Abstract
Background
Immune checkpoint inhibitors (ICIs) have been used to treat many cancers, but ICIs are rarely administered for malignant tumours coexisting with inflammatory bowel disease.
Methods and results
We report a 77-year-old man experiencing an ulcerative colitis (UC) flare-up after receiving nivolumab as third-line therapy for multiple metastases of renal cell carcinoma. Mild UC (proctitis form) had been diagnosed at age 59 years and remission was maintained for 17 years with only a low dose of 5-ASA. After nivolumab treatment, the patient developed diarrhoea, bloody stools and was hospitalised. Computed tomography revealed inflammation involving the entire colon and endoscopy revealed severe UC exacerbation. Histological analysis showed UC findings and also increased crypt apoptosis which is unusual for inflammatory bowel diseases, while being typical of ICI-induced colitis. As with ICI-induced colitis, this exacerbation was strongly suggested to have been caused by nivolumab, although remission was achieved by increasing the 5-ASA dose to 4000 mg without prednisolone.
Conclusion
The administration of ICI for UC is not as yet sufficiently safe and further research is required.
Keywords: Nivolumab, Immune checkpoint inhibitors (ICIs), Ulcerative colitis (UC), Inflammatory bowel disease (IBD), 5-Aminosalicylic acid (5-ASA)
Introduction
Nivolumab and other immune checkpoint inhibitors (ICIs), which have shown high efficacy against a variety of cancers in recent years, promise long-term survival and even recovery. ICIs are also associated with unique adverse events that are different from those associated with conventional chemotherapy [1, 2]. Immune-related adverse events (irAEs) are attributed to various autoimmune responses that can occasionally become severe and may even be fatal [3–5]. Among them, irAE-associated colitis is reported to closely resemble ulcerative colitis (UC) in endoscopic features and treatment responses [6–9]. A recent report confirmed the efficacy of concurrent administration of infliximab and ICIs [10].
Recently, the number of elderly-onset UC patients has been rising [11]. In elderly patients, the proportion of comorbidities including malignancy unrelated to inflammatory bowel disease (IBD) is high [12]. For these reasons, the number of IBD patients with a comorbid malignancy requiring ICI treatment is expected to increase. However, patients with autoimmune diseases such as IBD have historically been excluded from clinical trials of ICIs, and there are few reports of programmed cell death protein-1 (PD-1) inhibitors administered to patients with a pre-existing form of IBD [13, 14]. Herein, we report an elderly patient with remission of a worsening UC flare-up after nivolumab administration.
Case presentation
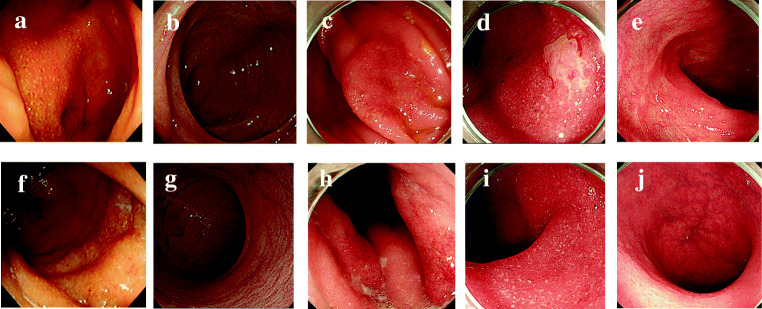
The patient was a 77-year-old man. At 59 years of age, he developed bloody faeces and was diagnosed with mild UC (proctitis form). UC was maintained at a Mayo score of 0 by treatment with 5-aminosalicylic acid (5-ASA) administered as a suppository and orally (Fig. 1a, b, f, g) [15]. At 60 years, he underwent a partial nephrectomy for right renal cell carcinoma; at 65 years, he underwent a total right nephrectomy for local recurrence. At 70 years, the patient developed lung metastasis. Interferon-α (3 million units twice/week) was administered for 3 years but was stopped after the onset of depression. At 73 years, he developed bone metastasis and underwent radiotherapy that failed to achieve a response, then further progressed to gastric metastasis. At 76 years and 3 months, axitinib (10 mg/day orally) was started as the second-line therapy. The lung and metastatic bone foci shrank, and pleural fluid decreased, but the patient developed severe general malaise, and loss of appetite followed by diarrhoea, and axitinib was thus stopped. At age 76 years and 6 months, we confirmed recovery of the patient’s general health and, after obtaining proper informed consent, started nivolumab (3 mg/kg every 2 weeks) as third-line therapy. After 3 months of nivolumab administration, the patient developed diarrhoea 6 times/day, and total colonoscopy revealed a flare-up of UC with a Mayo endoscopic subscore (MES) of 2, extending to the ascending colon from the rectum (Fig. 1c, h) [15]. Symptoms diminished after a temporary cessation of nivolumab; hence, nivolumab was restarted the following month. After 3 months of nivolumab re-administration, the patient developed diarrhoea 8 times/day and also bloody stools. This UC was given a Mayo score of 9, the diarrhoea was judged to be grade 3 according to the CTCAE ver.5, and the patient was hospitalised [16]. Computed tomography revealed inflammation throughout the colon. Endoscopy performed after hospitalisation revealed a more severe exacerbation than before, with an MES of 3 (Fig. 1d, i).
Fig. 1.
Colonoscopic findings from onset to after discharge. a–e Sigmoid colon to appendix. f–j Rectum. a, f Age 59 years (at onset). b, g Age 68 years (during maintenance of remission). c, h Age 76 years and 9 months (3 months after starting nivolumab). d, i Age 77 years and 1 month (3 months after restarting nivolumab). e, j Age 77 years and 3 months (after stopping nivolumab and increasing the dose of 5-ASA)
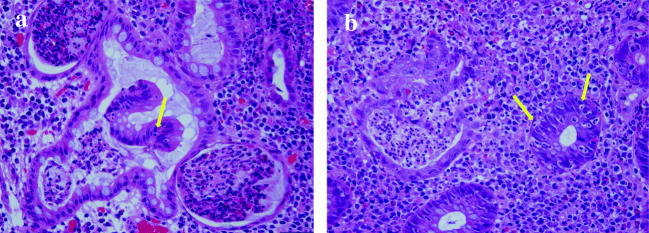
Histological analysis revealed erosion, reduced goblet cells, irregular duct layout, cryptitis, crypt abscesses and chronic inflammatory cell infiltrate in the stroma. These findings were consistent with UC flare-up, but after recognising increasing apoptosis, we considered the possibility of PD-1 inhibitor-induced enterocolitis to also be high (Fig. 2a, b). Although the diarrhoea was grade 3, we had to consider the advanced age of this UC patient. Hence, we first stopped nivolumab, increased 5-ASA to 4000 mg/day from 1500 mg/day and then observed rapid improvement of symptoms. Without prednisolone administration, the patient was discharged on day 15 of hospitalisation. Following discharge, he continued taking 5-ASA orally at 4000 mg/day with no decrease in renal function and maintained a Mayo score of 0. Another total colonoscopy performed 2 months later revealed continued remission with an MES of 0 (Fig. 1e, j). However, the lung metastasis enlarged, and new liver metastasis was also discovered. We considered restarting nivolumab with concurrent infliximab or vedolizumab, but given the absence of definitive evidence for treating ICI-induced colitis in a patient with UC and because a reduction in tumour size had been achieved with axitinib, we chose to administer low-dose axitinib (5 mg/day orally) as second-line therapy. Although diarrhoea appeared 3 times/day after initiating axitinib administration, the mucosa showed continued remission on endoscopy 6 months later, and this treatment is ongoing.
Fig. 2.
Histological findings after restarting nivolumab included reduced goblet cells, irregular duct layout, cryptitis, crypt abscesses, chronic inflammatory cell infiltrate in the stroma and moderate apoptosis (yellow arrow). Hematoxylin and eosin (H&E) staining × 400. a Rectum; b sigmoid colon
Discussion
We have presented a patient with a worsening UC flare-up due to nivolumab treatment. In this patient, the pre-existing UC was of the mild proctitis type, and remission had been maintained for 17 years with only a low dose of 5-ASA. We had difficulty in differentiating this flare-up from ICI-induced colitis; however, as the patient was elderly with pre-existing UC, prednisolone was not administered, and remission was once again achieved by stopping nivolumab and increasing the dose of 5-ASA.
In the few years since the development and approval of ICIs, they have brought about substantial changes in cancer treatment, and we expect that ICI indications will be expanded to include a wide range of malignancies [1, 2]. However, we must consider whether irAEs are becoming a clinical problem [3–5].
Given the mechanism of action of ICIs, there are concerns over an increasing incidence of irAEs and exacerbation of IBD and other autoimmune diseases. This concern has led to very few UC patients being treated with ICIs, and thus very few reports describing such cases.
Including the present case, ICI administration has been confirmed in 10 non-postoperative UC patients. Outcomes after treatment were flare-up in 5 patients (50%) and perforation in one (Table 1) [13, 14, 17–20]. Compared with the reported incidence rates of ICI-induced colitis which range from 0.7 to 22% for ICI monotherapy, the incidence of UC flare-up is high [4, 21].
Table 1.
Reports on the administration of immune checkpoint inhibitors for non-operated ulcerative colitis patients
| Case | Author | Name of ICIs | Age (years) | Sex (M/F) | Type of cancer | Treatment before flare-up | Flare-up | Treatment for flare-up |
|---|---|---|---|---|---|---|---|---|
| 1 | Hijikata et al. [17] | PD-1 (nivolumab) | 52 | F | Epipharyngeal carcinoma | 5-ASA | None | None |
| 2 | Kahler et al. [18] | CTLA-4 (ipilimumab) | NS | NS | Melanoma | 5-ASA | Colitis (grade 3) | Steroids |
| 3 | Kahler et al. [18] | CTLA-4 (ipilimumab) | NS | NS | Melanoma | None | None | None |
| 4 | Leonardi et al. [13] | PD-(L)1 | NS | NS | Non-small-cell lung carcinoma | Not stated | Non | None |
| 5 | Leonardi et al. [13] | PD-(L)1 | NS | NS | Non-small-cell lung carcinoma | Not stated | None | None |
| 6 | Leonardi et al. [13] | PD-(L)1 | NS | NS | Non-small-cell lung carcinoma | Not stated | None | None |
| 7 | Bergqvist et al. [19] | CTLA-4 (ipilimumab) | 48 | F | Melanoma | Vedolizumab | Diarrhoea (grade 3) | Steroids |
| 8 | Gutzmer et al. [14] | PD-1 (nivolumab) | 51 | F | Melanoma | Budesonide, sulfasalazine | Diarrhoea (grade 3) | Steroids |
| 9 | Bostwick et al. [20] | CTLA-4 (ipilimumab) | 61 | M | Melanoma | IFX → AZA |
1) Colitis (grade 3) 2) Perforation |
1) Steroids IFX → AZA 2) Colectomy |
| 10 | Present case | PD-1 (nivolumab) | 77 | M | Renal cell carcinoma | 5-ASA | Diarrhoea (grade 3) | 5-ASA dose increase |
ICIs, immune checkpoint inhibitors; PD-1, programmed cell death protein-1; PD-L1, programmed death protein ligand-1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; NS, not stated; 5-ASA, 5-aminosalicylic acid; AZA, azathioprine; IFX, infliximab
Currently, the number of elderly UC patients is increasing globally. Numbers of comorbidities, rates of comorbid infection and perioperative mortality from emergency surgery are also reportedly rising among elderly UC patients, and case fatality rates are correspondingly increasing [11, 22, 23]. As there is likewise an increasing rate of comorbid malignancies unrelated to IBD among elderly UC patients and a rising number of non-operated cases, patients requiring ICI therapy are predicted to increase. Furthermore, when ICIs cause enterocolitis in a UC patient, there is difficulty in strictly differentiating between UC exacerbation and ICI-induced colitis, because the latter often causes endoscopic and histological findings similar to those of UC [6, 7]. In the present case, in addition to the findings of UC, histological analysis showed increased crypt apoptosis which is unusual for IBD, while being typical of ICI-induced colitis [24, 25]. Furthermore, mucosal remission was maintained despite the diarrhoea associated with axitinib. We thus considered nivolumab to be a major contributor to UC relapse.
When enterocolitis occurs after administering drugs to elderly UC patients for comorbid recurrent progressive cancer, rapid therapeutic intervention is first required to prevent UC from flaring up or becoming more severe [11, 12, 22, 23].
Treatment guidelines for ICIs strongly recommend steroids or early administration of infliximab or vedolizumab for ICI-induced colitis. However, this information conflicts with treatment guidelines for elderly IBD patients, for whom steroids or infliximab may increase mortality and the incidence of infection [8, 9, 26, 27]. There is only a single case report of vedolizumab failing to prevent ICI-induced colitis in a UC patient after the administration of a CTCL-4 inhibitor [19].
By contrast, 5-ASA produces a dose-dependent effect, is very safe and is used throughout the world in many IBD patients [28, 29]. Kubo et al. reported improvement of PD-1 inhibitor-induced enterocolitis with only 5-ASA [30]. Our patient also achieved remission with only an increase in the dose of 5-ASA, raising the possibility of 5-ASA being a therapeutic option for flare-ups in UC patients after ICI therapy, especially in patients for whom steroid therapy carries high risk or is even contraindicated.
The administration of ICI for UC is not as yet sufficiently safe and further research is required.
Elderly UC patients who have a recurrent, progressive cancer need flexible treatment strategies that maintain remission of UC and aim for improving the long-term prognosis, which is the primary goal of cancer treatment.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA, Atkins MB, Leming PD, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5(10):1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshino K, Nakayama T, Ito A, Sato E, Kitano S. Severe colitis after PD-1 blockade with nivolumab in advanced melanoma patients: potential role of Th1-dominant immune response in immune-related adverse events: two case reports. BMC Cancer. 2019;19:1019. doi: 10.1186/s12885-019-6138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, De Felice KM, Loftus EV, Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42:406–417. doi: 10.1111/apt.13281. [DOI] [PubMed] [Google Scholar]
- 5.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/jco.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canete F, Manosa M, Lobaton T, Mesonero F, Rodriguez-Lago I, Cabre E, Cabriada JL, Lopez-Sanroman A, Domenech E. Nivolumab-induced immune-mediated colitis: an ulcerative colitis look-alike-report of new cases and review of the literature. Int J Colorectal Dis. 2019;34:861–865. doi: 10.1007/s00384-019-03268-4. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi R, Araki T, Mitsuyama K, Tokito T, Ishii H, Yoshioka S, Kuwaki K, Mori A, Yoshimura T, Tsuruta O, et al. The characteristics of nivolumab-induced colitis: an evaluation of three cases and a literature review. BMC Gastroenterol. 2018;18:135. doi: 10.1186/s12876-018-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson JA, Schneider BJ, Brahmer J, Armand P, Davies M, Ernstoff MS, Sosman JA, Johnson-Chilla A, Zuccarino-Catania G, Engh A. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), management of immunotherapy-related toxicities, Version 1. J Natl Compr Canc Ne. 2020;18:231–241. [Google Scholar]
- 9.Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, Bresalier RS, Charabaty A, Dadu R, Jazaeri A, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7:93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent therapy with immune checkpoint inhibitors and TNFalpha blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer. 2019;7:226. doi: 10.1186/s40425-019-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi HY, Chan FK, Leung WK, Li MK, Leung CM, Sze SF, Ching JY, Lo FH, Tsang SW, Shan EH, et al. Natural history of elderly-onset ulcerative colitis: results from a territory-wide inflammatory bowel disease registry. J Crohns Colitis. 2016;10:176–185. doi: 10.1093/ecco-jcc/jjv194. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis. 2009;15:182–189. doi: 10.1002/ibd.20628. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi GC, Gainor JF, Altan M, Kravets S, Dahlberg SE, Gedmintas L, Azimi R, Rizvi H, Riess JW, Hellmann MD, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36:1905–1912. doi: 10.1200/jco.2017.77.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutzmer R, Koop A, Meier F, Hassel JC, Terheyden P, Zimmer L, Heinzerling L, Ugurel S, Pfohler C, Gesierich A, et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer. 2017;75:24–32. doi: 10.1016/j.ejca.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/nejm198712243172603. [DOI] [PubMed] [Google Scholar]
- 16.Common Terminology Criteria for Adverse Events(CTCAE), version 5.0. US Department of Health and Human Services, 2017. https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 07 May, 2020.
- 17.Hijikata Y, Matsubara Y, Ota Y, Lim LA, Tani K, Hirata Y, Yotsuyanagi H (2020) Safe use of nivolumab in a patient with epipharyngeal carcinoma and preexisting ulcerative colitis: a histologically proven case report. Intern Med. 10.2169/internalmedicine.3901-19 [DOI] [PMC free article] [PubMed]
- 18.Kahler KC, Eigentler TK, Gesierich A, Heinzerling L, Loquai C, Meier F, Meiss F, Pfohler C, Schlaak M, Terheyden P, et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol Immunother. 2018;67:825–834. doi: 10.1007/s00262-018-2134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, Carneiro A, Marsal J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581–592. doi: 10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostwick AD, Salama AK, Hanks BA. Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. J Immunother Cancer. 2015;3:19. doi: 10.1186/s40425-015-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, Collins M, Chaput N, Robert C, Carbonnel F, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056–2067. doi: 10.1136/gutjnl-2018-316948. [DOI] [PubMed] [Google Scholar]
- 22.Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D’Inca R, Bossa F, Angelucci E, Biancone L, Gionchetti P, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30–35. doi: 10.1016/j.cgh.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Ikeuchi H, Uchino M, Matsuoka H, Bando T, Hirata A, Takesue Y, Tomita N, Matsumoto T. Prognosis following emergency surgery for ulcerative colitis in elderly patients. Surg Today. 2014;44:39–43. doi: 10.1007/s00595-013-0563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists’ perspective. J Clin Pathol. 2018;71:665–671. doi: 10.1136/jclinpath-2018-205143. [DOI] [PubMed] [Google Scholar]
- 25.Yanai S, Nakamura S, Kawasaki K, Toya Y, Akasaka R, Oizumi T, Ishida K, Sugai T, Matsumoto T (2019) Immune checkpoint inhibitor-induced diarrhea: clinicopathological study of 11 patients. Dig Endosc. 10.1111/den.13555 [DOI] [PubMed]
- 26.Toruner M, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Sturm A, Maaser C, Mendall M, Karagiannis D, Karatzas P, Ipenburg N, Sebastian S, Rizzello F, Limdi J, Katsanos K, et al. European Crohn’s and Colitis Organisation topical review on IBD in the elderly. J Crohns Colitis. 2017;11:263–273. doi: 10.1093/ecco-jcc/jjw188. [DOI] [PubMed] [Google Scholar]
- 28.Hiwatashi N, Suzuki Y, Mitsuyama K, Munakata A, Hibi T. Clinical trial: effects of an oral preparation of mesalazine at 4 g/day on moderately active ulcerative colitis. A phase III parallel-dosing study. J Gastroenterol. 2011;46:46–56. doi: 10.1007/s00535-010-0308-3. [DOI] [PubMed] [Google Scholar]
- 29.Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012;5:113–123. doi: 10.1586/ecp.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubo K, Kato M, Mabe K. Nivolumab-associated colitis mimicking ulcerative colitis. Clin Gastroenterol Hepatol. 2017;15:A35–A36. doi: 10.1016/j.cgh.2017.03.026. [DOI] [PubMed] [Google Scholar]