus, cannot be made available publicly. Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan has approved our access to the dataset. Researchers interested in accessing this dataset can submit a formal application to the Ministry of Health and Welfare to request access (https://dep.mohw.gov.tw/DOS/cp-2516-3591-113.html). All data generated or analysed during this study are included in this article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Po-Wei Chen and Peter Pin-Sung Liu.
Contributor Information
Huei-Kai Huang, Email: drhkhuang@gmail.com.
Ching-Hui Loh, Email: twdoc1960@gmail.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-70285-7.
References
- 1.James SL, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhuang XY, Wong DFK, Cheng C-W, Pan S-M. Mental health literacy, stigma and perception of causation of mental illness among Chinese people in Taiwan. Int. J. Soc. Psychiatry. 2017;63:498–507. doi: 10.1177/0020764017719303. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y-J. Unmet needs for the management of depression. Taiwan. J. Psychiatry. 2019;33:122. doi: 10.4103/TPSY.TPSY_25_19. [DOI] [Google Scholar]
- 5.Rabins PV. Depressive symptoms in ophthalmology patients. JAMA Ophthalmol. 2016;134:1015. doi: 10.1001/jamaophthalmol.2016.2367. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Wu X, Lin X, Lin H. The prevalence of depression and depressive symptoms among eye disease patients: A systematic review and meta-analysis. Sci. Rep. 2017;7:46453. doi: 10.1038/srep46453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pop-Jordanova N, Ristova J, Loleska S. Depression in ophthalmological patients. Pril. Makedonska Pril. Makedonska Akad. Nauk. Umet. Oddelenie Med. Nauk. 2014;35:53–58. doi: 10.2478/prilozi-2014-0007. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Wu S. Deep learning for identifying environmental risk factors of acute respiratory diseases in Beijing, China: Implications for population with different age and gender. Int. J. Environ. Health Res. 2019;00:1–12. doi: 10.1080/09603123.2019.1597836. [DOI] [PubMed] [Google Scholar]
- 9.Han JH, Lee HJ, Jung J, Park EC. Effects of self-reported hearing or vision impairment on depressive symptoms: A population-based longitudinal study. Epidemiol. Psychiatr. Sci. 2019;28:343–355. doi: 10.1017/S2045796018000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank CR, Xiang X, Stagg BC, Ehrlich JR. Longitudinal associations of self-reported vision impairment with symptoms of anxiety and depression among older adults in the United States. JAMA Ophthalmol. 2019;137:793–800. doi: 10.1001/jamaophthalmol.2019.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu R-R, Jian S, Yang M, Guan H-J. Prevalences and causes of vision impairment in elderly Chinese: A socioeconomic perspective of a comparative report nested in Jiangsu Eye Study. Int. J. Ophthalmol. 2016;9:1051–1056. doi: 10.18240/ijo.2016.07.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Sun HP, Wang P, Xu Y, Pan CW. Cataract and depressive symptoms among older Chinese adults. Optom. Vis. Sci. 2016;93:1479–1484. doi: 10.1097/OPX.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 13.McGwin G, Li J, McNeal S, Owsley C. The impact of cataract surgery on depression among older adults. Ophthalm. Epidemiol. 2003;10:303–313. doi: 10.1076/opep.10.5.303.17323. [DOI] [PubMed] [Google Scholar]
- 14.Hodge W, et al. The consequences of waiting for cataract surgery: A systematic review. Can. Med. Assoc. J. 2007;176:1285–1290. doi: 10.1503/cmaj.060962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feeny S, Posso A, McDonald L, Chuyen TTK, Tung ST. Beyond monetary benefits of restoring sight in Vietnam: Evaluating well-being gains from cataract surgery. PLoS One. 2018;13:1–12. doi: 10.1371/journal.pone.0192774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meuleners LB, Hendrie D, Fraser ML, Ng JQ, Morlet N. The impact of first eye cataract surgery on mental health contacts for depression and/or anxiety: A population-based study using linked data. Acta Ophthalmol. 2013;91:445–450. doi: 10.1111/aos.12124. [DOI] [PubMed] [Google Scholar]
- 17.Freeman EE, et al. Cataract-related vision loss and depression in a cohort of patients awaiting cataract surgery. Can. J. Ophthalmol. 2009;44:171–176. doi: 10.3129/i09-001. [DOI] [PubMed] [Google Scholar]
- 18.Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am. J. Ophthalmol. 2008;146:404–409. doi: 10.1016/j.ajo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Mitsonis CI. Anxiety and depression in cataract surgery: A pilot study in the elderly. Psychol. Rep. 2006;99:257. doi: 10.2466/pr0.99.1.257-265. [DOI] [PubMed] [Google Scholar]
- 20.To KG, et al. The impact of cataract surgery on depressive symptoms for bilateral cataract patients in Ho Chi Minh City, Vietnam. Int. Psychogeriatr. 2014;26:307–313. doi: 10.1017/S1041610213001907. [DOI] [PubMed] [Google Scholar]
- 21.Fraser ML, Meuleners LB, Lee AH, Ng JQ, Morlet N. Vision, quality of life and depressive symptoms after first eye cataract surgery. Psychogeriatrics. 2013;13:237–243. doi: 10.1111/psyg.12028. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, et al. Illness uncertainty, anxiety and depression in Chinese patients with glaucoma or cataract. Sci. Rep. 2018;8:11671. doi: 10.1038/s41598-018-29489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harwood RH, et al. Falls and health status in elderly women following first eye cataract surgery: A randomised controlled trial. Br. J. Ophthalmol. 2005;89:53–59. doi: 10.1136/bjo.2004.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahl HW. The psychological challenge of late-life vision impairment: Concepts, findings, and practical implications. J. Ophthalmol. 2013;2013:20. doi: 10.1155/2013/278135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nirmalan PK, et al. Relationship between vision impairment and eye disease to vision-specific quality of life and function in rural India: The aravind comprehensive eye survey. Investig. Ophthalmol. Vis. Sci. 2005;46:2308–2312. doi: 10.1167/iovs.04-0830. [DOI] [PubMed] [Google Scholar]
- 26.Brésin AP, Lafuma A, Fegnani F, Mesbah M, Berdeaux G. Blindness, low vision, and other handicaps as risk factors attached to institutional residence. Br. J. Ophthalmol. 2004;88:1330–1337. doi: 10.1136/bjo.2003.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon GJ, Rahman I, Menon SJ, Dutton GN. Complex visual hallucinations in the visually impaired. Surv. Ophthalmol. 2003;48:58–72. doi: 10.1016/S0039-6257(02)00414-9. [DOI] [PubMed] [Google Scholar]
- 28.Santos-Bueso E, et al. Prevalence and clinical characteristics of charles bonnet syndrome in Madrid, Spain. Eur. J. Ophthalmol. 2014;24:960–963. doi: 10.5301/ejo.5000483. [DOI] [PubMed] [Google Scholar]
- 29.Essue BM, et al. A multicenter prospective cohort study of quality of life and economic outcomes after cataract surgery in Vietnam: The VISIONARY study. Ophthalmology. 2014;121:2138–2146. doi: 10.1016/j.ophtha.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y-Y, et al. The association between glaucoma and risk of depression: A nationwide population-based cohort study. BMC Ophthalmol. 2018;18:146. doi: 10.1186/s12886-018-0811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh CY, et al. Taiwan’s national health insurance research database: Past and future. Clin. Epidemiol. 2019;11:349–358. doi: 10.2147/CLEP.S196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Chu Y-T, Wu S-C, Lee Y-C, Lai M-S, Tam S-C. Assessing measures of comorbidity using national health insurance databases. Taiwan J. Public Health. 2010;29:191–200. [Google Scholar]
- 34.Lin S-M, Yang S-H, Liang C-C, Huang H-K. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: A population-based cohort study. Osteoporos. Int. 2018;29:153–162. doi: 10.1007/s00198-017-4262-2. [DOI] [PubMed] [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. Cataract surgery and age-related cognitive decline: A 13-year follow-up of the English Longitudinal Study of Ageing. PLoS One. 2018;13:e0204833. doi: 10.1371/journal.pone.0204833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 2009;38:1228–1234. doi: 10.1080/03610910902859574. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used in this study is managed by the Taiwan Ministry of Health and Welfare, and, thus, cannot be made available publicly. Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan has approved our access to the dataset. Researchers interested in accessing this dataset can submit a formal application to the Ministry of Health and Welfare to request access (https://dep.mohw.gov.tw/DOS/cp-2516-3591-113.html). All data generated or analysed during this study are included in this article and its supplementary information files.