Abstract
Biotechnological strategies are needed to produce larger quantities of biomass and phytochemicals. In this study, callus cultures of Fagonia indica were elicited with different concentrations of chemically and biologically synthesized silver nanoparticles (chem- and bioAgNPs) to compare their effects on biomass, total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity of the extracts from callus. The results revealed that bioAgNPs being more biocompatible produced the highest biomass initially on day 10 (FW = 4.2152 ± 0.13 g; DW = 0.18527 ± 0.01 g) and day 20 (FW = 7.6558 ± 0.10 g; DW = 0.3489 ± 0.01 g) when supplemented in media as 62.5 µg/mL and 250 µg/mL, respectively. Initially, the highest TPC (319.32 ± 8.28 µg GAE/g of DW) was recorded on day 20 in chemAgNPs (31.25 µg/mL) induced callus as compared to TPC = 302.85 ± 3.002 µg GAE/g of DW in bioAgNPs-induced callus. Compared to the highest values of TFC (108.15 ± 2.10 µg QE/g of DW) produced in 15.6 µg/mL chemAgNPs-induced callus on day 20, TFC produced in bioAgNPs (62.5 µg/mL) was 168.61 ± 3.17 µg GAE/g of DW on day 10. Similarly, chemAgNPs-induced callus (62.5 µg/mL) showed the highest free radical scavenging activity (FRSA) i.e. 87.18% on day 20 while bioAgNPs (125 µg/mL) showed 81.69% FRSA on day 20 compared to highest among control callus (63.98% on day 40). The highest total antioxidant capacity of chemAgNPs-(125 µg/mL) induced callus was 330.42 ± 13.65 µg AAE/g of DW on day 20 compared to bioAgNPs-(62.5 µg/mL) induced callus (312.96 ± 1.73 µg AAE/g of DW) on day 10. Conclusively, bioAgNPs are potent elicitors of callus cultures of F. indica.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00851-w) contains supplementary material, which is available to authorized users.
Keywords: AgNPs, F. indica, Elicitation, Callus cultures, Phenolic compounds
Introduction
The low yield of pharmaceutically important phytochemicals and high market demand has created interest in biotechnological approaches to enhance the yield of secondary metabolites (Karuppusamy 2009). Over the years, different strategies have been used to enhance the production of medicinally important phytochemicals. In vitro culture including callus cultures, cell suspensions cultures, and organ cultures is an attractive technology for enhanced production of important phytochemicals irrespective of geography and season (Khan et al. 2016). Fagonia indica, a member of family Zygophyllaceae, has been researched in vitro as an emerging medicinal plant to produce important phenolic compounds (Khan et al. 2018a). The plant possesses various medicinal activities such as anticancer, antimicrobial, antitumor, and antioxidant activities (Eman et al. 2010; Waheed et al. 2012). Biotechnology strategies to enhance the production of phenolic compounds have been in practice since long now. Elicitation of in vitro cultures has proven one of the most effective strategies to produce important phytochemicals such as phenolic compounds in higher quantities (Ramirez-Estrada et al. 2016). New types of elicitors, both biotic and abiotic are added through new research that shows promising results (Khan et al. 2018a, b). Among recently applied elicitors, nanomaterials fascinated researchers because of their smaller size, easy penetration, and thus easy manipulation of the cell’s machinery to produce higher quantities of phytochemicals (Sanzari et al. 2019). Nanomaterials have been applied to plant tissue culture for different purposes including growth, organogenesis and transformation (Kim et al. 2017) and elicitation of secondary metabolites in different plants (Ali et al. 2019).
Silver nanoparticles (AgNPs) have been applied to different plants for their physiological as well as molecular-level effects on plant growth and secondary metabolism (Nair and Chung 2014; Verma et al. 2018). Besides, they are also known for their potential to influence biomass production, plant cell growth, and secondary metabolism in plant cell cultures especially callus cultures (Khan et al. 2019). AgNPs of both chemical and biological origin are synthesized through different routes (Prabhu and Poulose 2012). Chemically synthesized AgNPs (chemAgNPs) have been applied to many different plants for their effects on growth and secondary metabolism including Calendula officinalis (Al-Oubaidi and Mohammed-Ameen 2014), Oryza sativa (Ejaz et al. 2018), Arabidopsis thaliana (Syu et al. 2014) and Brassica juncea (Sharma et al. 2012). However, like other abiotic chemical elicitors, chemAgNPs also pose a threat to plant growth as well as the environment when given in higher concentrations. The issues include leakage of silver ions, and cell death as well as the release of AgNPs to the environment (Khan et al. 2019). Toxicity concerns and safety issues are surrounding the exposure of plant tissue cultures to nanomaterials (Kim et al. 2017). This suggests that biologically synthesized AgNPs (bioAgNPs) may be an important alternative to chemAgNPs because of their biological origin and thus biocompatibility and lesser toxicity to plants as well as the environment (Sehnal et al. 2019). Being of biological origin makes bioAgNPs a variant of biotic elicitors. This means they are essentially biological in origin and they can be more friendly to plants cells in terms of growth as well as having the silver component to trigger secondary metabolism. Plants such as F. indica contain alkaloids, saponins, terpenoids, sterols, flavonoids, and phenolic compounds which can play an effective role in the synthesis of AgNPs. BioAgNPs and chemAgNPs can be compared for their effects on plant growth, physiology, and secondary metabolism. The current study aimed to assess the effects of bioAgNPs in comparison with chemAgNPs on biomass accumulation, secondary metabolite production and antioxidant activity in F. indica callus cultures.
Materials and methods
Seed germination and callus induction
The seeds of F. indica were collected from plants in Lower Dir District of Province Khyber Pakhtunkhwa, Pakistan. The seeds were sterilized through mercuric chloride having a concentration of 0.1 g/100 mL. The process was performed in a biological safety cabinet (BioBase MSC Ltd. Class II, China). The tips of seeds were finely nicked using a cutter and then soaked on filter paper. After this, two seeds were transferred to each of 100 mL flasks containing MS basal medium (with 3% sucrose and 0.8% agar (w/v)). The flasks were then kept for germination in the growth chamber (BioBase Bjpx-A1500c, Ltd. China) 25 ± 2 °C under 16 h light/8 h dark.
For callus initiation, the protocol of Khan et al. (2016) was followed. Briefly, approximately 0.5 cm stem cuttings were aseptically cut from 30-day old F. indica plantlets grown in vitro, and transferred to a flask containing autoclaved MS medium (with 3% sucrose and 0.8% (w/v) agar) supplemented with Thidiazuron (TDZ = 1 mg/mL). The flasks were transferred to the growth chamber (25 ± 2 °C under 16 h light/8 h dark) for callus initiation. The callus cultures were maintained in a plant growth chamber for 40 days.
Preparation of biogenic silver nanoparticles
BioAgNPs were prepared according to the protocol of Adil et al. (2019). Briefly, silver nitrate solution (4 mM) was mixed in equal volumes with an extract from callus cultures of F. indica. The reaction was allowed to proceed in the sunlight illuminated area for 3 h. The synthesized AgNPs were then washed through repeated centrifugation (five times at 13,000 rpm for 10 min) and were sonicated for 30 min at 20 °C. The presence of AgNPs was then confirmed by UV–Vis spectroscopy and was characterized through transmission electron microscopy. Morphology analysis from various TEM images was performed through Image-J software. Finally, the reaction mixtures centrifuged to store the pelleted AgNPs for further application. For comparison with bioAgNPs, chemAgNPs were acquired from Sigma-Aldrich (Merck). The chemAgNPs (specific surface: 5.0 m2/g, diameter: < 100 nm, order code 576832-5G, Sigma–Aldrich, Steinheim, Germany) were obtained ready-made for application to callus cultures of F. indica to compare their effects on biomass accumulation, and secondary metabolism with bioAgNPs. The chemAgNPs for biological applications are synthesized through methods involving reducing and stabilizing chemicals of non-biological origin (Oldenburg and Saunders 2020).
Supplementation of medium with AgNPs
A stock solution of both chemAgNPs and bioAgNPs was prepared as 10 mg/mL in distilled water. The mixture was properly sonicated (Time = 1.5 h, Voltage = 40 kHz and temperature = 30 °C in Powersonic 405, Hwashin Technology Company, Korea) to prepare fine colloids of chem- and bioAgNPs. MS medium supplemented with TDZ (1 mg/mL) was prepared for callus growth according to the protocol in “Characterization of silver nanoparticles”. Different volumes of the stock solution were added to different sets of media to prepare the final concentrations of both types of AgNPs in media as 250 µg/mL, 125 µg/mL, 62.5 µg/mL, 31.25 µg/mL and 15.6 µg/mL. Along with this, media having no AgNPs were kept as control. During the transfer of callus to the media, 400 mg (0.4 g) of the F. indica callus was transferred aseptically (in biosafety cabinet) to each flask containing media supplemented with either of the AgNPs. The flasks were then kept in the growth chamber.
Growth kinetics and fresh/dry weight determination
Three flasks containing calli from all treatments were harvested after 10, 20, and 40 days for estimation of fresh weight (FW) and dry weight (DW). For FW, calli were carefully removed from cultures flask and were soaked on filter paper for 3 min. The final FW was recorded through analytical balance. For determination of dry weight (DW), the callus samples were kept at 51 °C in a dry oven for 24 h. The dried callus was ground to form fine powder with mortar and pestle. The fine powder was stored for the extraction of secondary metabolites.
Analytical scheme
Callus extracts preparation
For the quantification of phenolic compounds, dried callus samples (100 mg) from each treatment were added with 800 µL methanol and kept on a rotatory shaker for 24 h. The mixtures were sonicated for 5 min (Voltage = 50/60 Hz and temperature = 30 °C in Powersonic 405, Hwashin Technology Company, Korea) and vortexed subsequently for 20 min. The sonication and shaking steps were repeated two times. Finally, the reaction mixtures were centrifuged at 13000 rpm for 5 min. The Eppendorf tubes were weighed and properly labeled for each treated mixtures and control. After centrifugation, supernatant from each tube was collected and pipetted to already weighed Eppendorf tubes. The liquid extract was let to evaporate in a tube for 2 days, to obtain the extract. The final weight of the Eppendorf tube with the extract was recorded to find out the exact weight of the extract. The extract was stored for further analysis.
Determination of total phenolic content (TPC)
The total phenolic content was measured according to the Folin–Ciocalteu method. A diluted stock solution of Folin–Ciocalteu reagent was prepared in deionized distilled water. 6 g of sodium carbonate was dissolved in 100 mL of distilled water to prepare its stock solution (6%). Besides, the stock solution of gallic acid having a concentration of 4 mg/mL was prepared in methanol. 20 μl of the sample was added to 96 wells microplate using a multi-channel micropipette. This was followed by the addition of 90 μL Folin–Ciocalteu reagent. The mixture was incubated at room temperature for 5 min. Then, 90 μL sodium carbonate (6%) was added to the mixture. 25, 20, 15, 10 and 5 μg/mL final concentration of gallic acid was used as a positive control for the process. The samples were then incubated at room temperature for 1 h. The absorbance was measured at a wavelength of 630 nm using a microplate reader (TSx800 Absorbance Reader, BioTek Inc., USA). All the procedures were carried out in triplicate.
Determination of total flavonoid content (TFC)
A stock solution of aluminum chloride (10%) and potassium acetate (1 M) were prepared using distilled water. A stock solution of quercetin having a concentration of 4 mg/mL was prepared in methanol. The aluminum chloride colorimetric method was used to determine total flavonoid content. 20 μL of the sample, 10 μL of aluminum chloride, 10 μL of potassium acetate and 160 μL of distilled water was added to 96 wells microplate. The mixture was incubated at room temperature for 30 min. 20 μL of MeOH was used as positive control while 40, 20, 10, 5, and 2.5 μg/mL final concentration of quercetin was used as a positive control for this procedure. The spectra were recorded at 430 nm using a microplate reader (TSx 800 Absorbance Reader, BioTek Inc., USA). All the procedures were carried out in triplicate.
Antioxidant assay
The antioxidant activity of the samples was determined through free radical, Diphenyl picryl hydrazyl (DPPH) scavenging activity. A stock solution of DPPH was prepared by dissolving 3.2 mg of DPPH reagent in 100 mL of methanol. A stock solution of ascorbic acid (4 mg/mL) was also prepared in methanol. After the stock solutions preparation, 20 μL of the sample was added to 96 wells microplate. This was followed by the addition of 180 μL DPPH reagent. The mixture was incubated for 1 h. 40, 20, 10 and 5 μg/mL of ascorbic acid final concentration was used as a positive control for this procedure. The result was analyzed at 517 nm wavelength on a microplate reader (TSx800 Absorbance Reader, BioTek Inc., USA).
Total antioxidant capacity
Phosphomolybdenum method was used to determine the total antioxidant capacity of the sample. The reagent used in this method was the total antioxidant capacity (TAC) reagent. This reagent contains 20 mM sodium phosphate, 4 mM ammonium molybdate, and 0.6 M sulfuric acid. Firstly, 20 μL of the sample was added to the 96 wells microplate. Then, 180 μL of total antioxidant capacity (TAC) reagent was added to the same microplates. The mixture was incubated in a water bath at 95 °C for 90 min. After incubation, the sample was allowed to cool at room temperature. For this assay, 40, 20, 10, 5 μg/mL of ascorbic acid final concentration was used as a positive control. The result analysis was done at 630 nm wavelength using a microplate reader (TSx800 Absorbance Reader, BioTek Inc., USA). All the steps were repeated 3 times.
Statistical analysis
A completely randomized design was used for all the experiments. The experiments were repeated twice, and each treatment was performed in triplicates. Statistical analyses were carried out using SPSS 20 and Microsoft Excel 2019. Linear regression analysis was employed for the significant mean difference. p < 0.05 was used to define significant results. All the figures were prepared through Origin 8.5.
Results
Characterization of silver nanoparticles
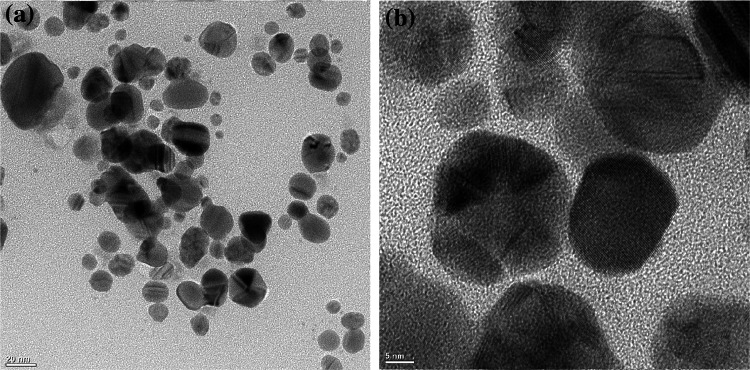
The synthesis of biologically fabricated AgNPs in this study was confirmed by reading the optical density through UV–visible spectroscopy. The characteristic surface plasmon resonance (SPR) peaks confirmed the synthesis of bioAgNPs through callus extract of F. indica (Fig. 1). AgNPs gives characteristics peaks between 400 and 500 nm. Higher absorbance i.e. OD between the wavelength range, λ400-500, is one of the specific properties of AgNPs. In our experiments, absorbance in this range was observed that directly confirms the presence of AgNPs. The bioAgNPs were further characterized by transmission electron microscopy (TEM). Analysis of TEM results also showed that AgNPs < 50 nm were synthesized through the callus extracts of F. indica (Fig. 2). The size distribution of AgNPs showed that most of the particles were spherical. The diameter of AgNPs synthesized through callus extract of F. indica varied between 2 and 36 nm with an average size of 15 nm.
Fig. 1.

UV-Vis spectroscopy-based surface plasmon resonance peaks of AgNPs obtained from callus extract of F. indica
Fig. 2.
Transmission electron microscopy (TEM) images of AgNPs synthesized via the extract from callus of F. indica and AgNO3 (2 mM), mixed in a 1:1 ratio (v/v). a TEM images of AgNPs and b high-resolution images showing characteristic lattice fringes of Ag
Growth kinetics and biomass accumulation
The fresh weight of the callus was recorded on days 10, 20, and 40 after initiation of the experiment. The callus showed impressive growth on MS media supplemented with TDZ (1 mg/mL) and different concentrations of AgNPs. Table 1 shows the measured data as the mean of triplicate data with ± standard error. As shown in the table, the highest FW value on day 10 was 2.75 ± 0.026 g in media supplemented with 62.5 µg/mL chemAgNPs. Compared to this on day 10, bioAgNPs (62.5 µg/mL) resulted in higher biomass accumulation (FW = 4.21 ± 0.13 g and DW = 0.185 ± 0.01 g). On day 10, the control callus accumulated as FW = 1.734 ± 0.29 g and DW = 0.10 ± 0.009 g. On day 10, it was observed that the biomass accumulation reached its maximum at 62.5 µg/mL of both types of AgNPs and then declined with an increase in concentration (Table 1). Furthermore, after day 20, callus produced the highest biomass in bioAgNPs (250 µg/mL) supplemented media (FW = 7.65 ± 0.10 g and DW = 0.34 ± 0.01 g) as compared to chemAgNPs (250 µg/mL) supplemented media (FW = 6.47 ± 1.75 g and DW = 0.30 ± 0.03 g).
Table 1.
Comparison of biomass accumulation after 10, 20, and 40 days of callus initiation from F. indica stem in MS medium supplemented with TDZ and different concentrations of chemically and biogenic AgNPs
| Concentrations of Ag-NPs (µg/mL) | Day 0 | Day 10 | Day 20 | Day 40 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FW(g) | FW (g) | DW (g) | FW (g) | DW (g) | FW (g) | DW (g) | ||||||||
| Chem-AgNPs | Bio-AgNPs | Chem-AgNPs | Bio-AgNPs | Chem-AgNPs | Bio-AgNPs | Chem-AgNPs | Bio-AgNPs | Chem-AgNPs | Bio-AgNPs | Chem-AgNPs | Bio-AgNPs | Chem-AgNPs | Bio-AgNPs | |
| Control | 0.4 | 0.4 | 1.7320 ± 0.29 | 0.1012 ± 0.009 | 4.6156 ± 0.03 | 0.2295 ± 0.005 | 19.1280 ± 0.75 | 0.61 ± 0.02 | ||||||
| 15.6 | 0.4 | 0.4 | 1.32 ± 0.07 | 1.7430 ± 0.14 | 0.075 ± 0.001 | 0.096 ± 0.09 | 3.30 ± 0.47 | 4.9948 ± 0.23 | 0.15 ± 0.02 | 0.2569 ± 0.01 | 18.0 ± 1.10 | 11.344 ± 0.58 | 0.63 ± 0.01 | 0.4451 ± 0.05 |
| 31.25 | 0.4 | 0.4 | 1.59 ± 0.13 | 2.1685 ± 0.25 | 0.077 ± 0.011 | 0.1153 ± 0.04 | 3.40 ± 0.08 | 5.0584 ± 0.03 | 0.17 ± 0.01 | 0.2623 ± 0.02 | 18.1 ± 3.33 | 12.8498 ± 0.64 | 0.66 ± 0.05 | 0.5103 ± 0.03 |
| 62.5 | 0.4 | 0.4 | 2.75 ± 0.02 | 4.2152 ± 0.13 | 0.13 ± 0.001 | 0.18527 ± 0.01 | 4.67 ± 0.65 | 5.7662 ± 0.04 | 0.21 ± 0.03 | 0.3394 ± 0.008 | 19.9 ± 1.27 | 17.1615 ± 1.05 | 0.75 ± 0.02 | 0.5346 ± 0.04 |
| 125 | 0.4 | 0.4 | 0.76 ± 0.02 | 3.4347 ± 0.15 | 0.064 ± 9.06 | 0.1037 ± 0.01 | 6.36 ± 0.46 | 7.0306 ± 0.33 | 0.27 ± 0.05 | 0.3369 ± 0.02 | 20.0 ± 2.28 | 16.4699 ± 0.73 | 0.85 ± 0.02 | 0.5869 ± 0.04 |
| 250 | 0.4 | 0.4 | 0.61 ± 0.32 | 1.0325 ± 0.08 | 0.046 ± 0.008 | 0.0591 ± 0.005 | 6.47 ± 1.75 | 7.6558 ± 0.10 | 0.30 ± 0.03 | 0.3489 ± 0.01 | 18.7 ± 1.08 | 14.7968 ± 0.27 | 0.70 ± 0.04 | 0.5682 ± 0.03 |
The bold values represent the highest values of FW and DW after 10, 20, and 40 days
Regression analysis of FW results shows that the effect of concentration of both bioAgNPs and chemAgNPs on FW is non-significant (p > 0.05) as compared to control. However, bioAgNPs seems to have a higher effect (p = 0.58) compared to chemAgNPs (p = 0.70). This was further verified by results from ANOVA. According to the value from Fisher’s test, the effect of bioAgNPs was higher (F = 106.3) than chemAgNPs (F = 100.97) on biomass accumulation as a result of different concentrations and time from culture initiation (Online resource 1). However, regression analysis of DW results has shown that the concentration of AgNPs had a significant effect on the accumulation of dry weight. Comparing the results of different type of AgNPs, chemAgNPs and bioAgNPs equally affected the dry weight (p < 0.05).
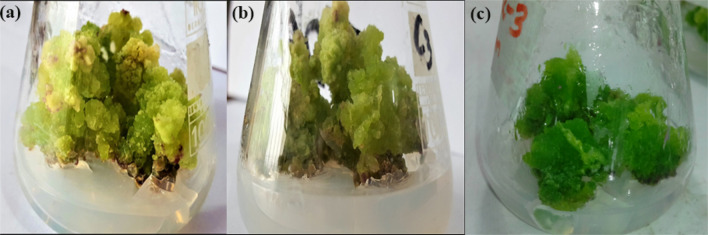
On day 20, the callus the biomass kept on increasing with the increase in concentrations of both types of AgNPs. Interestingly, after 40 days of culture, the highest biomass (FW = 20.0 ± 2.28 and DW = 0.85 ± 0.02 g) was recorded in media having 125 µg/mL chemAgNPs compared to control (FW = 19.12 ± 0.75 g and DW = 0.85 ± 0.02 g). However, for bioAgNPs supplemented media, the highest biomass i.e. FW = 17.16 ± 1.05 g and DW = 0.53 ± 0.01 g was produced in media containing 62.5 µg/mL bioAgNPs. In addition to this, the effect of chemAgNPs and bioAgNPs as compared to control was visually prominent in terms of color, and texture of the callus (Fig. 3). The biomass accumulation after 40 days was comparable to that of control.
Fig. 3.
Representative picture of comparison of a chemAgNPs-induced callus and b bioAgNPs-induced callus and c control callus biomass (without AgNPs) of F. indica
Total phenolic content in callus cultures of F. indica
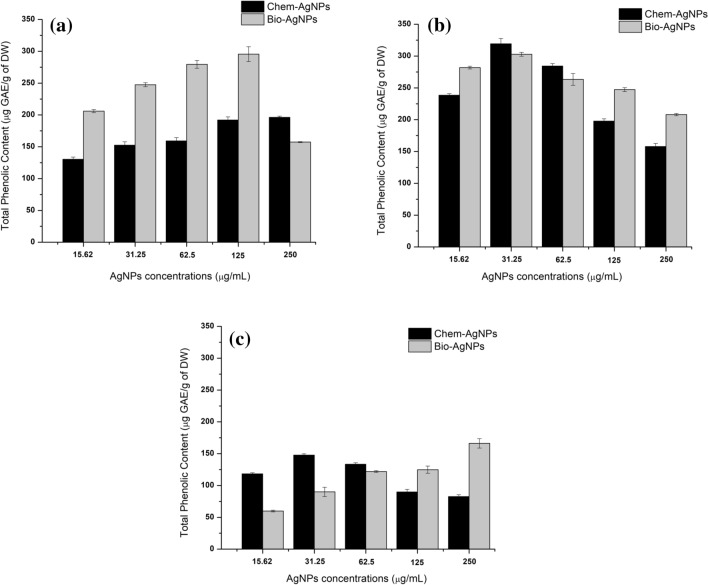
The total phenolic content was recorded temporally in callus on days 10, 20, and 40. The highest TPC (319.32 ± 8.28 µg GAE/g of DW) was produced after 20 days in callus supplemented with chemAgNPs (31.25 µg/mL) as compared to bioAgNPs (302.85 ± 3.002 µg GAE/g of DW). After 10 days, TPC increased with increasing concentration in both types of samples, peaking in bioAgNPs at a concentration of 125 µg/mL (TPC = 295.65 ± 11.66 µg GAE/g of DW) compared to the highest TPC (196.24 ± 1.96 µg GAE/g of DW) in callus supplemented with chemAgNPs (250 µg/mL). While the control callus produced 110.44 ± 3.45 µg GAE/g of DW when recorded on day 10 of initiation, TPC declined at higher concentrations (250 µg/mL) in bioAgNPs based callus (Fig. 4a).
Fig. 4.
Comparison of the effects of different concentrations of chemAgNPs and bioAgNPs on TPC accumulation in callus cultures of F. indica after a 10 days, b 20 days, and c 40 days of initiation. The values are given as mean ± standard error of three replicates
For control callus that produced TPC amounting to 169.803 ± 9.56 µg GAE/g of DW on day 20, both types of AgNPs triggered a higher amount to TPC. However, in contrast to day 10, the quantity of TPC peaked as the concentrations of AgNPs reached 31.25 µg/mL (Fig. 4b). Similarly, it was observed that the quantities of TPC decreased with time and comparatively lower quantities of TPC were observed on day 40. The highest quantities of TPC (166.21 ± 7.33 µg GAE/g of DW) observed in callus grown in 250 µg/mL bioAgNPs after 40 days was 166.21 ± 7.33 µg GAE/g of DW. In contrast to this, the highest TPC in response to chemAgNPs was 147.64 ± 2.19 µg GAE/g of DW when callus was grown in 31.25 µg/mL chemAgNPs. Further, it was seen that on day 40, while the TPC increased with increasing concentrations of bioAgNPs, there was a decrease in TPC with increasing chemAgNPs (Fig. 4c). Statistical analysis of TPC production in callus cultures showed that after 10 days, the effect of bioAgNPs on TPC was insignificant (p > 0.05) as compared to the effect of chemAgNPs (p = 0.045). However, after 20 days, the effect of bioAgNPs became significant (p = 0.013) compared to chemAgNPs (p = 0.085). While after 40 days, bioAgNPs continued to impose a significant effect (p = 0.027) on TPC compared to chemAgNPs (p = 0.085) (Online resource 2).
Quantification of total flavonoid content
The total flavonoid content was also recorded temporally in callus on days 10, 20, and 40.
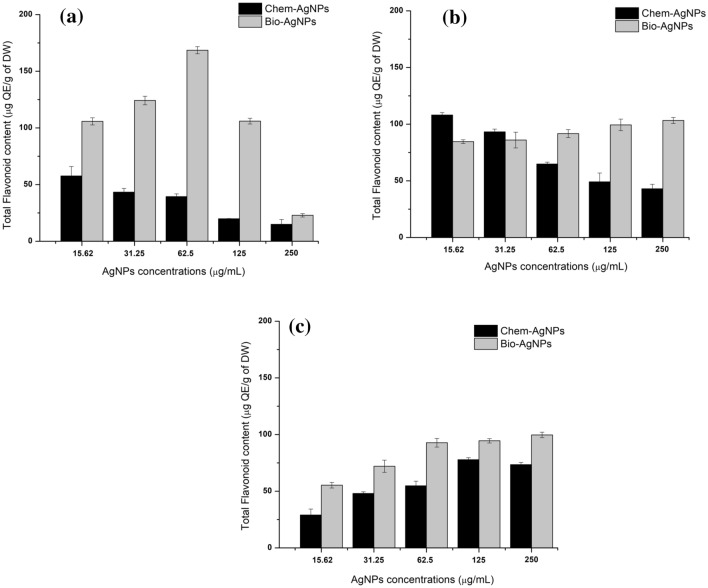
The highest TFC (168.61 ± 3.17 µg QE/g of DW) was produced on day 10 in callus supplemented with bioAgNPs (31.25 µg/mL). The TFC recorded in callus grown in media supplemented with chemAgNPs was 57.65 ± 8.33 µg QE/g of DW while the control callus produced 111.05 ± 3.45 µg QE/g of DW when recorded on day 10 of initiation. It was observed that the production of TFC on day 10 peaked at 62.5 µg/mL bioAgNPs and then decreased with increasing concentrations. However, in chemAgNPs based callus, TFC decreased with increasing concentrations (Fig. 5a).
Fig. 5.
Comparison of the effects of different concentrations of chemAgNPs and bioAgNPs on TFC accumulation in callus cultures of F. indica after a 10 days, b 20 days, and c 40 days of initiation. The values are given as mean ± standard error of three replicates
Furthermore, on day 20, chemAgNPs triggered higher TFC (108.15 ± 2.10 µg QE/g of DW) at a concentration of 15.6 µg/mL as compared to the highest TFC in bioAgNPs (103.26 ± 2.63 µg QE/g of DW) when they were administered at 250 µg/mL. While the control callus that was devoid of AgNPs, it produced lesser TFC (87.75 ± 2.89 µg QE/g of DW) on day 20. It was observed that the trend was the same as that recorded after 10 days, such that the quantity of TFC declined as the concentrations of chemAgNPs increased while an increase was observed with increasing the concentrations of bioAgNPs (Fig. 5b). After 40 days, like TPC, it was observed that the quantities of TFC decreased with time and comparatively lower quantities of TFC were observed on day 40. The highest quantities of TFC (99.66 ± 2.40 µg QE/g of DW) was observed in callus grown in 250 µg/mL bioAgNPs (250 µg/mL) compared to highest TFC in chemAgNPs based callus (77.82 ± 1.80 µg QE/g of DW) when it was grown in 125 µg/mL chemAgNPs. As was the case after 20 days, the control callus produced lesser TFC (52.421 ± 3.05 µg QE/g of DW). Interestingly, however, it was seen that on day 40, the TFC increased with increasing concentrations of both bioAgNPs, and chemAgNPs (Fig. 5c). Statistical analysis of TFC results showed that after 10 days, the effect of bioAgNPs on TFC was slightly insignificant (p = 0.06) as compared to the effect of chemAgNPs (p = 0.036). However, after 20 days, the effect of bioAgNPs became significant (p = 0.016) compared to chemAgNPs (p = 0.065) (Online resource 2). Overall results, Fisher’s test revealed that bioAgNPs imposed a significant effect on TFC compared to chemAgNPs.
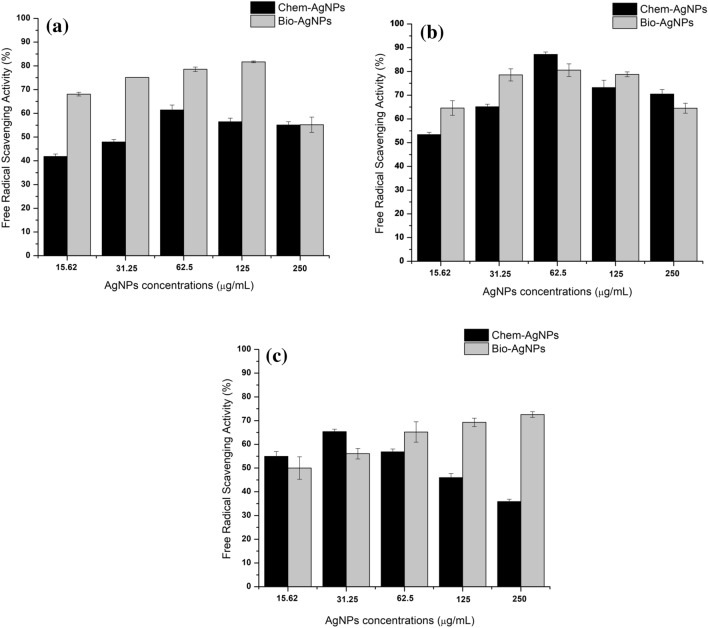
Free radical scavenging activity of chem- and bioAgNPs based callus
The free radical (DPPH) scavenging activity i.e. the antioxidant activity of both types of callus was recorded. Overall, highest FRSA (87.18%) was recorded in callus supplemented with chemAgNPs (62.5 µg/mL) on day 20 compared to 80.56% FRSA in callus supplemented with bioAgNPs (125 µg/mL) (Fig. 6b). On day 10, FRSA (81.69%) was observed in callus grown in 125 µg/mL bioAgNPs compared to control with lower FRSA (41.68%). The callus that was grown in chemAgNPs also showed lower FRSA (61.43%) at day 10 when supplemented with 62.5 µg/mL. FRSA increased with increasing concentration of AgNPs in media and peaked at 125 µg/mL AgNPs. At 250 µg/mL AgNPs caused a decrease in FRSA (Fig. 6a).
Fig. 6.
Comparison of the effects of different concentrations of chem-AgNPs and bio-AgNPs on free radical scavenging activity (%) in callus cultures of F. indica after a 10 days, b 20 days, and c 40 days of initiation. The values are given as mean ± standard error of three replicates
The FRSA decreased with time such that on day 40, the highest FRSA recorded was 72.55% which was produced in bioAgNPs based callus when provided with 250 µg/mL bioAgNPs. In comparison to this, chemAgNPs triggered the highest FRSA (65.36%) when given in a concentration of 31.25 µg/mL. The control callus showed comparatively lower FRSA (63.98%) than both types of AgNPs. However, on day 40, increasing bioAgNPs concentrations increased the FRSA while increasing chemAgNPs concentration beyond 31.25 µg/mL decreased the FRSA (Fig. 6c).
Statistical analysis of FRSA revealed that although overall AgNPs had an insignificant effect on FRSA compared to control, the P values of bioAgNPs based FRSA results (p = 0.177) showed that the effect of bioAgNPs was higher compared to chemAgNPs (p = 0.77) (Online resource 3).
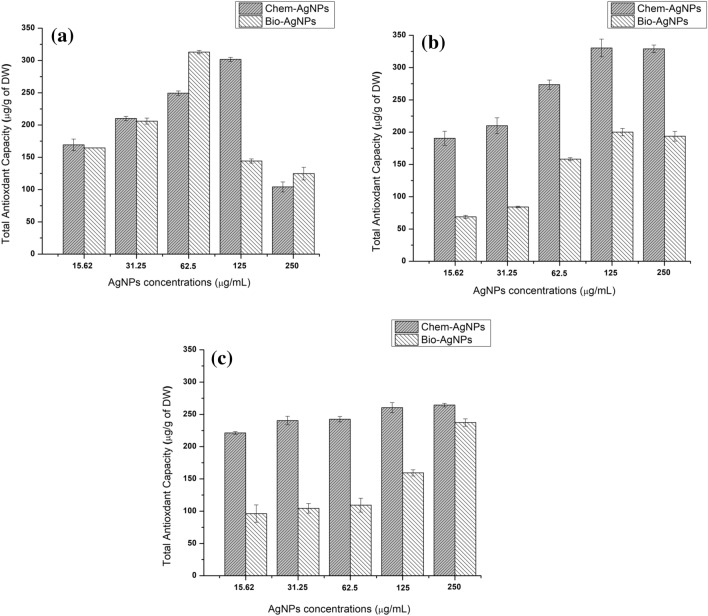
Total antioxidant capacity in chem- and bioAgNPs based callus
The TAC on days 10, 20, and 40 of callus initiation are shown in Fig. 7. On day 10, the highest value (312.96 ± 2.73 µg AAE/g of DW) was observed in 62.5 µg/mL bioAgNPs based callus. In chemAgNPs based callus, the highest TAC recorded was 301.65 ± 3.10 µg AAE/g of DW when the media was supplemented with 125 µg/mL chemAgNPs. In control callus, the TAC recorded was 306.42 ± 5.99 µg AAE/g of DW on day 10 of callus culture initiation. It was observed that high concentrations (250 µg/mL) of both types of AgNPs resulted in a decline in TAC (Fig. 7a). On day 20, the highest TAC value recorded in chemAgNPs based callus was 330.42 ± 13.65 µg AAE/g of DW compared to the 200.076 ± 5.66 µg AAE/g of DW for both types of AgNPs given in concentrations of 125 µg/mL. It was also observed that the TAC of callus grown in both types of AgNPs increased with increasing concentration till 125 µg/mL with a very slight decrease at 250 µg/mL (Fig. 7b). In chemAgNPs based callus, the TAC decreased with time such that on day 40 the highest value of TAC in 250 µg/mL chemAgNPs based callus was 264.40 ± 2.77 µg AAE/g of DW. However, the TAC of bioAgNPs based callus increased to 237.38 ± 5.73 µg AAE/g of DW on day 40 when supplemented with 250 µg/mL chemAgNPs. The control callus showed TAC of 270.2691 ± 2.24 µg AAE/g of DW on day 40. On day 40, it was observed that the higher the concentration of both types of AgNPs, the higher was the TAC of callus cultures (Fig. 7c).
Fig. 7.
Comparison of the effects of different concentrations of chemAgNPs and bioAgNPs on total antioxidant capacity in callus cultures of F. indica after a 10 days, b 20 days, and c 40 days of initiation. The values are given as mean ± standard error of three replicates
Discussion
Various studies have reported that the interaction of nanoparticles with plants brings many physiological and biochemical changes during plant growth. Such changes were reported to depend on the size and concentration of nanoparticles as well as their nature (Khodakovskaya et al. 2012). In this study, we evaluated the physiological and biochemical effects of chemAgNPs in comparison with bioAgNPs on callus cultures of F. indica. F. indica is known to be rich in significant bioactive compounds such as phenolic compounds and flavonoids (Khan et al. 2018a).
On day 10 of callus culture initiation, the higher biomass produced by bioAgNPs can be linked to the biocompatibility of AgNPs synthesized through extract of the same plant F. indica. The bioAgNPs are capped by plant-based compounds which can play an important role in the growth of the cell. Secondary metabolites, such as phenolics, flavonoids, and amines indirectly play a physiological role and may be able to affect plant cell growth (Yoshioka et al. 2004). Similarly, the decline of growth with an increase in concentrations of both types of AgNPs might be due to toxicity and lesser adaptability of the plant cells to AgNPs (Sanzari et al. 2019). With passing time, the callus cells might have become better adapted to the AgNPs because the highest biomass in both chemAgNPs and bioAgNPs based callus was produced at higher concentrations (250 µg/mL). The slightly lesser response of chemAgNPs compared to bioAgNPs (Table 1) could be viewed in the light of the fact that exogenous application of nanomaterial could be toxic for plant cells (Yan and Chen 2019). Our results have shown that bioAgNPs have shown friendly effects on biomass accumulation in callus cultures of F. indica. Control callus (lacking AgNPs) resulted in comparably high biomass after 40 days of callus culture initiation, which is following our previous study (Khan et al. 2016). This is because TDZ increases multiplication areas by breaking the apical dominance through physiological changes (Guo et al. 2011). Furthermore, when different concentrations of both types of AgNPs (chemAgNPs and bioAgNPs) were used in combination with TDZ, the color of callus changed from green to yellowish-green (Fig. 3). The shift in color might be due to the interaction of nanoparticles with callus and elicitation of defense response of the cells that produce important secondary metabolites phenolic compounds (Chung et al. 2018).
Furthermore, the production of TPC in callus cultures also followed a temporal pattern. Initially, bioAgNPs produced a higher quantity of TPC compared to chemAgNPs and TDZ-only callus cultures. It can be observed that as the concentration of bioAgNPs increased, the biomass accumulation declined while the TPC increased. BioAgNPs seems to be acting like a two-edged sword at the initial stages of the callus culture. It increases the biomass at lower concentrations (Table 1) and increases the TPC as the concentration increases (Fig. 4a). Similarly, chemAgNPs might have proved toxic for the plant cells leading to cell death and thus decreased growth as well as lesser TPC (Kim et al. 2017). However, with time, chemAgNPs in lower concentrations produced the highest quantity of TPC. This might be because the plant cells started to cope with the toxicity caused by chemAgNPs and thus produced higher quantities of secondary metabolites such as phenolic compounds, flavonoids, and alkaloids. For instance, at optimum concentrations, chemically reduced AgNPs were shown to enhance the quality of biochemical aspects including the production of phenolics and flavonoids in Trigonella foenum-graecum (Sadak 2019).
Nano elicitors have been reported to significantly enhance the production of antioxidant compounds i.e. phenolic compounds and flavonoids (Hong et al. 2008). Similarly, it has been reported that AgNPs led to enhanced taxol production in Corylus avellana cell suspension culture (Jamshidi et al. 2016). Similarly, observing the pattern after 40 days of callus culture initiation in our study, it can be suggested that prolonged exposure of callus to AgNPs beyond 20 days decreases the TPC in callus cultures. This can be correlated with biomass accumulation at this stage which increased with time of exposure as the callus adapted to them. Similarly, TFC accumulation also followed the same pattern of decreasing with time. Initially, bioAgNPs produced the highest quantities of TFC compared to chemAgNPs which became comparable in both types of AgNPs after time passed. After 40 days of callus cultures, TFC declined in callus in response to the supplementation of chem- and bioAgNPs. However, bioAgNPs produced higher quantities of TFC than chemAgNPs on day 40 of callus cultures. The reason for the decline in TPC and TFC and enhanced growth after prolonged exposure to AgNPs might be associated with the reason that in many cases there exists an inverse relationship of biomass accumulation and secondary metabolism (Isah 2019). Phytochemicals such as phenolic compounds and flavonoids are secondary metabolites that are produced in a response to different stresses. These phytochemicals usually contribute to the antioxidant potential of plants.
This can also be seen in the results of antioxidant assay. FRSA was higher in chemAgNPs and bioAgNPs supplemented callus cultures. Oxidative stress due to AgNPs brings its phytotoxic effects, which is the result of excess amounts of reactive oxygen species (ROS) due to AgNPs. To cope with the damaging effects of ROS, antioxidant defense mechanisms, ascorbate peroxidase (APX), catalase (CAT), guaiacol peroxidase (GPX), and superoxide dismutase (SOD) are activated in plant cells (Yan and Chen 2019). This directly implies that the plant cells in our experiments were in stress due to the introduction of AgNPs initially and thus activated their secondary metabolism. During stress conditions, higher quantities of reactive free radicals are produced, which directly affects cells, tissues, and their process by forming complex chain reactions (Sreelatha and Padma 2009). It has been reported that phenols and flavonoids serve as natural antioxidants and reduce or inhibit the activity of free radicals (Ali et al. 2019).
Conclusions
Overall, bioAgNPs showed favorable results in terms of biomass accumulation and triggering phenolic compounds and flavonoids. The intermediate concentrations of chemAgNPs also showed improved production of TPC and TFC. This study also revealed increased antioxidant activity with various concentrations of bioAgNPs. It can be suggested that maximum cell growth and enhanced production of secondary metabolites can be attained at optimal concentrations of bioAgNPs. The use of green synthesized AgNPs is promising because of lesser toxicity issues associated with it during synthesis as well as application. Further research is required to understand the molecular mechanism of green synthesized AgNPs in comparison to chemAgNPs in secondary metabolism and cell growth.
Electronic supplementary material
Online resource 1: Electronic supplementary material containing details on statistical analysis of Fresh and Dry weight of callus cultures supplemented with chem- and bioAgNPs at different concentrations.
Online resource 2: Electronic supplementary material containing details on statistical analysis of TPC and TFC of callus cultures supplemented with chem- and bioAgNPs at different concentrations.
Online resource 3: Electronic supplementary material containing details on statistical analysis of DPPH of callus cultures supplemented with chem- and bioAgNPs at different concentrations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the Department of Biotechnology, University of Malakand for continuous support in research activities.
Authors’ contribution
SB, AZ and TK contributed equally to this research and should be considered equal principle authors. SB, and AZ did the research work and wrote the manuscript. TK conceived the idea and supervised the work. NZ provided the resources and guided analytical steps of the research. WA analyzed the data and critically reviewed the work. TK wrote the manuscript and added to its technical part.
Funding
This research Project was conducted as a part of general graduate research and was partially funded by the Higher Education Commission research project, NRPU 6649.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shabana Begum, Ayesha Zahid and Tariq Khan have contributed equally to this study and should be considered principle authors equally.
Contributor Information
Shabana Begum, Email: shabanauom332@gmail.com.
Ayesha Zahid, Email: ayeshayosafzai410@gmail.com.
Tariq Khan, Email: tariqkhan@uom.edu.pk.
Nadir Zaman Khan, Email: nadir.zaman@uom.edu.pk.
Waqar Ali, Email: waqarali@uom.edu.pk.
References
- Adil M, Khan T, Aasim M, Khan AA, Ashraf M. Evaluation of the antibacterial potential of silver nanoparticles synthesized through the interaction of antibiotic and aqueous callus extract of Fagonia indica. AMB Express. 2019;9:75. doi: 10.1186/s13568-019-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Mohammad S, Khan MA, Raja NI, Arif M, Kamil A, Z-U-R Mashwani. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif Cells Nanomed Biotechnol. 2019;47:715–724. doi: 10.1080/21691401.2019.1577884. [DOI] [PubMed] [Google Scholar]
- Al-Oubaidi HKM, Mohammed-Ameen AS. The effect of (AgNO3) NPs on increasing of secondary metabolites of Calendula officinalis L. in vitro. Int J Pharm Pract. 2014;5:267–272. [Google Scholar]
- Chung I-M, Rajakumar G, Thiruvengadam M. Effect of silver nanoparticles on phenolic compounds production and biological activities in hairy root cultures of Cucumis anguria. Acta Biol Hung. 2018;69:97–109. doi: 10.1556/018.68.2018.1.8. [DOI] [PubMed] [Google Scholar]
- Ejaz M, Raja NI, Ahmad MS, Hussain M, Iqbal M. Effect of silver nanoparticles and silver nitrate on growth of rice under biotic stress. IET Nanobiotechnol. 2018;12:927–932. doi: 10.1049/iet-nbt.2018.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eman AA, Gehan HA, Yassin M, Mohamed S. Chemical composition and antibacterial activity studies on callus of Fagonia arabica L. Academia Arena. 2010;2:91–106. [Google Scholar]
- Guo B, Abbasi BH, Zeb A, Xu L, Wei Y. Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotechnol. 2011;10:8984–9000. doi: 10.5897/AJB11.636. [DOI] [Google Scholar]
- Hong Y, Lin S, Jiang Y, Ashraf M. Variation in contents of total phenolics and flavonoids and antioxidant activities in the leaves of 11 Eriobotrya species. Plant Foods Hum Nutr. 2008;63:200–204. doi: 10.1007/s11130-008-0088-6. [DOI] [PubMed] [Google Scholar]
- Isah T. Stress and defense responses in plant secondary metabolites production. Biol Res. 2019;52:39. doi: 10.1186/s40659-019-0246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi M, Ghanati F, Rezaei A, Bemani E. Change of antioxidant enzymes activity of hazel (Corylus avellana L) cells by AgNPs. Cytotechnology. 2016;68:525–530. doi: 10.1007/s10616-014-9808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppusamy S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res. 2009;3:1222–1239. [Google Scholar]
- Khan T, Abbasi BH, Khan MA, Shinwari ZK. Differential effects of thidiazuron on production of anticancer phenolic compounds in callus cultures of Fagonia indica. Appl Biochem Biotechnol. 2016;179:46–58. doi: 10.1007/s12010-016-1978-y. [DOI] [PubMed] [Google Scholar]
- Khan T, Abbasi BH, Khan MA. The interplay between light, plant growth regulators and elicitors on growth and secondary metabolism in cell cultures of Fagonia indica. J Photochem Photobiol B Biol. 2018;185:153–160. doi: 10.1016/j.jphotobiol.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Khan T, Ullah MA, Garros L, Hano C, Abbasi B. Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J Photochem Photobiol B Biol. 2018 doi: 10.1016/j.jphotobiol.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Khan MA, Khan T, Mashwani Z-U-R, Riaz MS, Ullah N, Ali H, Nadhman A. Chapter Two—Plant cell nanomaterials interaction: growth, physiology and secondary metabolism. In: Verma SK, Das AK, editors. Comprehensive analytical chemistry. Amsterdam: Elsevier; 2019. pp. 23–54. [Google Scholar]
- Khodakovskaya MV, De Silva K, Biris AS, Dervishi E, Villagarcia H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano. 2012;6:2128–2135. doi: 10.1021/nn204643g. [DOI] [PubMed] [Google Scholar]
- Kim DH, Gopal J, Sivanesan I. Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv. 2017;7:36492–36505. doi: 10.1039/C7RA07025J. [DOI] [Google Scholar]
- Nair PMG, Chung IM. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere. 2014;112:105–113. doi: 10.1016/j.chemosphere.2014.03.056. [DOI] [PubMed] [Google Scholar]
- Oldenburg SJ, Saunders AE (2020) Silver nanomaterials for biological applications. Merck. https://www.sigmaaldrich.com/technical-documents/articles/materials-science/silver-nanomaterials.html. Accessed 15 June 2020
- Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 2016;21:182. doi: 10.3390/molecules21020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadak MS. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum) Bull Natl Res Centre. 2019;43:38. doi: 10.1186/s42269-019-0077-y. [DOI] [Google Scholar]
- Sanzari I, Leone A, Ambrosone A. Nanotechnology in plant science: to make a long story short. Front Bioeng Biotech. 2019;7:120. doi: 10.3389/fbioe.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnal K, et al. An assessment of the effect of green synthesized silver nanoparticles using sage leaves (Salvia officinalis L.) on germinated plants of maize (Zea mays L.) Nanomaterials. 2019;9:1550. doi: 10.3390/nano9111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Bhatt D, Zaidi M, Saradhi PP, Khanna P, Arora S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol. 2012;167:2225–2233. doi: 10.1007/s12010-012-9759-8. [DOI] [PubMed] [Google Scholar]
- Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr. 2009;64:303–311. doi: 10.1007/s11130-009-0141-0. [DOI] [PubMed] [Google Scholar]
- Syu Y-Y, Hung J-H, Chen J-C, Chuang H-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem. 2014;83:57–64. doi: 10.1016/j.plaphy.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Verma SK, Das AK, Patel MK, Shah A, Kumar V, Gantait S. Engineered nanomaterials for plant growth and development: a perspective analysis. Sci Total Environ. 2018;630:1413–1435. doi: 10.1016/j.scitotenv.2018.02.313. [DOI] [PubMed] [Google Scholar]
- Waheed A, Barker J, Barton SJ, Owen CP, Ahmed S, Carew MA. A novel steroidal saponin glycoside from Fagonia indica induces cell-selective apoptosis or necrosis in cancer cells. Eur J Pharm Sci. 2012;47:464–473. doi: 10.1016/j.ejps.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Yan A, Chen Z. Impacts of silver nanoparticles on plants: a focus on the phytotoxicity and underlying mechanism. Int J Mol Sci. 2019;20:1003. doi: 10.3390/ijms20051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Inokuchi T, Fujioka S, Kimura Y. Phenolic compounds and flavonoids as plant growth regulators from fruit and leaf of Vitex rotundifolia. Zeitschrift fur Naturforschung C J Biosci. 2004;59:509–514. doi: 10.1515/znc-2004-7-810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.