Abstract
The RP-HPLC based comparative quantification of some important redox sensitive phenolic acids and flavonoids revealed overall greater elicitation of chalcone synthase related flavonoid biosynthetic pathway, concomitant with the greater utilization of cinnamic acid for the seedlings of the salinity resistant rice cultivar Patnai as compared to susceptible cultivar IR29 grown under post imbibitional salinity stress (PISS). When compared, the cultivar Patnai further exhibited significantly better antioxidant-coupled redox-regulation by up regulating ascorbate–glutathione pathway and reducing the expression of oxidative deterioration under PISS as compared to its counterpart, the cultivar IR29. A model for redox homeostasis in which complementation of action of ROS scavenging secondary metabolites with enzymatic antioxidant defense at metabolic interface necessary for maintenance of the redox homeostasis to combat salinity stress has been proposed.
Keywords: Salinity stress, Phenolic acids, Flavonoids, Ascorbate–glutathione pathway, Redox regulation, Rice
Introduction
Salinity induced loss of metabolic coordination with uncoupling of different metabolic pathways always leads to loss of redox homeostasis due to changes in ROS-antioxidant interaction dynamics (Miller et al. 2010; Hazmana et al. 2016; Bhattacharjee 2019). ROS-antioxidant interaction dynamics is not only significant in down -regulating oxidative damages but also in triggering signaling role of ROS under stress (Bhattacharjee and Dey 2018; Bhattacharjee 2019). Generally, plants are equipped with an array of anti-oxidative defense system, both enzymatic and non- enzymatic, not only to prevent oxidative damage, but to tightly regulate the endogenous titer of ROS under salinity (Miller et al. 2010; Bhattacharjee 2019). Out of several important anti-oxidative pathways, ascorbate–glutathione cycle with other hydrogen peroxide processing system has been regarded as the most potent one for combating oxidative stress and restoring redox homeostasis (Miller et al. 2010; Bhattacharjee and Dey 2018; Chakrabarty et al. 2019; Bhattacharjee 2019).
Though ascorbate and glutathione remain as major water soluble redox active molecule and occupy the center of the “Redox Hub” (Foyer and Noctor 2011; Bhattacharjee 2019) for their ability to buffer the endogenous concentration of ROS H2O2, the role of the most diverse group of secondary metabolite phenolics cannot be ruled out. Phenolics, being the products of secondary metabolism involving phenyl propanoid, shikimic acid and pentose phosphate pathway have added advantage for their ability to reduce the source of ROS by inhibiting Fenton type reaction apart from quenching ROS (Minh et al.2016; Bhattacharjee 2019; Dey and Bhattacharjee 2020). So, it may be extremely interesting to study the choice of plant towards anti-oxidative defense for their restoration of redox homeostasis under salinity stress, particularly at a time when they are in active growth phase.
Germplasm of rice exhibits variations in their redox regulatory mechanisms and hence their sensitivity towards salinity stress (Zagorchev et al. 2016). Plants exposed to salinity stress thus obviously leave the imprints of ROS regulatory mechanism and oxidative deterioration events. Techniques to measure and assess redox metabolic fingerprints (in terms of utilization of ascorbate–glutathione cycle and polyphenolic compounds) and redox status (in terms of pro oxidant/antioxidant ratio and oxidation by-products of membrane lipid and protein) are of immense importance and hence are exploited in the present study in context of salinity tolerance of two contrasting rice germplasm differing in sensitivity towards salinity (Oryza sativa L, cultivars IR29, Patnai). Further, the differential accumulation of chalcone synthase related flavonoids and cinnamic acid-dependent phenolic acids in the experimental cultivars are also investigated to understand the nature of elicitation of this secondary metabolite pathway under salinity induced oxidative stress.
Materials and methods
Salinity treatment and assessment of germination phenotypes
Seeds of two indica rice cultivars (Oryza sativa L., Cultivars Patnai, IR29), differing in sensitivity towards NaCl salinity stress (Mukta et al. 2017) were collected from CRRI Cuttack, Orissa, India. Seeds of each cultivar were washed with distilled water and surface sterilized with 0.2% HgCl2 solution for 5 min. Finally, sterilized seeds were washed thrice in distilled water and imbibed at sterile distilled water in darkness at 25° ± 2 °C, for 24 h. Thereafter, water imbibed seeds were plated to impose post-inbibitional salinity stress at 150 mM and 250 mM NaCl concentrations for 24 h at 25 °C with 14 h photoperiod (270 µm m−1 S−1) and 65 ± 2% relative humidity. After the imposition of post-imbibitional salinity stress, germinating seeds were allowed to grow for next 72 h in environmental chamber maintained at temperature 25° ± 2 °C, relative humidity 65 ± 2% and 14 h photoperiod with 270 µm m−1 S−1 illumination. For studying ROS-antioxidant interaction dynamics, 120 h old tissues (actual age of the seedling since imbibitions and 72 h post-treatment development) were used.
Quantitative assessment of phenolic acids and flavonoids by RP-HPLC
Samples were prepared through soxhlet mediated hydroethanoic extraction of dry powdered seedlings followed by rotary vacuum evaporation for concentrating the samples (Dey and Bhattacharjee 2020). For HPLC study, 20 µl solution was taken. HPLC analyses were performed using Dionex Ultimate 3000 liquid chromatograph including a diode array detector (DAD) with 5 cm flow cell and with Chromeleon system manager as data processor. Separation was achieved by a reversed-phase Acclaim C18 column (5 micron particle size, 250 × 4.6 mm) according to the process of Aditya and Bhattacharjee (2017). For the preparation of standard stock solutions of twenty-one phenolic acids and flavonoids like Gallic acid, Protocatechuic acid, Gentisic acid, p-Hydroxy benzoic acid, Catechin, Chlorogenic acid, Vanillicacid, Caffeic acid, Syringic acid, p-Coumaric acid, Ferullic acid, Cinnamic acid, Salicylic acid, Naringinin, Rutin, Ellagic acid, Myricetin, Quercetin, Naringenin, Apigenin and Kaempferol, methanol was used as solvent to get a final concentration of 10 μg ml−1. All standard solutions were filtered through HPLC filter (0.45 mm membrane filter, Milipore).
Estimation of total phenol and flavonoids
For determination of total phenol and flavonoids, the methods of Chang et al. (2002) were followed.
In-situ localization of H2O2 by laser confocal microscopy
The sample preparation was done by following the method of Kaur et al. (2016). 1 cm pieces of root segments of 3 days old seedlings of experimental rice cultivars were dipped immediately in 10 µM H2DCFDA solution and kept at room temperature. After 2 h, samples were washed thrice with autoclaved milliQ water and slides were prepared with 20% glycerol. Accumulation of H2O2 in roots was identified by DCFDA staining and Confocal microscopy using Leica application suite X software (microscope model number was Leica TCS SP8, laser scanning mode 488 nm, emission at 505–530 nm, objective used was 20X) in differentially grown seedlings (3 days old) raised from post-imbibitional salinity stressed condition. Green fluorescence indicated presence of H2O2.
Estimation of O2−. and H2O2 and “total ROS”
For the determination of Superoxide, the method of Chaitanya and Naithani (1994) was followed. Hydrogen peroxide was extracted and estimated following the procedure of MacNevin and Uron (1953) using titanic sulfate.
Total ROS in vivo assay was performed spectroflurometrically by placing seedling tissue (50 mg) in 8 mL 40 mM TRIS–HCl buffer (pH 7) in presence of 100 µM 2′,7′-dichloroflorescindiacetate (DCFDA, Sigma) at 30 °C. Supernatant was removed after 60 min and fluorescence was monitored in a spectroflurometer (Hitachi, Model F-4500 FL Spectrophotometer) with excitation at 488 nm and emission at 521 nm. (Simontacchi et al.1993).
DPPH (2,2′-diphenyl-1-pycryl hydrazyl) free radical scavenging activity
For determination of DPPH free radical scavenging activity, the process of Mensor et al. (2001) was followed.
FRAP assay
This was carried out according to Benzie and Strain (1996).
ABTS decolorization assay
ABTS [2,2′ azinobis (3-ethylbenzthiazoline)-6-sulfonic acid] free radical decolourization assay was done according to Re et al. (1999).
Determination of total thiol content
Total thiol content was assayed in acid soluble extracts (0.2 g FW/ml) as described by Tietze (1969).
Estimation of reduced ascorbate and glutathione
Determination of reduced ascorbate and glutathione content was performed according to the process of Hodges et al. (2001). Total glutathione contents were determined by the absorbance at 570 nm according to the method of Bhattacharjee and Dey (2018). The contents of glutathione (reduced form) were estimated from the standard curve of 0–20 nmol glutathione.
Assessment of the activities of APOX, DHAR, GR, CAT
Ascorbate peroxidase (APOX, EC 1.11.1.11) activity was determined according to Nakano and Asada (1981).
Dehydroascorbate reductase (DHAR, EC 1.8.5.1) activities were determined according to Song et al. (2005). Glutathione reductase (GR, EC 1.6.4.2) activity was measured according to Schaedle and Bassham (1977). For the extraction and estimation of catalase (CAT, EC 1.11.1.6), the process of Snell and Snell (1971) was followed.
Assessment of biomarker of oxidative stress
Oxidative damage to protein was estimated as the content of carbonyl groups following the procedures of Jiang and Zhang (2001). The membrane lipid peroxidations of tissues were estimated in terms of malondialdehyde accumulation. To estimate MDA content, the TBA (thiobarbituric acid) test was performed using the procedure of Heath and Packer (1968). Hydroperoxides was estimated according to the process of Nourooz-Zadeh et al. (1995) with necessary modifications. For the extraction and estimation of conjugated diene, the process of Buege and Aust (1978) was followed. Lipoxygenase activity (LOX, EC 1.13. 11.12) was estimated following the procedure of Peterman and Siedow (1985).
Determination of germination and early growth phenotypes
For studying the impact of PISS on germination and early growth phenotypes of two experimental cultivars (Patnai and IR29), Relative growth index (RGI), Relative germination performance (RGP), Coefficient of velocity of germination (CVG), Mean germination time (MGT), Germination rate index (GRI), Mean daily germination (MDG), Germination energy (GE) and Time in hour for attaining 50% germination (T50) were assessed and compared according to Rubio-Casal et al. (2003) and Bhattacharjee and Dey (2018).
Results
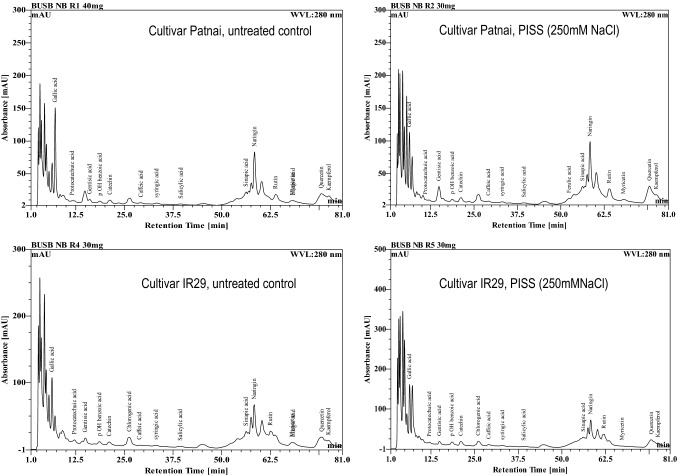
RP-HPLC data of PISS-raised seedlings of the cultivar Patnai exhibited enhanced accumulation, of gentisic acid, P-OH benzoic acid, caffeic acid, syringic acid and salicylic acid (Fig. 1, Table 1). On the contrary, there seemed to be a significant down-regulatory trend in the accumulation of protocatechuic acid, gentisic acid, and salicylic acid for the PISS raised cultivar IR29 (Fig. 1, Table 1). Ellagic acid, which was present in untreated control seedlings, was found to disappear completely in PISS-raised seedlings of IR29 (Fig. 1, Table 1). RP-HPLC based comparative analysis of six important flavonoids revealed significantly greater accumulation of catechine, naringenin, rutin in the cultivar patnai as compared to the cultivar IR29 (Fig. 1, Table 1). Further, under PISS, seedlings of IR29 exhibited significant down-regulation (49.4%) in accumulation of redox-sensitive flavonoid quercetin over their untreated control, which was otherwise up-regulated substantially (159%) for the cultivar Patnai (Fig. 1, Table 1). Though, both the cultivars exhibited accumulation of naringenin and rutin in the PISS-raised seedlings, but when compared, the cultivar Panai exhibited significantly higher accumulation than its counterpart IR29 (Table 1). Kaempferol, on the other hand exhibited down regulation in PISS raised seedlings of both the experimental cultivars.
Fig. 1.
RP-HPLC chromatogram of important phenolic acids and flavonoids extracted from 120 h old untreated control and PISS-raised (250 mM NaCl stress) seedling of rice (Oryza sativa L. Cultivar Patnai & IR29)
Table 1.
Post imbibitional salinity stress (PISS) induced changes in endogenous titer of some impotant redox sensitive phenolic acids and flavonoids along with the total pool of phenolics and flavonoids in two indica rice cultivars differing in sensitivity towards NaCl salinity stress (Oryza sativa L. Cultivars Patnai & IR29)
| Rice cultivars | Treatment | Total phenol (µg g−1dm) | Accumulation of redox sensitive phenolic acids (mg/100 g dm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | Protocatechuic acid | Gentisic acid | p OH benzoic acid | Chlorogenic acid | Caffeic acid | Syringic acid | Salicylic acid | Cinnamic acidc a | Ellagic acid | |||
| Oryza sativa L. Cultivar Patnai | Untreated control | 389.4 ± .08 | 31.654 | 0.16727 | 24.527 | 1.709 | _ | 0.5818 | 0.8909 | 3.0363 | 2.5270 | 0.1818 |
| PISS (250 mM NaCl) |
477.87 ± .25** (+22.72%) |
30.2726 (-4.36%) |
0.01818 (-8.68%) |
480.872 (+1860%) |
2.7363 (+60%) |
_ |
0.72727 (+25%) |
1.0545 (+18.36%) |
5.09 (+67.66%) |
3.6727 (+45.33%) |
0.2695 (+48.23%) |
|
| Oryza sativa L. Cultivar IR29 | Untreated control | 366.6 ± .15 | 31.36 | 3.381 | 173.5 | 2.818 | 284 | 0.509 | 1.054 | 5.745 | 105.9 | 20.98 |
| PISS (250 mM NaCl) |
390.42 ± .26** (+6.49%) |
45.4 (+44%) |
1.32 (-60.9%) |
107.7 (-37%) |
6.52 (+131%) |
267 (7%) |
1.928 (+278%) |
1.236 (+17.26%) |
5.527 (-3.79%) |
128.10 (+20%) |
– | |
| Rice cultivars | Treatment | Total flavonoids (µg g−1dm) | Accumulation of redox sensitive flavonoids (mg/100 g dm) | |||||
|---|---|---|---|---|---|---|---|---|
| Catechin | Naringin | Rutin | Myricetin | Quercetin | Kaempferol | |||
| Oryza sativa L. Cultivar Patnai | Untreated control | 384.075 ± .016 | 45.1 | 635.8 | 258.4 | 93.8 | 311.3 | 47.4 |
| PISS (250 mM NaCl) |
334.6 ± .017** (-13.06%) |
80.8 (+79%) |
1067.6 (+67%) |
598.7 (+131%) |
26.4 (-71%) |
806.9 (+159%) |
7.3 (-84%) |
|
| Oryza sativa L. Cultivar IR29 | Untreated control | 292.4 ± .32 | 57 | 331.9 | 52.6 | 123.5 | 535.2 | 8.8 |
| PISS (250 mM NaCl) |
113.85 ± .18** (-61%) |
117.6 (+106%) |
355.6 (+7.14%) |
75.77 (+44%) |
19.3 (-84%) |
270.7 (-49.4%) |
8.4 (-4.5%) |
|
Values are mean of three independent replicates ± standard error. * or ** represent the values which are significantly different at 0.01, 0.05 levels respectively, assessed through student t test. Values with in parenthesis represents % increment or reduction under PISS over control
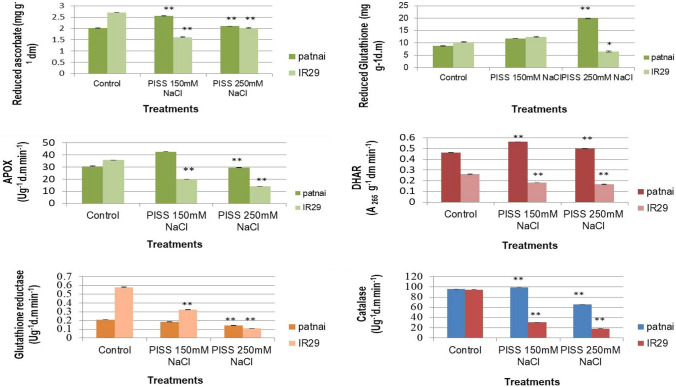
The redox state of the low molecular weight antioxidants as ascorbate and glutathione were found to be more inclined toward reduced state under different magnitude of PISS for the salt resistant cultivar Patnai compared to sensitive cultivar IR29 (Fig. 2). The cultivar IR 29 suffered significant loss of redox homeostasis due to the loss of reduced form of ascorbate and glutathione compared to Patnai, particularly under higher magnitude of PISS (Fig. 2). Post-imbibitional salinity stress of varied extent also exhibited significant variation in the activities of the enzymes of ascorbate–glutathione cycle in both the experimental cultivars (Fig. 2). The cultivar Patnai exhibited significantly greater up-regulation in the activities of APOX and DHAR. The enzyme GR maintained its activity steadily for the cultivar Patnai, even though higher magnitude (250 mM) of salinity stress was imposed (Fig. 2).
Fig. 2.
Efficiency of hydrogen peroxide processing system [assessed in terms of activities of enzymes of ascorbate–glutathione pathway (APOX, DHAR, and GR), CAT and accumulation of reduced glutathione and ascorbate] of untreated control and PISS (150 mM and 250 mM NaCl) raised 120 h old seedlings of two contrasting rice genotypes (Oryza sativa L. Cultivars of Patnai & IR29). Values are mean of three independent replicates ± standard error. * or ** represent the values which are significantly different level, at 0.01, 0.05, level, assessed through student t test
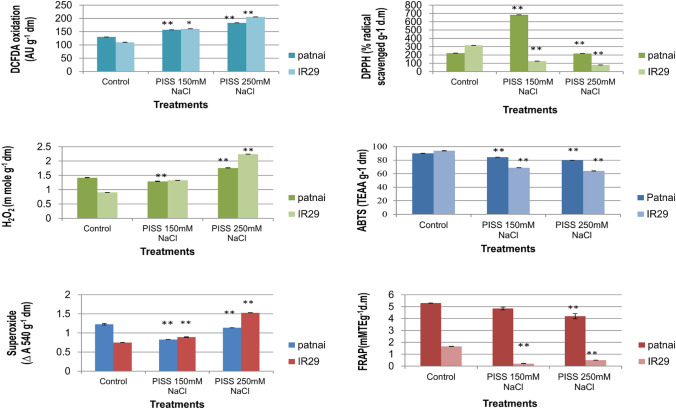
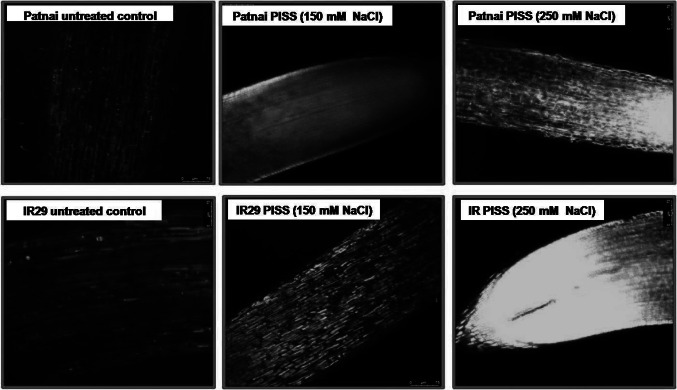
When the antioxidant competence (assessed through ABTS, DPPH, FRAP radical scavenging assay technique) was assessed and compared between the seedlings raised from post-imbibitional salinity stress of both the experimental rice cultivars, it conclusively exhibited the better antioxidant maintenance capacity or radical scavenging property in the seedlings of the salt resistant cultivars Patnai, otherwise which was found significantly reduced for the salt sensitive rice cultivar IR29 (Fig. 3). A comparison of ROS accumulation between Patnai and IR29 convincingly revealed greater accumulation of ROS [both at individual level (O2− and H2O2) and cumulative level (DCFDA oxidation)] for the salt susceptible cultivar IR29 compared to salt resistant cultivar Patnai (Fig. 3). Further, the cultivar Patnai exhibited significantly lower accumulation of ROS at cellular level as being detected histochemically by laser confocal microscopy under the same magnitude of PISS as compared to IR 29 (Fig. 4).
Fig. 3.
Changes in redox status (assessed in terms of accumulation of total ROS, H2O2, O2− and ABTS, FRAP, DPPH radical scavenging property) of PISS (150 mM and 250 mM NaCl) raised 120 h old seedlings of two contrasting cultivars of rice (Oryza sativa L. Cultivars of Patnai & IR29) differing in sensitivity towards salinity stress. Values are mean of three independent replicates ± standard error. * or ** represent the values which are significantly different level, at 0.01, 0.05, level, assessed through student t test
Fig. 4.
Accumulation of ROS in roots of untreated control and PISS-raised 120 h old seedlings identified by DCFDA staining and Confocal microscopy using Leica application suite X software (microscope model number was Leica TCS SP8, laser scanning mode 488 nm, emission at 505–530 nm, objective used was 20X) in PISS (150 mM and 250 mM NaCl) grown seedlings (5 days old). Green fluorescence indicates presence of ROS
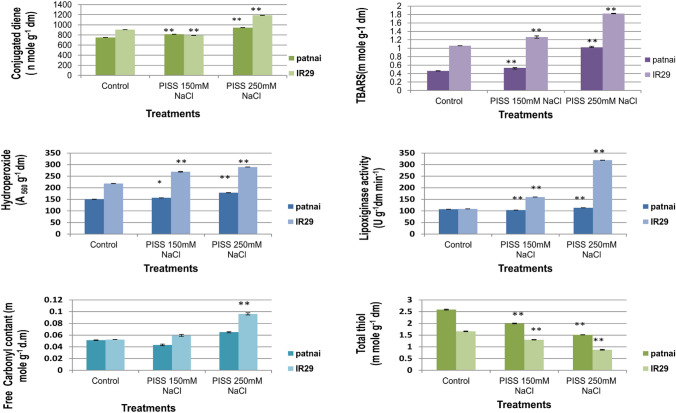
In general, a substantive rise in accumulation of redox biomarkers (hydroperoxide, TBARS, conjugated diene and free carbonyl content) for the cultivar IR29 as compared with the cultivar Patnai under similar extent of PISS (Fig. 5) was noticed. Lipoxygenase activity (responsible for enzymatic membrane lipid peroxidation) was found to be significantly higher for the cultivar IR29 as compared to Patnai (Fig. 5) under all doses of post imbibitional salinity stress. The germination phenotypes and early growth performances strongly correlated the data of accumulation of redox sensitive polyphenolic compound accumulation, efficacy of ascorbate–glutathione system and the status of redox biomarkers of the germinating tissue by large. The salt resistant cultivar Patnai, in general, exhibited better germination and early growth performances (RGI, RGP, CVG, MGT, GRI, MDG, GE and T50 value) in comparison to its counterpart IR29 under similar condition of post imbibitional salinity stress (Table 2), substantiating the central role of redox regulatory ability in the early growth process.
Fig. 5.
Assessment of redox biomarkers [assessed in terms of status of membrane lipid peroxidation (accumulation of hydroperoxide, conjugated diene, thiobarbituric acid reactive substances and lipoxygenase activity), protein oxidation (free carbonyl content) and total thiol content] of PISS-raised 120 h old seedlings of two contrasting rice genotypes differing in sensitivity towards salinity stress (Oryza sativa L. Cultivars of Patnai & IR29). Values are mean of three independent replicates ± standard error. * or ** represent the values which are significantly different level, at 0.01, 0.05, level, assessed through student t test
Table 2.
Germination and early growth performances of untreated control and PISS raised seedling of two contrasting rice genotypes (Oryza sativa L. Cultivars of Patnai & IR29)
| Experimental rice cultivars | Germination and early growth performances | ||||
|---|---|---|---|---|---|
| Treatment | RGI% | RGP% | CVG | MGT | |
| Patnai | Control | 100 ± .81 | 100 ± 0.47 | 25 ± 0.26 | 4 ± 0.25 |
| PISS 150 mM NaCl | 100.596 ± .10 | 100 ± 0.94 | 25 ± 0.26 | 4 ± 0.41 | |
| PISS 250 mM NaCl | 97.401 ± .17* | 92 ± 0.47 | 20.85 ± 0.14** | 3.097 ± 0.08* | |
| IR29 | Control | 100 ± 0.24 | 100 ± 0.24 | 25 ± 0.17 | 4 ± 0.26 |
| PISS 150 mM NaCl | 98.286 ± 0.15 | 80 ± 0.56** | 22.71 ± 0.14 | 3.628 ± 0.10 | |
| PISS 250 mM NaCl | 95.83 ± 0.38* | 72 ± 0.46** | 18.285 ± 0.15** | 2.77 ± 0.21* | |
| Experimental rice cultivars | Germination and early growth performances | ||||
|---|---|---|---|---|---|
| Treatment | GRI | MDG | GE | T50 | |
| Patnai | Control | 59.8174 ± 0.22 | 25 ± 0.16 | 100 ± 0.13 | 24 ± 0.47 |
| PISS 150 mM NaCl | 59.8174 ± 0.21 | 25 ± 0.40 | 100 ± 0.23 | 24 ± 0.5 | |
| PISS 250 mM NaCl | 48.07 ± 0.13** | 20 ± 0.27** | 80 ± 0.26* | 30 ± 0.5 | |
| IR29 | Control | 59.8174 ± 0.19 | 25 ± 0.16 | 100 ± 0.19 | 24 ± 0.82 |
| PISS 150 mM NaCl | 56.2987 ± 0.31** | 22 ± 0.29** | 88 ± 0.10** | 30 ± 0.084 | |
| PISS 250 mM NaCl | 42.88 ± 0.25** | 18 ± 0.11** | 72 ± 0.12** | 48 ± 0.04 | |
Values are mean of three independent replicates ± standard error. * or ** represent the values which are significantly different level, at 0.01, 0.05, level, assessed through student t test
Relative growth index (RGI), Relative germination performance (RGP), Coefficient of velocity of germination (CVG), Mean germination time (MGT), Germination rate index (GRI), Mean daily germination (MDG), Germination energy (GE) and Time in hour for attaining 50% germination (T50)
Discussion
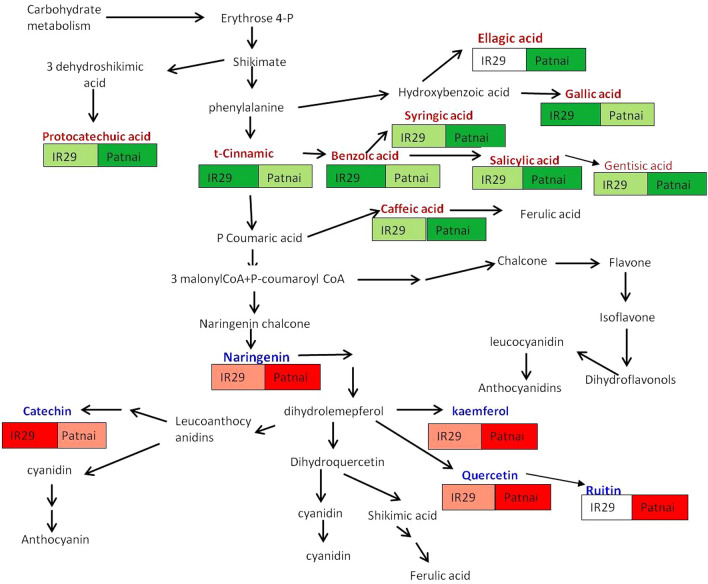
An intricate look into the biosynthetic pathway of important redox-sensitive flavonoids and phenolic acids studied for the experimental rice cultivars raised from salinity stress revealed clearly the greater utilization of cinnamic acid for the cultivar Patnai, augmenting the production of gentisic acid and syringic acid via salicylic acid (Fig. 6). Comparatively, greater accumulation of cinnamic acid and benzoic acid in IR29 might have been due to their lesser utilization for the production of syrringic acid, salicylic acid and gentisic acid (Fig. 6). Better utilization of shikimate for greater elicitation of protocatechuric acid via 3-hydroshikkimic acid has also been noticed for PISS-raised seedlings of salinity resistant cultivar Patnai (Fig. 6). Accumulation of chalcone synthase related flavonoid biosynthesis, i.e. naringenin and dihydrolemeferol- derived flavonoids catechin, quercetin and rutin revealed better elicitation of all for the salinity resistant rice cultivar Patnai when compared with its counterpart IR29 (Fig. 6). The RP-HPLC based data not only revealed greater elicitation of chalcone synthase related flavonoid biosynthetic pathway for the cultivar patnai, but also agreed well with the greater utilization of cinnamic acid in salt resistant rice germplasm. The greater reduction in the pool of kaemferol under PISS for the cultivar patnai may be due to the utilization of dihydrolemferol towards synthesis of quercetin and rutin (Fig. 6). Fini et al. (2011), Apel and Hirt (2004), Fiorani et al. (2005) put forward their views of significance of these classes of polyphenolic compounds for complementing their radical scavenging property along with enzymatic antioxidants for combating severe oxidative stress (Mishra et al. 2013; Pandey et al.2012; Lee et al. 2014). In fact, some workers also reported significant up-regulation of the enzymes phenylalanine lyase (the entry point enzyme of phenylpropanoid pathway) and chalcone synthase (first enzyme of committed flavonoid biosynthesis) under severe oxidative stress triggered by abiotic stress, as complementary mechanism of redox regulation (Fiorani et al. 2005; Lee et al. 2014). There are even evidences that flavonoids are excellent substrates for class III peroxidases for reducing H2O2, where reduced form of ascorbate functions for recycling of flavonoid radicals to their active reduced state (Fini et al. 2011).
Fig. 6.
Biosynthetic pathway of important redox-sensitive flavonoids and phenolic acids studied for the experimental rice cultivars raised from PISS highlighting differential utilization of cinnamic acid and chalcone synthase-related polyphenolic compound biosynthesis for the experimental rice cultivars (Cultivars Patnai & IR29). Color intensity within blocks under individual phenolic compounds represents their relative abundance
The elicitation of flavonoids like catechin, naringenin, ruitin, quercetin for the PISS raised seedlings of Patnai might have significant contribution in up-regulation of the activities of Halliwell-Asada pathway enzymes, particularly GR & DHAR (Lee et al. 2014, Hossain et al.2013, Anjum et al.2011), resulting better processing of ROS and mitigation of oxidative damages. So, the impact of remarkably better elicitation of polyphenolic compounds in PISS-raised seedlings of salt tolerant cultivar Patnai, apart from their direct redox buffering, might have been associated with the protection of the enzymes of Ascorbate–Glutathione cycle from oxidative damages, causing significant up-regulation in their activities as compared to the sensitive cultivar IR29 (Lee et al. 2014; Minh et al. 2016). Therefore, the maintenance of reduced state of ascorbate and glutathione pool involving ascorbate glutathione pathway for the salt resistant cultivar Patnai is closely associated with competent polyphenolic compound- associated redox regulation (Hossain et al. 2013; Zagorchev et al. 2016; Bhattacharjee and Dey 2018; Chakrabarty et al. 2019).
Appraisal of sensitive redox biomarkers, estimated in terms of the parameters of oxidative lipid and protein strongly stand for the fact that the indica rice cultivar, capable of simultaneously better elicitation of redox-sensitive polyphenolic compounds and efficacy of ASC-GSH system, possesses better redox regulatory property and hence suffers comparatively less oxidative deterioration induced by PISS in experimental cultivars (Faize et al. 2011; Basu et al. 2010; Zivcak et al. 2016; Tari et al. 2010). The significantly better capacity for elicitation of flavonoids and phenolic acids as well as the efficacy of ASC-GSH system evidently play pivotal role under PISS not only to combat oxidative damage to the juvenile tissue but to tightly regulate the endogenous redox cue necessary for germination and seedling establishment (Ali 2012; Castrillón-Arbeláez and Fryer 2016).
We suggest the role of polyphenolic compounds as one of the most promising secondary metabolite (produced through elicitation of chalcone synthase and better utilization of cinnamic acid) that complement with ascorbate–glutathione system for better redox-regulation and mitigation of salinity stress during early germination in rice. Further, the application of these metabolic redox parameters for screening of salt resistant rice cultivars is found to be extremely significant though not always decisive.
Acknowledgements
NB acknowledges The University of Burdwan, West Bengal, India, for Research & Instrumentation facility of UGC- CAS, Govt. of India to the Department of Botany (No. F.5-13/012 (SAP-II), University of Burdwan.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aditya M, Bhattacharjee S. GC-MS based evidences of rich foliar antioxidant potential of seed amaranth (Amaranthus hypochondriacus L., Accession No. IC47434) Ann Pharmacol Pharm. 2017;2(18):01–02. [Google Scholar]
- Ali MB. Secondary metabolites and environmental stress in plants: biosynthesis, regulation, and function. In: Ahmad P, Wani MR, editors. Physiological mechanisms and adaptation strategies in plants under changing environment. New York: Springer; 2012. pp. 55–85. [Google Scholar]
- Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci. 2011;197:177–185. [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Basu S, Roychowdhury A, Saha P, Sengupta DN. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010;60:51–59. [Google Scholar]
- Benzie IFE, Strain JJ. The ferric reducing ability of plasma (FRAP) as measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S (2019) ROS and oxidative stress: origin and implication. In: Reactive oxygen species in plant biology, Springer Nature, pp 1–31
- Bhattacharjee S, Dey N. Redox metabolic and molecular parameters for screening drought tolerant indigenous aromatic rice cultivars. Physiol Mol Biol Plants. 2018;24:7–23. doi: 10.1007/s12298-017-0484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Castrillón-Arbeláez PA, Fryer JPG. Secondary metabolites in Amaranthus spp.—a genomic approach to understand its diversity and responsiveness to stress in marginally studied crops with high agronomic potential. London: Intech Open Science; 2016. pp. 185–227. [Google Scholar]
- Chaitanya KSK, Naithani SC. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability of Shorea robusta Gaertn.f. New Phytol. 1994;126:623–627. [Google Scholar]
- Chakrabarty A, Banik N, Bhattacharjee S. Redox-regulation of germination during imbibitional oxidative and chilling stress in an indica rice cultivar (Oryza sativa L., Cultivar Ratna) Physiol Mol Biol Plants. 2019;25:649–665. doi: 10.1007/s12298-019-00656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Dey N, Bhattacharjee S. Accumulation of polyphenolic compounds and osmolytes under dehydration stress and their implication in redox regulation in four indigenous aromatic rice cultivars. Rice Sci. 2020;27(4):329–344. [Google Scholar]
- Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, et al. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599–2613. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal Behav. 2011;6:709–711. doi: 10.4161/psb.6.5.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani F, Umbach AL, Siedow JN. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiol. 2005;139:1795–1805. doi: 10.1104/pp.105.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazmana M, Hause B, Eiche E, Riemann M, Nick P. Different forms of osmotic stress evokes qualitatively different responses in rice. Comprehensive physiological analyses and reactive oxygen species profiling in drought tolerant rice genotypes under salinity stress. J Plant Physiol. 2016;202:45–56. doi: 10.1016/j.jplph.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photo-oxidation in isolated chloroplasts: kinetics and stoichiometry of fatty acid oxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hodges DM, Forney CF, Wisme WV. Antioxidant responses in harvested leaves of two cultivars of spinach differing in senescence rates. J Soc Hortic Sci. 2001;126:611–617. [Google Scholar]
- Hossain MA, Mostofa GM, Fujita M. Heat-shock positively modulates oxidative protection of salt and drought-stressed mustard (Brassica campestris L.) seedlings. J Pl Sci Mol Breed. 2013;2:1–13. [Google Scholar]
- Jiang M, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001;42(11):1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- Kaur N, Sharma I, Kirat K, Pati PK. Detection of reactive oxygen species in Oryza sativa L. (Rice) Bio-protocol. 2016;6:2061. doi: 10.21769/bioprotoc.2061. [DOI] [Google Scholar]
- Lee SY, Damodaran PN, Roh KS. Influence of salicylic acid on rubisco and rubisco activity in tobacco plant grown under sodium chloride in vitro. Saudi J Biol Sci. 2014;21:417–426. doi: 10.1016/j.sjbs.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNevin WM, Uron PF. Spectrum of hydrogen peroxide from organic hydroperoxides. Anal Biochem. 1953;25:1760–1761. [Google Scholar]
- Mensor LI, Menezes FS, Leitao GG, Reis AS, dosSantos T, Coube CS, Leito SG, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Minh LT, Khang DT, Ha PTT, Tuyen PT, Minh TN, Quan NV, Xuan TD. Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.) Int Lett Nat Sci. 2016;57:1–10. [Google Scholar]
- Mishra A, Kumar S, Pandey AK. Scientific validation of the medicinal efficacy of Tinospora cordifolia. Sci World J Article. 2013;11–12:292934. doi: 10.1155/2013/292934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukta S, Hossain SN, Nasiruddin KM, Islam MM. Screening of rice land races of coastal areas for salt tolerance at seedling stage using molecular markers. Asian J Biotech. 2017;09(02):71–79. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nourooz-Zadeh J, Tajaddini-Sarmadi J, Birlouez-Aragon I, Wolff SP. Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay. J Agric Food Chem. 1995;43:17–21. [Google Scholar]
- Pandey K, Mishra AK, Mishra A. Antifungal and anti-oxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamo mumtamala. Cell Mol Biol. 2012;58:142–147. [PubMed] [Google Scholar]
- Peterman T, Siedow NJ. Immunological comparison of lipoxigenase isoenzyme-1 and -2 with soyabean seedling lipoxigenases. Arch Biochem Biophys. 1985;38:476–483. doi: 10.1016/0003-9861(85)90190-0. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrinni N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activities applying an improved ABTS radical action decolonization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rubio-Casal AE, Castillo JM, Lucue CJ, Figueroa ME. Influence of salinity on germination and seed viability of two primary colonizers of Mediterranean salt plants. J Arid Environ. 2003;53:145–152. [Google Scholar]
- Schaedle M, Bassham JA. Chloroplast glutathione reductase. Plant Physiol. 1977;59:1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simontacchi M, Caro A, Fraga CG, Puntarulo S. Oxidative stress affects-tocopherol content in soyabean embryonic axes upon imbibitions. Plant Physiol. 1993;103:943–953. doi: 10.1104/pp.103.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell FD, Snell CT. Colorimetric methods of analysis. New York: Van Nostard Reinford Co; 1971. [Google Scholar]
- Song SQ, Cheng HY, Long CL, Jiang XC. Guides to seed biology research. Beijing: Science Press; 2005. pp. 97–100. [Google Scholar]
- Tari I, Kiss G, Deer AK, Csiszar J, Erdei L, Galle A, Gemes K, Horváth F, Poor P, Szepesi A, Simon LM. Salicylic acid increased aldose reductase activity and sorbitol accumulation in tomato plants under salt stress. Biol Plant. 2010;54:677–683. [Google Scholar]
- Tietze F. enzymatic method for quantitative determination of nano gram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Zagorchev L, Teofanova D, Odjakova M (2016) Ascorbate–glutathione cycle: controlling the redox environment for drought tolerance. In: Drought stress tolerance in plants volume 1: physiol and biochem. Springer pp 187–226
- Zivcak M, Brestic M, Sytar O (2016) Osmotic adjustment and plant adaptation to drought. In: Drought stress tolerance in plants volume 1: physiol and biochem. Springer, pp 105–144