Abstract
Soil waterlogging is a common problem in some agricultural areas, including regions under soybean (Glycine max) cultivation. In waterlogged soils, soil O2 depletion occurs due to aerobic microorganisms and plants, affecting the metabolic and physiological processes of plants after suffering anoxia in their root tissue. Another harmful factor in this situation is the exponential increase in the availability of iron (Fe) in the soil, which may result in absorption of excess Fe. The present study sought to evaluate the response mechanisms in soybean leaves ‘Agroeste 3680’ by physiological and biochemical analyses associating them with the development of pods in non-waterlogged and waterlogged soil, combined with one moderate and two toxic levels of Fe. Gas exchange was strongly affected by soil waterlogging. Excess Fe without soil waterlogging reduced photosynthetic pigments, and potentiated this reduction when associated with soil waterlogging. Starch and ureide accumulation in the first newly expanded trifoliate leaves proved to be response mechanisms induced by soil waterlogging and excess Fe, since plants cultivated under soil non-waterlogged soil at 25 mg dm−3 Fe showed lower contents when compared to stressed plants. Thus, starch and ureide accumulation could be considered efficient biomarkers of phytotoxicity caused by soil waterlogging and excess Fe in soybean plants. The reproductive development was abruptly interrupted by the imposition of stresses, leading to a loss of pod dry biomass, which was largely due to the substantial decrease in the net photosynthetic rate, as expressed by area (A), the blockage of carbohydrate transport to sink tissues and an increase of malondialdehyde (MDA). The negative effect on reproductive development was more pronounced under waterlogged soil.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00845-8) contains supplementary material, which is available to authorized users.
Keywords: Anoxia, Ferrous ion, Starch, Ureides, Glycine max
Introduction
Soybean is responsible for providing more than half of the world’s vegetable oil and two-thirds of it as protein meal, making it the most important grain legume crop (Divito et al. 2015). However, several regions under soybean cultivation are affected by soil waterlogging (Maekawa et al. 2011; Syed et al. 2015; Sartori et al. 2015), leading to a decrease in grain yield and economic losses (Linkemer et al. 1998; Yin and Komatsu 2017). According to Bailey-Serres et al. (2012) and Hirabayashi et al. (2013), climate change will increase the frequency and severity of this stressor, due to high rainfall and poor soil drainage, which may consequently negatively affect the cultivation of rain-fed plants in various regions of the world.
Excessive water affects the physical structure of soil (Rodríguez-Gamir et al. 2011). In addition, O2 dissolved in the water-filled pore spaces has a low diffusion rate compared to air (Dat et al. 2004; Yin and Komatsu 2017). Consequently, one of the main effects of soil waterlogging is the scarcity of O2, which is increased over time as a result of O2 depletion by aerobic microorganisms and plants, leading to an anoxic condition (Mohanty 2003).
The root tissue of plants under anoxia switches the pathway of energy production from oxidative phosphorylation to glycolysis and fermentation (Mohanty 2003), resulting in a severe decline in the energy status of root cells. The overall yield of adenosine triphosphate (ATP) produced during fermentation is only two molecules of ATP per glucose molecule, rather than the 38 molecules produced during oxidative phosphorylation (Sairam et al. 2009). Martínez-Alcántara et al. (2012) determined that the metabolic and physiological effects of soil waterlogging on plants are mainly derived from this energy change experienced by plants. According to these authors, initially, there is a reduction in water flux from roots, followed by alterations in water use, nutrient uptake and dry weight partitioning. In addition, the transport of ureides via the xylem is inhibited in soybean plants (Puiatti and Sodek 1999), resulting from lower N2-fixation activity. At the leaf level, one of the first detectable effects is decreased CO2 availability due to stomatal limitation, which is considered the initial negative effect of waterlogging on photosynthesis, and a process instigated to prevent water loss to the environment (Zhang et al. 2016; Yan et al. 2018).
Another key point to consider is that the microorganisms present in anoxic soils are anaerobes and facultative anaerobes. These microorganisms utilise alternative electron acceptors to maintain their metabolism (Maranguit et al. 2017). In this context, Fe-rich soils under anaerobic conditions are made phytotoxic to plants when insoluble Fe3+ oxides are reduced to soluble Fe2+ forms and released into the water present in the soil (Frei et al. 2016; Maranguit et al. 2017). Excess Fe in plant tissue can displace the cell redox balance towards a pro-oxidant state, affecting different morphological, physiological, and biochemical aspects in plants, generating oxidative stress (De Oliveira Jucoski et al. 2013; Müller et al. 2017), mainly by the action of hydroxyl radicals (HO∙) via the Fenton reaction (Hell and Stephan 2003; Pereira et al. 2013).
There are no previous reports on soil waterlogging associated with excess Fe in soybean crops, although tolerance is often a product of tolerance to anaerobiosis and to toxicities of the excessively available elements (Singh and Setter 2017). Therefore, the present study sought to evaluate the response mechanisms in soybean leaves ‘Agroeste 3680’ by physiological and biochemical analyses associating them with the development of pods in non-waterlogged and waterlogged soil, combined with one moderate and two toxic levels of Fe.
Materials and methods
Experimental site
The experiment was carried out under greenhouse conditions at the College of Agricultural and Technological Sciences, São Paulo State University (UNESP), São Paulo State, Brazil (21° 29′ S, 51° 2′ W; 396 m above sea level). The greenhouse used was an arch type, covered with a transparent light diffusing plastic film, with a thickness of 1000 microns. The sides of the greenhouse were covered by a Sombrite screen, with a 50% light-holding capacity.
Experimental design and treatments
The experimental design was completely randomised and arranged in a 2 × 3 factorial scheme, with two water levels in the soil (without waterlogging and with waterlogging) and three soil Fe levels (25, 125 and 500 mg dm−3). Each pot contained three plants, giving a final population of nine plants per treatment.
Cultivation conditions
The soil was a dystrophic Oxisol (Santos et al. 2018). The sample was collected at a depth of 0.0–0.2 m, forming a composite sample. It was crumbled, air-dried and sieved (4.0 mm), and shown to have the following chemical attributes: pH (CaCl2) 4.6, organic matter 14 g dm−3, P (resin) 4 mg dm−3, K 2.1 mmolc dm−3, Ca 7 mmolc dm−3, Mg 5 mmolc dm−3, S 5 mg dm−3, B 0.09 mg dm−3, Cu 0.6 mg dm−3, Fe 0.25 mg dm−3, Mn 16.7 mg dm−3, Zn 1 mg dm−3, potential acidity (H + Al) 18 mmolc dm−3, Al 4 mmolc dm−3, sum of bases 14.1 mmolc dm−3, cation exchange capacity 32.1 mmolc dm−3, and base saturation 44%.
Based on the chemical analysis, soil liming was carried out to raise the base saturation to 70% (Quaggio et al. 1985), by adding analytical reagent grade CaCO3 and MgCO3 at a ratio of 3:1. The soil containing carbonate salts was incubated for 30 days in pots at a humidity of 80% of the field capacity to allow it to equilibrate. The soil was then air-dried for seven days. The pots used were polypropylene with a capacity of 4 dm3, lined with a polystyrene blanket to prevent soil loss during the experiment.
Experimental process
The following fertilisation was carried out per pot: 10 mg dm−3 N as CO(NH2)2, 200 mg dm−3 P as Ca(H2PO4)2·H2O, 150 mg dm−3 K as K2SO4, 0.5 mg dm−3 B as H3BO3, 0.05 mg dm−3 Co as CoCl2·H2O), 1.0 mg dm−3 Cu as CuSO4·5H2O, 0.05 mg dm−3 Mo as H2MoO4, 0.05 mg dm−3 Ni as NiSO4·7H2O, 5.0 mg dm−3 Mn as MnSO4, and 2.0 mg dm−3 Zn as ZnSO4. The K supply was split into three equal applications, which were supplied before sowing, and at the V2 and R1 phenological stages (Fehr et al. 1971). Fe, as FeCl3·6H2O, was supplied at 0, 100 and 475 mg dm−3 to the soil to give one natural level of 25 mg dm−3 Fe and two high levels of 125 and 500 mg dm−3 Fe, respectively.
After four days, 10 soybean seeds were sown at a depth of 3 cm, having previously been inoculated with the N2-fixing bacterium Bradyrhizobium japonicum, strains SEMIA 587 and 5019. At the V1 phenological stage (Fehr et al. 1971), the seedlings were thinned to three representative seedlings per pot, selecting those with greater vigour and homogeneity of size.
At the R3 phenological stage (Fehr et al. 1971), the soil was waterlogged for a period of 10 days, totalling 70 days of experimental conduction from the germination of soybean seeds. The pots undergoing a waterlogged soil treatment were placed in larger pots with non-draining bottoms, and the water level in these was maintained at 2 cm above the soil surface of the inner pot.
During the experimental conduction, the replenishment of evapotranspired water for the plots was achieved using suspended micro-sprinklers, which were activated twice a day (morning and afternoon). In this way, soil humidity was maintained at 80% of the field capacity, except during the 10 days of stress in the pots where the soil was waterlogged. In these days, all plants were irrigated manually, respecting the water levels of each plot.
Measurements of gas exchange
On the 10th day of stress imposition, gas exchange evaluations were carried out using a LCpro portable infrared gas analyser (ADC Bioscientific Ltd., Hoddesdon, United Kingdom) on the first newly expanded trifoliate leaf (counting from the apex) of two plants from each pot. Evaluations were performed on a clear day between 10:00 and 11:30 a.m. Photosynthetically active radiation (PAR) was standardised to an artificial saturating light of 1000 µmol m−2 s−1, 380 μmol CO2 mol−1 air and a chamber temperature of 28 °C, according to Reis et al. (2017).
The net photosynthetic rate (A, µmol CO2 m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1) and transpiration rate (E, mmol H2O m−2 s−1) was obtained. Water use efficiency (WUE, μmol CO2 mmol−1 H2O) and instantaneous carboxylation efficiency (EiC, mol air−1) were estimated from Eqs. 1 and 2, respectively.
| 1 |
| 2 |
where Ci (µmol CO2 mol ar−1) is the internal CO2 level in the substomatal chamber.
Material storage for biochemical analysis
At the end of the stresses imposition, the first newly expanded trifoliate leaves (counting from the apex) were collected and frozen in liquid N2. The samples were packaged and labelled in aluminium foil and stored at −80 °C until analysed as described below.
Analysis of photosynthetic pigments
Methods for determining photosynthetic pigment levels (chlorophylls and carotenoids) were based on those described by Lichtenthaler and Wellburn (1983). Leaves from two plants in each pot were assayed. Fresh leaf tissue (0.5 g) was ground in liquid N2 with a pestle and mortar. Five ml 80% acetone was added to the samples and they were then centrifuged at 1500 rpm for 5 min. Following extraction, the absorbance (ABS) of the solvent was read at wavelengths specific to the different pigments by spectrophotometry. Chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Tchl) and carotenoid (CAR) contents were expressed as μg ml−1, and calculated according to Eqs. 3–6:
| 3 |
| 4 |
| 5 |
| 6 |
Analysis of carbon and nitrogen compounds in leaves
Leaves from two plants of each pot were analysed. Fresh leaf tissue (0.5 g) was ground in liquid N2 with a pestle and mortar. Based on the method of Bieleski and Turner (1966), compounds were extracted for 24 h with 0.5 ml MCW solution (60% methanol, 25% chloroform and 15% water). The samples were centrifuged at 3000 rpm for 15 min, and the supernatant and precipitate were collected.
One volume of chloroform and 1.5 volumes of water were added to 4 volumes of the resulting supernatant. After phase separation over 24 h, the aqueous (upper) phase was used for the quantification of compounds. The sucrose and total carbohydrate content (mg g−1 fresh weight, FW) were determined by the anthrone methods of Umbreit et al. (1957) and Yemm and Willis (1954), respectively, using glucose as a standard. The total ureide, allantoate and allantoin contents were determined by the phenylhydrazine method of Vogels and Van Der Drift (1970), using allantoin as a standard. For the determination of allantoate, the initial alkaline hydrolysis step was omitted, and allantoin was calculated by the difference to the total ureides assay. Ammonia and nitrate were determined by the phenol-hypochlorite method of McCullough (1967) and the salicylic acid method described by Cataldo et al. (1975), respectively. The total ureides, allantoate and allantoin, nitrate and ammonia contents were expressed as µmol g−1 FW.
From the residue obtained after centrifugation of the initial MCW extract (precipitate), the protein content was determined, as described by Bradford (1976), using bovine serum albumin as a standard. The starch content was also determined by the perchloric acid/anthrone method (Yemm and Willis 1954; Umbreit et al. 1957). Protein and starch contents were expressed as mg g−1 FW.
Analysis of concentrations of hydrogen peroxide and lipid peroxidation
Hydrogen peroxide (H2O2) concentrations were determined by reaction with potassium iodide (KI), according to Alexieva et al. (2001). Leaves from two plants of each pot were assayed. Fresh leaf tissue (0.25 g) was ground in liquid N2 with a pestle and mortar, to which 3 ml of 0.1% trichloroacetic acid (TCA) in 20% polyvinyl polypyrrolidone (PVPP) was added. After complete homogenisation, the samples were centrifuged at 10,000 rpm for 10 min at 4 °C in a refrigerated centrifuge. For the reaction, 0.2 ml of supernatant was added to 0.2 ml 100 mM potassium phosphate buffer pH 7.5 and 800 μl 1 M KI solution. The samples were kept on ice for 1 h, and then absorbance readings were taken at 390 nm. The H2O2 concentration was calculated based on a standard curve of H2O2, and the results were expressed in nmol g−1 FW.
Lipid peroxidation was evaluated by determining the concentration of metabolites reactive to 2-thiobarbituric acid (TBA), mainly malondialdehyde (MDA), according to the method of Heath and Packer (1968). The initial procedures for MDA measurement were the same as those described above for H2O2 measurements. Following centrifugation, 0.25 ml of supernatant was added to 1 ml 20% TCA solution containing 0.5% thiobarbituric acid (TBA). The samples were kept in a dry bath at 95 °C for 30 min and then on ice for 20 min. Subsequently, the samples were centrifuged at 10,000 rpm for 5 min in order to separate residues formed during heating. Samples were read at two wavelengths, 535 nm and 600 nm, and MDA concentrations were calculated according to Eq. 7:
| 7 |
Fe accumulation in shoots and partitioned biomass production
At the end of the exposure to stresses, plants were separated into shoots and roots. The material was oven dried at 65 °C for 72 h, and the pods were harvested from the shoots in order to assess the deleterious effect of the stresses on their development. Subsequently, shoot (SDW), pod (PDW) and root (RDW) dry weights were determined and expressed as g plant−1.
To determine the Fe accumulation in the shoot (FEAS), the samples were subjected to digestion with nitric-perchloric acid solution (3:1) at 200 °C (Malavolta et al. 1997) after being ground in a Wiley-type mill. Subsequently, Fe concentrations were determined by atomic absorption spectrophotometry. FEAS (µg plant−1) was calculated by multiplying Fe concentrations by the SDW.
Data analysis
Data were initially subjected to the Shapiro–Wilk normality test (p < 0.05), followed by analysis of variance (ANOVA) using the F test (p ≤ 0.05). The traits were compared using the Tukey test (p < 0.05). Pearson’s correlation (p < 0.05) with multiple comparisons at 1% probability was calculated using the corrplot package. All statistical analysis of the data was performed using protocols developed in the R software (R Development Core Team 2019).
Results
Gas exchange
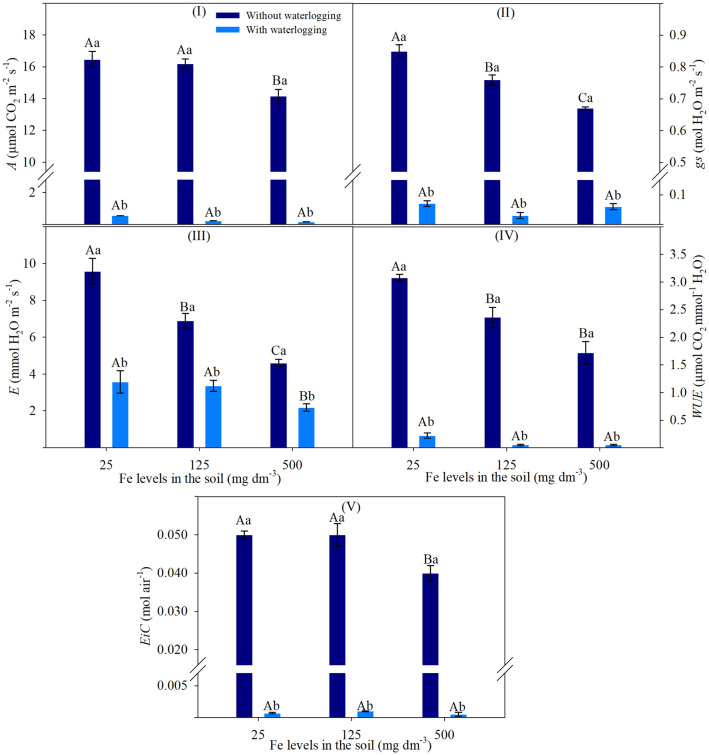
The traits of A, gs, E, WUE and EiC showed a significant effect of double interaction on ANOVA (Supplementary Table S1).
At Fe levels of 125 and 500 mg dm−3, plants cultivated without soil waterlogging showed reduced gs by 10 and 22% and E by 28 and 51%, respectively (Fig. 1II, III). The A and EiC traits were less sensitive and reduced only at 500 mg dm−3 Fe, decreasing by 14 and 17%, respectively (Fig. 1I, V). Levels of 125 and 500 mg dm−3 Fe resulted in similar reductions for WUE, by on average 46% (Fig. 1IV).
Fig. 1.
Net photosynthetic rate (I), stomatal conductance (II), transpiration rate (III), water use efficiency (IV) and instantaneous carboxylation efficiency (V) of soybean leaves based on the significance of ANOVA by factorial analysis (p ≤ 0.05), comprised of soils with two levels of water (without waterlogging and with waterlogging) and three iron levels (25, 125 and 500 mg dm−3). Different letters indicate significant differences according to the Tukey test (p < 0.05). Uppercase letters compare the conditions of waterlogging between different iron levels in the soil, while lowercase letters compare the conditions of soil waterlogging with the same iron levels. Vertical bars represent the standard deviation (n = 6 plants)
The increase of Fe levels associated with soil waterlogging showed no significant differences for A, gs, WUE and EiC (Fig. 1I, II, IV, V), while E decreased at 500 mg dm−3 Fe (Fig. 1III). The traits presented in Fig. 1 showed a marked reduction in soil waterlogging at all Fe levels relative to non-waterlogged soil (Fig. 1I–V).
Content of photosynthesis pigments
Chl a, Chl b, Tchl and CAR contents showed a significant effect of double interaction on ANOVA (Supplementary Table S1).
Similar decreases in Chl a, Chl b, Tchl and CAR contents were observed at 125 and 500 mg dm−3 Fe without soil waterlogging, resulting in average reductions of 24, 25, 25 and 20%, respectively. Similar behaviour was also found in soil waterlogging for contents of Chl b (− 24%), Tchl (− 14%) and CAR (− 20%; Fig. 2I–IV). In contrast, Chl a content was less sensitive, showing a reduction of 14% at 500 mg dm−3 Fe, while plants at 25 and 125 mg dm−3 Fe did not differ for this pigment (Fig. 2I).
Fig. 2.
Chlorophyll a (I), chlorophyll b (II), total chlorophyll (III) and carotenoid (IV) contents of soybean leaves based on the significance of ANOVA by factorial analysis (p ≤ 0.05), comprised of soils with two levels of water (without waterlogging and with waterlogging) and three iron levels (25, 125 and 500 mg dm−3). Different letters indicate significant differences according to the Tukey test (p < 0.05). Uppercase letters compare the conditions of waterlogging between different iron levels in the soil, while lowercase letters compare the conditions of soil waterlogging with the same iron levels. Vertical bars represent the standard deviation (n = 6 plants)
At each Fe level, plants showed a lower photosynthetic pigment content under soil waterlogging. At a natural level of Fe, Chl a, Chl b, Tchl and CAR showed a reduction of 50, 51, 50 and 43%, respectively, while the average reduction was 41%, 51%, 46% and 34%, respectively, with an excess of Fe (125 and 500 mg dm−3), as shown in Fig. 2I–IV.
Carbon compounds in leaves
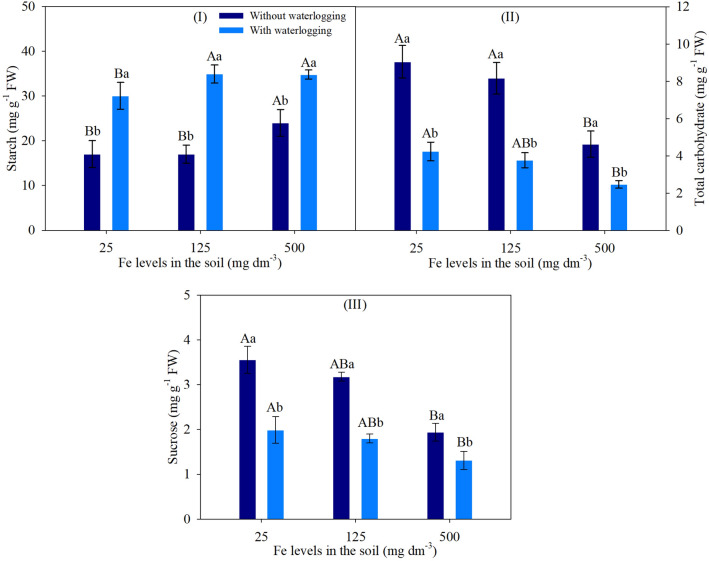
Starch, sucrose and total carbohydrate contents showed a significant effect of double interaction on ANOVA (Supplementary Table S1).
An increase in starch content was observed in non-waterlogged soil at 500 mg dm−3 Fe and waterlogged soil at 125 and 500 mg dm−3 Fe, with increases of 41, 16 and 16%, respectively. In addition, soil waterlogging provided higher starch contents at all Fe levels (Fig. 3I). In contrast, total carbohydrate and sucrose contents decreased in plants cultivated at 500 mg dm−3 Fe under both soil water conditions, and were lower under soil waterlogging, with a tendency towards less variation with increasing Fe levels compared to plants in non-waterlogged soils. These variations led to a reduction in total carbohydrate and sucrose contents by 49 and 45% (without waterlogging), and 41 and 34% (with waterlogging), respectively, between levels of 25 and 500 mg dm−3 Fe (Fig. 3II, III).
Fig. 3.
Starch (I), total carbohydrate (III) and sucrose (II) contents of soybean leaves based on the significance of ANOVA by factorial analysis (p ≤ 0.05), comprised of soils with two levels of water (without waterlogging and with waterlogging) and three iron levels (25, 125 and 500 mg dm−3). Different letters indicate significant differences according to the Tukey test (p < 0.05). Uppercase letters compare the conditions of waterlogging between different iron levels in the soil, while lowercase letters compare the conditions of soil waterlogging with the same iron levels. Vertical bars represent the standard deviation (n = 6 plants)
Nitrogen compounds in leaves
As shown by ANOVA (Supplementary Table S1), ammonia content was not affected by any factor, and its content in leaf tissue was null (0 nmol g−1 FW). Total ureides, allantoate and allantoin contents showed a significant single effect for soil waterlogging conditions and Fe levels. On the other hand, nitrate and protein contents showed a significant effect of double interaction.
Regardless of Fe levels, total ureides, allantoate and allantoin contents increased in plants on soil waterlogging, by 24, 18 and 31%, respectively. At 500 mg dm−3 Fe, total ureides, allantoate and allantoin contents showed increases of 28, 18 and 19%, respectively, compared to plant averages at 25 and 125 mg dm−3 Fe (Fig. 4I–III), regardless of the waterlogging conditions.
Fig. 4.
Total ureides (I), allantoate (II), allantoin (III), nitrate (IV) and protein (V) contents of soybean leaves based on the significance of ANOVA by factorial analysis (p ≤ 0.05), comprised of soils with two levels of water (without waterlogging and with waterlogging) and three iron levels (25, 125 and 500 mg dm−3). Different letters indicate significant differences according to the Tukey test (p < 0.05). Isolated uppercase letters compare the conditions of soil waterlogging regardless of iron levels, while isolated lowercase letters compare iron levels regardless of soil waterlogging. Uppercase letters compare the conditions of waterlogging between different iron levels in the soil, while lowercase letters compare the conditions of soil waterlogging with the same iron levels. Vertical bars represent the standard deviation (n = 6 plants)
Nitrate content was not changed in plants cultivated under increasing Fe levels without soil waterlogging. On the other hand, plants at 125 and 500 mg dm−3 Fe in waterlogged soils showed a similar reduction of 33% on average (Fig. 4IV). At 25 mg dm−3 Fe, nitrate content showed an increase of 38% for waterlogged soil, compared to plants in soil without waterlogging, while levels in plants at 125 and 500 mg dm−3 Fe did not differ between the two soil water conditions (Fig. 4IV).
Protein content showed an increase of 86% in non-waterlogged soil at 500 mg dm−3 Fe. On the other hand, in waterlogged soil, an increase in protein content occurred in plants cultivated at 25 mg dm−3 Fe, showing a 57% increase compared to higher levels of Fe (Fig. 4V). At 25 mg dm−3 Fe, protein content increased substantially on soil waterlogging relative to non-waterlogging soil, while it did not differ between the two water conditions at levels of 125 and 500 mg dm−3 Fe (Fig. 4V).
Hydrogen peroxide and lipid peroxidation concentrations
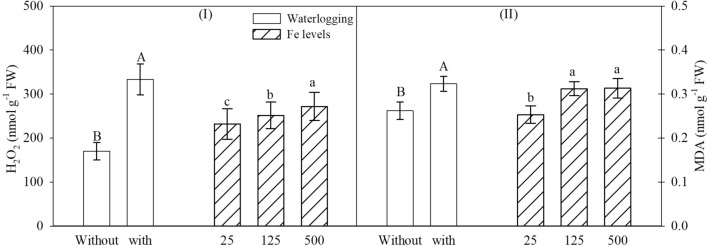
H2O2 and MDA concentrations showed a significant single effect for soil waterlogging conditions and Fe levels on ANOVA (Supplementary Table S1).
Regardless of Fe levels, H2O2 and MDA concentrations increased by 94 and 23%, respectively, in plants in waterlogged soil. The greatest increase for H2O2 was observed at 500 mg dm−3 Fe, while a similar increase was found for MDA at 125 and 500 mg dm−3. The increases were 17 and 23% for H2O2 and MDA concentrations, respectively (Fig. 5I, II), regardless of the waterlogging conditions.
Fig. 5.
Hydrogen peroxide (a) and malondialdehyde (b) concentrations of soybean leaves based on the significance of ANOVA by factorial analysis (p ≤ 0.05), comprised of soils with two levels of water (without waterlogging and with waterlogging) and three iron levels (25, 125 and 500 mg dm−3). Different letters indicate significant differences according to the Tukey test (p < 0.05). Isolated uppercase letters compare the conditions of soil waterlogging regardless of iron levels, while isolated lowercase letters compare iron levels regardless of soil waterlogging. Vertical bars represent the standard deviation (n = 6 plants)
Fe accumulation in shoots and partitioned biomass production
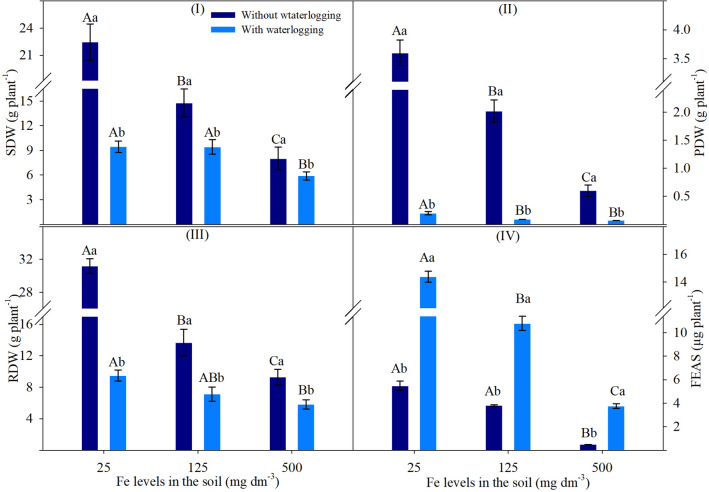
The traits of SDW, PDW, RDW and FEAS showed a significant effect of double interaction on ANOVA (Supplementary Table S1).
Plants cultivated in non-waterlogged soil showed severe decreases in SDW and RDW with increasing Fe levels. Upon soil waterlogging, these traits showed less variation, being reduced only at 500 mg dm−3 Fe. In addition, SDW and RDW showed the lowest values in waterlogged soil compared to non-waterlogged soil, especially for RDW, and both SDW and RDW showed a tendency towards less variation with increasing Fe levels (Fig. 6I, III).
Fig. 6.
Shoot dry weight (I), pod dry weight (II), root dry weight (III) and iron accumulation in the shoots (IV) of soybean leaves based on the significance of ANOVA by factorial analysis (p ≤ 0.05), comprised of soils with two levels of water (without waterlogging and with waterlogging) and three iron levels (25, 125 and 500 mg dm−3). Different letters indicate significant differences according to the Tukey test (p < 0.05). Uppercase letters compare the conditions of waterlogging between different iron levels in the soil, while lowercase letters compare the conditions of soil waterlogging with the same iron levels. Vertical bars represent the standard deviation (n = 6 plants for FEAS; n = 9 plants for dry weights)
BDW decreased with increasing Fe levels in both water conditions. This effect was more pronounced in non-waterlogged soil, resulting in reductions of 44 and 94% at 125 and 500 mg dm−3 Fe, respectively. In waterlogged soil, BDW showed a similar reduction, averaging 60% at both these Fe levels. At each Fe level, plants showed lower values in waterlogged soils, recording decreases of 94 (25 mg dm−3), 95 (125 mg dm−3) and 65% (500 mg dm−3) compared to non-waterlogged soils (Fig. 6II). FEAS showed a 91% decrease at 500 mg dm−3 Fe (without waterlogging), and a 25 and 74% decrease at 125 and 500 mg dm−3 Fe (with waterlogging), respectively. The highest values were observed in waterlogged soil (Fig. 6IV).
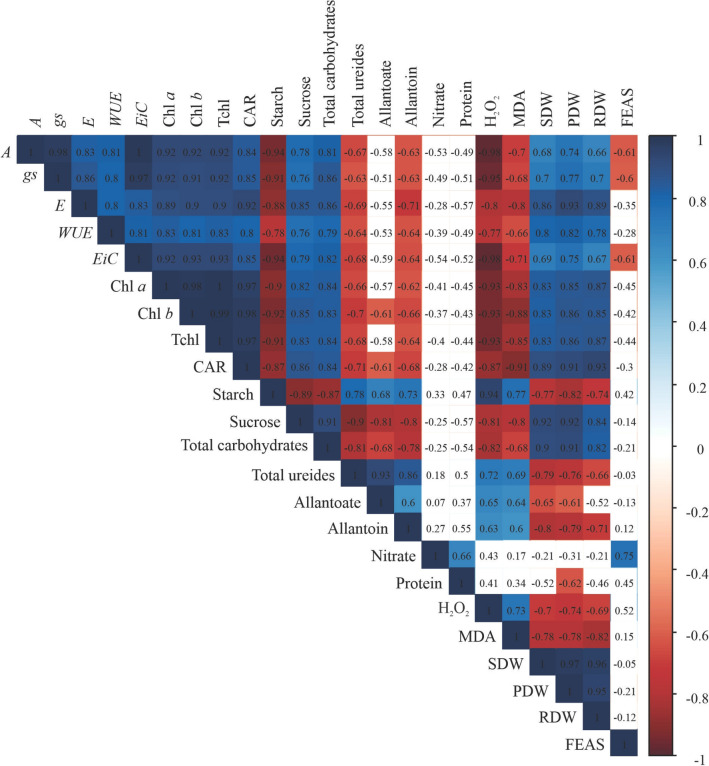
In Fig. 7, most traits showed significant correlations. However, nitrate, protein and FEAS were the traits that had the smallest relationship with the others.
Fig. 7.
Pearson’s correlation (p < 0.05) with multiple comparisons at 1% probability between traits. The squares that received the white colour belong to the category of non-significant correlative values. Net photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E), water use efficiency (WUE), instantaneous carboxylation efficiency (EiC), Chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Tchl), carotenoids (CAR), hydrogen peroxide (H2O2), malondialdehyde (MDA), shoot dry weight (SDW), pod dry weight (PDW), root dry weight (RDW), Fe accumulation in the shoot (FEAS)
Discussion
Gas exchange was affected by soil waterlogging and excess Fe
Photosynthesis is a primary physiological process for plant survival and growth (Yan et al. 2018). In sensitive species, soil waterlogging and excess Fe are stresses that directly affect photosynthesis, the measurements of which can determine the degree of tolerance shown by species. Reductions in gs can limit the influx of CO2 for photosynthesis. In addition, limitations may also occur in the carboxylation reactions, which leads to reduced photosynthesis, and which are commonly used to infer the cumulative effects of biochemical limitations (Müller et al. 2017).
In non-waterlogged soil, gs showed a progressive decrease with increasing Fe levels (Fig. 1II); however, changes in A and EiC were minor under these conditions (Fig. 1I, IV), suggesting that the behaviour observed in A was more closely associated with biochemical than stomatal limitations. Similar results were obtained with rice (Oryza sativa) by Müller et al. (2017). The decrease in gs in the current study was probably linked to the increase in FEAS (Fig. 6a), which was corroborated by the negative correlation recorded in Fig. 7. According to Dufey et al. (2009) and Pereira et al. (2013), stomatal closure is a late response to increased Fe uptake, rather than a defence mechanism in rice. Consequently, with the reduction of gs, E was reduced (Fig. 1II, III); however, it was not enough to optimise the WUE (Fig. 1V).
In waterlogged soil, gas exchange was substantially reduced (Fig. 1I–V). The natural level of Fe in the soil provided values similar to the two highest levels of Fe for traits associated with gas exchange, except for a reduction in E detected at 500 mg dm−3 Fe (Fig. 1III). These responses showed that soil waterlogging strongly affected the gas exchange of soybean at the R3 phenological stage. However, it is important to emphasise that the effects of waterlogging are complex, and vary depending on genotype, environmental conditions, growth stage and the duration of waterlogging (Tian et al. 2019).
Soil waterlogging and excess Fe reduced photosynthetic pigments
Damage to PSI and PSII can be inferred by the contents of Chl a and Chl b, respectively, since chlorophyll content varies within the photosystems (Wientjes et al. 2017). Under excess Fe in non-waterlogged soil, the contents of Chl a and Chl b (Fig. 2A, B) behaved similarly to that of Tchl (Fig. 4III), indicating that decreases recorded in these situations were similar between chlorophylls. However, the reduction of chlorophyll content at 125 mg dm−3 Fe did not reduce A (Fig. 1I), indicating that even with lower electron flow, A was maintained.
Soil waterlogging associated with excess Fe enhanced damage to Chl b content, which was more affected than Chl a content, mainly at 125 mg dm−3 Fe. In addition, the decline in Chl b content was not compensated for by Tchl content (Fig. 2I–III). Thus, it was assumed that a combination of these stresses impaired light energy utilisation and dissipation, and caused a reduction in antenna size relative to reaction centres, indicating greater photooxidative and oxidative damage to photosystems (Xu et al. 2015). On the other hand, soybean plants cultivated under natural levels of Fe in waterlogged soil showed a similar decrease between Chl a and Chl b content. In addition, the observed reductions in chlorophyll content did not determine a change in A (Fig. 1I), since this trait did not show differential behaviour with increasing Fe levels. Previous studies on soil waterlogging have reported notably greater damage to Chl b content in Helianthus tuberosus (Yan et al. 2018), Vigna radiata (Sairam et al. 2009) and Gossypium hirsutum (Kuai et al. 2014).
Carotenoids participate in light-absorption and protect the antenna complex from oxidative damage, due to their ability to extinguish reactive oxygen species (ROS) that are formed naturally during the photosynthetic process (Pinto et al. 2003). The variations observed in chlorophyll and carotenoid contents (Fig. 2I–IV) were possibly related to oxidative stress, as verified by MDA and H2O2 concentrations (Fig. 5I, II), which was corroborated by their significant correlation (Fig. 7).
Carbon and nitrogen compounds: starch and ureides accumulated in leaves
Starch is the main storage product in plants (Kuai et al. 2014). Its accumulation (Fig. 3I) suggested a blockage of carbohydrate transport to sink tissues and the decreased export of triose phosphates to the cytoplasm, resulting in lower contents of sucrose and total carbohydrates (Fig. 3II, III). The lower dry weight of partitioned biomass under stresses (Fig. 6II–IV) supported this hypothesis. Previous studies have shown similar results; for example, Wample and Davis 1983 found that sucrose translocation from the leaves to roots was blocked in waterlogged soil, resulting in an accumulation of starch in the leaf tissue of sunflower (Helianthus annuus). Kuai et al. (2014) reported that sucrose transport from the subtending leaves to the boll in cotton (Gossypium hirsutum) was irreversibly blocked after three days of soil waterlogging.
Ureides accumulation in soybean leaves under water deficit has previously been described (Vadez et al. 2000; King and Purcell 2005). These authors suggested that the accumulation of ureides could have a regulatory role, participating in an N-feedback inhibition of nitrogenase. However, a study by Gil-Quintana et al. (2013) using a split-root system rejected this hypothesis, since ureide content increased in the stressed nodules later than the inhibition of N2-fixation. It remains to be determined whether the accumulation of ureides in leaf tissue under soil waterlogging is a signal for the inhibition of N2-fixation, which opens up avenues for further research in this segment. The influence of Fe levels on ureides accumulation in soybean leaves may be related to Mn2+ availability. Izaguirre-Mayoral and Sinclair (2005) found that high Fe levels resulted in decreased Mn levels in soybean leaves, and proposed that the inhibition of allantoate amidohydrolase activity is caused by deficiency of its Mn2+ co-factor. This enzyme hydrolyses allantoate to ureideglycolate (Watanabe et al. 2014). Thus, allantoate accumulation may prevent stored allantoin in peroxisomes from moving to the endoplasmic reticulum, where ureides catabolism occurs.
According to Oliveira et al. (2013), nitrate uptake by soybean plants is lower under hypoxia than under normoxia, and the location at which it is metabolised is changed, occurring in the leaves under hypoxia and in the roots under normoxia. The nitrate content found in soybean leaves in this study did not differ between the two water conditions at levels of 125 and 500 mg dm−3 Fe; however, there was an increase of its content in plants exposed to soil waterlogging compared to those in non-waterlogged soil at 25 mg dm−3 Fe. FEAS (Fig. 6IV) showed a significant correlation with nitrate (Fig. 7), suggesting the influence on nitrate dynamics in soybean plants. In addition, protein increase in this cultivation situation (non-waterlogged soil at 25 mg dm−3 Fe) may be related to the formation of chelators or other substances to attenuate ROS.
Toxicity and partitioned biomass weights
Plants cultivated in waterlogged soil had higher FEAS values (Fig. 6IV), which were potentially toxic to plants, as this catalyses H2O2 decomposition, generating HO∙ (Fenton reaction). Fe3+ produced by this reaction can be reduced to Fe2+ by O∙−2 (Haber–Weiss reaction), allowing Fe2+ to participate in the Fenton’s reaction again (Becana et al. 1998; Hell and Stephan 2003). In addition, waterlogged soil can affect the dynamics of the functioning of the photosynthetic apparatus, leading to the leakage of ions from the electron transport chain to O2, inducing the over-production of 1O2 and H2O2 in PSII (Barbosa et al. 2014; Zheng et al. 2017). In addition, the reduced ferredoxin electron can be transferred to O2, instead of to NADP, generating O∙−2 at the acceptor side of PSI (Barbosa et al. 2014; Yan et al. 2018). These events might explain the higher H2O2 and MDA concentrations in soybean leaves due to soil waterlogging and excess Fe (Fig. 5I, II), which was associated with a loss of dry biomass (Fig. 7).
Soybean plants had severely decreased dry biomass with stress imposition (Fig. 6I–III). In particular, soil waterlogging was what most impacted PDW (Fig. 6III), being extremely harmful to soybeans at this phenological stage. This progressive loss of biomass and plant performance was a result of the physiological and biochemical changes documented in the current study (Figs. 1, 2, 3, 4, 5); however, other factors may have also contributed: (I) a reduction in soil redox and consequently a reduction in uptake capacity tending towards a decrease in plant N and P concentrations (Dat et al. 2004); (II) a change from oxidative phosphorylation to glycolysis and fermentation due to the absence of O2 in the root tissue (Sairam et al. 2009; Martínez-Alcántara et al. 2012); and (III) the ability of plants to mitigate excessive Fe uptake through exclusion mechanisms and/or by storing/remobilising absorbed Fe (Müller et al. 2017).
Conclusion
Gas exchange was strongly affected by soil waterlogging. Excess Fe without soil waterlogging reduced photosynthetic pigments, and potentiated this reduction when associated with soil waterlogging.
Starch and ureide accumulation in the first newly expanded trifoliate leaves proved to be response mechanisms induced by soil waterlogging and excess Fe, since plants cultivated under soil non-waterlogged soil at 25 mg dm−3 Fe showed lower contents when compared to stressed plants. Thus, starch and ureide accumulation could be considered efficient biomarkers of phytotoxicity caused by soil waterlogging and excess Fe in soybean plants.
The reproductive development was abruptly interrupted by the exposure to stresses, leading to a loss of pod dry biomass, which was largely due to the substantial decrease in A, the blockage of carbohydrate transport to sink tissues, and an increase in MDA. The negative effect on reproductive development was more pronounced under waterlogged soil.
Future studies should seek to understand the role of starch and ureide accumulation in soybean leaves induced by these stresses. In addition, new studies should be directed to elucidate the strategies used by soybean plants to avoid Fe toxicity under anoxia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) and the São Paulo Research Foundation (FAPESP; grant number #2018/17380-4), for scholarships supporting the first author.
Abbreviations
- ATP
Adenosine triphosphate
- A
Net photosynthetic rate
- gs
Stomatal conductance
- E
Transpiration rate
- EiC
Instantaneous carboxylation efficiency
- WUE
Water use efficiency
- Chl a
Chlorophyll a
- Chl b
Chlorophyll b
- Tchl
Total chlorophyll
- CAR
Carotenoids
- PSI
Photosystem I
- PSII
Photosystem II
- HO∙
Hydroxyl
- 1O2
Singlet oxygen
- H2O2
Hydrogen peroxide
- O∙−2
Superoxide anion
- ROS
Reactive oxygen species
- MDA
Malondialdehyde
- SDW
Shoot dry weight
- PDW
Pod dry weight
- RDW
Root dry weight
- FEAS
Fe accumulation in the shoots
- ABS
Absorbance
- ANOVA
Analysis of variance
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LA, van Dongen JT. Making sense of low oxygen sensing. Trends Plant Sci. 2012;17:129–138. doi: 10.1016/j.tplants.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Barbosa MR, Silva MMA, Willadino L, Ulisses C, Camara TR. Geração e desintoxicação enzimática de espécies reativas de oxigênio em plantas. Ciência Rural. 2014;44:453–460. doi: 10.1590/S0103-84782014000300011. [DOI] [Google Scholar]
- Becana M, Moran JF, Iturbe-Ormaetxe I. Iron-dependent oxygen free radical generation in plants subjected to environmental stress: toxicity and antioxidant protection. Plant Soil. 1998;201:137–147. doi: 10.1023/A:1004375732137. [DOI] [Google Scholar]
- Bieleski RL, Turner NA. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966;17:278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cataldo DA, Maroon M, Schrader LE, Youngs VL. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal. 1975;6:71–80. doi: 10.1080/00103627509366547. [DOI] [Google Scholar]
- Dat JF, Capelli N, Folzer H, Bourgeade P, Badot PM. Sensing and signalling during plant flooding. Plant Physiol Biochem. 2004;42:273–282. doi: 10.1016/j.plaphy.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Divito GA, Echeverría HE, Andrade FH, Sadras VO. Diagnosis of S deficiency in soybean crops: performance of S and N: S determinations in leaf, shoot and seed. Field Crops Research. 2015;180:167–175. doi: 10.1016/j.fcr.2015.06.006. [DOI] [Google Scholar]
- Dufey I, Hakizimana P, Draye X, Lutts S, Bertin P. QTL mapping for biomass and physiological parameters linked to resistance mechanisms to ferrous iron toxicity in rice. Euphytica. 2009;167:143–160. doi: 10.1007/s10681-008-9870-7. [DOI] [Google Scholar]
- Fehr WR, Caviness CE, Burmood DT, Pennington JS. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill1. Crop Sci. 1971;11:929. doi: 10.2135/cropsci1971.0011183X001100060051x. [DOI] [Google Scholar]
- Frei M, Tetteh RN, Razafindrazaka AL, Fuh MA, Wu LB, Becker M. Responses of rice to chronic and acute iron toxicity: genotypic differences and biofortification aspects. Plant Soil. 2016;408:149–161. doi: 10.1007/s11104-016-2918-x. [DOI] [Google Scholar]
- Gil-Quintana E, Larrainzar E, Seminario A, Díaz-Leal JL, Alamillo JM, Pineda M, Arrese-Igor C, Wienkoop S, González EM. Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. J Exp Bot. 2013;64:2171–2182. doi: 10.1093/jxb/ert074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hell R, Stephan UW. Iron uptake, trafficking and homeostasis in plants. Planta. 2003;216:541–551. doi: 10.1007/s00425-002-0920-4. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Roobavannan M, Sujan K, Lisako K, Dai Y, Satoshi W, Hyungjun K, Shinjiro K. Global flood risk under climate change. Nat Clim Change. 2013;3:816–821. doi: 10.1038/nclimate1911. [DOI] [Google Scholar]
- Izaguirre-Mayoral ML, Sinclair TR. Soybean genotypic difference in growth, nutrient accumulation and ultrastructure in response to manganese and iron supply in solution culture. Ann Bot. 2005;96:149–158. doi: 10.1093/aob/mci160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucoski GO, Cambraia J, Ribeiro C, Oliveira JA, Paula SO, Oliva MA. Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. Acta Physiol Plant. 2013;35:1645–1657. doi: 10.1007/s11738-012-1207-4. [DOI] [Google Scholar]
- King CA, Purcell LC. Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol. 2005;137:1389–1396. doi: 10.1104/pp.104.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai J, Liu Z, Wang Y, Meng Y, Chen B, Zhao W, Oosterhuis DM. Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton boll and its relationship with boll weight. Plant Sci. 2014;223:79–98. doi: 10.1016/j.plantsci.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- Linkemer G, Board JE, Musgrave ME. Waterlogging effects on growth and yield components in late-planted soybean. Crop Sci. 1998;38:1576. doi: 10.2135/cropsci1998.0011183X003800060028x. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Shimamura S, Shimada S. Effects of short-term waterlogging on soybean nodule nitrogen fixation at different soil reductions and temperatures. Plant Prod Sci. 2011;14:349–358. doi: 10.1626/pps.14.349. [DOI] [Google Scholar]
- Malavolta E, Vitti GC, Oliveira SA. Avaliação do estado nutricional de plantas: princípios e aplicações. 2. Piracicaba: Potafos; 1997. p. 319p. [Google Scholar]
- Maranguit D, Guillaume T, Kuzyakov Y. Effects of flooding on phosphorus and iron mobilization in highly weathered soils under different land-use types: short-term effects and mechanisms. CATENA. 2017;158:161–170. doi: 10.1016/j.catena.2017.06.023. [DOI] [Google Scholar]
- Martínez-Alcántara B, Jover S, Quiñones A, Forner-Giner MA, Rodríguez-Gamir J, Legaz F, Primo-Millo E, Iglesias DJ. Flooding affects uptake and distribution of carbon and nitrogen in citrus seedlings. J Plant Physiol. 2012;169:1150–1157. doi: 10.1016/j.jplph.2012.03.016. [DOI] [PubMed] [Google Scholar]
- McCullough H. The determination of ammonia in whole blood by a direct colorimetric method. Clin Chim Acta. 1967;17:297–304. doi: 10.1016/0009-8981(67)90133-7. [DOI] [PubMed] [Google Scholar]
- Mohanty B. Contrasting effects of submergence in light and dark on pyruvate decarboxylase activity in roots of rice lines differing in submergence tolerance. Ann Bot. 2003;91:291–300. doi: 10.1093/aob/mcf050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C, Silveira SFS, Daloso DM, Mendes GC, Merchant A, Kuki KN, Oliva MA, Loureiro ME, Almeida AM. Ecophysiological responses to excess iron in lowland and upland rice cultivars. Chemosphere. 2017;189:123–133. doi: 10.1016/j.chemosphere.2017.09.033. [DOI] [PubMed] [Google Scholar]
- Oliveira HC, Freschi L, Sodek L. Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency. Plant Physiol Biochem. 2013;66:141–149. doi: 10.1016/j.plaphy.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Pereira EG, Oliva MA, Rosado-Souza L, Mendes GC, Colares DS, Stopato CH, Almeida AM. Iron excess affects rice photosynthesis through stomatal and non-stomatal limitations. Plant Sci. 2013;201–202:81–92. doi: 10.1016/j.plantsci.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Pinto E, Sigaud-Kutner TCS, Leitão MAS, Okamoto OK, Morse D, Colepicolo P. Heavy metal-induced oxidative stress in algae 1. J Phycol. 2003;39:1008–1018. doi: 10.1111/j.0022-3646.2003.02-193.x. [DOI] [Google Scholar]
- Puiatti M, Sodek L. Waterlogging affects nitrogen transport in the xylem of soybean. Plant Physiol Biochem. 1999;37:767–773. doi: 10.1016/S0981-9428(00)86690-5. [DOI] [Google Scholar]
- Quaggio JA, van Raij B, Malavolta E. Alternative use of the SMP-buffer solution to determine lime requirement of soils. Commun Soil Sci Plant Anal. 1985;16:245–260. doi: 10.1080/00103628509367600. [DOI] [Google Scholar]
- Reis AR, Barcelos JPQ, Osório CRWS, Santos EF, Lisboa LAM, Santini JMK, Santos MJD, Furlani Junior E, Campos M, Figueiredo PAM, Lavres J, Gratão PL. A glimpse into the physiological, biochemical and nutritional status of soybean plants under Ni-stress conditions. Environ Exp Bot. 2017;144:76–87. doi: 10.1016/j.envexpbot.2017.10.006. [DOI] [Google Scholar]
- Rodríguez-Gamir J, Ancillo G, Carmen González-Mas M, Primo-Millo E, Iglesias DJ, Forner-Giner MA. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol Biochem. 2011;49:636–645. doi: 10.1016/j.plaphy.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Sairam RK, Dharmar K, Chinnusamy V, Meena RC. Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata) J Plant Physiol. 2009;166:602–616. doi: 10.1016/j.jplph.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Santos HG, Jacomine PKT, Anjos LHC, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JA, Cunha TJF, Oliveira JB. Sistema Brasileiro de Classificação de Solos. 5. Rio de Janeiro: Embrapa; 2018. p. 531. [Google Scholar]
- Sartori GMS, Marchesan E, De David R, Donato G, Coelho LL, Aires NP, Aramburu BB. Sistemas de preparo do solo e de semeadura no rendimento de grãos de soja em área de várzea. Ciência Rural. 2015;46:492–498. doi: 10.1590/0103-8478cr20150676. [DOI] [Google Scholar]
- Singh SP, Setter TL. Effect of Waterlogging on Element Concentrations, Growth and Yield of Wheat Varieties Under Farmer’s Sodic Field Conditions. Proc Natl Acad Sci India Sect B: Biol Sci. 2017;87:513–520. doi: 10.1007/s40011-015-0607-9. [DOI] [Google Scholar]
- Syed NH, Prince SJ, Mutava RN, Patil G, Li S, Chen W, Babu V, Joshi T, Khan S, Nguyen HT. Core clock, SUB1 and ABAR genes mediate flooding and drought responses via alternative splicing in soybean. J Exp Bot. 2015;66:7129–7149. doi: 10.1093/jxb/erv407. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Tian L, Li J, Bi W, Zuo S, Li L, Li W, Sun L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agric Water Manag. 2019;218:250–258. doi: 10.1016/j.agwat.2019.03.054. [DOI] [Google Scholar]
- Umbreit WW, Kingsley GR, Schaffert RR, Siplet H. A colorimetric method for transaminase in serum or plasma. J Lab Clin Med. 1957;49:454–459. [PubMed] [Google Scholar]
- Vadez V, Sinclair TR, Serraj R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol Plant. 2000;110:215–223. doi: 10.1034/j.1399-3054.2000.110211.x. [DOI] [Google Scholar]
- Vogels GD, Van Der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970;33:143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Wample RL, Davis RW. Effect of flooding on starch accumulation in chloroplasts of sunflower (Helianthus annuus L.) Plant Physiol. 1983;73:195–198. doi: 10.1104/pp.73.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Matsumoto M, Hakomori Y, Takagi H, Shimada H, Sakamoto A. The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism: purine catabolism in ABA-mediated stress tolerance. Plant Cell Environ. 2014;37:1022–1036. doi: 10.1111/pce.12218. [DOI] [PubMed] [Google Scholar]
- Wientjes E, Philippi J, Borst JW, van Amerongen H. Imaging the Photosystem I/Photosystem II chlorophyll ratio inside the leaf. Biochimica et Biophysica Acta (BBA) Bioenergetics. 2017;1858:259–265. doi: 10.1016/j.bbabio.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Xu S, Lin D, Sun H, Yang X, Zhang X. Excess iron alters the fatty acid composition of chloroplast membrane and decreases the photosynthesis rate: a study in hydroponic pea seedlings. Acta Physiol Plant. 2015;37:212. doi: 10.1007/s11738-015-1969-6. [DOI] [Google Scholar]
- Yan K, Zhao S, Cui M, Han G, Wen P. Vulnerability of photosynthesis and photosystem I in Jerusalem artichoke (Helianthus tuberosus L.) exposed to waterlogging. Plant Physiol Biochem. 2018;125:239–246. doi: 10.1016/j.plaphy.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Komatsu S. Comprehensive analysis of response and tolerant mechanisms in early-stage soybean at initial-flooding stress. J Proteom. 2017;169:225–232. doi: 10.1016/j.jprot.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Lu H, Kong X, Dai J, Li Z, Dong H. Growth, lint yield and changes in physiological attributes of cotton under temporal waterlogging. Field Crops Res. 2016;194:83–93. doi: 10.1016/j.fcr.2016.05.006. [DOI] [Google Scholar]
- Zheng XD, Zhou JZ, Tan DX, Wang N, Wang L, Shan DQ, Kong J. melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh seedlings by maintaining aerobic respiration, photosynthesis and ros migration. Front Plant Sci. 2017;08:1. doi: 10.3389/fpls.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.