Abstract
Adolescent girls and young women (AGYW) are a priority population for HIV prevention in high-burden settings. We evaluated psychosocial characteristics, behavioral risk factors for HIV, and pre-exposure prophylaxis (PrEP) awareness and uptake among AGYW seeking contraceptive services at four public sector family planning (FP) clinics offering integrated PrEP delivery in Kisumu, Kenya. From October 2018 to June 2019, we approached all AGYW (aged 15–24 years) seeking contraception to participate in a survey following receipt of FP services and PrEP screening. Overall, 470 AGYW were screened for PrEP at their FP visit by facility staff and subsequently enrolled in the survey. Median age was 22 years (interquartile range 20–23), 22% of AGYW were in school, and 55% were married. The most frequent forms of contraception were implants and injectables (41% each). Over a third of AGYW (36%) reported low social support, 13% had symptoms of moderate to severe depression, and 3% reported intimate partner violence. Three-quarters (75%) of AGYW reported recent condomless sex and 42% suspected that their primary partner had other sexual partners. Most AGYW (89%) had previously heard of PrEP; 76% had at least one PrEP eligibility criterion as per national guidelines; however, only 4% initiated PrEP at their current FP visit. PrEP initiators more frequently had high HIV risk perception than noninitiators (85% vs. 10%, p < 0.001). Low perceived HIV risk (76%) and pill burden (51%) were common reasons for declining PrEP among AGYW with HIV behavioral risk factors. PrEP counseling should be tailored to AGYW to guide appropriate PrEP decision-making in this important population.
Keywords: pre-exposure prophylaxis, PrEP, HIV prevention, women, adolescents, Africa
Introduction
For young women in eastern and southern Africa, HIV incidence rates remain unacceptably high.1 In September 2015, the World Health Organization (WHO) recommended oral tenofovir-based pre-exposure prophylaxis (PrEP) for persons with high HIV acquisition risk as part of a comprehensive HIV prevention strategy.2 Subsequently, in July 2016, the Kenya Ministry of Health (MOH) released guidelines recommending PrEP for all HIV-uninfected persons with substantial ongoing risk of HIV infection, including adolescent girls and young women (AGYW) as a priority population.3 PrEP implementation is progressing in HIV high-burden regions, and Kenya has led efforts to expand implementation with >55,000 individuals initiating PrEP as of January 2020.4 However, only a small minority of PrEP users worldwide are women of reproductive age.
PrEP implementation at delivery points frequently accessed by populations with high HIV risk could improve yield of PrEP promotion efforts. The most recent Kenyan Demographic and Health Survey reports that 60% of Kenyan women seek contraceptives through public sector family planning (FP) clinics5 and are routinely screened for HIV behavioral risk factors. Therefore, FP clinics could be an important platform for reaching AGYW with PrEP services. In the PrEP Implementation for Young Women and Adolescents (PrIYA) Program, we previously demonstrated the feasibility of integrated PrEP delivery for AGYW within routine FP clinics in Kenya.6 In the PrIYA Program, a PrEP-trained nurse was assigned to pilot integration of PrEP into routine services and to provide PrEP to AGYW attending the clinic. We found that 16% of AGYW with HIV risk factors accepted PrEP when offered by these PrEP-specialized nurses assigned to FP settings.6 Other studies among Kenyan AGYW report more modest PrEP uptake (<5%).7,8 Additional evaluations of PrEP outcomes in real-world FP clinics without additional research or demonstration project staffing are needed to guide PrEP implementation for AGYW.
In a follow-on survey conducted after completion of the PrIYA Program, we evaluated psychosocial characteristics, behavioral risk factors for HIV, and PrEP awareness and uptake among AGYW seeking FP services and who were screened for PrEP within a fully programmatic context (i.e., only MOH staff and no PrIYA Program-dedicated nurses) at four former PrIYA sites in Kisumu, Kenya.
Methods
Study setting and population
The PrIYA Program was a 2-year implementation project that integrated delivery of PrEP into routine maternal child health (MCH) and FP systems to reach AGYW at high risk for HIV acquisition.6,9 In collaboration with the Department of Health and Sanitation, Kisumu County, and the National AIDS and STI Control Programme (NASCOP), PrIYA was implemented from June 2017 to October 2018 in 16 facilities in Kisumu County, Kenya, which has an adult HIV prevalence of 19.9%.10–12 To evaluate psychosocial characteristics, behavioral risk factors for HIV, and PrEP uptake among AGYW seeking routine FP services, we conducted a follow-on study at a subset of former PrIYA sites that were continuing to deliver PrEP under programmatic conditions. Four public sector facilities were purposively selected based on having the highest monthly enrollment of new FP clients.
All HIV-uninfected women at the four facilities were approached after receipt of their routine FP services from October 2018 to June 2019. Those who were between 15 and 24 years old, had ever been screened for PrEP (either that day or previously), and were receiving FP services at the facility were eligible for enrollment. All eligible women who were interested in participating and provided written informed consent were enrolled. Women screened for PrEP the same day as the survey were included in this current analysis. We excluded women who were not screened for PrEP due to stock-outs, lack of PrEP providers that day, or other programmatic issues.
Data collection procedures
Trained study nurses administered surveys in Kiswahili, Dholuo, or English using tablet-based questionnaires. We surveyed participants about demographics, partnership characteristics, sexual and reproductive behaviors, perceived HIV risk, HIV risk behaviors, psychosocial factors, and experiences being offered and/or using PrEP. Before survey implementation, study staff field-tested the data collection instrument; questionnaire items or translations were refined as needed.
Behavioral HIV risk assessment
We evaluated participants for HIV behavioral risk factors using a standardized risk assessment tool used by the Kenya MOH to screen for PrEP, which includes the following behavioral characteristics: partner HIV status, condomless sex, engagement in transactional sex, and being forced to have sex in the last 6 months.3 HIV risk behaviors were also assessed using an empirical risk score validated to predict risk of HIV acquisition among young women in sub-Saharan African settings;13 characteristics included in the risk score were age <25 years (risk score of 2), not living with a spouse/partner (1), any alcohol use within the past 30 days (1), receiving financial support from a partner (1), having a partner with other sexual partners (2) or not knowing if a partner has other sexual partners (1), and having a curable sexually transmitted infection (STI) (1). We utilized the modified version of this risk score (having an STI was excluded since we did not assess STI status).13 High HIV risk is defined by an HIV risk score of ≥5 (corresponding to 5–15% HIV incidence in cohorts of African women).13 Risk scores of ≤4 correspond to HIV incidence of 0–5% and are considered low HIV risk. We also assessed self-perceived risk for HIV acquisition on a 4-point Likert scale by asking participants “What is your gut feeling about how likely you are to get infected with HIV?” with possible responses of very likely, somewhat likely, very unlikely, or extremely unlikely.14 Women self-reported their decision to initiate PrEP or decline PrEP during their clinic visit that day, which was confirmed with clinic records.
Assessment of psychosocial characteristics
We assessed depressive symptoms using the Center for Epidemiologic Studies Depression Scale (CESD-10), which is supported for reliable use in sub-Saharan Africa.15,16 We defined having moderate to severe depressive symptoms as CESD-10 scores of 10 or greater.15,16 Intimate partner violence was assessed with the four-item Hurt, Insult, Threaten, and Scream Scale (HITS),17 defining intimate partner violence with a cutoff of 10 or greater (absolute range: 4–20). We evaluated social support with the 18-item Medical Outcomes Study Social Support Survey (MOS-SSS).18 We defined inadequate social support as scores below 72 as such scores meant participants perceived that they were unable to receive social support at least most of the time for each scenario. Self-efficacy to take a daily oral medication was assessed by asking participants to rank on a 0–10 scale (0 = cannot do it at all, 10 = completely certain can do it) their response to this question: “How confident are you that you can integrate a daily medication into your daily routine?” We defined high self-efficacy as scores of 5 or greater, indicating they felt moderate to complete certainty that they could take a daily oral pill.
Statistical analyses
We used descriptive statistics to determine the frequency of demographic and psychosocial characteristics, pregnancy history and FP use, PrEP awareness and attitudes, and HIV behavioral risk.13 We used univariable Poisson regression models, clustering by facility, to compare frequency of HIV risk behaviors, psychosocial characteristics, and PrEP attitudes and beliefs among AGYW who initiated or declined PrEP. Multi-variable models were not performed as precise estimates were not possible due to strata sizes <10. Analyses were performed using STATA 15.0.
Considerations for human subjects
The Kenyatta National Hospital-University of Nairobi Ethics Research Committee and University of Washington Human Subjects Review Committee reviewed and approved the study protocol, informed consent forms, and data collection tools. We also obtained approval from the Kisumu County Department of Health and health administrators within the health facilities involved. All study participants received a KSH 300 (∼USD 3) reimbursement for their time and were welcome to end the interview at any point during the survey.
Results
Study population characteristics
Overall, 470 AGYW were screened for PrEP during their FP visit and were included in the analysis (77% of total study participants) (Fig. 1). Among these AGYW, the median age was 22 years (interquartile range [IQR] 20–23), 11% (50/470) were employed, and the median completed education was 12 years (IQR 10–12) (Table 1). Most AGYW had a current primary sexual partner (389/470, 83%), 55% were married, and the majority had at least one prior pregnancy (367/470, 78%). Over half of the AGYW initiated an FP method on the day of study participation (256/470, 55%) and 45% were refilling. The most common contraceptive methods in use were injectables and implants (each 41%); fewer AGYW used oral contraceptive pills (13%), intrauterine contraceptive devices (3%), or condoms alone (2%).
FIG. 1.
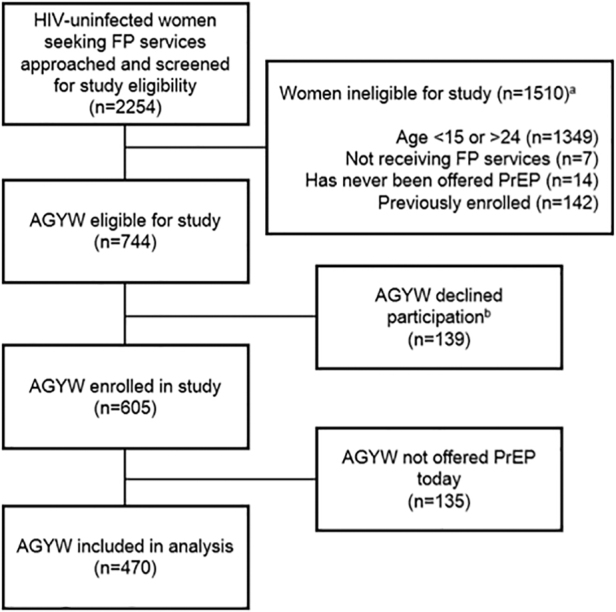
Flow chart of participant inclusion in the analysis among HIV-uninfected AGYW seeking FP services in Western Kenya. aCategories describing reasons for ineligibility are not mutually exclusive. bReasons for declining enrollment were not captured. Anecdotally, FP clients not having time to complete the survey was frequently reported as a reason for declining by study staff. cAGYW who were not screened for PrEP (n = 135) due to PrEP stock-outs or lack of PrEP providers were excluded from the current analysis. AGYW, adolescent girls and young women; FP, family planning; PrEP, pre-exposure prophylaxis.
Table 1.
Characteristics of Adolescent Girls and Young Women Seeking Family Planning Services Who Were Screened for PrEP (N = 470)
| Characteristicsa | N or median (% or IQR) |
|---|---|
| Demographic characteristics | |
| Age (years) | 22.0 (20.0–23.0) |
| Age ≤18 years | 44 (9.4%) |
| Married/living with a partner | 260 (55.3%) |
| Currently in school (n = 468) | 103 (22.0%) |
| Completed education (years) | 12.0 (10.0–12.0) |
| Regularly employed (n = 467) | 50 (10.7%) |
| Has a current partner | 389 (82.8%) |
| Pregnancy history and FP use | |
| Ever been pregnant before | 367 (78.1%) |
| Number of pregnancies (n = 367) | 1.0 (1.0–2.0) |
| Number of living children (n = 366) | 1.0 (1.0, 2.0) |
| FP status today | |
| Initiating | 256 (54.5%) |
| Refilling | 180 (38.3%) |
| Not initiating/refilling | 15 (3.2%) |
| Discontinuing | 19 (4.0%) |
| FP methodb (n = 450) | |
| Injectableb | 184 (40.9%) |
| IUCD | 14 (3.1%) |
| Implant | 184 (40.9%) |
| Condoms | 10 (2.2%) |
| OCP | 58 (12.9%) |
| HIV behavioral risk factors | |
| Partner HIV status (n = 389) | |
| Negative | 310 (79.7%) |
| Unknown | 75 (19.3%) |
| Positive | 4 (1.0%) |
| ≥4 Total lifetime sexual partners | 57 (12.1%) |
| Had condomless sexc | 353 (75.1%) |
| Engaged in transactional sexc | 22 (4.7%) |
| Forced to have sex against their willc | 24 (5.1%) |
| Experienced intimate partner violenced (n = 388) | 11 (2.8%) |
| High self-perceived HIV riske | 64 (13.6%) |
| Partner age difference ≥10 years (n = 358) | 33 (9.2%) |
| HIV risk score factors | |
| Any alcohol use (past 30 days) | 68 (14.5%) |
| Partner provides financial support | 460 (97.9%) |
| Primary partner has other partners (n = 387) | |
| No | 226 (58.4%) |
| Yes | 30 (7.8%) |
| Do not know | 131 (33.9%) |
| High behavioral HIV riskf | 111 (23.6%) |
| Psychosocial characteristics | |
| Low social supportg (n = 469) | 170 (36.2%) |
| Depressive symptomsh (n = 372) | 49 (13.2%) |
| Low self-efficacy to take daily medicationi (n = 466) | 162 (34.8%) |
| PrEP awareness, attitudes, and acceptability | |
| Heard of PrEP before today | 416 (88.5%) |
| Ever screened for PrEP before today | 311 (66.2%) |
| Initiated PrEP today | 20 4.3% |
| Knows someone who is taking PrEP | 94 (20.0%) |
| PrEP is for people who are promiscuous | |
| Completely disagree | 253 (53.8%) |
| Disagree | 205 (43.6%) |
| Neutral/unsure | 5 (1.1%) |
| Agree/completely agree | 7 (1.5%) |
Denominator is n = 470 unless otherwise noted.
FP method currently in use after receipt of FP services today. Women in Kenya using injectable contraceptives almost exclusively use DMPA.19
Within the last 6 months.
We evaluated intimate partner violence using the 4-item HITS Scale, defining intimate partner violence as scores of 10 and above (IPV: HITS score ≥10 = Yes, HITS score <10 = No).
We evaluated self-perceived HIV risk by asking “What is your gut feeling about how likely you are to get infected with HIV?” with possible responses of “very likely,” “somewhat likely,” “very unlikely,” or “extremely unlikely.” (high self-perceived HIV risk: very/somewhat likely = Yes, extremely/very unlikely = No).
We evaluated HIV risk using the Balkus et al. HIV risk scoring: age <25 = 1, married = 2, any alcohol = 1, partner provides financial support = 1, and partner has other partners: yes = 2 and do not know = 2. Scores of ≥5 correspond to 5–15 incident HIV cases per 100 person-years in cohorts of African women; risk scores of ≤4 correspond to <5 incident HIV cases per 100 person-years. (high HIV risk: HIV risk score ≥5 = Yes, HIV risk score <5 = No).
We evaluated social support using the 18-item Medical Outcomes Study Social Support Survey (MOS-SSS); scores of 72 and above indicate that respondents feel they are able to receive social support most of the time for each scenario item (low social support: MOS-SSS score <72 = Yes, MOS-SSS ≥72 = No).
We evaluated depressive symptoms using CESD-10 scores; scores of 10 and above denote high likelihood of moderate or severe depression (symptoms of moderate to severe depression: CESD-10 score ≥10 = Yes, CESD-10 score <10 = No).
We evaluated self-efficacy to take a daily oral medication by asking participants to rank on a 0–10 scale (0 = cannot do it at all, 10 = completely certain can do it) their response to the question: “How confident are you that you can integrate a daily medication into your daily routine?”
CESD-10, 10-Item Center for Epidemiologic Studies Depression Scale-Revised; DMPA, depomedroxyprogesterone acetate; FP, family planning; HITS, Hurt, Insult, Threaten, and Scream; IQR, interquartile range; PrEP, pre-exposure prophylaxis.
HIV risk behaviors and psychosocial characteristics
Three-quarters (353/470, 75%) of AGYW screened for PrEP had sex without a condom within the last 6 months and 19% reported not knowing the HIV status of their current partner; 1% reported knowing that their partner was HIV positive. Few were involved in transactional sex (22/470, 5%) or were forced to have sex against their will (24/470, 5%). More than one-third of AGYW (161/387, 42%) with current partners were unsure or suspected that their primary sexual partner had other sexual partners. Overall, 76% (356/470) of AGYW had at least one PrEP eligibility criterion as per NASCOP guidelines. Almost one-quarter of AGYW (111/470, 24%) were at high risk of acquiring HIV based on having an HIV risk score of ≥5.14 Over one-third of AGYW (36%) reported having low social support, 13% had moderate to severe depressive symptoms, and few (3%) experienced intimate partner violence.
PrEP awareness
Among 470 AGYW who were screened for PrEP, the majority (416/470, 89%) had previously heard of PrEP before their current FP visit and 20% reported personally knowing someone who uses PrEP. Among AGYW with any PrEP eligibility criteria (n = 356) and those with HIV risk scores ≥5 (n = 111), 10% and 8%, respectively, had not previously heard of PrEP before their current FP visit. The frequency of PrEP awareness was lower among AGYW ≤18 years old compared with those over 18 years [prevalence ratio (PR): 0.89, 95% confidence interval (CI): 0.80–0.99, p-value = 0.025]. AGYW with regular employment more frequently had heard of PrEP before their FP visit than unemployed AGYW (PR = 1.12, 95% CI 1.0–1.26, p = 0.05). AGYW who had engaged in transactional sex in the last 6 months also had higher prevalence of PrEP awareness (PR = 1.08, 95% CI 1.02–1.15, p = 0.009) as did those who had engaged in condomless sex in the last 6 months (PR = 1.09, 95% CI 1.0–1.19, p = 0.05) compared with AGYW not reporting those behaviors. No other characteristics were associated with PrEP awareness.
PrEP uptake
Twenty (4%) AGYW accepted PrEP when offered (20/470) (Table 2). The prevalence of high behavioral HIV risk (HIV risk scores ≥5) was 1.74-fold higher (40% vs. 23%, PR = 1.74, 95% CI 1.24–2.46, p = 0.001) among AGYW who initiated PrEP than those who did not. However, nearly a quarter (111/359, 24%) of AGYW who declined PrEP had HIV risk scores ≥5. The frequency of individual HIV risk factors was higher among those who initiated PrEP compared with those who declined (Table 2), including condomless sex in the past 6 months (90% vs. 74%, PR = 1.21, 95% CI 1.02–1.44, p = 0.033), alcohol use in the past 30 days (30% vs. 14%, PR = 2.17, 95% CI 1.43–3.33, p < 0.001), experiencing IPV (12% vs. 2%, PR = 4.85, 95% CI 1.03–22.73, p = 0.045), and having a partner who has other sexual partners (75% vs. 32%, PR = 2.31, 95% CI 1.83–2.92, p < 0.001). PrEP initiators also more frequently reported having a partner of unknown/positive HIV status than AGYW who declined PrEP (55% vs. 15%, PR = 6.05, 95% CI1.83–20.01, p = 0.003); 2 (50%) of the 4 AGYW who reported having an HIV-positive partner declined PrEP. The frequency of other HIV risk factors did not differ by PrEP initiation status.
Table 2.
Frequency of Behavioral HIV Risk Factors, Psychosocial Characteristics, and PrEP Attitudes Among AGYW Seeking Routine FP Services in Western Kenya Who Initiated or Declined PrEP (n = 470)
| Characteristic | Initiated PrEP todaya |
PR (95% CI)a | p | |
|---|---|---|---|---|
| Yes (n = 20) n (%) | No (n = 450) n (%) | |||
| Behavioral HIV risk factors | ||||
| Has partner of unknown/positive HIV status | 11 (55.0) | 68 (15.1) | 6.05 (1.83–20.01) | 0.003* |
| ≥4 Total lifetime sexual partners | 5 (25.0) | 52 (11.6) | 2.16 (0.73–6.43) | 0.165 |
| Had condomless sexb | 18 (90.0) | 335 (74.4) | 1.21 (1.02–1.44) | 0.033* |
| Engaged in transactional sexb | 0 (0.0) | 22 (4.9) | — | — |
| Forced to have sex against their willb | 1 (5.0) | 23 (5.1) | 0.98 (0.06–15.06) | 0.987 |
| Experienced intimate partner violencec (n = 388) | 2 (11.8) | 9 (2.4) | 4.85 (1.03–22.73) | 0.045* |
| High self-perceived HIV riskd | 17 (85.0) | 47 (10.4) | 1.68 (1.41–1.99) | <0.001* |
| Partner age difference ≥10 years (n = 358) | 6 (40.0) | 27 (7.9) | 6.57 (2.35–18.370 | <0.001* |
| HIV risk score factors (Balkus et al.e) | ||||
| Unmarried/not living with partner | 5 (25.0) | 205 (45.6) | 0.55 (0.31–0.98) | 0.041* |
| Alcohol use (past 30 days) | 6 (30.0) | 62 (13.8) | 2.18 (1.43–3.33) | <0.001* |
| Partner does not provide financial support | 1 (5.0) | 9 (2.0) | 2.50 (0.56–11.23) | 0.232 |
| Primary partner has other partners | 15 (75.0) | 146 (32.4) | 2.31 (1.83–2.92) | <0.001* |
| High behavioral HIV riske | 8 (40.0) | 103 (22.9) | 1.75 (1.24–2.46) | 0.001* |
| Psychosocial characteristics | ||||
| Low social support | 14 (70.0) | 156 (34.7) | 2.01 (1.63–2.48) | <0.001* |
| Depressive symptoms | 9 (60.0) | 40 (11.2) | 5.36 (2.62–10.95) | <0.001* |
| High self-efficacy to take daily medicationf | 20 (100.0) | 284 (63.7) | 1.57 (1.17–2.12) | 0.003* |
| PrEP attitudes and beliefs | ||||
| Heard of PrEP before today | 12 (60.0) | 404 (89.8) | 0.67 (0.43–1.04) | 0.074 |
| Knows someone on PrEP | 3 (15.0) | 91 (20.2) | 0.74 (0.09–6.43) | 0.786 |
| Thinks PrEP is for people who are promiscuous | 0 (0.0) | 7 (1.6) | — | — |
| Thinks PrEP will cause more risky sex | 2 (10.0) | 27 (6.0) | 1.67 (0.34–8.19) | 0.530 |
| Thinks PrEP is only for sex workers or people with HIV-infected partner(s) | 0 (0.0) | 3 (0.7) | — | — |
Tabulations reported for PrEP initiation today (yes/no) and other characteristics are reported as column percentages. PRs were estimated using Poisson regression, clustered by facility.
Within the last 6 months.
We evaluated intimate partner violence using the 4-item HITS Scale, defining intimate partner violence as scores of 10 and above (IPV: HITS score ≥10 = Yes, HITS score <10 = No).
We evaluated self-perceived HIV risk by asking “What is your gut feeling about how likely you are to get infected with HIV?” with possible responses of “very likely,” “somewhat likely,” “very unlikely,” or “extremely unlikely.” (high self-perceived HIV risk: very/somewhat likely = Yes, extremely/very unlikely = No).
We evaluated behavioral HIV risk using the Balkus et al. HIV risk scoring: age <25 = 1, married = 2, any alcohol = 1, partner provides financial support = 1, and partner has other partners: yes = 2 and do not know = 2. Scores of ≥5 correspond to 5–15 incident HIV cases per 100 person-years in cohorts of African women; risk scores of ≤4 correspond to <5 incident HIV cases per 100 person-years. (high HIV risk: HIV risk score ≥5 = Yes, HIV risk score <5 = No).
We evaluated self-efficacy to take a daily oral medication by asking participants to rank on a 0–10 scale (0 = cannot do it at all, 10 = completely certain can do it) their response to the question: “How confident are you that you can integrate a daily medication into your daily routine?”
Significance level ≤5%.
AGYW, adolescent girls and young women; CI, confidence interval; FP, family planning; HITS, Hurt, Insult, Threaten, and Scream; PR, prevalence ratio; PrEP, pre-exposure prophylaxis.
AGYW who initiated PrEP more frequently reported having low social support (70% vs. 35%, PR = 2.01, 95% 1.63–2.48, p < 0.001) and depressive symptoms (60% vs. 11%, PR = 5.36, 95% CI 2/62–10.95) compared with AGYW who declined PrEP. All PrEP initiators reported high self-efficacy to take a daily medication compared with 64% of PrEP decliners (p = 0.003). We found no differences in PrEP attitudes and beliefs between AGYW who chose to initiate PrEP and those who declined.
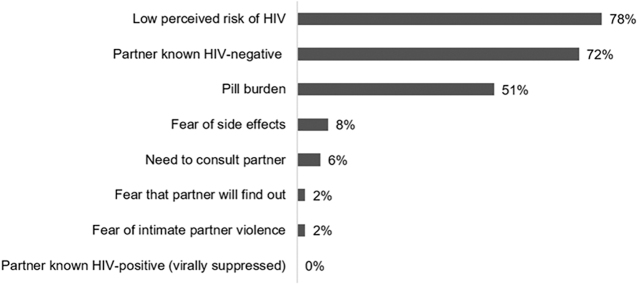
Among AGYW with high behavioral HIV risk (HIV risk scores ≥5) who declined PrEP (n = 111), the three most common reasons for declining were low perceived risk of HIV (78%), thinking their partner was HIV negative (72%), and pill burden (51%). AGYW with high HIV risk reported pill burden as a reason for declining PrEP with higher frequency than those with low HIV risk (51% vs. 28%, p < 0.001). Other reasons for declining PrEP were similar regardless of the HIV risk level (Fig. 2).
FIG. 2.
Reasons for declining PrEP among AGYW with high behavioral HIV risk who declined PrEP (n = 111). Behavioral HIV risk was defined using the Balkus et al. HIV risk scoring: age <25 = 1, married = 2, any alcohol = 1, partner provides financial support = 1, and partner has other partners: yes = 2 and do not know = 2. Scores of ≥5 correspond to 5–15 incident HIV cases per 100 person-years in cohorts of African women; risk scores of ≤4 correspond to <5 incident HIV cases per 100 person-years, (high HIV risk: HIV risk score ≥5). AGYW, adolescent girls and young women; PrEP, pre-exposure prophylaxis.
Discussion
In this survey of AGYW seeking FP services at public sector clinics in Kenya, we found high PrEP awareness and frequent behavioral risk factors for HIV, yet AGYW very infrequently initiated PrEP. Almost 90% of AGYW had previously heard of PrEP before their current FP visit, supporting the high reach of national PrEP sensitization efforts.20 Similar to other studies among Kenyan AGYW, recent condomless sex was very frequent (75%), which has implications for both HIV and STI incidence.6 However, <5% of AGYW initiated PrEP and 23% of PrEP decliners had high behavioral HIV risk scores. Some reasons for declining (e.g., pill burden and low HIV risk perception) could potentially be addressed through targeted counseling or implementation strategies. As PrEP programs scale-up in FP settings, it will become increasingly important to enhance PrEP uptake through approaches tailored to AGYW, including counseling on HIV risk and strategies to mitigate pill burden.6,21,22
In our study, 24% of overall AGYW had behavioral HIV risk scores ≥5, which are scores that translate to an estimated annualized HIV incidence of 5–15%.14 The recently completed ECHO (Evidence for Contraceptive Options and HIV Outcomes) randomized trial found an alarmingly high HIV incidence rate (4.3%) among AGYW recruited through FP clinics in eSwatini, Kenya, South Africa, and Zambia despite an individualized HIV prevention package provided to all participants and country-wide HIV treatment and prevention programs.23 Importantly, women were recruited for the ECHO trial based on geography, but no other characteristics of HIV risk, such as transactional sex, history of STIs, or other self-reported behaviors. Therefore, the potential HIV incidence among FP clients with behavioral risk factors in this setting could be even higher. In our study, >75% of AGYW recently engaged in condomless sex. Our results underscore that AGYW clients within the FP setting frequently have behavioral risk factors for HIV and provision of HIV prevention services within FP, which meet the needs, values, and preferences of AGYW, is urgently needed.
Although PrEP initiators more frequently had HIV behavioral risk factors than AGYW who declined PrEP, uptake was still low (7%) among AGYW with HIV risk scores ≥5 in our study. Similar to other studies among adolescents in HIV high-burden settings,24 self-perceived HIV risk was misaligned with behavioral risk factors as 78% of PrEP decliners with HIV risk scores ≥5 cited low perceived risk as a reason for declining. Misaligned HIV risk perception affects PrEP uptake25 and could potentially be influenced by targeted interventions. Two ongoing studies are incorporating same-day STI testing (as an objective marker for HIV risk) before PrEP counseling in Kenya and South Africa to strengthen cues to action by improving the accuracy of HIV risk perception and to increase PrEP uptake among women.26,27 Similar approaches could be tested within FP clinics given that the high frequency of condomless sex that we found also has implications for STIs. A study in Zimbabwe is testing an educational intervention to improve the accuracy of HIV risk perception among AGYW to increase uptake of HIV prevention services.28 More studies are needed that test strategies to increase motivation for PrEP uptake among AGYW, including improving alignment of perceived and actual HIV risk.
We previously found that pill burden was a common reason for declining and discontinuing PrEP in the parent PrIYA Program,6,10 similar to other studies29,30 and our current survey. Several novel PrEP agents are being tested, including long-acting injectable cabotegravir (CAB-LA).31 If successful, CAB-LA could obviate some adherence issues in the future. However, forthcoming results could take considerable time to translate into routine practice. In the meantime, approaches that can support daily pill taking, such as SMS adherence counseling tools or peer counseling groups,32–34 could encourage PrEP use. We also found that PrEP initiators more frequently reported low social support and symptoms of depression, which are associated with having HIV risk behaviors.35–38 Approaches that incorporate psychosocial support along with PrEP could provide multiple mechanisms to positively influence HIV prevention among AGYW.
There was lower PrEP uptake in this fully programmatic evaluation than in the parent PrIYA Program, which included PrEP-dedicated staff who provided PrEP in the FP or MCH clinics (4% vs. 16%).6 It is likely that without PrEP program-dedicated staff, screening or counseling on PrEP was less comprehensive and resulted in lower PrEP uptake. Options to streamline and simplify PrEP counseling and provision in busy FP clinics may be necessary to increase PrEP uptake. In addition, the current study only included AGYW (women over 24 years of age were excluded). In the parent PrIYA Program, AGYW had significantly lower PrEP uptake than women ≥24 years of age,6 suggesting that even in an environment with program-dedicated staff, AGYW have lower PrEP uptake. Our current results highlight the need to tailor PrEP decision-making support to AGYW. Additionally, future studies should incorporate evaluation of the quality of services in purely programmatic settings.
Studies in Kenya and South Africa among young pregnant women highlighted community-level HIV stigma as a deterrent to PrEP uptake, fearing that others would confuse their PrEP use with antiretroviral treatment for HIV.39,40 Our study did not assess perceived HIV stigma as a potential reason for declining PrEP, yet this may have influenced the low uptake of PrEP among these AGYW. We assessed other reasons for declining PrEP such as concerns for a partner's negative reaction (e.g., need to consult a partner, fear that a partner will find out, and fear of intimate partner violence) since this was commonly stated as a reason for not initiating PrEP by young, pregnant Kenyan women in a prior study.39 Our results were not consistent with this qualitative finding among pregnant women.
Our study has limitations. We sampled AGYW seeking FP services from public facilities, thus our results may not generalize to AGYW seeking FP services from other locations (e.g., retail pharmacies). We are unable to establish temporal relationships between behavioral HIV risk factors, psychosocial characteristics, and PrEP attitudes due to the cross-sectional design. Our study did not screen for STIs. However, by using an empirical risk score for HIV that did not include STI as a risk factor,14 we were able to quantify HIV risk with reliability. The use of self-reported information about relationship characteristics and HIV risk factors may have introduced reporting bias. Future studies could use medical records to confirm partner HIV status. Evaluations among AGYW should collect information about where and how AGYW have heard of PrEP to inform programmatic efforts to increase PrEP uptake in this high-risk group.
In this survey among AGYW within routine FP settings, AGYW frequently had behavioral risk factors for HIV, but infrequently initiated PrEP. PrEP counseling should be tailored to inform HIV risk perception among AGYW and guide appropriate PrEP decision-making in this high-risk population.
Acknowledgments
The authors thank the PrIYA study team and clients for their time and contributions. The authors thank the Kenyan Ministry of Health nationally and the Kisumu County Department of Health, as well as the facility heads and in-charges, for their collaboration.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was funded by the US National Institutes of Health (R01HD094630 and R01HD100201). The PrEP Implementation for Young Women and Adolescents (PrIYA) Program was funded by the US Department of State as part of the DREAMS Innovation Challenge (Grant No. 37188-1088 MOD01), managed by the JSI Research & Training Institute, Inc. The PrIYA team was supported by the University of Washington's Center for AIDS Research (CFAR) (P30 AI027757) and the Global Center for Integrated Health of Women, Adolescents, and Children (Global WACh).
References
- 1. UNAIDS. Fact Sheet–Global AIDS Update 2019. 2019. Available at: https://www.unaids.org/sites/default/files/media-asset/UNAIDS-FactSheet-en.pdf (Last accessed June30, 2020)
- 2. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015. Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf (Last accessed December30, 2017). [PubMed]
- 3. Kenya Ministry of Health. Guidelines on the use of antiretroviral drugs for treating and preventing HIV infection in Kenya. 2016. Available at: http://emtct-iatt.org/wp-content/uploads/2016/09/Guidelines-on-Use-of-Antiretroviral-Drugs-for-Treating-and-Preventing-HI.…pdf (Last accessed December12, 2017).
- 4. AVAC. Global PrEP Tracker. October 23, 2018. Available at: https://www.prepwatch.org/resource/global-prep-tracker/ (Last accessed January19, 2019).
- 5. Kenya National Bureau of Statistics. Republic of Kenya Demographic and Health Survey. 2015. Available at: https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf
- 6. Mugwanya KK, Pintye J, Kinuthia J, et al. Integrating preexposure prophylaxis delivery in routine family planning clinics: A feasibility programmatic evaluation in Kenya. PLoS Med 2019;16:e1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oluoch L, Mugo N, Roxby A, et al. Low uptake of pre-exposure prophylaxis among Kenyan adolescent girls at risk of HIV. Conference on retroviruses and opportunistic infections (CROI). Seattle, WA: Abstract 964;2019. Available at: https://www.croiconference.org/abstract/low-uptake-pre-exposure-prophylaxis-among-kenyan-adolescent-girls-risk-hiv/ (Last accessed June30, 2020).
- 8. Dunbar M, Kripke K, Haberer J, et al. Understanding and measuring uptake and coverage of oral pre-exposure prophylaxis delivery among adolescent girls and young women in sub-Saharan Africa. Sex Health 2019;15:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinuthia J, Pintye J, Abuna F, et al. Pre-exposure prophylaxis uptake and early continuation among pregnant and post-partum women within maternal and child health clinics in Kenya: Results from an implementation programme. Lancet HIV 2019;7:e38–e48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kenya Ministry of Health–National AIDS and STDs Control Programme (NASCOP). Kenya County Profiles 2016. Available at: https://nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-County-Profiles-2016.pdf (Last accessed June30, 2020).
- 11. Gumbe A, McLellan-Lemal E, Gust DA, et al. Correlates of prevalent HIV infection among adults and adolescents in the Kisumu incidence cohort study, Kisumu, Kenya. Int J STD AIDS 2015;26:929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akinyi B, Odhiambo C, Otieno F, et al. Prevalence, incidence and correlates of HSV-2 infection in an HIV incidence adolescent and adult cohort study in western Kenya. PLoS One 2017;12:e0178907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balkus JE, Brown E, Palanee T, et al. An Empiric HIV Risk Scoring Tool to predict HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2016;72:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Napper LE, Fisher DG, Reynolds GL. Development of the perceived risk of HIV scale. AIDS Behav 2012;16:1075–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baron EC, Davies T, Lund C. Validation of the 10-item Centre for Epidemiological Studies Depression Scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry 2017;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kilburn K, Prencipe L, Hjelm L, Peterman A, Handa S, Palermo T. Examination of performance of the Center for Epidemiologic Studies Depression Scale Short Form 10 among African youth in poor, rural households. BMC Psychiatry 2018;18:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sherin KM, Sinacore JM, Li XQ, Zitter RE, Shakil A. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med 1998;30:508–512 [PubMed] [Google Scholar]
- 18. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–714 [DOI] [PubMed] [Google Scholar]
- 19. Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med 2015. Jan 22;12(1):e1001778. doi: 10.1371/journal.pmed.1001778. PMID: ; 12(1): PMC4303292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masyuko S, Mukui I, Njathi O, et al. Pre-exposure prophylaxis rollout in a national public sector program: The Kenyan case study. Sex Health 2018;15:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Decker MR, Miller E, McCauley HL, et al. Recent partner violence and sexual and drug-related STI/HIV risk among adolescent and young adult women attending family planning clinics. Sex Transm Infect 2014;90:145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Celum CL, Delany-Moretlwe S, Baeten JM, et al. HIV pre-exposure prophylaxis for adolescent girls and young women in Africa: From efficacy trials to delivery. J Int AIDS Soc 2019;22(Suppl 4):e25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Consortium EfCOaHOET. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. Lancet 2019;394:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaida A, Dietrich JJ, Laher F, et al. A high burden of asymptomatic genital tract infections undermines the syndromic management approach among adolescents and young adults in South Africa: Implications for HIV prevention efforts. BMC Infect Dis 2018;18:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warren EA, Paterson P, Schulz WS, et al. Risk perception and the influence on uptake and use of biomedical prevention interventions for HIV in sub-Saharan Africa: A systematic literature review. PLoS One 2018;13:e0198680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. NIH Research Portfolio Online Reporting Tools (RePORT). Enhancing integrated delivery of PrEP within antenatal care with STI testing and expedited partner treatment. 2019. Available at: https://projectreporter.nih.gov/project_info_description.cfm?aid=9679456&icde=47174673 (Last accessed October26, 2019).
- 27. NIH Research Portfolio Online Reporting Tools (RePORT). Evaluation of Pre-Exposure Prophylaxis (PrEP) in Pregnant and Breastfeeding Women (PrEP-PP) (NCT03902418). 2019. https://clinicaltrials.gov/ct2/show/NCT03902418?term=Coates&draw=2&rank=10 (Last accessed October26, 2019)
- 28. Thomas R, Skovdal M, Galizzi MM, et al. Improving risk perception and uptake of pre-exposure prophylaxis (PrEP) through interactive feedback-based counselling with and without community engagement in young women in Manicaland, East Zimbabwe: Study protocol for a pilot randomized trial. Trials 2019;20:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kesler MA, Kaul R, Myers T, et al. Perceived HIV risk, actual sexual HIV risk and willingness to take pre-exposure prophylaxis among men who have sex with men in Toronto, Canada. AIDS Care 2016;28:1378–1385 [DOI] [PubMed] [Google Scholar]
- 30. Meyers K, Wu Y, Brill A, Sandfort T, Golub SA. To switch or not to switch: Intentions to switch to injectable PrEP among gay and bisexual men with at least twelve months oral PrEP experience. PLoS One 2018;13:e0200296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017;390:1499–1510 [DOI] [PubMed] [Google Scholar]
- 32. Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: A substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013;10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muwonge TR, Ngure K, Katabira E, et al. Short Message Service (SMS) surveys assessing pre-exposure prophylaxis (PrEP) adherence and sexual behavior are highly acceptable among HIV-uninfected members of serodiscordant couples in East Africa: A mixed methods study. AIDS Behav 2019;23:1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Psaros C, Haberer JE, Katabira E, et al. An intervention to support HIV preexposure prophylaxis adherence in HIV-serodiscordant couples in Uganda. J Acquir Immune Defic Syndr 2014;66:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nduna M, Jewkes RK, Dunkle KL, Shai NP, Colman I. Associations between depressive symptoms, sexual behaviour and relationship characteristics: A prospective cohort study of young women and men in the Eastern Cape, South Africa. J Int AIDS Soc 2010;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jewkes R, Dunkle K, Nduna M, et al. Factors associated with HIV sero-status in young rural South African women: Connections between intimate partner violence and HIV. Int J Epidemiol 2006;35:1461–1468 [DOI] [PubMed] [Google Scholar]
- 37. Lundberg P, Rukundo G, Ashaba S, et al. Poor mental health and sexual risk behaviours in Uganda: A cross-sectional population-based study. BMC Public Health 2011;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smit J, Myer L, Middelkoop K, et al. Mental health and sexual risk behaviours in a South African township: A community-based cross-sectional study. Public Health 2006;120:534–542 [DOI] [PubMed] [Google Scholar]
- 39. Pintye J, Beima-Sofie KM, Makabong OP, et al. HIV-Uninfected Kenyan adolescent and young women share perspectives on using preexposure prophylaxis during pregnancy. AIDS Patient Care STDS 2018;32:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vazquez L, Moll AP, Kacin A, Ndlovu NE, Shenoi SV. Perceptions of HIV preexposure prophylaxis among young pregnant women from Rural KwaZulu-Natal, South Africa. AIDS Patient Care STDS 2019;33:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]