Abstract
Abstract
Chickpea (Cicer arietinum) belonging to the Fabaceae family is a major legume crop and is a good source of protein and carbohydrates. Industrialization has resulted in soil contamination with heavy metals such as cadmium. Adsorption of cadmium by plants can lead to reduced yields and heavy metal toxicity. In the current study, changes in the anatomical, morphological features and biochemical properties of the chickpea plant were evaluated. Two indexes DWSTI and PHSTI were determined. Anatomically, there was a change in the number of xylem poles within the root structure which was most significant at treatments of 125 μg cadmium. There was also a noticeable change in leaf pigmentation, the total phenolics and soluble protein in the plant. Cadmium levels were elevated attaining concentrations of 0.21, 0.40 and 0.52 mg per gram dry weight in plants exposed to 62, 125 and 250 μg/g Perlit cadmium after a period of 30 days. A noticeable increase in the level of cadmium in the plant was observed. Two PCS genes, glutathione gamma-glutamylcysteinyltransferase 1 and glutathione gamma-glutamylcysteinyltransferase and four FC genes, 4 proteins and 4 mRNA were detected in chickpeas. Bioinformatics tools were utilized to predict enzyme structure and binding sites. Chickpea may be classified as a cadmium hyperaccumulator and may be considered for use in phytoremediation. This study provides a better understanding with regards to the response of chickpeas to cadmium and the genetic mechanism by which the plant regulates heavy metal toxicity.
Graphic abstract
Keywords: Cicer arietinum, Cadmium, Phytochelatin, Ferrochelatase
Introduction
Chickpea (Cicer arietinum L.) is a leguminous plant belonging to the Fabaceae family, subfamily Faboideae. It is a good source of proteins and carbohydrates. Dried pulses are produced in over 150 countries which equates to approximately 36% of the entire land mass of the world. Elevated levels of heavy metals have been observed in the soil of urbanized and industrial areas as a result of industrialization and the utilization of fossil fuels. A direct consequence is that agricultural lands have become contaminated and crops grown on these lands will lead to the introduction of heavy metals into the food chain that can be toxic to those who consume them. Additionally, crops grown on soils with heavy metal contamination exhibit poor nutritional value and can lead to deficiency diseases when consumed (Ali et al. 2015; Li et al. 2018). The level of uptake of heavy metals by the plant is dependent on several factors such as the actual element, soil type, plant family and species as well as the organ directly affected by the metal toxicant. Research has shown that plants exposed to metal toxicants undergo changes in their anatomy, physiology, morphology, biochemical composition and developmental features in order to combat the consequences of heavy metal toxicity. There is evidence of loss in leaf pigmentation, altered enzymatic activity and anatomic features as well as modifications in metabolism and protein structure. Phytochelatin (PCs) are low molecular peptides rich in the amino acid cysteine having the general structural formula (γ-Glu-Cys) n-Gly (n = 2–7). Phytochelatin synthase (PCS, EC 2.3.2.15) catalyzes the biosynthesis of PCs utilizing glutathione as substrate. They are thought to be involved in metal detoxification as cadmium is able to complex with the free thiol groups present. PCs-Cd complexes (2500–3600 Da) have the ability to traverse tonoplasts and are sequestered in vacuoles thereby allowing for cadmium detoxification. In the Arabidopsis plant, two PCS genes, PCS1 and PCS2 play an integral role in metal-dependent transpeptidation and make significant contributions to the plants ability to tolerate cadmium toxicity. Currently, the PCs biosynthetic pathway is believed to be of utmost importance in enabling plants to tolerate cadmium stress (Picault et al. 2006). Although there appears to be some inconsistency in the data with regards to the effect of PC accumulation on cadmium tolerance, various studies have reiterated that GSH plays an important role serving both as a substrate in the synthetic pathway of PC and as a ROS scavenger (Li et al. 2006). Ferrochelatase-1 (FC1, EC4.99.1.1) AtFC1 is induced by various biotic and abiotic stressors. Currently, most studies are primarily focused on identifying the role FC plays in regulating plant growth, maturation and metabolism with a few investigating the possible role of FCs in responding to abiotic stress in plants (Woodson et al. 2011). A novel function of AtFC1 was recently reported in which AtFC1 was induced in the presence of cadmium. Overexpression of AtFC1 in Arabidopsis resulted in enhanced cadmium tolerance, with AtFC1 mutations leading to the plants becoming more sensitive to cadmium. AtFC1 activated genes involved in PCs synthesis, suggesting that cadmium-induced AtFC1 may be able to modulate cadmium tolerance via the PCs synthesis-mediated pathway. There are no prior reports on the role of FC in regulating cadmium toxicity in chickpea (Song et al. 2017). Chickpea is an important protein source in the diet and is highly consumed in various countries. It is extensively cultivated in adjacent industrial and urban environments. There is therefore the possibility of cadmium infiltrating the food chain (Wallace et al. 2016). Identification of genes involved in the response of chickpeas to cadmium provides further clarity regarding the genetic manipulation of plants. In this study, the morphometric and biochemical response of chickpea to cadmium stress was evaluated and bioinformatics information regarding the PCS and FCS genes in chickpea investigated. The PCS gene is well known regarding its response to cadmium. Only a few studies report on this gene in chickpea. A few have reported on the response of the FC gene to cadmium. For the first time, we present in silico data on CaFC, an unknown gene in response to cadmium.
Materials and method
Propagation and cadmium exposure
Chickpea (Cicer arietinum L.) plantlets were sown at Tabriz University, Iran. The seeds were planted in Cocopeat and Perlite (autoclaved) in a greenhouse controlled plant growth chamber at 25 °C (days)/20–21 °C (night) and 16 h light/8 h dark period (Photoperiod: Light intensity; 555–560 μmol m−2 s−1, relative humidity 60–75%) in Oct. to Nov. 2018. After germinating, 3 plants at the leaf stage were selected and planted (diameter, 12 cm and height 15 cm) under sterile conditions. A hydroponic nutrient solution, (Hoagland solution), was utilized and plantlets treated with cadmium chloride in replicates of three at four different concentrations (control, 62, 125, and 250 μg/g perlite) for 10 days. Pot capacity was determined, and Hoagland’s solution applied to the plants based on their maximal capacity. After attaining the three-leaf stage, cadmium chloride treatment was applied to the plants. At the end of 5 days, the pots were washed with distilled water to thoroughly remove cadmium and the treatment applied once again. During treatment intervals, the pots were kept in distilled water.
Anatomy study
Samples from each plant (mature leaf, 3rd node of stem and 1–2 ml root tip) were fixed in ethyl alcohol-formaldehyde-acetic acid (F.A.A). Samples were dissected and stained in Safranin-Fast green. Plant tissue cross-section anatomy was evaluated from micro-photographs (Bryan 1955).
Determination of tolerance index
Stress tolerance indices were calculated using the equations (Amin et al. 2014):
Chlorophyll (a and b) and carotenoid content
Plant pigmentation was determined utilizing the Lichtenthaler and Welburn method (1987). Filtrate absorbance was determined spectrophotometrically (646 and 663 nm) and pigment concentration calculated (Lichtenthaler 1987).
Cadmium determination and bioaccumulation factor
Cadmium content of plant samples was determined by atomic absorption spectroscopy. The Bioaccumulation Factor (BAF) was determined based on the formula (Arnot and Gobas 2006): BAF = metal concentration (μg g−1 dry weight) of leaves/metal concentration (μg ml−1) in nutrient solution. BAF may be further classified as hyperaccumulators, accumulator and excluder in samples that accumulated metal ions > 1 μg/g, and < 1, respectively.
Total soluble protein
The Bradford assay was utilized to determine the soluble protein with the standard, bovine serum albumin (BSA). The absorbance was recorded at 595 nm and a standard curve generated (Bradford 1976).
Phenolic content
Plant-sample (0.1 g) was extracted with methanol (2 ml, 80%) in a Chinese mortar. The extract was centrifuged (10,000 rpm, 5 min) and the resulting supernatant was used to measure the total phenolic content. To the supernatant (100 µl), distilled water (2.8 ml), sodium carbonate (2%, 2 ml) and Folin reagent (100 µl) were added. The resulting mixture was vortexed and incubated at ambient temperature for 30 min. The absorbance of the solutions was measured at 720 nm and compared to the control. Gallic acid was used as standard. The data was expressed as mg gallic acid per gram fresh plant weight (Lamien-Meda et al. 2005).
Flavonoid content
Plant sample (0.1 g) was extracted with methanol (2 ml, 80%) in a Chinese mortar. The extract was centrifuged (10,000 rpm, 5 min) and the resulting supernatant used to measure the total flavonoid content. To the supernatant (500 µl), aluminum chloride (10%, 100 µl), potassium acetate (1 mM, 100 µl) and distilled water (2 ml) were added. The mixture was vortexed and kept at ambient temperature for 40 min. The absorbance of the solutions was measured at 415 nm. Quercetin was used as standard. Data was expressed as mg quercetin per gram fresh plant weight (Lamien-Meda et al. 2005).
In silico study of PCS and FCs in chickpea
A search of the NCBI gene database utilizing the keywords “phytochelatin synthase” and “ferrochelatase” was conducted to predict genes in Cicer arientum. Two genes in the same locus were found for PCS and four genes on three different chromosomes for FC. Align Sequences Nucleotide BLAST and Align Sequences Protein BLAST were used to estimate the similarity between the genes and their homologs in Arabidopsis. The 3D structure of the enzyme, binding and ligand binding sites were predicted utilizing PHYRE 2, and COACH software. The cell compartment in which PCS and FC could potentially act was predicted from several software that included LOCTREE3, DeepLoc-1.0 and Plant-MPLOC server (Chou and Shen 2010; Baca et al. 2010; Goldberg et al. 2014; Nielsen et al. 2017).
Statistical analyses
A completely randomized design with 3 replicates was used for the experiments. Data analysis was performed with the SPSS 20.0 software package (SPSS Inc., Chicago, IL, USA). Experimental data was reported as the mean ± SD. One-way ANOVA was used to determine differences between the means followed by the post hoc Tukey test. The level of significance was set at P < 0.05.
Results and discussion
Anatomic study
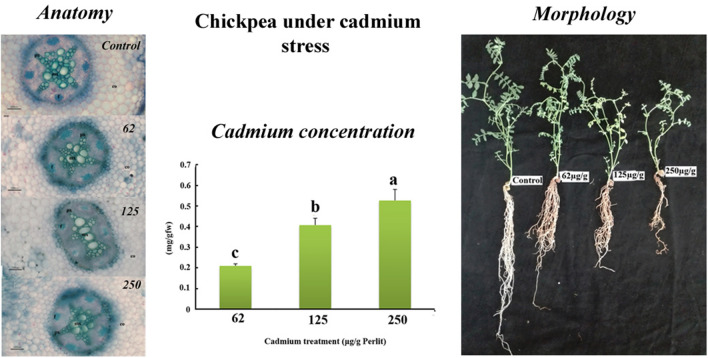
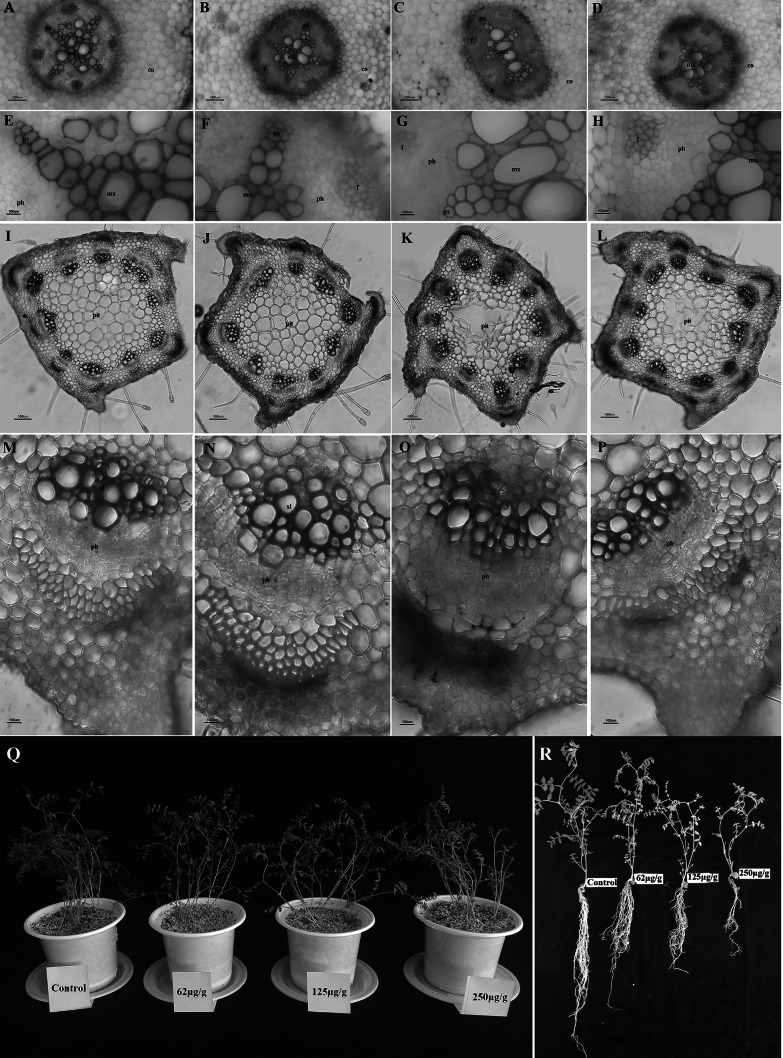
Exposure of root tissue to cadmium resulted in anatomical changes which were quantifiable. Treatment with cadmium (125 μg) showed differences in the anatomical structure of the root. The number of xylem poles changed from 4 to approximately 5. In the controls, expansion of the xylem bundles was more pronounced but gradually decreased with increasing cadmium concentration. These observations may be attributed to a decrease in root diameter. Placement of large metaxylem at stress levels of 125 μg cadmium was almost linear. In the other treatments it was relatively concentrated in the center of the vascular cylinder. Changes were also observed in the endoderm layer. In the controls suberanization occurred in radial walls which is typical for dicotyledons. Treatments with 62 μg cadmium, resulted in the suberin being drawn into tangential walls, which increased absorption efficiency. At stress levels of 125 and 250 μg cadmium the levels of suberin decreased. The number of cortex layers was not distinct between samples. At a stress level of 62 μg cadmium, suberin increased. Suberanization potentially causes closure of the apoplastic route preventing entry of heavy elements and serving as a barrier mechanism (Fig. 1a–h). The exoderm and endoderm are apoplastic barriers that play a critical role in the protection of the plant from a variety of stress factors. One possible defense mechanism employed by B. decumbens to regulate the uptake of metals is that of enhancing the thickness of these tissues. Typically, high quantities of root exodermal and endodermal tissue suggests that the plant is highly tolerant to heavy metal exposure (Lux et al. 2004). This mechanism is common in plants protecting them from tension. At a plant stress level of 125 μg cadmium the plant appears to utilize an anatomical mechanism to try to reduce the entry of heavy metals by changing the arrangement of the vessels. Reduced lignin synthesis and the number of xylem elements was visible at stress levels of 250 μg cadmium. In contrast to observations made in chickpea, the roots of maize and soybean when treated with cadmium had a wider diameter due to parenchyma cortex cells increasing in size (Vaculík et al. 2012). Observations in the soybean cortex may be due to decreased mitotic activity as well as enhanced resistance to the radial flow of water and solutes. Research conducted on Zea mays found that cadmium stimulates changes in root tissue dimension and various cells. The differences are species dependent and varied based on the actual tissue and levels of cadmium in the rhizosphere (Lux et al. 2004). Exposure of soybean roots to cadmium resulted in damage and enlargement of vascular tissue, uneven thickening of cell walls and darkened endodermic cells which may be due to the retention of cadmium ions by ammonium functional groups within the cell wall. Observed changes in the anatomical features of soybean root and the inhibitory effect of cadmium on plant growth is multifaceted with decreased absorption of nutrient and moisture, decreased transpiration rates which result in stunted shoot growth. Other contributing factors to the complex process could be hormonal in origin (Sharma and Laxmi 2016).
Fig. 1.
A–P: Transverse sections of chickpea stem and root under cadmium stress. A: Control root, B: 62 μg/g Root, C: 125 μg/g Root, D: 250 μg/g Root, E: Control root, F: 62 μg/g Root, G: 125 μg/g Root, H: 250 μg/g Root, I: Control stem, J: 62 μg/g Stem, K: 125 μg/g Stem, L: 250 μg/g Stem, M: Control stem, N: 62 μg/g Stem, O: 125 μg/g Stem, P: 250 μg/g Stem (Mv = metaxylem vessel; Pv = protoxylem vessel; Pit: Pith; Co: Cortex; Ph: Phloem; Fib: Fiber). Q–R: Morphometric features of chickpea (Cicer arietinum L.) under cadmium stress conditions
Tolerance index under cadmium stress
To gain a better understanding of morphological responses induced in chickpea seedlings by cadmium stress, two indexes DWSTI and PHSTI were measured (Fig. 2a, b). Figure 2a, b displayed that at higher cadmium treatments the percentage of tolerance in chickpea was lowered. Highest levels (79.43%) of DWSTI was detected at 62 μg/g Perlit and the lowest (35.77%) at 250 μg/g Perlit. Correspondingly, maximum PHSTI value (92.40%) was recorded at 62 μg/g Perlit and the lowest (60.43%) at 250 μg/g Perlit (Fig. 2a, b). Cadmium ions disrupted cellular mitosis and disturbed the growth process of roots and stems. In the leaves, photosynthesis was disrupted in different ways, causing structural changes, suppressing chlorophyll formation, blocking electron transport, and stomata closure thereby reducing the effectiveness of carbon dioxide, and interrupting plant development. Some researchers have also reported a continuous reduction in root and shoot dry mass with increased cadmium exposure (Sharma and Dubey 2005).
Fig. 2.
Effect of cadmium on the contents of A: PLSTI, B: PDSTI (%), C: Chlorophyll a (Chla) (mg g−1 FW), D: Chlorophyll b (Chlb) (mg g−1 FW), E: Carotenoids (mg g−1 FW), F: Protein content (mg g−1 FW), G: Phenolics compounds (mg gallic acid equivalent (GAE) g−1 extract), H: Flavonoid content (mg quercetin g−1 extract), I: Cadmium concentration (aerial organs), J: Cadmium Bioaccumulation Factor in chickpea (Cicer arietinum L.) growing under normal and cadmium concentrations. Bars represent the means of three replicates (±SE). Bars with different letters are significantly different (P < 0.05) according to LSD multiple range test
Pigmentation
Chlorophyll content significantly decreased under cadmium stress. Continued exposure to high concentrations of cadmium (250 μg/g Perlit) reduced Chla and Chlb significantly to approximately 76.78 and 8.70% of the control, respectively (Fig. 2c, d). Similarly, cadmium resulted in a decline in the levels of carotenoids as compared to the control after 30 days (Fig. 2c, d). This reduction in pigmentation was expected, as seedlings under stress were completely yellow. A decline in chlorophyll content has been reported in response to various abiotic stresses in plants. The observed decrease in the levels of chlorophyll in plants under cadmium exposure is believed to be as a result of (a) Inhibition of enzymes that are intimately involved in the biosynthetic pathway of chlorophyll, such as δ-aminolevulinic acid dehydratase (ALA-dehydratase) (Singh et al. 2011) and protochlorophyllide reductase;(b) Impaired supply of the cations, Mg2+, Fe2+, and Zn2+. Similar findings have been reported for cyanobacteria, unicellular chlorophytes (Chlorella) and plants (Picea abies, Zea mays, Quercus palustrus, Acer rubrum, Helianthus annuus and Prunus dulcis) exposed to heavy metals. The loss of chlorophyll under cadmium stress is an adaptation mechanism. Reduced chlorophyll leads to a decrease in excited electrons during photosynthesis with damage from ROS formation also being reduced. Carotenoids are low molecular weight lipophilic antioxidants in the chloroplast that protect chloroplastic membranes from oxidative stress. In addition to their structural role, carotenoids have the ability to absorb light disabling ROS directly or indirectly through the quenching of exited chlorophyll and prevention of ROS formation. Damage to photosynthesis occurs due to elevated levels of lipid peroxidation and a decrease in chlorophyll. Replacement of the central magnesium ion in chlorophyll by heavy metals is another injury inhibiting the trapping of light and photosynthesis thereby resulting in a loss of chlorophyll and photosynthetic activity. Decreased chlorophyll levels could be as a direct result of reduced photosynthetic activity and reduction of carbon stabilization at high heavy metal concentrations. Chlorophyll degradation and reduced rates of photosynthesis is a common feature in plants exposed to heavy metals especially cadmium. These findings are consistent with our results regarding the stress response of leaves exposed to cadmium (Godinho et al. 2018).
Soluble protein content
Cadmium treatment (62 and 125 μg/g) resulted in a decline in the soluble protein of chickpea to 68.35% and 49.60%, respectively. At concentrations of 250 μg/g cadmium, total soluble protein increased to approximately 66.22% of the control (Fig. 2f). Abiotic stress could compromise the de novo synthesis of various proteins while promoting others (Ericson and Alfinito 1984). The overall trend was that of a general decline in protein content. These results are supported by Costa and Spitz (1997) who detected a decline in total soluble protein in Lupine exposed to heavy metal stress. Romero-Puertas (2002) stated that the reduction in protein content in Lupine is possibly as a result of protein degradation due to enhanced protease activity (Romero-Puertas et al. 2002). Some researchers believe that heavy metals cause lipid peroxidation due to ROS formation during oxidative stress, which may be the predominant cause for reduction of protein in plants exposed to heavy metal stress. Proteins involved in primary carbon metabolism and photosynthetic machinery such as Rubisco, are reduced during cadmium stress. Increased protein content at high levels of cadmium may be due to the production of proteins involved in stress response and translation. Micro-array analysis of rice undergoing cadmium stress revealed that several genes encoding the cytochrome P450 family of proteins as well as other proteins were released during stress and expressed differently. Proteins associated with stress were strongly induced due to cadmium exposure (Nishizawa and Satoshi 2009). Levels of GST enzyme was significantly enhanced in the leaves and root system of Cu-stressed wheat seedlings (Li et al. 2013). In Brassica napus there was a down-regulation of ribosomal proteins confirming that cadmium induced toxicity impacts the regulation and/or synthesis of proteins (Ali et al. 2015).
Phenol and flavonoid compounds
An increase in the total phenolic content was observed in plants exposed to cadmium. Highest phenolic content was observed at treatments consisting of 250 μg/g Perlit cadmium (Fig. 2g). Manquián-Cerda et al. (2018) showed that under cadmium stress Vaccinium corymbosum L. produced phenolic compounds with reducing capability (Manquián-Cerda et al. 2018). Our results compares well with Manquián-Cerda et al. (2018) who illustrated that increased phenolic content in blueberries improved their tolerance to cadmium stress. The total level of flavonoid compounds in the controls was however similar to the levels observed in treated plants. No increase in flavonoids was observed (Fig. 2h). Plants exposed to cadmium might reduce the synthesis or release of flavonoids by an unidentified mechanism to escape a potentially dangerous situation which could be triggered by the production of phenoxyl radicals. Some researchers also believe that a lack of increase in phenolic or flavonoid compounds could be as a result of excess cadmium damaging antioxidative reaction mechanisms (Manquián-Cerda et al. 2018). A similar scenario was observed in Spartina densiflora. When exposed to moderate levels of cadmium, the plant was able to produce metabolites such as ascorbic acid and glutathione. The results from this study revealed that phenolic compounds play an important role in protecting the plant from cadmium exposure. The plant appears to utilize chelated compounds to form flavonoids thereby reducing heavy metal stress (Lone et al. 2008; Yang et al. 2017).
Cadmium determination and Bioaccumulation factor
The cadmium content of the aerial organs in chickpea undergoing cadmium stress is shown in Fig. 2i. This reveals that the cadmium content of chickpea significantly increased when exposed to cadmium stress. Cadmium content attained levels of 0.21, 0.40 and 0.52 mg per gram dry weight in plants exposed to 62, 125 and 250 μg/g Perlit cadmium after 30 days (Fig. 2i). Treatment with different concentrations of cadmium resulted in BAF values greater than 1. Investigations revealed that with increasing cadmium concentrations in low and medium stress conditions, changes in the BAF did not occur. By increasing the levels of cadmium to high stress, the factor significantly decreased while remaining higher than one. Chickpea is therefore considered as a cadmium hyperaccumulator plant (Fig. 2j). Chickpeas treated with high concentrations of cadmium had impaired and defective root systems (reduced root length) and changes in root tissue color (Fig. 1q, r). There was also a decline in root uptake. Further studies are required to validate the maximum quantity of cadmium that may be absorbed by chickpeas. Studies on the effect of cadmium stress on soybean plants revealed that growth parameters were severely affected with soybeans transporting cadmium well into their aerial organs. Unlike other cadmium-sensitive legumes, soybean is resistant to cadmium toxicity. Rivera-Becerril et al. (2002) studied three genotypes of cadmium-stressed chickpea. Their results revealed that the cadmium content of the root was approximately 20 to 50 times the amount of accumulated cadmium in aerial organs with the cadmium content of sections of chickpea ranging from 20 to 30 μg/g dry weight (Rivera-Becerril et al. 2002). Similar results were reported for peanut pods (Paul et al. 2018). In the study, chickpea was identified as a suitable plant to be utilized in the phytoremediation of contaminated soils (Paul et al. 2018). High levels of cadmium in the different chickpea genotypes revealed the importance of genetic variation in cadmium resistance. Research has shown that due to their use in complex planting systems and in the nitrogen and phosphorus stabilization of soil, legumes can cause cadmium contamination in neighboring plants. In the study it was shown that there was an increase in the cadmium content of tomato, pea, pakchoi and cabbage planted in close proximity to soybeans. Another study found high levels of heavy metals such as cadmium, mercury and lead in Lupine and other plants. Chubukova et al. (2015) showed that cadmium affected chickpea and bean with Rhizobium stimulating its nodulation. Liu et al. (2012) recently discussed the effects of cadmium on legumes and its potential for bioremediation. It was proposed that legumes may be utilized in the efficient removal of cadmium from contaminated soils (Liu et al. 2012).
Bioinformatics study of genes involved in heavy metal stress in chickpea
The tolerance of chickpea to cadmium is related to the presence of genes involved in mitigating heavy metal stress. The identification of genes involved in enhancing the plant’s resistance is important in understanding the mechanisms involved in mitigating cadmium contamination. Through genetic engineering plants with high resistance to heavy metal toxicity may be developed.
Study of PCS genes in chickpea
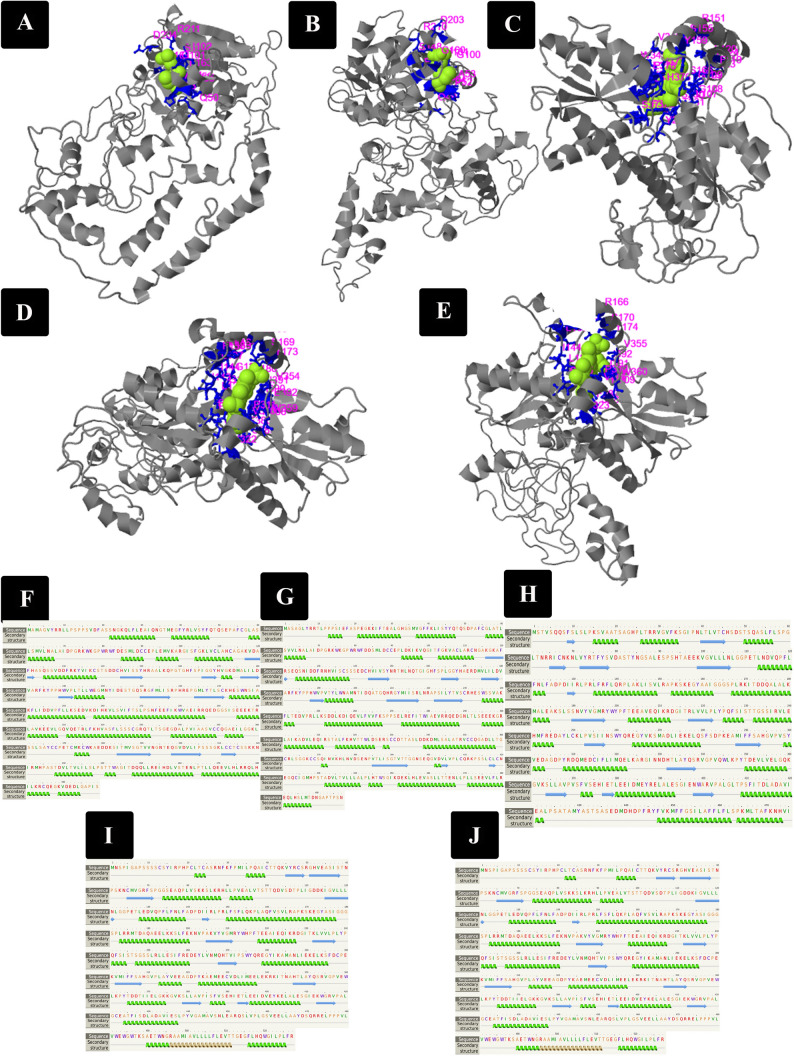
Bioinformatics study on the PCS protein in chickpea using the NCBI database revealed two PCS genes, glutathione gamma-glutamylcysteinyltransferase 1 and glutathione gamma-glutamylcysteinyltransferase 3-like, located on chromosome Ca3, 31444–31468 k with accession numbers XP_004493799 and XP_004493800 respectively. These genes are located beside the glycolipid transfer protein 1-like gene. The genes contained 499 and 497 amino acids respectively and show similarity in their mRNA (Query cover: 64%, Ident: 70.03% and E value: 5e–118) and protein structure (Query cover: 96%, Ident: 58.69% and E value: 0). A comparative study of these genes with PCS 1 and PCS 2 in Arabidopsis showed that the first gene (XP_004493799) had a higher similarity (Table 1). Glutathione gamma-glutamylcysteinyltransferase 1 and glutathione gamma-glutamylcysteinyltransferase 3-like contained 8 and 9 exons respectively (Fig. 3a, b). PCS1 has an alpha helix (49%), beta strand (12%) and disordered (22%) whereas PCS3 had an alpha helix (44%), beta strand (11%) and disordered (22%) (Fig. 4f, g). The α-helix predominates and varies based on solvent-accessibility (Engel and DeGrado 2004). Propensity towards the β-sheet conformation differs significantly. Three-dimensional (3D) structures were first predicted with Phyre2 web-based services (not shown) and the COACH software used to predict the substrate. Based on predictions from the COACH meta-server (Fig. 4) both PCSs enzymes can bind to L-gamma-glutamylcysteine or gammaglutamylcysteine (3GC) as substrate. Predictions were based on “2bu3a”, a “Transferase” enzyme in Nostoc (Fig. 4a, b and Appendix). Consensus binding residues are illustrated in pink. The C-score for PCS3 was greater than that of PCS1. The residues involved in formation of the binding site are illustrated in Fig. 4a, b and the Appendix. Based on LocTree3, both enzymes had a high probability (87% and 84%, respectively), of being present within the nucleus. DeepLoc-1.0 showed both enzymes as soluble and located in the cytoplasm whereas Plant-MPLOC server predicted that the enzymes were present in the mitochondrion/nucleus and nucleus, respectively. Determining the actual location of these enzymes will provide more clarity regarding their mechanism of action and cellular pathway when plants are experiencing heavy metal stress. The PCS gene has been identified in monocotyledon, dicotyledonous, coniferales and fungi. There is limited information regarding PCS in animals. In plants, the protein sequence of this enzyme ranges between 200 and 500 amino acids with 400 to 500 amino acids being most prevalent. Most known species (Arabidopsis, rice, alfalfa, sorghum, potatoes, corn and lotus) have at least two homologous PCS genes. The number of exons ranges between 6 and 10 exons. PCS from lettuce has as 8–9 exons. In the PCS enzyme, details regarding the C-terminal is less known. The N-terminal is well protected in various species. There is an abundance of cysteine in PCS enzymes. Ontogeny studies show that PCS is involved in multiple cellular pathways, inclusive of signaling, defense, stress, phytochemistry and heavy metal detoxification. Variation in the structure of PCS proteins has led to functional differences. Protected residues in the PCS enzyme, include Cys 56, His 162, Asp 180 in Arabidopsis and other plants, including lettuce and Nostoc. These residues are involved in the catalytic activity of the enzyme. Bioinformatics data on the PCS genes in chickpea is consistent with that of other plants (Yamazaki et al. 2018; Bundy and Kille 2014).
Table 1.
Comparison of the proteins sequences of PC1, PC3, FC1 and three FC2 with their homologous in Arabidopsis by Blast tool (Basic Local Alignment Search Tool)
| Parameters | ID AED95055.1 phytochelatin synthase 1 (PCS1) [Arabidopsis thaliana] | ID NP_001322971.1phytochelatin synthase 2 (PCS2) [Arabidopsis thaliana] | ||||||
|---|---|---|---|---|---|---|---|---|
| Sequences producing significant alignments | Description | Length | Query cover | E value | Ident | Query cover | E value | Ident |
| XP_004493799 | Glutathione gamma-glutamylcysteinyltransferase 1 | 499 | 99% | 0 | 63.86% | 99% | 0 | 59.04% |
| XP_004493800 | Glutathione gamma-glutamylcysteinyltransferase 3-like | 497 | 96% | 0 | 54.21% | 96% | 5e−165 | 49.21% |
| ID CAA73809.1 ferrochelatase-I [Arabidopsis thaliana] | ID OAP09354.1 FC2 [Arabidopsis thaliana] | |||||||
| XP_004510373 | Ferrochelatase-1, chloroplastic/mitochondrial [Cicer arietinum] | 475 | 99% | 0 | 70.45% | 71% | 0 | 67.58% |
| XP_004503011.1 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 527 | 75% | 0.0 | 70.64% | 94% | 0.0 | 76.83% |
| XP_004503842 | ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 527 | 72% | 0 | 71.18% | 98% | 0 | 74.35% |
| XP_004503866.1 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 527 | 72% | 0.0 | 71.18% | 99% | 0.0 | 74.35% |
Fig. 3.
Exon-intron structure analysis of PCSs and FCs in chickpea was performed utilizing Genomic regions, transcripts, and products in NCBI. Introns and exons were represented by black lines and yellow boxes, respectively, and upstream/downstream blue boxes. A: PC1 and B: PC3 included 8 and 9 exon respectively. C: FC1 (LOC101507238), D: FC2 (LOC101515447), E: FC2 (LOC101509199) and F: FC2 (LOC101494913) with 10, 11, 11 and 11 exons respectively
Fig. 4.
A–E: An overview of the 3D model of chickpea PCSs and FC generated by the Coach Software. The structures were predicted using coordinate templates represented in Appendix. The software was utilized to identify the binding site sequence in the 3D model of chickpea PCSs and FC. HEM, PP9 and 3GC were identified in the binding site of the 3D model of FC1, FC2 and PCSs, respectively. Consensus binding residues are represented by a pink color utilizing the COACH Software. Their exact positions are given in the Appendix. A: XP_004493799 (PC1), B: XP_004493800 (PC3), C: XP_004510373.1 (FC1), D: XP_004503842.1 (FC2) and E: XP_004503011.1 (FC2). F–J: The secondary structure of predicted proteins predicted by Phyro2 software. Green helices represent α-helices, blue arrows indicate β-strands and faint lines indicate coil. F: XP_004493799 (PC1), G: XP_004493800 (PC3), H: XP_004510373.1 (FC1), I: XP_004503842.1 (FC2) and J: XP_004503011.1 (FC2). Green helices represent α-helices, blue arrows indicate β-strands and faint lines indicate coil. The ‘SS confidence' line indicates the confidence in the prediction, with red being high confidence and blue low confidence
FC1 and FC2s genes in chickpea
A search for FC genes in chickpea using the NCBI database revealed the presence of 4 genes, 4 proteins and 4 mRNA (Table 1 and 2). One was recorded as FC1 and the others as FC2. The four genes included ferrochelatase-1, chloroplastic/mitochondrial (LOC101507238) on Chromosome Ca7, NC_021166.1 (31789492..31793600), ferrochelatase-2, chloroplastic-like (LOC101515447) on Chromosome Ca6, NC_021165.1 (6275626..6280973), ferrochelatase-2, chloroplastic-like (LOC101509199) on Chromosome Ca5, NC_021164.1 (47931155..47935844), and ferrochelatase-2, chloroplastic-like [Cicer arietinum (chickpea) (LOC101494913) on Chromosome Ca6, NC_021165.1 (6545229..6550577). Gene sequences of FC1 and FC2 and their mRNA sequences were compared utilizing Blast. FC1 mRNA had a length of 1428 nucleotides and the three FC2s, 1584 nucleotides. Comparison of the sequence of FC1 with FC2 mRNA showed Query cover: 67–68%, Ident: 71.05–71.47% and E value: 0. A comparison of the three FC2s showed that XM_004502954.2 was similar to XM_004503785.3 and XM_004503809.3 with Query cover: 96%, Ident: 85.74% and E value: 0. XM_004503785.3 and XM_004503809.3 are also similar (Table 2). FC1 (XP_004510373) is located on chromosome ca7 (Location 101507238-475) with 9 exons whereas the FC2s are located on chromosomes ca5 and ca6. Three genes of FC2 represented as XP_004503011.1, XP_004503842 and XP_004503866.1, are located on Ca5 (Location 101509199-527), Ca6 (Location 101515447-527) and Ca6 (Location 101494913-527) with 10, 11 and 11 exons respectively. FC1 on Ca7 was located between uncharacterized LOC113787575 and protein ROOT HAIR DEFECTIVE 3 genes. FC2 on Ca5 was located after TOM1-like protein 5 gene with glycosyltransferase At3g07620 and serine/threonine-protein kinase SRK2A-like genes possibly being located after it. FC2 on Ca6 was located between serine/threonine-protein kinase SRK2A-like and TOM1-like protein 5 genes while FC2 on Ca6 was located between up and down unread region and TOM1-like protein 5 gene (Fig. 3c–f). A closer look at the position of the FC2 genes shows that the adjacent gene is probably glycosyltransferase At3g07620 gene which is not present near FC2 on Ca6, whereas the other FC2 on Ca6 with 11 exons has only a part of the TOM1-like protein 5 gene adjacent. It seems that a locus is repeated on the chromosome, which may be due to the crossing over of chromatids. The FC1 gene has 475 amino acids and the FC2s 527 amino acids. Comparison of the sequence of FC1 with FC2s proteins using Blast, showed Query cover: 71%, Ident: 73.08–73.67% and E value: 0. A closer examination of the 3 FC2s sequences showed that they are quite similar. A comparison of the three FC2s showed that the similarity of XP_004503011.1 with both XP_004503842 and XP_004503866.1 was Query cover: 71%, Ident: 73.67% and E value: 0. The two proteins XP_004503842 and XP_004503866 are also quite similar (Table 2). A Blast comparison of the protein sequences of FC1 (XP_004510373) and the three FC2s (XP_004503011.1, XP_004503842 and XP_004503866.1) in chickpea with their homologs in Arabidopsis FC1 (CAA73809.1) and FC2 (OAP09354.1) revealed that FC1 in chickpea is similar to FC1 in Arabidopsis with Query cover: 99%, Ident: 70.45% and E value: 0. The FC2s in chickpeas are similar to FC2 Arabidopsis with Query cover: 94–99%, Ident: 74.35–76.83% and E value: 0 (Table 3). A closer examination revealed that except for approximately 80 of the initial nucleotides, the rest of their structure was conserved in Arabidopsis and chickpea. A Blast comparison of these genes with human homologues showed XP_004510373.1 (FC1), XP_004503842.1(FC2) and XP_004503011.1 with ferrochelatase precursor [Homo sapiens] having similarity Query cover: 60–74%, Ident: 39.29–42.81% and E value: 2e–72–4e–75. The central conserved motifs may be essential for function. Ferrochelatase appears to possess a structurally conserved core similar to that observed in enzymes from bacteria, plants and mammals. Two ferrochelatase proteins, FC1 and FC2 have been identified in Arabidopsis thaliana. Most organisms express only one ferrochelatase. Microarray analysis suggests that FC2 could be involved in stress response (Zhao et al. 2017). Despite FC1 and FC2 showing significant similarity (83%) and identity (69%) at the amino acid level, they are not. This is due to segmental duplication in the A. thaliana genome. They actually belong to two distinctive groups of plant ferrochelatases. FC2 messenger RNAs (mRNAs) are light inducible and accumulate predominantly in the stems, flowers and leaves of plants. The FC1 gene is also light inducible and is expressed in all plant tissues, including roots, at comparable levels (Zhao et al. 2017; Espinas et al. 2016). Induction of FC1 promoter activity was pronounced when the plant was exposed to norflurazon (an inhibitor of carotenoid biosynthesis), viral infection and wounding, with repression of FC2 promoter activity in most cases. cDNA microarray expression analysis also provided confirmation regarding FC1 transcription induction in response to wounding with FC1 up-regulation being initiated by chemicals generating reactive oxygen species (ROS) and application of cytoplasmic protein synthesis inhibitors. In humans, Proto IX overproduction due to FC mutants can be critical leading to severe liver damage (Goodwin et al. 2006). Disruption of heme biosynthesis in humans typically results in critical metabolic disorders. Mutations of the FC gene can lead to Erythropoietic protoporphyria (EPP), which in most cases is manifested as cutaneous photosensitivity (Yoshida et al. 2018). Similarly, a reduction in FC activity in Nicotiana tabacum as a result of transcriptional down-regulation of its FC2-type FC by antisense RNA causes the accumulation of Proto IX (photosensitizing substrate) and necrotic lesions on the leaves. In a FC1 knock-down mutant, heme levels decreased in seedlings and roots. Chlorophyll and carotenoid pigmentation, as well as the efficacy of photosystem II (PSII), were however not largely affected. These FC1 plants were cultivated using heterotrophic conditions. FC1 mutants grown under soil conditions are yet to be characterized. It is unclear as to how A. thaliana plants possess the ability to adapt to decreased levels or the total loss of FC2-type protein. The results suggest that FC2 play a greater role than FC1 in responding to abiotic and biotic stressors. Additionally, FC2-1 knock-down plants had altered photosynthetic machinery and accumulation of protochlorophyllide (Pchlide), a photosensitizer, in the dark, suggesting the presence of a (fluorescent) flu phenotype of the FC2 mutant. It has been proposed that heme is regulatory, controlling transcription and intercellular signaling in yeast, animals, higher plants and Chlamydomonas (Woodson et al. 2011). Investigation of the secondary structure of FC in chickpea with Phyr2 showed that these proteins have differences in their folding, β-sheet and α-helical content. FC1 included α helix (44%), β strand (16%) and disordered (16%), while FC2 ca5 consisted of an α helix (44%), β strand (10%) TM helix (6%) and disordered (23%) and FC2 ca2 had an α helix (43%), β strand (14%), TM helix (3%) and disordered (20%) (Fig. 4h, j). Conformations exhibiting the greatest level of stability and by extension the most frequently detected are that of the α-helix and β-pleated sheet. The α-helix exhibits more flexibility than the β sheet which is more rigid. The α-helix is therefore able to facilitate enzyme functionality at low temperatures. Proteins within these organisms possess less hydrophobicity and covalency. The flexibility of the α-helix as compared to the β sheet may enhance enzyme flexibility. Higher levels of α-helix in cold active enzymes may facilitate catalysis at lower temperatures (Veno et al. 2019). COACH meta-server results showed that chickpea FC1 was able to bind to PP9 and that FC2s links to PP9 and HEM as substrate. COACH results are presented in the Appendix. “Ferrochelatase” for FC1 and “the homospermidine synthase hss”, “DCCD-modified PsbS ferrochelatase” and “ferrochelatase” for FC2s, were the classifications used in predicting the 3D structure. “Saccharomyces cerevisiae”, “ Homo sapiens” and “Bacillus subtilis” were also employed as models in predicting ligand and substrate interactions in chickpea FC1, as well as “Legionella pneumophila”, “Spinacia oleracea”, “Saccharomyces cerevisiae” and “Homo sapiens” for FC2s. The ligand site of the predicted PCSs and FC1, enzyme in chickpea was identified utilizing the COACH software (Fig. 4). Consensus binding residues are illustrated in pink (Fig. 4c–e and Appendix). I-TASSER (Iterative Threading ASSEmbly Refinement), a protein modeling bioinformatics tool, utilizes a hierarchical approach allowing for the prediction of protein structure and functionality (Khor et al. 2015). Target sequences were initially threaded throughout a representative PDB structure library (with a pair-wise sequence identity cut-off of 70%) to detect potential folds. Eight hundred non homologous single-domain proteins were directly collected from the PDB library, which possessed a pair-wise sequence identity < 30% with sizes ranging from 50 to 300 residues (Pandit et al. 2006; Kim et al. 2009). The cell compartment of these enzymes was identified using different software. Based on DeepLoc-1.0 software, XP_004510373.1(FC1) is plastid and soluble whereas XP_004503842.1(FC2) and XP_004503011.1 are plastid membrane. The Plant-MPLOC server showed the three enzymes as chloroplast and mitochondrial. Utilizing LocTree3, XP_004510373.1(FC1), XP_004503842.1(FC2) and XP_004503011.1 had a high probability (95%, 96% and 95% respectively), of being within the chloroplast. A characteristic feature of FC2 is the presence of a hydrophobic C-terminal extension which is inclusive of a putative carboxyl terminal Chla/b-binding (CAB) domain with a conserved Chl-binding motif also occurring in the sequence of cyanobacterial ferrochelatase and in FC2-type 1 ferrochelatases from other higher plants. The CAB domain while not critical for catalytic activity is required for ferrochelatase dimerization in the cyanobacterium Synechocystis sp. PCC 6803. Based on the diversity of expression patterns for the two FC genes the concept of subcellular localization is favorable. The two isoforms supply heme to heme-dependent proteins and respond to intrinsic and extrinsic factors. In vitro experimental data with plant organelles supports FC2 translocation to chloroplasts and illustrated the dual targeting of FC1 to both mitochondria and chloroplasts. Separation of the envelope and thylakoid membranes from cucumber revealed a differential distribution of FC2 with preference for accumulation in thylakoid membranes. Both FC isoforms may be involved in heme biosynthesis for proteins, which could be present in various cellular compartments. FC2 contains a C-terminal extension, referred to as the light-harvesting complex (LHC) motif. LHC resembles a transmembrane domain of light-harvesting chlorophyll-binding proteins (LHCPs). It is proposed that this peptide domain participates in Chl binding. Masuda et al. (2003) reported that most FC activity occurred within the chloroplast with minimal FC activity in the mitochondria. It may be inferred based on the distribution pattern of subcellular heme synthesis that the predominating plastid FC activity is that of supplying plastidic and cytoplasmic proteins with heme. A single FC gene encodes a plastid-localized FC protein in Chlamydomonas reinhardtii (Li et al. 2006) A red algal FC isoform, was only identified in mitochondrial extracts. Prior studies utilizing FC antibodies showed the presence of FC2 in chloroplast membranes. Several attempts with both stable and transient expression of FC-GFP (green fluorescent protein) gene constructs showed both FC1 and FC2 fusion products being present only in plastids. FC1–GFP fusion proteins in planta appeared to be exclusively translocated to plastids (Masuda et al. 2003). The assignment of both isoforms to the two organelles and/or the sub organellar membranes of chloroplasts is however still debatable in photosynthetic eukaryotes. Phylogeny strongly supports that angiosperms evolved two FC genes with encoded proteins differing in their primary structure. Alignment of two FC sequences from tobacco revealed 62% identity. The identity of both isoforms increased to 76% when the C-terminal extension of FC2 was excluded from the alignment. Based on the high sequence similarity between the two plant isoforms and their structural diversity when compared with animal FC, it has been proposed that the two plant isogenes did not originate from two phylogenetically distant genomes (Oborník and Green 2005). Evolution of the two plant FC genes by gene duplication, has resulted in distinctive expression profiles in different plant organelles under varied growth conditions. In the synthesis of heme, the FC gene products exhibit various functionality. Based on the diverging transcript levels of both genes, prior recommendations support FC2 providing heme for heme-dependent proteins in photosynthesis, with tobacco FC1 expression being greater in non-photosynthetic tissue. Our results correlate with that observed in Arabidopsis, pea and cucumber. Considering that both FCs serve divergent heme pools in various plant organelles, both FC isoforms may be associated with different sub organellar compartments in plastids. Specific membrane distribution of both FC isoforms has been documented. Investigation of FC activity indicates that plastids are the main site for heme biosynthesis in photosynthetic and non-photosynthetic pea cells (Pisum sativum L.) CsFeC2, the N-terminal transit peptide of CsFeC1, targeted fusion protein only in plastids, and not mitochondria. In the study, it was shown that AtFC1 was transcriptionally activated by cadmium exposure. AtFC1 could positively regulate plant tolerance to cadmium stress. The current research enhances current knowledge regarding the role of FC1 in regulating plant response to cadmium stress. It also provides a basis for continued research of downstream genes. AtFC1, a cadmium stress-responsive gene, enables the plant to withstand cadmium toxicity (Sjödin et al. 2016).
Table 2.
Comparison of the mRNA sequences of FC1 (XM_004510316.3) and three FC2 (XM_004502954.2, XM_004503785.3 and XM_004503809.3) together by Blast tool (Basic Local Alignment Search Tool)
| Parameters | XM_004510316.3 | XM_004502954.2 | XM_004503785.3 | XM_004503809.3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequences producing significant alignments | Description | Length | Query cover | E value | Ident | Query cover | E value | Ident | Query cover | E value | Ident | Query cover | E value | Ident |
| XM_004510316.3 | Ferrochelatase-1, chloroplastic/mitochondrial [Cicer arietinum] | 1428 | 68% | 1e−133 | 71.05% | 67% | 5e−137 | 71.47% | 67% | 5e−137 | 71.47% | |||
| XM_004502954.2 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 1584 | 68% | 1e−133 | 71.05% | 96% | 0.0 | 85.74% | 96% | 0.0 | 85.74% | |||
| XM_004503785.3 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 1584 | 67% | 5e−137 | 71.47% | 96% | 0.0 | 85.74% | 100% | 0.0 | 100% | |||
| XM_004503809.3 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 1584 | 67% | 5e−137 | 71.47% | 96% | 0.0 | 85.74% | 100% | 0.0 | 100% | |||
Table 3.
Comparison of the proteins sequences of FC1 (XP_004510373) and three FC2 (XP_004503011.1, XP_004503842 and XP_004503866.1) with their homologs in Arabidopsis FC1 (CAA73809.1) and FC2 (OAP09354.1) by Blast tool (Basic Local Alignment Search Tool)
| Parameters | ID CAA73809.1 ferrochelatase-I [Arabidopsis thaliana] | ID OAP09354.1 FC2 [Arabidopsis thaliana] | ||||||
|---|---|---|---|---|---|---|---|---|
| Sequences producing significant alignments | Description | Length | Query cover | E value | Ident | Query cover | E value | Ident |
| XP_004510373 | Ferrochelatase-1, chloroplastic/mitochondrial [Cicer arietinum] | 475 | 99% | 0 | 70.45% | 71% | 0 | 67.58% |
| XP_004503011.1 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 527 | 75% | 0.0 | 70.64% | 94% | 0.0 | 76.83% |
| XP_004503842 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 527 | 72% | 0 | 71.18% | 98% | 0 | 74.35% |
| XP_004503866.1 | Ferrochelatase-2, chloroplastic-like [Cicer arietinum] | 527 | 72% | 0.0 | 71.18% | 99% | 0.0 | 74.35% |
Conclusion
Cadmium toxicity results in changes in the morphological and anatomical features of chickpea plants. Levels of chlorophyll and carotenoid significantly decreased in response to cadmium stress. There was a decline in the level of soluble protein which may be due to enhanced protease activity. Total phenolic content of the plants increased. There was however no observed increase in the level of flavonoids during cadmium stress. A noticeable increase in the level of cadmium in the plant was observed. Chickpea may be classified as a cadmium hyperaccumulator and may be considered for use in phytoremediation. Two PCS genes, glutathione gamma-glutamylcysteinyltransferase 1 and glutathione gamma-glutamylcysteinyltransferase were detected in chickpeas. The genes show similarity in their mRNA and amino acid structure. A comparison of these genes with the PCS1 and PCS2 genes in Arabidopsis revealed that glutathione gamma-glutamylcysteinyltransferase 1 had a greater similarity. Glutathione gamma-glutamylcysteinyltransferase 1 and glutathione gamma-glutamylcysteinyltransferase contain 8 and 9 exons respectively. Based on predictions from the COACH meta-server, both PCSs enzymes can bind to L-gamma-glutamylcysteine or gammaglutamylcysteine (3GC) as substrate. The location of the PCS1 and PCS3 enzymes is inconclusive. LocTree3 software predicted that both enzymes were present within the nucleus. DeepLoc-1.0 software showed both enzymes as being soluble and located in the cytoplasm whereas the Plant-MPLOC server showed the enzymes as mitochondrion/nucleus and nucleus, respectively. Four FC genes, 4 proteins and 4 mRNA were detected in chickpeas. One was recorded as FC1 and three as FC2s. The length of FC1 mRNA was 1428 nucleotides and FC2s 1584 nucleotides. FC1 has 9 exons and the FC2s 10, 11 and 11 exons respectively. The FC1 protein had 475 amino acids and the three FC2s, 527 amino acids. The three FC2s are very similar. FC1 in chickpeas is similar to FC1 in Arabidopsis and FC2s in chickpeas are also similar to FC2 in Arabidopsis. Results from the current research provide further insight into the role of FC1 in mediating plant response to cadmium stress. FC1 contributes to the plant’s tolerance to cadmium toxicity. This study provides a better understanding regarding the response of chickpeas to cadmium and the genetic mechanism by which the plant can regulate heavy metal toxicity. This information can be useful in the genetic engineering and genetic manipulation of plants to mitigate heavy metal contamination.
Acknowledgements
This work was funded by a grant from the Shahid Chamran University of Ahvaz Research Council and Tabriz University Research Council. Last but not least, an emotional, heartfelt thank you to my late uncle Dr. Reza Oboodi for his unending support and guidance to the author (Dr. Maryam Kolahi). I hope he is somehow watching this from somewhere with a satisfactory smile.
Appendix
See Table 4.
Table 4.
Features of PCSs and FCs determined in chickpea, accuracy of the predicted model-based on C-score
| Power of 3D structure prediction % | Template | Subject | Organism | First ligand | Others ligand | |
|---|---|---|---|---|---|---|
| XP_004493799_1PCS1 | %55 |
C2btwA C4ry2A D2bu3a1 |
“Alr0975” “peptidase-containing ABC transporter PCAT1” “Primitive phytochelatin synthase” |
Nostoc” “Clostridium thermocellum” “Nostoc” |
3GC 0.22 | POP 0.04 ‘SF4 0.11 |
| XP_004493800.1PCS 3 like | %48 |
D1cjaa C2btwA C4ry2A D2bu3a1 |
“Actin-fragmin kinase” “Alr0975” “peptidase-containing ABC transporter PCAT1” “Primitive phytochelatin synthase” |
“Physarum polycephalum” “Nostoc” “Clostridium thermocellum” “Nostoc” |
3GC 0.29 | MPD 0.06 ‘NAG 0.06 |
| XP_004503842.1FC 2 | 74% |
C2ph5A C4ri3A D1lbqa D2hrca1 |
“The homospermidine synthase hss” “DCCD-modified PsbS “Ferrochelatase” “ferrochelatase” |
Legionella pneumophila “Spinacia oleracea “Saccharomyces cerevisiae” “Homo sapiens” |
HEM 0.51 | BCT 0.07 ‘CO 0.06 ‘FE 0.07 ‘PP9 0.39 |
| XP_004510373.1FC1 ca 6 | 76% |
D1lbqa D2hrca1 D2hk6a1 |
“Ferrochelatase” “Ferrochelatase” “Ferrochelatase” |
“Saccharomyces cerevisiae” “Homo sapiens” “Bacillus subtilis” |
PP9 0.97 | BCT 0.01 ‘FES 0.04 ‘SAL 0.06 ‘CO 0.08 |
| XP_004503011.1FC 2 ca5 | 75% |
C2ph5A C4ri3A D1lbqa D2hrca1 |
“The homospermidine synthase hss” “DCCD-modified PsbS “ferrochelatase” “Ferrochelatase” |
Legionella pneumophila “Spinacia oleracea “Saccharomyces cerevisiae” “Homo sapiens” |
PP9 0.93 | HO1 0.02 ‘SAL 0.05 ‘BCT 0.06 ‘CO 0.09 |
%Power of 3D structure prediction, classification, template organism, C-score for substrate, other ligands determined by iterative implementation of the threading assembly refinement (COACH) to construct the protein
HEM, protoporphyrin IX containing FE heme B; PP9, protoporphyrin; FE, iron(3+); BCT, hydrogencarbonate; CO, cobalt(2 +); SAL, salicylic acid; FES, DI-MU-sulfido-diiron; HO1, protoporphyrin IX 2,4-disulfonic acid; NAG, aldehydo-N-acetyl-D-glucosamine; MPD, 2-methylpentane-2,4-diol; 3GC, gamma-glutamylcysteine; SF4, tetra-MU3-sulfido-tetrairon; POP, diphosphate(2-)
Funding
Not applicable’ for that section.
Compliance with ethical standards
Conflict of interest
The authors report no declarations of interest and that they are responsible for the content and writing of the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE. 2015;10:e0123328. doi: 10.1371/journal.pone.0123328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin H, Arain BA, Amin F, Surhio MA. Analysis of growth response and tolerance index of Glycine max (L.) Merr. under hexavalent chromium stress. Adv Life Sci. 2014;1:231–241. [Google Scholar]
- Arnot JA, Gobas FAPC. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev. 2006;14(4):257–297. doi: 10.1139/a06-005. [DOI] [Google Scholar]
- Baca A, Kornfeind P, Preuschl E, Bichler S, Tampier M, Novatchkov H. A server-based mobile coaching system. Sensors (Basel) 2010;10:10640–10662. doi: 10.3390/s101210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryan JHD. Differential staining with a mixture of safranin and fast green FCF. Stain Technol. 1955;30(4):153–157. doi: 10.3109/10520295509114456. [DOI] [PubMed] [Google Scholar]
- Bundy JG, Kille P. Metabolites and metals in Metazoa—What role do phytochelatins play in animals? Metallomics. 2014;6:1576–1582. doi: 10.1039/C4MT00078A. [DOI] [PubMed] [Google Scholar]
- Chou KC, Shen HB. Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE. 2010;5:e11335–e11335. doi: 10.1371/journal.pone.0011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubukova OV, Postrigan BN, Andrei B, Chemeris A. The effect of cadmium on the efficiency of development of legume-rhizobium symbiosis. Biol Bull. 2015;42:458–462. doi: 10.1134/S1062359015050040. [DOI] [PubMed] [Google Scholar]
- Costa G, Spitz E. Influence of cadmium on soluble carbohydrates, free amino acids, protein content of in vitro cultured Lupinus albus. Plant Sci. 1997;128:131–140. doi: 10.1016/S0168-9452(97)00148-9. [DOI] [Google Scholar]
- Engel DE, DeGrado WF. Amino acid propensities are position-dependent throughout the length of α-helices. J Mol Biol. 2004;337:1195–1205. doi: 10.1016/j.jmb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ericson MC, Alfinito SH. Proteins produced during salt stress in tobacco cell culture. Plant Physiol. 1984;74:506–509. doi: 10.1104/pp.74.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinas NA, Kobayashi K, Sato Y, Mochizuki N, Takahashi K, Tanaka R, Masuda T. Allocation of heme Is differentially regulated by ferrochelatase isoforms in Arabidopsis cells. Front Plant Sci. 2016;7:1326. doi: 10.3389/fpls.2016.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho DP, Serrano HC, Da Silva AB, Branquinho C, Magalhaes S. Effect of cadmium accumulation on the performance of plants and of herbivores that cope differently with organic defenses. Front Plant Sci. 2018;9:1723. doi: 10.3389/fpls.2018.01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg T, Hecht M, Hamp T, Karl T, Yachdav G, Ahmed N, Altermann U, Angerer P, Ansorge S, Balasz K, Bernhofer M, Betz A, Cizmadija L, Do KT, Gerke J, Greil R, Joerdens V, Hastreiter M, Hembach K, Herzog M, Kalemanov M, Kluge M, Meier A, Nasir H, Neumaier U, Prade V, Reeb J, Sorokoumov A, Troshani I, Vorberg S, Waldraff S, Zierer J, Nielsen H, Rost B. LocTree3 prediction of localization. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RG, Kell WJ, Laidler P, Long CC, Whatley SD, McKinley M, Badminton MN, Burnett AK, Williams GT, Elder GH. Photosensitivity and acute liver injury in myeloproliferative disorder secondary to late-onset protoporphyria caused by deletion of a ferrochelatase gene in hematopoietic cells. Blood. 2006;107:60–62. doi: 10.1182/blood-2004-12-4939. [DOI] [PubMed] [Google Scholar]
- Khor BY, Tye GJ, Lim TS, Choong YS. General overview on structure prediction of twilight-zone proteins. Theor Biol Med Model. 2015;12:15. doi: 10.1186/s12976-015-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Keyes T, Straub JE. Replica exchange statistical temperature Monte Carlo. J Chem Phys. 2009;130:124112. doi: 10.1063/1.3095422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamien-Meda A, Lamien C, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Li J, Guo J, Xu W, Ma M. Enhanced cadmium accumulation in transgenic tobacco expressing the phytochelatin synthase gene of Cynodon dactylon L. J Integr Plant Biol. 2006;48:928–937. doi: 10.1111/j.1744-7909.2006.00314.x. [DOI] [Google Scholar]
- Li G, Peng X, Xuan H, Wei L, Yang Y, Guo T, Kang G. Proteomic analysis of leaves and roots of common wheat (Triticum aestivum L.) under copper-stress conditions. J Proteome Res. 2013;12:4846–4861. doi: 10.1021/pr4008283. [DOI] [PubMed] [Google Scholar]
- Li C, Yang X, Xu Y, Li L, Wang Y. Cadmium detoxification induced by salt stress improves cadmium tolerance of multi-stress-tolerant Pichia kudriavzevii. Environ Pollut. 2018;242:845–854. doi: 10.1016/j.envpol.2018.07.058. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HKBTM. Plant cell membranes. Cambridge: Academic Press; 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes; pp. 350–382. [Google Scholar]
- Liu L, Zhang Q, Hu L, Tang J, Xu L, Yang X, Yong JWH, Chen X. Legumes can increase cadmium contamination in neighboring crops. PLoS ONE. 2012;7:e42944–e42944. doi: 10.1371/journal.pone.0042944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone MI, He Z, Stoffella PJ, Yang X. Phytoremediation of heavy metal polluted soils and water: progresses and perspectives. J Zhejiang Univ Sci B. 2008;9:210–220. doi: 10.1631/jzus.B0710633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Šottníková A, Opatrná J, Greger M. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol Plant. 2004;120:537–545. doi: 10.1111/j.0031-9317.2004.0275.x. [DOI] [PubMed] [Google Scholar]
- Manquián-Cerda K, Cruces E, Escudey M, Zúñiga G, Calderón R. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol Environ Saf. 2018;150:320–326. doi: 10.1016/j.ecoenv.2017.12.050. [DOI] [PubMed] [Google Scholar]
- Masuda T, Suzuki T, Shimada H, Ohta H, Takamiya K. Subcellular localization of two types of ferrochelatase in cucumber. Planta. 2003;217:602–609. doi: 10.1007/s00425-003-1019-2. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Almagro Armenteros JJ, Sønderby CK, Sønderby SK, Winther O. DeepLoc: prediction of protein subcellular localization using deep learning. Bioinformatics. 2017;33:3387–3395. doi: 10.1093/bioinformatics/btx431. [DOI] [PubMed] [Google Scholar]
- Nishizawa ION, Satoshi MK. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil. 2009;325:97–108. doi: 10.1007/s11104-009-0116-9. [DOI] [Google Scholar]
- Oborník M, Green BR. Mosaic origin of the heme biosynthesis pathway in photosynthetic eukaryotes. Mol Biol Evol. 2005;22:2343–2353. doi: 10.1093/molbev/msi230. [DOI] [PubMed] [Google Scholar]
- Pandit SB, Zhang Y, Skolnick J. TASSER-Lite: an automated tool for protein comparative modeling. Biophys J. 2006;91:4180–4190. doi: 10.1529/biophysj.106.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul PJ, Samineni S, Thudi M, Sajja SB, Rathore A, Das RR, Khan AW, Chaturvedi SK, Lavanya GR, Varshney RK, Gaur PM. Molecular mapping of QTLs for heat tolerance in chickpea. Int J Mol Sci. 2018;19:2166. doi: 10.3390/ijms19082166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picault N, Cazale AC, Beyly A, Cuiné S, Carrier P, Luu DT, Forestier C, Peltier G. Chloroplast targeting of phytochelatin synthase in Arabidopsis: effects on heavy metal tolerance and accumulation. Biochimie. 2006;88(11):1743–1750. doi: 10.1016/j.biochi.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Rivera-Becerril F, Calantzis C, Turnau K, Caussanel JP, Belimov AA, Gianinazzi S, Strasser RJ, Gianinazzi-Pearson V. Cadmium accumulation and buffering of cadmium-induced stress by arbuscular mycorrhiza in three Pisum sativum L. genotypes. J Exp Bot. 2002;53:1177–1185. doi: 10.1093/jexbot/53.371.1177. [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Palma JM, Gómez M, Del Río LA, Sandalio LM. Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ. 2002;25:677–686. doi: 10.1046/j.1365-3040.2002.00850.x. [DOI] [Google Scholar]
- Sharma P, Dubey RS. Lead toxicity in plants. Braz J Plant Physiol. 2005;17(1):35–52. doi: 10.1590/S1677-04202005000100004. [DOI] [Google Scholar]
- Sharma M, Laxmi A. Jasmonates: emerging players in controlling temperature stress tolerance. Front Plant Sci. 2016;6:1129. doi: 10.3389/fpls.2015.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Gautam N, Mishra A, Gupta R. Heavy metals and living systems: an overview. Indian J Pharmacol. 2011;43:246–253. doi: 10.4103/0253-7613.81505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin S, Öhrfelt A, Brinkmalm G, Zetterberg H, Blennow K, Brinkmalm A. Targeting LAMP2 in human cerebrospinal fluid with a combination of immunopurification and high resolution parallel reaction monitoring mass spectrometry. Clin Proteomics. 2016;13:4. doi: 10.1186/s12014-016-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Feng SJ, Chen J, Zhao WT, Yang ZM. A cadmium stress-responsive gene AtFC1 confers plant tolerance to cadmium toxicity. BMC Plant Biol. 2017;17:187. doi: 10.1186/s12870-017-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veno J, Rahman RNZRA, Masomian M, Ali MSM. Kamarudin NHA. Insight into improved thermostability of cold-adapted Staphylococcal lipase byglycine to cysteine mutation. Molecules. 2019;24(17):3169. doi: 10.3390/molecules24173169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TC, Murray R, Zelman KM. The nutritional value and health benefits of chickpeas and hummus. Nutrients. 2016;8:766. doi: 10.3390/nu8120766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JD, Perez-Ruiz JM, Chory J. Heme synthesis by plastid Ferrochelatase I regulates nuclear gene expression in plants. Curr Biol. 2011;21:897–903. doi: 10.1016/j.cub.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ueda Y, Mukai A, Ochiai K, Matoh T. Rice phytochelatin synthases OsPCS1 and OsPCS2 make different contributions to cadmium and arsenic tolerance. Plant Direct. 2018;2:e00034. doi: 10.1002/pld3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AM, Cheng ZY, Pu HQ, Cheng N, Li HY, Liu SM, Ding J, Li JS, Hu XB, Ren XW, Zheng TZ, Bai YN. Heavy metal assessment among Chinese nonferrous metal-exposed workers from the Jinchang cohort study. Biomed Environ Sci. 2017;30:530–534. doi: 10.3967/bes2017.070. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Hagiwara S, Watanabe T, Nishida N, Ida H, Sakurai T, Komeda Y, Yamao K, Takenaka M, Enoki E, Kimura M, Miyake M, Kawada A, Kudo M. Erythropoietic protoporphyria-related hepatopathy successfully treated with phlebotomy. Intern Med. 2018;57:2505–2509. doi: 10.2169/internalmedicine.0673-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WT, Feng SJ, Li H, Faust F, Kleine T, Li LN, Yang ZM. Salt stress-induced Ferrochelatase 1 improves resistance to salt stress by limiting sodium accumulation in Arabidopsis thaliana. Sci Rep. 2017;7:14737. doi: 10.1038/s41598-017-13593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]