Our study demonstrated that a small persister population exhibits significantly higher heat tolerance than normal cells and that this small fraction contributes to the heat tolerance of the total bacterial population. This study also demonstrated that indole, known to inhibit persister formation, and its derivatives are very promising candidates to reduce the heat tolerance of not only normal bacterial cells but also persister cells.
KEYWORDS: heat tolerance, indole, persister cells, toxin-antitoxin systems
ABSTRACT
YafQ is an endoribonuclease toxin that degrades target gene transcripts such as that of tnaA, a gene encoding tryptophanase to synthesize indole from tryptophan. DinJ is the cognate antitoxin of YafQ, and the YafQ-DinJ system was reported to regulate persister formation by controlling indole production in Escherichia coli. In this study, we investigated the role of YafQ-DinJ, indole production, and persister population in bacterial heat tolerance. yafQ (ΔyafQ), dinJ (ΔdinJ), and tnaA (ΔtnaA) single-gene knockout mutants showed approximately 10-fold higher heat tolerance than wild-type (WT) E. coli BW25113. Persister fractions of all mutants were slightly larger than that of the WT. Interestingly, these persister cells showed an approximately 100-fold higher heat tolerance than normal cells, but there was no difference among the persister cells of all mutants and the WT in terms of heat tolerance. Indole and its derivatives promoted a drastic reduction of bacterial heat tolerance by just 10 min of pretreatment, which is not sufficient to affect persister formation before heat treatment. Surprisingly, indole and its derivatives also reduced the heat tolerance of persister cells. Among the tested derivatives, 5-iodoindole exhibited the strongest effect on both normal and persister cells.
IMPORTANCE Our study demonstrated that a small persister population exhibits significantly higher heat tolerance than normal cells and that this small fraction contributes to the heat tolerance of the total bacterial population. This study also demonstrated that indole, known to inhibit persister formation, and its derivatives are very promising candidates to reduce the heat tolerance of not only normal bacterial cells but also persister cells.
INTRODUCTION
Antimicrobials are generally fed to livestock to prevent infections by pathogens, but unfortunately consumption of such livestock poses a severe threat to human health due to the development of drug-resistant bacteria. Even vegetables can be causative when they grow with manure containing fecal contamination by drug-resistant bacteria (1). Drug resistance in bacteria normally occurs due to gene mutations, which can be inherited from one generation to the next (2). Strictly controlled usage of antimicrobials in agriculture and livestock production can prevent emergence of drug resistance because drug resistance generally arises through unnecessary and uncontrolled overdoses. Conversely, so-called persister cells represent an uninherited, sneaky bacterial population that is more difficult to combat, since they evade antimicrobial attack. Persister cells are genetically identical to parental cells but are somehow in a metabolically inactive state; therefore, they exhibit remarkable tolerance against antibiotics targeting cellular activities such as cell division or protein synthesis (3, 4).
The toxin-antitoxin (TA) system is a key regulation factor of stress tolerance and persister formation (5–7). The TA system consists of a toxin and the corresponding antitoxin encoded in a single operon. The toxin inhibits bacterial metabolic systems such as DNA replication, transcription, and translation or destabilizes bacterial membrane homeostasis (7, 8). The antitoxin encoded in front of the toxin gene is a dedicated immune protein or mRNA for its toxin. In the case of the type II TA system, antitoxin functions as a protein that inhibits toxin by forming a protein complex during nonstress conditions while environmental stresses such as heat shock, oxidative stress, antibiotic attacks, or starvation trigger proteolytic degradation of antitoxin, resulting in free cytosolic toxin that induces a nongrowing state to survive stress conditions (9). Several type II TA systems have been demonstrated to be involved in persister formation, and HipAB was the first one to be discovered (10–12). Toxin HipA inhibits translation by phosphorylation of the elongation factor EF-Tu, preventing its interaction with tRNA. Toxin RelE is the best-characterized ribosome-dependent RNA interferase, which cleaves mRNA at the A site of the ribosome with site preferences (13). YafQ is also characterized as a ribosome-dependent mRNA interferase that targets the mRNAs of several genes, such as those of elongation factors or tryptophanase (14–16). These toxins eventually interfere with translation, resulting in drug-tolerant persister cell formation. Indole production in Escherichia coli is directly regulated by the YafQ-DinJ system, in which YafQ degrades the mRNA of tnaA, which encodes tryptophanase that metabolizes tryptophan (Trp) to indole (16). Furthermore, it was demonstrated that indole production controlled the persister formation of E. coli, and pretreatment with a high concentration of indole (∼2.0 mM) before antibiotic treatments significantly reduced the persister population.
Although many studies have described the practical issues and fundamental drug resistance mechanisms of bacterial persister cells, no study so far has discussed the tolerance of persister cells to physical stresses, such as heat, acidic, and oxidative stresses, except the study of bet hedging in Saccharomyces cerevisiae by Levy et al., in which persister cells of S. cerevisiae showed more heat tolerance than normal cells (17). Therefore, this study aimed to investigate the role of the persister population and the YafQ-DinJ toxin-antitoxin system and its regulating indole production in bacterial heat tolerance.
RESULTS
Heat tolerance, injury, and recovery of the ΔyafQ and ΔdinJ mutants.
To investigate the role of the YafQ-DinJ system in E. coli heat tolerance, yafQ (ΔyafQ) and dinJ (ΔdinJ) single-gene knockout mutants and wild-type (WT) E. coli BW25113 were exposed to heat stress (55°C) in 5 ml of tryptic soy broth (TSB) in test tubes followed by recovery culture for 4 h. The cell viabilities were determined before and after heat treatment and once every hour during recovery culture by plating on tryptic soy agar (TSA) and deoxycholate hydrogen sulfide lactose (DHL) agar plates. Both intact and heat-injured cells could form colonies on TSA plates, while only intact cells could form colonies on DHL agar plates, because a biological surfactant, sodium deoxycholate, inhibited the growth of heat-injured cells. Therefore, the difference between the CFU of TSA and DHL was evaluated as the number of injured cells in this study. According to the viable counts on TSA, cell amounts of all three tested strains were approximately 109 CFU/ml before heat treatments.
The viable count of the WT on DHL agar did not show a difference compared to the count on TSA, whereas the counts of the ΔyafQ and ΔdinJ mutants on DHL showed lower tendencies than those on TSA even without heat stress (Fig. 1A) (time [t] = −1). The differences between TSA and DHL counts of the ΔyafQ and ΔdinJ mutants were 0.28 ± 0.19 and 0.25 ± 0.14 log CFU/ml, respectively, which indicates that more than 50% of their cells were somehow already injured without heat stress. After heat treatment as shown in Fig. 1A (t = 0), the intact cell count of WT (on DHL) decreased to 3.65 ± 0.32 log CFU/ml, while the total cell count including intact and injured cells (on TSA) decreased to 5.76 ± 0.54 log CFU/ml, indicating that more than 99% of the survivors were injured but culturable by this heat treatment. The TSA count of the ΔyafQ mutant also decreased (6.07 ± 1.00 log CFU/ml) similarly; however, the DHL count (4.78 ± 0.35 log CFU/ml) after heat treatment was significantly higher than that of the WT (3.65 ± 0.32 log CFU/ml). In other words, approximately 1% of surviving WT cells were intact cells while approximately 12% of surviving ΔyafQ mutant cells were intact, indicating that the surviving ΔyafQ mutant cells were more heat tolerant than those of the WT. Conversely, compared to WT, the ΔdinJ mutant showed higher viable counts on both TSA and DHL (7.24 ± 0.50 and 5.16 ± 0.61 log CFU/ml, respectively), and the ratio of intact cells in the surviving cells was approximately 1%, indicating the higher heat tolerance of the total population of the ΔdinJ mutant.
FIG 1.
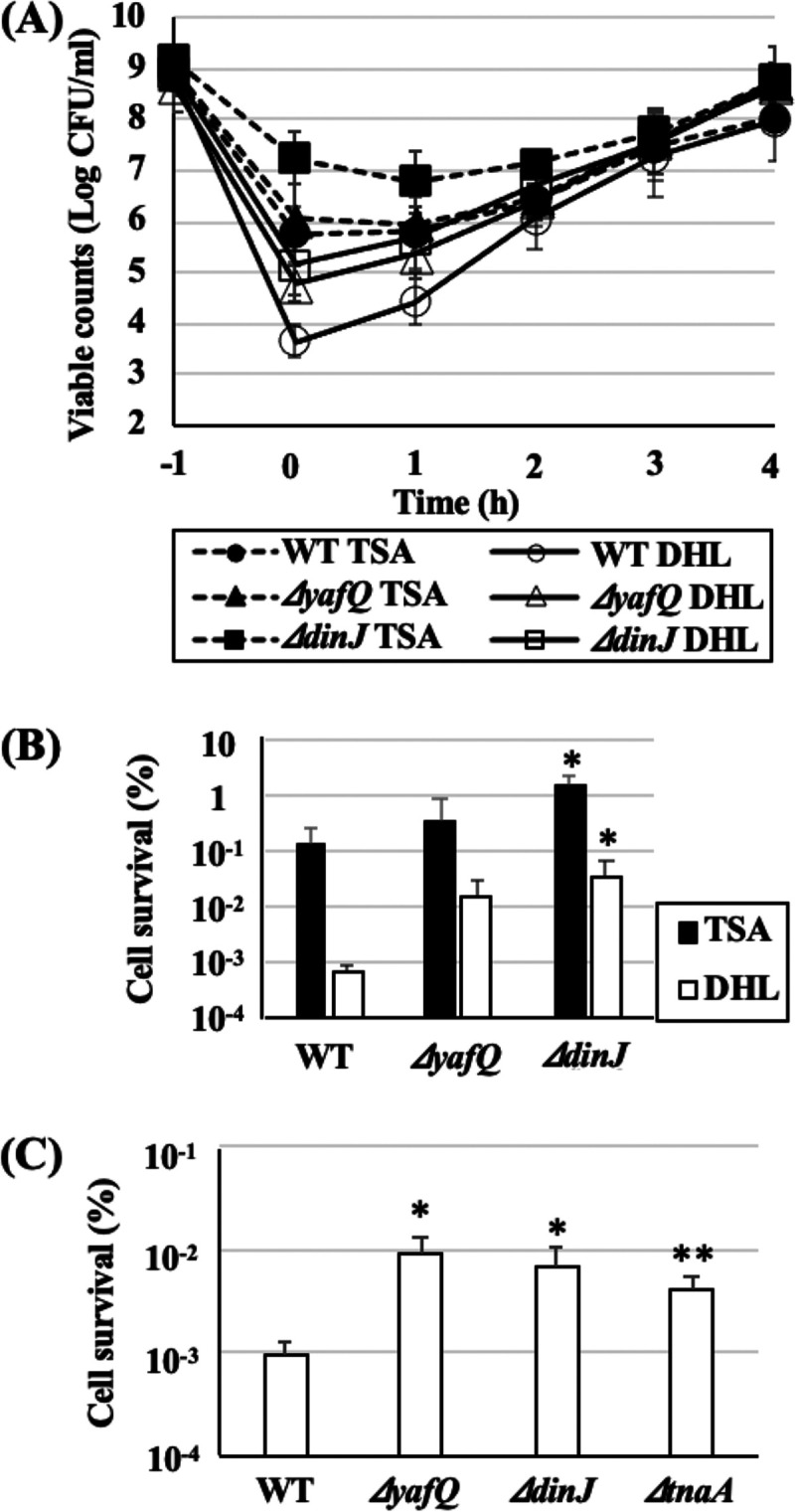
Heat tolerance, injury, and recovery of E. coli BW25113 and its single-gene knockout mutants. (A) E. coli BW25113 (WT), the ΔyafQ mutant, and the ΔdinJ mutant were cultured in TSB at 37°C overnight and subsequently subjected to heat treatments in test tubes (60°C, 10 min). The viable counts of WT and single-gene knockout mutants during heat treatments and recovery cultures were determined. Data points at t = −1 and 0 h mean viable counts before and just after heat treatments. (B) Heat tolerance of each strain was determined as a ratio of the number of the cells that survived heat treatment (t = 0) to the number of untreated (before heat treatment, t = −1) cells. (C) Heat tolerances of intact cells were determined by viable counts on DHL plates before and after heat treatments with the thermal cycler method. Data points represent means of results from at least three independent experiments, and error bars indicate standard deviations. Significant differences between the data for WT and each knockout mutant strain on TSA or DHL plates were analyzed by t test (*, P < 0.1; **, P < 0.05).
Heat tolerance was also evaluated as cell survival rate (%) on both TSA and DHL plates after heat treatment (Fig. 1B). Both ΔyafQ and ΔdinJ mutants exhibited higher survival rates after heat treatment compared to that of the WT; particularly, the survival rates of their intact cells were more than 10-fold greater than the survival rate of intact cells in the WT. As shown in Fig. 1A, during recovery culture, only DHL counts of the three strains had increased, while TSA counts were the same for the first 1 h, which means that only injured cells had recovered but no cell divisions had occurred during this period. DHL and TSA counts were at the same level at 2 h, and thereafter both counts simultaneously increased, indicating that complete recovery from heat injury was achieved at 2 h and cell division restarted spontaneously. Recovery behaviors of the ΔyafQ and ΔdinJ mutants were similar to that of WT.
Persister isolation and heat tolerance of persister cells.
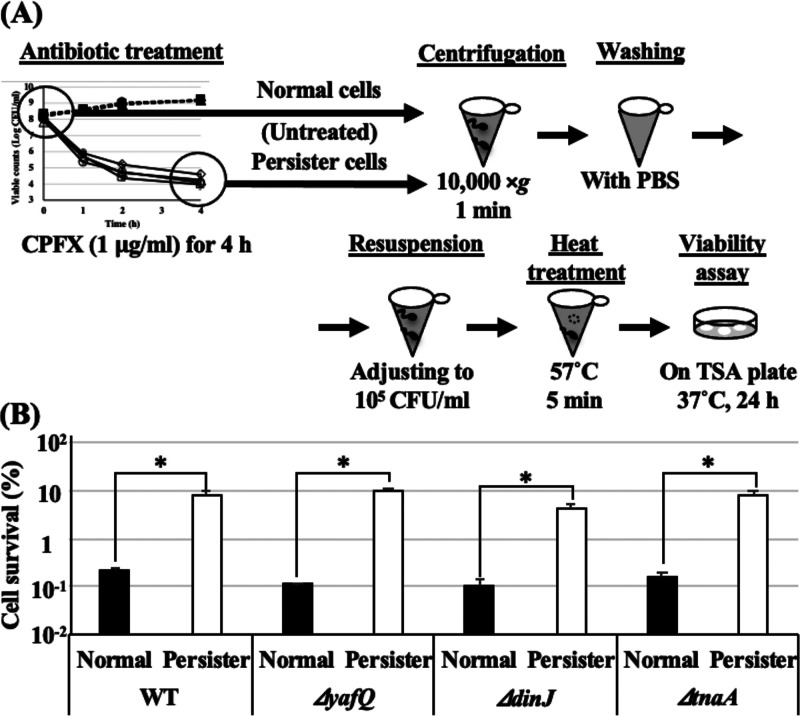
As shown in Fig. 1B and C, the ΔyafQ and ΔdinJ mutants and the tnaA single-gene knockout mutant (ΔtnaA) exhibited tendencies of higher heat tolerance than WT. Therefore, to confirm whether persister frequency in total population affects the difference in heat tolerance, persister cells of all strains were isolated and their heat tolerances were examined. In order to determine the viable counts of both intact and injured but recoverable cells, only TSA was used for viable counts in the following experiments. All strains were treated with ciprofloxacin (CPFX; 1.0 μg/ml) for 4 h, and viable counts were determined once every hour (Fig. 2A). After 4 h treatment, killing curves reached plateau phases where only persister cells survived, so these surviving cells were collected, and the cell densities were readjusted for the subsequent heat tolerance assay. All deletion mutants showed tendencies toward higher persister frequencies than that of the WT ([1.15 ± 0.22] × 10−2%) (Fig. 2B). The viable counts of CPFX-untreated cells (normal cells) of all strains after heat treatments with thermal cycler (57°C for 5 min) were below the detection limit (102 CFU/ml). Therefore, the viable counts of all untreated strains after heat treatments were considered to be less than 102 CFU/ml for the calculation of cell survival rates, while all CPFX-treated persister cells exhibited survival rates >100-fold higher than those of the normal parental cells (Fig. 3). Interestingly, there was no difference in the survival rates among the persister cells of the WT and all deletion mutants.
FIG 2.
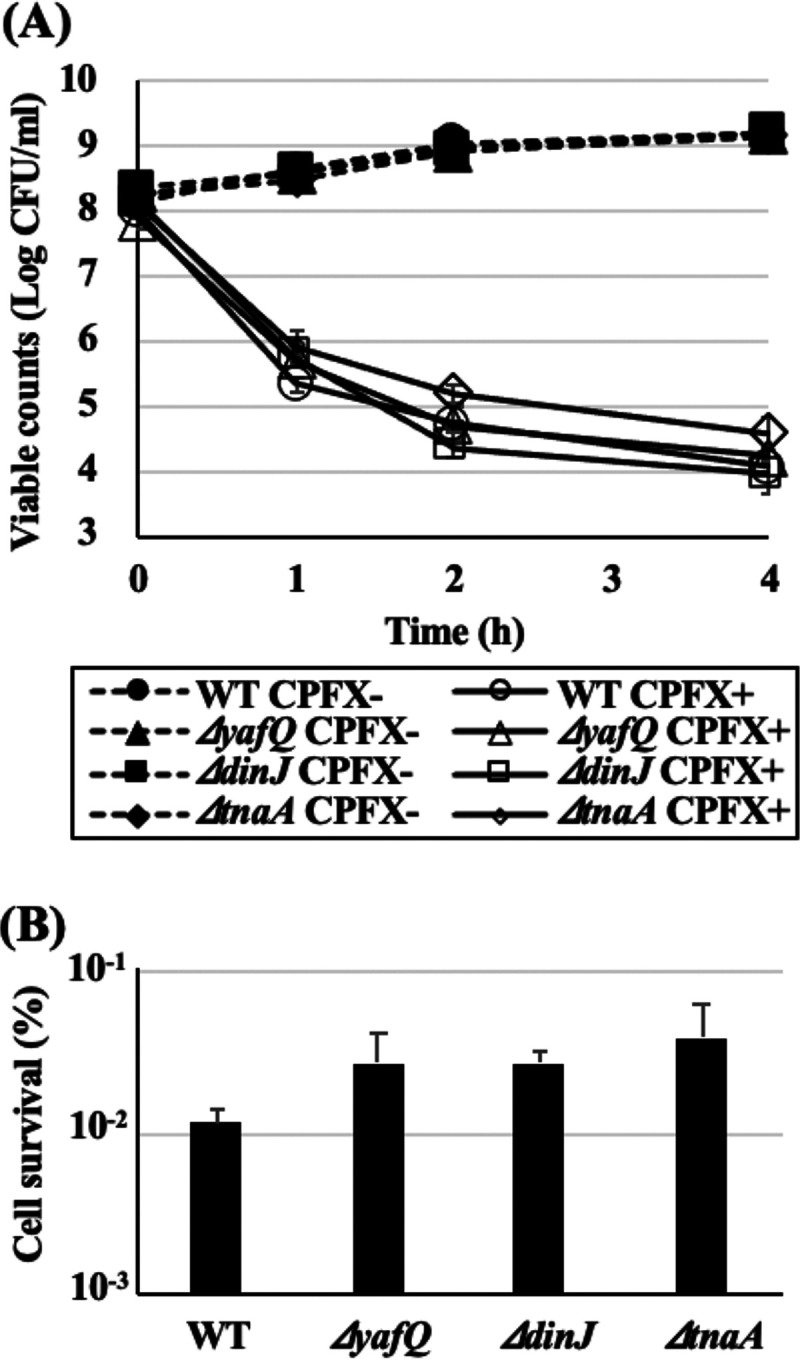
Persister cells were isolated by 4-h treatments with CPFX. (A) Precultures of WT, the ΔyafQ mutant, the ΔdinJ mutant, and the ΔtnaA mutant were diluted in fresh LB to obtain 108 to 109 CFU and subjected to CPFX treatments (1.0 μg/ml, 37°C, 120 rpm). Viable count was monitored during CPFX treatments. Data points at 0 h mean numbers of viable counts before treatment. (B) Persister frequency was determined by calculating the ratio of the cells that survived 4 h CPFX treatment to the number of cells before treatment. Data points represent means of results from at least three independent experiments, and error bars indicate standard deviations. Significant differences between the data for WT and each knockout mutant strain were analyzed by t test. P values for the ΔdinJ, ΔyafQ, and ΔtnaA mutants were 0.19, 0.52, and 0.24, respectively.
FIG 3.
Heat tolerance of persister cells was a great deal higher than that of intact cells. (A) Scheme illustrating the setup for persister isolation and heat tolerance assay. (B) Isolated persister cells and normal (untreated) cells of WT and the ΔyafQ, ΔdinJ, and ΔtnaA mutants were adjusted to be approximately 105 CFU/ml in fresh TSB and subjected to heat tolerance assay with the thermal cycler method (57°C for 5 min). The viable counts of normal cells after heat treatments were below the detection limit (102 CFU/ml); therefore, their viable counts after heat treatments were considered as 102 CFU/ml for the calculation of the cell survival rates here. Data points represent means of results from at least three independent experiments, and error bars indicate standard deviations. Significant differences between the data for normal and persister cells were analyzed by t test (*, P < 0.05).
Effect of tryptophan on persister formation and heat tolerance.
Additional tryptophan (Trp) in culture medium increases indole production and reduces persister formation of E. coli (16). To confirm the contribution of persister cells to heat tolerance of E. coli, persister frequency and heat tolerance with additional Trp were examined in the WT and the ΔtnaA mutant. Indole production of WT increased from 108 ± 4.69 to 293 ± 14.0 μM/optical density at 600 nm (OD600), and the persister frequency decreased from (1.15 ± 0.22) × 10−2% to (0.29 ± 0.01) × 10−2% in a medium with additional Trp compared to that in a medium without additional Trp (Fig. 4A). In addition, the survival rate of the cells cultured with additional Trp (0.88% ± 0.22%) was significantly lower than that of cells cultured without additional Trp (3.37% ± 0.44%) (Fig. 4B). On the contrary, there was no difference in either persister frequency or heat tolerance between ΔtnaA mutant cells cultured with and without additional Trp. Indole production of the ΔtnaA mutant was lower than the detection limit (0.01 mM) both with and without additional Trp. These results confirm that the highly heat-tolerant persister fraction contributes to the heat tolerance of the total bacterial population, and indole controls the heat tolerance via persister frequency.
FIG 4.
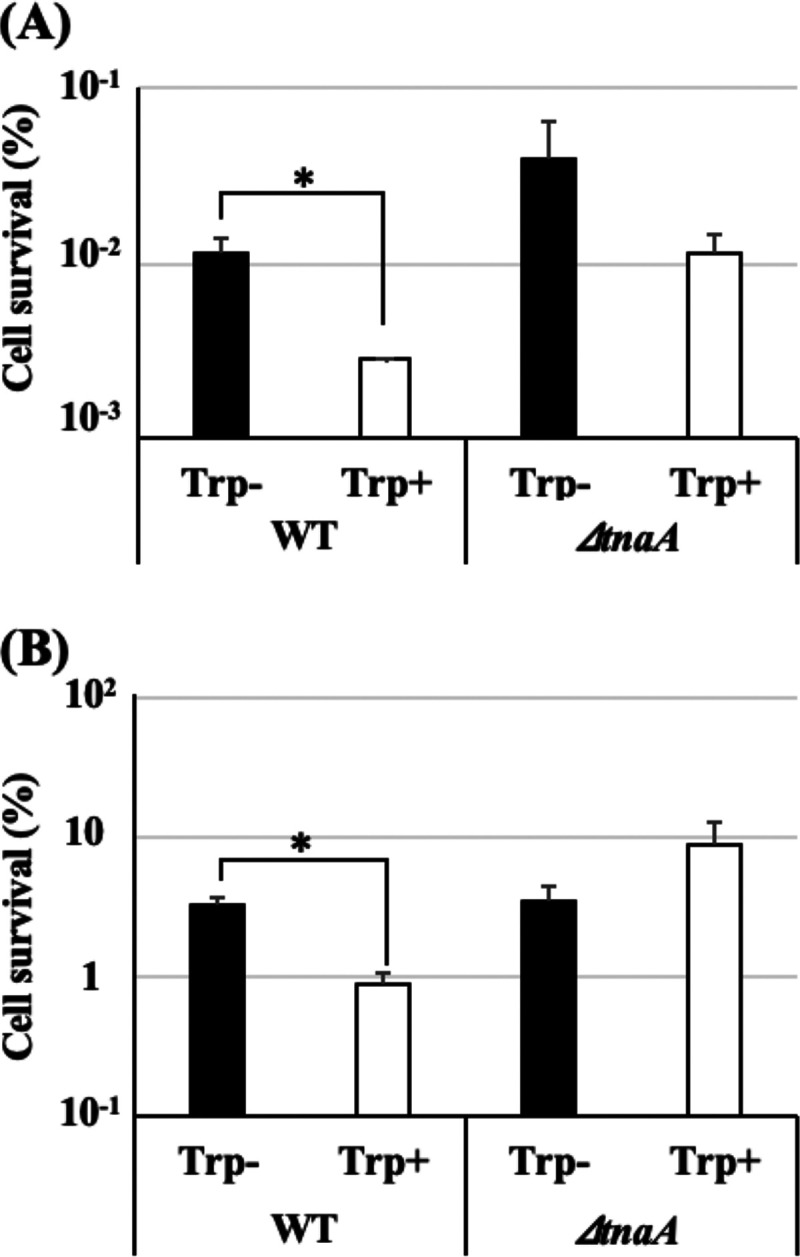
Additional Trp reduced persister frequency and heat tolerance of E. coli. WT and the ΔtnaA mutant were cultured at 37°C with aeration for 24 h in TSB with or without additional Trp (4.0 mM) and subsequently resuspended in fresh LB to 108 to 109 CFU/ml and subjected to CPFX treatments to determine persister frequencies (A) or diluted in fresh TSB to be approximately 108 CFU/ml and subjected to heat tolerance analysis using the thermal cycler method (57°C, 5 min) (B). Data points represent means of results from at least three independent experiments, and error bars indicate standard deviations. Significant differences between Trp− and Trp+ data were analyzed by t test (*, P < 0.05).
Direct effect of indole on heat tolerance.
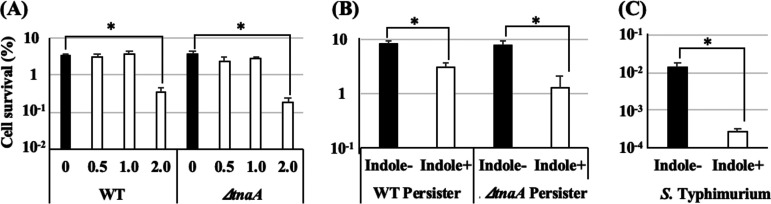
Hu et al. demonstrated that an addition of a certain amount of indole strictly reduced persister formation (16). Similarly, in our study, highly heat tolerant persister cells were controlled by the synthesized indole, and these cells could survive under heat stress conditions where normal cells could not. Therefore, the direct effect of indole on the heat tolerance of bacterial cells was investigated. To avoid persister-mediated effects on heat tolerance, indole was added 10 min before heat treatments, as this time period was not sufficient to change the persister frequency. As shown in Fig. 5A, 10-min indole (2.0 mM) pretreatment reduced the survival rates of both the WT and the ΔtnaA mutant to less than one-tenth of the survival rates of untreated cells. However, there was no significant effect of indole at a concentration less than 1.0 mM. Interestingly, 10 min of indole pretreatment also significantly reduced the survival rates of persister cells of both the WT (from 8.17% ± 1.54% to 3.92% ± 0.83%) and the ΔtnaA mutant (from 7.92% ± 1.68% to 1.34% ± 0.78%) (Fig. 5B). Furthermore, indole also greatly reduced the survival rate of indole-nonproducing Salmonella enterica serovar Typhimurium NBRC12529 ([1.39 ± 0.41] × 10−2% to [0.26 ± 0.06] × 10−3%). (Fig. 5C).
FIG 5.
Indole can directly reduce bacterial heat tolerance. (A) Precultures of WT and the ΔtnaA mutant were diluted in fresh TSB to obtain approximately 108 CFU/ml and subjected to heat tolerance analysis (55°C, 5 min) with several final concentrations of indole (approximately 0 to 2.0 mM). (B) Isolated persister cells of WT and the ΔtnaA mutant were resuspended in fresh TSB with or without 2.0 mM indole to obtain approximately 105 CFU/ml and subjected to heat tolerance assay (57°C, 5 min). (C) The preculture of S. Typhimurium was diluted in fresh TSB with or without 2.0 mM indole to obtain approximately 108 CFU/ml and subjected to heat tolerance analysis (55°C, 5 min). Data points represent means of results from at least three independent experiments, and error bars indicate standard deviations. Significant differences between indole− and indole+ data were analyzed by t test (*, P < 0.05).
Effect of indole derivatives on heat tolerance.
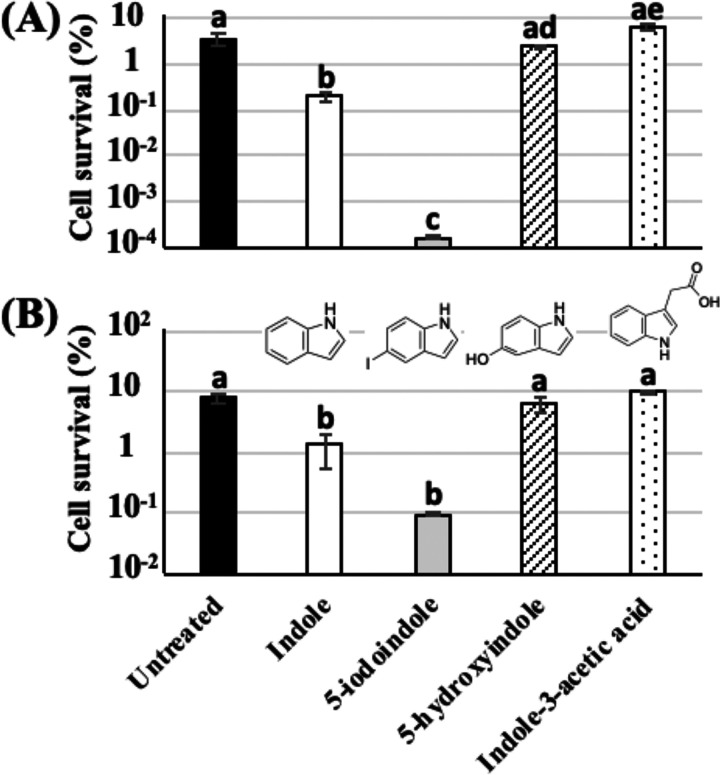
Among the great variety of indole derivatives, halogenated indoles are capable of eradicating persister cells and biofilm (18). Therefore, we investigated the potential of three indole derivatives in reducing heat tolerance of both normal and persister cells. Since 5-iodoindole has antibacterial activity against E. coli, we first treated normal cells of E. coli with 0.5, 1.0, or 2.0 mM 5-iodoindole to determine the optimal concentration for the heat tolerance assay. A significant reduction of viable count was obtained with 1.0 and 2.0 mM 5-iodoindole treatment, but no reduction was obtained in the case of the 0.5 mM treatment (data not shown). Therefore, subsequent heat tolerance assays were performed with 0.5 mM 5-iodoindole and 2.0 mM indole, 5-hydroxyindole, and indole-3-acetic acid. As shown in Fig. 6A, 5-iodoindole exhibited a stronger effect on the heat tolerance of normal cells even at a concentration less than that of indole. The survival rate of cells treated with 5-iodoindole ([1.75 ± 0.20] × 10−4%) was approximately 0.005% of the survival rate of untreated cells (3.54% ± 0.95%) and approximately 0.09% of the survival rate of indole-treated cells (0.19% ± 0.04%). However, 5-hydroxyindole and indole-3-acetic acid did not reduce the heat tolerance of normal cells. In addition, 5-iodoindole also exhibited a higher potential for reducing the heat tolerance of persister cells compared to that of indole (Fig. 6B), although the difference was not as clear as that observed in normal cells (Fig. 6A). The survival rate of persister cells treated with 5-iodoindole (0.10% ± 0.01%) was approximately 1.26% of untreated cells (7.92% ± 1.68%) and approximately 7.5% of indole-treated cells (1.34% ± 0.78%). However, the other two derivatives did not show any difference compared to untreated cells.
FIG 6.
Effects of indole derivatives on heat tolerance of the ΔtnaA mutant. (A) The preculture of the ΔtnaA mutant was diluted in fresh TSB with indole (2.0 mM), 5-iodoindole (0.5 mM), 5-hydroxyindole (2.0 mM), or indole-3-acetic acid (2.0 mM) to obtain approximately 108 CFU/ml and subjected to heat tolerance assays (55°C, 5 min). (B) Isolated persister cells of the ΔtnaA mutant were resuspended in fresh TSB with indole (2.0 mM), 5-iodoindole (0.5 mM), 5-hydroxyindole (2.0 mM), or indole-3-acetic acid (2.0 mM) to obtain approximately 105 CFU/ml and subjected to heat tolerance assays (57°C, 5 min). Data points represent means of results from at least three independent experiments, and error bars indicate standard deviations. Significant differences among all of the data were analyzed by the Tukey’s test, and bars with different letters are statistically different (P < 0.05).
DISCUSSION
Indole plays many important roles in bacterial physiologies, such as biofilm formation, virulence, spore formation, acid resistance, and persister formation (19–23). The conversion of tryptophan to indole by tryptophanase (TnaA) is the only indole synthesis pathway in bacteria, and over 85 bacterial species, including both Gram-negative and Gram-positive bacteria, utilize this pathway (24–26). The concentration of excreted indole into a liquid medium can normally reach 0.5 to 1.0 mM, and the presence of sufficient amounts of Trp can increase its production (25). The secreted indole acts as an intercellular signaling molecule, such as quorum sensing (QS) molecules, in microbial communities (23, 26, 27). Various bacteria coexist in nature; therefore, indole-nonproducing bacteria can encounter a significant amount of indole produced by indole-producing bacteria, and indole in a broad range of concentrations can have harmful or beneficial effects on individual bacteria (21, 23, 26, 28). In this study, we demonstrated that the persister fraction in total bacterial population considerably contributed to bacterial heat tolerance, and indole played a key role in regulating bacterial heat tolerance with or without influencing persister frequency.
YafQ-DinJ is a type II toxin-antitoxin system and is known to regulate persister formation by controlling indole production. Overexpressed YafQ, an endoribonuclease-type toxin, strictly reduced tnaA expression and, consequently, indole production and persister formation (16). As shown in Fig. 1, the ΔyafQ mutant, the ΔdinJ mutant, and the indole-nonproducing ΔtnaA mutant exhibited higher heat tolerance than the WT strain. The higher heat tolerances of these three mutants could be explained by their higher persister frequencies (Fig. 2) and the higher heat tolerance of those persister cells (Fig. 3). The higher persister frequency and heat tolerance of the ΔtnaA mutant may be due to the absence of indole production, as the enhanced indole production in the WT by additional Trp significantly reduced persister frequency and heat tolerance (Fig. 4).
As antitoxin strongly inhibits the effects of toxin, the phenotype of the antitoxin knockout mutant can be influenced by free toxin, while the phenotype of the toxin knockout mutant can show contrary or no influence. However, both the ΔyafQ and ΔdinJ mutants somehow exhibited higher persister frequencies and heat tolerances than the WT (Fig. 1 and 2). Moreover, indole productions by the ΔyafQ mutant (181 ± 4.83 μM/OD600) and the ΔdinJ mutant (148 ± 15.47 μM/OD600) were significantly higher than that by the WT (108 ± 4.69 μM/OD600). Higher indole production of the ΔyafQ mutant might be attributed to the lack of YafQ, which results in nonribosomal degradation of tnaA transcripts. Contradictorily, however, this increased indole production did not reduce the persister frequency and heat tolerance of the ΔyafQ mutant compared to that of the WT. Higher indole production by the ΔdinJ mutant seems to be inconsistent with its higher persister frequency and heat tolerance compared to that of the WT. In 2012, Vega et al. demonstrated that addition of 0.5 mM indole to culture medium increased the persister frequency of the ΔtnaA mutant (23). The extracellular indole concentrations of overnight culture supernatants of the WT and the ΔyafQ and ΔdinJ mutants were 0.41 ± 0.04 mM, 0.54 ± 0.38 mM, and 0.57 ± 0.05 mM, respectively. These results indicate that, in the range of these low concentrations, enhanced indole production of the ΔyafQ and ΔdinJ mutants could increase their persister frequencies and heat tolerances. Conversely, additional Trp enhanced indole production to approximately 1.0 mM, which was enough to reduce the persister frequency according to the study of Hu et al. (16); therefore, the heat tolerance of the total bacterial population was also reduced subsequently (Fig. 4).
The direct effect of indole on bacterial heat tolerance was also evaluated in this study, and the results indicated that indole somehow directly acts on bacterial cells to reduce heat tolerance without influencing the number of persister cells (Fig. 5). As described above, different concentrations of indole can have different effects, such as enhancing biofilm formation and reducing or increasing persister frequency. The concentration of extracellular indole required to reduce heat tolerances of the WT and the ΔtnaA mutant was 2.0 mM; this concentration of indole could also reduce the heat tolerances of their persister cells and S. Typhimurium cells. These results suggest that the decrease in heat tolerance by additional Trp (Fig. 4) was caused by reduction in persister formation by indole production and not by the direct effect of indole on heat tolerance, as the indole concentration of the culture supernatant with additional Trp was only 1.01 ± 0.04 mM, which is less than the effective indole concentration (2.0 mM) required to directly reduce heat tolerance. In 2013, Kim et al. demonstrated that 1.0 mM indole inhibited the refolding of denatured protein in vitro (29), which could explain the direct effect of indole on bacterial heat tolerance. In addition, it has been demonstrated that a high concentration of indole (2.0 to 5.0 mM) prevented cell division of E. coli by modulating membrane potential (30), which could be the mechanism involved in the reduction of heat tolerance demonstrated in this study. Conversely, the halogenated indole 5-iodoindole exhibited remarkable reduction in the heat tolerances of both normal and persister cells at a concentration at which it did not show antimicrobial activity (i.e., 0.5 mM) (Fig. 6). It has already been demonstrated that 1.0 to 2.0 mM 5-iodoindol inhibited the growth of E. coli and Staphylococcus aureus and also eradicated the persister cells when used in combination with antibiotics (18). However, pretreatment time before heat treatment in our study was only 10 min, while the persister eradication effect of 5-iodoindole was detected after 3 h of incubation, indicating that, like indole, 5-iodoindole also directly reduced heat tolerance. Interestingly, 5-hydroxyindole did not reduce heat tolerance, which may suggest the importance of hydrophobicity of indole in interacting with denatured proteins and preventing their refolding.
In conclusion, a small fraction of persister cells regulated by TA systems and indole production possessed remarkably high heat tolerance and contributed to the heat tolerance of the total bacterial population. Therefore, this small persister population cannot be neglected in bacterial heat tolerance and food safety. Furthermore, as indole can reduce bacterial heat tolerance with or without influencing persister cells, indole and its derivatives can enhance the antimicrobial effect of heat treatments or other treatments for food production or hygienic purposes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type (WT) strain Escherichia coli BW25113 and the ΔyafQ, ΔdinJ, and ΔtnaA knockout mutants (Keio collection) were obtained from National BioResource Project (NBRP). Salmonella enterica serovar Typhimurium NBRC 12529 was purchased from the Institute for Fermentation, Osaka (IFO). Tryptic soy broth (TSB) (BD) and Luria-Bertani (LB) broth (BD) were used for heat treatment and persister isolation, respectively. Tryptic soy agar (TSA) (BD), LB agar (Nacalai Tesque, Japan), and deoxycholate hydrogen sulfide lactose (DHL) agar (Nissui Pharmaceutical, Japan) were used for determination of cell viability. Phosphate-buffered saline (PBS; pH 7.4) was used for cell dilution and washing steps.
All strains were refreshed from glycerol stocks at −80°C by overnight culture at 37°C with aeration, subsequently streaked on TSA plates, and incubated overnight at 37°C. For refreshing cultures of knockout mutants from −80°C stocks, 5.0 μg/ml of kanamycin (Nacalai Tesque, Japan) was added to TSA or TSB. A single colony from each plate was transferred to fresh TSB or LB broth, precultured overnight at 37°C with aeration, and subsequently used for the experiments. To investigate the influence of tryptophan (Trp), a single colony from each plate was transferred to TSB containing 4.0 mM Trp (TSBW), cultured at 37°C with aeration for 24 h, and subsequently used for the experiments.
Cell viability assay.
Cell viability was determined by counting the number of colonies (CFU) on selective (DHL) or nonselective (TSA) agar plates. In this study, the number of colonies on DHL plates represented the number of intact cells and the number on TSA plates represented the number of intact and injured but recoverable cells. Therefore, the number of heat-injured cells was determined as CFUTSA − CFUDHL and evaluated as log(CFUTSA/CFUDHL). An LB agar plate was used to determine the cell viability for persister cells. Colony counts were performed using the spotting method as described previously with certain modifications (31, 32). Briefly, the samples were serially diluted with PBS and subsequently spot plated (10 μl) or spread plated (100 μl) on plates. The detection limit for spot plating and spread plating was 100 and 10 CFU/ml, respectively. The plates were incubated at 37°C for 24 h; then, the number of colonies was counted.
Isolation of persister cells.
LB precultures of all strains were diluted in fresh LB, and OD600 was adjusted to 0.4 (∼108 to ∼109 CFU/ml). For TSB and TSBW precultures, cells were collected by centrifugation (10,000 × g, 1 min, 25°C), washed with PBS, and subsequently resuspended in fresh LB to an OD600 of 0.4 (∼108 to ∼109 CFU/ml). Ciprofloxacin (CPFX) (Nacalai Tesque, Japan) was added to adjusted cells at a final concentration of 1.0 μg/ml, and cells were incubated at 37°C with aeration (120 rpm) for 4 h. After 0, 1, 2, and 4 h of incubation, cells were collected from 500 μl of cultures by centrifugation and resuspended in 500 μl of PBS. The cells were washed, again centrifuged, resuspended in 500 μl of PBS, and cell viability was determined by plating LB agar (CFU/ml). Persister frequency was calculated as the ratio of cells that survived 4 h CPFX treatment to the parental population before antibiotic treatment. The cells that survived 4 h CPFX treatment were collected by centrifugation, washed with PBS, and subsequently used for heat tolerance assays.
Heat tolerance assay.
Heat treatments were performed using two methods, the test tube method and the thermal cycler method. The test tube method was used only for heat injury and recovery assays. Briefly, TSB preculture was diluted in fresh TSB and to obtain an OD600 of 0.1 (∼108 CFU/ml). Five milliliters of this cultures was placed in a test tube and incubated in a water bath (120 rpm) at 25°C for 10 min, followed by 60°C for 10 min, and finally 25°C for 10 min; next, the test tube was incubated at 37°C for 4 h (120 rpm) to allow recovery of heat-injured cells. Cell viability was determined at each time point (before heat treatment and at 0, 1, 2, 3, and 4 h after heat treatment).
Other heat treatments were carried out using the Thermal Cycler Dice Touch (TaKaRa, Japan) with a 0.2-ml PCR tube (Nippon Genetics, Japan). Briefly, the preculture was diluted in fresh TSB to obtain an OD600 of 0.1 (∼108 CFU/ml). Aliquots of 75 μl were placed in PCR tubes and subjected to heat treatment in the thermal cycler (25°C for 10 min, 55°C for 5 min, 25°C for 10 min). For heat tolerance assay of persister cells, isolated persister cells were resuspended in fresh TSB to obtain a cell density of approximately 105 CFU/ml, and heat treatments were performed using a temperature of 57°C instead of 55°C. CPFX-untreated normal cells could spontaneously involve persister fractions less than 0.1% of the total population. The influence of persister cells in the normal cell population was insignificant in this study, as the cell density of normal cells was adjusted to be approximately 105 CFU/ml where the number of persister cells was below the detection limit (102 CFU/ml). Cell viability was evaluated before and after heat treatments.
Evaluating the effects of indole and its derivatives on heat tolerance.
To evaluate the effects of indole and its derivatives on heat tolerance, precultures of WT and the ΔtnaA mutant in TSB were diluted in fresh TSB with 0.5 to 2.0 mM indole (Tokyo Chemical Industry, Japan), 5-iodoindole (Sigma-Aldrich), 5-hydoroxyindole (Tokyo Chemical Industry, Japan), or indole-3-acetic acid (Tokyo Chemical Industry, Japan) and applied to the following heat tolerance assays. Persister cells of WT and the ΔtnaA mutant were also resuspended in fresh TSB with indole or its derivatives and applied to the following heat tolerance assays. The final concentration of indole was 2.0 mM for the heat tolerance assay of indole-nonproducing Salmonella enterica serovar Typhimurium NBRC 12529.
Indole measurement.
Indole measurements were carried out according to previous reports (20) with certain modifications. Briefly, standard solutions of indole in the range of 0 to 800 μM concentrations were prepared in butanol (Nacalai Tesque, Japan). Cell-free supernatants were prepared by centrifuging 1 ml of cultured samples (OD600 = 0.1). In order to measure the indole productions, 50 μl of supernatant was mixed with 400 μl of butanol and 50 μl of Kovac’s reagent (Merck), while 50 μl of standard solution was mixed with 350 μl of butanol, 50 μl of fresh TSB, and 50 μl of Kovac’s reagent. After incubation at 25°C for 5 min, the absorbance of standard solutions and samples at 590 nm was measured, and indole concentrations were calculated from the established standard curve. When the concentration of indole was higher than 800 μM, the supernatants were optimally diluted with fresh TSB to obtain a concentration within the range of the standard curve.
Statistical analysis.
Data points of all experiments represent means of the results from at least three independent experiments, and error bars indicate standard deviations. Significance of the differences was determined using the t test or Tukey’s test in Excel.
REFERENCES
- 1.Woolhouse MEJ, Ward MJ. 2013. Sources of antimicrobial resistance. Science 341:1460–1461. doi: 10.1126/science.1243444. [DOI] [PubMed] [Google Scholar]
- 2.McEwen SA, Collignon PJ. 2017. Antimicrobial resistance: a one health perspective. Microbiol Spectr 6:ARBA-0009-2017. doi: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pontes MH, Groisman EA. 2019. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci Signal 12:eaax3938. doi: 10.1126/scisignal.aax3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis K. 2010. Persister cells. Annu Rev Microbiol 64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 5.Harms A, Maisonneuve E, Gerdes K. 2016. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354:aaf4268. doi: 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- 6.Maisonneuve E, Gerdes K. 2014. Molecular mechanisms underlying bacterial persisters. Cell 157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Page R, Peti W. 2016. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol 12:208–214. doi: 10.1038/nchembio.2044. [DOI] [PubMed] [Google Scholar]
- 8.Unterholzner SJ, Poppenberger B, Rozhon W. 2013. Toxin-antitoxin systems: biology, identification, and application. Mob Genet Elements 3:e26219. doi: 10.4161/mge.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan WT, Espinosa M, Yeo CC. 2016. Keeping the wolves at bay: antitoxins of prokaryotic type II toxin-antitoxin systems. Front Mol Biosci 3:9. doi: 10.3389/fmolb.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moyed HS, Bertrand KP. 1983. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol 155:768–775. doi: 10.1128/JB.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher MA, Balani P, Min J, Chinnam NB, Hansen S, Vulić M, Lewis K, Brennan RG. 2015. HipBA–promoter structures reveal the basis of heritable multidrug tolerance. Nature 524:59–64. doi: 10.1038/nature14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen SV, Turnbull KJ, Roghanian M, Bærentsen R, Semanjski M, Brodersen DE, Macek B, Gerdes K. 2019. Serine-threonine kinases encoded by split hipA homologs inhibit tryptophanyl-tRNA synthetase. mBio 10:e01138-19. doi: 10.1128/mBio.01138-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen K, Zavialov AV, Pavlov MY, Elf J, Gerdes K, Ehrenberg M. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112:131–140. doi: 10.1016/s0092-8674(02)01248-5. [DOI] [PubMed] [Google Scholar]
- 14.Harrison JJ, Wade WD, Akierman S, Vacchi-Suzzi C, Stremick CA, Turner RJ, Ceri H. 2009. The chromosomal toxin gene yafQ is a determinant of multidrug tolerance for Escherichia coli growing in a biofilm. Antimicrob Agents Chemother 53:2253–2258. doi: 10.1128/AAC.00043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maehigashi T, Ruangprasert A, Miles SJ, Dunham CM. 2015. Molecular basis of ribosome recognition and mRNA hydrolysis by the E. coli YafQ toxin. Nucleic Acids Res 43:8002–8012. doi: 10.1093/nar/gkv791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Kwan BW, Osbourne DO, Benedik MJ, Wood TK. 2015. Toxin YafQ increases persister cell formation by reducing indole signalling. Environ Microbiol 17:1275–1285. doi: 10.1111/1462-2920.12567. [DOI] [PubMed] [Google Scholar]
- 17.Levy SF, Ziv N, Siegal ML. 2012. Bet hedging in yeast by heterogeneous, age-correlated expression of a stress protectant. PLoS Biol 10:e1001325. doi: 10.1371/journal.pbio.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Kim YG, Gwon G, Wood TK, Lee J. 2016. Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Expr 6:123. doi: 10.1186/s13568-016-0297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Maeda T, Hong SH, Wood TK. 2009. Reconfiguring the quorum-sensing regulator SdiA of Escherichia coli to control biofilm formation via indole and N-acylhomoserine lactones. Appl Environ Microbiol 75:1703–1716. doi: 10.1128/AEM.02081-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirakawa H, Kodama T, Takumi-Kobayashi A, Honda T, Yamaguchi A. 2009. Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157:H7. Microbiology 155:541–550. doi: 10.1099/mic.0.020420-0. [DOI] [PubMed] [Google Scholar]
- 21.Kim YG, Lee JH, Cho MH, Lee J. 2011. Indole and 3-indolylacetonitrile inhibit spore maturation in Paenibacillus alvei. BMC Microbiol 11:119. doi: 10.1186/1471-2180-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirakawa H, Hayashi-Nishino M, Yamaguchi A, Nishino K. 2010. Indole enhances acid resistance in Escherichia coli. Microb Pathog 49:90–94. doi: 10.1016/j.micpath.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Vega NM, Allison KR, Khalil AS, Collins JJ. 2012. Signaling-mediated bacterial persister formation. Nat Chem Biol 8:431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T, Snell EE. 1972. Reversibility of the tryptophanase reaction: synthesis of tryptophan from indole, pyruvate, and ammonia. Proc Natl Acad Sci U S A 69:1086–1090. doi: 10.1073/pnas.69.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Young KD. 2013. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 159:402–410. doi: 10.1099/mic.0.064139-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Lee J. 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Jayaraman A, Wood TK. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Park W. 2015. Indole: a signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J Microbiol 53:421–428. doi: 10.1007/s12275-015-5273-3. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Hong H, Heo A, Park W. 2013. Indole toxicity involves the inhibition of adenosine triphosphate production and protein folding in Pseudomonas putida. FEMS Microbiol Lett 343:89–99. doi: 10.1111/1574-6968.12135. [DOI] [PubMed] [Google Scholar]
- 30.Chimerel C, Field CM, Piñero-Fernandez S, Keyser UF, Summers DK. 2012. Indole prevents Escherichia coli cell division by modulating membrane potential. Biochim Biophys Acta 1818:1590–1594. doi: 10.1016/j.bbamem.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieuwerts S, De Bok FAM, Mols E, De Vos WM, Van Hylckama Vlieg J. 2008. A simple and fast method for determining colony forming units. Lett Appl Microbiol 47:275–278. doi: 10.1111/j.1472-765X.2008.02417.x. [DOI] [PubMed] [Google Scholar]
- 32.Gayán E, Govers SK, Michiels CW, Aertsen A. 2016. Severely heat injured survivors of E. coli O157:H7 ATCC 43888 display variable and heterogeneous stress resistance behavior. Front Microbiol 7:1845. doi: 10.3389/fmicb.2016.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]