Abstract
OBJECTIVE:
To clarify the function of microRNA-15a in the spinal cord injury (SCI) and its potential mechanism.
PATIENTS AND METHODS:
The plasma levels of microRNA-15a and signal transducer and activator of transcription 3 (STAT3) in SCI patients were determined by quantitative Real Time-Polymerase Chain Reaction (qRT-PCR). The correlation between the expressions of microRNA-15a and STAT3 was analyzed. The in vitro SCI model was established in H2O2-induced C8-D1A and C8B4 cells, and in vivo SCI model was established in mice by hitting T10. The mRNA and protein expressions of tumor necrosis factor-ɑ (TNF-ɑ) and interleukin 6 (IL-6) were detected in the SCI model. The apoptosis was examined by flow cytometry or TUNEL staining, respectively. The motor function of mouse hindlimb was evaluated using the Basso Beattie Bresnahan (BBB) standard scale. The target gene of microRNA-15a was predicted by bioinformatics and further verified by dual-luciferase reporter gene assay. The expression changes of target genes in C8-D1A and C8B4 cells with microRNA-15a overexpression or knockdown were examined by qRT-PCR and Western blot. Finally, rescue experiments were performed to evaluate the regulatory effects of microRNA-15a and STAT3 on cell apoptosis.
RESULTS:
MicroRNA-15a was lowly expressed in plasma of SCI patients, while STAT3 was highly expressed with a negative correlation to microRNA-15a. Identically, microRNA-15a was lowly expressed in H2O2-induced C8-D1A and C8B4 cells, and STAT3 was highly expressed. MicroRNA-15a overexpression downregulated mRNA and protein levels of TNF-ɑ and IL-6 in C8-D1A and C8B4 cells. BBB score was markedly low in SCI mice relative to controls. SCI mice injected with microRNA-15a mimics had higher BBB score than those injected with negative control. Besides, SCI mice with microRNA-15a overexpression had downregulated expressions of STAT3, TNF-ɑ, and IL-6 in the impaired spinal cord tissues, as well as lower apoptotic rate. Through bioinformatics, we found binding sites between STAT3 and microRNA-15a. Their binding conditions were further verified by dual-luciferase reporter gene assay. Moreover, STAT3 expression was negatively regulated by microRNA-15a. Finally, rescue experiments showed that STAT3 overexpression could reverse the regulatory effects of microRNA-15a on expressions of TNF-ɑ and IL-6, as well as apoptosis.
CONCLUSIONS:
MicroRNA-15a expression decreases in the SCI model, which participates in the process of SCI by regulating inflammatory response and cell apoptosis via targeting STAT3.
Keywords: MicroRNA-15a, STAT3, Inflammation, Apoptosis, SCI
Introduction
Spinal cord injury (SCI) resulted from a fall from height, traffic accidents, sports injuries, and violent injuries. It is manifested as high disability, high cost, and low mortality which mainly occurs in young and middle-aged people1,2. SCI poses a huge burden on affected patients and their families. The pathological process of SCI is a complex dynamic process involving multiple stages of primary injury, secondary injury, and chronic phase of injury3. Secondary injuries on blood-spinal cord barrier, ischemic edema, inflammatory response, lipid peroxidation, and impaired ion pathways lead to neuronal death and apoptosis in residual nerve cells and adjacent tissues4,5. The effective intervention approaches for SCI are still lacked nowadays. Current approaches mainly focus on preventing the deterioration of secondary injury at post-SCI. However, the specific mechanism of secondary injury at post-SCI remains unclear and brings great difficulties to the clinical treatment of SCI.
Signal transducer and activator of transcription 3 (STAT3) is a kind of DNA binding protein consisting of 750-795 amino acids. It is widely expressed in different types of tissues and cells, showing a close relationship with central nervous system diseases, tumors, and cardiovascular diseases6,7. As a member of the JAK-STAT pathway family, STAT3 exerts crucial functions in cellular behaviors. In the central nervous system, STAT3 is mainly distributed in astrocytes and neurons, which is responsible for neuronal proliferation and differentiation, and nerve regeneration8,9. Scholars10,11 have shown that astrocyte activation and glial scar formation triggered by nerve damage require the involvement of the STAT3 pathway. The activated STAT3 mediates SCI-induced inflammation12. In addition, Dai et al13 have observed that acute SCI can abnormally activate the JAK2/STAT3 pathway, leading to the occurrence of apoptosis. Therefore, the explorations on the STAT3 pathway are important for the treatment of SCI.
MicroRNA is a non-coding RNA containing about 20-25 nucleotides and specifically recognizes and binds to the 3’UTR of the target mRNA. It causes degradation or translational inhibition of the target mRNA and further regulates cellular behaviors14. A large number of microRNAs are abnormally expressed at post-SCI, further regulating ischemic edema, inflammatory reaction, and neuronal necrosis15–17. MiRNA-494 promotes the functional recovery in SCI rats by regulating the PTEN/AKT/mTOR pathway and inhibiting apoptosis18. The overexpression of miRNA-21 may exert a neuroprotective effect on SCI by downregulating the pro-apoptotic proteins Faslg and PDCD419. However, the role of microRNA-15a in SCI has not been studied. In this study, we focused on exploring the role of microRNA-15a in SCI, so as to provide novel targets for the treatment and rehabilitation of SCI.
Patients and Methods
Patients
Plasma samples were collected from 20 SCI patients and 20 healthy controls from March 2017 to March 2018 in Danyang People’s Hospital of Jiangsu Province. Sample collection received an informed consent by the patients and was approved by the Hospital’s Ethics Committee.
Establishment of the SCI Model in Mice
This study was approved by the Animal Ethics Committee of Nantong University Animal Center. Forty-eight Sprague-Dawley (SD) rats (weighing 180-220 g, male) were applied to this study. The mice were anesthetized with 3% phenobarbital (30 mg/kg, i.p.). After the skin was disinfected, the mouse was placed on the surgical table with a thermostatic pad. The mice were cut open at T10. The sclera and lamina of T9-T11 were removed to fully expose the dura mater. SCI was made by a hitting weighing 9 g from the height of 10 mm. After surgery, 0.05 mg/kg buprenorphine was subcutaneously administered for analgesia. The mice were observed (for) drinking water, eating, the skin around the wound and urination. 10 mg/kg negative control, microRNA-15a mimics or the same volume of normal saline were administered in mice through the tail vein. At 4 weeks, the mice were sacrificed and subjected to cardiac perfusion using 10% methanol. Impaired spinal cord tissues were harvested and preserved in liquid nitrogen.
Cell Culture
C8D1A and C8B4 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA), and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Rockville, MD, USA) containing 10% fetal bovine serum (FBS; Gibco, Rockville, MD, USA), penicillin 100 U/mL and 100 pg/mL streptomycin. The cells were placed in a 37°C, 5% C02 incubator. The medium was replaced every two days. In vitro SCI model was established by 10 μM H2O2 induction for 12 h in cells.
Cell Transfection
The cells with good growth were inoculated into the cell culture plate, and transfection was performed when the cell density reached 50%-60%. The cells were transfected with microRNA-15a mimics, microRNA-15a inhibitor, pcDNA-STAT3 or negative control using Lipofectamine 3000 3000 (Invitrogen, Carlsbad, CA, USA). The medium was replaced at 6 h. The transfected cells were harvested after 24 h for other experiments. The oligonucleotide sequences and plasmids used in the experiments were provided by GenePharma (Shanghai, China).
Behavioral Observation
After SCI model established at 1 h, 1 d, 1 w, 2 w, 3 w, and 4 w, BBB score was evaluated by two researchers blinded to the experimental grouping. The average BBB score was calculated from two independent records.
RNA Extraction and qRT-PCR
Total RNA in tissues or cells was extracted by TRIzol method (Invitrogen, Carlsbad, CA, USA). The content of the RNA sample was determined by the acid protease apparatus and then diluted with diethyl pyrocarbonate (DEPC) water (Beyotime, Shanghai, China). The complementary deoxyribose nucleic acid (cDNA) was synthesized according to the instructions of TaKaRa (Otsu, Shiga, Japan) RNA PCR Kit. QRT-PCR parameters were: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 60 s. The primers used in this study were as follows: MicroRNA-15a, F: 5′-CACCCCTAGTTCAGTTCTGCA-3′, R: 5′-CTGGGCACAGGCGGTCAG-3′; STAT3, F: 5′-CAGCAGCTTGACACACGGTA-3′, R: 5′- AAACACCAAAGTGGCATGTGA-3′, Tumor necrosis factor-α (TNF-α), F: 5′-GTCGCTACCGTCGTGACTTC-3′, R: 5′-CAGACATGCACCTACCCAGC-3′; Interleukin 6 (IF-6), F: 5′-ACTCACCTCTTCAGAACGAATTG-3′, R: 5′-CCATCTTTGGAAGGTTCAGGTTG-3′; Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), F: 5′-AGGTCGGTGTGAACGGATTTG-3′, R: 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Western Biot
The total protein from cells was extracted using radioimmunoprecipitation assay (RIPA) (Beyotime, Shanghai, China) and loaded for electrophoresis. After transferring on a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) at 300 mA for 100 min, it was blocked in 5% skim milk for 2 h, incubated with primary antibodies at 4°C overnight and secondary antibodies for 2 h. The bands were exposed by enhanced chemiluminescence (ECL) and analyzed by Image J Software (NIH, Bethesda, MD, USA). ”
Cell Apoptosis Assay
Cells were washed with phosphate-buffered saline (PBS) twice, digested and fixed in pre-cold 70% ethanol at 4°C for 30 min. Subsequently, the cells were induced with 5 mU of Annexin V-FITC (fluorescein isothiocyanate) and 1 ml of PI (50 mg/mU) for 5 min. The apoptosis was determined using flow cytometry (Becton-Dickinson, Franklin Fakes, NJ, USA).
Terminal-Deoxynucleoitidyl Transferase Mediated Nick End Labeling (TUNEL)
The tissues were dehydrated and embedded, and the sections were routinely dewaxed, washed, hydrated, and fixed strictly in accordance with the TUNEF Apoptosis Kit (Sigma-Aldrich, St. Fouis, MO, USA). Five randomly selected fields in each sample were observed, and at least 100 cells were counted in each field (magnification 400×). AI = number of apoptotic cells / total cell number × 100%.
Duai-Luciferase Reporter Gene Assay
The possible binding sites of microRNA-15a on the STAT3 were predicted by TargetScan (http://www.targetscan.org/). The predicted sequence was inserted into the luciferase reporter plasmid, and the reporter vector STAT3 WT was established. Meanwhile, the predicted binding sequence in STAT3 was mutated to establish STAT3 MUT. STAT3 WT or STAT3 MUT and microRNA-15a mimics were co-transfected in cells using Fipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). After 48 h of transfection, the cells were harvested for the determination of the activity.
Statistical Analysis
Statistical Product and Service Solutions (SPSS) 20.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA) was used for all statistical analysis. The data were expressed by . The t-test was used to compare the differences between two different groups. Pearson’s correlation coefficient analysis was conducted to evaluate the expression relationship. p<0.05 was considered statistically significant.
Results
MicroRNA-15a was Lowly Expressed, and STAT3 Was Highly Expressed in SCI Model
MicroRNA-15a is closely related to a variety of diseases and can regulate inflammatory process20,21. To investigate the expression changes and the possible roles of microRNA-15a in SCI, we first examined the plasma level of microRNA-15a in SCI patients. It is found that microRNA-15a was lowly expressed in the plasma of SCI patients (Figure 1A). Subsequently, we determined the STAT3 expression, a crucial gene in inflammation at post-SCI. Both mRNA and protein levels of STAT3 were higher in SCI patients than in healthy controls (Figure 1B, 1C). Meanwhile, an in vitro SCI model was established in H2O2-induced C8-D1A and C8B4 cells. Consistent with the expression patterns of microRNA-15a and STAT3 in SCI patients, the cellular level of microRNA-15a was lower, and STAT3 was higher in H2O2-induced C8-D1A and C8B4 cells (Figure 1D–1F). These results suggested that microRNA-15a and STAT3 were involved in the process of SCI.
Figure 1.
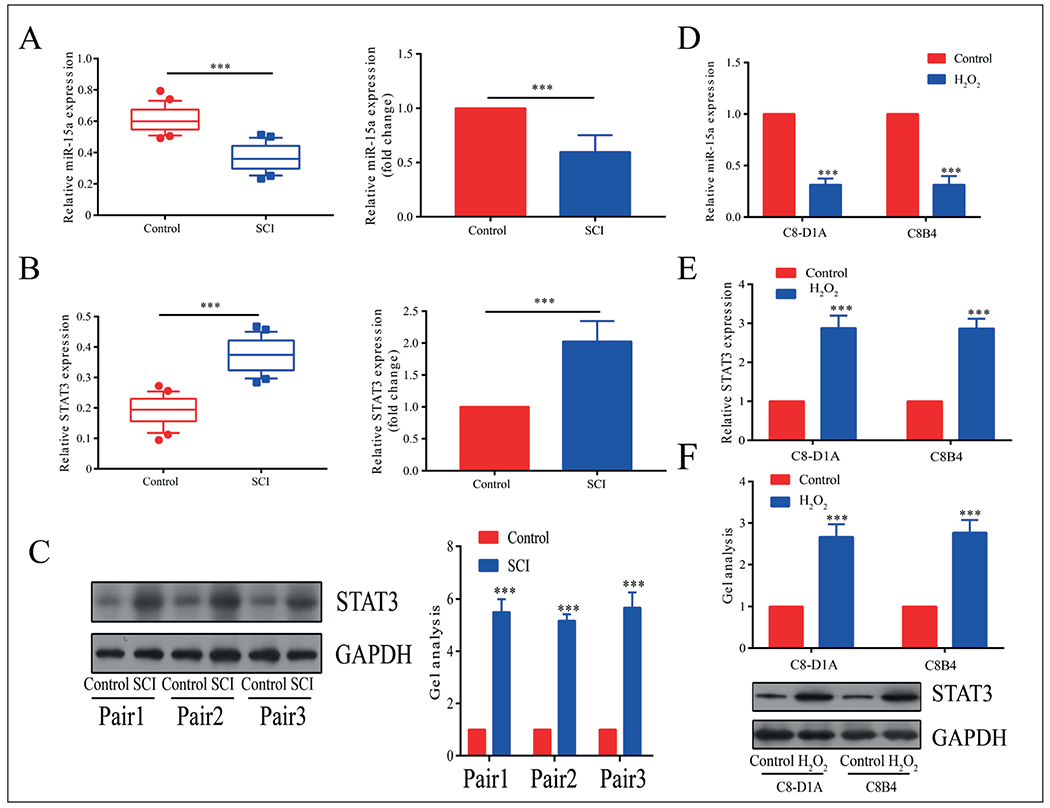
MiR-15a was lowly expressed and STAT3 was highly expressed in the SCI model. A, MiR-15a was lowly expressed in plasma of SCI patients than controls. B, STAT3 was highly expressed in plasma of SCI patients than controls and was highly expressed in SCI models. C, In the SCI animal models, the protein level of STAT3 was highly expressed than in controls. D, MiR-15a was lowly expressed in H2O2-induced C8-D1A and C8B4 cells than controls. E, F, STAT3 was highly expressed in H2O2-induced C8-D1A and C8B4 cells than controls. *p<0.05, **p<0.01, ***p<0.001, &No significance.
Overexpression of MicroRNA-15a Alleviated Inflammation and Apoptosis in In Vitro SCI Model
To explore the specific role of microRNA-15a in SCI, we first constructed microRNA-15a mimics and negative control. Transfection of microRNA-15a mimics in C8-D1A, and C8B4 cells markedly upregulated microRNA-15a expression (Figure 2A). We found that both mRNA and protein levels of TNF-α and IL-6 were downregulated in H2O2-induced C8-D1A and C8B4 cells overexpressing microRNA-15a (Figure 2B–2E). Furthermore, the transfection of microRNA-15a mimics inhibited apoptosis in C8-D1A and C8B4 cells (Figure 2F). These results suggested that microRNA-15a inhibited inflammation and apoptosis at post-SCI.
Figure 2.
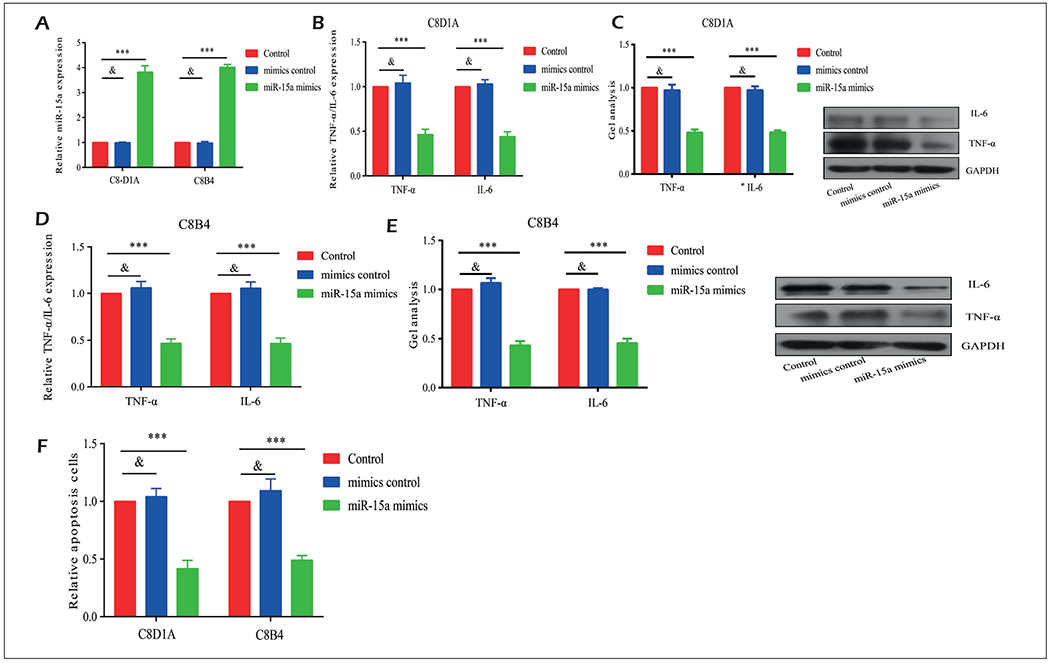
Overexpression of miR-15a alleviated inflammation and apoptosis in in vitro SCI model. A, Transfection of miR-15a mimics in C8-D1A and C8B4 cells markedly upregulated miR-15a expression. B-E, Both mRNA and protein levels of TNF-α and IL-6 were downregulated in H2O2-induced C8D1A and C8B4 cells overexpressing miR-15a. F, Transfection of miR-15a mimics inhibited apoptosis in C8-D1A and C8B4 cells. *p<0.05, **p<0.01, ***p<0.001, &No significance
Overexpression of MicroRNA-15a Alleviated Inflammation and Apoptosis in In Vivo SCI Model
Subsequently, we evaluated the in vivo function of microRNA-15a in SCI. BBB score was markedly lower in SCI mice injected with negative control or microRNA-15a mimics relative to those in the sham group, suggesting the successful construction of the SCI model in mice (Figure 3A). In particular, the SCI mice with microRNA-15a overexpression presented a higher BBB score than in controls, indicating a protective role of microRNA-15a in neuronal behaviors at post-SCI. MicroRNA-15a was highly expressed in the impaired spinal cord of SCI mice (Figure 3B). Besides, both mRNA and the protein levels of STAT3 were downregulated in SCI mice with microRNA-15a overexpression (Figure 3C, 3D). Consistent with in vitro results, SCI mice with microRNA-15a overexpression had lower levels of TNF-α and IL-6 relative to controls (Figure 3E, 3F). TUNEL staining showed fewer apoptotic cells in the SCI mice injected with microRNA-15a mimics than controls (Figure 3G). The above data all demonstrated the in vivo effect of microRNA-15a on inhibiting inflammation and apoptosis at post-SCI.
Figure 3.
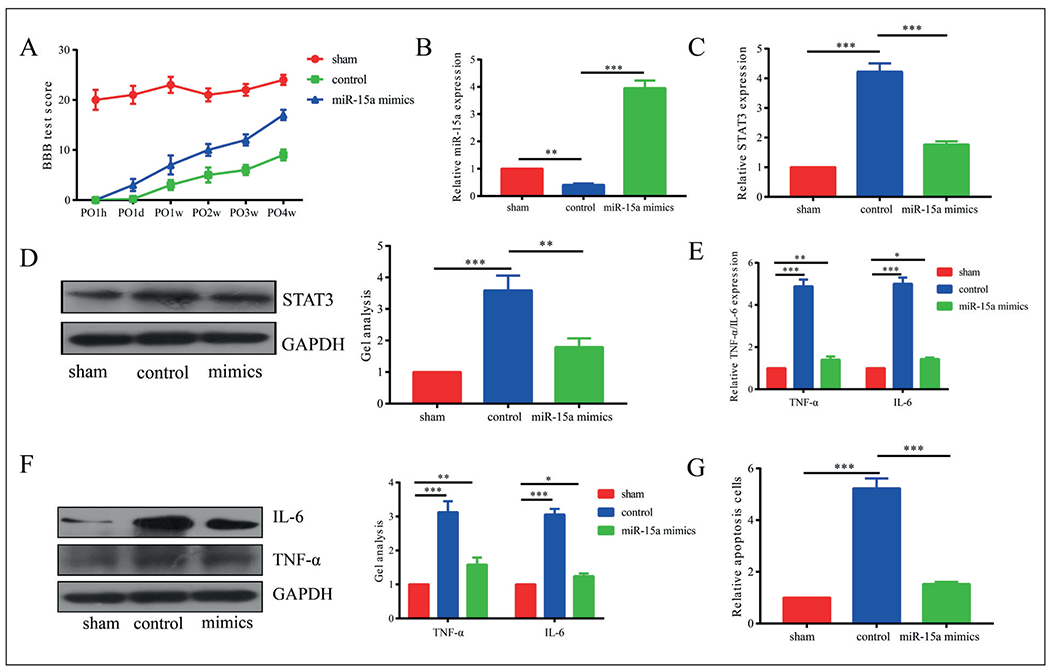
Overexpression of miR-15a alleviated inflammation and apoptosis in in vivo SCI model. A, BBB score was markedly lower in SCI mice injected with negative control or miR-15a mimics relative to those in sham group. B, MiR-15a was highly expressed in the impaired spinal cord of SCI mice than controls. C, D, Both mRNA and protein levels of STAT3 were downregulated in SCI mice with miR-15a overexpression. E, F, SCI mice with miR-15a overexpression had lower levels of TNF-α and IL-6 relative to controls. G, TUNEL staining showed fewer apoptotic cells in SCI mice injected with miR-15a mimics than controls. *p<0.05, **p<0.01, ***p<0.001, &No significance.
STAT3 Was the Target Gene of MicroRNA-15a
To explore the specific mechanism of microRNA-15a in SCI, we predicted the target gene of microRNA-15a by bioinformatics. The potential binding sites were found between STAT3 and microRNA-15a (Figure 4A). Based on the binding sequences, STAT3-WT and STAT3-MUT were constructed. The luciferase activity markedly decreased in cells co-transfected with microRNA-15a mimics and STAT3-WT, whereas those co-transfected with microRNA-15a mimics and STAT3-MUT did not show a significant change (Figure 4B, 4C). Hence, we proved that STAT3 was the target gene of microRNA-15a. Both qRT-PCR and Western blot showed that STAT3 expression was negatively regulated by microRNA-15a (Figure 4D, 4E).
Figure 4.
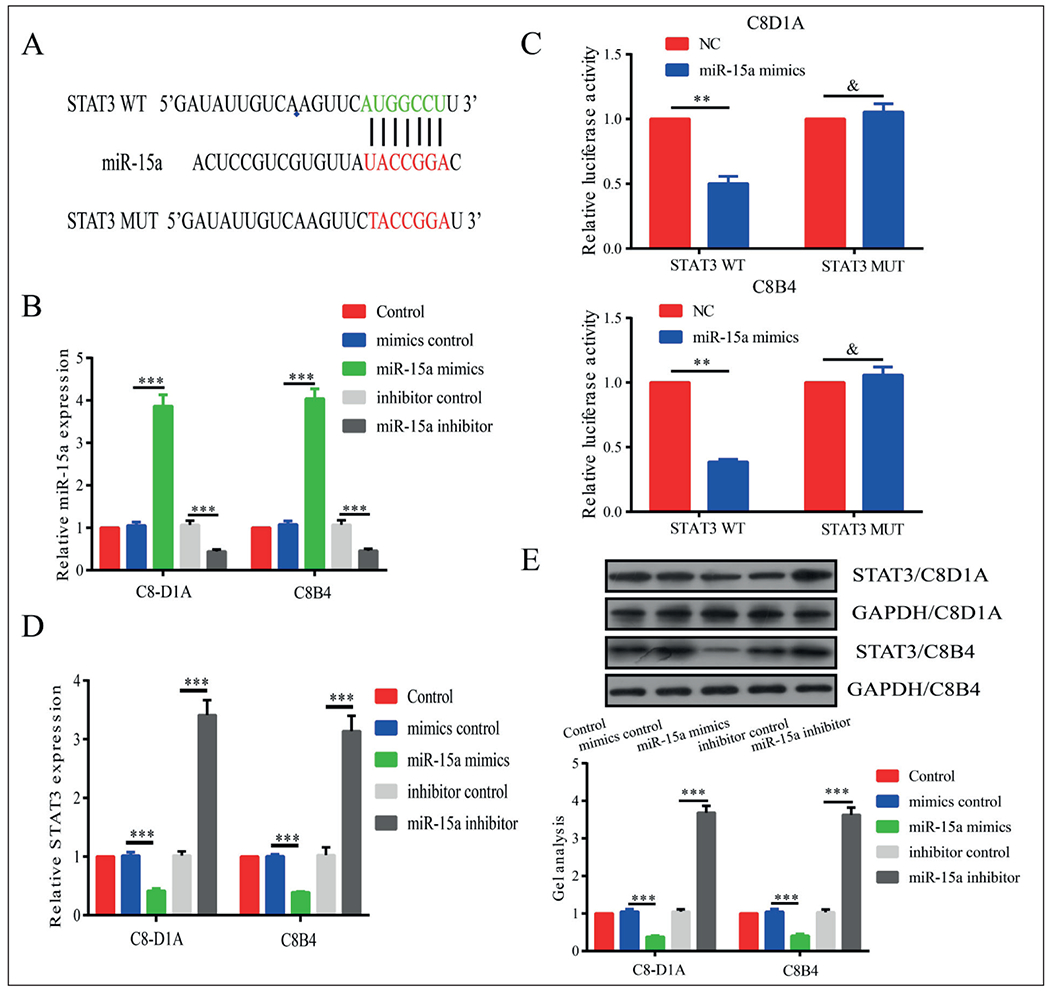
STAT3 was the target gene of miR-15a. A, Potential binding sites between STAT3 and miR-15a. B, Transfection efficacy of miR-15a mimics and inhibitor. C, Luciferase activity markedly decreased in cells co-transfected with miR-15a mimics and STAT3-WT, whereas those co-transfected with miR-15a mimics and STAT3-MUT did not show significant change. D, E, Both qRT-PCR and Western blot showed that STAT3 expression was negatively regulated by miR-15a. *p<0.05, **p<0.01, ***p<0.001, &No significance.
MicroRNA-15a Exerted its Function through Targeting STAT3
Since STAT3 has been proved to be the target gene of microRNA-15a, and its expression was negatively regulated by microRNA-15a, we speculated whether the regulatory effects of microRNA-15a on SCI was dependent on STAT3. The transfection efficacy of pcDNA-STAT3 was detected in C8-D1A and C8B4 cells (Figure 5A). Moreover, the downregulated levels of TNF-α and IL-6 by transfection of microRNA-15a mimics were partially reversed by STAT3 overexpression (Figure 5B, 5C). The inhibited apoptosis due to microRNA-15a overexpression was greatly accelerated after transfection of pcDNA-STAT3 (Figure 5D). Therefore, we believed that microRNA-15a inhibited inflammation and apoptosis in SCI through degrading STAT3.
Figure 5.
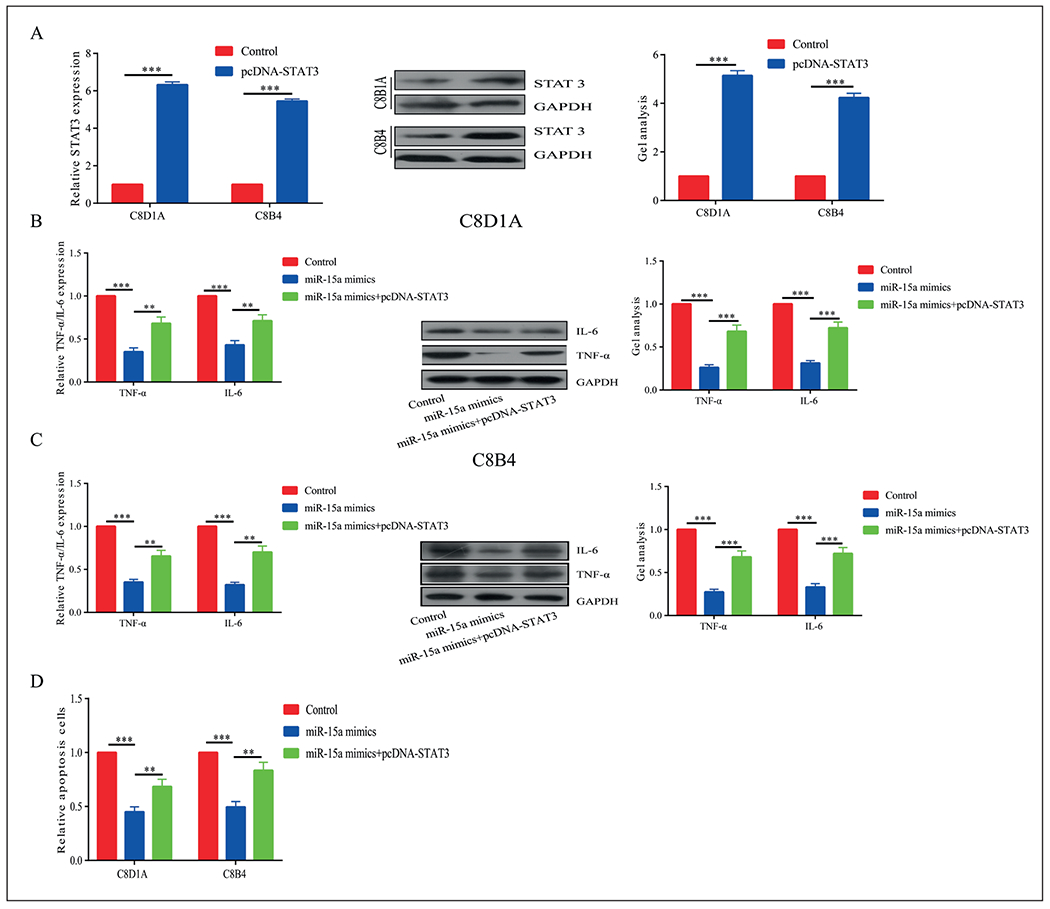
MiR-15a exerted its function by targeting STAT3. A, Transfection efficacy of pcDNA-STAT3. B, C, Downregulated levels of TNF-a and IL-6 by transfection of miR-15a mimics were partially reversed by STAT3 overexpression. D, The inhibited apoptosis due to miR-15a overexpression was greatly accelerated after transfection of pcDNA-STAT3. *p<0.05, **p<0.01, ***p<0.001, &No significance.
Discussion
SCI is common in traffic, labor, and sports accidents, which is a serious disabling injury to the central nervous system22. Patients with cervical SCI often experience severe injuries and extremity dysfunction, posing a catastrophic impact on their life quality. Several pathways are involved in the SCI-induced secondary injury. Recovery and clinical outcomes of SCI are closely related to inflammatory response, neuronal apoptosis, and oxidative stress at post-SCI. Hence, the prevention of inflammation and apoptosis contributes to the improving the prognosis of SCI22.
In recent years, a variety of microRNAs has been identified in regulating the development and recovery of SCI. The upregulation of miR-133b markedly affects axonal regeneration through downregulating RhoA expression, which contributes to post-SCI repair23. The up-regulation of miR-486 at post-SCI inhibits the expressions of NeuroD6, GPx3, and TxnLI, leading to aggravated motor neuron degeneration due to the dysregulated active oxygen scavenging system24. Therefore, it is particularly important to study the role of microRNAs in the development of SCI. Previous studies have pointed out the role of microRNA-15a in other diseases. For example, microRNA-15a-5p inhibits peritoneal dialysis-induced inflammation and fibrosis of peritoneal mesothelial cells through targeting VEGF-A25. However, the role of microRNA-15a in SCI is still rarely studied.
In this study, we detected the low expression of microRNA-15a in plasma of SCI patients and H2O2-induced in vitro SCI model. Meanwhile, STAT3 was highly expressed in SCI. The overexpression of microRNA-15a downregulated TNF-α and IL-6 in C8-D1A and C8B4 cells, and inhibited apoptosis confirming that microRNA-15a had a regulatory effect on inflammation and apoptosis26. To verify whether microRNA-15a had a similar function in the SCI mice, we injected microRNA-15a mimic or negative control into the tail vein of SCI mice. BBB was higher in SCI mice with microRNA-15a overexpression than in controls. Moreover, expressions of STAT3, TNF-α, and IL-6 decreased in the impaired spinal cord of mice. Apoptosis was reduced in SCI mice with microRNA-15a overexpression as well. We believed that microRNA-15a exerted a repair function in SCI mice.
JAK-STAT pathway is regulated by a variety of cytokines, showing a vital role in cellular behaviors. STAT3 is the most active molecule in the STAT family, which is capable of regulating cell growth, apoptosis, and cell cycle. The activation of STAT3 regulates inflammation and apoptosis at post-SCI7. In this paper, we indicated the binding of STAT3 to microRNA-15a through dual-luciferase reporter gene assay. Moreover, STAT3 overexpression reversed the inhibitory effect of overexpressed microRNA-15a on the mRNA and protein expressions of TNF-α and IL-6, and apoptosis. It is concluded that microRNA-15a inhibited inflammation and apoptosis at post-SCI through targeting and downregulating STAT3. Whether STAT3 is involved in the in vivo repair function of microRNA-15a in SCI mice, however, needs to be further investigated.
Conclusions
We revealed that microRNA-15a expression decreases in the SCI model, which participates in the process of SCI through regulating inflammatory response and cell apoptosis via targeting STAT3.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31700444), Social Development Guide Project of Zhenjiang (FZ2015074 and FZ2015075) and Science and Technological Development Special Foundation of Danyang (SF201510).
Footnotes
Conflict of Interest
The Authors declare that they have no conflict of interests.
References
- 1).Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 2014; 6: 309–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, Garshick E. Traumatic spinal cord injury in the United States, 1993-2012. JAMA 2015; 313: 2236–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).James ND, McMahon SB, Field-Fote EC, Bradbury EJ. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol 2018; 17: 905–917. [DOI] [PubMed] [Google Scholar]
- 4).Ahuja CS, Wilson JR, Nori S, Kotter M, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers 2017; 3: 17018. [DOI] [PubMed] [Google Scholar]
- 5).Zheng Z, Wang ZG, Chen Y, Chen J, Khor S, Li J, He Z, Wang Q, Zhang H, Xu K, Fanghua G, Xiao J, Wang X. Spermidine promotes nucleus pulposus autophagy as a protective mechanism against apoptosis and ameliorates disc degeneration. J Cell Mol Med 2018; 22: 3086–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Song S, Min Η, Niu M, Wang L, Wu Y, Zhang B, Chen X, Liang Q, Wen Y, Wang Y, Yi L, Wang H, Gao Q. S1PR1 predicts patient survival and promotes chemotherapy drug resistance in gastric cancer cells through STAT3 constitutive activation. EBioMedicine 2018; 37: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Dang M, Zeng X, Chen B, Wang H, Li H, Liu Y, Zhang X, Cao X, Du F, Guo C. Soluble receptor for advance glycation end-products inhibits ischemia/reperfusion-induced myocardial autophagy via the STAT3 pathway. Free Radio Biol Med 2019; 130: 107–119. [DOI] [PubMed] [Google Scholar]
- 8).Madaro L, Passafaro M, Sala D, Etxaniz U, Lugarini F, Proietti D, Alfonsi MV, Nicoletti C, Gatto S, De Bardi M, Rojas-Garcia R, Giordani L, Marinelli S, Pagliarini V, Sette C, Sacco A, Puri PL. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol 2018; 20: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Renault-Mihara F, Mukaino M, Shinozaki M, Kumamaru H, Kawase S, Baudoux M, Ishibashi T, Kawabata S, Nishiyama Y, Sugai K, Yasutake K, Okada S, Nakamura M, Okano H. Regulation of RhoA by STAT3 coordinates glial scar formation. J Cell Biol 2017; 216: 2533–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Oliva AJ, Kang Y, Sanchez-Molano J, Furones C, Atkins CM. STAT3 signaling after traumatic brain injury. J Neurochem 2012; 120: 710–720. [DOI] [PubMed] [Google Scholar]
- 11).Okada S, Nakamura M, Katoh Η, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 2006; 12: 829–834. [DOI] [PubMed] [Google Scholar]
- 12).Zong S, Zeng G, Fang Y, Peng J, Tao Y, Li K, Zhao J. The role of IL-17 promotes spinal cord neuroinflammation via activation of the transcription factor STAT3 after spinal cord injury in the rat. Mediators Inflamm 2014; 2014: 786947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Dai J, Xu LJ, Han GD, Sun HL, Zhu GT, Jiang HT, Yu GY, Tang XM. MicroRNA-125b promotes the regeneration and repair of spinal cord injury through regulation of JAK/STAT pathway. Eur Rev Med Pharmacol Sci 2018; 22: 582–589. [DOI] [PubMed] [Google Scholar]
- 14).Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature 2009; 460: 529–533. [DOI] [PubMed] [Google Scholar]
- 15).He J, Zhao J, Peng X, Shi X, Zong S, Zeng G. Molecular mechanism of miR-136-5p targeting NF-kappaB/A20 in the IL-17-mediated inflammatory response after spinal cord injury. Cell Physiol Biochem 2017; 44: 1224–1241. [DOI] [PubMed] [Google Scholar]
- 16).Li XQ, Chen FS, Tan WF, Fang B, Zhang ZL, Ma H. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J Neuroinflammation 2017; 14: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG. MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 2018; 24: 221–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Zhu H, Xie R, Liu X, Shou J, Gu W, Gu S, Che X. MicroRNA-494 improves functional recovery and inhibits apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal cord injury. Biomed Pharmacother 2017; 92: 879–887. [DOI] [PubMed] [Google Scholar]
- 19).He F, Ren Y, Shi E, Liu K, Yan L, Jiang X. Overexpression of microRNA-21 protects spinal cords against transient ischemia. J Thorac Cardiovasc Surg 2016; 152: 1602–1608. [DOI] [PubMed] [Google Scholar]
- 20).Shang J, He Q, Chen Y, Yu D, Sun L, Cheng G, Liu D, Xiao J, Zhao Z. MiR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J Cell Physiol 2019; 234: 9746–9755. [DOI] [PubMed] [Google Scholar]
- 21).Lu WJ, Zeng LL, Wang Y, Zhang Y, Liang HB, Tu XQ, He JR, Yang GY. Blood microRNA-15a correlates with IL-6, IGF-1 and acute cerebral ischemia. Curr Neurovasc Res 2018; 15: 63–71. [DOI] [PubMed] [Google Scholar]
- 22).Kim HY, Kumar H, Jo MJ, Kim J, Yoon JK, Lee JR, Kang M, Choo YW, Song SY, Kwon SP, Hyeon T, Han IB, Kim BS. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett 2018; 18: 4965–4975. [DOI] [PubMed] [Google Scholar]
- 23).Yu YM, Gibbs KM, Davila J, Campbell N, Sung S, Todorova TI, Otsuka S, Sabaawy HE, Hart RP, Schachner M. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci 2011; 33: 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).UlTTENBOGAARD M, BAXTER KK, CHIARAMELLO A. The neurogenic basic helix-loop-helix transcription factor NeuroD6 confers tolerance to oxidative stress by triggering an antioxidant response and sustaining the mitochondrial biomass. ASN Neuro 2010; 2: e00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Shang J, He Q, Chen Y, Yu D, Sun L, Cheng G, Liu D, Xiao J, Zhao Z. MiR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J Cell Physiol 2019; 234: 9746–9755. [DOI] [PubMed] [Google Scholar]
- 26).Griesinger AM, Josephson RJ, Donson AM, Mulcahy LJ, Amani V, Birks DK, Hoffman LM, Furtek SL, Reigan P, Handler MH, Vibhakar R, Foreman NK. Interleukin-6/STAT3 pathway signaling drives an inflammatory phenotype in group A ependymoma. Cancer Immunol Res 2015; 3: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Song Y, Zeng Z, Jin C, Zhang J, Ding B, Zhang F. Protective effect of ginkgolide B against acute spinal cord injury in rats and its correlation with the Jak/STAT signaling pathway. Neurochem Res 2013; 38: 610–619. [DOI] [PubMed] [Google Scholar]


