Abstract
Human body surfaces, such as the skin, intestines, and respiratory and urogenital tracts, are colonized by a large number of microorganisms, including bacteria, fungi, and viruses, with the gut being the most densely and extensively colonized organ. The microbiome plays an essential role in immune system development and tissue homeostasis. Gut microbiota dysbiosis not only modulates the immune responses of the gastrointestinal (GI) tract but also impacts the immunity of distal organs, such as the lung, further affecting lung health and respiratory diseases. Here, we review the recent evidence of the correlations and underlying mechanisms of the relationship between the gut microbiota and common respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), lung cancer, and respiratory infection, and probiotic development as a therapeutic intervention for these diseases.
1. Introduction
Chronic respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD), as well as respiratory virus infection, are often accompanied by gastrointestinal diseases or symptoms [1–3]. Patients with gastrointestinal diseases, such as inflammatory bowel disease (IBD) and gastroesophageal reflux, are prone to develop pulmonary dysfunction and have an increased incidence of respiratory disease [4, 5]. These connections suggest a vital communication between the gut and lung. The human microbiome is believed to contribute to homeostasis and disease and is responsible for the interactions between these two mucosal sites. Changes in microbial composition or/and diversity will not only directly affect the colonized organ itself but also impact distant organs and systems [6]. In particular, gut microbiota dysbiosis is associated with various diseases, such as allergies, autoimmune diseases, diabetes, obesity, and cancer [7]. Recently, an increasing amount of evidence has indicated that the gut microbiota is closely related to respiratory health and disease, playing a crucial role in the development of asthma, COPD, cystic fibrosis (CF), lung cancer, and respiratory infection [8–11]. In this review, we summarize the recent findings involving the relationship and mechanisms underlying the relationship between the gut microbiota and common respiratory diseases, including asthma, COPD, CF, lung cancer, respiratory infection, and other respiratory diseases, and the use of probiotics for improving or treating these diseases.
2. The Gut and Airway Microbiome
The human gastrointestinal (GI) tract harbors approximately 1014 bacteria consisting up to 1000 different species [12], and the advance of sequencing technology has rendered the gut microbiota the most widely studied microbiome of the human body. However, the study of the airway microbiota is still in its infancy when compared with that of the gut microbiota. At the phylum level, Firmicutes and Bacteroidetes account for more than 90% of the gut microbial community [13]. The microbiota of the upper and lower respiratory tracts are distinct, with more Firmicutes and Actinobacteria in the nostril and more Firmicutes, Proteobacteria, and Bacteroidetes in the oropharynx [14], whereas there are more Bacteroidetes and Firmicutes in the lung [15]. At the genus level, Bacteroides, Faecalibacterium, and Bifidobacterium are enriched in the gut [16], while Prevotella, Veillonella, and Streptococcus are the prominent genera in the lung [15]. Although the gut and respiratory microbiota exhibit compositional differences, the epithelia of both the GI and respiratory tracts develop from a common embryonic structure, the anatomical structures and functions of the two mucosal sites are similar, and early-life microbial colonization of the gut and lung exhibits similarities. Therefore, accumulating evidence has highlighted the relationship and crosstalk between the gut and lung, referred to as the gut-lung axis [10, 17, 18].
The gut microbiota is affected by many factors, such as drugs, diet, mode of delivery, and feeding practices, which may play a role in susceptibility to respiratory diseases (Figure 1). For example, early-life acid-suppressive medications and antibiotic use, fast food consumption, caesarian-section delivery, and formula feeding are correlated with an increased risk of asthma, while a higher fiber intake, vaginal delivery, and breastfeeding are negatively correlated with asthma [19–25]. In addition, diet, smoke, and drugs, such as antibiotic and immunosuppressant, can also influence rheumatoid arthritis (RA) and the gut microbiota [26, 27].
Figure 1.
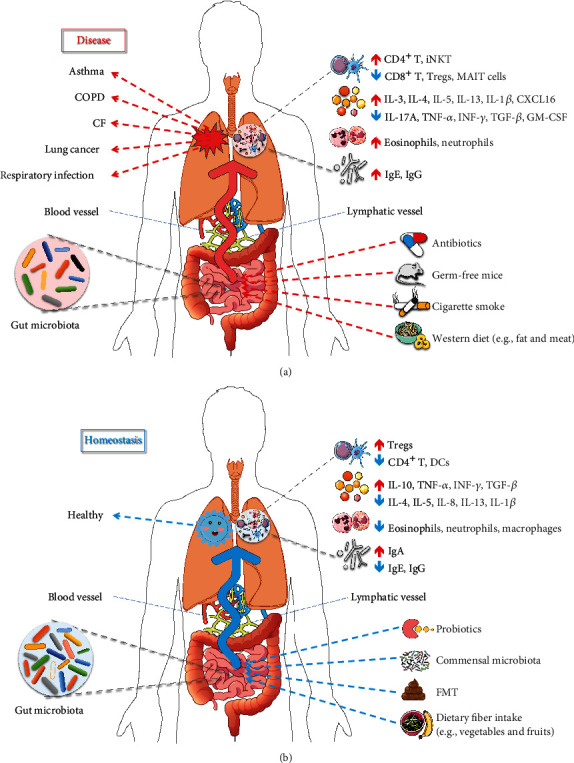
The role of gut microbiota in respiratory disease and homeostasis. The dysbiosis of gut microbiota contributes to respiratory diseases (a), while a healthy gut microbiota plays a protective role in the lung (b). The gut microbiota is influenced by several factors, including antibiotics, probiotics, cigarette smoke, diets, and fecal microbiota transplantation (FMT), and is associated with lung health and disease by regulating the respiratory immunity and inflammation through the blood and lymphatic system. ↑: increase; ↓: decrease.
3. The Gut Microbiota and Respiratory Diseases
3.1. Asthma
Asthma is a chronic airway inflammatory disease characterized by reversible airflow restriction and airway hyperresponsiveness. The increasing morbidity and mortality of asthma have made it a serious threat to human health. According to the “hygiene hypothesis,” early-life exposure to specific microbiota constituents is essential for the development and maturity of the immune system, and their absence may increase the susceptibility to asthma and allergic diseases [28]. With the advance of sequencing technologies, an increasing number of studies have revealed a close relationship between the gut microbiota and asthma. The gut microbiota is different between healthy controls (HCs) and asthmatic individuals and is associated with the development of asthma.
Higher microbial diversity is often regarded as beneficial. A recent study showed a connection between low gut microbial diversity in early life and asthma in childhood [29]. Breastfeeding may protect against asthma and allergic disease in children, and gut bacterial diversity was lower in formula-fed infants than in breastfed infants [30]. In addition to microbial diversity, specific gut bacteria have also been found to be closely related to asthma. For instance, Clostridium and Eggerthella lenta were more abundant in the gut of asthma patients than in that of HCs [31]. Furthermore, the decrease in Bifidobacterium, Akkermansia, and Faecalibacterium abundances and the increase in Candida and Rhodotorula abundances increased a child's risk of developing allergies and asthma [32], and intestinal colonization by Clostridium difficile at 1 month of age was associated with asthma at 6-7 years of age [33]. Therefore, we speculate that the well-balanced commensal microbiota in the GI tract may be beneficial to host health and that reduced microbial diversity may be a marker for underlying pathologic conditions.
Noting the altered gut microbiota in asthma patients, researchers have attempted to modulate the pulmonary immune response as well as prevent and treat asthma through improving the gut microbiota. Arrieta et al. revealed a significant reduction in Lachnospira, Veillonella, Faecalibacterium, and Rothia abundances in the gut of asthmatic infants. Furthermore, inoculations of these bacteria in germ-free (GF) mice ameliorated airway inflammation and prevented asthma development [34]. Similar results were obtained in both murine and human studies in which oral administration of Lactobacillus rhamnosus, Lactobacillus casei, and Bifidobacterium breve potentially prevented and treated allergies and asthma [35–37]. Another study indicated that probiotic intervention for pregnant women and their infants who were at a high risk of allergy could protect caesarian-delivered children from allergic disease [38]. Additionally, a double-blind, randomized, placebo-controlled trial of 160 asthmatic children suggested that Lactobacillus can reduce asthma severity and improve asthma control [39].
However, other studies have drawn opposite conclusions, finding that probiotics do not have significant benefits in asthmatic children [40]. In these randomized controlled trials (RCTs), probiotic supplementation (such as with Lactobacillus and Bifidobacterium) for 7 weeks to 6 months was found to have no preventive or therapeutic effects on children with a high risk of asthma or on asthmatic patients. No significant differences in the outcome measures were observed between the probiotic and placebo groups, including the incidence of asthma, clinical outcomes (asthma-related events, quality of life, respiratory tract infections, antibiotic use, and asthma exacerbations), and pulmonary function (fraction of exhaled nitric oxide (FeNO) and forced expiratory volume in 1 s (FEV1)) [41–46]. Although probiotics had no significantly beneficial effects on asthma, the possibility of preventing and treating asthma cannot be denied. In addition, fecal microbiota transplantation (FMT) is another way to improve the gut microbiota, but its clinical application is currently limited in asthma. Additional studies are required to confirm the clinical stability and safety of both probiotic supplementation and FMT. In summary, the gut microbiota is closely associated with asthma, and its imbalance is related to an increased risk and severity of asthma, suggesting that appropriate gut microbiota intervention may be a feasible way to prevent and treat asthma.
Probiotics are also used in patients with autoimmune diseases, such as RA, which have been shown to be associated with the gut microbiota [47]. An early study reported that Lactobacillus salivarius, Lactobacillus iners, and Lactobacillus ruminis were increased in the gut of untreated RA patients, suggesting that a relationship potentially exists between the Lactobacillus community and the development of RA [48]. RCTs have demonstrated beneficial the effects of Lactobacillus acidophilus, Lactobacillus casei, or Bifidobacterium bifidum in RA patients [49, 50]. In contrast, Pineda et al. found that Lactobacillus rhamnosus and Lactobacillus reuteri did not clinically improve RA [51]. Probiotics have been shown to regulate immune system function and affect inflammation in a strain-specific manner. Lactobacilli are probiotic bacteria; however, different Lactobacillus species have different effects on RA, and some Lactobacillus species may cause arthritis [52]. For example, Lactobacillus casei plays an important role in inducing arthritis, while Lactobacillus fermentum does not induce arthritis [53]. Therefore, regarding probiotic supplementation, it is highly important to choose a bacterial strain and dosage that are safe and beneficial.
3.2. COPD
COPD, a common chronic, preventable, and treatable respiratory disease, is characterized by persistent airflow limitation and increased airway inflammation. Worldwide, COPD has been a major public health problem because of its high prevalence, morbidity, and mortality. Although much evidence has shown a coexistence of COPD and chronic gastrointestinal diseases such as IBD, few studies have reported the gut microbiota in COPD patients. Smoking is the principal cause of developing COPD and is associated with the microbial community and immune response of the GI tract [54]. The gut microbiota changes along with different cigarette smoking statuses [55]; Biedermann et al. found an altered gut microbiota in healthy smokers compared with that in nonsmokers, and they further observed an increase in Actinobacteria and Firmicutes and a decrease in Bacteroidetes and Proteobacteria abundances after smoking cessation [56]. In a mouse study, Lachnospiraceae sp. was increased in the gut after smoke exposure [54]. Although few studies have identified the direct association between the gut microbiota and COPD, there is evidence that the gut microbiota may play a vital role in COPD induced by cigarette smoke.
Similarly, there have been relatively few studies about probiotics that revealed the connection between the gut microbiota and COPD. For example, intragastric supplementation with Lactobacillus rhamnosus and Bifidobacterium breve in mice with COPD attenuated airway inflammation and alveolar damage [57]. In vitro, these two probiotics showed a similar anti-inflammatory effect on cigarette smoke-induced inflammation in human macrophages [58]. In the future, additional studies conducted on COPD patients are required to investigate and confirm the role of probiotics in COPD and to provide new therapeutic strategies for COPD.
3.3. CF
CF, a common autosomal recessive disease that affects mainly the lungs, is primarily driven by cystic fibrosis transmembrane conductance regulator (CFTR) mutation. The GI tract also strongly presents CFTR dysfunction and is among the earliest parts of the body affected in CF patients, suggesting a close link between the gut and lung. In CF patients, the gut microbiota was significantly altered, with reduced bacterial abundance, richness, and diversity and different microbial compositions compared to those in HCs [59–61]. For example, increased abundances of Staphylococcus, Streptococcus, and Veillonella dispar and decreased abundances of Bacteroides, Bifidobacterium adolescentis, and Faecalibacterium prausnitzii were observed in the gut of CF patients compared with those of HCs [62]. Importantly, the gut microbiota, which was reported to be associated with CFTR variants [63], seems to be essential for the pathophysiology and development of CF. A murine study indicated that the loss of functional CFTR was associated with augmentation of pathogenic bacteria, such as Mycobacteria and Bacteroides fragilis [64]. Moreover, several cross-sectional studies revealed a certain relationship between the gut microbiota and lung function, disease exacerbation, and severity of CF patients [65–67].
In recent years, numerous RCTs have shown that restoration of the gut microbiota followed by probiotic supplementation is related to improvement of CF, further strengthening the idea that the gut microbiota can influence airway inflammation in CF. Lactobacillus administration caused a reduction in bacterial density and an increase in microbial diversity in the gut [68], as well as beneficial effects on exacerbation risk and quality of life in CF patients [69]. However, some inconsistent results were also yielded; for example, Van Biervliet et al. found no significant differences in pulmonary function and disease exacerbations between probiotic and placebo groups [70]. Therefore, according to a meta-analysis, fastidiously designed and adequate RCTs are needed to assess the safety and efficacy of probiotics and to ascertain the specific probiotic strains or dose that can be of significant benefit for CF patients [71].
3.4. Lung Cancer
Lung cancer is one of the malignant tumors with the fastest growth of morbidity and mortality and has become the greatest threat to human health. Antibiotics are believed to alter the gut microbiota, and a large demographic study found that exposure to certain antibiotics, such as penicillin, cephalosporins, or macrolides, was associated with an increased risk of lung cancer [72], which suggested a close correlation between the gut microbiota and lung cancer. Using 16S rRNA sequencing, researchers found no significant difference in alpha diversity but a difference in gut microbiota beta diversity between patients with lung cancer and HCs [73, 74]. Moreover, at the phylum and genus levels, lung cancer patients had an increased abundance of Enterococcus and a reduced level of the phylum Actinobacteria and genus Bifidobacterium, and these microbial communities might be potential biomarkers for lung carcinogenesis [73].
Recently, several studies have indicated that the gut microbiota also contributes to the effect of lung cancer therapeutics. Patients with non-small-cell lung cancer (NSCLC) who responded to antiprogrammed death 1 (PD-1) immunotherapy (responders) harbored a higher gut microbial diversity than those who did not respond (nonresponders), and gut microbial diversity was positively associated with progression-free survival (PFS) [75]. Another study revealed that responders showed increased abundances of Akkermansia muciniphila, Ruminococcus, Eubacterium, and Alistipes and decreased abundances of Bifidobacterium and Parabacteroides in the gut compared with those of nonresponders [76]. In addition, antibiotic use can influence the efficacy of lung cancer therapy. Previous studies found that antibiotics before and during antitumor therapy significantly reduced the clinical benefit (PFS and overall survival) of antitumor drugs in patients with NSCLC [77, 78]. Furthermore, FMT into GF or antibiotic-treated mice can ameliorate antitumor effects and clinical activity of antitumor drugs [79]. Similarly, supplementation with Enterococcus hirae and Barnesiella intestinihominis can prolong PFS in mice with advanced lung cancer undergoing chemoimmunotherapy [80]. Taken together, the gut microbiota markedly influences the outcome of antitumor therapy for lung cancer, suggesting a potential strategy to improve the clinical outcomes of patients with lung cancer by modulating the gut microbiota. However, a large number of clinical studies are required to confirm the effectiveness and safety of FMT and probiotics.
3.5. Respiratory Infection
Respiratory infection is the most common infectious disease and is a leading cause of morbidity and mortality worldwide. The gut commensal microbiota provides essential benefits to pulmonary mucosal immunity and plays protective roles in respiratory infection by distally driving host responses to pneumonia [81]. Depletion or absence of the gut microbiota is believed to influence the host immune response. Schuijt et al. found that microbiota-depleted mice showed increased bacterial dissemination, inflammation, organ damage, and mortality compared with control mice, and FMT reversed the gut microbiota diversity and enhanced the host defense against pneumonia [82]. In addition, the gut microbiota differed between patients with respiratory infection and HCs. It was reported that certain gut microbiota, such as Enterococcaceae, was associated with community-acquired pneumonia (CAP) [83], and respiratory syncytial virus and influenza virus infection resulted in a dysbiotic gut microbiota in mice [84]. Many studies have suggested that oral administration of probiotics can not only protect against bacterial pneumonia [85] but also contribute to accelerated recovery from respiratory viral infection [86, 87], further emphasizing the crucial role of the gut microbiota in respiratory infection.
Tuberculosis (TB) typically affects the lungs (pulmonary TB), causing approximately 10 million cases and over 1 million deaths per year worldwide, with the heaviest toll in low- and middle-income countries [88]. Likewise, the gut commensal microbiota can protect against early lung colonization by Mycobacterium tuberculosis (Mtb) [89]. Disruption of the gut microbiota with antibiotics increased the burden and dissemination of Mtb, and FMT reconstituted the gut microbiota and restored TB containment by reducing the Mtb burden [90]. The gut microbiota was significantly different between patients with TB and HCs. At the phylum level, Actinobacteria and Proteobacteria, which contain many pathogenic species, were enriched in the gut of TB patients, while Bacteroidetes, which contains a variety of beneficial commensal microbiota species, was decreased in TB patients compared to those in HCs [91]. At the genus level, several butyrate and propionate-producing bacteria, such as Faecalibacterium, Roseburia, Eubacterium, and Phascolarctobacterium, were more abundant in TB patients than in HCs [92]. Similar to antibiotic and antitumor therapy, anti-TB treatment also has dramatic effects on the gut microbiota. Patients who underwent standard HRZE (isoniazid, rifampicin, pyrazinamide, and ethambutol) therapy exhibited a perturbed gut microbiota, with a depletion of Ruminococcus, Eubacterium, Lactobacillus, and Bacteroides and an increase in Erysipelatoclostridium and Prevotella abundances [93], and HRZ(E)-induced dysbiosis was long lasting in both mice and humans [94]. Furthermore, studies on probiotics suggested that supplementation with Lactobacillus can restore anti-Mtb immunity in the lungs [95]. Taken together, these findings indicate that the gut microbiota may contribute to the pathophysiology of TB.
3.6. Other Respiratory Diseases
In addition to the above common respiratory diseases, other respiratory disorders, such as ILD, acute respiratory distress syndrome (ARDS), acute lung injury (ALI), and ventilator-associated pneumonia (VAP), also show a certain correlation with the gut microbiota. For example, ILD is characterized by progressive fibrosis and respiratory failure, and changes in the gut microbiota have been reported in patients with silicosis and pulmonary fibrosis [96]. ARDS/ALI is the most common form of organ failure in critically ill patients, and VAP, which is among the most common infections in mechanically ventilated patients, has a high mortality rate [97, 98]. Previous studies revealed that gut-associated bacteria and pathogens were enriched in the bronchoalveolar lavage fluid (BALF) of patients with ARDS, which suggested gut-lung translocation [99, 100]. Additionally, the GI microbiota contributes to the development of ALI in mice [101], and FMT can significantly reduce ALI inflammation [102]. RCTs of ventilated patients suggested that patients treated with probiotics had a decreased incidence of microbiologically confirmed VAP, as well as reduced durations of intensive care unit [103] and hospital stays [104]. A meta-analysis also found an association between probiotic supplementation and reduced VAP incidence, suggesting a clinical benefit of probiotics for ventilated patients [105].
4. The Complex Interactions between the Gut and Lungs
Cigarette smoking, which is a risk factor for many diseases, can not only change the lung microbiota but also affect the gut microbiota [55, 106]. Lung mucosal exposure to cigarette smoke may be involved in the development of autoantibodies associated with RA, such as peptidylarginine deiminase (PAD) 2 [107], suggesting that the lungs could be a site of autoimmunity generation in RA. Scher et al. found that the lung microbiota in RA patients was similar to that in sarcoidosis patients, characterized by reduced alpha diversity and decreased abundance of Actinomyces and Burkholderia [108]. The notion that the gut microbiota influences the local and systemic immune systems is not novel, and the role of the gut microbiota in autoimmune diseases, including RA, is currently well characterized [47]. Since the lungs and gut are both mucosal sites that are exposed to environmental factors, it is possible that both organs share microbiota and that the microbiota can induce local and systemic immunity/inflammation in both organs. This evidence suggests that the gut/lung microbiota may potentially drive the initiation of autoimmune diseases.
Previous studies have found an altered function and structure in intestinal mucosa in asthma patients and increased intestinal permeability in COPD patients [109, 110], further supporting the hypothesis that a link exists between the gut and lungs. Moreover, a growing number of studies have suggested that an immunological relationship exists between the gut and lungs [111, 112]. The gut microbiota can shape local intestinal and systemic immunity and the lung mucosa, thereby affecting respiratory diseases. Taken together, the complex interaction between the gut and lungs is likely to be mediated by locally resident microbiota.
5. Possible Mechanisms of the Gut Microbiota in Respiratory Diseases
The gut commensal microbiota contributes to influencing and maintaining body homeostasis by regulating the immune response of both the GI system and distal organs. The possible mechanisms include the regulation of extraintestinal T cell populations, development of oral immune tolerance through regulatory T cells (Tregs), production of short-chain fatty acids (SCFAs), and regulation of systemic inflammation [113]. The immune cells and cytokines induced by the gut microbiota and its metabolites, such as SCFAs, can enter systemic circulation through the blood and lymphatic system, which regulate the immune and inflammatory responses in the lung and further influence respiratory health and disease (Figure 1). For example, exaggerated allergic airway inflammation in GF mice was correlated with increased T helper 2 (Th2) cytokine (IL-4 and IL-5) and IgE levels in the lung [114]. The commensal gut microbiota can enhance host defense against bacterial pneumonia by increasing IL-17A levels and upregulating pulmonary granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling [115]. In antibiotic-treated mice, a greatly increased mortality due to respiratory viral infection was related to a decreased abundance of Tregs in the respiratory and GI tracts [116], and increased pulmonary colonization by Mtb was associated with a significantly reduced accumulation of mucosal-associated invariant T (MAIT) cells in the lungs [89]. Table 1 summarizes our current understanding of the possible mechanisms of the gut microbiota acting on common respiratory diseases.
Table 1.
The possible mechanisms underlying the effects of the gut microbiota on respiratory diseases.
| Respiratory diseases | Alterations in the gut microbiota | Possible mechanisms | References |
|---|---|---|---|
| Asthma | Gut microbiota disrupted by antibiotics | Exacerbate Th2 responses by increasing the infiltration of inflammatory cells and the production of inflammatory cytokines (IL-4 and IL-13). | [117, 118] |
| Reduce Treg abundance in the lung. | [119] | ||
| Exaggerate Th1/Th17 adaptive immune responses in the lung. | [120] | ||
| GF mice | Elevate the total number of eosinophils, number of CD4+ T cells, and level of Th2 cytokines and alter the number and phenotype of conventional DCs in the airways. | [114] | |
| Increase CXCL16 expression and accumulate iNKT cells in the gut and lungs. | [121] | ||
| Probiotics | Reverse the Th1/Th2 imbalance: increase the levels of the anti-inflammatory cytokine IL-10 while reduce the levels of proinflammatory cytokines such as IL-4, IL-5, and IL-13. | [122–125] | |
| Increase PPARγ expression of DCs in the lung. | [126] | ||
| Increase lung CD4+ T cell and CD4+Foxp3+ Treg abundance while decrease activated CD11b+ DC abundance. | [37] | ||
| Decrease MMP9 expression in the BALF and serum and inhibit inflammatory cell infiltration into the lung. | [36] | ||
|
| |||
| COPD | Cigarette smoke | Alter mucin gene expression and cytokine production in the gut; increase Muc2, Muc3, and Muc4 expression; and increase CXCL2 and IL-6 expression while decrease IFN-γ and TGF-β expression. | [54] |
| Inhibit the NK-κB pathway by reducing p65 phosphorylation and IκBα in the gut. | [127] | ||
| Probiotics | Suppress macrophage inflammation by inducing the expression of IL-1β, IL-6, IL-10, IL-23, TNF-α, CXCL-8, and HMGB1. | [58] | |
| Increase NK cell activity and the number of CD16+ cells. | [128] | ||
|
| |||
| CF | Probiotics | Reduce IL-8 production by intestinal cells. | [129] |
| Reduce the level of the gut inflammatory marker calprotectin. | [68] | ||
| Antibiotic treatment | Augment the proportions of Th17, CD8+ IL-17+, and CD8+ IFNγ+ lymphocytes and IL-17-producing γδ T cells. | [130] | |
|
| |||
| Lung cancer | Gut microbiota disrupted by antibiotics | Upregulate the expression of VEGFA and downregulate the expression of BAX and CDKN1B while reduce IFN-γ, GZMB, and PRF1 produced by CD8+ T cells. | [131] |
| Suppress CTX-induced Th17 responses and reduce the abundance of tumor-infiltrating CD3+ T cells and Th1 cells. | [132] | ||
| FMT | Accumulate CCR9+CXCR3+CD4+ T cells into the tumor microenvironment. | [79] | |
| Probiotics | Upregulate the mRNA levels of IFN-γ, GZMB, and PRF1. | [131] | |
| Boost CTX-induced anticancer Th1 and Tc1 responses and promote the infiltration of IFN-γ+γδT cells into cancer lesions. | [80] | ||
|
| |||
| Respiratory infection | Commensal gut microbiota | SFB promotes pulmonary Th17 immunity as demonstrated by increased IL-22 and IL-22+ TCRβ+ cell levels. | [133] |
| Protect against Mtb infection by improving the activity of MAIT cells in the lungs. | [89] | ||
| Regulate virus-specific CD4 and CD8 T cell and antibody responses. | [134] | ||
| Contribute to the accumulation of IL-22-producing ILC3s in newborn lung. | [81] | ||
| Induce NF-κB activation in the lung through TLR4. | [135, 136] | ||
| Gut microbiota disrupted by antibiotics | Reduce pulmonary GM-CSF production through IL-17A signaling. | [115] | |
| Reduce MAIT cell and IL-17A levels. | [89] | ||
| Reduce mincle expression on lung DCs. | [95] | ||
| Decrease bacterial killing activity of alveolar macrophages while increase the levels of proinflammatory cytokines such as IL-6 and IL-1β in the lung. | [136] | ||
| GF mice | Decrease proinflammatory cytokine (TNF-α and CXCL1) levels and neutrophil influx while produce large amounts of IL-10 in the lungs. | [137] | |
| FMT | Normalize the pulmonary TNF-α and IL-10 levels. | [82] | |
| Probiotics | Activate the TLR-signaling pathway through the protein Mal. | [85] | |
| Enhance the mRNA expression of IFN-γ, IL-12a, IL-2rb, IL-12rb1, PRF1, Klrk1, CD247, and TNF-α in the lung. | [138] | ||
|
| |||
| ALI | FMT | Reduce TNF-α, IL-1β, and IL-6 levels by downregulating the TGF-β1/Smads/ERK signaling pathway. | [102] |
ALI: acute lung injury; BAX: Bcl-2 associated X; CDKN1B: cyclin-dependent kinase inhibitor 1B; CTX: cyclophosphamide; CXCL: C-X-C motif chemokine ligand; DCs: dendritic cells; ERK: extracellular signal-regulated kinase; FMT: fecal microbiota transplantation; GF mice: germ-free mice; GM-CSF: granulocyte-macrophage colony-stimulating factor; GZMB: granzyme B; HMGB1: high-mobility group box 1; IFN-γ: interferon-gamma; IκBα: inhibitor of NK-κB α; Klrk1: killer cell lectin-like receptor subfamily K, member 1; MAIT cells: mucosal-associated invariant T cells; Mal: MyD88 (myeloid differentiation primary response protein) adaptor protein; MMP9: matrix metalloproteinase 9; Muc: mucin; NF-κB: nuclear factor kappa-B; PPARγ: peroxisome proliferator-activated receptor gamma; PRF1: perforin; SFB: segmented filamentous bacteria; TLR: toll-like receptor; TNF-α: tumor necrosis factor alpha; Tregs: regulatory T cells; VEGFA: vascular endothelial growth factor A.
Currently, the mechanisms of probiotic regulation of lung health and disease have become a research hotspot since there is increasing evidence that probiotics have protective and therapeutic effects on respiratory diseases by optimizing microbial balance in the GI tract (Table 1). Oral administration of probiotics contributes to regulating respiratory immune responses through numerous signaling pathways. For example, Bifidobacterium bifidum can stimulate the Th1/Th2 balance and upregulate IFN-γ, IL-4, and IL-12 secretion in the spleen [139]; Escherichia coli can reduce respiratory inflammatory cell recruitment as well as Th2 and Th17 responses [140]; Enterococcus faecalis suppresses Th17 cell development in the lung, spleen, and gut [141]; and Lactobacillus plantarum can reduce the numbers of lung innate immune cells (macrophages and neutrophils) and levels of cytokines (IL-6 and TNF-α) in the BALF and induce an immunosuppressive Treg response in the lungs [142]. Despite these effects, the precise mechanisms underlying probiotic effects on the lung and many aspects of the probiotic regulation of immune responses remain largely unknown.
6. Conclusions
Increasing evidence suggests an important and complex crosstalk between the gut and lung, as well as between the gut microbiota and host immunity. Gut microbial dysbiosis is believed to be associated with the etiology or/and development of common respiratory diseases, such as asthma, COPD, CF, lung cancer, and respiratory infection. To date, the understanding of the mechanism involving the gut-lung axis is still in its infancy and remains to be further elucidated. Future research into modification and improvement of the gut microbiota and into the balance of gut and lung immunity through diet, probiotics, and FMT is necessary to improve our understanding of the role of gut microbiota in the lung and to provide effective and new therapeutic strategies for respiratory diseases.
Acknowledgments
We thank American Journal Experts (AJE) for English language editing. This work was supported by the Guangzhou Healthcare Collaborative Innovation Major Project (201604020012), the Science and Technology Planning Project of Guangdong Province (2016A020215107), the Open Project of the State Key Laboratory of Respiratory Disease (SKLRD2016OP014), and the Open Projects Program of Guangdong Provincial Key Laboratory of Occupational Disease Prevention and Treatment (2017B030314152).
Conflicts of Interest
The authors declare no conflict of interests.
References
- 1.Labarca G., Drake L., Horta G., et al. Association between inflammatory bowel disease and chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulmonary Medicine. 2019;19(1):186–186. doi: 10.1186/s12890-019-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vutcovici M., Brassard P., Bitton A. Inflammatory bowel disease and airway diseases. World Journal of Gastroenterology. 2016;22(34):7735–7741. doi: 10.3748/wjg.v22.i34.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Li F., Wei H., Lian Z. X., Sun R., Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. Journal of Experimental Medicine. 2014;211(12):2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massart A., Hunt D. P. Pulmonary manifestations of inflammatory bowel disease. The American Journal of Medicine. 2020;133(1):39–43. doi: 10.1016/j.amjmed.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Blanco A. J., Vélez A. G., Solís-García G., Posadas A. S., Alonso S. B., Cimadevilla J. L. R. Comorbidities and course of lung function in patients with congenital esophageal atresia. Archivos Argentinos de Pediatría. 2020;118(1):25–30. doi: 10.5546/aap.2020.eng.25. [DOI] [PubMed] [Google Scholar]
- 6.Feng Q., Chen W.-D., Wang Y.-D. Gut microbiota: an integral moderator in health and disease. Frontiers in Microbiology. 2018;9:p. 151. doi: 10.3389/fmicb.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gholizadeh P., Mahallei M., Pormohammad A., et al. Microbial balance in the intestinal microbiota and its association with diabetes, obesity and allergic disease. Microbial Pathogenesis. 2019;127:48–55. doi: 10.1016/j.micpath.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Budden K. F., Gellatly S. L., Wood D. L. A., et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nature Reviews. Microbiology. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 9.Hayden H. S., Eng A., Pope C. E., et al. Fecal dysbiosis in infants with cystic fibrosis is associated with early linear growth failure. Nature Medicine. 2020;26(2):215–221. doi: 10.1038/s41591-019-0714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bingula R., Filaire M., Radosevic-Robin N., et al. Desired turbulence? Gut-lung axis, immunity, and lung cancer. Journal of Oncology. 2017;2017:15. doi: 10.1155/2017/5035371.5035371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumas A., Bernard L., Poquet Y., Lugo-Villarino G., Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cellular Microbiology. 2018;20(12, article e12966) doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 12.Gerard P. Gut microbiota and obesity. Cellular and Molecular Life Sciences. 2016;73(1):147–162. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S., Covington A., Pamer E. G. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunological Reviews. 2017;279(1):90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemon K. P., Klepac-Ceraj V., Schiffer H. K., Brodie E. L., Lynch S. V., Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. MBio. 2010;1(3):p. e00129. doi: 10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson R. P., Erb-Downward J. R., Martinez F. J., Huffnagle G. B. The microbiome and the respiratory tract. Annual Review of Physiology. 2016;78(1):481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robles Alonso V., Guarner F. Probiotic Bacteria and Their Effect on Human Health and Well-Being. Vol. 107. Karger Publishers; 2013. Intestinal microbiota composition in adults; pp. 17–24. [Google Scholar]
- 17.Frati F., Salvatori C., Incorvaia C., et al. The role of the microbiome in asthma: the gut(-)lung axis. International Journal of Molecular Sciences. 2019;20(1):p. E123. doi: 10.3390/ijms20010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsland B. J., Trompette A., Gollwitzer E. S. The gut-lung axis in respiratory disease. Annals of the American Thoracic Society. 2015;12(Supplement 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 19.Mitre E., Susi A., Kropp L. E., Schwartz D. J., Gorman G. H., Nylund C. M. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatrics. 2018;172(6, article e180315) doi: 10.1001/jamapediatrics.2018.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni J., Friedman H., Boyd B. C., et al. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatrics. 2019;19(1):p. 225. doi: 10.1186/s12887-019-1594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cepeda A. M., the ISAAC Phase III Latin America Group, Thawer S., et al. Diet and respiratory health in children from 11 Latin American countries: evidence from ISAAC phase III. Lung. 2017;195(6):683–692. doi: 10.1007/s00408-017-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusconi F., Zugna D., Annesi-Maesano I., et al. Mode of delivery and asthma at school age in 9 European birth cohorts. American Journal of Epidemiology. 2017;185(6):465–473. doi: 10.1093/aje/kwx021. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadizar F., Vijverberg S. J. H., Arets H. G. M., et al. Breastfeeding is associated with a decreased risk of childhood asthma exacerbations later in life. Pediatric Allergy and Immunology. 2017;28(7):649–654. doi: 10.1111/pai.12760. [DOI] [PubMed] [Google Scholar]
- 24.Dreher M. L. Whole fruits and fruit fiber emerging health effects. Nutrients. 2018;10(12):p. 1833. doi: 10.3390/nu10121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loverdos K., Bellos G., Kokolatou L., et al. Lung microbiome in asthma: current perspectives. Journal of Clinical Medicine. 2019;8(11):p. 1967. doi: 10.3390/jcm8111967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Zhang D., Jia H., et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nature Medicine. 2015;21(8):895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 27.Diamanti A. P., Rosado M. M., Laganà B., D’Amelio R. Microbiota and chronic inflammatory arthritis: an interwoven link. Journal of Translational Medicine. 2016;14(1):233–233. doi: 10.1186/s12967-016-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley D. The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Current Opinion in Allergy and Clinical Immunology. 2014;14(5):390–396. doi: 10.1097/ACI.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 29.Abrahamsson T. R., Jakobsson H. E., Andersson A. F., Björkstén B., Engstrand L., Jenmalm M. C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clinical and Experimental Allergy. 2014;44(6):842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 30.Oddy W. H. Breastfeeding, childhood asthma, and allergic disease. Annals of Nutrition and Metabolism. 2017;70(2):26–36. doi: 10.1159/000457920. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., Li F., Liang B., et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiology. 2018;18(1):p. 114. doi: 10.1186/s12866-018-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujimura K. E., Sitarik A. R., Havstad S., et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nature Medicine. 2016;22(10):1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Nimwegen F. A., Penders J., Stobberingh E. E., et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. Journal of Allergy and Clinical Immunology. 2011;128(5):948–955.e3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Arrieta M.-C., Stiemsma L. T., Dimitriu P. A., et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science Translational Medicine. 2015;7(307, article 307ra152) doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 35.Kozakova H., Schwarzer M., Tuckova L., et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cellular & Molecular Immunology. 2016;13(2):251–262. doi: 10.1038/cmi.2015.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C. T., Chen P. J., Lee Y. T., Ko J. L., Lue K. H. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. Journal of Microbiology, Immunology, and Infection. Wei Mian Yu Gan Ran Za Zhi. 2016;49(5):625–635. doi: 10.1016/j.jmii.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Raftis E. J., Delday M. I., Cowie P., et al. Bifidobacterium breve MRx0004 protects against airway inflammation in a severe asthma model by suppressing both neutrophil and eosinophil lung infiltration. Scientific Reports. 2018;8(1):p. 12024. doi: 10.1038/s41598-018-30448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kallio S., Kukkonen A. K., Savilahti E., Kuitunen M. Perinatal probiotic intervention prevented allergic disease in a caesarean-delivered subgroup at 13-year follow-up. Clinical and Experimental Allergy. 2019;49(4):506–515. doi: 10.1111/cea.13321. [DOI] [PubMed] [Google Scholar]
- 39.Huang C.-F., Chie W.-C., Wang I.-J. Efficacy of Lactobacillus administration in school-age children with asthma: a randomized, placebo-controlled trial. Nutrients. 2018;10(11, article e1678):p. 1678. doi: 10.3390/nu10111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J., Zhang Y., He C., Dai J. Probiotics supplementation in children with asthma: a systematic review and meta-analysis. Journal of Paediatrics and Child Health. 2018;54(9):953–961. doi: 10.1111/jpc.14126. [DOI] [PubMed] [Google Scholar]
- 41.Davies G., Jordan S., Brooks C. J., et al. Long term extension of a randomised controlled trial of probiotics using electronic health records. Scientific Reports. 2018;8(1, article 7668) doi: 10.1038/s41598-018-25954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt R. M., Pilmann Laursen R., Bruun S., et al. Probiotics in late infancy reduce the incidence of eczema: a randomized controlled trial. Pediatric Allergy and Immunology. 2019;30(3):335–340. doi: 10.1111/pai.13018. [DOI] [PubMed] [Google Scholar]
- 43.Rose M. A., Stieglitz F., Köksal A., Schubert R., Schulze J., Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clinical and Experimental Allergy. 2010;40(9):1398–1405. doi: 10.1111/j.1365-2222.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 44.Stockert K., Schneider B., Porenta G., Rath R., Nissel H., Eichler I. Laser acupuncture and probiotics in school age children with asthma: a randomized, placebo-controlled pilot study of therapy guided by principles of traditional Chinese medicine. Pediatric Allergy and Immunology. 2007;18(2):160–166. doi: 10.1111/j.1399-3038.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 45.Kukkonen A. K., Kuitunen M., Savilahti E., Pelkonen A., Malmberg P., Mäkelä M. Airway inflammation in probiotic-treated children at 5 years. Pediatric Allergy and Immunology. 2011;22(2):249–251. doi: 10.1111/j.1399-3038.2010.01079.x. [DOI] [PubMed] [Google Scholar]
- 46.Smith T. D. H., Watt H., Gunn L., Car J., Boyle R. J. Recommending oral probiotics to reduce winter antibiotic prescriptions in people with asthma: a pragmatic randomized controlled trial. Annals of Family Medicine. 2016;14(5):422–430. doi: 10.1370/afm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clemente J. C., Manasson J., Scher J. U. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:p. j5145. doi: 10.1136/bmj.j5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Zou Q., Zeng B., Fang Y., Wei H. Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Current Microbiology. 2013;67(2):170–176. doi: 10.1007/s00284-013-0338-1. [DOI] [PubMed] [Google Scholar]
- 49.Zamani B., Golkar H. R., Farshbaf S., et al. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. International Journal of Rheumatic Diseases. 2016;19(9):869–879. doi: 10.1111/1756-185X.12888. [DOI] [PubMed] [Google Scholar]
- 50.Alipour B., Homayouni-Rad A., Vaghef-Mehrabany E., et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. International Journal of Rheumatic Diseases. 2014;17(5):519–527. doi: 10.1111/1756-185X.12333. [DOI] [PubMed] [Google Scholar]
- 51.de los Angeles Pineda M., Thompson S. F., Summers K., de Leon F., Pope J., Reid G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Medical Science Monitor. 2011;17(6):Cr347–Cr354. doi: 10.12659/msm.881808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simelyte E., Rimpiläinen M., Zhang X., Toivanen P. Role of peptidoglycan subtypes in the pathogenesis of bacterial cell wall arthritis. Annals of the Rheumatic Diseases. 2003;62(10):976–982. doi: 10.1136/ard.62.10.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simelyte E., Rimpiläinen M., Lehtonen L., Zhang X., Toivanen P. Bacterial cell wall-induced arthritis: chemical composition and tissue distribution of four Lactobacillus strains. Infection and Immunity. 2000;68(6):3535–3540. doi: 10.1128/IAI.68.6.3535-3540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allais L., Kerckhof F. M., Verschuere S., et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environmental Microbiology. 2016;18(5):1352–1363. doi: 10.1111/1462-2920.12934. [DOI] [PubMed] [Google Scholar]
- 55.Lee S. H., Yun Y., Kim S. J., et al. Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study. Journal of Clinical Medicine. 2018;7(9):p. 282. doi: 10.3390/jcm7090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biedermann L., Zeitz J., Mwinyi J., et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3, article e59260) doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verheijden K. A. T., van Bergenhenegouwen J., Garssen J., Bezemer G. F. G., Kraneveld A. D., Folkerts G. Treatment with specific prebiotics or probiotics prevents the development of lung emphysema in a mouse model of COPD. European Journal of Pharmacology. 2011;668:e12–e13. doi: 10.1016/j.ejphar.2011.09.220. [DOI] [Google Scholar]
- 58.Mortaz E., Adcock I. M., Ricciardolo F. L. M., et al. Anti-inflammatory effects of Lactobacillus rahmnosus and Bifidobacterium breve on cigarette smoke activated human macrophages. PloS One. 2015;10(8, article e0136455) doi: 10.1371/journal.pone.0136455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L., Somerset S. The clinical significance of the gut microbiota in cystic fibrosis and the potential for dietary therapies. Clinical Nutrition. 2014;33(4):571–580. doi: 10.1016/j.clnu.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Dayama G., Priya S., Niccum D. E., Khoruts A., Blekhman R. Interactions between the gut microbiome and host gene regulation in cystic fibrosis. Genome Medicine. 2020;12(1):p. 12. doi: 10.1186/s13073-020-0710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y., Leong L. E. X., Keating R. L., et al. Opportunistic bacteria confer the ability to ferment prebiotic starch in the adult cystic fibrosis gut. Gut Microbes. 2018;10(3):367–381. doi: 10.1080/19490976.2018.1534512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Enaud R., Hooks K. B., Barre A., et al. Intestinal inflammation in children with cystic fibrosis is associated with Crohn’s-like microbiota disturbances. Journal of Clinical Medicine. 2019;8(5):p. 645. doi: 10.3390/jcm8050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vernocchi P., Del Chierico F., Russo A., et al. Gut microbiota signatures in cystic fibrosis: loss of host CFTR function drives the microbiota enterophenotype. PloS One. 2018;13(12, article e0208171) doi: 10.1371/journal.pone.0208171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch S. V., Goldfarb K. C., Wild Y. K., Kong W., De Lisle R. C., Brodie E. L. Cystic fibrosis transmembrane conductance regulator knockout mice exhibit aberrant gastrointestinal microbiota. Gut Microbes. 2014;4(1):41–47. doi: 10.4161/gmic.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coffey M. J., Nielsen S., Wemheuer B., et al. Gut microbiota in children with cystic fibrosis: a taxonomic and functional dysbiosis. Scientific Reports. 2019;9(1):p. 18593. doi: 10.1038/s41598-019-55028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoen A. G., Li J., Moulton L. A., et al. Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. The Journal of Pediatrics. 2015;167(1):138–147.e3. doi: 10.1016/j.jpeds.2015.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burke D. G., Fouhy F., Harrison M. J., et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiology. 2017;17(1):p. 58. doi: 10.1186/s12866-017-0968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.del Campo R., Garriga M., Pérez-Aragón A., et al. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: A double blind prospective study. Journal of Cystic Fibrosis. 2014;13(6):716–722. doi: 10.1016/j.jcf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Van Biervliet S., Declercq D., Somerset S. Clinical effects of probiotics in cystic fibrosis patients: a systematic review. Clinical Nutrition ESPEN. 2017;18:37–43. doi: 10.1016/j.clnesp.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Van Biervliet S., Hauser B., Verhulst S., et al. Probiotics in cystic fibrosis patients: a double blind crossover placebo controlled study: pilot study from the ESPGHAN Working Group on Pancreas/CF. Clinical Nutrition ESPEN. 2018;27:59–65. doi: 10.1016/j.clnesp.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Coffey M. J., Garg M., Homaira N., Jaffe A., Ooi C. Y. Probiotics for people with cystic fibrosis. Cochrane Database of Systematic Reviews. 2020;1(1) doi: 10.1002/14651858.cd012949.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boursi B., Mamtani R., Haynes K., Yang Y. X. Recurrent antibiotic exposure may promote cancer formation--another step in understanding the role of the human microbiota? European Journal of Cancer. 2015;51(17):2655–2664. doi: 10.1016/j.ejca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhuang H., Cheng L., Wang Y., et al. Dysbiosis of the gut microbiome in lung cancer. Frontiers in Cellular and Infection Microbiology. 2019;9:p. 112. doi: 10.3389/fcimb.2019.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W. Q., Zhao S. K., Luo J. W., et al. Alterations of fecal bacterial communities in patients with lung cancer. American Journal of Translational Research. 2018;10(10):3171–3185. [PMC free article] [PubMed] [Google Scholar]
- 75.Jin Y., Dong H., Xia L., et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. Journal of Thoracic Oncology. 2019;14(8):1378–1389. doi: 10.1016/j.jtho.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Biancheri P., Divekar D., Watson A. J. M. Could fecal transplantation become part of PD-1-based immunotherapy, due to effects of the intestinal microbiome? Gastroenterology. 2018;154(6):1845–1847. doi: 10.1053/j.gastro.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 77.Hakozaki T., Okuma Y., Omori M., Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncology Letters. 2019;17(3):2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Derosa L., Hellmann M. D., Spaziano M., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Annals of Oncology. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Routy B., Le Chatelier E., Derosa L., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 80.Daillère R., Vétizou M., Waldschmitt N., et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45(4):931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 81.Gray J., Oehrle K., Worthen G., Alenghat T., Whitsett J., Deshmukh H. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Science Translational Medicine. 2017;9(376, article eaaf9412) doi: 10.1126/scitranslmed.aaf9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuijt T. J., Lankelma J. M., Scicluna B. P., et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren X., Gamallat Y., Liu D., et al. The distribution characteristics of intestinal microbiota in children with community-acquired pneumonia under five years of age. Microbial Pathogenesis. 2020;142, article 104062 doi: 10.1016/j.micpath.2020.104062. [DOI] [PubMed] [Google Scholar]
- 84.Groves H. T., Cuthbertson L., James P., Moffatt M. F., Cox M. J., Tregoning J. S. Respiratory disease following viral lung infection alters the murine gut microbiota. Frontiers in Immunology. 2018;9:p. 182. doi: 10.3389/fimmu.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vieira A. T., Rocha V. M., Tavares L., et al. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 51A. Microbes and Infection. 2016;18(3):180–189. doi: 10.1016/j.micinf.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Waki N., Yajima N., Suganuma H., et al. Oral administration of Lactobacillus brevis KB290 to mice alleviates clinical symptoms following influenza virus infection. Letters in Applied Microbiology. 2014;58(1):87–93. doi: 10.1111/lam.12160. [DOI] [PubMed] [Google Scholar]
- 87.Kawahara T., Takahashi T., Oishi K., et al. Consecutive oral administration of Bifidobacterium longum MM-2 improves the defense system against influenza virus infection by enhancing natural killer cell activity in a murine model. Microbiology and Immunology. 2015;59(1):1–12. doi: 10.1111/1348-0421.12210. [DOI] [PubMed] [Google Scholar]
- 88.WHO. Global tuberculosis report 2019. World Health Organization; 2019. [Google Scholar]
- 89.Dumas A., Corral D., Colom A., et al. The host microbiota contributes to early protection against lung colonization by Mycobacterium tuberculosis. Frontiers in Immunology. 2018;9:p. 2656. doi: 10.3389/fimmu.2018.02656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khan N., Vidyarthi A., Nadeem S., Negi S., Nair G., Agrewala J. N. Alteration in the gut microbiota provokes susceptibility to tuberculosis. Frontiers in Immunology. 2016;7:p. 529. doi: 10.3389/fimmu.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo M., Liu Y., Wu P., et al. Alternation of gut microbiota in patients with pulmonary tuberculosis. Frontiers in Physiology. 2017;8:p. 822. doi: 10.3389/fphys.2017.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maji A., Misra R., Dhakan D. B., et al. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environmental Microbiology. 2018;20(1):402–419. doi: 10.1111/1462-2920.14015. [DOI] [PubMed] [Google Scholar]
- 93.Wipperman M. F., Fitzgerald D. W., Juste M. A. J., et al. Antibiotic treatment for tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed. Scientific Reports. 2017;7(1):p. 10767. doi: 10.1038/s41598-017-10346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Namasivayam S., Maiga M., Yuan W., et al. Longitudinal profiling reveals a persistent intestinal dysbiosis triggered by conventional anti-tuberculosis therapy. Microbiome. 2017;5(1):p. 71. doi: 10.1186/s40168-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Negi S., Pahari S., Bashir H., Agrewala J. N. Gut microbiota regulates mincle mediated activation of lung dendritic cells to protect against Mycobacterium tuberculosis. Frontiers in Immunology. 2019;10:p. 1142. doi: 10.3389/fimmu.2019.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Y., Chen L., Sun G., Li Y., Huang R. Alterations in the gut microbiota of patients with silica-induced pulmonary fibrosis. Journal of Occupational Medicine and Toxicology. 2019;14(1):p. 5. doi: 10.1186/s12995-019-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chacko B., Peter J. V., Tharyan P., John G., Jeyaseelan L. Pressure-controlled versus volume-controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) The Cochrane Database of Systematic Reviews. 2010;1(1):CD008807–CD008807. doi: 10.1002/14651858.cd008807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Metersky M. L., Kalil A. C. Management of ventilator-associated pneumonia. Clinics in Chest Medicine. 2018;39(4):797–808. doi: 10.1016/j.ccm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Dickson R. P., Singer B. H., Newstead M. W., et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nature Microbiology. 2016;1(10):p. 16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panzer A. R., Lynch S. V., Langelier C., et al. Lung microbiota is related to smoking status and to development of acute respiratory distress syndrome in critically ill trauma patients. American Journal of Respiratory and Critical Care Medicine. 2018;197(5):621–631. doi: 10.1164/rccm.201702-0441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kapur R., Kim M., Rebetz J., et al. Gastrointestinal microbiota contributes to the development of murine transfusion-related acute lung injury. Blood Advances. 2018;2(13):1651–1663. doi: 10.1182/bloodadvances.2018018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li B., Yin G.-F., Wang Y.-L., Tan Y.-M., Huang C.-L., Fan X.-M. Impact of fecal microbiota transplantation on TGF-β1/Smads/ERK signaling pathway of endotoxic acute lung injury in rats. 3 Biotech. 2020;10(2):p. 52. doi: 10.1007/s13205-020-2062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peled J. U., Devlin S. M., Staffas A., et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. Journal of Clinical Oncology. 2017;35(15):1650–1659. doi: 10.1200/JCO.2016.70.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahmoodpoor A., Hamishehkar H., Asghari R., Abri R., Shadvar K., Sanaie S. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: a prospective double-blind randomized controlled trial. Nutrition in Clinical Practice. 2019;34(1):156–162. doi: 10.1002/ncp.10191. [DOI] [PubMed] [Google Scholar]
- 105.Weng H., Li J.-G., Mao Z., et al. Probiotics for preventing ventilator-associated pneumonia in mechanically ventilated patients: a meta-analysis with trial sequential analysis. Frontiers in Pharmacology. 2017;8:p. 717. doi: 10.3389/fphar.2017.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li K.-j., Chen Z.-l., Huang Y., et al. Dysbiosis of lower respiratory tract microbiome are associated with inflammation and microbial function variety. Respiratory Research. 2019;20(1):272–272. doi: 10.1186/s12931-019-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Makrygiannakis D., Hermansson M., Ulfgren A. K., et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Annals of the Rheumatic Diseases. 2008;67(10):1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 108.Scher J. U., Joshua V., Artacho A., et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome. 2016;4(1):p. 60. doi: 10.1186/s40168-016-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vieira W. A., Pretorius E. The impact of asthma on the gastrointestinal tract (GIT) Journal of Asthma and Allergy. 2010;3:123–130. doi: 10.2147/jaa.s10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rutten E. P. A., Lenaerts K., Buurman W. A., Wouters E. F. M. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145(2):245–252. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 111.Segal L. N., Blaser M. J. A brave new world: the lung microbiota in an era of change. Annals of the American Thoracic Society. 2014;11(Supplement 1):S21–S27. doi: 10.1513/annalsats.201306-189mg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anand S., Mande S. S. Diet, microbiota and gut-lung connection. Frontiers in Microbiology. 2018;9:p. 2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Samuelson D. R., Welsh D. A., Shellito J. E. Regulation of lung immunity and host defense by the intestinal microbiota. Frontiers in Microbiology. 2015;6:p. 1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herbst T., Sichelstiel A., Schär C., et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. American Journal of Respiratory and Critical Care Medicine. 2011;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 115.Brown R. L., Sequeira R. P., Clarke T. B. The microbiota protects against respiratory infection via GM-CSF signaling. Nature Communications. 2017;8(1):p. 1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grayson M. H., Camarda L. E., Hussain S.-R. A., et al. Intestinal microbiota disruption reduces regulatory T cells and increases respiratory viral infection mortality through increased IFNγ production. Frontiers in Immunology. 2018;9:1587–1587. doi: 10.3389/fimmu.2018.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cait A., Hughes M. R., Antignano F., et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunology. 2018;11(3):785–795. doi: 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- 118.Yang X., Feng H., Zhan X., et al. Early-life vancomycin treatment promotes airway inflammation and impairs microbiome homeostasis. Aging. 2019;11(7):2071–2081. doi: 10.18632/aging.101901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adami A. J., Bracken S. J., Guernsey L. A., et al. Early-life antibiotics attenuate regulatory T cell generation and increase the severity of murine house dust mite-induced asthma. Pediatric Research. 2018;84(3):426–434. doi: 10.1038/s41390-018-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Russell S. L., Gold M. J., Reynolds L. A., et al. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. Journal of Allergy and Clinical Immunology. 2015;135(1):100–109.e5. doi: 10.1016/j.jaci.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 121.Olszak T., An D., Zeissig S., et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen J. C., Tsai C. C., Hsieh C. C., Lan A., Huang C. C., Leu S. F. Multispecies probiotics combination prevents ovalbumin-induced airway hyperreactivity in mice. Allergologia et Immunopathologia. 2018;46(4):354–360. doi: 10.1016/j.aller.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 123.Fonseca V. M. B., Milani T. M. S., Prado R., et al. Oral administration of Saccharomyces cerevisiae UFMG A-905 prevents allergic asthma in mice. Respirology. 2017;22(5):905–912. doi: 10.1111/resp.12990. [DOI] [PubMed] [Google Scholar]
- 124.Juan Z., Zhao-Ling S., Ming-Hua Z., et al. Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology. 2017;22(5):898–904. doi: 10.1111/resp.12985. [DOI] [PubMed] [Google Scholar]
- 125.Spacova I., Petrova M. I., Fremau A., et al. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy. 2018;74(1):100–110. doi: 10.1111/all.13502. [DOI] [PubMed] [Google Scholar]
- 126.Hsieh M.-H., Jan R.-L., Wu L. S.-H., et al. Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells. Journal of Molecular Medicine (Berlin, Germany) 2018;96(1):39–51. doi: 10.1007/s00109-017-1598-1. [DOI] [PubMed] [Google Scholar]
- 127.Wang H., Zhao J. X., Hu N., Ren J., du M., Zhu M. J. Side-stream smoking reduces intestinal inflammation and increases expression of tight junction proteins. World Journal of Gastroenterology. 2012;18(18):2180–2187. doi: 10.3748/wjg.v18.i18.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reale M., Boscolo P., Bellante V., et al. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. British Journal of Nutrition. 2012;108(2):308–314. doi: 10.1017/S0007114511005630. [DOI] [PubMed] [Google Scholar]
- 129.Antosca K. M., Chernikova D. A., Price C. E., et al. Altered stool microbiota of infants with cystic fibrosis shows a reduction in genera associated with immune programming from birth. Journal of Bacteriology. 2019;201(16) doi: 10.1128/jb.00274-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bazett M., Bergeron M. E., Haston C. K. Streptomycin treatment alters the intestinal microbiome, pulmonary T cell profile and airway hyperresponsiveness in a cystic fibrosis mouse model. Scientific Reports. 2016;6(1):p. 19189. doi: 10.1038/srep19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gui Q. F., Lu H. F., Zhang C. X., Xu Z. R., Yang Y. H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genetics and Molecular Research. 2015;14(2):5642–5651. doi: 10.4238/2015.May.25.16. [DOI] [PubMed] [Google Scholar]
- 132.Viaud S., Saccheri F., Mignot G., et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gauguet S., D'Ortona S., Ahnger-Pier K., et al. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infection and Immunity. 2015;83(10):4003–4014. doi: 10.1128/IAI.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ichinohe T., Pang I. K., Kumamoto Y., et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsay T. B., Yang M. C., Chen P. H., Hsu C. M., Chen L. W. Gut flora enhance bacterial clearance in lung through toll-like receptors 4. Journal of Biomedical Science. 2011;18(1):p. 68. doi: 10.1186/1423-0127-18-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen L. W., Chen P. H., Hsu C. M. Commensal microflora contribute to host defense against Escherichia coli pneumonia through toll-like receptors. Shock. 2011;36(1):67–75. doi: 10.1097/SHK.0b013e3182184ee7. [DOI] [PubMed] [Google Scholar]
- 137.Fagundes C. T., Amaral F. A., Vieira A. T., et al. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. Journal of Immunology. 2012;188(3):1411–1420. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 138.Yoda K., He F., Miyazawa K., Kawase M., Kubota A., Hiramatsu M. Orally administered heat-killed Lactobacillus gasseri TMC0356 alters respiratory immune responses and intestinal microbiota of diet-induced obese mice. Journal of Applied Microbiology. 2012;113(1):155–162. doi: 10.1111/j.1365-2672.2012.05316.x. [DOI] [PubMed] [Google Scholar]
- 139.Mahooti M., Abdolalipour E., Salehzadeh A., Mohebbi S. R., Gorji A., Ghaemi A. Immunomodulatory and prophylactic effects of Bifidobacterium bifidum probiotic strain on influenza infection in mice. World Journal of Microbiology & Biotechnology. 2019;35(6):p. 91. doi: 10.1007/s11274-019-2667-0. [DOI] [PubMed] [Google Scholar]
- 140.Secher T., Maillet I., Mackowiak C., et al. The probiotic strain Escherichia coli Nissle 1917 prevents papain-induced respiratory barrier injury and severe allergic inflammation in mice. Scientific Reports. 2018;8(1):11245–11245. doi: 10.1038/s41598-018-29689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang B., An J., Shimada T., Liu S., Maeyama K. Oral administration of Enterococcus faecalis FK-23 suppresses Th17 cell development and attenuates allergic airway responses in mice. International Journal of Molecular Medicine. 2012;30(2):248–254. doi: 10.3892/ijmm.2012.1010. [DOI] [PubMed] [Google Scholar]
- 142.Vareille-Delarbre M., Miquel S., Garcin S., et al. Immunomodulatory effects of Lactobacillus plantarum on inflammatory response induced by Klebsiella pneumoniae. Infection and Immunity. 2019;87(11, article e00570) doi: 10.1128/iai.00570-19. [DOI] [PMC free article] [PubMed] [Google Scholar]


