Abstract
BACKGROUND:
Tuberculosis (TB) and diabetes mellitus are still of much public health concern. Screening of TB patients for diabetes will ensure early case detection, better management of diabetes, and better TB treatment outcome. The objective of this study was to determine the prevalence and associated factors of diabetes in TB patients and their impact on treatment outcome of TB.
MATERIALS AND METHODS:
This was a longitudinal follow-up study of registered TB patients under the Revised National Tuberculosis Control Program in all five TB units of Bhopal district. Participants were contacted and the interview was conducted. The blood sugar of all TB patients was checked, and they were followed up to assess the treatment outcome from October 2014 to September 2017. Data were analyzed using SPSS (version 16.0. Chicago, SPSS Inc.). Logistic regression was done to find the factors for diabetes in TB patients. The Chi-square tests were used to find the difference in treatment outcomes and assess the relative risk for poor outcome in diabetic TB patients.
RESULTS:
Of total 662 TB patients, 82 (12.39%) were diagnosed as diabetic. Age >50 years, males, higher body mass index, pulmonary TB, patients on Category II treatment, and history of smoking were found to be predictors of diabetes in TB patients. The treatment outcome of TB was more unfavorable (defaulter, failure, and death) in diabetic TB patients (16.17%) than in nondiabetic TB patients (5.8%) (risk ratio = 2.78, 1.469–5.284 confidence interval).
CONCLUSION:
The high prevalence of diabetes and the unfavorable treatment outcome in diabetic TB patients make screening and management of diabetes at an early-stage crucial for a better outcome in TB patients.
Keywords: Diabetes, treatment outcome, tuberculosis
Introduction
Tuberculosis (TB) and diabetes mellitus (DM) are both still of much public health concern despite various efforts to reduce their incidence. With the epidemiological transition, the burden of disease from infectious diseases is supposed to be declining and the burden from noncommunicable diseases on the rise. However, nearly 95% of patients suffering from TB and 70% of patients with diabetes live in low- and middle-income countries, especially in Southeast Asia.[1] The global rise in diabetes poses a huge challenge in the combat against TB. The TB capital, India, accounts for about a quarter of the global TB burden. It also has the highest number of people with diabetes globally, with as many as 60 million people suffering from type 2 diabetes.[2] For a developing country like India, this has severe implications on its health system owing to the double burden from a high prevalence of TB and diabetes.[2]
The association between diabetes and TB was suggested by researchers as early as the 1930s. Many researchers studied the impact of this duo (TB-diabetes) and considered it a major public health concern.[3] Diabetes accounts for 14.8% (range: 7.1%–23.8%) of pulmonary TB and 20.2% (8.3%–41.9%) of smear-positive TB, as per a study conducted by Stevenson et al.[4] The studies conducted in multiple settings in which TB patients were screened for DM found that the prevalence of diabetes ranged from 1.9% to 44% in these patients.[5,6,7,8] According to the World Health Organization (WHO) reports, diabetes triples a person's risk of developing TB, and globally, 15% of TB cases may be linked to diabetes.[9]
Diabetes may adversely affect TB treatment outcomes and reduce the likelihood of a favorable outcome and increase drug resistance, the risk of relapse, and death.[10,11,12,13,14] Screening for DM in TB patients provides an opportunity for early detection and timely intervention for better treatment outcomes.[15,16] The importance of routine implementation of bidirectional screening of the two diseases was realized after the joint work done by the WHO and International Union against Tuberculosis and Lung Disease, which strongly recommended surveillance of diabetes in TB in all countries including India in primary health-care settings.[17] Although Indian health systems have implemented policies for the screening of diabetes in TB patients and vice versa, a joint response is needed to improve the reporting and monitoring mechanism.
The literature available in this domain is predominantly of retrospective studies which rely heavily on medical records for the diagnosis of DM. The current study was conducted with the rationale of filling these lacunae in the literature by assessing the factors associated with diabetes and TB and the treatment outcomes to provide more evidence for the importance of screening for DM in TB patients to counter a dual epidemic.
Materials and Methods
The current study was a facility-based longitudinal follow-up study carried out on registered TB cases under the Revised National Tuberculosis Control Program (RNTCP) in TB units (five TUs) of Bhopal district from October 2014 to September 2017. We included all patients aged 18 years and above with established diagnosis of TB during the period of October 2014 to September 2016, excluding seriously ill patients such as those with TB meningitis, septicemia, etc., and pregnant patients. Type 1 diabetics who were hard to find in the community, there being no proper representation of that group, were excluded from the study.
The sample size was calculated, knowing that the difference in the prevalence of diabetes in TB patients and the general population was around 3%. The alpha (type 1 error) was set at 0.01, and the power was set at 80%. The sample size estimated was rounded off to 600 after accounting for a 10% attrition rate.
Null hypothesis (Ho): Diabetes prevalence in TB patients is equal to that found in the general population (8%). Alternate hypothesis (H1): The prevalence of diabetes in TB patients is significantly more (11%) than in the general population.
The study participants were selected by a simple random technique using a random number table from already constructed sampling frame. A line listing of all TB patients of age 18 or above currently on DOTS (Directly Observed Treatment Shortcourse) therapy was made using TB register under RNTCP in Bhopal district.
The day of DOTS therapy was ascertained for all selected patients. Participants were contacted and the interview was conducted after obtaining informed consent by means of predesigned and pretested pro forma. This consisted of sociodemographic profile and TB status of patients with the type of TB, category, and duration of treatment together with blood glucose level, duration, and treatment history of diabetes. Patients were screened and diagnosed as diabetic with fasting blood sugar level ≥126 mg/dl or random blood glucose level ≥200 mg/dl in patients with classical symptoms of hyperglycemia and prediabetes with fasting blood sugar level between 100 and 125 mg/dl, according to the American Diabetes Association guidelines at the time of recruitment.[18] Further, all participants were contacted after their treatment was completed, and the results of their treatment were recorded from the patient's register in the TB unit.
The variables of interest were the treatment outcomes by the RNTCP guidelines: the number of cured patients, treatment completed, failure, defaulters, death, transfers out, etc.[19] A TB patient with bacteriologically confirmed TB at the beginning of treatment who was smear or culture negative in the last month of treatment and on at least one previous occasion was considered cured. A TB patient was considered as having completed treatment, if they had completed treatment without the evidence of failure, but with no record of sputum smear or culture results in the last month of treatment either because tests were not done or because results are unavailable, and was negative on at least one previous occasion. Treatment failure was when a patient's sputum smear or culture was positive at month 5 or later during treatment. A patient who received anti-TB treatment for more than 1 month and who had not taken anti-TB drugs for 2 months or more consecutively after the start of the treatment was considered to have defaulted. Loss-to-follow-up patients are those who did not start treatment or whose treatment was interrupted for 2 consecutive months or more. A TB patient is considered to have died if death was by any cause before the start of or during treatment. A patient is considered “not evaluated” if they transferred out to another treatment unit or if the treatment outcome was not known to the reporting unit.
Ethical approval from the institutional ethical committee was obtained, and informed written consent was obtained from all patients in the study.
Data were entered in Microsoft Excel 2007 and analyzed using Epi Info 7. All categorical variables were analyzed using the Chi-square test; P < 0.05 was considered as statistically significant. Multivariate logistic regression analysis was done on variables found to be significant in the univariate regression analysis. Risk ratios (RRs) were calculated to assess the treatment outcome of diabetic and nondiabetic TB patients.
Results
The total number of TB patients interviewed was 662, 352 of whom were male (mean age: 39.4 years) and 310 female (mean age: 33.5 years). Most of the patients had pulmonary TB (n = 462; 69.7%) as compared to extrapulmonary TB (n = 200; 30.3%). Around 536 (80.9%) were on Category I treatment and 126 (19.1%) on Category II treatment. Out of the total number of TB patients, 82 (12.4%) were diagnosed as type 2 diabetic; 28 of them (4.2%) were newly diagnosed and 108 (16.3%) were prediabetic [Table 1].
Table 1.
Characteristics of tuberculosis patients by diabetes status
| Variables | TB patients |
|
|---|---|---|
| With DMN (%) | Without DM N (%) | |
| Total patients | 82 (12.4) | 580 (87.6) |
| Age group (years) | ||
| ≤50 | 34 (6.3) | 499 (93.7) |
| >50 | 48 (37.2) | 81 (62.8) |
| Gender | ||
| Male | 53 (15.1) | 299 (84.95) |
| Female | 29 (9.3) | 281 (90.6) |
| Education status | ||
| Illiterate | 10 (15.8) | 53 (84.12) |
| Literate | 72 (12.0) | 527 (87.9) |
| Socioeconomic status$ | ||
| Lower | 14 (14.0) | 86 (86.0) |
| Middle | 67 (12.7) | 458 (87.2) |
| Upper | 1 (2.7) | 36 (97.3) |
| BMI | ||
| ≤25 | 42 (8.1) | 475 (91.9) |
| >25 | 40 (27.6) | 105 (72.4) |
| Smoking | ||
| Yes | 40 (17.1) | 194 (82.9) |
| No | 42 (9.8) | 386 (90.2) |
| Alcohol consumption | ||
| Yes | 14 (14.1) | 85 (85.9) |
| No | 68 (12.1) | 495 (87.9) |
| Family history of DM | ||
| Yes | 23 (16.2) | 119 (88.8) |
| No | 59 (11.3) | 461 (88.7) |
| Site of TB | ||
| Pulmonary | 71 (15.4) | 391 (84.6) |
| Extrapulmonary | 11 (5.5) | 189 (94.5) |
| Treatment category | ||
| Category 1 | 51 (9.5) | 485 (90.5) |
| Category 2 | 31 (24.6) | 95 (75.4) |
Socio-economic status $; According to Modified BG Prasad Classification. BMI=Body mass index, TB=Tuberculosis, DM=Diabetes mellitus
Elderly (>50 years), male, and obese TB patients were found to be significantly more associated with diabetes than female, young, and nonobese patients. In the current study, socioeconomic status, literacy, smoking, and alcohol status were not significantly associated with the presence of diabetes in TB patients. Those who had pulmonary TB and patients on type II treatment were prone to diabetes. Besides, in multivariate logistic regression, age, type of TB, treatment category, and body mass index (BMI) remained significant [Table 2].
Table 2.
Univariate and multivariable regression analysis showing factors associated with the presence of diabetes in tuberculosis patients
| Variable | Diabetic tuberculosis patients N (%) | Crude OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value |
|---|---|---|---|---|---|
| Age (>50 years) | 48 (37.2) | 8.69 (5.28-14.31) | <0.001 | 9.27 (5.24-16.407) | <0.001 |
| Sex (male) | 53 (15.1) | 1.71 (1.06-2.77) | 0.027 | 0.92 (0.44-1.94) | 0.833 |
| BMI (>25) | 40 (27.6) | 4.3 (2.66-6.97) | <0.001 | 2.11 (1.18-3.761) | 0.011 |
| Smoking (yes) | 40 (17.1) | 1.89 (1.18-3.02) | 0.007 | 1.159 (0.55-2.42) | 0.694 |
| Pulmonary TB | 71 (15.3) | 3.02 (1.56-5.83) | <0.001 | 2.02 (0.989-4.42) | 0.054 |
| Treatment category (II) | 31 (24.6) | 3.1 (1.88-5.1) | <0.001 | 3.27 (1.80-5.942) | 0.0001 |
Literacy status, socioeconomic status, family history of diabetes - Not significant in univariate logistic regression. OR=Odds ratio, CI=Confidence interval, BMI=Body mass index, TB=Tuberculosis
Of total 662 TB patients, 38% were cured, 45.7% were treatment completed, 3.2% were failures, 1.3% dead, and 9.1% were either transferred out or had not completed their treatment at the study period. The treatment outcome of diabetic TB patients was poor as there were more failure, relapse, and defaulter cases. There was also a significant difference in TB outcomes between TB-DM and nondiabetic TB patients [Table 3 and Figure 1]. The relative risk of the unfavorable treatment outcome (defaulter, failure, and death) of DM-TB patients was 2.78 (1.469–5.284) as compared to nondiabetic TB patients [Table 4].
Table 3.
Comparison of outcome of tuberculosis treatment in patients with diabetes and without diabetes
| Treatment output | TotalN (%) | TB patients with DMN (%) | TB patients without DMN (%) | Chi-square test |
|---|---|---|---|---|
| Cure | 257 (38.8) | 24 (29.3) | 233 (40.2) | χ2=18.8, df=5, P=0.003 |
| Treatment complete | 303 (45.8) | 33 (40.2) | 270 (46.6) | |
| Failure | 21 (3.2) | 6 (7.3) | 15 (2.6) | |
| Defaulted | 12 (1.8) | 2 (2.4) | 10 (1.7) | |
| Death | 9 (1.4) | 3 (3.7) | 6 (1.0) | |
| Not evaluated* | 60 (9.0) | 14 (17.1) | 46 (7.9) |
*Transferred out and those who not completed their treatment at study period. TB=Tuberculosis, DM=Diabetes mellitus
Figure 1.
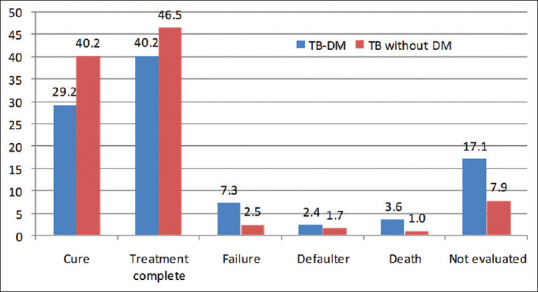
Distribution of study participants according to their tuberculosis treatment outcomes (in %)
Table 4.
Relative risk of unfavorable outcomes in tuberculosis patients with diabetes
| Outcome | TotalN (%) | TB patients with DMN (%) | TB patients without DMN (%) | RR (95% CI) |
|---|---|---|---|---|
| Unfavorable | 42 (6.9) | 11 (16.2) | 31 (5.8) | 2.78 (1.46-5.28), P=0.002 |
| Favorable | 560 (93.1) | 57 (83.8) | 503 (94.2) | |
| Total | 602 (100) | 68 (11.2) | 534 (88.7) |
RR=Risk ratio, CI=Confidence interval, TB=Tuberculosis, DM=Diabetes mellitus
Discussion
The aim of the present study was to find the prevalence of diabetes in patients with TB and its impact on treatment outcome of the disease. Most of the various studies done on the same domain to elicit this impact have been record based. This study was the first of its kind in this area. It was a prospective study in which the patient was followed from the start of treatment till completion, and the interview was done in a person. We found a higher prevalence (12.4%) of diabetes in TB patients and poor treatment outcome in DM-TB patients compared to nondiabetic TB patients (RR 2.78) registered under RNTCP in Bhopal district.
The overall prevalence of diabetes in our study was 12.4% and of prediabetes was 16.3%. Similar results were reported in earlier studies globally, and from India, the prevalence of diabetes in TB patients ranged from 9.5% to 44%.[5,6,7,20,21,22,23,24] Similar to other studies, the prevalence of diabetes in TB patients in our study was significantly higher in the older age group (>50 years), males, smokers, and overweight (BMI >25) patients.[5,6,7,20,21,22,23,24,25,26,27,28,29] In the current study, similar to a study in the United States, the socioeconomic status was not significantly associated with the presence of diabetes in TB patients.[30] We also found that diabetes was significantly more common in patients with pulmonary TB and type II treatment category patients. This is supported by earlier studies in various parts of India.[21,24,31,32,33]
A prospective study on the treatment outcome of TB in India showed the prevalence of DM in TB patients as 15.8% with TB-DM patients: failure as 5.6, default as 5.6, and death rate as 2.8, whereas in the present study, the prevalence was 12.4% and TB-DM: failure as 7.3, default as 2.4, and death rate as 3.6; the prevalence and poor outcome rates were similar as the study population and sample size were nearly equal.[34] The finding of the current study supported studies in the United States and China, where patients with DM had 2.0% higher odds of death than patients without DM, and treatment failure occurred in 4.1% of patients without diabetes, but 6.7% of patients with diabetes.[24,35] The study in the United States also found that 9% died of TB or other causes while undergoing TB treatment, whereas in the current study, it was 1.4% due to TB. The inclusion of other causes might have led to an increased rate of recorded deaths in the US study.[35] A systematic review of the impact of diabetes on TB outcomes found that the patients with diabetes had a risk ratio (RR) for the combined outcome of failure and death of 1.5, whereas it was 2.9 in the present study.[36] Various theories have been proposed for poor TB outcome as a result of DM such as immunologic dysfunction, i.e., alterations in monocyte chemoattraction, alveolar macrophage activity, type I cytokine phenotype, and higher mycobacterial load in DM patients, which lead to a delay in microbial clearance and, in turn, affect treatment success.[11,12,20,35,36,37,38] The unfavorable outcome in TB patients may be due to poor diabetic control, so rigorous glycemic control is necessary for the management of TB-DM.
Conclusion
Diabetes and TB co-epidemic remains a major challenge in TB control globally. In the current study, a higher prevalence of diabetes in TB patients, as well as an unfavorable treatment outcome in diabetic TB patients, was found. This study shows that it is vital and feasible to screen TB patients for diabetes since we were able to implement a screening program within the routine system with existing staff. The nationwide TB and DM programs have already given a special emphasis on this duo to initiate a joint response to provide the much-needed care on both issues at both organizational and clinical levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006;43:848–54. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]
- 3.Root HF. The association of diabetes and tuberculosis. N Engl J Med. 1934;210:127–47. [Google Scholar]
- 4.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: The impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: A systematic review. PLoS One. 2017;12:e0175925. doi: 10.1371/journal.pone.0175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakrishnan S, Vijayan S, Nair S, Subramoniapillai J, Mrithyunjayan S, Wilson N, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One. 2012;7:e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Shenoy VP, Bairy I, Srinivasa H, Mukhopadhyay C. Diabetes mellitus and HIV as co-morbidities in tuberculosis patients of rural south India. J Infect Public Health. 2011;4:140–4. doi: 10.1016/j.jiph.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Tuberculosis & Diabetes. [Last accessed on 15 Jul 2018]. Available from: https://www.who.int/tb/publications/diabetes_tb.pdf .
- 10.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 11.Wang CS, Yang CJ, Chen HC, Chuang SH, Chong IW, Hwang JJ, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–10. doi: 10.1017/S0950268808000782. [DOI] [PubMed] [Google Scholar]
- 12.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135:483–91. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan B, Du J, Lan X, Zhou M, Wang J, Wang W. Effect of type 2 diabetes mellitus on sputum negative conversion and treatment effects of multi-drug-resistant tuberculosis. Biomed Res. 2017;28:3917–22. [Google Scholar]
- 14.Shewade HD, Jeyashree K, Mahajan P, Shah AN, Kirubakaran R, Rao R, et al. Effect of glycemic control and type of diabetes treatment on unsuccessful TB treatment outcomes among people with TB-diabetes: A systematic review. PLoS One. 2017;12:e0186697. doi: 10.1371/journal.pone.0186697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon CY, Harries AD, Baker MA, Hart JE, Kapur A, Lönnroth K, et al. Bi-directional screening for tuberculosis and diabetes: A systematic review. Trop Med Int Health. 2010;15:1300–14. doi: 10.1111/j.1365-3156.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 16.Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–99. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 17.WHO/HTM/TB/2011.15. Geneva: World Health Organization; 2011. [Last accessed on 15 Jul 2018]. WHO, International Union against Tuberculosis and Lung Disease. Collaborative Framework for Care and Control of Tuberculosis and Diabetes. Available from: https://apps.who.int/iris/handle/10665/44698 . [PubMed] [Google Scholar]
- 18.American Diabetes Association. 2.Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 19.Muruganathan A, Thomas A, Muniyandi M, Chandrasekaran V. Revised National Tuberculosis Control Programme (RNTCP) J Indian Med Assoc. 2010;108:868–70. [PubMed] [Google Scholar]
- 20.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006;10:74–9. [PubMed] [Google Scholar]
- 21.Raghuraman S, Vasudevan KP, Govindarajan S, Chinnakali P, Panigrahi KC. Prevalence of diabetes mellitus among tuberculosis patients in urban Puducherry. N Am J Med Sci. 2014;6:30–4. doi: 10.4103/1947-2714.125863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna A, Lohya S, Sharath BN, Harries AD. Characteristics and treatment response in patients with tuberculosis and diabetes mellitus in New Delhi, India. Public Health Action. 2013;3:S48–50. doi: 10.5588/pha.13.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.India Tuberculosis-Diabetes Study Group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013;18:636–45. doi: 10.1111/tmi.12084. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Xiao H, Sugawara I. Tuberculosis complicated by diabetes mellitus at Shanghai pulmonary hospital, China. Jpn J Infect Dis. 2009;62:390–1. [PubMed] [Google Scholar]
- 25.Aftab H, Ambreen A, Jamil M, Garred P, Petersen JH, Nielsen SD, et al. High prevalence of diabetes and anthropometric heterogeneity among tuberculosis patients in Pakistan. Trop Med Int Health. 2017;22:465–73. doi: 10.1111/tmi.12842. [DOI] [PubMed] [Google Scholar]
- 26.Sarker M, Barua M, Guerra F, Saha A, Aftab A, Latif AH, et al. Double trouble: Prevalence and factors associated with tuberculosis and diabetes comorbidity in Bangladesh. PLoS One. 2016;11:e0165396. doi: 10.1371/journal.pone.0165396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair S, Kumari AK, Subramonianpillai J, Shabna DS, Kumar SM, Balakrishnan S, et al. High prevalence of undiagnosed diabetes among tuberculosis patients in peripheral health facilities in Kerala. Public Health Action. 2013;3:S38–42. doi: 10.5588/pha.13.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumpatla S, Sekar A, Achanta S, Sharath BN, Kumar AM, Harries AD, et al. Characteristics of patients with diabetes screened for tuberculosis in a tertiary care hospital in South India. Public Health Action. 2013;3:S23–8. doi: 10.5588/pha.13.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naik B, Kumar AM, Satyanarayana S, Suryakant MD, Swamy NM, Nair S, et al. Is screening for diabetes among tuberculosis patients feasible at the field level? Public Health Action. 2013;3(Suppl 1):S34–7. doi: 10.5588/pha.13.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez A, Brown HS, 3rd, Restrepo BI. Association between tuberculosis and diabetes in the Mexican border and non-border regions of Texas. Am J Trop Med Hyg. 2006;74:604–11. [PMC free article] [PubMed] [Google Scholar]
- 31.Dave P, Shah A, Chauhan M, Kumar AM, Harries AD, Malhotra S, et al. Screening patients with tuberculosis for diabetes mellitus in Gujarat, India. Public Health Action. 2013;3:S29–33. doi: 10.5588/pha.13.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guptan A, Shah A. Tuberculosis and diabetes: An Appraisal. Ind J Tub. 2000;47:3–8. [Google Scholar]
- 33.Achanta S, Tekumalla RR, Jaju J, Purad C, Chepuri R, Samyukta R, et al. Screening tuberculosis patients for diabetes in a tribal area in South India. Public Health Action. 2013;3:S43–7. doi: 10.5588/pha.13.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui AN, Khayyam KU, Sharma M. Effect of diabetes mellitus on tuberculosis treatment outcome and adverse reactions in patients receiving directly observed treatment strategy in India: A prospective study. Biomed Res Int. 2016;2016:7273935. doi: 10.1155/2016/7273935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: Analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18:187–201. [PubMed] [Google Scholar]
- 38.Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, et al. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2005;139:57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


