Abstract
Context
Interleukin 6 (IL-6) contributes to bone remodeling in preclinical studies. Clinical trials investigating the role of IL-6 in bone remodeling are limited.
Objective
To investigate if IL-6 regulates bone remodeling in humans.
Design
Plasma concentrations of the bone resorption marker carboxy-terminal type I collagen crosslinks (CTX) and of the bone formation marker procollagen type 1 N-terminal propeptide (P1NP) were measured during a mixed-meal tolerance test (MMTT) in 3 placebo-controlled human studies.
Participants
Five healthy individuals participated in study 1; 52 obese individuals, in study 2; and 10 healthy individuals, in study 3.
Interventions
Study 1 was a single-blinded crossover study consisting of a 1-h infusion of saline (placebo) or the IL-6 receptor antibody tocilizumab followed by an exercise bout. Study 2 was a randomized, double-blinded 12-week exercise training intervention study. Participants received infusions of saline or tocilizumab. Study 3 was a randomized, double-blinded, crossover study consisting of 30 min infusion of saline or IL-6.
Main outcomes measures
Effect of IL-6 on CTX levels.
Results
CTX was significantly (P < 0.01) decreased during MMTTs in all 3 studies. Treatment with tocilizumab did not affect exercise or meal induced changes in plasma CTX or P1NP concentrations acutely (study 1) or after a 12-week treatment period (study 2). Exogenous IL-6 had no effect on CTX or P1NP plasma concentrations (study 3).
Conclusions
IL-6 may not regulate bone remodeling in humans.
Keywords: bone resorption, bone formation, interleukin-6
Interleukin-6 (IL-6) is cytokine secreted from numerous cell types including muscle cells in response to exercise [1]. IL-6 has been reported to regulate glucose homeostasis through gut hormone responses [2] and modulation of gastric emptying [3].
The role of IL-6 in bone metabolism is debated [4]. Given that individuals with diabetes have impaired bone structure (bone quality is impaired in type 2 diabetes and bone mineral density (BMD) is lowered in type 1 diabetes) [5] and systemic IL-6 levels are elevated in patients with diabetes [6], it has been speculated that IL-6 may be a regulator of bone metabolism in humans [7]. Several studies have reported IL-6 as a regulator of bone metabolism [7-12]. Indeed, both in vitro [12,13] and in vivo studies [8-11] have reported an effect of IL-6 on bone resorption, and 3 epidemiological studies have found differential associations of IL-6 with bone loss in humans [7,14,15]. Yet, knowledge regarding a role for IL-6 in regulating bone metabolism in humans is limited.
To address this, we measured bone resorption and bone formation markers, carboxy-terminal type I collagen crosslinks (CTX) and procollagen type 1 N-terminal propeptide (P1NP) in healthy and obese individuals in 3 different human intervention studies. We first explored if endogenous IL-6 contributes to exercise and meal induced changes in plasma CTX and P1NP levels. A single bout of exercise has been reported to increase markers of bone resorption in humans [16] and long-term exercise training has been shown to increase bone formation marker osteocalcin in an IL-6 dependent manner [17]. In contrast to exercise, food intake reduces markers of bone resorption [18].
We first assessed the role of IL-6 in regulating CTX and P1NP in a crossover designed study using the IL-6 receptor antibody tocilizumab in healthy individuals during an acute exercise bout and following a mixed-meal tolerance test (MMTT). Second, to track if chronic lack of IL-6 signaling alters bone metabolism in response to exercise training we measured CTX, P1NP, and BMD in abdominally obese individuals randomized to exercise or no exercise in combination with the IL-6 receptor antibody tocilizumab or placebo. Finally, we infused exogenous IL-6 into healthy individuals to test if acutely elevated levels of IL-6 affect fasting and postprandial levels of markers of bone resorption. The present study is explorative in its nature and based on data from previously published independent human studies [3,19]. The study was powered based on the primary outcome (changes in plasma levels of CTX after a single dose of rhIL-6 [15 ug] in healthy individuals during a MMTT; study 3).
Materials and Methods
The present manuscript is based on data from 3 individual studies (studies 1, 2, and 3). Data from all 3 studies have previously been published (PMID 29731419; PMID 30595477; PMID 29720225; PMID 31710522; PMID 31636570; PMID 31268469). The studies adhered to the latest versions of the Declaration of Helsinki and were approved by the scientific-ethical committee of the Capital Region of Denmark as H-15004576 (study 1), H-16018062 (study 2), and H-1-2013-059 (study 3). Study 2 was registered at Clinicaltrials.gov as NCT02901496.
Study designs
Study 1.
Briefly, study 1 was performed in a placebo-controlled, single-blinded manner. Ten healthy males went through 2 study visits with a minimum of 7 days apart. Data from this study have previously been published (PMID 29731416; study 4). In the present study, samples from 5 participants were included. Participant characteristics are provided in Table 1. Participants were included after an initial screening procedure and instructed to avoid vigorous physical activity and alcohol for 48 h preceding study days.
Table 1.
Baseline Characteristics
| Study 2 | ||||||
|---|---|---|---|---|---|---|
| Study 1 | No Exercise + Placebo | No Exercise + Tocilizumab | Exercise + Placebo | Exercise + Tocilizumab | Study 3 | |
| Male/female (n) | 5/0 | 1/11 | 5/8 | 3/11 | 3/10 | 10/0 |
| Age (years) | 21 (2) | 48 (12) | 44 (12) | 39 (13) | 44 (12) | 24 (1) |
| Body weight (kg) | 75 (4) | 95 (20) | 99 (17) | 92 (14) | 96 (15) | 84 (6) |
| Body mass index (kg/m2) | 22.3 (0.9) | 33.0 (6.3) | 32.9 (4.9) | 33.0 (4.9) | 33.0 (4.7) | 24.1 (2.1) |
| Bone mineral density (g/cm2) | 1.271 (0.06) | 1.273 (0.082) | 1.260 (0.063) | 1.250 (0.088) | 1.257 (0.080) | - |
| Whole body lean mass (kg) | 66.5 (4.3) | 47.4 (9.1) | 52.7 (11.1) | 45.4 (7.3) | 49.9 (8.1) | 66.7 (4.7) |
| VO2 max (mL/min/kg) | 56 (4) | 27 (5) | 29 (5) | 28 (5) | 29 (6) | 50 (5) |
| HbA1c (mmol/mol) | 34.0 (1.9) | 36.5 (3.8) | 37.2 (2.7) | 33.6 (3.0) | 34.0 (3.0) | 32.8 (2.5) |
| CRP (mg/L) | 1.20 (0.45) | 3.59 (3.09) | 2.01 (1.53) | 3.46 (3.51) | 2.50 (2.57) | <1 |
| Calcium (mmol/L) | 2.34 (0.11) | 2.28 (0.07) | 2.29 (0.08) | 2.29 (0.07) | 2.40 (0.35) | 2.31 (0.08) |
| Phosphate (mmol/L) | 0.85 (0.11) | — | — | — | — | 0.99 (0.11) |
Data are presented as mean (standard deviation).
Abbreviations: CRP = C-reactive protein. HbA1c = hemoglobin A1c.
Participants arrived fasted (>10 h) at morning at the research facility (Centre for Physical Activity Research [CFAS]). Body weight and vital signs were assessed, and participants were placed in bed. Bilateral antecubital venous lines for infusion and blood sampling were placed. Fasting blood samples were drawn at time point −60 min followed by a 1-h infusion of 100 mL saline (placebo) or 8 mg/kg body weight of the IL-6 receptor antibody tocilizumab (RoActemra, Roche Pharma) in 100 mL saline. A dose considered to fully block IL-6 signaling [20,21]. Due to the long wash out of tocilizumab, participants received saline during visit 1 and tocilizumab during visit 2 but were unaware of the order of administration. Blood was sampled at the end of the infusion (time point 0), and immediately thereafter participants performed a 1-h treadmill run at 75% of VO2 max. Blood was sampled every 20 min (time points 20, 40, and 60) while running. Running was followed by a 2-h MMTT, and blood samples were drawn at 75, 90, 120, 150, and 180 min and analyzed for CTX and P1NP.
Study 2.
Study 2 was a randomized, placebo-controlled, double-blinded exercise training study including 53 abdominally obese men and women. Participants were eligible if they were abdominally obese (waist to height ratio ≥ 0.5 and/or waist circumference ≥ 88/102 for women and men, respectively) [22] and physically inactive [23,24] but otherwise healthy. Data from this study have previously been published [19]. In the present study, samples from 52 participants were included. Participant characteristics are provided in Table 1. Participants were instructed not to change free-living physical activity and eating habits during the study period and to avoid exercise and alcohol 48 h prior to study days. Participants were block-randomized (1:1:1:1:1) into (i) no exercise + placebo, (ii) no exercise + tocilizumab, (iii) exercise + placebo, (iv) exercise + tocilizumab, or (v) resistance training + placebo as parallel groups. Participants allocated to the resistance training + placebo group were not included in the analysis of the present study. Participants received intravenous infusions of saline (placebo) or 8 mg/kg body weight of the IL-6 receptor antibody tocilizumab (Roche Pharma) every 4 weeks and 3 times in total. This dose of tocilizumab completely blocks IL-6 signaling [25]. The exercise training was aerobic high-intensity interval training performed on an ergometer bike. All training sessions took place at our research facility (CFAS) and were supervised. The exercise intensity was progressively increased over the 12-week training period. Before and after the training intervention all participants went through several study visits including medical screening, dual X-ray absorptiometry, and a MMTT (PMID 29720225). Blood samples obtained at −10, 0, 30, 60, 90, and 120 min relative to the start of the MMTT have been analyzed for CTX and P1NP.
Study 3.
Briefly, study 3 was a randomized, placebo-controlled, double-blinded, crossover study including healthy young males. Data from this study have previously been published (studies 1 and 3 in [3]). Thirteen healthy males went through 2 study visits with a minimum of 7 days apart. Samples from 10 participants have been analyzed. Subject characteristics are provided in Table 1. Participants were included following an initial screening procedure and instructed to avoid vigorous physical activity and alcohol for 48 h preceding the study days. Participants arrived fasted (>10 h) at the research facility (CFAS). Body weight and vital signs were assessed, and participants were placed in bed. Bilateral antecubital venous lines for infusion and blood sampling were placed. On the study days, blood was sampled in the fasting state (time point −30 min) and following 30 minutes infusion of saline (placebo) or 15μg rhIL-6 (time point 0). Furthermore, blood was sampled at 10, 15, 30, 60, 90, and 120 min relative to the start of the MMTT and have been analyzed for CTX and P1NP.
Measurements of CTX and total procollagen type 1 N-terminal propeptide
For measurement of CTX and P1NP, blood was sampled into ethylenediamine tetra-acetate tubes and plasma CTX was measured using the IDS-iSYS CTX (CrossLaps®) assay (Immunodiagnostic Systems, plc, Tyne and Wear, UK). Plasma P1NP was measured using the IDS-iSYS intact P1NP assay (Immunodiagnostic Systems). Both assays were carried out on a dedicated automated analyzer, iSYS (Immunodiagnostic Systems) according to the manufacturer’s instructions. Both assays are chemiluminescence immunoassays. For each assay the sample aliquots were kept frozen at −80°C until the day of analysis. None of the samples had previously been thawed, and all analyses were performed immediately after thawing the samples. All samples were analyzed using a single batch of each assay. Assay performance was verified using the manufacturers’ control specimens. The intermediary precisions expressed as coefficients of variation for CTX were 5.3% (at CTX concentration 213 ng/L), 3.4% (869 ng/L), and 3.5% (2113 ng/L) for iSYS. For P1NP the intermediary precisions were 5.4% (18.96 µg/L), 6.5% (48.48 µg/L), and 6.1% (122.10 µg/L) for iSYS.
Statistical considerations
This study is a post-hoc analysis performed on 3 previously published studies not designed to investigate postprandial changes in bone resorption markers. We performed a power calculation based on the number of study participants (only completers) and the variability in bone resorption markers (study 3): A power of 85% was calculated based on an effect size of 5% in the primary outcome (decremental area under the curve from 0 to 120 min of CTX), a variation of 10%, an alpha coefficient of 0.05 using a paired t-test. Study 3 was chosen as we here used a supraphysiologic dosage of IL-6 and therefore anticipated this setup to have, if any, the largest effect on bone markers (CTX).
Intention-to-treat analyses was not performed. Statistical differences between 2 groups or effect of intervention (crossover design) were assessed by unpaired or paired t-test, respectively, whereas for the cases of repeated measures (3 variables: time + group + study day), we used mixed effects modeling (generalized linear model). All calculations were performed using STAT14 (SE) (SAS, Cary, NC, US). For illustrations, GraphPad Prism version 8.0 for Windows (GraphPad Software, La Jolla, CA, US) was used. P < 0.05 was considered significant. For reporting of significant differences between interventions, we applied the current guidelines by American Statistical Association as which recommends effect size measures and the corresponding calculated 95% confidence intervals (CI) [26].
All calculations were based on absolute concentrations of CTX (ng/L) and P1NP (µg/L). Data are shown as mean ± standard error of the mean unless otherwise indicated.
Results
No effect of endogenous IL-6 on bone resorption marker CTX and bone formation marker P1NP during exercise and a liquid meal in healthy individuals (study 1)
To study the role of endogenous IL-6 in regulating markers of bone turnover during an acute exercise bout and a MMTT, the IL-6 receptor antibody tocilizumab or saline were infused after an overnight fast in five healthy participants (−60 min to 0 min) (Fig. 1). The dose of tocilizumab is considered to fully block IL-6 receptors by the time of the MMTT [20,21]. Tocilizumab had no significant effect on plasma concentrations of CTX (P = 0.28) or P1NP (P = 0.16) compared to saline.
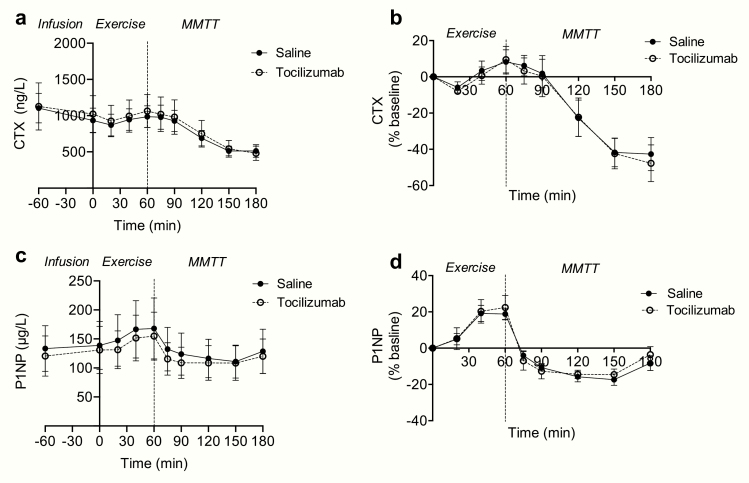
Figure 1.
Effect of IL-6 receptor blockade (with tocilizumab) on CTX and P1NP during an acute exercise bout and a post exercise meal. Measurements during an acute exercise bout (time point 0-60 min) and a postexercise MMTT (time point 60-180 min) with and without IL-6 receptor blockade (with tocilizumab) on (A) plasma CTX and (C) plasma P1NP concentrations. (B) Plasma CTX and (D) plasma P1NP concentrations expressed as percentage change from baseline (time point 0). Data represent mean values; error bars indicate SEM. N = 5. Filled circles placebo (saline), open circles IL-6 receptor blockade (with tocilizumab).
During exercise (time point 0 to 60 min), plasma concentrations of CTX and P1NP increased numerically; however, tocilizumab did not affect exercise-induced changes in plasma concentrations of CTX (AUC0-60min CTX: Mean of differencessaline vs tozilizumab: −196 ng/L [95% CI: −3310 to 2916 ng/L], P = 0.86) or P1NP (AUC0-60min P1NP: Mean of differencessaline vs tozilizumab: −95 µg/L [95% CI: −819 to 624 µg/L], P = 0.73).
Plasma concentrations of CTX and P1NP decreased significantly during the postexercise MMTT; however, treatment with tocilizumab did not affect meal-induced changes in plasma concentrations of CTX (difference of the area under the curve [dAUC] CTX: 60-180 min: mean of differences: −4723 ng/L [95% CI: −21 070 to 11 624 ng/L], P = 0.46) or P1NP (dAUC P1NP: 60–180 min: mean of differences: 469 µg/L [95% CI: −1707 to 2646 µg/L], P = 0.58). In conclusion, this small sample size provides no indications that IL-6 receptor blockade influences exercise and meal-induced regulation of CTX and P1NP.
No significant effect of IL-6 receptor blockade alone or in combination with exercise training on plasma concentrations of CTX in abdominally obese humans (study 2)
To study long-term effects of IL-6 receptor blockade, alone and in combination with exercise training, on bone resorption, we investigated how 12 weeks of endurance exercise with and without IL-6 receptor blockade influenced plasma levels of CTX during a MMTT. First, to test if 12 weeks of aerobic exercise regulates bone resorption, we compared plasma concentrations of CTX in the no exercise + placebo group to the exercise + placebo group (Fig. 2). Plasma concentrations of CTX during fasting were numerically higher, but not statistically different (P = 0.10) in the exercise + placebo group compared to no exercise + placebo. Postprandial plasma concentrations of CTX were similar when comparing the exercise + placebo group to the no exercise + placebo group (P = 0.45).
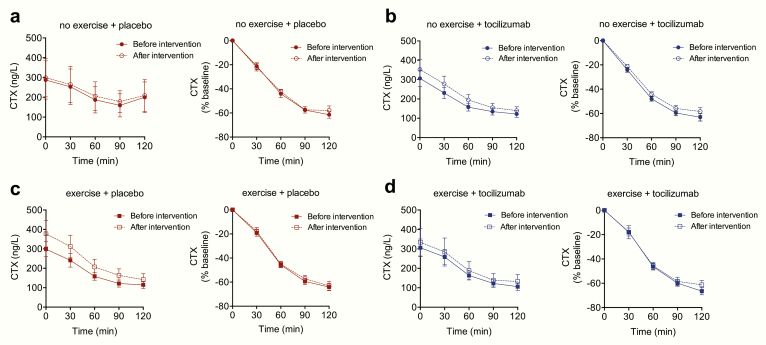
Figure 2.
Effect of exercise training in combination with IL-6 receptor blockade (with tocilizumab) on CTX. Measurements of plasma CTX during an MMTT before and after a 12-week exercise training intervention in (A) the no exercise + placebo group, (B) no exercise + tocilizumab group, (C) exercise + placebo group, and (D) exercise + tocilizumab group. Left panels represent plasma concentrations of CTX, right panels show CTX concentrations as percentage change from baseline. Data represent mean values; error bars indicate SEM. N = 12-14.
We then compared effects of tocilizumab alone by comparing no exercise + placebo to no exercise + tocilizumab treated individuals. Plasma concentrations of CTX during fasted and postprandial conditions were not significantly (P = 0.93, P = 0.56) altered by tocilizumab compared to placebo (saline infusion) (Fig. 2).
Finally, to test if there is a role for exercise-induced IL-6 in regulating plasma CTX concentrations, we compared exercise + placebo to exercise + tocilizumab treated individuals. No significant difference (P = 0.66) in fasting plasma concentrations of CTX was observed when comparing exercise + placebo to exercise + tocilizumab.
Plasma concentrations of CTX decreased significantly during the MMTT in all 4 groups (P < 0.05). A maximum decrease in CTX of ~60% was observed 120 min after meal intake. No significant differences in meal-induced inhibition of CTX were observed when comparing the decremental AUCs between the 4 groups (P > 0.4). In summary, there was no effect of 12 weeks aerobic exercise training or of IL-6 receptor blockade on plasma levels of CTX.
Interleukin-6 blockade alone or in combination with exercise training has no effect on plasma concentrations of P1NP in abdominally obese humans (study 2)
To study long-term effects of IL-6 receptor blockade, alone and in combination with exercise training, on bone formation, we investigated how 12 weeks of endurance exercise with and without IL-6 receptor blockade influenced plasma levels of P1NP during a MMTT. First, to test if 12 weeks of aerobic exercise regulates bone formation, we compared plasma concentrations of P1NP in the no exercise + placebo group to the exercise + placebo treated group (Fig. 3). Plasma concentrations of P1NP during fasting were numerically higher, but not statistically different (P = 0.10) in the exercise + placebo group compared to no exercise + placebo.
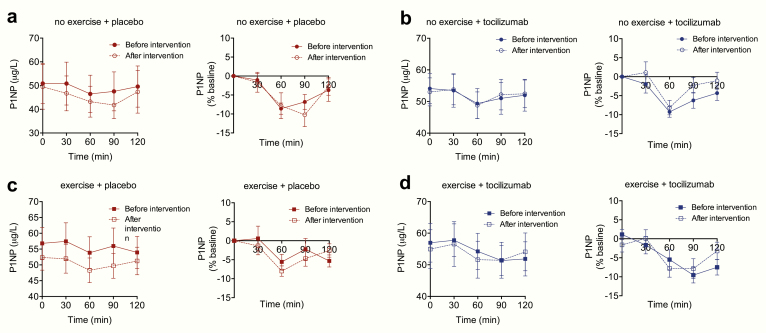
Figure 3.
Effect of exercise training in combination with IL-6 receptor blockade (with tocilizumab) on P1NP. Measurements of plasma P1NP during an MMTT before and after a 12-week exercise training intervention in (A) the no exercise + placebo group, (B) no exercise + tocilizumab group, (C) exercise + placebo group and (D) exercise + tocilizumab group. Left panels represent plasma concentrations of P1NP, right panels show P1NP concentrations as percentage change from baseline. Data represent mean values; error bars indicate SEM. N = 12-14.
Postprandial plasma concentrations of P1NP were however similar when comparing the exercise + placebo group to the no exercise + placebo group (P = 0.52).
We then compared effects of tocilizumab alone by comparing no exercise + placebo to no exercise + tocilizumab treated individuals. Plasma concentrations of P1NP under fasted and postprandial conditions were not significantly (P = 0.87, P = 0.39) altered by tocilizumab compared to placebo (saline infusion) (Fig. 3).
Finally, to test if there is a role for exercise-induced IL-6 in regulating plasma P1NP concentrations, we compared exercise + placebo to exercise + tocilizumab treated individuals. No significant differences (P = 0.91) in fasting plasma concentrations of CTX were observed when comparing exercise + placebo to exercise + tocilizumab.
Plasma concentrations of P1NP decreased significantly during the MMTT in all 4 groups; (P < 0.05). A maximum decrease in P1NP of ~10% was observed 60 min after meal intake. No significant differences in meal-induced inhibition of P1NP were observed when comparing the decremental AUCs between the four groups (P > 0.2). In summary, there was no effect of 12 weeks aerobic exercise or of IL-6 receptor blockade on plasma levels of P1NP.
No effect of IL-6 receptor blockade on bone mineral density in abdominally obese humans after a 12-week exercise training intervention (study 2)
Bone mineral density remained unchanged in all 4 groups following the 12-week intervention. In the no exercise + placebo group, BMD was 1.273 ± 0.023 g/cm2 before the intervention and 1.280 ± 0.026 g/cm2 after the intervention (P = 0.42). In the no exercise + tocilizumab group, BMD was 1.260 ± 0.016 g/cm2 before and 1.270 ± 0.016 g/cm2 after the intervention (P = 0.62). Exercise had no effect on BMD; in the exercise + placebo group, BMD levels were, respectively, 1.250 ± 0.023 g/cm2 and 1.258 ± 0.024 g/cm2 before and after the intervention (P = 0.93) and in the exercise + tocilizumab group BMD levels were, respectively, 1.257 ± 0.021 g/cm2 and 1.264 ± 0.023 g/cm2 before and after the intervention respectively (P = 0.29).
No effect of exogenous IL-6 on bone resorption marker CTX and bone formation marker P1NP during a liquid meal in healthy individuals (study 3)
To investigate if exogenous IL-6 regulates the resorption marker CTX and the formation marker P1NP we infused rhIL-6 (15 µg) or saline (placebo) over a 30-min period (−30 to 0 min) into healthy participants. The 30 min infusion was followed by a 2-h liquid MMTT. Infusion of rhIL-6 increased systemic concentrations of IL-6 from 0.47 ± 0.02 pg/mL to 148.2 ± 13.8 pg/mL. Plasma concentrations of CTX and P1NP were unchanged by the infusion of rhIL-6 (PCTX = 0.58; PP1NP = 0.64) (Fig. 4).
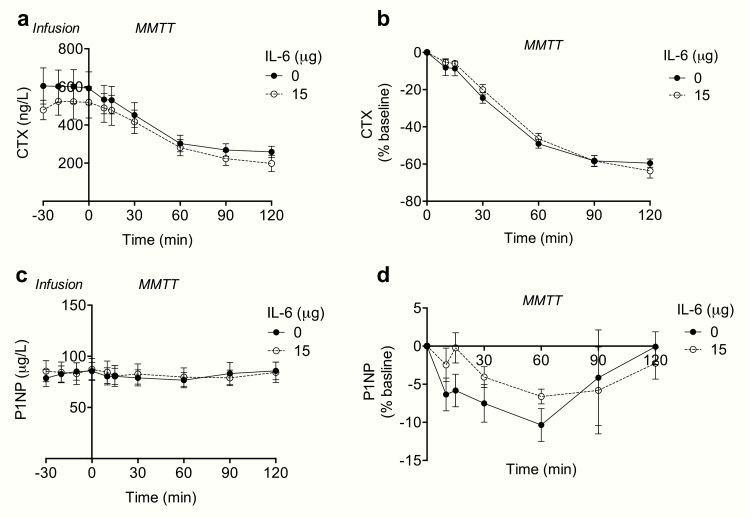
Figure 4.
CTX and P1NP in response to exogenous IL-6 and a liquid meal. Measurements of (A) plasma CTX and (C) plasma P1NP in response to infusion of saline (0 μg) and rhIL-6 (15 μg) (time point 0-30 min) and in response to a liquid meal (MMTT) (time point 0-120 min). (B) Plasma CTX and (D) plasma P1NP concentrations expressed as percentage change from baseline (time point 0). Data represent mean values; error bars indicate SEM. N = 10.
Whereas there was no significant effect of the meal on plasma concentrations of P1NP (P = 0.32), plasma concentrations of CTX decreased significantly (P < 0.05) during the MMTT (Fig. 4). Infusion of IL-6 had no significant effect on meal-induced suppression of CTX (dAUC0-120 min: 23 739 ± 5862) compared to placebo CTX (dAUC0-120 min: 27 599 ± 5607 ng/L, P = 0.27) (Fig. 4).
Discussion
This exploratory study, based on previously published work, investigated whether IL-6 is involved in regulating bone resorption and bone formation in healthy individuals. We used the IL-6 receptor antibody tocilizumab to block endogenous IL-6 signaling both acutely (study1) and long term (study 2), and we infused rhIL-6 to increase plasma concentrations of IL-6 (study 3). To test if IL-6 signaling contributes to bone metabolism during physiological conditions, we studied bone resorption and formation markers in response to acute exercise followed by a MMTT (study 1), during a MMTT before and after 12 weeks of exercise training (study 2), and during a MMTT in combination with infusion of rhIL-6 (study 3). This combination of study designs provided a solid foundation to study whether IL-6 plays a role in regulating bone metabolism in humans.
Blocking endogenous IL-6 signaling acutely and long-term (12 weeks) had no significant effect on the 2 markers of bone remodeling CTX and P1NP in healthy humans. Neither basal levels of CTX and P1NP nor exercise- and meal-induced changes in CTX and P1NP were influenced by IL-6 receptor blockade. Unexpectedly and in contrast to previous exercise studies [27], there was no effect of the 12-week training intervention on markers of bone remodeling; this was also, not surprisingly, reflected by unchanged BMD. Because cycling is nonweight-bearing and impact-free, the training load in the present study 2 was likely not sufficient to provide an osteogenic stimulus [28]. Importantly and relevant for those patients treated with tocilizumab, the results suggest that IL-6 receptor blockade appears to have no effect on plasma levels of CTX and P1NP. Of note, samples from study 2 were recently analyzed for osteocalcin [17]. The analysis showed that 12 weeks of exercise training increased levels of osteocalcin and, moreover, that combining exercise training with IL-6 receptor blockade prevented exercise from increasing osteocalcin levels. In the paper by Subrata et al, mice unable to initiate an IL-6 response to exercise showed a reduced capacity to exercise, an effect which was shown to involve osteocalcin. In humans, however, we currently do not have data to support that exercise capacity is determined by endogenous IL-6, but it has not been the primary question of any human study and remains to be thoroughly studied.
Based on the pharmacokinetics of tocilizumab, we anticipate that IL-6 receptors were completely blocked in both the acute and long-term study [20,21]. We did not measure tocilizumab levels in the circulation; instead, in the acute study increased plasma levels of IL-6 were reflective of an impaired IL-6 receptor-dependent clearance of IL-6 and thus efficient blockade [3,29]. In the long-term study, the efficiency of tocilizumab was evaluated by reduced levels of high-sensitive C-reactive protein [19,30,31].
Raising the systemic concentration of IL-6 through infusion of rhIL-6 had no significant effect on plasma levels of CTX and P1NP alone and also no effect on the meal-induced suppression of CTX and P1NP. The infusion of rhIL-6 was short-lasting (30 min) and plasma levels of IL-6 returned to baseline within 60 min. Thus, circulating concentrations of IL-6 remained elevated for a short time period, and it cannot be excluded that increasing IL-6 over a longer period may affect markers of bone remodeling potentially by recruitment of preosteoclast. On the other hand, our study was powered (study 3 only) to detect a 5% change in CTX levels during a >100-fold increase in plasma concentrations of IL-6, which is why we find it unlikely that IL-6 plays a major role in the acute regulation of bone resorption. As we did not perform sample size calculations on studies 1 and 2 (due to statistical requirements of choosing a single primary outcome), the generalizability of these 2 studies may therefore be reduced. Another potential statistical limitation is that we cannot conclude if gender-specific differences in the effects of IL-6 exist base on these data.
The present study relates to the physiology of CTX and P1NP by including exercise, exercise training, and postprandial conditions and it covers both loss of function and gain of function of IL-6.
Despite the explorative nature of the study we consider it a thorough study with a negative finding. Of note, study participants were all healthy; some were lean (study 1 and 3) and some obese (study 2), and it would possibly be relevant to examine if endogenous IL-6 plays a role in regulating bone remodeling in other populations such as in patients with osteoporosis or conditions where circulating levels of IL-6 are chronically elevated such as in postmenopausal women.
Limitations
One limitation of this study is that the assessments of CTX and P1NP are explorative outcomes in all 3 included studies. Also, the number of participants is low, and it may be viewed as a limitation that all study participants were healthy and not representative of people with bone diseases. Only completers were included for biochemical analyses, and we did therefore not perform intention-to-treat statistical analyses. We did not perform any direct measurement of the efficiency of the IL-6 receptor blockade. Finally, we only included measurement of 2, but well characterized [32], markers of bone metabolism, CTX and P1NP.
Conclusion
Bone resorption and bone formation assessed by changes in CTX and P1NP, respectively, remained unchanged following short-term and long-term IL-6 receptor blockade. Moreover, increasing circulating levels of IL-6 acutely (through infusion of rhIL-6) had no effect on plasma CTX and P1NP. Thus, in conclusion, these explorative studies indicate that IL-6 does not seem to play a major role in regulating bone resorption and bone formation in healthy humans.
Acknowledgments
Financial Support: Study 2 was funded by TrygFonden. The Centre for Physical Activity Research (CFAS), where all the studies were performed, is supported by a grant from TrygFonden, and the Centre of Inflammation and Metabolism (CIM) is a member of DD2—the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724). L.L.L. was partly financed by a grant from the Danish Diabetes Academy, which is supported by the Novo Nordisk Foundation. R.H.C. was supported by the Danish Heart Foundation (grant number 16-R107-A6704-22970). H.E. was funded by the Independent Research Fund Denmark and the Novo Nordisk Foundation. N.J.W.A was funded by the Novo Nordisk Foundation, NNF Center for Protein Research and Rigshospitalet.
Additional Information
Disclosure summary: The authors have no conflict of interest to declare
Data Availability: The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. Faseb J. 2002;16(11):1335-1347. [DOI] [PubMed] [Google Scholar]
- 2. Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lang Lehrskov L, Lyngbaek MP, Soederlund L, et al. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 2018;27(6):1201-1211.e3. [DOI] [PubMed] [Google Scholar]
- 4. Manolagas SC. Role of cytokines in bone resorption. Bone. 1995;17(2):S63-S67. [DOI] [PubMed] [Google Scholar]
- 5. Hygum K, Starup-Linde J, Langdahl BL. Diabetes and bone. Osteoporos Sarcopenia. 2019;5(2):29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114-S124. [DOI] [PubMed] [Google Scholar]
- 7. Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, Pfeilschifter J.. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86(5):2032-2042. [DOI] [PubMed] [Google Scholar]
- 8. Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. Embo J. 1994;13(5):1189-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88-91. [DOI] [PubMed] [Google Scholar]
- 10. Grey A, Mitnick MA, Masiukiewicz U, et al. A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology. 1999;140(10):4683-4690. [DOI] [PubMed] [Google Scholar]
- 11. Li X, Zhou ZY, Zhang YY, Yang HL. IL-6 contributes to the defective osteogenesis of bone marrow stromal cells from the vertebral body of the glucocorticoid-induced osteoporotic mouse. PloS One. 2016;11(4):e0154677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol. 2002;169(6):3353-3362. [DOI] [PubMed] [Google Scholar]
- 13. Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145(10):3297-3303. [PubMed] [Google Scholar]
- 14. Kania DM, Binkley N, Checovich M, Havighurst T, Schilling M, Ershler WB. Elevated plasma levels of interleukin-6 in postmenopausal women do not correlate with bone density. J Am Geriatr Soc. 1995;43(3):236-239. [DOI] [PubMed] [Google Scholar]
- 15. McKane WR, Khosla S, Peterson JM, Egan K, Riggs BL. Circulating levels of cytokines that modulate bone resorption: effects of age and menopause in women. J Bone Miner Res. 1994;9(8):1313-1318. [DOI] [PubMed] [Google Scholar]
- 16. Welsh L, Rutherford OM, James I, Crowley C, Comer M, Wolman R. The acute effects of exercise on bone turnover. Int J Sports Med. 1997;18(4):247-251. [DOI] [PubMed] [Google Scholar]
- 17. Chowdhury S, Schulz LC, Palmisano B, et al. Muscle derived interleukin-6 increases exercise capacity by signaling in osteoblasts. J Clin Invest. 2020;130(6):2888-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30(6):886-890. [DOI] [PubMed] [Google Scholar]
- 19. Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2019;29(4):844-855.e3. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Peck R. Clinical pharmacology of tocilizumab for the treatment of patients with rheumatoid arthritis. Expert Rev Clin Pharmacol. 2011;4(5):539-558. [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Chen YC, Terao K. Clinical pharmacology of tocilizumab for the treatment of polyarticular-course juvenile idiopathic arthritis. Expert Rev Clin Pharmacol. 2017;10(5):471-482. [DOI] [PubMed] [Google Scholar]
- 22. Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. Bmj. 1995;311(6998):158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garber CE, Blissmer B, Deschenes MR, et al. ; American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359. [DOI] [PubMed] [Google Scholar]
- 24. Tremblay MS, Aubert S, Barnes JD, et al. ; SBRN Terminology Consensus Project Participants Sedentary behavior research network (SBRN): terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song SN, Yoshizaki K. Tocilizumab for treating rheumatoid arthritis: an evaluation of pharmacokinetics/pharmacodynamics and clinical efficacy. Expert Opin Drug Metab Toxicol. 2015;11(2):307-316. [DOI] [PubMed] [Google Scholar]
- 26. Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am Stat. 2019;73(sup1):1-19. [Google Scholar]
- 27. Wallace JD, Cuneo RC, Lundberg PA, et al. Responses of markers of bone and collagen turnover to exercise, growth hormone (GH) administration, and GH withdrawal in trained adult males1. J Clin Endocrinol Metab. 2000;85(1):124-133. [DOI] [PubMed] [Google Scholar]
- 28. Olmedillas H, González-Agüero A, Moreno LA, Casajus JA, Vicente-Rodríguez G. Cycling and bone health: a systematic review. BMC Med. 2012;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uchiyama Y, Yoshida H, Koike N, et al. Anti-IL-6 receptor antibody increases blood IL-6 level via the blockade of IL-6 clearance, but not via the induction of IL-6 production. Int Immunopharmacol. 2008;8(11):1595-1601. [DOI] [PubMed] [Google Scholar]
- 30. Unver N, McAllister F. IL-6 family cytokines: key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018;41:10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15(6):R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET; National Bone Health Alliance Bone Turnover Marker Project Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28(9):2541-2556. [DOI] [PubMed] [Google Scholar]