Abstract
The evolution of the COVID-19 pandemic can be monitored through the detection of SARS-CoV-2 RNA in sewage. Here, we measured the amount of SARS-CoV-2 RNA at the inflow point of the main waste water treatment plant (WWTP) of Montpellier, France. We collected samples 4 days before the end of lockdown and up to 70 days post-lockdown. We detected increased amounts of SARS-CoV-2 RNA at the WWTP from mid-June on, whereas the number of new COVID-19 cases in the area started increasing a couple of weeks later. Future epidemiologic investigations shall explain such asynchronous finding.
Keywords: COVID-19, Virus, PCR, Health Surveillance
1. Introduction
SARS-CoV-2 is the etiologic agent responsible for the current coronavirus disease 2019 (COVID-19) pandemic. Wastewater-based epidemiology represents an attractive strategy to surveil the evolution of virus circulation in populations [2,6], contributing to cost-effective virus control without infringing on individual liberties. About half of symptomatic patients persistently shed SARS-CoV-2 RNA in their feces at levels going up to 108 RNA copies per stool sample [3,[11], [12], [13],15,16], which means that a single patient can shed billions of SARS-CoV-2 RNA copies in wastewater at a time. Moreover, an asymptomatic child was recently reported as negative for SARS-CoV-2 RNA based on throat swab specimen, while his stools were positive [10], suggesting that symptomatic and asymptomatic persons are likely to release SARS-CoV-2 RNA in city sewerages. Of note, subgenomic viral RNA was not detected in stools [12], e.g. no infectious virus is shed (although this finding remains to be further investigated).
Several reports indicate that SARS-CoV-2 RNA was readily detected in wastewater, and it is proposed that such approach could anticipate the occurrence of novel COVID-19 outbreaks in low prevalence regions [1,4,5,7,8,14]. The end of the stringent lockdown (that occurred in France on May 11th) is therefore an adequate time to measure the re-emergence of the virus through the monitoring of wastewater. Here, we collected effluent composite samples (using a 24-h automatic sampler) in wastewater upstream of the main wastewater treatment plant (WWTP) of Montpellier metropolitan area located in Lattes, France, which receives the wastewater from ≈ 470,000 inhabitants. The sampling dates were May 7th, 18th, 26th, June 4th, 15th, 25th, and July 20th to monitor SARS-CoV-2 RNA expression levels during lockdown and up to 70 days after its end. During this period, the virus was still circulating in the area, but the incidence was relatively low (number of newly diagnosed COVID-19 patients per day <20).
Collected wastewater was processed as follows: on the day of water collection, samples were maintained at 4 °C for transport and immediately cleared by centrifugation at 4500g for 30 min at 4 °C. The supernatant was passed through a 40 μm cell strainer (Corning) to remove large floating components. At this stage, the samples were frozen at −20 °C for later analyses with samples collected at other timepoints. Upon thawing, RNAs were concentrated on a Vivaspin 50 kDa MWCO filter membrane (Sartorius). Starting from 50 ml of water, the sample was concentrated down a hundred times to an adjusted volume of 500 μl. RNA extraction was performed using the NucleoSpin RNA Virus kit (Macherey-Nagel), including harsh lysis conditions (lysis buffer enriched in guanidinium isothiocyanate heated at 70 °C for 5 min). RT-qPCR was performed on 10 μl of purified RNA using the highly sensitive TaqPath One-Step RT-qPCR, CG master mix (ThermoFisher Scientific). The N1 and N3 primer/probe sets designed by the Center for Disease Control (CDC) were used to detect SARS-CoV-2 RNA and a standard curve was run in parallel using a positive control plasmid (Integrated DNA Technologies) coding for the nucleoprotein (N) of SARS-CoV-2. Using RNA extracted from Vero E6 cells either non-infected or infected with SARS-CoV-2 in vitro, we showed that the N1 and N3 primer/probe sets recognized solely the RNA from infected cells (Table 1). A recent study used a Dengue virus (DENV) sequence surrogate to determine PCR efficiency [5]. However, autochthonous cases of Dengue virus have been reported in Montpellier area (European Centre for Disease Prevention and Control) and thus, we could not use this RNA sequence for control, as it could be contained naturally in the wastewater of Montpellier (through infected mosquito eggs for instance). In contrast, Ebola virus has not reached Europe, and hopefully never will, and therefore, no Ebola RNA would “contaminate” our PCR efficiency reading. Here, we used a sensitive primer/probe set previously described [9] that target the complementary DNA of the VP40-encoding RNA of Ebola virus (Zaire strain) to assess the RT-qPCR efficiency intrinsic to each sample. First, we showed that the Ebola standard (Ebo Std) primer/probe set was not detecting RNA from SARS-CoV-2-infected Vero E6 cells (Table 1). Using water samples at the inlet or the at t WWTP of the Montpellier metropolitan area collected on June 15th, we found that the Ebo Std primer/probe set gave no signal while the primer/probes N1, N3, and RLP27 (targeting the human rlp27 gene) returned positive signals (Table 1). In comparison, the nearby WWTP of Murviel-lès-Montpellier, treating the wastewater from 2000 inhabitants, and a Montpellieran household in which no one was sick nor had symptoms, were negative for SARS-CoV-2 RNA on May 29, 2020. Collectively, these data show that N1 and N3 are relatively sensitive and specific for SARS-CoV-2 RNA detection.
Table 1.
Specificity of the primer/probe sets from in vitro SARS-CoV-2 infected cells. The Table shows the cycle threshold (Ct) of individual RT-qPCR reaction. The table shows representative data performed in duplicates from at least two independent experiments. “No Ct” indicates that no signal was detected over 40 cycles. nt: not tested.
| Primer/probe set | N1 | N3 | Ebo Std | RLP27 |
|---|---|---|---|---|
| RNA from non-infected cells | No Ct | No Ct | No Ct | nt |
| RNA from SARS-CoV-2 infected cells | 19 | 17.4 | No Ct | nt |
| Water samples collected upstream of the Montpellier WWTP | 36.4 | 37.6 | No Ct | 30.9 |
| No Ct | 37.3 | No Ct | 30.2 | |
| Water samples collected upstream of the Murviel-lès-Montpellier WWTP | No Ct | nt | nt | 39.0 |
| No Ct | nt | nt | 34.5 | |
| Sample collected in Montpellier household wastewater | No Ct | nt | nt | 24.6 |
| No Ct | nt | nt | 27.5 |
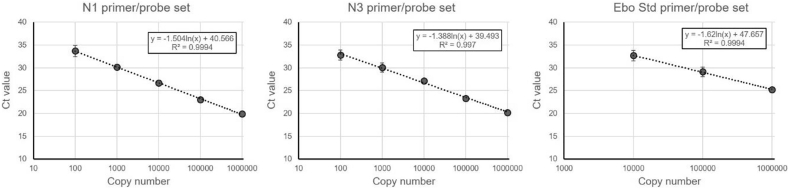
A synthetic RNA sequence of 91 nucleotides (gcagaucgaauccacucaggccaaucuauggugaaugucauaucgggccccaaagugcuaaugaaguuuggcuuccucuaggugucggcag) derived from the VP40-coding gene of Ebola virus and recognized by the above-mentioned Ebo Std primer/probe set was purchased (Integrated DNA Technologies) to be used as an internal normalization standard. Standard curves were run using the N control plasmid (N1 and N3 primer/probe sets) or the Ebo Std RNA (Ebo Std primer/probe set) to estimate copy numbers (Fig. 1). Of note, the Ebo Std primer/probe set was less sensitive than N1 and N3, probably because the Ebo Std synthetic template RNA is relatively fragile and short (91 nucleotides).
Fig. 1.
Standard curves of the N1 (left), N3 (middle) and Ebo Std (right) primer/probe sets showing the cycle threshold (Ct) value at indicated template copy number. The mean +/− SD of duplicates from a representative experiment is shown performed at least three times.
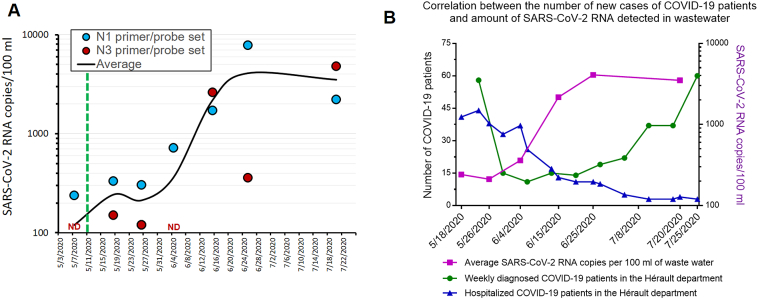
Next, we measured the SARS-CoV-2 RNA levels using N1 and N3 primer/probe sets in wastewater collected upstream of the main WWTP of the Montpellier metropolitan area on May 7th, 18th, 26th, June 4th, 15th, 25th, and July 20th (Fig. 2A). Our data highlights that the wastewater from the three later dates (June 15th, 25th, and July 20th) contained about fifty-fold more SARS-CoV-2 RNA than from previous dates. We confirmed that our RT-qPCR reaction was working properly for each sample using Ebo Std. Sample-to-sample variation of PCR efficiency was negligible compared to the magnitude of the SARS-CoV-2 RNA increase observed, as depicted by the similar curves of raw versus corrected datasets (Supplementary Fig. S1). Similarly, inlet water flowrate entering the WWTP was varying by less than 25% and rain precipitations (Montpellier wastewater network is partially unitary) were also very low (between 0 and 2 mm of rain on the 24 h preceding sample collection). These variables were not incorporated in the calculations because they are negligibly impacting the shape of the curve. Moreover, the calculations required for thorough data normalization are not trivial.
Fig. 2.
SARS-CoV-2 RNA detection in the Montpellier wastewater and number of COVID-19 cases. (A) The number of SARS-CoV-2 RNA copies was measured using either the N1 or N3 primer/probe sets. Each dot corresponds to the concentration of SARS-CoV-2 RNA in wastewater measured from the sum of the RNA copy number calculated from two RNA extractions and four RT-qPCR reactions performed in two individual runs. The first five samples were treated in parallel and therefore, differences in these samples cannot be attributed to experimental variation. Sterile water wells were included in duplicates for each primer/probe set in each plate as negative control and returned “No Ct” (not shown on the graph). PCR efficiency, rain precipitation and flowrates were negligible and not considered in the calculation. The green dotted line indicates the end of the strict lockdown in France. The black line corresponds to the smoothened average of the N1 and N3 primer/probe sets. ND: not detected. (B) The graph shows the number of new positive patients in the Hérault department over a 7-day period (green; left axis) as a function of time (source: SI-DEP; Santé Publique France) and on the right Y axis, the average SARS-CoV-2 RNA levels measured with the N1 and N3 primer/probe sets (purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
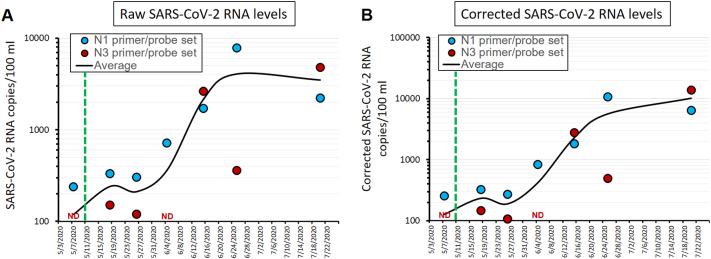
The following are the supplementary data related to this article.Supplementary Fig. S1.
Supplementary Figure S1. Comparison of raw versus corrected SARS-CoV-2 RNA levels. (A) Cruve of the raw SARS-CoV-2 RNA levels (as presented in main Figure 2A). (B) SARS-CoV-2 RNA levels corrected for PCR efficiency intrinsic to each timepoint.
We then put in perspective the amount of SARS-CoV-2 RNA in Montpellier wastewater (Fig. 2B; purple line) with the number of new COVID-19 cases weekly recorded in the Hérault department (> 40% inhabitant living in the Montpellier metropolitan area). Interestingly, an 3–4 times increase in the number of newly diagnosed COVID-19 patients was starting early July compared to May (Fig. 2B; green line). Of note, the outlier 58 newly diagnosed COVID-19 patients from week 17–23 May is surprisingly high, which may be linked to readjustments following the creation of a new virological testing database as of May 13 (the former public database reporting only 25 cases). In contrast to new COVID-19 cases, the number of hospitalized COVID-19 patients kept decreasing over the same period (Fig. 2B; blue line). This later observation is less relevant to measure the immediacy of the epidemic's resurgence as this parameter suffers high patient-to-patient variability.
The surge in the number of new COVID-19 patients started roughly 2–3 weeks after the increase of SARS-CoV-2 RNA levels in wastewater (Fig. 2B). Our data are reminiscent of a recent Spanish study, in which the authors could detect SARS-CoV-2 RNA in wastewater several weeks before the first COVID-19 cases were reported [8]. However, they could not see a correlation between SARS-CoV-2 RNA levels in wastewater and the number of newly diagnosed COVID-19 patients. Along the same lines, Medema & colleagues showed a correlation between the cumulative cases of COVID-19 and SARS-CoV-2 RNA although the data were not correlated as a function of time [5]. A work in Paris, France is ongoing to further determine the temporal correlation between wastewater SARS-CoV-2 RNA levels and COVID-19 epidemiological features [14].
In conclusion, we report effective detection of SARS-CoV-2 RNA in the wastewater of Montpellier area upstream of the treatment plant and identified an increase of the amount of detected viral RNA mid-June, associated with a rising number of newly identified COVID-19 cases in the department. Although a delayed correlation may exit, further investigations are required to better characterize the intricate relationship between these two variables. Indeed, we are unable at this stage to determine whether this increase announces an upcoming relapse of the epidemics in the area or relates to intrinsic SARS-CoV-2 RNA variations associated with uneven virus shedding (from patient-to-patient and depending on the stage of the disease for a given patient). Moreover, various other parameters might also impact these results, such as people from distant clusters moving to second homes and tourist accommodation, the chronic underestimation of prevalence rates, or local variability in the geographical pattern of virus spread. These hypotheses are non-exclusive and future multiparametric investigations are required to routinely use wastewater surveillance as a powerful predictive tool for ongoing and future epidemic outbreaks.
Authors' contributions
J.T established our consortium and initiated the project. J.T and R.G designed the study. R.D provided epidemiological datasets and geomatics expertise. N.A.M provided the rain precipitation dataset and hydrology expertise. E.P, W·B, M.D. and R.G performed and optimized the technical experimentations. R.G wrote the manuscript and J.T, R.D, N.A.M and W·B edited and commented on the manuscript. All authors agree on the final version of the manuscript.
Funding
This work was funded by the CNRS. The funder had no role in the design of the study.
Author statement
J.T established our consortium and initiated the project. J.T and R.G designed the study. R.D provided epidemiological datasets and geomatics expertise. N.A.M provided the rain precipitation dataset and hydrology expertise. E.P, W·B, M.D. and R.G performed and optimized the technical experimentations. R.G wrote the manuscript and J.T, R.D, N.A.M and W·B edited and commented on the manuscript. All authors agree on the final version of the manuscript.
Declaration of Competing Interest
None declared.
Acknowledgements
We thank Jean Verdier and Nima Machouf for their helpful comments and suggestions, and Yonis Bare for technical assistance. The strain BetaCoV/France/IDF0372/2020 was supplied by the National Reference Centre for Respiratory Viruses hosted by Institut Pasteur (Paris, France) and headed by Dr. Sylvie van der Werf.
Contributor Information
Julie Trottier, Email: Julie.trottier@cnrs.fr.
Raphael Gaudin, Email: raphael.gaudin@irim.cnrs.fr.
References
- 1.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., Yang J., Ye G., Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 4.La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environmental Science & Technology Letters. 2020;0 doi: 10.1021/acs.estlett.0c00357. null. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien E., Xagoraraki I. A water-focused one-health approach for early detection and prevention of viral outbreaks. One Health. 2019;7 doi: 10.1016/j.onehlt.2019.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732 doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randazzo W., Truchado P., Cuevas-Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ro Y.T., Ticer A., Carrion R., Jr., Patterson J.L. Rapid detection and quantification of Ebola Zaire virus by one-step real-time quantitative reverse transcription-polymerase chain reaction. Microbiol. Immunol. 2017;61:130–137. doi: 10.1111/1348-0421.12475. [DOI] [PubMed] [Google Scholar]
- 10.Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N., Wu W.J., Yuan C., Yu M.L., Li P., Yan J.B. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brunink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wurtzer S., Marechal V., Mouchel J., Maday Y., Teyssou R., Richard E., Almayrac J., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. MedRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., Zhao J., Huang J., Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]