Graphical abstract
Keywords: Chloroquine, COVID-19, DFT, Drug delivery, Fullerene
Highlights
-
•
DFT calculations have been performed to study the interactions between doped C60 with chloroquine.
-
•
Adsorption of chloroquine on the doped C60 fullerene is more thermodynamically favorable.
-
•
AlC59…chloroquine complex shows the most negative binding energy.
Abstract
Chloroquine (CQ) has been reported as an effective drug in the control of COVID-19 infection. Since C60 fullerene has been considered as a drug delivery system, the interaction between pristine fullerene and chloroquine drug and also the interaction between B, Al, Si doped fullerene and chloroquine drug have been investigated based on the density functional theory calculations. The results of this study show that the doped fullerene, especially Al and Si doped fullerene could be the better drug delivery vehicles for chloroquine drug because of their relatively better energetic and electronic properties with chloroquine.
1. Introduction
In December 2019, in Wuhan City of China, a novel pandemic corona virus disease similar to human coronaviruses, SARS and MERS pneumonia, was identified which called COVID-19. This virus as pandemic putting the whole world on high alarm and on the basis of the World Health Organization (WHO), more than 14 million cases of the virus and about 610,000 deaths were reported, over the world till July 2020. The mean age of patients was 59 years and 56% were male. It is significant that few of the first cases observed in children and approximately half observed in adults aged 60 or over. The COVID-19 infection induced clusters of intense respiratory diseases like severe serious respiratory syndrome coronavirus and caused ICU admission and high fatality. Information about the epidemiology, origin, clinical spectrum of disease and duration of human transmission, are major gaps about this illness, up to date. The start of severe acute respiratory syndrome (SARS) was approximately 18 years ago, and the genetic sequences are nearly indistinguishable and assign 79.6% of sequence identity with SARS-CoV. This resemblance can be beneficial in a theoretical comparative investigation through medicines that have shown effectiveness in contradiction of SARS. Until now, with the increase of coronavirus epidemic in the whole world, the various clinical research groups in the whole world centralize on studying the therapeutic options for the therapy of COVID-19 [1], [2], [3], [4]. On the basis of some clinical investigations, chloroquine (CQ) which has been recognized as anti-malarial drug for many years, has been reported as highly effective drug in the control of COVID-19 infection in vitro [1], [2], [3], [4], [5]. The chloroquine drug, also has been used in the treatment of illnesses such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), primary Sjogren syndrome and antiphospholipid syndrome (APS) [3], [6], [7], [8], [9]. The studies of the preclinical in vitro showed the antiviral effects and prophylactic of chloroquine in contradiction of SARS-CoV-2 (COVID-19). The mechanism of action of chloroquine in contradiction of COVID-19 has not fully clarified, up till now. On the other hand, an extended utilization of these drugs seems potentially perilous and enhanced the danger of cardiac death. So, various treatment regimens attempt to study on efficient in vivo application of these medicines [4], [10], [11], [12], [13].
In order to decrease the side effects and to enhance therapeutic efficiency of chloroquine and to enhance specific cellular uptake and decrease nonspecific collection in the vivo tissues, the use of nanomedicine as an important field of nanotechnology in pharmaceuticals and the design of nano drug delivery systems on the basis of nanomaterials, which can control the drug release in vivo and enhance performance encapsulation and absorption, is essential [3], [4], [14].
In recent years, carbon based nanomaterials such as fullerene, carbon nanotubes (CNTs), graphene and also boron nitride nanomaterials have been widely considered in many applications in materials science and nanotechnology such as drug delivery systems because of their unique chemical and physical properties [15], [16], [17], [18], [19], [20], [21], [22]. Drug delivery system as one of the most important subject in therapy, employs nanomaterials to improve the drug solubility in addition to in vivo life time, and ultimately lead to design of harmless and effective drug nano delivery systems [23], [24]. Because carbon nanomaterials could be reacted to specific irritant, due to their electrical conductivity, high mechanical strength, electrochemical sensing and actuation properties, they could be categorized into smart materials [25], [26], [27].
Among nanomaterials, fullerenes have received much attention as appropriate carbon-based nanomaterials for drug delivery because of their suitable properties such as unique spherical structure, hydrophobic characteristic, versatile chemical, physical and biological properties, unique biological activities such as antioxidant capacity, efficient drug loading and fewer side effects in biological media [14], [23], [28]. Although C60 fullerenes are barely soluble in water, but, various methods such as, encapsulation, chemical functionalization of fullerene with a variety of particular groups and using dopant atoms can led to the increase of the solubility in water [14], [29], [30], [31]. The interaction of C60 fullerene and its derivatives with different drugs has been widely investigated because of the possible application of fullerene in drug delivery [32], [33], [34]. In spite of the prominent advantages of expensive experimental methods, the application of computational approaches in order to comprehend the mechanism and the nature of these interactions, has been gradually extended because the empirical methods are expensive and time consuming [23], [32].
Different computational studies have frequently been done on the interaction between fullerenes and different drugs [14], [23], [28], [32], [35], [36], [37], [38], [39]. Since interaction between chloroquine and C60 fullerenes have not been studied computationally to date, the aim of this work is to perform density functional theory (DFT) calculations to test the ability of fullerene to adsorb chloroquine as a favorable candidate for carrying this drug.
On the other hands, the dipole moment of free C60 fullerene is zero. Hence, discovery an important way to improve the electronic and magnetic properties of fullerene ia an important work. There are many computational reports which show that functionalization and doping can improve the electronic and magnetic properties of nanostructures [14], [19], [21], [23], [38], [39], [40], [41], [42], [43], [44]. The efficiency of B, Al and Si dopants in C60 fullerenes has been previously reported [23]. All the dopant atoms, such as B, Al and Si, have more metallic characteristic relative to carbon and doping B, Al and Si in C60 fullerene reduce the Eg of C60. Also, replacing of B, Al and Si atoms instead of one of the carbon atoms of C60 fullerene can increase adsorption energy from −4.1 kcal/mol in pristine fullerene to the range about −12 to −45 kcal/mol in the doped fullerene [23]. It is notable that there are not any reports on the interaction of chloroquine and B, Al and Si doped fullerenes based on DFT calculations. Therefore, in the present work, the interactions of chloroquine drug with B, Al and Si doped fullerenes, in addition to adsorption of chloroquine on pristine C60, have been investigated using DFT calculations to investigate the improvement of the drug delivery properties by these nano vehicles. This study can offer the opportunity to do more researches in the field of developing performance of drug delivery systems [40], [41]. Some of important chemical properties such as, solvation energy (Es), binding energy (Eb), band gap energy (Eg) (HOMO – LUMO), chemical hardness (η) and electrophilicity indexes (w) have been calculated in this work. Also, calculations in water media have been performed in order to investigate the solvent effect on the structure and electronic properties.
2. Computational details
Density functional theory (DFT) method has been used in this work to investigate the interaction and specifically the formation probability of a stable structure between chloroquine drug and pristine, B-, Si-, and Al-doped C60 fullerenes. To appraise the most stable structure of chloroquine:C60 and its energy, the optimization of the different configurations of the drug and C60 fullerene in gas phase and water media have been carried out at B3LYP functional and 6-31G* basis set, implemented in Gaussian 09 program [45].
Relative stability with respect to the nanostructures–drug interactions of the complexes have been performed primarily based on the binding or adsorption energies. Binding energies (Eb) have been calculated by the following equation:
where in the above equation, Ecomplex represents the total electronic energy of the functionalized fullerenes with the attached drugs after geometry optimization. EC60, EXC59 and Edrug are defined as the optimized energies of the related compounds [14], [23].
Counterpoise (cp) correction is advised in the binding energy calculations, in order to destroy the basis set superposition errors (BSSE) [38], [46]. The counterpoise corrected binding energy of these complexes is:
where, EBSSE is defined as basis set superposition errors energy. In the following, vibrational frequencies calculations have been performed at the same level of theory and no imaginary mode was obtained which validate that all of the stationary points correspond to correct minima on the potential energy surface. The investigated structures are neutral (charge Q = 0) and have been stabilized by multiplicity (M = 2S + 1, where S is the total spin) of one [19], [20], [40].
The electrophilicity index (ω) is one of the main parameters which measures the stabilization in energy when the system gets an extra electronic charge from the environment. In this work, electrophilicity index as a descriptor for the charge transfer direction, which higher value of ω means higher electrophilic power of the compound, has been calculated using the following equation:
which in the above equation, μ represents electronic chemical potential and η is defined as chemical hardness of the ground state [14], [23].
The maximum electronic charge ΔNmax of an electrophile that can accept from the environment have been defined as the following formula:
which electrophilicity index, on the basis of ΔNmax can be written as:
in the above equation, χ is defined as electronegativity of a system. Therefore, the maximum electronic charge ΔNmax in terms of electrophilicity index (ω) and electronegativity (χ) can be calculated by:
Therefore, the amount of charge transfer between two systems, for example, between drug and doped fullerenes, can be defined on the basis of electrophilicity, which is called electrophilicity-based charge transfer (ECT) which can be computed as:
which in the above equation, If ECT > 0, A acts as electron acceptor and if ECT < 0, A acts as an electron donor [14], [47].
In order to study the solvation effects on the stabilities and electronic properties of the structures and also calculations of solvation energies (Esolv), which can be obtained using geometrically optimization our DFT structures in the gas phase and in water solvent, calculations in the water solvent with the same levels of theory by the conductor polarizable continuum model (CPCM) have been carried out, too [14], [23], [48], [49], [50]. Also, in the first of calculations, natural population analysis (NPA) and molecular electrostatic potential (ESP) have been performed at the same method in order to study quantitative investigations of charge distributions and better perceivable perception of the nature of interactions [14], [23].
3. Results and discussion
3.1. Molecular electrostatic potential (ESP) and NPA analyses
In order to study the adsorption behavior of chloroquine drug on pristine C60 fullerene and doped fullerene by B, Si and Al dopant atoms, DFT calculations have been carried out at B3LYP levels of theory by 6-31G(d) basis set. In the first, electrostatic potentials map (ESP) on the molecular surfaces of a single chloroquine has been computed and showed in Fig. 1 . Since, red and blue colors in the ESP map show more negative and positive regions, respectively [14], we can conclude that nitrogen atoms of the drug molecules which are shown with red color, might be considered as the possible active site for the interaction with the fullerene and doped fullerenes.
Fig. 1.
Molecular electrostatic potentials map (ESP) of chloroquine drug.
The natural atomic charges for B-, Al-, and Si-doped C60 fullerene complexes with chloroquine drug calculated with B3LYP/6-31G(d) method in water media and also molecular electrostatic potential (ESP) map which is an appropriate method to investigate the charge distributions of the compounds as three dimensional, have been analyzed. The values of natural atomic charges of some atoms with more negative and more positive charges which are listed in Table 1 , show that in all these complexes, the N atoms of the chloroquine molecule show the highest negative atomic charges among the other atoms and B, Al, and Si show the highest positive atomic charges. In AlC59…CQ and SiC59…CQ complexes, the N atom of the molecules which is linked to the Al and Si, has the most negative atomic charges and Si atom has the highest positive atomic charge among B, Al and Si in these complexes.
Table 1.
The values of natural atomic charges of some atoms with more negative and more positive charges of B-, Al-, and Si-doped C60 fullerene complexes with chloroquine drug calculated with B3LYP/6-31G(d) method in water media.
| Atom | BC59…CQ | Atom | AlC59…CQ | Atom | SiC59…CQ |
|---|---|---|---|---|---|
| N 61 | −0.52221 | N 61 | −0.52236 | N 61 | −0.52282 |
| N 62 | −0.54556 | N 62 | −0.53896 | N 62 | −0.52465 |
| N 63 | −0.47965 | N 63 | −0.66341 | N 63 | −0.67420 |
| C 64 | −0.47750 | C 64 | −0.47944 | C 64 | −0.48028 |
| C 65 | −0.47579 | C 65 | −0.47605 | C 65 | −0.47603 |
| C 67 | −0.26429 | C 67 | −0.26409 | C 67 | −0.26427 |
| C 69 | −0.27068 | C 69 | −0.27064 | C 69 | −0.27060 |
| C 70 | −0.27056 | C 70 | −0.27054 | C 70 | −0.27033 |
| C 71 | 0.28351 | C 71 | 0.29023 | C 71 | 0.30333 |
| C 75 | −0.35679 | C 75 | −0.35505 | C 75 | −0.34986 |
| B 108 | 0.62176 | Al 108 | 1.66607 | Si 108 | 1.92518 |
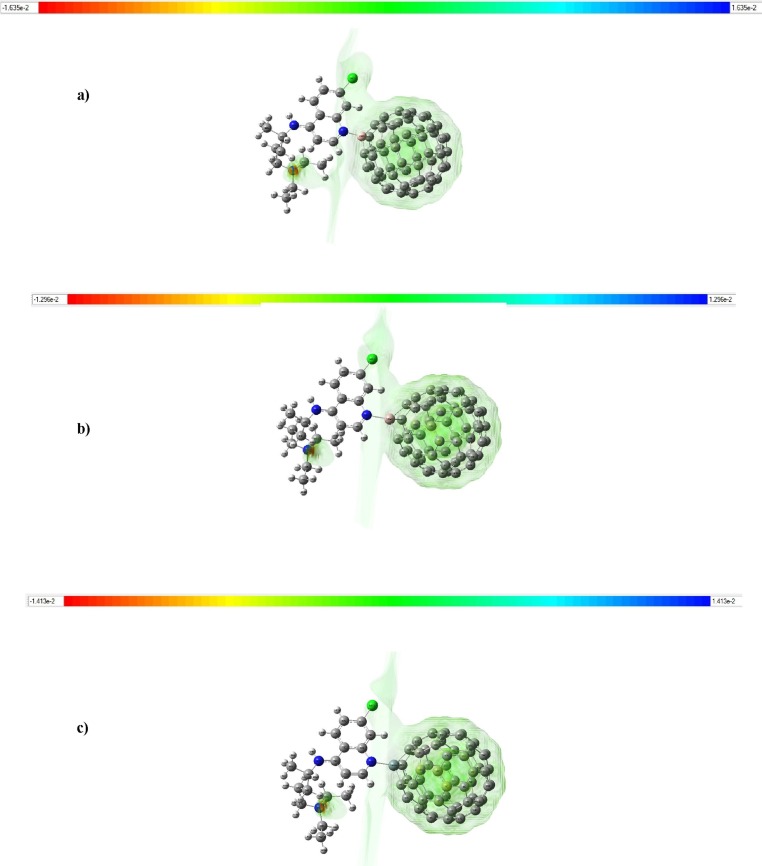
Also, according to the electrostatic potentials map of the doped C60 fullerene complexes which have been shown in Fig. 2 , the active sites for BC59, AlC59 and SiC59 are boron, aluminum and silicon atoms, respectively due to the positive charge distribution around these dopant atoms which are shown with blue/green color. According to Fig. 2, the color code of B-, Al-, and Si-doped C60 fullerene complexes are between −1.635e−2 and +1.635e−2 ; −1.296e−2 and +1.296e−2 ; and −1.413e−2 and +1.413e−2, respectively. These data show that the electrostatic potential values order among these complexes is: BC59…CQ > SiC59…CQ > AlC59…CQ.
Fig. 2.
Electrostatic potentials map of a) BC59…CQ b) AlC59…CQ and c) SiC59…CQ complexes.
3.2. Optimization, energies and electronic properties of the structures
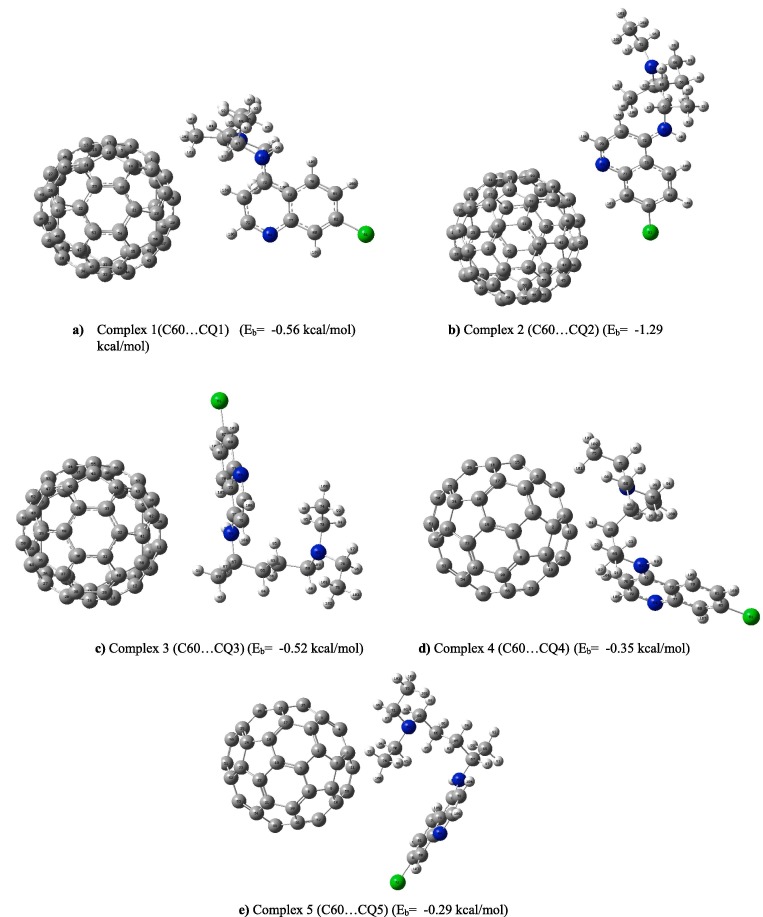
Five different geometric configurations have been investigated to find the better possible interaction positions of chloroquine drug and C60, which have been shown in Fig. 3 . In the complexes a, b and c, three different N atoms of chloroquine drug approaches to one of the C atom of C60 fullerene, respectively. In the complexes d and e, C65 atom and C74 atom of chloroquine approaches to one of the C atom of fullerene, respectivelly. On the basis of obtained binding energies for all these configurations, which have been reported in Fig. 3, three possible configurations on the basis of different active sites, which are the N atoms in chloroquine, were chosen for chloroquine interaction with the side-wall of fullerene. After optimization of single drug, C60 fullerene and complexes of pristine C60 and three configurations of this drug, the values of binding energy with BSSE corrections between pristine fullerene and different configurations of this drug, have been calculated in the gas phase and water solvent and listed in Table 2 .
Fig. 3.
The optimized structure of different configurations of chloroquine drug and C60 fullerene.
Table 2.
Energetic parameters and electronic properties of chloroquine, fullerene and different complexes of pristine C60 fullerene and CQ in the gas phase and water media.
| Structure | EHOMO (eV) | ELUMO (eV) | Eg (eV) | ΔEg (%) | η (eV) | ω (eV) | ECT | µD (Debye) | α | Eb (kcal/mol) | Esol (kcal/mol) | ΔG (kcal/mol) | ΔH (kcal/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gas | |||||||||||||
| Chloroquine (CQ) | −5.41 | −1.05 | 4.36 | – | 2.18 | 2.39 | – | 6.25 | 211.7 | – | −7.72 | – | – |
| C60 | −6.50 | −3.56 | 2.94 | – | 1.47 | 8.63 | – | 0.0 | 437.9 | – | −1.1 | – | – |
| C60…CQ1 | −5.38 | −3.66 | 1.72 | 41.42 | 0.86 | 11.86 | −3.76 | 6.11 | 646.2 | −1.59 | −8.52 | – | – |
| C60…CQ2 | −5.44 | −3.36 | 2.07 | 29.29 | 1.03 | 9.33 | −2.75 | 7.34 | 655.8 | −2.59 | −6.79 | – | – |
| C60…CQ3 | −5.40 | −3.54 | 1.86 | 36.72 | 0.93 | 10.76 | −3.32 | 5.40 | 643.8 | −1.10 | −8.24 | – | – |
| water | |||||||||||||
| Chloroquine (CQ) | −5.41 | −1.27 | 4.14 | – | 2.07 | 2.69 | – | 8.67 | 220.6 | – | −7.72 | – | – |
| C60 | −6.40 | −3.46 | 2.93 | – | 1.46 | 8.29 | – | 0.0 | 439.3 | – | −1.1 | – | – |
| C60…CQ1 | −5.41 | −3.49 | 1.91 | 34.80 | 0.95 | 10.34 | −3.03 | 8.63 | 721.5 | −0.56 | −8.52 | −0.81 | −1.19 |
| C60…CQ2 | −5.39 | −3.47 | 1.91 | 34.70 | 0.95 | 10.26 | −3.01 | 8.80 | 732.6 | −1.29 | −6.79 | −1.98 | −2.20 |
| C60…CQ3 | −5.40 | −3.47 | 1.92 | 34.39 | 0.96 | 10.22 | −2.99 | 8.53 | 719.3 | −0.52 | −8.24 | −0.76 | −0.97 |
According to Table 2, the order of the absolute value of binding energies or adsorption energies for different configurations is E2 > E1 > E3 in the both gas and solvent phases. It means that the most negative binding energy is observed for complex 2 and therefore this complex is the most stable structure among the other complexes. The optimized structure of different configurations of this drug and fullerene in the solvent phase are shown in Fig. 3. The binding energy of the most stable structure of pristine fullerene and chloroquine in complex 2 was obtained −2.59 and −0.56 kcal/mol for the gas phase and water solvent, respectively. Geometric parameters of pristine fullerene and the chloroquine practically unchanged upon the interaction and relatively small adsorption energy indicated that interaction between the pristine and chloroquine mostly happens through noncovalent in nature and this weak interaction shows that there is a physisorption between C60 and chloroquine [14], [23].
Also, other electronic properties such as the values of EHOMO, ELUMO, HOMO-LUMO energy gap (ELUMO-EHOMO), the percentage value of the difference in the Eg energies of C60 and C60… CQ (ΔEg), chemical hardness (η) which is obtained by η = (ELUMO − EHOMO)/2, electrophilicity index (ω), electrophilicity-based charge transfer (ECT), dipole moments (μ) and solvation energies (Esol) which can be computed according to the equation: Esol = Esolvent − Egas, have been calculated for single drug, C60 and complexes of different configurations of this drug and pristine C60 are gathered in Table 2.
The calculated values of EHOMO, ELUMO and HLG (ELUMO − EHOMO) of the studied molecules show that the HOMO energy levels of all structures expect complex 1 in water media are slightly higher than those of in the gas phase. Also, the LUMO energy levels of C60 and complex 3 increases slightly in solvent phase compared to the gas phase. Hence, the HLG values of complex 2 in water media is smaller than the HLG values of complex 2 in the gas phase. According to the results, we can conclude that the adsorption of drug on pristine C60 decreases the values of HLG (Eg) and the value of this decrease for complex 2 is smaller than the other complexes. It means that the Eg of C60 by interacting with chloroquine drug in configuration 2 changed less compared to the other configurations of this drug. Therefore, we can concluded that complex 2 is an electronically harmless interaction and the nanocarrier would not significantly change the drug properties [23]. In order to study the electronic sensitivity and to correlate Eg energy and the electrical conductivity, the following formula has been generally used:
where in this equation, k is the Boltzmann’s constant and it is clear that the electrical conductivity shows an exponential relation with Eg energy [14]. The ΔEg values in Table 2 which were obtained by calculating the percentage value of the difference in the Eg energies of C60 and C60… CQ, shows that conductivitiy of the complex 2 has been increased in water solvent compared to gas phase.
Besides, the values of chemical hardness and electrophilicity index which can be applied to estimate the chemical stability and reactivity of chemical compounds [14] which are listed in Table, 1 show that chemical hardness values of complex 2 decreases from gaseous to water media whereas electrophilicity indexes of this structure increases in water media. Increases of electrophilicity index of complexes compared to the single drug and C60, means that interaction of drug with fullerene produced more electrophilic characteristic for the examined structures [23].
Calculated dipole moments of these structures show that the values of dipole moment in water media are higher than those of in gas phase. Fullerene with μ = 0 does not show any polarity because of the absence of charge separation. The dipole moments of fullerene increase significantly after complexation with drug because of the perturbation in the electron density. Among the complexes of C60 and different configurations of this drug, complex 2 shows the greatest dipole moment in both phases relative to the other compounds. It is remarkable that the dipole moments of single drug is almost comparable with the dipole moments of their related complexes with fullerene. The results of dipole moments show that adsorption of chloroquine on fullerene as complex 2, increases the polarity of the whole system which is a favorite property for drug delivery in biological systems [28], [38]. The amount of electrophilicity-based charge transfer (ECT) of these structures show that since charge transfer rates are smaller than zero, ΔN < 0 and therefore ECT < 0, drug acts as electron donor and fullerene acts as electron acceptor and the charge flows from drug to fullerene.
The solvation energy which is defined as the difference between the optimized energies in water and gaseous phases is a quantity for measurment of the solubility of a material in a solvent. Negative values of the solvation energy shows that the reaction is spontaneous. The more negative solvation energy, the larger the degree of solubility. The values of solvation energies listed in Table 2 show that all the complexes have been stabilized in the existence of water solvent. It means that the solubility of all complexes can be enhanced when the drug are adsorbed on the C60 surface within the solvent [23], [35].
On the basis of the obtained parameters, we can conclude that complex 2 can be selected as the most stable structure to investigate the effect of doped C60 fullerene with B, Si and Al dopant atoms on the adsorption behavior of chloroquine drug.
3.3. Adsorption of chloroquine on doped fullerenes
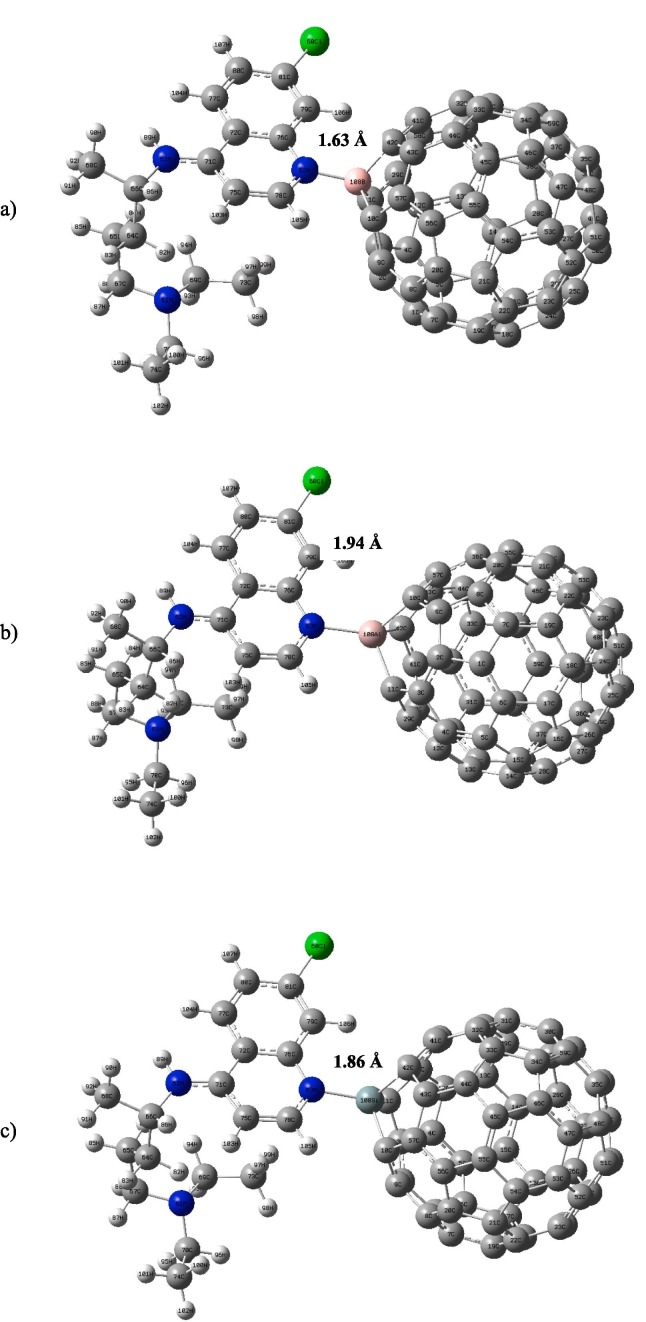
Since chemical functionalization of fullerene by dopant atoms can led to enhance of the solubility in water, one of carbon atoms on C60 in complex 2 which is the most stable structure, has been replaced by the dopant atoms as B, Al and Si and the obtained complexes have been optimized in the gas phase and water media at the same level of theory. B atom is smaller in size compared to carbon atom and the other dopants are bigger in size. According to natural atomic charges of these structures, the active sites for BC59, AlC59 and SiC59 are boron, aluminum and silicon atoms, respectively due to the positive charge distribution around these dopant atoms. The optimized structures of investigated complexes in the gas phase have been shown in Fig. 4 . All the dopant atoms caused specified deformations at the point where they have been doped in the fullerene cage. To investigate a more numerical evaluation, distance between the N atom of chloroquine drug and the C atom of pristine C60 and also distance between the N atom of chloroquine and the dopant atoms in doped fullerenes, which has been presented in Fig. 4, have been considered. For complex 2, the distance between the C atom of pristine C60 in the hexagona ring and N atom of chloroquine has been reported as 3.34 Å. When one carbon is replaced by B, Al and Si, the distance between the dopant and N atom of chloroquine, after optimization have been decreased and reported as 1.63, 1.94 and 1.86 Å, respectively. This results show that the deformation due to the dopant atoms becomes more noticeable when dopant atom is larger in size.
Fig. 4.
The optimized structures of a) BC59…CQ b) AlC59…CQ and c) SiC59…CQ complexes.
The values of binding energies, solvation energies and electronic properties of the investigated structures which have been calculated in both gas and solvent phases, have been gathered in Table 3 .
Table 3.
Energetic parameters and electronic properties of the B-, Si- and Al-doped C60 fullerene and chloroquine at the most stable complex in the gas phase and water media.
| Structure | EHOMO (eV) | ELUMO (eV) | Eg (eV) | ΔEg (%) | η (eV) | ω (eV) | ECT | µD (Debye) | α | Eb (kcal/mol) | Esol (kcal/mol) | ΔG (kcal/mol) | ΔH (kcal/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gas | |||||||||||||
| BC59…CQ2 | −4.89 | −3.35 | 1.54 | 47.39 | 0.77 | 10.99 | −3.84 | 8.57 | 701.1 | −43.67 | −10.73 | – | – |
| AlC59…CQ2 | −5.48 | −4.34 | 1.13 | 61.41 | 0.56 | 21.29 | −7.18 | 18.11 | 713.3 | −67.39 | −15.37 | – | – |
| SiC59…CQ2 | −4.57 | −3.03 | 1.53 | 47.76 | 0.76 | 9.41 | −3.46 | 24.78 | 713.8 | −53.25 | −19.06 | – | – |
| water | |||||||||||||
| BC59…CQ2 | −5.25 | −3.71 | 1.53 | 47.63 | 0.76 | 13.07 | −4.21 | 23.50 | 1098.3 | −45.73 | −10.73 | −30.6 | −34.5 |
| AlC59…CQ2 | −5.25 | −3.66 | 1.58 | 46.01 | 0.79 | 12.54 | −4.01 | 29.32 | 1107.6 | −69.91 | −15.37 | −54.8 | −60.1 |
| SiC59…CQ2 | −5.05 | −3.09 | 1.96 | 33.29 | 0.98 | 8.47 | −2.54 | 32.83 | 1116.8 | −63.21 | −19.06 | −40.2 | −48.6 |
The negative values of binding energies (Eb) for MC59…chloroquine (M: B, Al and Si) complexes, display that the adsorbate chloroquine shows an exothermic interaction with doped fullerene. Among these complexes, AlC59…chloroquine complex shows the most negative binding energy value as −67.39 and −69.91 kcal/mol in gas phase and water solvent, respectively. The Eb energies in solvent phase are more negative compared to the gaseous phase indicating more strongly interacted complexes in water solvent. Also, the order of binding energies of these complexes in the both phases show that there is a stronger interaction between the doped fullerenes and chloroquine drug compared to the pristine C60 and drug. It means that the weak interaction of C60 and chloroquine drug was changed to strong chemisorption by doping the fullerene. Also, among these doped fullerene complexes, Al doped in C60 shows the strongest interaction with chloroquine drug due to the higher binding energy in magnitude and B atom doped in C60 shows the smallest interaction with chloroquine drug. We can conclude that the B atom due to its smaller size relative to the other atoms causes the smallest deformations compared to the other dopant atoms inserted in fullerene and hence the values of binding energy of this complex is smaller than that of the other complexes.
The values of solvation energies reported in Table 3 show that the order of solubilities of these complexes in water solvent are SiC59…chloroquine > AlC59…chloroquine > BC59…chloroquine because of the highest absolute value of solvation energy of SiC59…chloroquine and the smallest absolute value of solvation energy of BC59…chloroquine. Comparing solvation energies pristine C60 complex with chloroquine drug and doped C60 complexes with chloroquine drug show that the dopant atoms increases the solubilities.
For BC59 and AlC59 compounds, Singly Occupied Molecular Orbital (SOMO) has been defined as the HOMO and Eg is reported as SOMO–LUMO energy gap. Since silicon and carbon have the same number of valence electrons, doping of C60 with silicon does not alter the net occupancy of the energy levels compared to B and Al dopants [23]. Comparing the results of Table 2 and Table 3 show that doping boron, silicon and aluminum in C60 decrease the energy gap (Eg) of the doped complexes relative to pristine C60 complex 2 in the gas phase and the order of the amount of this decrease is AlC59…chloroquine > SiC59… chloroquine > BC59… chloroquine. It can concluded that B atom due to its smaller size has a smaller effect on decreases of Eg of these complexes. The Eg also can be specified as an indicator of reactivity and kinetic stability [14]. The value of Eg of AlC59…chloroquine complex was calculated as the smallest one with 1.13 eV in the gas phase. But, in water media, BC59…chloroquine complex showed the smallest Eg with 1.53 eV. Therefore, we can conclude that AlC59…chloroquine complex in the gas phase and BC59…chloroquine complex in the solvent phase have higher reactivity according to the smallest chemical hardness.
Besides, the values of electrophilicity index, the maximum electronic charge ΔNmax and electrophilicity-based charge transfer (ECT) of the MC59…chloroquine complexes in the gas phase are higher than the C60…chloroquine complex. The order of obtained values for these parameters is AlC59…chloroquine > BC59…chloroquine > SiC59…chloroquine and BC59…chloroquine > AlC59…chloroquine > SiC59…chloroquine, in the gas phase and water media, respectively, which these results are in agreement with the analysis of Eg and chemical hardness results of these complexes.
The ΔEg values listed in Table 3, which were computed by calculating the percentage value of the difference in the Eg energies of MC59 and MC59…chloroquine, show that conductivities of the MC59…chloroquine complexes have been enhanced relative to the C60…chloroquine complex. Also, conductivity of the AlC59…chloroquine complex is higher than the other complexes in the gas phase due to the higher ΔEg value, which is accordance with the higher reactivity of AlC59…chloroquine complex in the gas phase.
The dipole moment of pristine C60 fullerene is zero because of the absence of charge separation. By replacing one C atom of C60 fullerene with B, Al and Si atoms (BC59, AlC59 and SiC59), the electron density at the B–fullerene, Al-fullerene and Si-fullerene interface has been perturbed, leading to dipole moments of 0.7 D, 6.28 D and 2.23 D in the BC59, AlC59 and SiC59 complexes, respectively. Therefore, the dipole moments increase by doping with these elements. The order of dipole moments can be related on the electropositivity of the dopant atoms. Al and Si being more electropositive than B, loses more charges to the C60 fullerene and gains more positive charges, that is accountable for high dipole moments of AlC59 and SiC59. The charge on Al in AlC59 and the charge on Si in SiC59 is higher than the charge on B in BC59. Boron (B) with less electropositive caracteristic compared to Al and Si, dose not show a high dipole moment as AlC59, and since dipole moment depends on the quantity of the charges along with separation between them [42], the B atom in BC59 with the lowest dipole moment, shows the lowest positive charge compared to the positive charges of Al and Si atoms in AlC59 and SiC59 complexes. Therefore, we can conclude that dopant atoms increase the electronic and magnetic properties of C60 in interaction with drugs. Among B, Al and Si dopant atoms, Al and Si dopants have higher affects on electronic and magnetic properties of C60 than B atom. The calculated dipole moments of the MC59…chloroquine complexes show that the dipole moments of these complexes in both phases are larger than C60…chloroquine complex. Among these complexes, SiC59…chloroquine complex shows the largest dipole moment in both phases compared to the other compounds. Also the calculated values of isotropic polarizability (α) in both phases for these complexes which are listed in Table 3, show that the polarizability values in water media are larger than those of in the gas phase and the polarizability value for SiC59…chloroquine is higher than that of the other complexes which is accordance with the order of dipole moment and deformation of these compounds. Also, the comparision of the polarizabilities of C60…chloroquine complexes, which have been shown in Table 2, and the polarizabilities of MC59…chloroquine complexes show that the polarizability of all doped C60 complexes is higher than that of the pure C60 complexes. These results show that the polarizabilities follow the same order as the dipole moment of these complexes.
Besides, the thermodynamic possibility of chloroquine drug adsorption on pristine C60 and B-, Al- and Si- doped C60 fullerene have been investigated. The enthalpy changes (ΔH) and the Gibbs free energy changes (ΔG) at P = 1 atm and T = 298.14 K have been computed by the results of vibrational frequency calculations in water media, are listed in Table 2 and Table 3. These values show that complex 2 and AlC59…CQ complex show the lowest Gibbs free energy and enthalpy changes, similar to the results of the binding energies. The values of ΔG and ΔH are −1.98 (−2.20) and − 54.8 (−60.1) kcal/mol for complex 2 and AlC59…CQ complex, respectively. The more value of ΔH compared to that of ΔG is related the entropic effect. The negative values of ΔG and ΔH show that all the adsorptions are exothermic. The values of ΔG and ΔH for chloroquine adsorption on the pristine C60 are less than those of chloroquine adsorption on doped C60 fullerene. This show that the adsorption of this drug on the doped C60 fullerene, especially on AlC59, is more thermodynamically favorable.
3.4. Vibrational properties
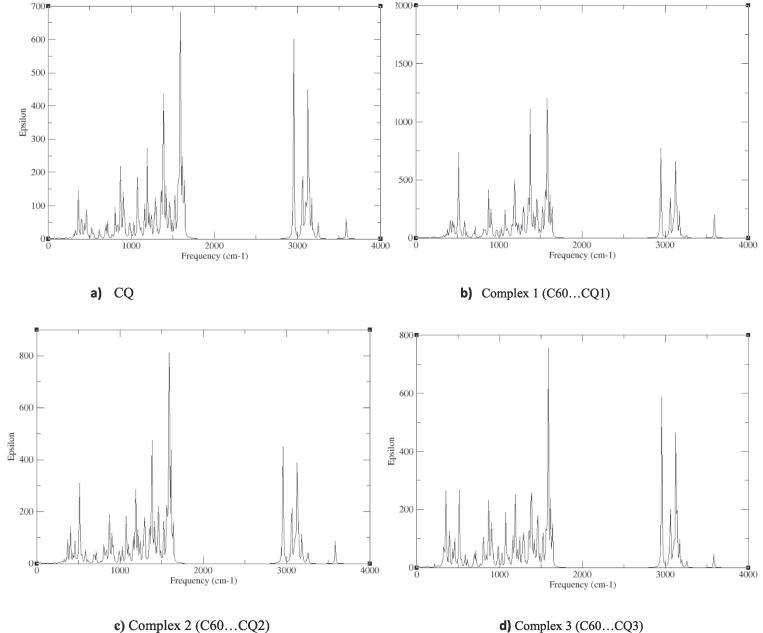
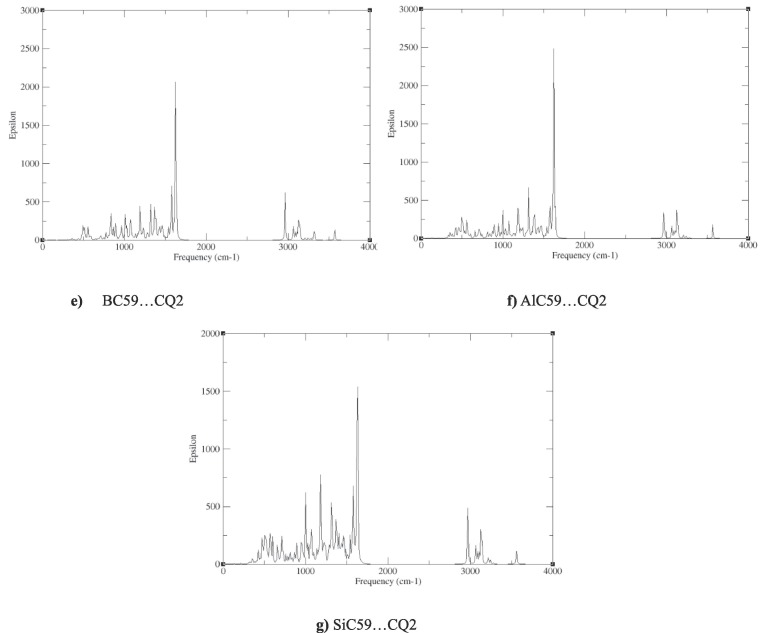
The calculated infrared (IR) spectra of chloroquine (CQ), three configurations of CQ:C60 (C60…CQ1), (C60…CQ2) and (C60…CQ3), and (BC59…CO2), (AlC59…CQ2) and (SiC59…CQ2), which have been obtained from vibrational frequency calculations to confirm the stability of the studied compounds, have been shown in Fig. 5 . For all the structures, there have been no imaginary vibrational modes observed, which indicates the structural stability of all these compounds. The IR spectra of chloroquine shows that the frequencies of the strongest IR bands of CQ and C60…CQ2 complex have been shown in the range 1300–1600 cm−1, which the two more obvious bands have been observed at 1588 and 1382 cm−1 which have been assosiated to the C12-N3 and C10-N2 stretching modes of chloroquine molecule, respectively. These figures show that after chloroquine adsorption, the frequencies of these two vibrational modes have not been changed obviously and remain virtually the same. In the case of BC59…CQ, AlC59…CQ and SiC59…CQ, the strongest IR band shifts to higher frequencies (nearly: 1620 cm−1) which have been related to the C—C and C—N stretching modes.
Fig. 5.
IR spectra for a) CQ, b) Complex 1 (C60…CQ1), c) Complex 2 (C60…CQ2), d) Complex 3 (C60…CQ3), e) BC59…CQ2, f) AlC59…CQ2, g) SiC59…CQ2.
4. Conclusions
In this study, DFT calculations have been performed to study the interactions between pristine C60 fullerene and also the B, Al, Si doped fullerene with chloroquine drug in order to investigate these compounds as drug delivery vehicles for treatment of COVID-19. Some important parameters have been calculated and reported in this work. The most negative binding energy among different configurations of this drug and pristine C60 is observed for complex 2, which shows that this complex is the most stable structure among the other complexes. The decreases of the Eg of C60 as a result of interacting with chloroquine drug in configuration 2 is smaller than that of the other configurations of this drug. So, complex 2 is an electronically harmless interaction and the nano vehicle would not significantly alter the drug properties and also shows that the conductivitiy of the complex 2 is higher than the other complexes. Increases of electrophilicity index of C60…CQ complexes relative to the single drug and C60, means that the interaction of drug with fullerene produced more electrophilic characteristic for the examined structures. The results of dipole moments show that adsorption of chloroquine on fullerene, increases the dipole moment of the whole system which is a preferred property for drug delivery in biological systems. The negative values of amount of electrophilicity-based charge transfer (ECT) of these structures show that drug acts as electron donor and fullerene acts as electron acceptor.
On the basis of the obtained results, complex 2 as the most stable structure have been considered to study the effect of doped C60 fullerene with B, Si and Al dopant atoms on the adsorption behavior of chloroquine drug. The most negative value of binding energy has been observed for AlC59…chloroquine complex which shows that there is a stronger interaction between Al doped fullerenes and chloroquine drug compared to the other complexes. It can be concluded that the weak interaction of C60 and chloroquine drug was altered to strong chemisorption by doping the fullerene. Comparing the solvation energies of pristine C60 complex with chloroquine drug and doped C60 complexes with chloroquine drug show that the dopant atoms increases the solubilities and among them, SiC59…chloroquine and AlC59…chloroquine complex have the higher solvation energies compared to the other complexes. The values of electrophilicity index and electrophilicity-based charge transfer (ECT) of the MC59…chloroquine complexes in the gas phase are higher than the C60…chloroquine complex. Also, conductivity of the AlC59…chloroquine complex is higher than the other complexes in the gas phase due to the higher ΔEg value, which is in agreement with the higher reactivity of AlC59…chloroquine complex in the gas phase. The calculated dipole moments of MC59…chloroquine complexes are larger than C60…chloroquine complexes and SiC59…chloroquine complex shows the highest dipole moment and polarizability compared to the other compounds. Also, vibrational analysis show that there is no imaginary vibrational modes, which indicates the structural stability of the compounds. Thermodynamic parameters such as the enthalpy changes (ΔH) and the Gibbs free energy changes (ΔG) show that complex 2 and AlC59…CQ2 complex show the lowest Gibbs free energy and enthalpy changes, similar to the results of the binding energies. The values of ΔG and ΔH for chloroquine adsorption on the doped C60 and pristine C60 fullerene show that the adsorption of chloroquine drug on the doped C60 fullerene is more thermodynamically favorable. On the basis of all the obtained results we can conclude that the doped C60 fullerene, particularly AlC59…chloroquine and SiC59…chloroquine, could be the better nano vehicles for chloroquine drug delivery compared to the pristine C60 fullerene due to their better reactivity, better electronic and magnetic properties and the more favorable thermodynamic properties.
CRediT authorship contribution statement
Samaneh Bagheri Novir: Conceptualization, Methodology, Software, Data curation, Writing - original draft, Investigation, Supervision, Writing - review & editing. Mohammad Reza Aram: Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The present study was carried out in — Iranian Center for Quantum Technologies (ICQTs). We would like to thank Development and Application of New Technologies Company (TAKFAN) for their support.
References
- 1.Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S., Yuen K.Y. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. Nature. 2020;579:507–513. [Google Scholar]
- 3.Colson P.h., Rolain J.M., Raoult D. Int. J. Antimicrob. Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah A., Kashyap R., Tosh P., Sampathkumar P., OHoro J.C. Mayo Clin. Proc. 2020;65:646–652. doi: 10.1016/j.mayocp.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Ntimicrob Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen J.S. Ann. Rheum. Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanouriakis A. Ann. Rheum. Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 8.Tektonidou M.G. Ann. Rheum. Dis. 2019;78:1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivino F.B. Dis. Clin. North. Am. 2016;42:531–551. doi: 10.1016/j.rdc.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Cell Discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecuit M. Mdecine et Maladies Infectieuses. 2020;50:229–230. doi: 10.1016/j.medmal.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Int. J. Antimicrob. Agents. 2020;55:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parlak C., Alver Ö. Chem. Phys. Lett. 2017;678:85–90. [Google Scholar]
- 15.Feng W., Luo W., Feng Y. Nanoscale. 2012;4:6118–6134. doi: 10.1039/c2nr31505j. [DOI] [PubMed] [Google Scholar]
- 16.Dubacheva G.V., Liang C.K., Bassani D.M. Coord. Chem. Rev. 2012;256:2628–2639. [Google Scholar]
- 17.Sazonova V., Yaish Y., Üstünel H., Roundy D., Arias T.A., McEuen P.L. Nature. 2004;431:284–287. doi: 10.1038/nature02905. [DOI] [PubMed] [Google Scholar]
- 18.Park S., Ruoff R.S. Nat. Nanotechnol. 2009;4:217–224. doi: 10.1038/nnano.2009.58. [DOI] [PubMed] [Google Scholar]
- 19.V. Rosiles González, A. Escobedo-Morales, D. Cortés Arriagada, Ma. De L. Ruiz Peralta, E. Chigo Anota, Appl. Nanosci. 9 (2019) 317–326.
- 20.Cano Ordaz J., Chigo Anota E., Salazar Villanueva M., Castro M. New J. Chem. 2017;41:8045–8052. [Google Scholar]
- 21.Chigo Anota E., Cocoletzi G.H. Physica E. 2014;56:134–140. [Google Scholar]
- 22.Chigo Anota E., Cocoletzi G.H., Sánchez Ramírez J.F. J. Mol. Model. 2013;19:4991–4996. doi: 10.1007/s00894-013-1999-1. [DOI] [PubMed] [Google Scholar]
- 23.KhodamHazrati M., Hadipour N.L. Phys. Lett. A. 2016;380:937–941. [Google Scholar]
- 24.Shi J., Votruba A.R., Farokhzad O.C., Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon S., Young Jang J., Ryoun Youn J., Jeong J.-H., Brenner H., Seok Song Y. Sci. Rep. 2013;3:3269–3275. doi: 10.1038/srep03269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishna V., Stevens N., Koopman B., Moudgil B. Nat. Nanotechnol. 2010;5:330–334. doi: 10.1038/nnano.2010.35. [DOI] [PubMed] [Google Scholar]
- 27.Yuna Y.H., Shanovb V.N., Schulza M.J., Tuc Y., Yarmolenkod S., Nerallad S., Pixleye S.K., Behbehanie M., Donge Zh., Heinemanf W.R., Brian Halsallf H., Bange A. Proc. SPIE. 2006;6172:617205. [Google Scholar]
- 28.Shariatinia Z., Shahidi S. J. Mol. Graph. Model. 2014;52:71–81. doi: 10.1016/j.jmgm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Youle R.J., Karbowski M. Nat. Rev. Mol. Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 30.Hazrati M.K., Hadipour N.L. Phys. Lett. A. 2016;380:937–941. [Google Scholar]
- 31.Bakry R., Vallant R.M., Najam-ul-Haq M., Rainer M., Szabo Z., Huck C.W., Bonn G.K. Int. J. Nanomedicine. 2007;2(4):639–649. [PMC free article] [PubMed] [Google Scholar]
- 32.Bashiri S., Vessally E., Bekhradnia A., Hosseinian A., Edjlali L. Vacuum. 2017;136:156–162. [Google Scholar]
- 33.Singh R., Lillard J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi J., Zhang H., Wang L., Li L., Wang H., Wang Z., Li Z., Chen C., Hou L., Zhang C. PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials. 2013;34:251–261. doi: 10.1016/j.biomaterials.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Gallo M., Favila A., Glossman-Mitnik D. Chem. Phys. Lett. 2007;447:105–109. [Google Scholar]
- 36.de Leon A., Jalbout A.F., Basiuk V.A. Chem. Phys. Lett. 2008;452:306–314. [Google Scholar]
- 37.Narayan P., Samanta, Kumar Das K. J. Mol. Graph. Model. 2017;72:187–200. doi: 10.1016/j.jmgm.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Shokuhi Rad A., Aghaeib S.M., Aalia E., Peyravic M. Diam. Relat. Mater. 2017;77:116–121. [Google Scholar]
- 39.Eslami M., Moradi M., Moradi R. Physica E. 2017;87:186–191. [Google Scholar]
- 40.Larkia S., Shakerzadehb E., Chigo Anotac E., Behjatmanesh-Ardakani R. Chem. Phys. 2019;526:110424–110430. [Google Scholar]
- 41.Farmanzadeh D., Keyhanian M. Theor. Chem. Acc. 2019;138:11–21. [Google Scholar]
- 42.ShokuhiRad A., Ayub Kh. Mater. Res. Bull. 2018;97:399–404. [Google Scholar]
- 43.Shokuhi Rad A., Aghaei S.M., Aali E., Peyravi M., Jahanshahi M. Appl. Organomet. Chem. 2017;32:1–10. [Google Scholar]
- 44.ShokuhiRad A., Ayub Kh. Com. Theor. Chem. 2017;1121:68–75. [Google Scholar]
- 45.M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery Jr., T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, Gaussian 03, Gaussian, Inc, Pittsburgh, PA, 2003.
- 46.Boys S.F., Bernardi F.D. Mol. Phys. 1970;19:553–566. [Google Scholar]
- 47.Padmanabhan J., Parthasarathi R., Subramanian V., Chattaraj P.K. J. Phys. Chem. A. 2007;111:1358–1361. doi: 10.1021/jp0649549. [DOI] [PubMed] [Google Scholar]
- 48.Cossi M., Rega N., Scalmani G., Barone V. J. Comput. Chem. 2003;24:669–681. doi: 10.1002/jcc.10189. [DOI] [PubMed] [Google Scholar]
- 49.Cossi M., Barone V., Cammi R., Tomasi J. Chem. Phys. Lett. 1996;255:327–335. [Google Scholar]
- 50.Barone V., Cossi M. J. Phys. Chem. A. 1998;102:1995–2001. [Google Scholar]