Abstract
Drought stress is an important environmental stress that clearly affect biological systems of plants. There is a possibility that growth regulators are able to protect plants under drought conditions. Ascorbic acid (AsA) plays a particular role on growth of plants and protects cells from oxidative damage caused by environmental stresses. This study emphasized the impacts of AsA on improving the drought tolerance of the pepper plants. Based on a factorial arrangement in a completely randomized design, the experiment had two factors. The first factor was drought: irrigation within the field capacity, moderate stress (irrigation within the 60% field capacity) and severe stress (irrigation within the 30% field capacity). The second factor was AsA: 0 mM sprayed with distilled water, 0.5 mM and 1 mM. The experiment had three replications. Drought stress inhibited plant growth parameters including fruit number, height, weight, yield, chlorophyll a and b, total chlorophyll, carotenoid contents, it caused improvement in activity of catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), proline content, anthocyanins, soluble sugars, malondialdehyde (MDA) and H2O2 in the leaves of sweet pepper. Application of AsA contributes to an increase in antioxidant enzymes activity such as SOD, CAT, POD and proline contents, chlorophyll a and b, total chlorophyll, carotenoids, soluble carbohydrates. However, it reduced the content of anthocyanins, MDA and H2O2. Based on this study, it can be suggested that ascorbic acid adjusted antioxidant activity, especially after it has been subjected to drought stress.
Keywords: Chlorophyll, Field capacity, Malondialdehyde, Superoxide dismutase
Introduction
Sweet pepper (Capsicum spp.) contains an incredible intra and inter-specific variety in color, shape, taste, fruit type and biochemical content (Sakaldas and Kaynas 2010). The fruits of pepper are an abundant sources of antioxidants nutrients such as provitamin A (carotenoids) which are important nutritional antioxidants in food (Yasuor et al. 2015). In various types of pepper, carotenoid pigments are responsible for yellow, orange and red colors. In the pepper fruits, levels of these compounds depend on many factors, including variety, maturity, growth and weather conditions (Hwang et al. 2012). However, this crop is particularly sensitive to soil water deficit. It is well-known that drought stress during the initial developmental and reproductive stages can reduce the number and size of buds and fruits (Campos et al. 2014).
Drought stress is a harmful non-biological factor that reduces growth and development of plants as well as yields (Rasheed et al. 2020). Most areas are classified as dry and semi-arid in Iran, therefore drought-resistant plants with high performance are essential (Ding et al. 2015). One of the most important scientific and economic issues in arid areas is to improve plant performance to mitigate the adverse conditions of drought (Penella et al. 2014).
Ascorbic acid (AsA) can improve plant growth and elevate yield through improvement of resistance to stress (Zhou et al. 2016). Also, AsA maintains plant’s water at an optimal amount in conditions of drought stress (Noman et al. 2015). Previous study addressed that AsA is involved in multiple physiological and biochemical steps from seed germination until senescence plants, for example, oxidative stress, cell division and enlargement, flowering, growing fruit signaling, resistance against invading pathogens, increasing yield and stress tolerance in plants (Latif et al. 2016). In the past, the research has been conducted to confirm incremental effect of AsA on the growth and quality of fruit in plants, which can be pointed out to flame seedless grapevine by El-Sayed et al. (2000); mangoes by Ahmed (2001); Washington Navel orange by Ragab (2002); banana by Mostafa (2004); white flame seedless grapevines by Wassel et al. (2007). Moreover, Maksoud et al. (2009) has shown that foliar application of AsA elevates yield and quality of fruit of olive trees. Yousef et al. (2009) reported that a month before the harvest of olive trees, a 90 ml AsA foliar spray improved the chemical properties of the fruit and had a positive effect on the characteristics of olive oil. Production of reactive oxygen species under stress conditions increases and internal protective activities may be insufficient (Arafa et al. 2007). There are various advanced defense systems in plants (Dolatabadian et al. 2008) which contains non enzymatic antioxidant compounds (ascorbic acid, salicylic acid, glutathione, tocopherols, etc.) and antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) for elimination of ROS (Mohammadi et al. 2020; Athar et al. 2008). Moreover, ASA in all plants exists as a natural antioxidant compound in normal conditions and under stress conditions would increase (Dolatabadian et al. 2008) and it plays a vital role in preserving the activity of enzymatic antioxidant like SOD, CAT, and POD (Arafa et al. 2007). The effect of AsA on the activity of antioxidant enzymes can be varied. Mekki et al. (2015) reported that AsA decreases the activities of SOD, CAT, of corn (Zea mays L) under drought stress and decreased hydrogen peroxide (H2O2) content and oxidative damage while Aroca (2006) confirmed that AsA increased the activity of SOD and Liu et al. (2014) explained that AsA increased the activity of POD in plums (Prunus domestica). It can be said that osmotic adjustment is an important process in postponing water stress, which takes place in conditions of water shortages through the accumulation of compatible solutes (Sun et al. 2013). Proline as an amino acid is one of the most important cytosolutes, and acts as a compatible solute and has been suggested as a general index for drought stress tolerance (Liu et al. 2011). Amin et al. (2009) reported that exogenous application of AsA under drought stress showed positive increase in proline content and led to stability of cell membrane and drought resistance. Therefore, since AsA is one of the most affordable plant growth regulators and has positive effects on plant growth at both conditions of stress and control, it can be used for increased resistance to drought stress in sweet pepper seedlings.
The objective of this study was to investigate the effect of a foliar spraying of AsA as agent to ameliorate the adverse effects of drought on growth, physiological and biochemical parameters of pepper seedlings.
Materials and methods
Plant materials and growth conditions
This research was carried out to assess influence of foliar application of different concentrations of AsA on biochemical and physiological attributes of sweet pepper (Capsicum annuum L.) under normal and drought conditions. The research was carried out in a greenhouse at the Faculty of Agriculture, University of Ilam. The study was conducted as a factorial experiment in a completely randomized design with two factors. The main factors were different levels of drought and AsA concentrations. Each treatment had three replications. It lasted 4 months, from seed cultivation to sampling. Seeds of sweet pepper were provided by the Faculty of Agriculture. The peppers seeds were surface sterilized with 1% sodium hypochlorite for 10 min before being washed with tap water for 1 min. Then, the seeds were sown in plastic pots that measured 20 cm in height and 23 cm in diameter. The pots were filling with a mixture of fine sand, sand, leaf mold and garden soil with a ratio of 1:1:1. After filling, each pot weighed 7 kg. The soil samples were analyzed for various soil properties (Table 1)
Table 1.
Physico-chemical properties of the experimental soil
| Characteristics | Units | Values |
|---|---|---|
| Moisture content | (%) | 32 |
| P | (ppm) | 3.47 |
| K | (ppm) | 33.63 |
| pH | 7.3 | |
| Sand | (%) | 22 |
| Clay | (%) | 11 |
| Silt | (%) | 67 |
| Soil texture | Silty loam | |
| EC | (ds/m) | 0.7 |
| Organic carbon | (%) | 0.42 |
| Total N | (%) | 0.04 |
The average temperature and relative humidity during plant growth were 18/25 °C (day/night), and 60–70%, respectively. In the early stages of plant growth, irrigation was complete. A few drops of Tween-20 (polyoxyethylene sorbitan monolaurate) were added as a surfactant to a solution of AsA to increase adhesion between leaves. The AsA treatment started at the fourth leaf stage. AsA was used at the concentration of 0, 0.5 and 1 mM. The AsA was sprayed onto the leaves so that both sides of each leaf became completely wet. The foliar spray was applied twice. The first instance of application was 72 h before the drought stress treatment. Accordingly, 72 h after the first foliar spray, all plants were exposed to three levels of drought stress: stress-free conditions (full irrigation, i.e. the control group), moderate stress (60% of field capacity) and severe stress (30% of field capacity). The second spray was applied 2 weeks after the drought stress began. The drought stress treatments were maintained until the end of the experiment. All pots were weighed on a daily basis.
In this experiment, 27 sweet pepper plants were harvested at 80% maturity (in the green stage). Leaf samples consisted of three replicates. Each replicate was obtained from three pots, and therefore a total of 9 plants existed in each treatment group. The samples were immediately placed in liquid nitrogen after harvest. They were stored in the freezer (− 80 °C) before measuring the biochemical properties, the physiological features and the activity of antioxidant enzymes.
Determination of fruit yield
The factors such as drought stress, high temperature greenhouse and susceptibility of the cultivar reduced plant growth, and economic performance therefore, fruit storage in the greenhouse has not been possible. The fruit harvest was carried out at four times. Pepper fruits were collected when they were green and fully grown. At harvest time, weight and number per plant as well as the total weight of fruits per plant were recorded and the total yield was calculated.
Determination of lipid peroxidation
MDA was evaluated by the Zhao et al. (1993) method. For this purpose, 0.25 g of pepper leaf tissue at 5 ml of 1% tricyclic acetic acid (TCA) was crushed and centrifuged at 5000 g for 10 min at 4 °C, then 1 ml of supernatant and 4 ml of 20% TCA containing 0.5% thiobarbituric acid was mixed together and the mixture was exposed to a temperature of 95 °C at 30 min, the mixture was immediately cooled and read with a spectrophotometer at 450, 532, and 600 nm. To determine the MDA, the following equation was used:
Estimation of soluble carbohydrates
Carbohydrates from pepper leaf tissue were extracted according to a method by Badour (1959). For this purpose, 1 ml of herbal extract and 9 ml of anthron sulfuric acid were mixed in a glass tube and heated to 100 °C at 7 min. The absorbance was read at 620 nm spectrophotometer. The results of this factor were presented as mg g−1 dry weight.
Estimation of proline amount
The amount of free proline was measured according to method by Bates et al. (1973) in the plant. To begin, 0.2 g of leaves samples were crushed by a mortar and pestle, then homogenized by centrifugation at 18,000 g at 15 min. This was followed by adding 2 ml to the test tube containing 2 ml glacial acetic acid and freshly prepared acid ninhydrin solution (1.25 g ninhydrin dissolved in 20 ml 6 m orthophosphoric acid and 30 ml glacial acetic acid). Then test tubes were placed at 100 °C for 1 h and cooled at 25 °C. In the next steps, 4 ml of toluene were added to the contents of the test tube and vortexed at 20 s. The above test tubes were kept vertically at 10 min until phase separation. The absorbance was read at 520 nm. The content of proline presented as µg g−1fresh weight.
Determination of chlorophyll and carotenoids
Lichtenthaler (1987) method was used to measure chlorophyll and carotenoids. For this purpose, 0.2 g of fresh leaves of pepper was crushed using 15 ml of acetone 80% and filtered. The absorbance was read at 470, 663, 646 nm. Chlorophyll concentration was determined by the equation below:
Determination of anthocyanins
According to Wagner (1979) method, 0.1 g fresh leaf from the tip of the shoots and root ends was ground and centrifuged in 10 ml acidic methanol (99:1 methylic alcohol: HCl). Then, the resulting solution was kept overnight in the dark and read by the spectrophotometer at 550 nm.
Determination hydrogen peroxide
The extraction and estimation H2O2 was evaluated by Velikova et al. (2000). For this purpose, 0.2 g of pepper seedlings leaves was crushed in a mortar with 3 ml of 0.1% (w/v) trichloroacetic acid (TCA) and then centrifuged at 12000 × g for 15 min. 0.5 ml of phosphate buffer (pH 7.0) was added to 0.5 ml of supernatant and 1 ml of 1 M KI was mixed. The absorbance was read at 390 nm. The absorption rate was also expressed as µmol g−1 FW.
Determination of AsA content
AsA content of pepper leaves was estimated by a modified procedure following a method by Luwe et al. (1993). Initially, pepper leaf samples (0.5 g) were crushed in liquid nitrogen by mortar and pestle. They were homogenized in ice-cold trichloroacetic acid (TCA, 1% w/v). Then, the solution was centrifuged at 12,000 rpm for 20 min at a temperature of 4 °C. This was followed by adding 50 µL potassium phosphate buffer mixture (0.95 ml, 100 mm, pH 7.0) and ascorbate oxidase (1 μl of 1 μl−1 unit) to the supernatant. Eventually, the absorbance was read at 265 nm.
Enzyme extraction method
To begin, 0.5 g of Pepper leaves were added to 5 ml of 100 mM phosphate buffer (pH 7.8) containing 5% w/v PVP and 1 ml EDTA in a mortar kept on ice bath to be homogeneous. Then, it was centrifuged at 10,000 g for 30 min at 4 °C and assayed the activity of antioxidant enzymes (Zhang et al. 2009).
Determination of catalase (CAT) enzyme activity
Catalase activity was assessed by Dhindsa et al. (1981) method. For this purpose, 3 ml of reaction mixture contained 0.1 ml of herbal extract, 15 mM phosphate buffer (pH 7.0) and 15 mM H2O2. The reaction was initiated by adding herbal extract. The absorbance changes of the solution were read at 240 nm every 40 s. One unit of catalase enzyme activity was expressed as the amount of enzyme essential to reduce the absorption unit of 0.1 with an optical density of 240 nm min−1.
Estimation of SOD enzyme activity
The SOD activity was evaluated by the Xu et al. (2008) method with some modification. In practice, 1000 µl of enzymatic extracts with 2.465 ml of 55 mM methionine, 100 mM of phosphate buffer (pH 7.8), 300 ml nitrobluetetrasolium 0.75 mM and 60 µl of 0.1 mM riboflavin were placed in a test tube and incubated in fluorescence light (40 µmol m−2 s−1) at 10 min. The absorbing solution read to 560 nm by a UV/visible spectrometer. One unit of SOD enzyme is determined as the amount of enzyme that inhibits 50% of photoreduction NBT.
Estimation of POD enzyme activity
POD activity was measured by the Zhou and Leul (1998) method. The reaction mixture contained 50 ml of enzyme extract, 0.4% H2O2, potassium phosphate buffer to pH 6.1 and 1% guaiacol. The absorbance was read at 470 nm. The activity of the enzyme was expressed as min−1 g−1fresh at 25 ± 2 °C.
Statistical analysis
In this experiment, all data were analyzed by SPSS software 18.1 and Duncan’s multiple range tests. The difference was considered P < 0.05. The analyses were carried out to determine significant differences between the means at a significance level of P < 0.05. Pearson’s correlation between different concentrations of AsA and various levels of drought stress were calculated by SPSS software.
Results
Plant height
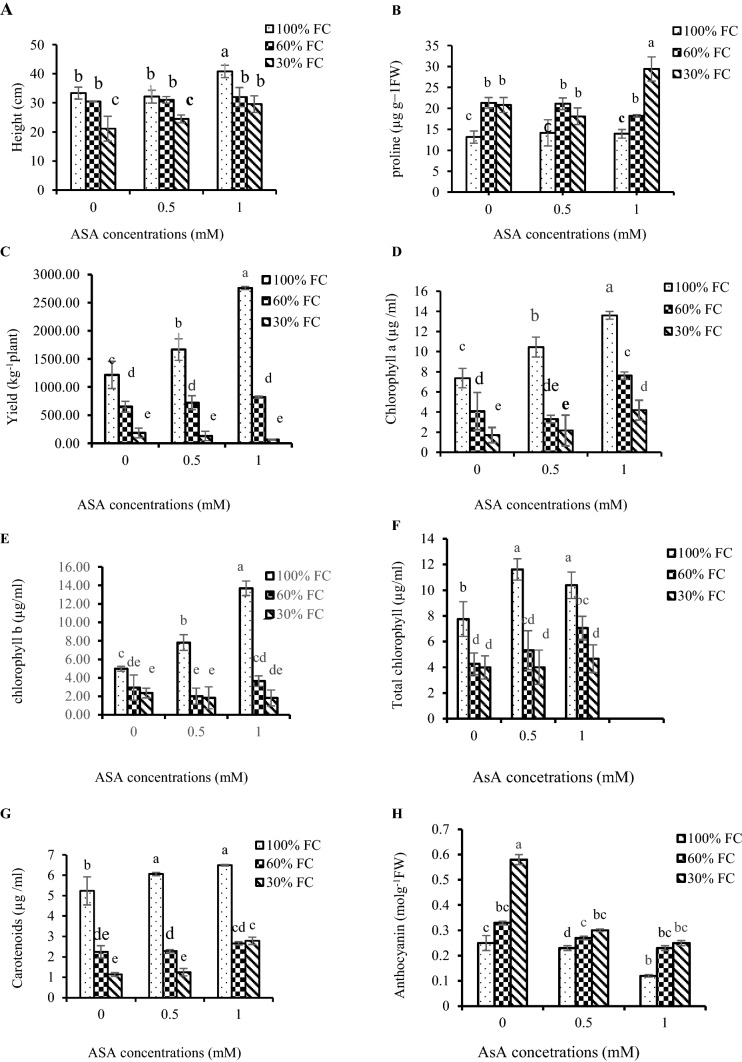
Drought stress and ascorbic acid treatments have affected plant height of pepper significantly (Table 2). A significant increase in plant height of pepper plant was observed when exposed to drought stress (Figs. 1, 2a).
Table 2.
Means comparison of ascorbic acid and drought stress effects on morphological parameters of pepper plants
| Treatments | Plant height (cm) | Fruit number | Fruit weight (g) | Total yield (kg/plant) |
|---|---|---|---|---|
| Ascorbic acid | ||||
| Control | 28.312b | 3.33b | 1.39b | 6.65c |
| 0.5 mM | 29.202b | 4.33b | 1.76a | 8.56b |
| 1 mM | 34.088a | 5.88a | 1.80a | 12.14a |
| Drought stress | ||||
| Control | 35.40a | 6.88a | 3.20a | 7.31a |
| 60% | 31.127b | 4.55b | 1.23b | 1.88b |
| 30% | 25.07b | 2.11c | 0.70c | 1.25c |
The same letters in each column indicate no significant difference at the 5% probability level in the Duncan test
Fig. 1.

The effect of three concentrations of ascorbic acid treatments (0, 0.5 and 1 mM) on plant height of pepper
Fig. 2.
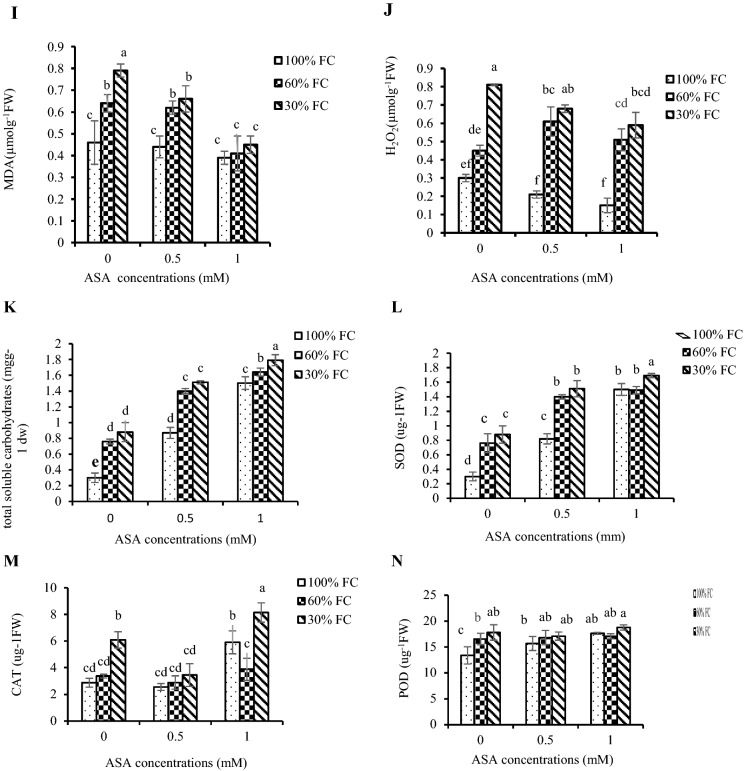
Interaction effects of drought stress and ascorbic acid treatment on a height, b proline, c yield, d chlorophyll a, e chlorophyll b, f total chlorophyll, g carotenoids, h anthocyanin in pepper leaves under drought stress, i MDA, j H2O2, k total soluble carbohydrates and l SOD, m CAT, n POD in pepper leaves under drought stress
Fruit yield
Both drought stress and ascorbic acid treatments have affected on fruits yield of pepper significantly (Table 2). The Ascorbic acid treatment showed that a significantly increased fruit yield of pepper during to drought stress (Fig. 2c).
Fruit yield component
The growth parameters of pepper plants were determined based on weight and number of fruit that showed significant variations due to AsA and drought levels. The pepper plants subjected to drought stress showed significant decrease of all morphological properties such as plant height, number of fruits and yield (Table 2). However, ascorbic acid significantly increased all the above parameters as compared with control plants and/or drought-stressed plants (Table 2).
Leaf chlorophyll and carotenoids content
Chlorophyll a, b, total and carotenoids content of pepper leaves were significantly affected by drought stress and AsA. The obtained data (Table 3) demonstrated that drought stress significantly decreased the content of chlorophyll a, b, total chlorophyll and carotenoids. The ascorbic acid treatment significantly increased the leaf chlorophyll and carotenoids content of pepper plants exposed to severe drought (Table 3). The content of Chlorophyll a, b, total and carotenoid decreased by increasing of drought intensity and increased with raising AsA (Fig. 2d–g).
Table 3.
Mean comparison of ascorbic acid and drought stress effects on photosynthetic pigments of pepper plants
| Treatments | Chlorophyll a (µg/ml) | Chlorophyll b (µg/ml) | Total chlorophyll (µg/ml) | Carotenoids (µg/ml) | Anthocyanin (mol g−1 FW) |
|---|---|---|---|---|---|
| Ascorbic acid | |||||
| Control | 4.39c | 2.60b | 5.69b | 2.88c | 0.39a |
| 0.5 mM | 5.31b | 5.25a | 6.63ab | 3.20b | 0.27b |
| 1 mM | 8.48a | 5.85a | 7.37a | 3.98a | 0.20c |
| Drought stress | |||||
| Control | 10.48a | 7.78a | 8.66a | 5.93a | 0.25c |
| 60% | 5.01b | 3.55b | 6.36b | 2.40b | 0.3ab |
| 30% | 2.69c | 2.37c | 4.69c | 1.73c | 0.31a |
The same letters in each column indicate no significant difference at the 5% probability level in the Duncan test
Anthocyanin content
The content of anthocyanin of plant leaves increased during drought stress. The foliar spray of ascorbic acid resulted in lesser anthocyanin (Table 3). Anthocyanin content significantly increased by increasing drought intensity and decreased with increasing AsA (Fig. 2h).
H2O2 content
The data of this study showed that drought stress effectively increased the leaf H2O2 contents as compared with control plants. Exogenous application of ascorbic acid decreased the H2O2 content in plants exposed to medium and severe drought stressed conditions (Table 4) and H2O2 content were raised with increasing of drought intensity and decreased with enhancing foliar spray AsA (Fig. 2j).
Table 4.
Means comparison of ascorbic acid and drought stress effects on biochemistry and physiological parameters of pepper plants
| Treatments | Proline (µg g−1 FW) | H2O2 (µmolg−1 FW) | MDA (µmol g−1 FW) | Total soluble carbohydrates (mg g−1 DW) | ASA (µmol g−1 FW) | SOD (Ug−1 FW) | POD (Ug−1 FW) | CAT (Ug−1 FW) |
|---|---|---|---|---|---|---|---|---|
| Ascorbic acid | ||||||||
| Control | 17.46c | 0.52a | 0.58a | 0.65c | 19.76c | 0.65c | 15.89b | 2.96c |
| 0.5 mM | 18.43b | 0.50ab | 0.57ab | 1.26b | 43.15b | 1.24b | 16.51b | 4.12b |
| 1 mM | 17.79b | 0.42b | 0.49c | 1.65a | 73.37a | 1.56a | 17.81a | 6a |
| Drought stress | ||||||||
| Control | 13.77c | 0.23c | 0.44c | 0.89c | 37.55c | 0.87c | 15.44c | 3.38b |
| 60% | 20.21b | 0.27a | 0.51b | 1.27b | 54.32a | 1.22b | 16.79a | 3.80b |
| 30% | 22.78a | 0.55b | 0.69a | 1.39c | 44.41b | 1.36a | 17.88a | 5.89a |
The same letters in each column indicate no significant difference at the 5% probability level in the Duncan test
MDA
This study showed that drought stress effectively increased the leaf MDA contents as compared with control plants. Exogenous application of ascorbic acid decreased the MDA content in plants exposed to medium and severe drought stressed conditions (Table 4) and MDA content were raised with increasing of drought intensity and decreased with enhancing foliar spray of AsA (Fig. 2i).
Changes in soluble carbohydrates contents
Drought significantly changed the concentration of soluble carbohydrates in pepper seedling. Soluble carbohydrates contents were increased by drought stress and ascorbic acid application further increased the soluble carbohydrates contents of plants exposed to medium and severe drought conditions (Table 4). In this experiment, with increase in both treatments, the carbohydrate solution was increased (Fig. 2k).
Proline
The results indicated that proline content was affected by drought and AsA foliar application (Table 4). The content of proline in free leaves increased with increasing drought stress in pepper, and its content increased significantly after application of ascorbic acid in leaves (Fig. 2b).
Ascorbic acid
Drought stress significantly increased AsA content in sweet pepper plants when compared with control plants (Table 4). Accordingly, the amount of AsA increased by higher levels of drought stress. The exogenous application of AsA increased the AsA content in the stressed and non-stressed sweet pepper plants (Table 4). The interaction between drought stress and ascorbic acid showed that ascorbic acid content in sweet pepper plants increases in response to higher levels of drought stress and ascorbic acid.
Antioxidant enzyme activities
Drought stress and AsA influenced activities of antioxidant enzymes of pepper plant leaf. The results showed that exogenous application of ascorbic acid and drought stress stimulated antioxidant enzymes activities in pepper leaves (Table 4). The activities of antioxidant enzymes were significantly increased with enhancing of drought stress intensity and ASA (Fig. 2l–n).
Correlation coefficient
Analyzing the coefficients of correlation between different traits involved in pepper production provides informative data about the relative effect of factors on each other. Correlation coefficients between the plant traits could determine whether selection for one trait may affect the other ones. Altogether, positive and negative correlations were found among different traits (Table 5). Meanwhile, correlation coefficient analysis was performed between morphological factors including plant height, fruit number, fruit weight, total yield which resulted in relatively high correlation. On the other hand, a significant positive correlation was obtained between proline, chlorophyll a, anthocyanin, carotenoids and also, there was significant negative correlation with H2O2.
Table 5.
Correlation coefficients (r) among pepper seedlings traits
| Plant height (cm) | Fruit number | Fruit weight (g) | Total yield (kg) | Proline (µg g−1 FW) | Chla (µg/ml) | Chlb (µg/ml) | Chlt (µg/ml) | |
|---|---|---|---|---|---|---|---|---|
| Plant height | 1 | |||||||
| Fruit number | 0.729** | 1 | ||||||
| Fruit weight | 0.747** | 0.816** | 1 | |||||
| Total yield | 0.681** | 0.856** | 0.954** | 1 | ||||
| Proline | 0.609** | 0.647** | 0.750** | 0.759** | 1 | |||
| Chla | 0.792** | 0.854** | 0.899** | 0.888** | 0.716** | 1 | ||
| Chlb | 0.148 | 0.28 | 0.496** | 0.496** | 0.158 | 0.382* | 1 | |
| Chlt | 0.204 | 0.315 | 0.416* | 0.408* | 0.147 | 0.359 | 0.899** | 1 |
| Carotenoid | 0.760** | 0.783** | 0.934** | 0.919** | 0.703** | 0.903** | 0.545** | 0.480* |
| Anthocyanin | 0.450 | 0.277 | 0.239 | 0.185 | 0.489** | 0.331 | − 0.568** | − 0.566** |
| MDA | − 0.167 | − 0.126 | − 0.344 | − 0.284 | − 0.291 | − 0.288 | − 0.140 | − 0.499** |
| CAT | − 0.139 | − 0.178 | − 0.269 | − 0.253 | − 0.008 | − 0.119 | − 0.427* | − 0.431* |
| POD | − 0.229 | − 0.264 | − 0.375 | − 0.337 | − 0.107 | − 0.200 | − 0.515** | − 0.630** |
| SOD | 0.051 | 0.009 | − 0.258 | − 0.265 | − 0.132 | − 0.025 | − 0.623** | − 0.626** |
| Total soluble | 0.059 | 0.025 | − 0.269 | − 0.267 | − 0.151 | − 0.010 | − 0.589** | − 0.573** |
| Carbohydrates | ||||||||
| H2O2 | − 0.759** | − 0.690** | − 0.834** | − 0.719** | − 0.819** | − 0.487** | − 0.448* | − 0.902** |
| Carotenoid (µg/ml) | Anthocyanin (mol g−1 FW) | MAD (µmol g−1 FW) | CAT (µg−1 FW) | POD (µg−1 FW) | SOD (µg−1 FW) | Total soluble carbohydrates (mg g−1 DW) | H2O2 (µmol g−1 FW) | |
|---|---|---|---|---|---|---|---|---|
| Plant height | ||||||||
| Fruit number | ||||||||
| Fruit weight | ||||||||
| Total yield | ||||||||
| Proline | ||||||||
| Chla | ||||||||
| Chlb | ||||||||
| Chlt | ||||||||
| Carotenoid | 1 | |||||||
| Anthocyanin | 0.218 | 1 | ||||||
| MDA | − 0.052 | − 0.52 | 1 | |||||
| CAT | − 0.115 | 0.499** | − 0.269 | 1 | ||||
| POD | − 0.353 | 0.340 | 0.194 | 0.504** | 1 | |||
| SOD | − 0.245 | 0.387* | 0.097 | 0.350 | 0.683** | 1 | ||
| Total soluble | − 0.234 | 0.339 | 0.116 | 0.357 | 0.688** | 0.988** | 1 | |
| Carbohydrates | ||||||||
| H2O2 | − 0.238 | 0.480* | 0.111 | 0.300 | 0.226 | 0.227 | 0.227 | 1 |
**High significant (1% level of probability)
Meantime, we found positive main correlations between chlorophyll a, with chlorophyll b, carotenoids and as well as significant negative correlation with H2O2.
In addition, chlorophyll b with total chlorophyll, carotenoids had a significant positive correlation and with hydrogen peroxide, anthocyanin, total soluble carbohydrates, CAT, POD, SOD, had a significant negative correlation. Also, total chlorophyll with carotenoids had significant positive correlation and this factor had significant negative correlation with hydrogen peroxide, anthocyanin, MDA, total soluble carbohydrates, CAT, POD, SOD and ultimately among enzymes, CAT enzyme with POD and SOD enzyme with total soluble carbohydrates and POD enzyme with SOD, total soluble carbohydrates significant positive correlations.
Discussion
Drought is one of the most important environmental stresses that inhibit the growth of the product and cause crop yield limitations of the product (Liu et al. 2016). Due to the osmotic effect of drought, it can be said that drought causes different responses in the cell such as inhibition of growth and synthesis of some non-toxic compounds, which are used to increase osmotic potential and metabolic processes, and ultimately increases the activity of some antioxidant enzymes (Turkan et al. 2005). Considering that the role of AsA in drought stress conditions in pepper plants is not known, therefore, it was decided in this research to investigate the physiological, biochemical and metabolic effects of ascorbic acid on pepper seedlings under drought stress conditions (Tables 2, 3 and 4).
AsA is a water-soluble molecule and is well known as an antioxidant that helps to detoxify active oxygen in cell (Liu et al. 2014). Ascorbic acid spray reduces the effects of drought stress in plants such as closure of the stomata, absorbing nutrients, total chlorophyll, protein synthesis, transfusion, the process of photosynthesis and growth of plant (Hafez and Gharib 2016).
Drought stress can be assessed by its impacts on fruit morphological properties, plant height, fruit yield, leaf chlorophyll and carotenoids content and increase of MDA, H2O2, soluble carbohydrates, proline, anthocyanin and antioxidant enzymes. In this study, it is found that AsA treatment could increase growth parameters and yield of pepper seedlings under drought stress (Table 2). The results of the recent study have been consistent with previous studies on olive trees as foliar spraying with ascorbic acid had favourable effects on growth characters and yield (El-Sayed et al. 2014).
Ascorbic acid in the pepper seedlings during the metabolic process in the plant is able to control the free radicals produced, which can increase plant resistance to stress and protect the side effects of the active oxygen (El-Sayed et al. 2014). Ragab (2002) also reported that ascorbic acid may be substituted for synthetic auxin. However, due to the effect of auxin, the role of ascorbic acid in plants can be explained. Cell membrane stability is an indicator of cellular damage caused by various biological stresses (Saneoka et al. 2004). Usually, MDA content reflects cell damage (Li et al. 2018) in plants. In the present study, drought stress significantly increased MDA content due to increased lipid peroxidation and cell membrane damage. However, AsA was able to significantly reduce these negative symptoms. By reducing the MDA concentration, AsA protects membranes from drought stress (Table 4). These results showed that AsA plays a role in maintaining cell membrane stability, and this is consistent with the findings for Hibiscus esculentus L. and Oryza sativa L. under drought (Amin et al. 2009; Guo et al. 2005).
The reduction of chlorophyll is common in drought stress conditions (Chen et al. 2016; Javadi et al. 2017), since there are several reports of the reduction of chlorophyll and carotene in environmental stresses (Aghaie et al. 2018; Koffler et al. 2014; Nxele et al. 2017). Reductions in chlorophyll content may be due to damage caused by tension in the biosynthesis of plant pigments or increased destruction pigmentation (Nematpour et al. 2020). Reducing photosynthetic pigments may result in increased synthesis of compatibility solutions, such as proline, because both of them are produced from similar precursors (Le Dily et al. 1993). One of the important antioxidant pigments in carotenoids is that they play a special protective role in stress conditions (Egert and Tevini 2002). Since carotenoid reduction is often associated with the destruction of chlorophyll pigments, it can be suggested that photodegradation and loss of photodegradation may be due to the destruction of carotene (Javadi et al. 2017). Our results are also consistent with the theory that shows the relationship between the photosynthetic pigment concentration: Chla, and total chla, as well as carotenoids in drought stress conditions. The concentration of photosynthetic pigments is strongly reduced under drought stress, although the use of appropriate concentrations of ascorbic acid decreases drought stress (Table 3).
Our result is consistent with the previous reports on wheat, cauliflower and basil where the plants sprayed with ascorbic acid showed significant increase in photosynthetic pigments content compared with control (Athar et al. 2008; Latif et al. 2016; Khalil et al. 2010).
Many drought-tolerant plant species contain anthocyanins which are believed to act as osmoregulators under drought stress (Chalker-Scott 1999), therefore, plant tissues that usually contain anthocyanins are resistant to drought stress (Sherwin and Farrant 1998). For example, in a study, drought resistance of purple cultivar is better than that of green cultivar (Bahler et al.1991). Anthocyanins increase the drought resistance in plants through potential water stability. In this case, anthocyanins are assumed to interfere with the osmotic regulation of the plants (Choinski and Johnson 1993; Chalker-Scott 2002). In present study, the results showed that drought subjected pepper seedlings, contained higher anthocyanins. On the other hand, when ascorbate was applied, anthocyanins content was reduced (Table 4). The results of this study are consistent with the findings of Halimeh et al. (2013) in Dracocephalum moldavica under drought stress.
Low molecular organic compounds, such as soluble carbohydrates, proline and other amino acids, can regulate the osmotic potential of the cell to improve water absorption under drought stress (Esmaeilpour et al. 2016) and protect enzymes, biological membranes and photosynthetic apparatus against oxidative damage (Anjum et al. 2012).
Recent study showed that pepper seedlings responded to drought exposure with accumulation of proline and soluble carbohydrates (Table 4) and this achievement agrees with previous reports on rice (Guo et al. 2005). In the present study, soluble carbohydrates and proline in plants under both treatments were higher than controls (Table 4). During oxidative stress, the accumulation of proline content with ascorbic acid treatment was increased and enhanced resistance against losing leaf water and plant growth rate under stress conditions occurred (Tasgin et al. 2003; Yazdanpana et al. 2011).
Active oxygen species such as H2O2, superoxide and singlet oxygen are produced due to water shortages in plants and have harmful effects on cell membrane stability and permeability (Ashraf et al. 2011). There are many reports that ascorbic acid is a free radical scavenger (Gill and Tuteja 2010). Due to lack of water, reactive oxygen species (ROS) are formed, resulting in damage to plants. The main source of ROS is the chloroplast of the plant cells, which causes the change in electron transfer redox and ultimately leads to the formation of oxygen species (Aghaie et al. 2018). Similar results as the elevation of H2O2 in pepper leaves under drought stress in comparison to control were recorded and application of AsA treatments reduced H2O2 in pepper leaves in drought-stressed plants (Table 4).
One of the general strategies for neutralizing the toxin in the plant is the use of several enzymatic and non-enzymatic methods that protect plants from damage caused by ROS (Sairam and Saxena 2000), including the superoxide dismutase enzyme (SOD) that converts superoxide to H2O2, catalase and peroxidase enzymes which also reduces H2O2 to H2O and O2 (Kadkhodaie et al. 2013; Anjum et al. 2012). Therefore, detoxification of the enzyme in the plant is related almost to the activity of the SOD enzyme and then to the stimulation of other antioxidant enzymes (Alscher et al. 2002). In this study, the magnitude of the activity of the SOD enzyme in chilli peptides has been increased gradually, with the previous reports of increased activity of this enzyme in drought stress conditions in tomato (Tahi et al. 2008, Aghaie et al. 2018) and wheat (Csiszar 2005).
CAT enzyme is a key enzyme in the glutathione-ascorbate cycle, which plays an important role in eliminating H2O2, which is caused by the SOD enzyme in various cell sections. The results indicate that the activity of the enzyme CAT also had similar changes in the activity of the SOD enzyme in pepper seedlings (Table 4). According to available evidence, these enzymes substantially detoxify H2O2 (Table 4). Ren et al. (2016) suggested that when plants are affected by drought stress, the catalase enzyme is responsible for the decomposition of H2O2. When seedlings of pepper treated with AsA and drought, increased activity of SOD, CAT and POD enzymes, it was showed that AsA increased the activity of these three enzymes under drought stress (Table 4). In this study, it can be concluded that with increasing of AsA, the activity of SOD, CAT and POD enzymes and H2O2 levels in pepper leaves increased and decreased, respectively. Therefore, the use of foreign ascorbic acid can keep plants resistant to drought by eliminating active oxygen species (Table 4). According to studies conducted in the past show use of AsA increases the activity of enzymatic and non-enzymatic antioxidants to counteract the harmful effects of various environmental stresses (Hafez and Gharib 2016; Bai et al. 2013).
Conclusions
Statistical analysis of this study showed that drought stress increased ROS levels and had negative effects on plant growth factors, however, when drought stress was applied with AsA, plant growth was improved and ROS levels decreased. Drought stress increased the activity of SOD, CAT, POD enzymes and soluble carotenoids and carbohydrates in pepper seedlings, but the effect of both AsA treatments and drought increased the activity of antioxidant enzymes compared to drought stress alone. Therefore, by increasing the activity of antioxidant enzymes, it can stimulate tolerance to drought stress in pepper.
Acknowledgements
We gratefully thank the University of Ilam for financial support.
Funding
This study was financially supported by University of Ilam.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aghaie P, Tafreshi SAH, Ebrahimi MA, Haerinasab M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci Hortic. 2018;232:1–12. doi: 10.1016/j.scienta.2017.12.041. [DOI] [Google Scholar]
- Ahmed AM. Studies for controlling malformation and improving yield and fruit quality of Hindy Bisinnara mangoes by using active dry yeast, ascorbic acid and sulphur. Minia J Agric Res Dev (Egypt) 2001;21(2):219–233. [Google Scholar]
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53(372):1331–1341. doi: 10.1093/jxb/53.372.1331. [DOI] [PubMed] [Google Scholar]
- Amin B, Mahleghah G, Mahmood HMR, Hossein M. Evaluation of interaction effect of drought stress with ascorbate and salicylic acid on some of physiological and biochemical parameters okra (Hibiscus esculentus L.) Res J Biol Sci. 2009;4(4):380–387. [Google Scholar]
- Anjum SA, Farooq M, Xie XY, Liu XJ, Ijaz MF. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci Hortic. 2012;140:66–73. doi: 10.1016/j.scienta.2012.03.028. [DOI] [Google Scholar]
- Arafa AA, Khafagy MA, El-Banna MF. Role of glycinebetaine and ascorbic acid in the alleviation of salt-stress induced micro-morphological damages in sweet pepper seedlings. J Biol Sci. 2007;7(6):879–887. doi: 10.3923/jbs.2007.879.887. [DOI] [Google Scholar]
- Aroca R. Exogenous catalase and ascorbate modify the effects of abscisic acid (ABA) on root hydraulic properties in Phaseolus vulgaris L. plants. J Plant Growth Regul. 2006;25(1):10–17. doi: 10.1007/s00344-005-0075-1. [DOI] [Google Scholar]
- Ashraf M, Akram NA, Al-Qurainy F, Foolad MR. Drought tolerance: roles of organic osmolytes, growth regulators, and mineral nutrients. In: Sparks DL, editor. Advances in agronomy. Cambridge: Academic Press; 2011. pp. 249–296. [Google Scholar]
- Athar HUR, Khan A, Ashraf M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp. 2008;63(1–3):224–231. doi: 10.1016/j.envexpbot.2007.10.018. [DOI] [Google Scholar]
- Badour SSA (1959) Analytically chemical investigation of the Kaliummangles in chlorella in comparison with other mangelezustanden. Doctoral dissertation, University of Goettingen
- Bahler BD, Steffen KL, Orzolek MD. Morphological and biochemical comparison of a purple-leafed and a green-leafed pepper cultivar. HortScience. 1991;26(6):736. [Google Scholar]
- Bai T, Ma P, Li C, Yin R, Ma F. Role of ascorbic acid in enhancing hypoxia tolerance in roots of sensitive and tolerant apple rootstocks. Sci Hortic. 2013;164:372–379. doi: 10.1016/j.scienta.2013.10.003. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. J Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Campos H, Trejo C, Peña-Valdivia CB, García-Nava R, Conde-Martínez FV, Cruz-Ortega MR. Stomatal and non-stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: delayed restoration of photosynthesis during recovery. Environ Exp. 2014;98:56–64. doi: 10.1016/j.envexpbot.2013.10.015. [DOI] [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol. 1999;70(1):1–9. doi: 10.1111/j.1751-1097.1999.tb01944.x. [DOI] [Google Scholar]
- Chalker-Scott L. Do anthocyanins function as osmoregulators in leaf tissues? Adv Bot Res. 2002;37:103–106. doi: 10.1016/S0065-2296(02)37046-0. [DOI] [Google Scholar]
- Chen YE, Liu WJ, Su YQ, Cui JM, Zhang ZW, Yuan M, Zhang HY, Shu Y. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol Plant. 2016;158(2):225–235. doi: 10.1111/ppl.12438. [DOI] [PubMed] [Google Scholar]
- Choinski JS, Jr, Johnson JM. Changes in photosynthesis and water status of developing leaves of Brachystegia spiciformis Benth. Tree Physiol. 1993;13(1):17–27. doi: 10.1093/treephys/13.1.17. [DOI] [PubMed] [Google Scholar]
- Csiszar J. Effect of osmotic stress on antioxidant enzyme activities in transgenic wheat calli bearing MsALR gene. Acta Biol. 2005;49(1–2):49–50. doi: 10.1631/jzus.2007.B0458. [DOI] [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32(1):93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- Ding L, Gao C, Li Y, Li Y, Zhu Y, Xu G, Shen Q, Kaldenhoff R, Kai L, Guo S. The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP) Plant Sci. 2015;234:14–21. doi: 10.1016/j.plantsci.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Dolatabadian A, Sanavy SM, Chashmi NA. The effects of foliar application of ascorbic acid (vitamin C) on antioxidant enzymes activities, lipid peroxidation and proline accumulation of canola (Brassica napus L.) under conditions of salt stress. J Agron Crop Sci. 2008;194(3):206–213. doi: 10.1111/j.1439-037X.2008.00301.x. [DOI] [Google Scholar]
- Egert M, Tevini M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum) Environ Exp. 2002;48(1):43–49. doi: 10.1016/S0098-8472(02)00008-4. [DOI] [Google Scholar]
- El-Sayed MA, Ahmed A, Ali AH (2000) Responses of “flame seedless” grapevine to application of ascorbic acid. In: 2nd conference on agricultural science, Assiut, Egypt. Iowa, USA, pp 317–340
- El-Sayed OM, El-Gammal OHM, Salama ASM. Effect of ascorbic acid, proline and jasmonic acid foliar spraying on fruit set and yield of Manzanillo olive trees under salt stress. Sci Hortic. 2014;176:32–37. doi: 10.1016/j.scienta.2014.05.031. [DOI] [Google Scholar]
- Esmaeilpour A, Van Labeke MC, Samson R, Boeckx P, Van Damme P. Variation in biochemical characteristics, water status, stomata features, leaf carbon isotope composition and its relationship to water use efficiency in pistachio (Pistacia vera L.) cultivars under drought stress condition. Sci Hortic. 2016;211:158–166. doi: 10.1016/j.scienta.2016.08.026. [DOI] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Guo Z, Tan H, Zhu Z, Lu S, Zhou B. Effect of intermediates on ascorbic acid and oxalate biosynthesis of rice and in relation to its stress resistance. Plant Physiol Biochem. 2005;43(10–11):955–962. doi: 10.1016/j.plaphy.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hafez EM, Gharib HS. Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Int J Plant Prod. 2016;10(4):579–596. doi: 10.22069/IJPP.2016.3051. [DOI] [Google Scholar]
- Halimeh R, Mahlagh G, Maryam P, Pazoki A. Effect of drought interactions with ascorbate on some biochemical parameters and antioxidant enzymes activities in Dracocephalum moldavica L. Middle East J Sci Res. 2013;13(4):522–531. doi: 10.5829/idosi.wasj.2013.27.07.126. [DOI] [Google Scholar]
- Hwang IG, Shin YJ, Lee S, Lee J, Yoo SM. Effects of different cooking methods on the antioxidant properties of red pepper (Capsicum annuum L.) Prev Nutr Food Sci. 2012;17(4):286. doi: 10.3746/pnf.2012.17.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi T, Rohollahi D, Ghaderi N, Nazari F. Mitigating the adverse effects of drought stress on the morpho-physiological traits and anti-oxidative enzyme activities of Prunus avium through β-amino butyric acid drenching. Sci Hortic. 2017;218:156–163. doi: 10.1016/j.scienta.2017.02.019. [DOI] [Google Scholar]
- Kadkhodaie A, Razmjoo J, Zahedi M. Peroxidase, ascorbate peroxidase and catalase activities in drought sensitive, intermediate and resistance sesame (Sesamum indicum L.) genotypes. Int J Agron Plant Prod. 2013;4(11):3012–3021. [Google Scholar]
- Khalil SE, Abd El- Aziz NG, Abou-Leila BH. Effect of water stress and ascorbic acid and spraying time on some morphological and biochemical composition of Ocimum basilicum plant. Am J Sci. 2010;6(12):33–44. [Google Scholar]
- Koffler BE, Luschin-Ebengreuth N, Stabentheiner E, Müller M, Zechmann B. Compartment specific response of antioxidants to drought stress in Arabidopsis. Plant Sci. 2014;227:133–144. doi: 10.1016/j.plantsci.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif M, Akram NA, Ashraf M. Regulation of some biochemical attributes in drought-stressed cauliflower (Brassica oleracea L.) by seed pre-treatment with ascorbic acid. J Hortic Sci Biotechnol. 2016;91(2):129–137. doi: 10.1080/14620316.2015.1117226. [DOI] [Google Scholar]
- Le Dily F, Billard JP, Saos JLE, Huault C. Effect of NaCl and gabaculine on chlorophyll and proline levels during growth of radish cotyledons. Plant Physiol Biochem. 1993;31:303–310. [Google Scholar]
- Li J, Arkorful E, Cheng S, Zhou Q, Li H, Chen X, Sun K, Li X. Alleviation of cold damage by exogenous application of melatonin in vegetatively propagated tea plant (Camellia sinensis (L.) O. Kuntze) Sci Hortic. 2018;238:356–362. doi: 10.1016/j.scienta.2018.04.068. [DOI] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Wilchek M, Bayer EA, editors. Methods in enzymology. Cambridge: Academic Press; 1987. pp. 350–382. [Google Scholar]
- Liu C, Liu Y, Guo K, Fan D, Li G, Zheng Y, Yuc L, Yang R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ Exp Bot. 2011;71(2):174–183. doi: 10.1016/j.envexpbot.2010.11.012. [DOI] [Google Scholar]
- Liu K, Yuan C, Chen Y, Li H, Liu J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci Hortic. 2014;176:45–53. doi: 10.1016/j.scienta.2014.06.027. [DOI] [Google Scholar]
- Liu Y, Liang H, Lv X, Liu D, Wen X, Liao Y. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol Biochem. 2016;100:113–129. doi: 10.1016/j.plaphy.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Luwe M, Takahama U, Heber U. Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves. Plant Physiol. 1993;101:969–976. doi: 10.1104/pp.101.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksoud MA, Saleh MA, El-Shamma MS, Fouad AA. The beneficial effect of biofertilizers and antioxidants on olive trees under calcareous soil conditions. World J Agric Res. 2009;5(3):350–352. [Google Scholar]
- Mekki BED, Hussien HA, Salem H. Role of glutathione, ascorbic acid and α-tocopherol in alleviation of drought stress in cotton plants. Int J ChemTech Res. 2015;8(4):1573–1581. [Google Scholar]
- Mohammadi M, Tavakoli A, Pouryousef M, Fard EM. Study the effect of 24-epibrassinolide application on the Cu/Zn-SOD expression and tolerance to drought stress in common bean. Physiol Mol Biol Plants. 2020;31:1–6. doi: 10.1007/s12298-020-00757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa EAM. Effect of spraying with ascorbic acid, vitamin B and active dry yeast on growth, flowering, leaf mineral status, yield and fruit quality of Grand Nain banana plants. Ann Agric Sci. 2004;49(2):643–659. [Google Scholar]
- Nematpour A, Eshghizadeh HR, Zahedi M, Gheysari M. Interactive effects of sowing date and nitrogen fertilizer on water and nitrogen use efficiency in millet cultivars under drought stress. J Plant Nutr. 2020;43(1):122–137. doi: 10.1080/01904167.2019.1659351. [DOI] [Google Scholar]
- Noman A, Ali S, Naheed F, Ali Q, Farid M, Rizwan M, Irshad MK. Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch Agron Soil Sci. 2015;61(12):1659–1672. doi: 10.1080/03650340.2015.1028379. [DOI] [Google Scholar]
- Nxele X, Klein A, Ndimba BK. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S Afr J Bot. 2017;108:261–266. doi: 10.1016/j.sajb.2016.11.003. [DOI] [Google Scholar]
- Penella C, Nebauer SG, San Bautista A, Lopez-Galarza S, Calatayud Á. Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: physiological responses. J Plant Physiol. 2014;171(10):842–851. doi: 10.1016/j.jplph.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Ragab MM (2002) Effect of spraying urea, ascorbic acid and NAA on fruiting of Washington Navel orange trees. Doctoral dissertation, University of Minia
- Rasheed R, Yasmeen H, Hussain I, Iqbal M, Ashraf MA, Parveen A. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol Mol Biol Plants. 2020;4:1. doi: 10.1007/s12298-019-00756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Sun LN, Zhang QY, Song XS. Drought tolerance is correlated with the activity of antioxidant enzymes in Cerasus humilis seedlings. Int Biomed Res. 2016 doi: 10.1155/2016/9851095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam RK, Saxena DC. Oxidative stress and antioxidants in wheat genotypes: possible mechanism of water stress tolerance. J Agron Crop Sci. 2000;184(1):55–61. doi: 10.1046/j.1439-037x.2000.00358.x. [DOI] [Google Scholar]
- Sakaldas M, Kaynas K. Biochemical and quality parameters changes of green sweet bell peppers as affected by different postharvest treatments. Afr J Biotechnol. 2010;9(48):8174–8181. doi: 10.5897/AJB10.1021. [DOI] [Google Scholar]
- Saneoka H, Moghaieb RE, Premachandra GS, Fujita K. Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environ Exp Bot. 2004;52(2):131–138. doi: 10.1016/j.envexpbot.2004.01.011. [DOI] [Google Scholar]
- Sherwin HW, Farrant JM. Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul. 1998;24(3):203–210. doi: 10.1023/A:1005801610891. [DOI] [Google Scholar]
- Sun J, Gu J, Zeng J, Han S, Song A, Chen F, Fang W, Jiang J, Chen S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci Hortic. 2013;161:249–258. doi: 10.1016/j.scienta.2013.07.015. [DOI] [Google Scholar]
- Tahi H, Wahbi S, El Modafar C, Aganchich A, Serraj R. Changes in antioxidant activities and phenol content in tomato plants subjected to partial root drying and regulated deficit irrigation. Plant Biosyst. 2008;142(3):550–562. doi: 10.1080/11263500802410900. [DOI] [Google Scholar]
- Taşgin E, Atici O, Nalbantoglu B. Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul. 2003;41(3):231–236. doi: 10.1023/B:GROW.0000007504.41476.c2. [DOI] [Google Scholar]
- Turkan I, Bor M, Ozdemir F, Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005;168(1):223–231. doi: 10.1016/j.plantsci.2004.07.032. [DOI] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- Wagner GJ. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassel AH, Abd El Hameed A, Gobara A, Attia M. Effect of some micronutrients, gibberllic acid and ascorbic acid on growth, yield and quality of white banaty seedless grapevines. Afr Crop Sci J. 2007;8:547–553. [Google Scholar]
- Xu PL, Guo YK, Bai JG, Shang WX. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol Plant. 2008;132(4):467–478. doi: 10.1111/j.1399-3054.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Yasuor H, Firer M, Beit-Yannai E. Protective structures and manganese amendments effects on antioxidant activity in pepper fruit. Sci Hortic. 2015;185:211–218. doi: 10.1016/j.scienta.2015.01.034. [DOI] [Google Scholar]
- Yazdanpanah S, Baghizadeh A, Abbassi F. The interaction between drought stress and salicylic and ascorbic acids on some biochemical characteristics of Satureja hortensis. Afr J Agric Res. 2011;6(4):798–807. doi: 10.5897/AJAR10.405. [DOI] [Google Scholar]
- Yousef ARM, Ayad HS, Saleh MMS. The beneficial effect of spraying some antioxidant vitamins on fruit quality, oil composition and improving oil characteristics of Picaul olive. World J Agric Res. 2009;5(S):871–880. [Google Scholar]
- Zhang W, Jiang B, Li W, Song H, Yu Y, Chen J. Polyamines enhance chilling tolerance of cucumber (Cucumis sativus L.) through modulating antioxidative system. Sci Hortic. 2009;122(2):200–208. doi: 10.1016/j.scienta.2009.05.013. [DOI] [Google Scholar]
- Zhao GQ, Zhao Q, Zhou X, Mattei MG, De Crombrugghe B. TFEC, a basic helix-loop-helix protein, forms heterodimers with TFE3 and inhibits TFE3-dependent transcription activation. Mol Cell Biol. 1993;13(8):4505–4512. doi: 10.1128/MCB.13.8.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Leul M. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul. 1998;26(1):41–47. doi: 10.1023/A:1006004921265. [DOI] [Google Scholar]
- Zhou X, Gu Z, Xu H, Chen L, Tao G, Yu Y, Li K. The effects of exogenous ascorbic acid on the mechanism of physiological and biochemical responses to nitrate uptake in two rice cultivars (Oryza sativa L.) under aluminum stress. Plant Growth Regul. 2016;35(4):1013–1024. doi: 10.1007/s00344-016-9599-9. [DOI] [Google Scholar]