Abstract
Finger millet is an important cereal that is grown in semi-arid and arid regions of East-Africa. Salinity stress is a major environmental impediment for the crop growth and production. This study aimed to understand the physiological and biochemical responses to salinity stress of six Kenyan finger millet varieties (GBK043137, GBK043128, GBK043124, GBK043122, GBK043094, GBK043050) grown across different agroecological zones under NaCl-induced salinity stress (100, 200 and 300 mM NaCl). Seeds were germinated on the sterile soil and treated using various concentrations of NaCl for 2 weeks. Early-seedling stage of germinated plants were irrigated with the same salt concentrations for 60 days. The results indicated depression in germination percentage, shoot and root growth rate, leaf relative water content, chlorophyll content, leaf K+ concentration, and leaf K+/Na+ ratios with increased salt levels and the degree of increment differed among the varieties. On the contrary, the content of proline, malonaldehyde, leaf total proteins, and reduced sugar increased with increasing salinity. At the same time, the leaf Na+ and Cl− amounts of all plants increased substantially with increasing stress levels. Clustering analysis placed GBK043094 and GBK043137 together and these varieties were identified as salt-tolerant based on their performance. Taken together, our findings indicated a significant varietal variability for most of the parameters analysed. The superior varieties identified could be used as promising genetic resources in future breeding programmes directed towards development of salt-tolerant finger millet hybrids. Further analysis at genomic level needs to be undertaken to better understand the genetic factors that promote salinity tolerance in finger millet.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00853-8) contains supplementary material, which is available to authorized users.
Keywords: Finger millet, Physiological and biochemical analyses, Salinity stress, Varietal differences
Introduction
Salinity is a major environmental constraint that adversely affects growth and productivity of many agricultural crops globally (Qadir et al. 2014). This abiotic stress mostly characterizes arid and semi-arid regions that experience low rainfall and scarcity of good quality water. Accumulation of soluble salts due to irrigation without proper water drainage systems, coupled with underlying rocks rich with high salt contents, leads to gradual salinization of arable land, thereby affecting soil characteristics (Asfaw et al. 2018). The problem is expected to aggravate further owing to the effects of climate change and rising sea levels as farmers will be forced to use saline water for irrigation in arid and semi-arid regions due to the adequate rain and shortage of good quality water (Neocleous et al. 2017). If existing salinity stress phenomenon continues to persist, it is estimated that more than 50% of the world’s arable land could be severely affected by salinity by the year 2050 (Machado and Serralheiro 2017).
Salinity stress triggers complex adaptive responses that function to synchronize the ions in order to ameliorate hyperosmolarity and reinstall cellular ionic homeostatic conditions (Chen et al. 2019; Ganie et al. 2019). Collectively, these coordinated and regulated responses inhibit growth and development and subsequently reduce crop yields. Combating salinity stress to increase crop production necessitates application of various parameters which underlie salinity tolerance. It is therefore imperative to identify the genetic resources with high tolerance levels and to understand their mechanisms of salinity tolerance. The response of plants to salinity is a quantitatively inherited trait and varies according to the species, varieties and genotypes and is also influenced by their interactions with the environment (Bertazzini et al. 2018; Shabala et al. 2013). Many reports have shown that short term salinity stress significantly affects the germination rate, seedling and root growth, levels of ion contents and relative water content, photosynthetic pigments, proline content, level of membrane lipid peroxidation as well as the amounts of reducing sugars and total protein (Dugasa et al. 2019; Sarabi et al. 2017; Kumar and Khare 2016). These physiological and biochemical indices have been used for classification of salt-sensitive and salt-tolerant varieties in plant breeding programmes. The most feasible approach to evaluate performance of plants against salinity under laboratory conditions is through assessing their physiological and biochemical responses on the application of different concentrations NaCl.
Finger millet [Eleusine coracana L. (Geartn)] is a valuable cereal crop cultivated in arid and semi-arid regions of Asia and Africa (Chivenge et al. 2015). The crop is well adapted to heat, drought and degraded soils. Its cereals have comparatively better antioxidant, nutraceutical properties and storage qualities than other cereals (Kumar et al. 2016). Like other members of the poaceae family, finger millet, is glycophytic in nature and hence is adversely affected by salinity stress (Ibrahim 2016; Hema et al. 2014). There is limited information which is available on the physiological and biochemical responses of different finger millet varieties of under salinity stress. Understanding these responses in finger millet is therefore of great importance for breeding salt resistant and tolerant crops. Owing to the wide disparity in agro-ecological regions across finger millet growing regions, several finger millet landraces exhibit an adaptation to a large range of environmental conditions. Such landraces represent valuable sources of useful genetic material that might be exploited to improve salinity tolerance of finger millet varieties adapted to distinct geographical zones affected by salinity. We therefore investigated the physiological and biochemical responses to salinity stress of six finger millet varieties under NaCl induced salinity stress.
Materials and methods
Plant material germination assay and salinity treatment
Six finger millet varieties (GBK043124, GBK043122, GBK043137, GBK043128, GBK043094 and GBK043050) were grown in different agroecological zones in Kenya as detailed in Supplementary Table 1. Ten healthy seeds were planted in round pots containing sterile soil to a depth of 1 cm and irrigated with 100, 200 and 300 mM NaCl at an interval of 3 days for 2 weeks. The control seeds were irrigated with distilled water. Germination rate was scored on the 17th treatment day. Germinated seedlings were grown for 2 weeks under greenhouse (25 ± 2 °C and 60–70% humidity, with a 16/8-h photoperiod provided sunlight). Seedlings were irrigated with 100, 200 and 300 mM NaCl for 21 days at an interval of 3 days. Control plants were watered with distilled water. Five replications were used for each set of treatment and 5 plants were randomly sampled and their shoot length and root length recorded.
Relative water content
A leaflet was removed from a plant on the 21st day and fresh weight (FW) measured. Each leaflet was immersed in deionized water for 4 h and wiped before taking its turgid weight (TW) measured. The leaflet was then dried in an oven for 24 h and its dry weight (DW) measured. Relative water content (RWC%) was calculated as: RWC = [(FW − DW)/(TW − DW)] × 100.
Determination of chlorophyll and proline contents
Chlorophyll a, b and total chlorophylls (a + b) were determined according to Arnon (1949). 0.2 g fresh leaves were taken from 21 days-old NaCl stressed plants and finely ground by vortexing. The extract was centrifuged for 3 min and absorbance of the supernatant was measured at 645 and 663 nm. Total chlorophyll content, mg/g fresh mass (FM), was calculated as: TC = 20.2(A645) + 8.02(A663) × V/1000 × W where V is the volume of total extract/l and W is the mass of material.
Measurement of proline content
Proline accumulation was determined as described by Bates et al. (1973). 50 mg fresh leaf was homogenized in 10 ml of 3% w/v sulphosalicylic acid. The homogenate was filtered and mixed solution of acidic ninhydrin. The reaction mixture was heated at 100 °C for 60 min and then cooled in ice for 5 min and toluene was added. Absorbance was measured at 520 nm and proline content [µmol/g fresh weight (F. WT)] was calculated from l-proline standard curve.
Lipid peroxidation assay
0.3 g of fresh leaves were homogenized in 0.1% (w/v) trichloroacetic acid and the homogenates were centrifuged for 15 min at 4 °C. The supernatant was mixed with 0.5 ml of 1.5 ml 0.5% thiobarbituric acid (TBA) diluted in 20% trichloroacetic acid (TCA) incubated at 95 °C for 25 min before incubating it on ice for 10 min. Absorbance was measured at 532 and 600 nm with 1% TBA in 20% TCA as control. Malondialdehyde (µmol/g FW) amount was calculated according to Heath and Packer (1968).
Estimation of reducing sugar
The amount of reducing sugar in shoots was determined as described by Johnson et al. (1964). Sugar was extracted from 1.0 g homogenized tissue using 80% ethanol at 95 °C, then centrifuged for 10 min. Resulting supernatant was dried for 2 h at 80 °C, before dissolving the residue in 10 ml of water 2.0 ml alkaline copper reagent. The mixture was heated at 100 °C for 10 min and cooled. 1.0 ml Nelson’s reagent was added and the volume was adjusted to 10 ml with water. Absorbance was taken at 520 nm. Reducing sugar (mg/g FW) amount was calculated using standard glucose curve.
Estimation of leaf total protein
Total sample protein was extracted using the acetone-trichloroacetic acid (TCA) precipitation (Damerval et al. 1986). 500 g of leaf tissue from each treatment was homogenized in 10% TCA in ice and incubated overnight at 4 °C. The homogenate was centrifuged at for 15 min at 4 °C and the pellet was washed with 100% acetone. The pigment-free pellet was first washed with 80% ethanol, ethanol/trichloromethane (3:1 v/v), then ethanol/ethoxyethane (3:1 v/v) and finally with ethoxyethane. Washed pellet was suspended in 0.1 N NaOH for protein estimation. Sample proteins were estimated at 750 nm using bovine serum albumin as standard.
Measurements of Na+ K+ and Cl− ion content in plant tissue
Mature leaves were powdered and ashed. The ashes were dissolved in 5 ml 30% ammonia, and further diluted with deionized water (Cheng et al. 2004). Concentrations of Na+ and K+ ions were measured using a flame atomic absorption spectrometry. Cl− ions were extracted from 1 g of plant material and amount was determined using Eaton et al. (1995) method.
Statistical analysis
A completely randomized block design with five replications for each experiment was used and the results represent mean ± standard error. ANOVA was performed using the Minitab statistical software 17 (Minitab Inc., State College, PA, USA) and differences between means were separated using the Fisher’s protected LSD test at a confidence level of 95%. Relationships between assessed features were performed by Pearson’s correlation. Principal component analysis and Cluster analysis were carried out using the FactoMineR package (Lê et al. 2008).
Results
The present study investigated the changes in growth parameters, relative water content, lipid peroxidation level, proline content, reducing sugar and total protein under NaCl induced salinity stress in six finger millet varieties. The parameters analyzed exhibited significant variations among the varieties.
Effect of salinity on shoot Na, K and Cl ion contents
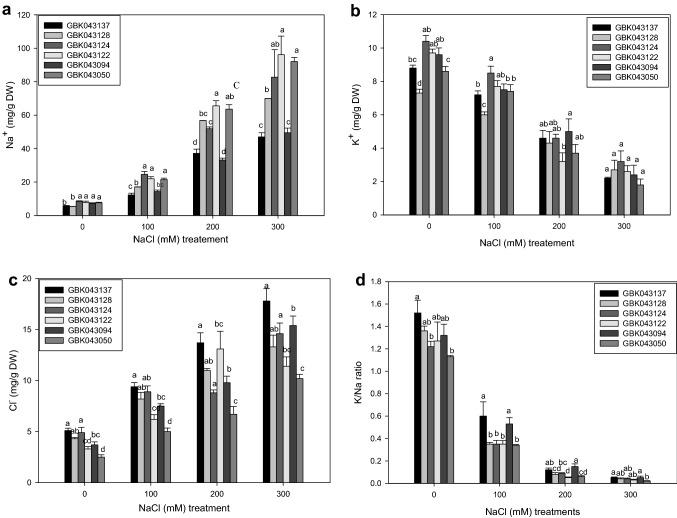
The salinity treatments, varieties and the synergy effects were significant for the concentrations of all leaf ions (Fig. 1a–c, Supplementary Table 2). As expected, the level of Na+ and Cl− in all varieties was higher under salt stress but differed in the degree of the increment. The gradual increase of salinity stress triggered a gradual rise of both ion concentration in finger millet leaves. The average levels of Na+ in leaves ranged from 5.37 to 7.82 mg/g DW for plants grown in control conditions and from 12.3 to 96.2 mg/g DW for salinity stressed plants (Fig. 1a). Under 300 mM NaCl stress treatments, the different varieties increased their Na+ ion concentration from 6.8- to 13.1-fold when compared to the controls. GBK043124, GBK043137 and GBK043094 displayed statically the minimum increase of Na+ under salinity stress (Fig. 1a). On the other hand, the leaf Cl− levels ranged from 2.5 to 5.1 mg/g DW for finger millet plants under control conditions and from 5.0 to 17.8 mg/g DW for plants under salinity stress (Fig. 1c). GBK043050 had the lowest concentration of Cl− under untreated and salinity stress treatments. GBK043124 had the least (3.0.5-fold) increase in Cl− ion concentration under salt treatment, while GBK043094 had the largest (4.2-fold) increase (Fig. 1c). In contrast, salinity stress induced significant reduction of K+ concentration in leaves of finger millet plants irrigated with three NaCl doses (Fig. 2). In comparison to control experiments, potassium ions concentration decreased by about 18.6, 53.3 and 72.6% in leaves of plants grown under 100, 200 and 300 mM NaCl respectively. GBK043094 upheld the highest concentration of K+ and had a 74.0% decline in K+ concentration while, GBK043050 had the highest decrease in K content (78.9%) under salinity conditions (Fig. 1b). The lowest potassium ion concentration under salinity was found in GBK043128 followed by GBK043124 (Fig. 1b). The leaf K+/Na+ ratios differed among the varieties of finger millet studied, ranging from 0.05 in both GBK043094 to 0.02 in GBK043050. Varieties, GBK043094 and GBK043137 presented the greatest K+/Na+ ratio under salinity stress owing to low concentration of Na+ in the leaves (Fig. 1d).
Fig. 1.
Effect of salinity stress on ion concentration of finger millet under salinity stress. a Na+ concentration, b K+ concentration, c Cl− concentration, d K+/Na+ ratio. For each NaCl treatment, values within sharing same letter comparing NaCl treatments are not significantly different at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
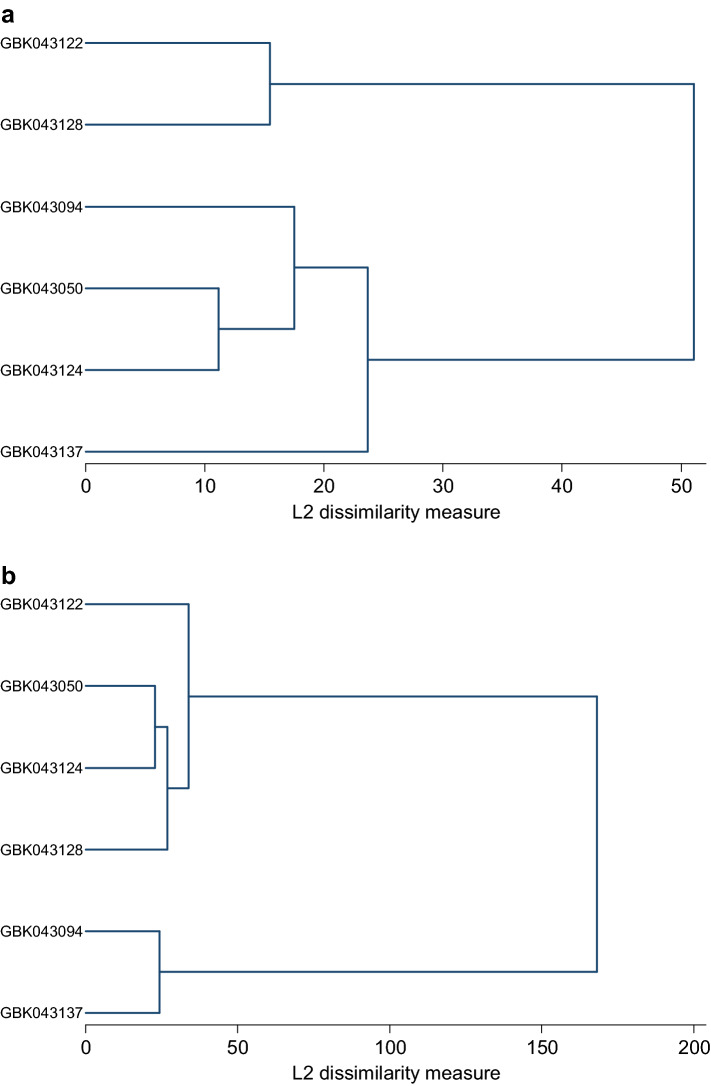
Fig. 2.
Dendrogram of the studied finger millet varieties, obtained by cluster analysis based on their physiological and biochemical characteristics under salinity stress. a 0 NaCl; b 100 mM NaCl; c 200 mM NaCl; d 300 mM NaCl
Effects of salt stress on seed germination
The effect of salinity stress on finger millet seeds germination, evaluated by the percentage of germinated seeds after 17 days, is as shown in Table 1. Our results indicated that for all varieties, the germination rate decreased with increase of the NaCl concentration and varied among the varieties. This decrease in germination rate was most profound at 200 mM and 300 mM NaCl concentrations where 0% germination rate were recorded for all the six varieties. In contrast, at moderate stress levels (100 mM NaCl), significant differences in germination profile was observed with GBK043122 having the highest germination rate (46.25%) compared to others whose germination rates ranged from 3.75 to 22.50%. The germination percentage under control conditions was also distinct among the six finger millet varieties and ranged from 90.00% for GBK043137 to 56.25% for GBK043122 (Table 1).
Table 1.
Effects of NaCl on germination rate of six finger millet varieties
| Variety | Germination rate (%) | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 90.0 ± 3.5a | 3.8 ± 3.8c | 0.0 ± 0.0a | 0.0 ± 0.0a |
| GBK043128 | 65.0 ± 3.5bc | 18.8 ± 2.4bc | 0.0 ± 0.0a | 0.0 ± 0.0a |
| GBK043124 | 80.0 ± 8.4a | 37.5 ± 7.8ab | 0.0 ± 0.0a | 0.0 ± 0.0a |
| GBK043122 | 56.3 ± 5.2c | 46.3 ± 9.7a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| GBK043094 | 63.8 ± 1.3bc | 22.5 ± 11.6bc | 0.0 ± 0.0a | 0.0 ± 0.0a |
| GBK043050 | 76.3 ± 3.7ab | 18.85 ± 5.5bc | 0.0 ± 0.0a | 0.0 ± 0.0a |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Growth characteristics in finger millet varieties under salt stress
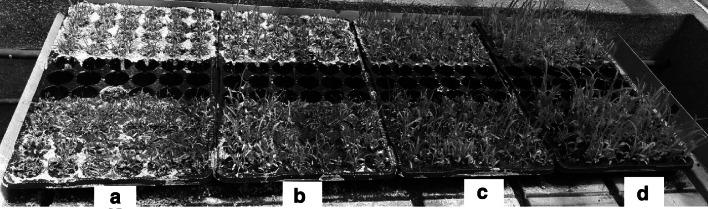
After phenotypic observation, chlorosis (yellowish color) was observed in all plants under salinity conditions. Leaf chlorosis, leaf scorching, slowed and delayed growth and enlargement of the leaves were distinctly observed in seedlings of all varieties under salinity stress. Plants growing under control conditions exhibited healthy leaves and normal shoot and root developmental stages (Fig. 3). The shoot length progressively retarded with increase in NaCl concentration (Table 2). Particularly, the shoot height of GBK043128 population was significantly reduced under severe salt stress conditions (300 mM NaCl) by about 72.1% while GBK043124 had the least shoot height reduction rate at 63.3% when compared to the control plants (Table 2). Significance variations on the effect of NaCl on shoot length were only observed at 200 mM NaCl concentration. Higher salt concentrations did not record any varietal difference on shoot length (Table 2). Similarly, increasing salinity stress resulted in gradual reductions in plant root lengths in all studied varieties ranging from 20.9% for GBK043137 to 36.1% for GBK043128 compared to their respective controls (Table 3). We also observed significant differences between varieties in root length values across the salt concentrations, signifying that increased salt stress adversely affected root length growth in the varieties at different degrees (Table 3).
Fig. 3.
Effect of salinity stress on growth of finger millet. a Seedling growth on 300 mM NaCl. b Seedling growth on 200 mM NaCl. c Seedling growth on 100 mM NaCl; d seedling growth on 0 mM NaCl
Table 2.
Effect of NaCl on growth of finger millet
| Variety | Seedlings shoot length (cm) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 3.8 ± 0.4ab | 2.5 ± 0.3a | 1.6 ± 0.2ab | 1.2 ± 0.1a |
| GBK043128 | 4.3 ± 0.2a | 2.6 ± 0.4a | 1.9 ± 0.2a | 1.2 ± 0.2a |
| GBK043124 | 3.0 ± 0.3b | 2.4 ± 0.2ab | 1.6 ± 0.3ab | 1.1 ± 0.1a |
| GBK043122 | 3.2 ± 0.4b | 2.5 ± 0.2a | 1.2 ± 0.2bc | 1.0 ± 0.0a |
| GBK043094 | 3.1 ± 0.3b | 2.3 ± 0.2ab | 1.0 ± 0.0c | 1.1 ± 0.1a |
| GBK043050 | 3.1 ± 0.2b | 1.7 ± 0.1b | 1.3 ± 0.2bc | 1.1 ± 0.1a |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Table 3.
Effect of NaCl on root growth
| Variety | Seedlings root length (cm) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 6.8 + 0.4b | 6.4 + 0.4ab | 5.9 + 0.2a | 5.4 + 0.2a |
| GBK043128 | 8.0 + 0.4a | 7.2 + 0.4a | 5.9 + 0.3a | 5.1 + 0.3ab |
| GBK043124 | 6.9 + 0.6b | 6.3 + 0.6ab | 5.6 + 0.4a | 5.0 + 0.46ab |
| GBK043122 | 6.7 + 0.2b | 6.2 + 0.5b | 5.5 + 0.3a | 4.9 + 0.2ab |
| GBK043094 | 6.8 + 0.5b | 6.2 + 0.7b | 5.9 + 0.4a | 5.4 + 0.4a |
| GBK043050 | 7.1 + 0.4b | 6.4 + 0.5ab | 5.6 + 0.2a | 4.8 + 0.3b |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Relative water content
The changes in leaf RWC along with increase in salinity stress are presented in Table 4. The RWC of all varieties under control conditions were similar ranging from 79.44 to 87.86%. Exposition to increasing salinity stress progressively reduced water potential of leaves in all varieties compared to their respective control plant leaves and they exhibited variation in their relative water content. Variety GBK043094 tolerated salinity stress better with the least reduction in relative water content under severe salinity stress (300 mM NaCl) compared to the others (Table 4).
Table 4.
Effect of NaCl on relative water content
| Variety | Seedlings relative water content (%) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 85.3 ± 4.1a | 71.5 ± 4.1a | 35.0 ± 3.9a | 37.1 ± 3.3b |
| GBK043128 | 87.9 ± 5.3a | 71.5 ± 4.1abc | 35.0 ± 3.9b | 26.8 ± 2.3c |
| GBK043124 | 84.8 ± 4.9a | 67.2 ± 3.4bc | 34.2 ± 5.0b | 28.2 ± 2.6c |
| GBK043122 | 83.0 ± 1.8a | 68.0 ± 1.9bc | 33.7 ± 3.3b | 46.7 ± 9.2c |
| GBK043094 | 82.1 ± 6.7a | 72.3 ± 3.7ab | 51.3 ± 6.1a | 46.7 ± 9.2a |
| GBK043050 | 79.4 ± 4.6a | 65.4 ± 4.8c | 33.2 ± 4.5b | 27.6 ± 3.7c |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Effects of salt stress on chlorophyll content
Analysis of total chlorophyll content demonstrated significant differences in photochemistry among varieties and the salt treatments (Table 5). More specifically, for all the varieties, the addition of NaCl elicited significant decrease in chlorophyll content compared to the non-saline treatments and inverse relationship between salinity stress and total chlorophyll content in all finger millet varieties was observed. In contrast, plants grown under normal conditions maintained a relatively high level of total chlorophyll content and interestingly, they did not have similar chlorophyll content. Under saline conditions, photosynthetic pigment of varieties GBK043137 and GBK043128 were found to be extremely reduced with reduction percentages of 48.2% and 39.5%, respectively. However, GBK043124 retained a relatively higher chlorophyll content compared to its respective control value, under 300 mM NaCl stress conditions (Table 5).
Table 5.
Effect of salinity stress on total chlorophyll content of finger millet varieties
| Variety | Seedlings chlorophyll content (mg/g FW) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 8.4 ± 0.4a | 8.1 ± 1.8a | 5.0 ± 0.4a | 4.4 ± 0.6a |
| GBK043128 | 9.1 ± 1.0b | 7.5 ± 1.5b | 6.4 ± 0.5b | 5.5 ± 0.1b |
| GBK043124 | 5.9 ± 0.1c | 6.8 ± 0.1c | 7.3 ± 0.2c | 5.5 ± 0.1c |
| GBK043122 | 7.3 ± 1.9d | 5.9 ± 0.1d | 6.1 ± 1.0d | 5.8 ± 0.7d |
| GBK043094 | 6.2 ± 0.5e | 5.0 ± 0.9 | 5.0 ± 0.8e | 4.7 ± 1.4e |
| GBK043050 | 5.1 ± 1.6f | 4.0 ± 0.9f | 5.0 ± 0.4f | 3.9 ± 0.7f |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Proline accumulation and lipid peroxidation assay
The effect of free proline content in the six finger millet varieties at early seedling growth stage under NaCl induced osmotic stress is shown in Table 6. Increasing salt concentrations from 100 to 200 and 300 mM NaCl application remarkably induced increased free proline content in the plants by an average of 39.8%, 54.2% and 66.4% respectively, relative to the levels in the control plants (Table 6). GBK043094 variety had the significantly highest proline content, followed by GBK043137, GBK043124 and GBK043122 while GBK043128 and GBK043050 had the lowest (Table 6). In unstressed plants, proline concentration was similar. As shown in Table 7, we observed continuous increment in malondialdehyde content in leaves of all varieties tested in response to salinity stress relative to their respective controls and the magnitude of response differed among the varieties. A continuous increase in the level of lipid peroxidation was observed with increasing level of salinity in all the varieties. The malondialdehyde levels was elevated to 20.7%, 31.3% and 51.2% at 100, 200 and 300 mM NaCl, respectively, as compared to unstressed plants (Table 7). Malondialdehyde content was significantly elevated in GBK043050, GBK043122, GBK043124 and GBK043128 under severe salinity stress (300 mM NaCl) treatments signifying higher rates of oxidative damage and lipid peroxidation whereas GBK043094 and GBK043137 had lower levels of malondialdehyde at corresponding salinity stress (Table 7).
Table 6.
Effect of salinity stress on free proline content of finger millet varieties
| Variety | Proline content (µg/g FW) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 200.9 ± 2.4a | 411.9 ± 13.4a | 529.3 ± 3.0ab | 655.2 ± 28.6b |
| GBK043128 | 224.3 ± 3.6a | 340.3 ± 33.9b | 471.3 ± 63.7bc | 571.3 ± 37.1c |
| GBK043124 | 208.4 ± 30.6a | 322.4 ± 34.0b | 417.9 ± 50.6c | 585.1 ± 86.6bc |
| GBK043122 | 234.5 ± 16.2a | 344.0 ± 18.2b | 433.2 ± 12.3c | 666.7 ± 2.1b |
| GBK043094 | 208.2 ± 14.4a | 401.6 ± 25.7a | 558.0 ± 12.9a | 801.9 ± 22.8a |
| GBK043050 | 212.8 ± 21.6a | 319.7 ± 7.5b | 404.4 ± 34.9c | 560.5 ± 53.4c |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Table 7.
Effect of salinity stress on Malondialdehyde content of finger millet varieties
| Variety | Malondialdehyde content (µg/g FW) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 1.94 ± 0.1a | 2.2 ± 0.3b | 2.4 ± 0.5b | 2.7 ± 0.4b |
| GBK043128 | 2.21 ± 0.3a | 2.9 ± 0.2ab | 2.9 ± 0.4ab | 3.6 ± 0.2a |
| GBK043124 | 2.67 ± 0.4a | 3.3 ± 0.3a | 3.4 ± 0.5a | 3.7 ± 0.6a |
| GBK043122 | 2.22 ± 0.4a | 2.8 ± 0.2ab | 3.3 ± 0.1a | 3.8 ± 0.4a |
| GBK043094 | 1.96 ± 0.2a | 2.3 ± 0.3b | 2.47 ± 0.2b | 2.8 ± 0.3b |
| GBK043050 | 2.19 ± 0.8a | 2.5 ± 0.9b | 3.0 ± 0.7ab | 3.9 ± 0.5a |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Reducing sugars and protein contents under NaCl stress
The impact of salinity treatment triggered substantial elevation in reducing sugar amounts in the stressed plants when compared to control the experiments (Table 8). Increasing salt concentration caused an increase in reducing sugar amounts in the stressed plant shoots and highest accretion of reducing sugar was found in 100 mM NaCl stress followed by 200 mM and 300 mM NaCl treatments. However, varietal differences were observed and the increment was remarkably highest in GBK043094, followed by GBK043050, GBK043137and GBK043122 while GBK043128 had the lowest amount (Table 8). Plants under control conditions had the lowest protein content ranging from 1.2 to 2.2 mg/g FW reducing whereas the highest reducing sugar content protein content of 4.5 to 6.5 mg/g FW was found in plants treated with 300 mM NaCl (Table 8). As shown in Table 9, increasing NaCl concentration had a substantial impact on the protein content of finger millet plants and the response was in a dose dependent relationship. A clear varietal difference was observed and significantly higher levels of protein were found in GBK043094, GBK043050 and GBK043122 than the rest, under control and also stress conditions (Table 9).
Table 8.
Effect of salt stress on reducing sugars on finger millet
| Variety | Reducing sugars content (mg/g FW) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 1.6 ± 0.4bc | 2.1 ± 0.6ab | 4.0 ± 0.8bc | 4.9 ± 0.9bc |
| GBK043128 | 1.2 ± 0.3c | 1.6 ± 0.3b | 3.7 ± 0.7bc | 4.6 ± 0.8c |
| GBK043124 | 1.3 ± 0.3c | 1.7 ± 0.3b | 3.3 ± 0.5c | 4.7 ± 0.3c |
| GBK043122 | 1.8 ± 0.4abc | 2.1 ± 0.4ab | 3.7 ± 0.5bc | 5.5 ± 0.2bc |
| GBK043094 | 2.2 ± 0.3a | 2.7 ± 0.2a | 5.0 ± 0.0a | 6.5 ± 0.5a |
| GBK043050 | 2.1 ± 0.4ab | 2.5 ± 0.4a | 4.4 ± 0.3ab | 5.8 ± 0.4ab |
Values within a column marked with different superscript in each column differ significantly different at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Table 9.
Effect of salt stress on total protein on finger millet
| Variety | Total protein content (mg BSA/g FW) under NaCl stress | |||
|---|---|---|---|---|
| 0 mM | 100 mM | 200 mM | 300 mM | |
| GBK043137 | 15.2 ± 1.3b | 34.4 ± 1.6b | 73.8 ± 7.3c | 95.7 ± 9.8b |
| GBK043128 | 15.3 ± 2.1b | 33.9 ± 3.0b | 73.1 ± 7.4c | 94.7 ± 8.0b |
| GBK043124 | 13.2 ± 1.9b | 32.1 ± 3.1b | 74.7 ± 7.1bc | 85.6 ± 4.1b |
| GBK043122 | 20.0 ± 2.2a | 42.5 ± 5.2a | 95.9 ± 4.1a | 111.9 ± 7.4a |
| GBK043094 | 20.5 ± 3.0a | 45.1 ± 5.7a | 90.5 ± 9.7a | 119.2 ± 6.5a |
| GBK043050 | 20.4 ± 1.2a | 43.3 ± 3.3a | 89.2 ± 11.5ab | 117.5 ± 5.4a |
Values within a column marked with different superscript in each column differ significantly at p < 0.05 [Fishers LSD]. Each value represented as mean ± SD are the mean of three replications
Cluster analysis
Clustering grouped the six finger varieties into two major clusters based on their potential characteristics under control and salinity stress conditions, respectively (Fig. 2a–d). Varieties grouped into specific classes indicate the presence of greater diversity among finger millets under different salinity stresses with varieties GBK043137 and GBK043094 showing greater tolerance to salinity stress.
Discussion
Plants tolerance to salinity stress is a complex trait which is ascribed to a plethora of related morphological, physiological and biochemical adaptive responses, which operate synergistically to lessen cell hyperosmolarity and the ensuing ion disequilibrium (Parihar et al. 2015). In this regard, screening and selection of finger millet varieties tolerant to salinity stress is essential in order to understand their adaptations under saline soils and for the successful production of finger millet in salinity prone areas. In this study, six finger millet varieties from different agroecological zones in Kenya were subjected to different levels of salinity stress, and our findings showed tremendous variabilities within the tested parameters.
Accumulation of ions in plant tissues, which is known to cause fluctuations of macronutrients is regularly used to evaluate the capability of a plant to resist salinity stress. The concentration of Na+ K+ and Cl− ions and the Na+/K+ ratio are vital features that are usually used for screening of salt tolerant plants (Sarabi et al. 2017). Leaf tissues were used in this study because they are more sensitive to salt and they start displaying toxicity much earlier compared to other plant organs (Munns and Tester 2008). Our findings revealed that the NaCl treatments increased the finger millet leaf Na+ and Cl− concentrations. On the contrary, salinity treatments caused decease of K+ ions in all varieties, probably due to membranes depolarization and the loss of Ca+ ion was due to the displacement by Na+ ions. Na+ and K+ ions have similar cellular effects despite the fact Na+ inhibits K+ absorption through binding and obstructing its transport system (Flowers and Yeo 1986). Several studies have reported that plants growing under high NaCl concentrations have low ratios of K+/Na+ ratio which is caused by deficiency of intracellular K+ (Dugasa et al. 2019; Cirillo et al. 2019; Sandhu et al. 2017; Sarabi et al. 2017). The same phenomenon was also observed in this study, where increment of NaCl concentration decreased the leaf K+/Na+ ratios. In our study, we observed a clear association between K+/Na+ ratio and salinity tolerance and varieties, GBK043137 and GBK043094 showed the highest K+/Na+ ratios under both control and NaCl treatments. However, these varieties were placed at the highest ranking for salinity tolerance index. Cellular influx of Cl− ions require energy in a reaction mechanism catalyzed by a Cl−/2H+ coupled antiporters and symporters. The Cl− ions are typically taken up freely with water uptake, and are therefore accumulated in leaf organs depending on the transpiration rate (Munns and Tester 2008). Like Na+, Cl− ions may also be sequestered in cell vacuoles. In our study, the concentration of Cl− in leaves was higher than that of Na+ and this may be justified by the partial control of Na+ at roots. Comparable results were also exhibited by melon (Sarabi et al. 2017) and cucumber (Colla et al. 2012).
Seed germination and seedling emergence are fundamental biological processes in plant growth and development cycles. Excellent seed germination and emergence are thus important for attainment of high yields. Increasing concentrations of salt adversely affects germination process (Laghmouchi et al. 2017; Anuradha and Rao 2001). In the present study, the germination percentage was delayed or constrained under salinity stress compared to control growth conditions. The observed decrease in germination rate under the salinity stress could be attributed to salt toxicity and changes in cellular osmotic potential. We found out that under higher salt concentration, the reduction in the germination rate was less for the salinity-tolerant variety (GBK043094), compared with most salt sensitive variety (GBK043050). This finding was in accordance to previous work in lettuce (Ahmed et al. 2019), alfalfa (Sandhu et al. 2017) and wheat (Tounsi et al. 2017) under saline conditions. In addition, a high degree of shoot growth depression in seedlings grown under salinity stress was clearly noticeable, more in the salt-sensitive varieties, which displayed reduced leaf area, leaf chlorosis, leaf burns and plant death which are symptoms associated with plant toxicity. Slow growth of both shoots and roots is an adaptive characteristic for plant survival under salinity conditions because this permits the plants to commit numerous resources to mitigate the stress (Soares et al. 2018). Retarded shoot growth under salinity stress could be ascribed to the reduction in osmotic potential due to extra concentration of Na+ and Cl− ions in the shoot and root zone resulting in a nutritional imbalance. It could also be due to the deviation of energy destined for growth and development, to exclude Na+ ions from cellular absorption and biosynthesis of solutes for preservation of cell turgor during hypertonic saline conditions. The observed reduction of leaf area under salinity treatments compared to control plants also suggests that salinity stress may affect plant growth through reduction in leaf area. Previous works have disclosed that salt tolerant plants display less growth retardation and have relatively higher growth rate compared to sensitive ones under salinity stress (Carillo et al. 2019; Hussain et al. 2018; Sarabi et al. 2017). Consequently, our findings suggest that GBK043128 and GBK043137 have a better capacity to sustain growth and development under salt treatments as compared to other finger millet varieties studied (Table 2). Further, roots play a key role in salt tolerance of plants as they are the first organs that control uptake and translocation of nutrients and salts throughout the plant. Due to their direct exposure to saline environment, root growth is also vulnerable to salt stress although the extend is lesser than that of the shoots (Munns and Tester 2008). Inhibition of root growth in plants adversely affects the survival and productivity of plants and therefore root growth under saline conditions may serve as good indicator in the first steps of screening for salinity tolerance programs. Growth of roots varies widely due to soil conditions because the status of all nutrient in plants is maintained from the soil with the help of roots. Root growth rate may be severely affected by saline soils and reduction may even be recorded in salt-tolerant plants. In agreement with previously published studies on the effects of salinity on root elongation (Cirillo et al. 2019; Dugasa et al. 2019), salinity treatments were found to cause stunted root growth. The growth-promoting effect under salinity stress could be due to an increase in the osmotic potential of the cells in the elongation zone coupled with enhanced cell division. The absorbed ions at this point could be quickly compartmentalized into the vacuoles without getting to the maximum capacity, thereby increasing the turgor within the cells and stimulating cell elongation. We also observed that the effects of salinity stress on root growth was much less compared to that of the shoot. This feature could be explained by the fact that roots are less affected by salinity due to transport of ions to other plant organs and hence the stressed roots were able to maintain osmotic balance.
Several studies suggest chlorophyll content is a biochemical marker of salt tolerance in plants (Ishikawa and Shabala 2019; Taïbi et al. 2016; Sairam et al. 2005). It is known that salt tolerant plants show increased or unchanged chlorophyll levels under salinity conditions whereas chlorophyll contents decreased in salt-sensitive plants (Stepien and Johonson 2009; Ashraf and Harris 2013). Decrease of chlorophyll content under salt stress is considered to be a result of slow synthesis or fast breakdown of the pigments in cells (Ashraf and Shahbaz 2003). The decrease in total chlorophyll content may also be observed due to ion accumulation and functional disorders observed during stoma opening and closing under salinity stress (Nawaz and Ashraf 2010). Another reason for the decrease of chlorophyll content under salt conditions is stated to be the rapid maturing of leaves (Yeo et al. 1991). In our study, statistically significant decrease in total chlorophyll content was observed with increasing salt concentration. Similar results were reported by Ashraf (1998) and Ali et al. (2004) who showed that total chlorophyll content of rice leaves was generally reduced under high salinity. While the other varieties recorded a decrease over the control plants, variety GBK043094 recorded unchanged total chlorophyll content with increase in stress (Table 4). These findings signified that salinity stress damaged the photochemical apparatus of the plant leaves. Our results also showed that reduction in chlorophyll content was variety specific. Some varieties had comparatively lesser quantum of negative variation in chlorophyll content, thus signifying their potential to grow and perform moderately well even under higher levels of salt stress. High salt concentration induced reduction of total chlorophyll content, suggesting that salt stress induces chlorophyll degradation and destruction of chloroplast structures.
All plants employ complex biochemical defensive mechanisms against oxidative injury of free radicals and reactive oxygen species (ROS) during abiotic stresses. Among these defense systems is the aggregation of compatible solutes such as proline, an osmoprotectant that preserves membrane integrity and mitigates oxidative burst in plant challenged by salt stress (Rao et al. 2013; Rasool et al. 2013). Proline exists in all plant organs, accumulating in greater proportions compared to other amino acids in salinity stressed plants (Islam et al. 2009). Although, the beneficial outcome of proline overproduction in plants during salinity stress have been explicated, the definite roles of proline accretion are still obscure (Islam et al. 2009; Verbruggen and Hermans 2008). Our study reported an increased concentration of free proline content in all six finger millet varieties, with GBK043094 and GBK043137 displaying higher free proline amounts at all salinity treatments suggesting that they are comparatively more tolerant to salinity stress than the rest and which may be related to their competitive ability under saline stress against oxidative stress. Based on these results, it is worth noting that increased concentration of free proline content in finger millet plants subjected to salinity treatments corresponded to improved salinity tolerance. Likewise, accumulation of protein compounds plays vital role in physiological responses of plant to salinity stress. Increased production of proteins and other nitrogen containing compound may induce the biosynthesis of osmotically active organic compounds including proteins with osmoprotective capacities, thereby conferring salinity resistance (Ashraf and Harris 2004). Generally, plants exposed to NaCl stress have comparatively reduced protein levels which often results to loss of cellular turgor. Just like in our case, reduction in the content of soluble protein was observed in maize plants subjected to salinity treatments (von Alvensleben et al. 2013). Contrary to other osmolytes such as proline and malondialdehyde which are present at very low amounts except when their biosynthesis is triggered by stress, compatible solutes such as reducing sugars are elements of metabolism with different cell functional roles, such as precursors of other metabolites, signalling molecules and major source of energy. Their levels are highly controlled by various systems to ensure cellular homeostasis. Reducing sugars therefore play a crucial role in plant cells osmotic adjustment during salinity stress. The high reducing sugar levels measured in plants with high salinity tolerant index clearly shows that sugar contributes to osmotic adjustment during salt treatments thus, cushioning the plants against the toxic effects of NaCl. These results are substantiated by a remarkable increase in sugar amounts in salt tolerant genotypes in pigeon pea (Awana et al. 2019), Juncus sp. (Al-Hassan et al. 2016) and wheat (Kerepesi and Galiba 2000).
Degradation of polyunsaturated fatty acids in plants yields malondialdehyde (MDA), a biomarker for determining the degree of lipid peroxidation and cellular membrane damage (Yang et al. 2018). Results from our study reveal that MDA content in stressed plants raised with increasing stress levels which corroborated with results exhibited in other plant species like Lycium ruthenicum (Li et al. 2019), wheat (Dugasa et al. 2019) and Cucumis melo L. (Sarabi et al. 2017). Our results indicated that some varieties of finger millet may tolerate saline environments more than others depending on the severity of the stress and do this by lowering the rate of lipid peroxidation and cell membrane damage. The salt-tolerant varieties have an efficient and effective antioxidant defence mechanism. The strong negative correlation witnessed between MDA and shoot height (r = − 0.6872, Supplementary Table 3), and root length (r = − 7555, Supplementary Table 3) affirms that the NaCl stress triggered lipid peroxidation is one of the reasons for the observed stunted shoot and root growth in finger millet plants. Salinity stress induces excessive production ROS without a concomitant scavenging mechanism which causes reduction in chlorophyll (Nxele et al. 2017), which is a key factor in growth and development of plants.
Lastly, it is imperative to note that this study was conducted in a laboratory set-up (artificial conditions) which may not mirror their complex natural environment under which the crop is grown. Nevertheless, the findings give suggestive index salinity tolerance to the studied finger millet varieties.
Conclusions
This study provides analysis of the effect of NaCl stress treatments on the physiological and biochemical underlying stress-response mechanisms of finger millet. The findings revealed significant variety specific salinity stress responses in finger millet., It can be concluded that progressive salinity stress treatments significantly reduced finger millet seed germination rate, growth and development by differentially modulating several biochemical responses among the finger millet varieties studied. From the responses of GBK043094 and GBK043137, we hypothesize that these varieties are promising genetic resources with considerable high salinity tolerance, they can be utilized for breeding programs of the crop towards enhanced salinity tolerance. Further analyses are required to explicate the genes and mechanisms of salinity tolerance observed in some of finger millet varieties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This project was funded by The World Academy of Sciences grant (Ref. No. 17-357 RG/BIO/AF/AC_I—FR3240297745) through the generous contribution of the Swedish International Development Cooperation Agency. The authors thank the Kenyatta University and Pwani University for providing laboratory facilities. The authors gratefully acknowledge Kenya Agricultural and Livestock Research Organization, Gene Bank, for providing the finger millet seeds.
Author contribution
AM, AN, WM designed and did the experiments performed data analyses. AM, WM wrote the draft manuscript. ES and RO revised and corrected the draft manuscript. WM conceptualized the idea and design, supervised the work and made critical review of the article.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Asunta Mukami and Wilton Mbinda have contributed equally to this work.
References
- Ahmed S, Ahmed S, Roy SK, Woo SH, Sonawane KD, Shohael AM. Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agric. 2019;4(1):361–373. doi: 10.1515/opag-2019-0033. [DOI] [Google Scholar]
- Al-Hassan M, del Pilar LM, Boscaiu M, Vicente O. Stress tolerance mechanisms in Juncus: responses to salinity and drought in three Juncus species adapted to different natural environments. Funct Plant Biol. 2016;43:949–960. doi: 10.1071/FP16007. [DOI] [PubMed] [Google Scholar]
- Ali Y, Aslam Z, Ashraf MY, Tahir GR. Effect of salinity on chlorophyll concentration, leaf area, yield and yield components of rice genotypes grown under saline environment. Int J Environ Sci Technol. 2004;1(3):221–225. doi: 10.1007/BF03325836. [DOI] [Google Scholar]
- Anuradha S, Rao SSR. Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (Oryza sativa L.) Plant Growth Regul. 2001;33:151–153. doi: 10.1023/A:1017590108484. [DOI] [Google Scholar]
- Arnon D. Copper enzyme in isolated chloroplast and chlorophyll expressed in terms of mg per gram. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaw E, Suryabhagavan KV, Argaw M. Soil salinity modeling and mapping using remote sensing and GIS: the case of Wonji sugar cane irrigation farm, Ethiopia. J Saudi Soc Agric Sci. 2018;17(3):250–258. doi: 10.1016/j.jssas.2016.05.003. [DOI] [Google Scholar]
- Ashraf MY. Effect of salinity on growth, chlorophyll content, and flag leaf area of rice (Oryza sativa L.) genotypes. Int Rice Res Notes. 1998;2:33–35. [Google Scholar]
- Ashraf MPJC, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- Ashraf M, Harris PJC. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51(2):163–190. doi: 10.1007/s11099-013-0021-6. [DOI] [Google Scholar]
- Ashraf M, Shahbaz M. Assessment of genotypic variation in salt tolerance of early CIMMYT hexaploid wheat germplasm using photosynthetic capacity and water relations as selection criteria. Photosynthetica. 2003;41(2):273–280. doi: 10.1023/B:PHOT.0000011961.33120.b6. [DOI] [Google Scholar]
- Awana M, Yadav K, Rani K, Gaikwad K, Praveen S, Kumar S, Singh A. Insights into salt stress-induced biochemical, molecular and epigenetic regulation of spatial responses in Pigeonpea (Cajanus cajan L.) Plant Growth Regul. 2019 doi: 10.1007/s00344-019-09955-4. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bertazzini M, Sacchi GA, Forlani G. A differential tolerance to mild salt stress conditions among six Italian rice genotypes does not rely on Na+ exclusion from shoots. Plant Physiol. 2018;226:145–153. doi: 10.1016/j.jplph.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Carillo P, Cirillo C, De Micco V, Arena C, de Pascale S, Rouphael Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric Water Manag. 2019;212:12–22. doi: 10.1016/j.agwat.2018.08.037. [DOI] [Google Scholar]
- Chen J, Zong J, Li D, Chen Y, Wang Y, Guo H, Li J, Li L, Guo A, Liu J. Growth response and ion homeostasis in two bermudagrass (Cynodon dactylon) cultivars differing in salinity tolerance under salinity stress. Soil Sci Plant Nutr. 2019;65:419–429. doi: 10.1080/00380768.2019.1631125. [DOI] [Google Scholar]
- Cheng W, Zhang G, Yao H, Dominy P, Wu W, Wang R. Possibility of predicting heavy-metal contents in rice grains based on DTPA-extracted levels in soil. Commun Soil Sci Plant Anal. 2004;35:2731–2745. doi: 10.1081/CSS-200036424. [DOI] [Google Scholar]
- Chivenge P, Mabhaudhi T, Modi A, Mafongoya P. The potential role of neglected and underutilised crop species as future crops under water scarce conditions in Sub-Saharan Africa. Int J Environ Res Public Health. 2015;12:5685–5711. doi: 10.3390/ijerph120605685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo C, De Micco V, Arena C, Carillo P, Pannico A, De Pascale S, Rouphael Y. Biochemical, physiological and anatomical mechanisms of adaptation of callistemon citrinus and viburnum lucidum to NaCl and CaCl2 salinization. Front Plant Sci. 2019;10:742. doi: 10.3389/fpls.2019.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla G, Rouphael Y, Rea E, Cardarelli M. Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci Hortic. 2012;135:177–185. doi: 10.1016/j.scienta.2011.11.023. [DOI] [Google Scholar]
- Damerval C, de Vienne D, Zivy M, Thiellement H. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis. 1986;7:52–54. doi: 10.1002/elps.1150070108. [DOI] [Google Scholar]
- Dugasa MT, Cao F, Ibrahim W, Wu F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol Plant. 2019;165:134–143. doi: 10.1111/ppl.12743. [DOI] [PubMed] [Google Scholar]
- Eaton AD, Clesceri LS, Greenberg AE. Standard methods for the examination of water and wastewater. Washington: American Public Health Association; 1995. [Google Scholar]
- Flowers TJ, Yeo AR. Ion relations of plants under drought and salinity. Funct Plant Biol. 1986;13(1):75–91. doi: 10.1071/PP9860075. [DOI] [Google Scholar]
- Ganie SA, Molla KA, Henry RJ, Bhat KV, Mondal TK. Advances in understanding salt tolerance in rice. Theor Appl Genet. 2019;132:851–870. doi: 10.1007/s00122-019-03301-8. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hema R, Vemanna RS, Sreeramulu S, Reddy CP, Senthil KM, Udayakumar M. Stable expression of mtlD gene imparts multiple stress tolerance in finger millet. PLoS ONE. 2014;9(6):e99110. doi: 10.1371/journal.pone.0099110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MI, Al-Dakheel AJ, Reigosa MJ. Genotypic differences in agro-physiological, biochemical and isotopic responses to salinity stress in quinoa (Chenopodium quinoa Willd.) plants: prospects for salinity tolerance and yield stability. Plant Physiol Biochem. 2018;129:411–420. doi: 10.1016/j.plaphy.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Ibrahim EA. Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol. 2016;192:38–46. doi: 10.1016/j.jplph.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Shabala S. Control of xylem Na+ loading and transport to the shoot in rice and barley as a determinant of differential salinity stress tolerance. Physiol Plant. 2019;165:619–631. doi: 10.1111/ppl.12758. [DOI] [PubMed] [Google Scholar]
- Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol. 2009;166(15):1587–1597. doi: 10.1016/j.jplph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Johnson G, Lambert C, Johnson DKD, Sunderwirth SG. Colorimetric determination of glucose, fructose, and sucrose in plant materials using a combination of enzymatic and chemical methods. J Agric Food Chem. 1964;12:216–219. doi: 10.1021/jf60133a007. [DOI] [Google Scholar]
- Kerepesi I, Galiba G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 2000;40:482–487. doi: 10.2135/cropsci2000.402482x. [DOI] [Google Scholar]
- Kumar V, Khare T. Differential growth and yield responses of salt-tolerant and susceptible rice cultivars to individual (Na+ and Cl−) and additive stress effects of NaCl. Acta Physiol Plant. 2016;38:1–9. doi: 10.1007/s11738-016-2191-x. [DOI] [Google Scholar]
- Kumar A, Metwal M, Kaur S, Gupta AK, Puranik S, Singh S, Singh M, Gupta S, Babu BK, Sood S, Yadav R. Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front Plant Sci. 2016;7:934. doi: 10.3389/fpls.2016.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghmouchi Y, Belmehdi O, Bouyahya A, Senhaji NS, Abrini J. Effect of temperature, salt stress and pH on seed germination of medicinal plant Origanum compactum. Biocatal Agric Biotechnol. 2017;10:156–160. doi: 10.1016/j.bcab.2017.03.002. [DOI] [Google Scholar]
- Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J stat softw. 2008;25(1):1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- Li Y, Zhang T, Zhang Z, He K. The physiological and biochemical photosynthetic properties of Lycium ruthenicum Murr in response to salinity and drought. Sci Hortic. 2019;256:1–9. doi: 10.1016/j.scienta.2019.05.057. [DOI] [Google Scholar]
- Machado RMA, Serralheiro RP. Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae. 2017;3(30):2–13. doi: 10.3390/horticulturae3020030. [DOI] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nawaz K, Ashraf M. Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J Agron Crop Sci. 2010;196(1):28–37. doi: 10.1111/j.1439-037X.2009.00385.x. [DOI] [Google Scholar]
- Neocleous D, Ntatsi G, Savvas D. Physiological, nutritional and growth responses of melon (Cucumis melo L.) to a gradual salinity built-up in recirculating nutrient solution. J Plant Nutr. 2017;40(15):2168–2180. doi: 10.1080/01904167.2017.1346673. [DOI] [Google Scholar]
- Nxele X, Klein A, Ndimba BK. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S Afr J Bot. 2017;108:261–266. doi: 10.1016/j.sajb.2016.11.003. [DOI] [Google Scholar]
- Parihar P, Singh S, Singh R, Singh VP, Prasad SM. Effect of salinity stress on plants and its tolerance strategies: a review. ESPR. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD. Economics of salt-induced land degradation and restoration. Nat Resour Forum. 2014;38:282–295. doi: 10.1111/1477-8947.12054. [DOI] [Google Scholar]
- Rao ES, Kadirvel P, Symonds RC, Ebert AW. Relationship between survival and yield related traits in Solanum pimpinellifolium under salt stress. Euphytica. 2013;190(2):215–228. doi: 10.1007/s10681-012-0801-2. [DOI] [Google Scholar]
- Rasool S, Ahmad A, Siddiqi TO, Ahmad P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiologiae Plant. 2013;35(4):1039–1050. doi: 10.1007/s11738-012-1142-4. [DOI] [Google Scholar]
- Sairam RK, Srivastava GC, Agarwal S, Meena RC. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant. 2005;49:85–91. doi: 10.1007/s10535-005-5091-2. [DOI] [Google Scholar]
- Sandhu D, Cornacchione MV, Ferreira JF, Suarez DL. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci Rep. 2017;7:1–18. doi: 10.1038/srep42958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabi B, Bolandnazar S, Ghaderi N, Ghashghaie J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: prospects for selection of salt tolerant landraces. Plant Physiol Biochem. 2017;119:294–311. doi: 10.1016/j.plaphy.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Shabala S, Hariadi Y, Jacobsen SE. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J Plant Physiol. 2013;170:906–914. doi: 10.1016/j.jplph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Soares AC, Geilfus CM, Carpentier SC. Genotype-specific growth and proteomic responses of maize towards salt stress. Front Plant Sci. 2018;9:1–15. doi: 10.3389/fpls.2018.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien P, Johnson GN. Contrasting responses of photosynthesis to salt stress in the glycophyte arabidopsis and the halophyte thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 2009;149(2):1154–1165. doi: 10.1104/pp.108.132407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot. 2016;105:306–312. doi: 10.1016/j.sajb.2016.03.011. [DOI] [Google Scholar]
- Tounsi S, Feki K, Hmidi D, Masmoudi K, Brini F. Salt stress reveals differential physiological, biochemical and molecular responses in T. monococcum and T. durum wheat genotypes. Physiol Mol Biol Plants. 2017;23:517–528. doi: 10.1007/s12298-017-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35(4):753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- von Alvensleben N, Stookey K, Magnusson M, Heimann K. Salinity tolerance of Picochlorum atomus and the use of salinity for contamination control by the freshwater cyanobacterium Pseudanabaena limnetica. PLoS ONE. 2013;8:e63569. doi: 10.1371/journal.pone.0063569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shahidi F, Tsao R. Biomarkers of oxidative stress and cellular-based assays of indirect antioxidant measurement. Meas Antioxid Act Capacity. 2018 doi: 10.1002/9781119135388.ch9. [DOI] [Google Scholar]
- Yeo AR, Lee λS, Izard P, Boursier PJ, Flowers TJ. Short- and long-term effects of salinity on leaf growth in rice (Oryza sativa L.) J Exp Bot. 1991;42(7):881–889. doi: 10.1093/jxb/42.7.881. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.