Abstract
Proper transport of metal and their homeostasis is very crucial for the growth and development of plants. Plants root are the primary organs which comes in contact with the stress and thus few modifications occurs, often determining the nutrient efficiency or sometimes as a stress tolerance mechanism. Plant utilizes two strategies for the uptake of iron viz, strategy I-reduction based and strategy II-chelation based. In this review we attempted for a better understanding of how the chelators acts in the mechanism of iron uptake from soils to plants and how iron is distributed in the plants.
Keywords: Iron homeostasis, Chelators, Iron uptake strategies, Iron translocation, Gene regulators
Introduction
For the proper growth and development of plants, the proper uptake and transport of metal across the plant system is very crucial. Iron (Fe) is an essential plant nutrient required in a trace quantity, which is responsible for various metabolic processes like photosynthesis, cell wall metabolism, and respiratory electron transport chain and also provides protection to the plants against various oxidative stress (Li et al. 2012; Dey et al. 2019). However, the excess concentration of Fe in plants can deleteriously affect the plant total growth causing phytotoxicity affecting leaf by forming yellow–brown spots on the surface of the leaf causing leaf chlorosis and inhibiting the plant growth as well as delimiting the nutritional values of the plants and via Fenton reaction can generate reactive oxygen species which can harm the total yield of the plants (Wang et al. 2000; Fang et al. 2001; Carrasco-Gil et al. 2018; Kar and Panda 2018). Earlier, it has been reported, toxicity of Fe reduced the chlorophyll content in plants (Li et al. 2012; Carrasco-Gil et al. 2018). Fe is never found in free elemental state but mostly in the form of Iron-oxides (Fe2O3/FeO) (Mengel and Kirkby 2001; Schulte 2002). Fe2+ is the preferred form of Fe for root absorption and it is also absorbed as Fe3+ chelate form (Briat et al. 2007; García et al. 2018). Fe is abundantly present in soil, mainly in its oxidized state i.e., Fe3+ with low solubility found especially in calcareous and alkaline soil where it is present in a low concentration and is too less to meet the plant requirement for various biological processes (Romheld 1987; Grillet et al. 2014). Due to the bioavailability of Fe in soils varies with the soil types, plants have adapted strategies for the mobilization and appropriate uptake of Fe needed in both Fe excess and deficient condition (Kobayashi and Nishizawa 2012). Thus, proper Fe uptake, transport, its acquisition and distribution within the plant is very crucial in maintaining Fe homeostasis in plants. Plants as an oxygenic photosynthetic organism has to face a dual challenge: one is to acquire Fe from an inorganic environment and other is to make it available in organic form which can be taken and utilized by plants to carry out the fundamental biological processes of life. Fe is taken up from soils following two mechanisms i.e., strategy I-reduction based and strategy II-chelation based (Marschner and Römheld 1994; Lingam et al. 2011; Naranjo-Arcos et al. 2017). In this review, we attempted to study how the chelators plays a crucial role in Fe uptake and how they can regulate in plants need for Fe management.
Iron chelation and solubilization at the rhizosphere
Iron uptake strategies
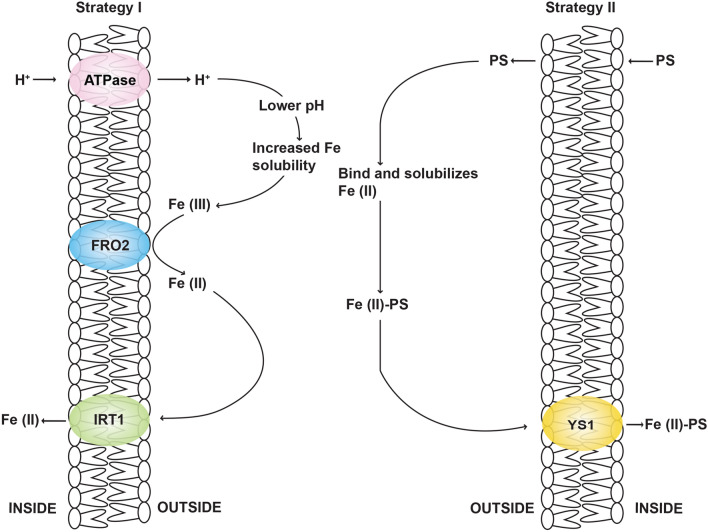
Non-graminaceous plants follow up the strategy I-reduction based, which includes proton release, acidifies the soil to solubilize Fe3+ leading to Fe reduction. FIT (FER-like iron deficiency-induced transcription factor) is known to play a pivotal role in the upregulation of the root-expressed clustered genes involved in the Fe acquisition. Action of FIT is upregulated upon Fe deficiency (Bauer et al. 2004; Ivanov et al. 2012; Naranjo-Arcos et al. 2017). Fe3+ is reduced to Fe2+ upon acidification by membrane bound ferric reductase oxidase (FRO) like FRO2. FRO genes are expressed in different parts of the plants and therefore they have a specific role in respective organs in reduction based Fe transport and are not limited to the root plasma membrane only (Jeong and Guerinot 2009; Kobayashi and Nishizawa 2012; Liang et al. 2017). Once Fe3+ are reduced to Fe2+ which is a substrate for transmembrane transport, they are then transported to the roots by a member of zinc regulated transporter (ZRT)-IRT-like protein (ZIP) family, IRT1. Fe3+-chelate reductase which are the integral membrane protein that transfer electrons from cytosolic NADPH to FAD found in the plasmalemma of the root epidermis (Hell and Stephan 2003). Both FRO2 and IRT1 are specifically expressed in root epidermis responsible for Fe uptake from soils and are important for plant growth (Jeong and Guerinot 2009). Arabidopsis thaliana AtIRT1, first Fe-transporter belonging to ZIP family whose expression is rapidly upregulated upon Fe-deficiency which are probably controlled by both root and shoot derived signals (Gayomba et al. 2015). Belonging to a broad substrate range along with high-affinity for Fe-transport, IRT1 is also able to transport various other divalent cations like Cd2+, Co2+, Zn2+, Mn2+ without disturbing the Fe concentration (Baxter et al. 2008; Naranjo-Arcos et al. 2017). Although marginally, another metal transporter NRAMP1 have also been playing a hidden role in Fe uptake from soils. However, its potential role in Fe uptake mechanism still needs better understanding (Schikora and Curie 2010). Fe is stored readily in non-reactive form in plastids encoded by Ferritin (FER) gene and may also be stored in the vacuole of the plant cell and the mobilization from this organelle is generally mediated by two proteins viz., NRAMP3 and NRAMP4 proteins (Aznar et al. 2015; Naranjo-Arcos et al. 2017). Grasses utilizes strategy II-chelation based, follows the uptake of Fe3+ chelated by mugineic acid-based phytosiderophores, resulting in Fe(III)-PS complex which are then transported via yellow stripe (YS) family into the roots. Grasses are able to take up Fe2+ in addition in Fe(III)-PS complex (Jeong and Guerinot 2009; Lin et al. 2016). YS1, yellow stripe gene from maize was first characterized in Poaceae and its function as Fe-transporter in strategy II for Fe transport (Curie et al. 2001). In Graminaceae, transport of Fe-phytosiderophore complexes helps in the uptake of Fe from the rhizosphere complexes through proteins from the YSL family (Nozoye et al. 2011; Aznar et al. 2015).
Solubilization and uptake of iron
Upon Fe deficiency, many studies have been carried out on root responses towards stress condition. Most of the dicot and fewer monocot species on Fe deficiency has a characteristic, where there is an increase in the activity of NADPH dependent reductase as well as an ATPase-driven proton efflux pump which enhances the rate of solubilization of inorganic Fe(III) in the rhizosphere increasing the reduction of Fe(III) to Fe(II) (Hell and Stephan 2003; Römheld and Marschner 1986). However, the roots of the grasses have an ability to solubilize and uptake Fe from the sparingly soluble inorganic Fe(III) by releasing chelators for Fe. These substances are characterized as non-protein amino acids such as mugineic acid and avenic acid. The chelating substances released by roots are termed as phytosiderophores that chelates Fe(III) in the rhizosphere and forms Fe(III)-PS complex which are imported by specific plasmalemma transporter proteins (Grotz and Guerinot 2006; Kobayashi et al. 2005; Kobayashi and Nishizawa 2012; Kar and Panda 2018). An onset of Fe shortage stimulates the release of phytosiderophores. In the xylem sap, citrate has been known to play a crucial role in chelating Fe and its trafficking (Brown and Chaney 1971; Aznar et al. 2015; Yokosho et al. 2016). FRD3, ferric reductase defective 3, belonging to MATE family facilitates the citrate efflux in the xylem. OsFRDL1 (FRD like gene) in rice also possesses similar functions of citrate efflux in the root pericycle cell, exhibiting its role for the efficient Fe translocation (Yokosho et al. 2009). PEZ1, dramatically lowers the amount of caffeic acid as well as protocatechuic acid in the xylem sap, responsible for the loading of these phenolic compounds facilitating the remobilization of apoplasmic Fe into the plant. FRD3, FRDL1, PEZ1 are the efflux Fe chelators which are present in their free forms (Kobayashi and Nishizawa 2012). By comparing the biochemical pathway of both Fe sufficient and deficient plants, most of the responsible genes were cloned in rice and barley. The secretion of MAs significantly increases in response to Fe deficient condition and the tolerance to this condition is strongly related to the amount of MAs produced and secreted, so that Fe(III)-PS complex can be formed which can be taken up by the roots of the plants. For instance, various crops like sorghum, rice, maize secretes deoxymugineic acid (DMA) in very low amounts and hence they are very susceptible to the Fe-deficiency. However, barley secretes comparatively large amount of numerous kinds of MAs including mugineic acid, 3-hydroxymugineic acid (HMA) and 3-epi-hydroxy-mugineic acid (epi-HMA) and they are relatively more tolerant to the low Fe availability (Negishi et al. 2002). Three molecules of methionine are involved in the biosynthesis that are integrated in one enzymatic step into nicotianamine (NA) which are catalyzed by nicotianamine synthase and it has been reported earlier that in all plant cells, nicotianamine acts as an Fe chelators and it is upregulated in roots of the strategy II plants in low Fe content, and plays a central role in Fe uptake mechanism (Scholz et al. 1992; Schuler et al. 2012). NA is the first intermediate in the process of biosynthesis of phytosiderophores, resulting in the uptake of Fe(III)-PS complex. NA is known to promote high content of Fe and its bioavailability in cereal grains and is produced by NA synthase (NAS) (Lee et al. 2009; Zheng et al. 2010). Any alteration in the activities of NAS gene can affect the NA content in plants which can result in the phenotypical changes related to the uptake as well as distribution of Fe (Takahashi et al. 2003; Klatte et al. 2009). NA is found in the phloem sap as well as it is also detected inside the root tip vacuoles where it functions in Zn chelation. Fe-NA complexes inside the phloem might chelate Fe (von Wirén et al. 1999; Weber et al. 2006; Rellán-Álvarez et al. 2008), it can also prevent precipitation of Fe and deliver it to the target sites. NA can also minimize Fe toxicity as the chelation can prevent ROS production (Becker et al. 1995; Hell and Stephan, 2003). However, the role of NA is still a matter of clarification in strategy I plants. But earlier studies have proved that NA can form more stable complex with Fe2+ than Fe3+. The precise mechanism of secretion of chelators is still unclear and needs better understanding. The cloning of mutant allele ys1 from maize helped in elucidating the uptake of Fe(III)-PS complex. Several YS1-like transporter has been reported to transport NA-iron complex, suggesting it as a ligand for Fe transport (Hell and Stephan 2003; Schuler et al. 2012). Fe2+ which is present inside the cell may be further chelated by NA, which is already present in strategy I and strategy II plants. One of the important mechanism regulating Fe homeostasis is vacuolar sequestration serving as Fe storage strategy. Several genes were reported to regulate Fe trafficking occuring between cytosol and vacuoles. Arabidopsis AtVIT1 (Vacuolar Iron Transporter 1), expressed in seeds serving as a vacuolar sequestration of Fe, indicating that the gene can be potentially used for Fe biofortification (Roschttardtz et al. 2009).
Rice, being a graminaceous species, possesses not only strategy II but involve a partial part of strategy I mechanism of Fe uptake (Ishimaru et al. 2006). It includes OsYSL15 for the uptake of Fe3+-PS complexes as well as it also possess OsIRT1 and OsIRT2 for the uptake of Fe2+ form of Fe, depending on their growth condition. OsYSL15 is the primary transporter which is upregulated during Fe-deficient condition for the uptake of Fe-PS from the rhizosphere. It is also present in the stele and developing seeds, where it plays a vital role in the process of Fe homeostasis (Morrissey and Guerinot 2009). Divalent metal transporter, OsIRT1 and OsIRT2 regulated by FIT is the primary transporter of Fe where Fe2+ is predominantly available (Hindt and Guerinot 2012; Kobayashi et al. 2014; Connorton et al. 2017; Kar and Panda 2018; Dey et al. 2019) (Fig. 1).
Fig. 1.
Strategic representaion of iron uptake in rice
Translocation of iron: from rhizosphere to shoots
Once the Fe is taken up from the rhizosphere into the root epidermis or exodermis, it is then transported to vascular bundles, where xylem and phloem plays their respective roles for the translocation of Fe. Both symplastic and apoplastic pathways are involved in this radial transport system. However, in latter pathway, casparian strips in the exodermis and endodermis comes into role for the Fe translocation (Fig. 2).
Fig. 2.
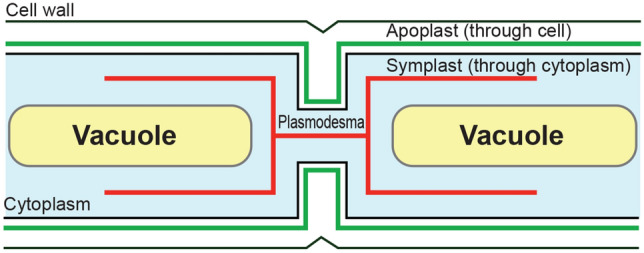
Apoplastic and symplastic pathway
Plants by utilizing the Fe uptake mechanism, chelates the major portion of both Fe3+ and Fe2+ to avoid the Fe toxic condition and to facilitate its transport into the plant shoot systems. Under Fe-deficient condition, the enzymes and transporter genes which are responsible for the Fe uptake are induced in the cortex and vascular bundles along with root epidermis and are thought to be involved in the process of Fe transport into the shoots (Kobayashi and Nishizawa, 2012; Ogo et al. 2014). Fe-DMA complex has been reported to be found in the phloem sap as the primary chemical form indicating that DMA can also help in the internal translocation of Fe along with uptake from the rhizosphere (Nishiyama et al. 2012). NA is the precursor of DMA and acts as a potential chelator of Fe and helps in the translocation of Fe by supressing their toxicity in the plants. In rice, the NAS enzymes (OsNAS1, OsNAS2, OsNAS3) are involved in the synthesis of NA. However, expression of OsNAS3 is low in comparison to OsNAS1 and OsNAS2 and is maily found in the vascular bundles, suggesting its potential role in the translocation of Fe in the shoots (Inoue et al. 2003). OsYSL2 transporter is responsible for phloem mediated Fe-distribution and Fe-NA transport across the plasma membrane and NA efllux transporter ENA1 and ENA2 are thought to be responsible for the extrusion of NA for the redistribution of Fe into the apoplast or intracellular compartments (Ishimaru et al. 2010; Ogo et al. 2014). As a principal chelator, NA plays crucial role in Fe transport and acquisition, either Fe is freely available or it bound to any target structures like Heme or stored as phytoferritin (Hell and Stephan 2003) (Table 1).
Table 1.
Functions of genes involved in iron uptake and transport
| Type | Genes | Functions | Response | References |
|---|---|---|---|---|
| Fe-uptake | FRO2 | Transfer electrons across the plasma membrane to reduce Fe3+ to Fe2+ in the rhizosphere | Strongly induced by low Fe | Connolly et al. (2003) |
| NAS | Chelators for metal cations like Fe II and III | Strongly induced by low Fe | Murata et al. (2006) and Bashir et al. (2006) | |
| NAAT | Transfer of amino group from NA to DMA | Strongly induced by low Fe | Murata et al. (2006) and Bashir et al. (2006) | |
| AHA2 | Generates a proton gradient that drives the active transport of nutrients by H(+)-symport | Induced by low Fe | Schmidt et al. (2000) and Gruber et al. (2013) | |
| IRT1 | Uptake of iron from the rhizosphere across the plasma membrane in the root epidermal layer | Strongly induced by low Fe | Santi and Schmidt (2009) | |
| PEZ1 | Efflux of phenolics to the rhizosphere for Fe III chelation | Marschner (1995) | ||
| YS1/YSL | Yellow strip like protein responsible for uptake of chelated Fe III into root cells | Strongly induced by low Fe | Römheld and Marschner (1986) and Curie et al. (2001) | |
| TOM1 | MAs efflux transporter | Strongly induced by low Fe | Durrett et al. (2007) | |
| Fe-translocation | FRD3 | Citrate efflux transporter | Induced/constitutive by low Fe | Li et al. (2015) and Majerus et al. (2009) |
| PEZ | Phenolics efflux transporter | Weekly induced by low Fe | Marschner (1995) | |
| TOM | MAs efflux transporter | Strongly induced by low Fe | Durrett et al. (2007) | |
| YS1/YSL | Yellow strip like protein responsible for uptake of chelated Fe III into root cells | Induced/repressed by low Fe | Römheld and Marschner (1986) and Curie et al. (2001) | |
| FRO | Ferric chelate reductase for Fe-translocation | Induced by low Fe | Connolly et al. (2003) | |
| IRT | Ferrous ion transporter for Fe-translocation | Induced by low Fe | Santi and Schmidt (2009) | |
| NRAMP | Ferrous ion transporter for Fe-translocation | Induced by low Fe | Tsukamoto et al. (2009) | |
| Fe-storage | Ferritin | High capacity iron storage and sequestration | Induced by Fe-excess | Jin et al. (2010) |
| Fe-compartmentalization | FRO | Ferric chelate reductase for chlorophyll Fe transport | Constitutive by low Fe | Connolly et al. (2003) |
| NRAMP | Fe transporter from vacuole into cytosol | Induced by low Fe | Tsukamoto et al. (2009) | |
| VIT1 | Vacuolar iron influx | Constitutive/repressed by low Fe response | Tsukamoto et al. (2009) | |
| Gene regulator | FER/FIT | Positive transcriptional regulator | Induced by low Fe | Santi and Schmidt (2009) |
| bHLH038/bHLH039 | Positive transcriptional regulator | Induced by low Fe | Ishimaru et al. (2011) | |
| PYE | Negative transcriptional regulator | Induced by low Fe | Curie et al. (2001) | |
| BTS | Putative transcriptional/posttranscriptional regulator | Induced by low Fe | Curie et al. (2001) | |
| EIN3/EIL1 | Ethylene signaling regulator | Induced by low Fe | Yuan et al. (2005) |
Microbial siderophores in Fe mobilisation in plants
There are low molecular mass biomolecules, less than 10 kDa, released from the microorganisms which acts as chelating agents for metal ions. These secreted molecules are known as microbial siderophores and has been reported to possess higher affinity for Fe3+ as compared to the phytosiderophores like mugineic acids (Sharma et al. 2018). The bacteria producing siderophores mostly resides in the rhizospheric region and increases the mobility and availability of metal ions through their secretions, contributing to phytoremediation (Schalk et al. 2011). In case of Fe, presence of siderophore bearing microbes in the rhizosphere leads to better morphological responses. Sah et al. (2017) has reported that higher amount of siderophore producing Pseudomonas aeruginosa strain when applied experimentally in the rhizosphere of Zea mays has brought better shoot and root growth along with increased cob length and grain number. Few plants develop adaptive responses under Fe limiting conditions by secreting phenolic compounds which in turn attracts the siderophore producing rhizobacteria (Jin et al. 2010). The mechanism of mobilisation of Fe using siderophores is still not clear. In another work, pollutant degrading bacteria, Cupriavidus necator produces siderphore which is named as cuprabactin utilised by the bacteria to overcome Fe limitation (Li et al. 2019). This work can be utilised in future as an approach to inoculate the microbe in rhizosphere for increasing Fe acquisition for plants. Other than Fe, microbes are also responsible in uptake of other important elements like copper, zinc and cadmium. Depending upon their chemical structures, siderophores are classified into different types: hydroxymate siderophore, catecholate siderophore and carboxylate siderophore. Mostly, hydroxymate siderophores are responsible for metal immobilisation of metals in plants (Ahmed and Holmström 2014). Pseudobacter putida producing pseudobactin is responsible for growth and yield of plants (Gamalero and Glick 2011). Although, not much studies have been made on metal transportation in plants through siderophores, few studies suggested ligand exchange between phytosiderophore and microbial siderophore is responsible for the same. It has also been reported about the involvement of soil microorganisms in regulation of signalling processes in plants leading to Fe acquisition (Ferreira et al. 2019).
Conclusion
Fe is an essential nutrient element required for the proper growth and development as well as to carry out various metabolic processes within the plant system. However, its toxicity or deficiency can have deleterious effects on plant growth. Plants have developed strategies to deal with the altered conditions for the Fe uptake so that plants are able to control Fe homeostasis by reacting to both Fe-excess and Fe-deficiency (Marschner and Römheld 1994; Lingam et al. 2011; Verma and Pandey 2017).
Fe uptake mechanism is classically divided into two strategies. Strategy I is predominant in eudicotyledonous and non-poaeceae monocot or non-graminaceous plants which are commonly known as “reduction based strategy”. It was made clear using A. thaliana that this strategy involves 3 step process on plasma membrane protein which are induced in the Fe-deficiency condition: the AHA2, Arabidopsis H+-ATPase releases protons which acidifies the soil surface by lowering soil pH, followed by the reduction of Fe3+ to Fe2+ by ferric reductase oxidase 2, FRO2 and finally the Fe2+ are imported into the root cells of the plant by high affinity ferrous Fe transporter 1, IRT1 (Santi and Schmidt 2009; Hell and Stephan 2003; Brumbarova et al. 2015; Kobayashi et al. 2019). Strategy II, which is commonly known as chelation based strategy has been well described in plant species belonging to the Poaecae family i.e., graminaceous plants. Rice, being a model plant has been used to describe various unknown factors which are involved in strategy II Fe uptake mechanism (Sisó-Terraza et al. 2016; Rajniak et al. 2018). Strategy II involves the secretion of phytosiderophores (PS) belonging to mugineic acid family, with the potential to chelate Fe3+ and forms Fe(III)-MA complexs. Enzymes such as nicotianamine synthase (NAS), nicotianamine aminotransferase (NAAT), deoxymugineic acid synthase (DMAS) are involved in the biosynthesis of all types of MAS from S-adenosyl methionine, resulting in deoxymugineic acid (DMA), which is the precursor of all phytosiderophores. After DMA binds to Fe3+ and forms the complex, they are then imported by the Yellow stripe (YS) or Yellow stripe-like (YSL) family members (Grotz and Guerinot 2006; Kobayashi et al. 2005; Kobayashi and Nishizawa 2012; Brumbarova et al. 2015; Connorton et al. 2017; Kobayashi et al. 2019).
Future prospects
In the context of abiotic stress in plants caused by Fe, thorough studies has been performed in the last decade to illustrate the homeostatic activity under both excess and deficient condition. The knowledge of function of chelators and their respective transporters contribute a major part in understanding Fe uptake in plants. Metabolomic approaches has made successful attempts to report the accumulation of natural Fe complex in xylem and phloem tissues, explaining their apoplastic and symplastic pathways involved in Fe transportation. Under deficient Fe scenario, efforts has always been taken to enhance the Fe quantity in the plants and studies of the chelators regarding the same remains primary. In recent years, extensive studies has been performed on understanding the homeostatic responses by many crop plants under both Fe excess and deficient condition through cellular, biochemical and genetic approaches. In large number of crop plants, next generation sequencing has been performed to understand the uptake strategies, transport and homeostasis aiming for novel genes and hypothesis of their functions (Do Amaral et al. 2016; Wu et al. 2017; Aung et al. 2018). Knowing the chelation and transportation, sets a foundation for further assessment of the processing mechanism. Although, large number of genes and signalling pathways has been reported regarding regulation, there are more steps to uncover the interconnection of signalling pathways in case of transportation of the metal inside the plants. Recent development in site targeted mutagenesis with CRISPR/Cas9 can be a better approach to explain proper functions of reported genes. By utilising functional genomic approach, functionality of the chelators and transporters can be altered in bringing biofortification in crop plants.
Acknowledgements
We sincerely acknowledge fellowship obtained from Department of Science and Technology-Innovation in Science Pursuit for Inspired Research (DST-INSPIRE), Government of India, No. DST/INSPIRE Fellowship/2016/IF160804, Dated 12.02.2018.
Abbreviations
- FIT
FER-like iron deficiency-induced transcription factor
- FRO
Ferric reductase oxidase
- ZRT
Zinc regulated transporter
- IRT
Iron regulated transporter
- YS
Yellow stripe
- YSL
Yellow stripe like
- FRD
Ferric reductase defective
- FRDL
Ferric reductase defective like
- MATE
Multidrug and toxic compound extrusion
- NADPH
Nicotinamide adenine dinucleotide phosphate
- FAD
Flavin adenine dinucleotide
- NRAMP
Natural resistance-associated macrophage protein
- PS
Phytosiderophores
- PEZ
Phenolic efflux zero
- DMA
Deoxymugineic acid
- MAs
Mugineic acid
- VIT1
Vacuolar iron transporter 1
- NAS
Nicotianamine synthase
- NAAT
Nicotianamine aminotransferase
- DMAS
Deoxymugineic acid synthase
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed E, Holmström SJ. Siderophores in environmental research: roles and applications. Microb Biotechnol. 2014;7(3):196–208. doi: 10.1111/1751-7915.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MS, Masuda H, Kobayashi T, Nishizawa NK. Physiological and transcriptomic analysis of responses to different levels of iron excess stress in various rice tissues. Soil Sci Plant Nutr. 2018;64(3):370–385. doi: 10.1080/00380768.2018.1443754. [DOI] [Google Scholar]
- Aznar A, Chen NW, Thomine S, Dellagi A. Immunity to plant pathogens and iron homeostasis. Plant Sci. 2015;240:90–97. doi: 10.1016/j.plantsci.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H. Cloning and characterisation of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem. 2006;43:32395–32402. doi: 10.1074/jbc.M604133200. [DOI] [PubMed] [Google Scholar]
- Bauer P, Thiel T, Klatte M, Bereczky Z, Brumbarova T, Hell R, Grosse I. Analysis of sequence, map position and gene expression reveals conserved essential genes for iron uptake in Arabidopsis and tomato. Plant Physiol. 2004;136:4169–4183. doi: 10.1104/pp.104.047233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter IR, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, Ml G, Salt DE. The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc Natl Acad Sci USA. 2008;105:12081–12086. doi: 10.1073/pnas.0804175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Fritz E, Manteuffel R. Subcellular localization and characterization of excessive iron in the nicotianamine-less tomato mutant chloronerva. Plant Physiol. 1995;108(1):269–275. doi: 10.1104/pp.108.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat JF, Curie C, Gaymard F. Iron utilization and metabolism in plants. Curr Opin Plant Biol. 2007;10(3):276–282. doi: 10.1016/j.pbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Brown JC, Chaney RL. Effect of iron on the transport of citrate into the xylem of soybean and tomatoes. Plant Physiol. 1971;47:836–840. doi: 10.1104/pp.47.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbarova T, Bauer P, Ivanov R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 2015;20(2):124–133. doi: 10.1016/j.tplants.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Carrasco-Gil S, Hernandez-Apaolaza L, Lucena JJ. Effect of several commercial seaweed extracts in the mitigation of iron chlorosis of tomato plants (Solanum lycopersicum L.) Plant Growth Regul. 2018;86(3):401–411. doi: 10.1007/s10725-018-0438-9. [DOI] [Google Scholar]
- Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003;133(3):1102–1110. doi: 10.1104/pp.103.025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connorton JM, Balk J, Rodríguez-Celma J. Iron homeostasis in plants: a brief overview. Metallomics. 2017;9(7):813–823. doi: 10.1039/c7mt00136c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat J-F, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409(6818):346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Dey S, Kar S, Regon P, Panda SK. Physiology and biochemistry of Fe excess in acidic Asian soils on crop soil. J Soil Sci Agroclimatogy. 2019;16(1):112–126. doi: 10.20961/stjssa.v16i1.30456. [DOI] [Google Scholar]
- Do Amaral MN, Arge LWP, Benitez LC, Danielowski R, da Silveira Silveira SF, da Rosa FD, de Oliveira AC, da Maia LC, Braga EJB. Comparative transcriptomics of rice plants under cold, iron, and salt stresses. Funct Integr Genomics. 2016;16(5):567–579. doi: 10.1007/s10142-016-0507-y. [DOI] [PubMed] [Google Scholar]
- Durrett TP, Gassmann W, Rogers EE. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007;144:197–205. doi: 10.1104/pp.107.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang WC, Wang JW, Lin CC, Kao CH. Iron induction of lipid peroxidation and effects on antioxidative enzyme activities in rice leaves. Plant Growth Regul. 2001;35(1):75–80. doi: 10.1023/A:1013879019368. [DOI] [Google Scholar]
- Ferreira MJ, Silva H, Cunha A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: a review. Pedosphere. 2019;29(4):409–420. doi: 10.1016/S1002-0160(19)60810-6. [DOI] [Google Scholar]
- Gamalero E, Glick BR. Mechanisms used by plant growth-promoting bacteria. In: Maheshwari DK, editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer; 2011. pp. 17–46. [Google Scholar]
- García MJ, Corpas FJ, Lucena C, Alcántara E, Pérez-Vicente R, Zamarreño ÁM, Bacaicoa E, García-Mina JM, Bauer P, Romera FJ. A shoot Fe signaling pathway requiring the OPT3 transporter controls GSNO reductase and ethylene in Arabidopsis thaliana roots. Front Plant Sci. 2018;9:1325. doi: 10.3389/fpls.2018.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayomba SR, Zhai Z, Jung HI, Vatamaniuk OK. Local and systematic signaling of iron status and its interactions with homeostasis of other essential elements. Front Plant Sci. 2015;6:716. doi: 10.3389/fpls.2015.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet L, Ouerdane L, Flis P, Hoang MTT, Isaure MP, Lobinski R, Curie C, Mari S. Ascorbate efflux as a new strategy for iron reduction and transport in plants. J Biol Chem. 2014;289(5):2515–2525. doi: 10.1074/jbc.M113.514828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta. 2006;1763(7):595–608. doi: 10.1016/j.bbamcr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gruber BD, Giehl RFH, Friedel S, von Wiren N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013;163:161–179. doi: 10.1104/pp.113.218453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R, Stephan UW. Iron uptake, trafficking and homeostasis in plants. Planta. 2003;216(4):541–551. doi: 10.1007/s00425-002-0920-4. [DOI] [PubMed] [Google Scholar]
- Hindt MN, Guerinot ML. Getting a sense for signals: regulation of the plant iron deficiency response. Biochim Biophys Acta. 2012;1823(9):1521–1530. doi: 10.1016/j.bbamcr.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2 and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 2003;36:366–381. doi: 10.1046/j.1365-313X.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Nishizawa NK. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ Plant J. 2006;45(3):335–346. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashu M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa NK. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010;62:379–390. doi: 10.1111/j.1365-313X.2010.04158.x. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Kakei Y, Shimo H, Bashir K, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. A rice phenolic efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J Biol Chem. 2011;286:24649–24655. doi: 10.1074/jbc.M111.221168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov R, Tiedemann J, Czihal A, Baumlein H. Transcriptional regulator AtET2 is required for the induction of dormancy during late seed development. J Plant Physiol. 2012;169:501–508. doi: 10.1016/j.jplph.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Jeong J, Guerinot ML. Homing in on iron homeostasis in plants. Trends Plant Sci. 2009;14:280–285. doi: 10.1016/j.tplants.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Jin CW, Li GX, Yu XH, Zheng SL. Plant Fe status affects the composition of siderophore-secreating microorganisms in the rhizosphere. Ann Bot. 2010;105:835–841. doi: 10.1093/aob/mcq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Panda SK. Iron homeostasis in rice: deficit and excess. Proc Natl Acad Sci India Sect B Biol Sci. 2018 doi: 10.1007/s40011-018-1052-3. [DOI] [Google Scholar]
- Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P. The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol. 2009;150(1):257–271. doi: 10.1104/pp.109.136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol. 2012;63(1):131–152. doi: 10.1146/annurev-arplant-042811-105522. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, Nakanishi H, Nishizawa NK. Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot. 2005;56(415):1305–1316. doi: 10.1093/jxb/eri131. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Itai RN, Nishizawa NK. Iron deficiency responses in rice roots. Rice. 2014;7(1):27. doi: 10.1186/s12284-014-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nozoye T, Nishizawa NK. Iron transport and its regulation in plants. Free Radic Biol Med. 2019;133:11–20. doi: 10.1016/j.freeradbiomed.2018.10.439. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeon US, Lee SJ, Kim YK, Persson DP, Husted S, Schjørring JK, Kakei Y, Masuda H, Nishizawa NK, An G. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc Natl Acad Sci. 2009;106(51):22014–22019. doi: 10.1073/pnas.0910950106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ma H, Jia P, Wang J, Jia L, Zhang T, Wei X. Ecotoxicology and Environmental Safety Responses of seedling growth and antioxidant activity to excess iron and copper in Triticum aestivum L. Ecotoxicol Environ Saf. 2012;86:47–53. doi: 10.1016/j.ecoenv.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Li G, Xu W, Kronzucker HJ, Shi W. Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis. J Exp Bot. 2015;66(7):2041–2054. doi: 10.1093/jxb/erv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhu L, Pan D, Li S, Xiao H, Zhang Z, Shen X, Wang Y, Long M. Siderophore-mediated iron acquisition enhances resistance to oxidative and aromatic compound stress in Cupriavidus necator JMP134. Appl Environ Microbiol. 2019;85(1):e01938-18. doi: 10.1128/AEM.01938-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Zhang H, Li X, Ai Q, Yu D. bHLH transcription factor bHLH115 regulates iron homeostasis in Arabidopsis thaliana. J Exp Bot. 2017;68(7):1743–1755. doi: 10.1093/jxb/erx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XY, Ye YQ, Fan SK, Jin CW, Zheng SJ. Increased sucrose accumulation regulates iron-deficiency responses by promoting auxin signaling in Arabidopsis plants. Plant Physiol. 2016;170(2):907–920. doi: 10.1104/pp.15.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingam S, Julia M, Tzvetina B, Thomas P, Claudia FS, Eddy B, Pascal G, Bauer P. Interaction between the bHLH transcription factor FIT and ETHYLENE INSENSITIVE3/ETHYLENE INSENSITIVE3-LIKE1 reveals molecular linkage between the regulation of iron acquisition and ethylene signaling in Arabidopsis. Plant Cell. 2011;23(5):1815–1829. doi: 10.1105/tpc.111.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus V, Bertin P, Lutts S. Abscisic acid and oxidative stress implications in overall ferritin synthesis by African rice (Oryza glaberrima Steud.) seedling sexposed to short term iron toxicity. Plant Soil. 2009;324:253–265. doi: 10.1007/s11104-009-9952-x. [DOI] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2. London: Academic press; 1995. [Google Scholar]
- Marschner H, Römheld V. Strategies of plants for acquisition of iron. Plant Soil. 1994;165:261–274. doi: 10.1007/BF00008069. [DOI] [Google Scholar]
- Mengel K, Kirkby EA. Principles of plant nutrition. Ann Bot. 2001;93(4):479–480. doi: 10.1093/aob/mch063. [DOI] [Google Scholar]
- Morrissey J, Guerinot ML. Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev. 2009;109(10):4553–4567. doi: 10.1021/cr900112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Ma JF, Yamaji N, Ueno U, Nomoto K, Iwashita T. A specific transporter for iron (III)-phytosiderophore in barley roots. Plant J. 2006;46:563–572. doi: 10.1111/j.1365-313X.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- Naranjo-Arcos MA, Maurer F, Meiser J, Pateyron S, Fink-Straube C, Bauer P. Dissection of iron signaling and iron accumulation by overexpression of subgroup Ib bHLH039 protein. Sci Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-11171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi T, Nakanishi H, Yazaki J, Kishimoto N, Fujii F, Shimbo K. cDNA microarray analysis of gene expression during Fe-deficiency stress in barley suggests that polar transport of vesicles is implicated in phytosiderophore secretion in Fe-deficient barley roots. Plant J. 2002;30(1):83–90. doi: 10.1046/j.1365-313X.2002.01270.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama R, Kato M, Nagata S, Yanagisawa S, Yoneyama T. Identification of Zn nicotianamine and Fe-2′-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.) Plant Cell Physiol. 2012;53:381–390. doi: 10.1093/pcp/pcr188. [DOI] [PubMed] [Google Scholar]
- Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem. 2011;286(7):5446–5454. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo Y, Kakei Y, Itai RN, Kobayashi T, Nakanishi H, Takahashi H, Nakazono M, Nishizawa NK. Spatial transcriptomes of iron-deficient and cadmium-stressed rice. New Phytol. 2014;201:781–794. doi: 10.1111/nph.12577. [DOI] [PubMed] [Google Scholar]
- Rajniak J, Giehl RF, Chang E, Murgia I, von Wirén N, Sattely ES. Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat Chem Biol. 2018;14(5):442–450. doi: 10.1038/s41589-018-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellán-Álvarez R, Abadía J, Álvarez-Fernández A. Formation of metal-nicotianamine complexes as affected by pH, ligand exchange with citrate and metal exchange. A study by electrospray ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(10):1553–1562. doi: 10.1002/rcm.3523. [DOI] [PubMed] [Google Scholar]
- Romheld V. What’s new in plant physiology: different strategies for iron acquisition in higher plants. Physologia Plantarum. 1987;70:231–234. doi: 10.1111/j.1399-3054.1987.tb06137.x. [DOI] [Google Scholar]
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986;80(1):175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschttardtz H, Conejero G, Curie C, Mari S. Identification of the endodermal vacuole as the iron storage compartment in the Arabidopsis embryo. Plant Physiol. 2009;151:1329–1338. doi: 10.1104/pp.109.144444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah S, Singh N, Singh R. Iron acquisition in maize (Zea mays L.) using pseudomonas siderophore. Biotech. 2017;7(2):121. doi: 10.1007/s13205-017-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009;183:1072–1084. doi: 10.1111/j.1469-8137.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- Schalk IJ, Hannauer M, Braud A. New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol. 2011;13(11):2844–2854. doi: 10.1111/j.1462-2920.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- Schikora A, Curie C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell. 2010;22:904–917. doi: 10.1105/tpc.109.073023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Tittel J, Schikora A. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol. 2000;122:1109–1118. doi: 10.1104/pp.122.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz G, Becker R, Pich A, Stephan UW. Nicotianamine: a common constituent of strategies I and II of iron acquisition in plants. J Plant Nutr. 1992;15:1649–1665. doi: 10.1080/01904169209364428. [DOI] [Google Scholar]
- Schuler M, Rellán-Álvarez R, Fink-Straube C, Abadía J, Bauer P. Nicotianamine functions in the phloem-based transport of iron to sink organs, in pollen development and pollen tube growth in Arabidopsis. Plant Cell. 2012;24(6):2380–2400. doi: 10.1105/tpc.112.099077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EE (2002) Soil and applied iron. In understanding plant nutrition. Cooperative extension publications. University of Wisconsin-Extension
- Sharma R, Bhardwaj R, Gautam V, Kohli SK, Kaur P, et al. Microbial siderophores in metal detoxification and therapeutics: recent prospective and applications. In: Egamberdieva D, Ahmad P, et al., editors. Plant microbiome: stress response, microorganisms for sustainability. 5. Singapore: Springer; 2018. pp. 337–350. [Google Scholar]
- Sisó-Terraza P, Luis-Villarroya A, Fourcroy P, Briat JF, Abadía A, Gaymard F, Abadía J, Álvarez-Fernández A. Accumulation and secretion of coumarinolignans and other coumarins in Arabidopsis thaliana roots in response to iron deficiency at high pH. Front Plant Sci. 2016;7:1711. doi: 10.3389/fpls.2016.01711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell. 2003;15(6):1263–1280. doi: 10.1105/tpc.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Nakanishi H, Uchida H, Watanabe S, Matsuhashi S. 52Fe translocation in barley as monitored by a poitron emitting tracer imaging system (PETIS): evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant Cell Physiol. 2009;50:48–57. doi: 10.1093/pcp/pcn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma L, Pandey N. Impact of iron stress on oxidative metabolism in green gram plants (Vigna radiata (L) Wilczek) J Glob Biosci. 2017;6(6):5120–5130. [Google Scholar]
- von Wirén N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, Leigh RA, Hider RC. Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol. 1999;119(3):1107–1114. doi: 10.1104/pp.119.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA. 2000;97(9):4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G, von Wirén N, Hayen H. Analysis of iron (II)/iron (III) phytosiderophore complexes by nano-electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom. 2006;20(6):973–980. doi: 10.1002/rcm.2402. [DOI] [PubMed] [Google Scholar]
- Wu LB, Ueda Y, Lai SK, Frei M. Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.) Plant Cell Environ. 2017;40(4):570–584. doi: 10.1111/pce.12733. [DOI] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol. 2009;149:297–305. doi: 10.1104/pp.108.128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Ma JF. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J Exp Bot. 2016;67(18):5485–5494. doi: 10.1093/jxb/erw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YX, Zhang J, Wang DW, Ling HQ. AtbHLH29 of Arabidopsis thaliana is a functional ortholog of tomato FER involved in controlling iron acquisition in strategy I plants. Cell Res. 2005;15:613–621. doi: 10.1038/sj.cr.7290331. [DOI] [PubMed] [Google Scholar]
- Zheng L, Cheng Z, Ai C, Jiang X, Bei X, Zheng Y, Glahn RP, Welch RM, Miller DD, Lei XG, Shou H. Nicotianamine, a novel enhancer of rice iron bioavailability to humans. PLoS ONE. 2010;5(4):e10190. doi: 10.1371/journal.pone.0010190. [DOI] [PMC free article] [PubMed] [Google Scholar]