Abstract
Salt stress is a major abiotic stress causing adverse effects on plant growth and development. The aim of this study was to investigate the effect of NaCl stress on growth, stress indicator parameters (lipid peroxidation, chlorophyll content and proline content), yield, and the expression of heat shock proteins genes (Hsp17.8, Hsp26.3, Hsp70 and Hsp101) of five Jordanian durum wheat (Triticum durum) landraces. Plants were irrigated with tap water as control or 200 mM NaCl. Significant differences among the 5 Triticum durum landraces in terms of growth parameters, stress indicator parameters, and expression of heat shock proteins genes were observed. Salt stressed landraces demonstrated decreased growth, increased levels of stress indicator parameters, and upregulation in Hsp17.8, Hsp26.3, Hsp70 and Hsp101 expression. Landraces T11 and M23 showed the highest growth, lowest levels of stress indicator parameters, and high expression of heat shock protein genes under NaCl stress. Whereas, J2 and A8 landraces showed the lowest growth, highest levels of stress indicator parameters and low expression of heat shock protein genes under NaCl stress. In conclusion, NaCl stress caused significant reduction in growth parameters, increased level of lipid peroxidation and proline content and upregulation in heat shock proteins gene expression levels. Growth, stress indicator parameters and gene expression results suggest that T11 and M23 landraces are the most NaCl stress tolerant landraces and could be used to enhance the gene pool in wheat breeding programs.
Keywords: Triticum durum, Salinity, HSP, Expression, Proline
Introduction
Plants are frequently exposed to variety of stresses in both natural and agricultural settings, adversely affecting their homeostasis, development and productivity (Mahajan and Tuteja 2005; Munns and Tester 2008; Munns and Gilliham 2015; Al Khateeb et al. 2017). It has been reported that about one-third of the world’s irrigated arable land is influenced by salinity (Deikman et al. 2012). In agricultural lands, salt stress is partly attributed to the accumulation of salts from irrigation water; however, evaporation and transpiration play roles in increasing soil salts concentration in terrestrial habitats (Yadav et al. 2011). Salt accumulation can reach levels damaging to plants, particularly for salt-sensitive species. Generally, salt stress affects plants in two phases. In the first phase, it causes osmotic stress that reduces water extractability, leading to growth retardation (Sudhakar et al. 2001; Abogadallah 2010). In the second phase, harmful levels of ions accumulate in plant cells, resulting in toxicity through enzyme inactivation, inhibition of protein synthesis, and disruption of the plasma membrane (Cicerali 2004; Munns and Tester 2008; Wallender and Tanji, 2012). The most significant and common ions that contribute to soil salinity are Na+ and Cl− (Yadav et al. 2011). Jordan is one of the water-short countries with a Mediterranean climate of long, dry and hot summers and mild wet winters (Black 2009). Consequently, the possibility of salt accumulation in agricultural lands and salt injury to crops exists, and this is currently a matter of concern (Rizhsky et al. 2002; Mittler 2006).
Plants have evolved multiple well-understood strategies to respond or adapt to salinity, thereby reducing salt injury. These include delayed germination or maturity, alterations in photosynthesis, build up of compatible solutes (osmolytes), changes in the membrane structure, induction of antioxidant enzymes, selective accumulation or exclusion of salt ions, control of ion uptake by roots and transport into leaves, and stimulation of hormones (Mitra 2001; Parida and Das 2005; Türkan and Demiral 2009; Yadav et al. 2011).
Heat shock proteins (HSPs), are ubiquitous group of conserved proteins, principally produced in response to a sudden rise in temperature (Al-Whaibi 2011), and are now known to be induced under a variety of environmental stresses. Five major families of HSPs, HSP100, HSP90, HSP70, HSP60, and small HSP (sHSP) have been discerned based on their approximate molecular weights in plant and animal cells (Vierling 1991; Boston et al. 1996; Bharti and Nover 2002; Wang et al. 2004; Kotak et al. 2007; Park and Seo 2015). Generally, HSPs function in cellular homeostasis in both prokaryotic and eukaryotic cells (Lindquist and Craig 1988; Wang et al. 2004). They help organisms withstand stress by acting as molecular chaperones, helping to prevent protein misfolding, and achieve proper folding of misfolded proteins. Consequently, this facilitates cell functioning and survival under stress (Sitia and Braakman 2003; Wang et al. 2004; Swindell et al. 2007; Hüttner and Strasser 2012). Prior studies suggested an involvement of HSPs in osmoprotection, ions transportation, scavenging of free-radicals, and embryogenesis (Wang et al. 2003). A recent study found that Triticum aestivum HSP23.9 planys an important role in plant respons to salt and heat stress. Furthermore, they observed a significant increase in T. aestivum HSP23.9 expression under these stresses (Wang et al. 2020). Another study, found an upregulation in T. aestivum HSP17.4, HSP17.7 and HSP19.1 genes under salt stress (Muthusamy et al. 2017).
Durum wheat (Triticum durum) is one of the foremost cereal crops that nourish the rising global population (Vasil 2007; Peng et al. 2011; Distelfed et al. 2014). It is an important staple food for over half of the world population in terms of total yield and calories produced. Durum wheat, although native to the Mediterranean region and South West Asia and grown mainly in arid and semi-arid regions, is now cultivated in more than 2.2 million km2 of land worldwide (Vasil 2007; Shiferaw et al. 2013; Distelfed et al. 2014; Hiei et al. 2014; Jones 2015; Yi et al. 2015). A significant drop in growth and yield of durum wheat under salt stress has been underscored in the literature, making the growing demand for this crop more challenging (Chen et al. 2007). Hence, research attempts have been undertaken to examine responses and tolerance of many wheat landraces to NaCl stress, a research theme of international concern (Lobell et al. 2011).
In this study, we measure expression of HSP genes in an attempt to understand the possible associations with NaCl stress in durum wheat and examine their role in plant response and tolerance. Growth, stress indicator parameters and HSP gene expression of salt-stressed landraces of T. durum were assessed aiming to recognize landrace(s) that can be successfully used by Jordanian farmers on a large scale, despite the extreme changes in weather.
Materials and methods
Plant materials
Five T. durum landraces (T11, J2, M23, R15 and D4) were collected from different regions in Jordan. Two experimental approaches were used. First, seeds of each landrace were grown under NaCl stress in Petri dishes. Secondly, seeds were grown in soil and irrigated with 200 mM solution of NaCl.
Petri dish experiments
Seeds were surface sterilized in 70% (v/v) ethanol containing 0.05% (v/v) Tween-20 three times; 5 min for the first time and 10 min for the second time, followed by washing with 70% (v/v) ethanol for 2 min. Finally, seeds were washed with sterile distilled water for three times (30 s each time). Then, seeds were divided into two groups and grown in sterile Petri dishes (each Petri dish contains 20 seeds of one landrace and for each landrace 4 replicates were used per salinity level.). The first group was moistened with distilled water (control group), and the second with 200 mM NaCl (treated group). Seeds were incubated in a growth chamber at 23 °C ± 1 °C and 16/8 h light/dark photoperiod using completely randomized design (CRD). On the 5th day, germination percentage was quantified as follows:
Phenotypic data including lengths of shoots and roots were assessed on the 7th day following planting. Similarly, stress indicator parameters (proline content and lipid peroxidation (LPO) level) and gene expression levels by RT-PCR were analyzed using 7 day old seedlings.
Greenhouse experiment
Seeds were sown in plastic pots containing 5 kg top soil in Yarmouk University greenhouse. All pots were initially irrigated with tap water for 10 days. Then, the control group was irrigated with tap water and the treated group was irrigated with 200 mM NaCl solution (500 mL for each pot twice a week) for one week. After that, each group was irrigated with the previous solutions but using 700 mL for each pot (twice a week) until the seeds were harvested. Four pots/landrace/salinity level were used, in each pot 3 plants were grown, pots arranged in CRD in the greenhouse.
Biochemical analysis
Proline content assay
Proline content was determined according to the method of Bates et al. (1973) with few modifications. Leaf tissue (0.25 g FW) was harvested and homogenized in 5 mL of 3% aqueous sulfosalicylic acid and the resulting homogenate was filtered through Whatman filter paper. One mL of filtrate was then transferred to a test tube containing 1 mL acid ninhydrin and 1 mL glacial acetic acid and incubated at 100 °C for 1 h. To terminate the reaction, test tubes were transferred into ice and 2 mL of toluene was added and mixed vigorously for 15–20 s. Afterward, test tubes were incubated at room temperature until two distinct phases were formed. The chromophore containing toluene layer was transferred to another test tube and the absorbance was measured photometrically at 520 nm using GENESYS 10S UV–VIS spectrophotometer (Thermo Fisher Scientific, Medison, USA). Proline content was determined using a standard curve in the range of 20–200 mg/mL and calculated on a fresh weight basis.
Chlorophyll content determination
Young leaf tissues (200 mg) was ground and mixed with 80% acetone, the mixture were incubated overnight at room temperature under continuous shaking, then the sample was filtered using filter paper to remove tissue debris, then absorbance was measured at 645 and 663 nm.
Lipid peroxidation content
To assess the level of LPO for all treatments, the product of LPO, malondialdehyde (MDA) was measured following Carmak and Horst’s protocol (1991) with modifications. Leaf tissue (0.25 g FW) for each treatment was independently homogenized in 5 mL of 0.1% trichloroacetic acid (TCA) and the homogenate was centrifuged at 15.000 g for 10 min. One mL of the supernatant was transferred to a test tube containing 4 mL 0.5% thiobarbituric acid (TBA) in 20% TCA. The mixture was then transferred to a water bath set at 95 °C for 30 min, cooled in ice, and then centrifuged at 10.000 g for 10 min. The supernatant was transferred to a test tube and the absorbance was read at 532 nm. A 0.5% TBA in 20% TCA solution was used as a blank and an extinction coefficient of 155/mM/cm was used to determine the MDA content.
Gene expression assessment (RT-PCR)
Real time RT-PCR analysis was conducted to evaluate the impact of salinity on expression pattern of heat shock protein genes; Hsp17.8, Hsp26.3, Hsp70 and Hsp101. Shoots of 7-day-old seedlings of T11, J2, M23, R15 and A8 landraces grown under standard and NaCl stress conditions (200 mM) were used for RNA extraction and RT-PCR analysis.
Total RNA was isolated from leaves using RNA extraction kit (RNeasy plant minikit, Qiagen) following the manufacturer’s instructions. The quantity of extracted RNA was measured by a nanodrop; the quality of extracted RNA was checked by mixing 5 µL of RNA with 2.5 µL of bromophenol blue and loaded on 1% agarose gel. The gel was run at 5 V/cm2 for 60 min. cDNA was synthesized using reverse transcriptase (Thermo scientific revertaid first strand cDNA synthesis). Sequence-specific primers (Table 1) were used to amplify the cDNA. α-tubulin was used as an internal control gene of T. durum.
Table 1.
Primers sequence of target genes used in gene expression analysis
| Gene name | Sequence |
|---|---|
| Hsp17.8 (F) | 5′-GCGGCCGCGAGAATGGAG-3′ |
| Hsp17.8 (R) | 5′-GCGGCACACGGCGGAGAT-3′ |
| Hsp101 (F) | 5′-TGGAGAGGAAGCGGATTCAG3′ |
| Hsp101 (R) | 5′-CTGCTTCAGCTTCCGGATCT3′ |
| Hsp26.3 (F) | 5′-CATGGCCCGTCTGCTGTCTCT-3′ |
| Hsp26.3 (R) | 5′-AGCACGCCGTTCTTCATCTCG-3′ |
| Hsp70 (F) | 5′-CCCAGCGCCAGGCCACTAAGGAC-3′ |
| Hsp70 (R) | 5′-CAAAGCGAGCCCGTGTGATGGTA-3′ |
| α-tubulin (F) | 5′ AGCGCCTTTGAGCCTTCGTCC-3′ |
| α-tubulin (R) | 5′-TCATCGCCCTCATCACCGTCC-3′ |
Statistical analysis
All experiments were repeated independently at least twice and results of one representative experiment are presented. At least four replicates were used for each treatment. Means were compared using one way ANOVA with Tukey’s significant difference test (P < 0.05).
Results
Effect of salinity on growth and morphological parameters
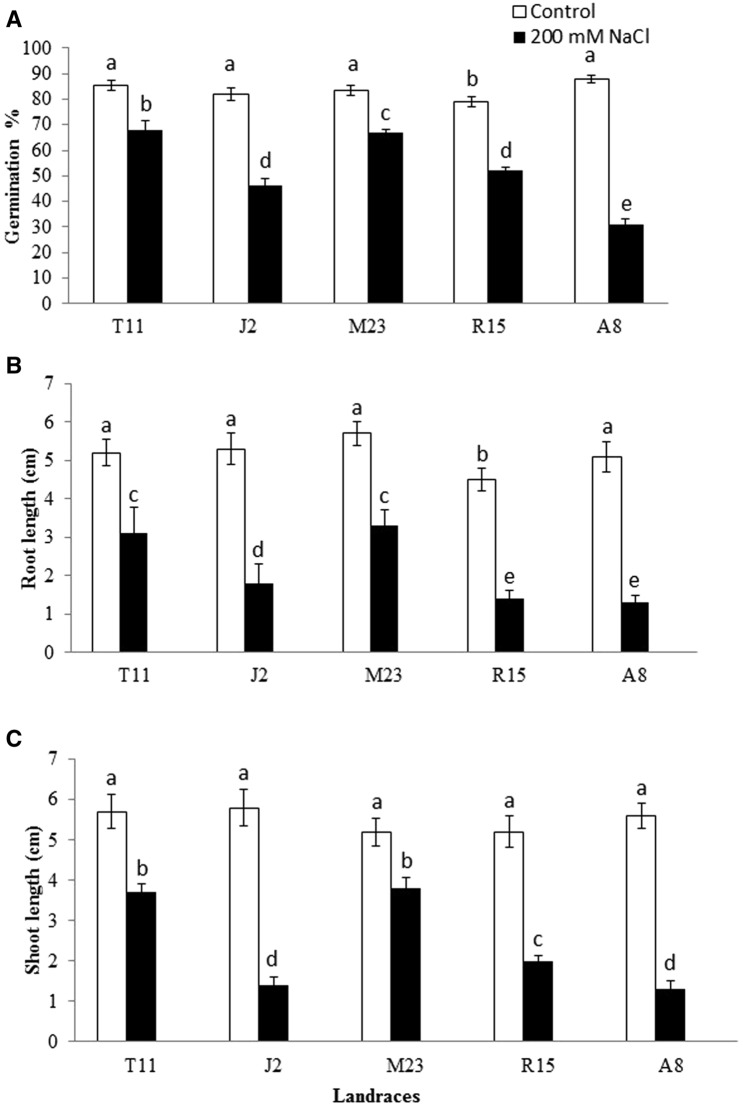
In general, germination percentages of all tested T. durum landraces were significantly decreased by salinity stress (P < 0.05) (Fig. 1a). A8 landrace significantly showed the lowest germination percentage compared to the other landraces (P < 0.05) (Fig. 1a). The highest germination percentage under NaCl stress was observed for T11 and M23 landraces compared to all other landraces, indicating that T11 and M23 are the most salt tolerant landraces at the germination stage (Fig. 1a). In addition, root lengths (cm) of all landraces were significantly decreased by NaCl stress compared to the control group (P < 0.05) (Fig. 1b). T11 and M23 landraces significantly showed the highest root length compared to other landraces (P < 0.05) under NaCl stress. On the other hand, the shortest roots were observed for A8 and R15 landraces under NaCl stress (Fig. 1b).
Fig. 1.
Germination percentage (a), root length (b) and shoot length (c) of 5 wheat (T. durum Desf.) landraces grown under salt stress (200 mM NaCl). Error bars denote standard error (n = 80). Means followed by different letters are significantly different at P < 0.05 according to Tukey’s test
Shoot length (cm) was significantly decreased by NaCl stress in all landraces (P < 0.05) (Fig. 1c). However, T11 and M23 landraces significantly showed the longest shoots under NaCl stress. On the other hand, A8 and J2 landraces significantly showed the shortest shoots at 200 mM NaCl compared to M23 and T11 landraces (P < 0.05) (Fig. 1c).
Results of phenotyping analysis indicats that T11 and M23 showed the lowest reduction in germination percentage, root length and shoot length under NaCl stress as compared to other landraces.
Effect of salinity on stress indicator parameters
Proline content (µg/mL) of 7-day-old T. durum seedlings grown under NaCl stress increased significantly in comparison to the control group (P < 0.05) (Fig. 2a). Specifically, at 200 mM NaCl, A8 and J2 landraces significantly accumulated the highest proline content in comparison to all other landraces (P < 0.05) (Fig. 2a). On the other hand, M23 landrace showed the lowest proline content (P < 0.05) (Fig. 2a).
Fig. 2.
Proline content (a), malondialdehyde (MDA) content (b) and chlorophyll content (c) of 5 wheat (T. durum Desf.) landraces grown under salt stress (200 mM NaCl). Error bars denote standard error (n = 4). Means followed by different letters are significantly different at P < 0.05 according to Tukey’s test
Furthermore, MDA content (mM/g protein) was significantly increased by salt stress for all studied T. durum landraces compared to their controls (P < 0.05) (Fig. 2b). J2 and A8 landraces significantly accumulated the highest level of MDA at 200 mM NaCl compared to the control groups and other landraces (P < 0.05) (Fig. 2b). On the other hand, the lowest MDA level was observed for M23 landrace.
Finally, chlorophyll content (µg/mg FW) for all landraces showed significant decrease under NaCl stress condition compared to the control group (P < 0.05) (Fig. 2c). However, among all landraces, M23 and T11 landraces significantly showed the highest chlorophyll content compared to all other landraces under NaCl stress. On the other hand, A8 and J2 landraces showed the lowest chlorophyll content in comparison with the other landraces (Fig. 2c).
Effect of salinity on heat shock proteins gene expression
As shown in Fig. 3a, HSP17.8 expression was significantly induced (P < 0.05) under NaCl stress compared to control for all studied landraces. The highest relative transcript level of HSP17.8 was observed in R15 and T11 landraces under NaCl stress, which was significantly higher (P < 0.05) than its control (510% and 591.5% relative to their control, respectively). On the other hand, J2 landrace showed the lowest relative transcript level of HSP17.8 under 200 mM NaCl concentration (248% relative to its control). HSP26.3 was significantly induced (P < 0.05) under NaCl stress compared to control in all landraces (Fig. 3b). The highest (P < 0.05) relative HSP26.3 transcript level was found in T11 landrace (611.11% relative to its control). On the other hand, J2 and A8 landraces showed the lowest (P < 0.05) relative transcript level of HSP26.3 under 200 mM NaCl concentration (200% and 218% relative to their control, respectively). Different HSP70 expression levels were observed among studied landraces under stress conditions. The hieghest HSP70 expression level was observed in T11 landrace (575% relative to its control). In contrast, the lowest HSP70 transcript level was observed in J2 landrace (210% relative to its control) (Fig. 3c). HSP101 was significantly induced (P < 0.05) under NaCl stress compared to control in all landraces. The highest (P < 0.05) HSP101 transcript level was found in T11 landrace under NaCl stress (345.8% relative to its control). In contrast, the lowest HSP101 transcript level was found in J2 and A8 landraces (242% and 213%, relative to their control) (Fig. 3d).
Fig. 3.
HSP 17.8 (a), HSP 26.3 (b) HSP 70 (c) and HSP 101 transcript level of 5 wheat (T. durum Desf.) landraces grown under salt stress (200 mM NaCl). Error bars denote standard error (n = 4). Means followed by different letters are significantly different at P < 0.05 according to Tukey’s test
Effect of salinity on T. durum yield components
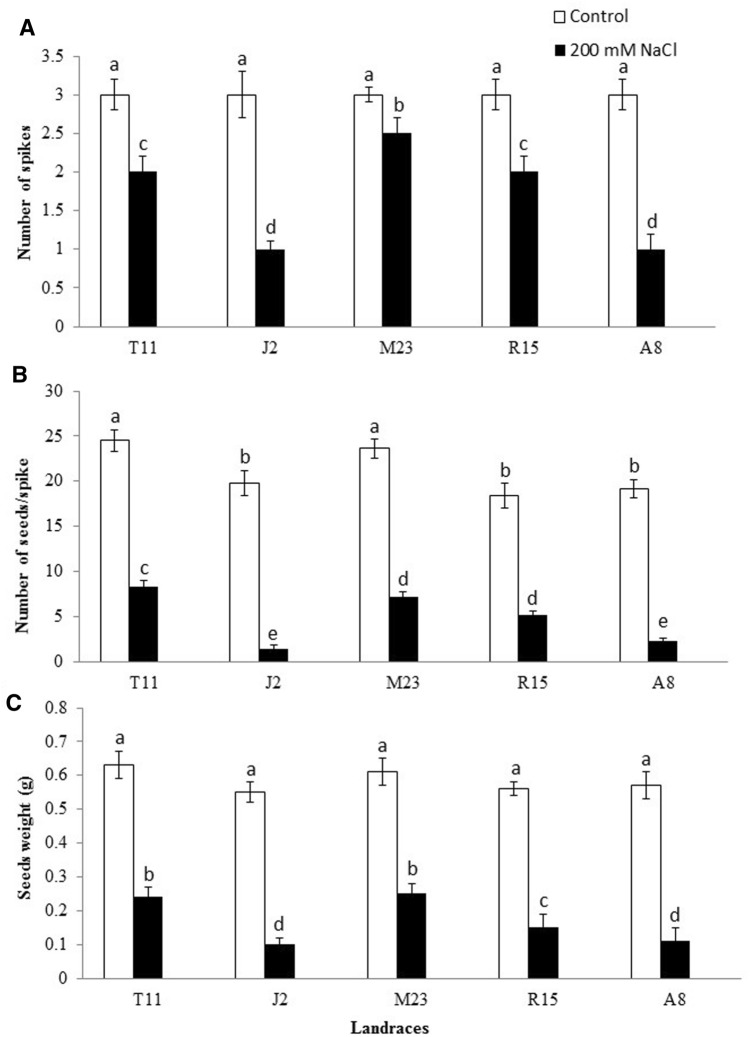
Spikes number/plant of all T. durum landraces was significantly decreased by the increase in NaCl concentration (P < 0.05) (Fig. 4a). However, J2 and A8 were the most negatively affected landraces at 200 mM NaCl stress compared to all other landraces (Fig. 4a). On the other hand, M23 was the least affected landrace compared to all other landraces, while R15 and T11 showed similar response in terms of spike number to salinity stress (Fig. 4a). Number of seeds/spike of all T. durum landraces was significantly decreased by salinity stress compared to their control (P < 0.05) (Fig. 4b). At 200 mM NaCl, T11 landrace was the least affected landrace in terms of seeds number/spike (P < 0.05) (33% compared to its control) (Fig. 4b). Wherease, J2 landrace was most affected by NaCl stress and produced the lowest seeds number/spike at 200 mM NaCl (6% relative to its control) (Fig. 4b). Results of the current study showed that seed weight (g) of all landraces was significantly decreased by salinity stress compared to their control (P < 0.05) (Fig. 4c). In particular, T11 and M23 landraces produced the heaviest seeds (more than 0.6 g) compared to other landraces under the same conditions, while J2 and A8 landraces produced the lightest seeds compared to the control and other landraces (Fig. 4c). Results of yield components analysis indicats that T11 and M23 showed the lowest reduction in number of seeds/spike, number of spikes/plant and seeds weight under NaCl stress as compared to other landraces.
Fig. 4.
Number of spikes (a), number of seeds/spike (b) and seeds weight (c) of 5 wheat (T. durum Desf.) landraces grown under salt stress (200 mM NaCl). Error bars denote standard error (n = 12). Means followed by different letters are significantly different at P < 0.05 according to Tukey’s test
Discussion
In this study, we found that NaCl stress decreased plant growth (Figs. 1a–c, 4a–c), which coincide with the general trend of plant growth retardation under saline conditions, and are consistent with previous results on durum wheat (Abdel-Ghani, 2009) and other plant species (Dolferus et al. 2011). This study also revealed varied growth responses to NaCl stress among the five T. durum landraces, with highest growth for landraces T11 and M23 and lowest for landraces J2 and A8. Therefore, plants from landraces T11 and M23 had highest germination %, root and shoot lengths, spike number, seed number and weight (Figs. 1a–c, 4a–c). Varied growth responses to NaCl stress of studied landraces may be attributed to differences among landraces due to genetic background and varied levels of tolerance and sensitivity to stress factors. The highest growth for landraces T11 and M23 might be because of adaptive mechanisms, which enhance their ability to tolerate stress, such as stomatal regulation, changes in hormonal balance, activation of antioxidant defense system and osmotic adjustment (Farooq et al. 2009). These results also suggest that landraces T11 and M23 have the genetic potential to tolerate salt stress in the Jordanian environment, at least at this stage of their growth.
Salt stress increased proline content (Fig. 2a) in all landraces with highest proline content in landraces J2 and A8 and lowest proline content in landraces T11 and M23. Changes in MDA content (Fig. 2b) in the same manner to proline, in all landraces, is indicative of a correlation between free radical generation and proline accumulation. These results also suggest that plants of landraces J2 and A8 experienced higher salt stress level and, therefore, higher accumulation of proline and MDA content. On the other hand, salinity tolerance of landraces T11 and M23 plants could be associated with the enhanced antioxidative capacity to scavenge reactive oxygen species and, thus, suppressed level of MDA and proline accumulation. Koca et al. (2007) reported a marked decrease in growth and increase in proline accumulation in two different cultivars of Sesamum indicum grown under salt stress. Similarly, high MDA level has previously been reported in leaves and roots of two maize genotypes under salt stress (de Azevedo Neto et al. 2006). In this study, a significant decrease in chlorophyll content in all tested T. durum landraces was observed; however, this decrease was lowest in landraces T11 and M23 and highest in the landrace A8 (Fig. 2c), consistent with trends observed for growth responses. Current results indicate that landraces T11 and M23 are more promising over others in tolerance to NaCl stress. The reduction of chlorophyll content in salt stressed plants observed in this study, especially in A8 plants, could be because of an increased cell or tissue damage represented by MDA accumulation (Cho and Park 2000).
Our results revealed similarity in the expression of the four HSP genes between plants exposed to NaCl stress (Fig. 3a–d). However, stress-mediated induction of these HSP genes was highest in the T11, M23 and R15 plants. HSPs are a tractable class of conserved proteins functioning in attaining cellular homeostasis and metabolic activities under frequent exposure to a diverse set of environmental stresses (Kim et al. 2014). Therefore, this stress-reducing effect observed for growth and stress indicator parameters, especially in T11 and M23 plants, could be related to the increased expression of the four genes. The results of the present study corroborate earlier investigations on bread wheat (Triticum aestivum L.) (Grigorova et al. 2011), which have also revealed the HSP genes (Hsp 17.8, Hsp 26.3, Hsp 70 and Hsp 101b) expressions to be increased in drought-tolerant wheat variety Katya than in the drought-sensitive Sadovo.
In conclusion, results showed that NaCl stress decreased plants growth and yield in all studied durum wheat landraces. However, growth parameters, stress indicator parameters and molecular results are in good correlation suggested that landraces T11 and M23 being relatively more tolerant to NaCl stress than other landraces. Findings from this study can be extrapolated to other plant species, including crops. The responses of measured parameters to salt stress were found to be genotype dependent, suggesting the importance of the genetic engineering to develop plant cultivars with high ability to withstand salt stress.
Acknowledgements
The authors would like to thank the deanship of research at Yarmouk University/Jordan for funding this Project (31/2015).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Ghani AH. Response of wheat varieties from semi-arid regions of Jordan to salt stress. J Agron Crop Sci. 2009;195(1):55–65. [Google Scholar]
- Abogadallah GM. Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav. 2010;5(4):369–374. doi: 10.4161/psb.5.4.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khateeb W, Al Shalabi A, Schroeder D, Musallam I. Phenotypic and molecular variation in drought tolerance of Jordanian durum wheat (Triticum durum Desf) landraces. Physiol Mol Biol Plants. 2017;23(2):311–319. doi: 10.1007/s12298-017-0434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Whaibi MH. Plant heat-shock proteins: a mini review. J King Saud Univ Sci. 2011;23(2):139–150. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Bharti K, Nover L. Heat stress-induced signalling. In: Scheel D, Wasternack C, editors. Plant signal transduction: frontiers in molecular biology. Oxford: Oxford University Press; 2002. pp. 74–115. [Google Scholar]
- Black E. The impact of climate change on daily precipitation statistics in Jordan and Israel. Atmos Sci Lett. 2009;10(3):192–200. [Google Scholar]
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32(1–2):191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83(3):463–468. [Google Scholar]
- Chen Z, Zhou M, Newman IA, Mendham NJ, Zhang G, Shabala S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol. 2007;34(2):150–162. doi: 10.1071/FP06237. [DOI] [PubMed] [Google Scholar]
- Cho UH, Park JO. Mercury-induced oxidative stress in tomato seedlings. Plant Sci J. 2000;156(1):1–9. doi: 10.1016/s0168-9452(00)00227-2. [DOI] [PubMed] [Google Scholar]
- Cicerali IN (2004) Effect of salt stress on antioxidant defence systems of sensitive and resistant cultivars of lentil (Lens culinaris M.). M.Sc. thesis, submitted to the Graduate School ofNatural and Applied Science of Middle EastTechnical University, Turkey
- de Azevedo Neto AD, Prisco JT, Filho JE, de Abreu CEB, Gomes-Filho E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot. 2006;56(1):87–94. [Google Scholar]
- Deikman J, Petracek M, Heard JE. Drought tolerance through biotechnology: improving translation from the laboratory to farmers’ fields. Curr Opin Biotechnol. 2012;23(2):243–250. doi: 10.1016/j.copbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Distelfed A, Avni R, Fischer AM. Senescence, nutrient remobilization, and yield in wheat and barley. J Exp Bot. 2014;65:3783–3798. doi: 10.1093/jxb/ert477. [DOI] [PubMed] [Google Scholar]
- Dolferus R, Ji X, Richards RA. Abiotic stress and control of grain number in cereals. Plant Sci. 2011;181(4):331–341. doi: 10.1016/j.plantsci.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S. Plant drought stress: effects, mechanisms and management. Agron Sustain Dev. 2009;29(1):185–212. [Google Scholar]
- Grigorova B, Vaseva II, Demirevska K, Feller U. Expression of selected heat shock proteins after individually applied and combined drought and heat stress. Acta Physiol Plant. 2011;33(5):2041–2049. [Google Scholar]
- Hiei Y, Ishida Y, Komari T. Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Front Plant Sci. 2014;5:628. doi: 10.3389/fpls.2014.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttner S, Strasser R. Endoplasmic reticulum-associated degradation of glycoproteins in plants. Front Plant Sci. 2012;3:67. doi: 10.3389/fpls.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HD. Wheat Biotechnology: current status and future prospects. In: Davey MR, Azhakanandam K, Daniell H, Silverstone A, editors. Recent advancements in gene expression and enabling technologies in crop plants. New York: Springer; 2015. pp. 263–290. [Google Scholar]
- Kim BM, Rhee JS, Jeong CB, Seo JS, Park GS, Lee YM, Lee JS. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp Biochem Physiol Part C J Toxicol Pharmacol. 2014;166:65–74. doi: 10.1016/j.cbpc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Koca H, Bor M, Ozdemir F, Turkan I. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot. 2007;60:344–351. [Google Scholar]
- Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD. Complexity of the heat stress response in plants. Curr Opin Plant Biol. 2007;10(3):310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22(1):631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J. Climate trends and global crop production since 1980. Science. 2011;333(6042):616–620. doi: 10.1126/science.1204531. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 2005;444(2):139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Mitra J. Genetics and genetic improvement of drought resistance in crop plants. Curr Sci. 2001;80(6):758–763. [Google Scholar]
- Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11(1):15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Munns R, Gilliham M. Salinity tolerance of crops-what is the cost? New Phytol. 2015;208(3):668–673. doi: 10.1111/nph.13519. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Muthusamy S, Dalal M, Chinnusamy V, Bansal K. Genome-wide identification and analysis of biotic and abiotic stress regulation of small heat shock protein (HSP20) family genes in bread wheat. J Plant Physiol. 2017;211:100–113. doi: 10.1016/j.jplph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf. 2005;60(3):324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Park CJ, Seo YS. Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J. 2015;31(4):323–333. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JH, Sun D, Nevo E. Domestication evolution, genetics and genomics in wheat. Mol Breed. 2011;28(3):281–301. [Google Scholar]
- Rizhsky L, Liang H, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. J Plant Physiol. 2002;130:1143–1151. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiferaw B, Smale M, Braun HJ, Duveiller E, Reynolds M, Muricho G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013;5(3):291–317. [Google Scholar]
- Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426(6968):891. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Sudhakar C, Lakshmi A, Giridarakumar S. Changesin the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001;161:613–619. [Google Scholar]
- Swindell WR, Huebner M, Weber AP. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 2007;8(1):125. doi: 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkan I, Demiral T. Recent developments in understanding salinity tolerance. Environ Exp Bot. 2009;67(1):2–9. [Google Scholar]
- Vasil IK. Molecular genetics improvement of cereals: transgenic wheat (Triticum aestivum L.) Plant Cell Rep. 2007;26(8):1133–1154. doi: 10.1007/s00299-007-0338-3. [DOI] [PubMed] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Biol. 1991;42(1):579–620. [Google Scholar]
- Wallender WW, Tanji KK. Nature and extent of agricultural salinity and sodicity. In: Wallender WW, Tanji KK, editors. Agricultural salinity assessment and management. 2. Reston: American Society of Civil Engineers; 2012. pp. 1–25. [Google Scholar]
- Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9(5):244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Gao X, Dong J, Tian X, Wang J, Palta JA, Xu S, Fang Y, Wang Z. Overexpression of the Heat-Responsive Wheat Gene TaHSP23.9 in transgenic Arabidopsis conferred tolerance to heat and salt stress. Front Plant Sci. 2020;11:243. doi: 10.3389/fpls.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Irfan M, Ahmad A, Hayat S. Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol. 2011;32(5):667. [PubMed] [Google Scholar]
- Yi H, Qiong W, Jian Z, Tao S, Guang-xiao Y, Guang-yuan H. Current status and trends of wheat genetic transformation studies in China. J Integr Agr. 2015;14(3):438–452. [Google Scholar]