Abstract
Purpose
Contrast sensitivity (CS) is predictive of various aspects of an individual's functional vision, such as recognizing faces and driving. Currently available CS charts are limited in terms of the spatial frequencies they can test and/or the contrast resolution of the targets they present. The traditional methods for measuring full CS functions (CSFs) are time consuming. The purpose of this study was to examine the feasibility of using the quick CSF method in a low vision population and to assess the relationships of CS with other visual functions, which can contribute to the understanding of the functional vision.
Methods
Static visual acuity, dynamic visual acuity, CS, global motion perception thresholds, and visual field were measured binocularly in 53 individuals with low vision. The number of participants who could complete each assessment was used to assess feasibility. The relationships between CS and other visual functions were assessed using linear regressions and multiple regressions.
Results
The quick CSF was quantifiable in 34 participants of the 42 with quantifiable visual acuities. The area under the log CSF—the summary statistic of CSF—was significantly correlated with static visual acuity and dynamic visual acuity (r = −0.79 and r = −0.63, respectively; P < 0.001).
Conclusions
The qCSF is capable of measuring CS in a wide range of visual impairment severities. area under the log CSF only correlates with measures of visual acuity.
Keywords: qCSF, low vision, dynamic visual acuity, motion perception
Visual acuity is the most common assessment of spatial vision completed in a routine eye examination.1 It is measured using high contrast letters, symbols, or gratings. Although it is an extremely useful measure to detect deficits in the visual system and to determine optical corrections, it does not provide information about the observer's perception of images with lower contrast. Another assessment of spatial vision, contrast sensitivity (CS) or the sensitivity to luminance variations, is shown to be more representative of visual functions performed daily, such as performance in driving and ability to perceive faces.2–7
The most widely used tool for measuring CS clinically is the Pelli-Robson chart, which is a printed, wall-mounted chart (measures 60 × 85 cm) that has two variations available.8 Another commonly used clinical CS test is the Mars letter CS chart, which is handheld (measures 23 × 36 cm), available in three versions, and has been reported to have equal or slightly better repeatability than the Pelli-Robson chart.9 Unfortunately, both of the charts can only measure CS at only one spatial frequency and are limited in terms of the contrast resolution of the targets they present. Research has demonstrated that some pathologies affect specific CS frequency ranges, such as amblyopia and Parkinson's disease, whereas many other pathologies, such as early glaucoma and multiple sclerosis affect CS at all spatial frequencies.4,10–13 Low vision results from a wide variety of pathologic causes; therefore, CS needs to be measured across a broad range of spatial frequencies.
These charts are also limited simply by being printed. The large size of the Pelli-Robson chart makes it difficult to illuminate the chart evenly, and the Mars chart can be affected by shadows, depending on how it is held. Furthermore, the contrast values of the printed text may change over time owing to exposure to light and handling. Furthermore, the limited numbers of letters and sequences of letters in printed charts may make memorizing easier for patients, which decreases the validity of repeated measurements with these charts, although there are no published reports confirming or denying the impact of letter memorization on printed chart validity.
One way to get around the limitations of printed charts is to measure the complete CS function (CSF), which consists of contrast detection thresholds calculated for multiple spatial frequencies, spaced on a logarithmic scale. Unfortunately, the precise CSF tests that are used in research facilities require up to 500 to 1000 trials and take about 30 to 60 minutes to complete,14 making them impractical for use in clinical settings. Computerized tests such as the Vistech CS chart (Vistech, Hartford, CT) that use sine wave grating patches to measure CS have been shown to have poor repeatability.15
The quick CSF (qCSF) method, developed by Lesmes et al.,16 estimates the full shape of the CSF precisely and in agreement with the CSFs obtained independently using the conventional methods by applying a Bayesian adaptive algorithm and incorporating a 10-alternative forced choice letter identification task.17 The qCSF method is accurate and precise when compared with traditional methods of CS measurement in clinical populations, such as patients with AMD, amblyopia, glaucoma, and persons with age-related vision changes (Ramulu PY, et al. IOVS 2015;56(7):ARVO E-Abstract 2225; Jia W, et al. IOVS 2014;55(13):ARVO E-Abstract 762; Lesmes LA, et al. IOVS 2012;53(14):ARVO E-Abstract 4358).18 The qCSF procedure results are less affected by changes in test conditions such as luminance and testing distance and have test-retest reliability of greater than 0.95 after 20 quick trials, which take approximately 4 minutes to complete.19 Thus, the qCSF procedure is an effective clinical tool for the measurement of CSF. However, there are some limitations present in the literature:20,21 the qCSF procedure was always tested in controlled environments and homogeneous populations. Furthermore, the logMAR visual acuities of the clinical populations tested were between 0.00 (normal visual acuity) and 1.10 logMAR, which does not include individuals with severe or profound visual impairments. Approximately 211 million individuals among the world population have moderate to severe vision impairment and have visual acuities ranging from more than 6/18 (0.50 logMAR) to less than 3/60 (2.00 logMAR) or worse. Therefore, it would be valuable to know if the qCSF procedure is capable of measuring CS in individuals with a wide range of visual impairment severities. Furthermore, if qCSF measurements are to be used as part of routine eye examinations, it would be helpful to investigate how performance on the qCSF relates to other visual functions, which are predictive of individuals’ functional vision, such as static visual acuity (SVA), dynamic visual acuity (DVA), global motion perception (MP), and visual field (VF) extent.22–25
The objectives of this study are to (1) test the feasibility of using the qCSF procedure in a low vision population with a broad range of visual function impairments, including visual acuities worse than 1.10 logMAR and variable VF losses, and (2) to assess the relationship of CSF measured using qCSF with other visual functions such as SVA, DVA, MP, and VF measured binocularly.
Methods
This study received ethics approval from a University of Waterloo Office of Research Ethics Committee and adhered to the tenets of the Declaration of Helsinki. This study was conducted as part of a larger project examining the classification of Para Nordic and Para Alpine skiers with visual impairments at the 2017 Para Nordic (Finsterau, Germany) and Para Alpine (Tarvisio, Italy) World Championship events, and at a 2018 Para Nordic World Cup event (Oberried, Germany). All participants who had visual impairments severe enough to qualify them to compete in Paralympic sport were informed and signed consent before enrollment in this observational study. None of the participants had familiarity with psychophysical procedures. Participants were asked to wear their habitual distance refractive correction for the study.
During the visit, participants’ SVA, DVA, translational MP (TMP), radial MP (RMP), CSF, and VF were assessed binocularly. SVA was measured using an Early Treatment Diabetic Retinopathy Study chart at 1 m and/or the Berkeley Rudimentary Vision Test at 0.25 to 1.00 m (as per the test protocol).1,26 Dynamic visual acuity was measured using the computer program moV& (V&mp Vision Suite, Waterloo, Canada), which used a single tumbling E letter that moved in a random walk trajectory at a speed of 1 m/s, and was presented on a high-definition television screen (50” or 60” display, 60 Hz refresh rate and 1920 × 1080 resolution, illuminance at 130–150 lux) at a distance of 1 m.27 The initial size of the letter presented was 0.60 log units bigger than the participant's static visual acuity to make sure that the subject started the test from a suprathreshold level, and the maximum letter size presentable on this screen was 2.60 logMAR at a distance of 1 m. Five targets were presented per level of visual acuity measured and the target display time was set to be unlimited to ensure adequate time to respond to the direction of the letter E. The translational (up or down movement) and radial (in or out movement) MP tests were designed to quantify how well local moving elements in visual scenes are integrated to create a global moving stimulus. We measured both TMP and RMP using random dot kinematograms that consisted of 100 individual, full-contrast, local dots that were equivalent to the size of the target detail of a 2.0 logMAR letter (Dalton K, et al. IOVS 2017;58(8):ARVO E-Abstract 4693). The test stimuli for the DVA, TMP, and RMP assessments were presented on high definition televisions (50” or 60” displays, 60 Hz refresh rate and 1920 × 1080 resolution). The illuminance on the screen was kept at 130 to 150 lux for all measurements.
CSF was measured using the qCSF procedure on an AST Platform. The AST platform consisted of a 46" NEC P463 screen with 1920 × 1080 resolution, calibrated to 90 cd/m2 background luminance. At a viewing distance of 4 m, the screen allows a display of stimuli in a spatial frequency range from 1.4 to 36.2 cycles per degree (cpd), which includes the entire set of frequencies mandated by the US Food and Drug Administration (1.5–18.0 cpd). However, for this study, we used a viewing distance of 1 m because most subjects had visual acuities of worse than 1.0 logMAR. At this distance, the screen allowed a display of spatial frequencies ranging from 0.35 to 9.00 cpd. It was possible to present contrast levels of down to 0.2% reliably.28 The area under log CSF (AULCSF), peak CS (peak CS), and the contrast acuity (spatial frequency where contrast threshold was 100%) were the summary statistics calculated by the software.18 AULCSF is calculated as the AULCSF curve, which quantifies the entire range of contrast visibility.29
Binocular VF was assessed using an arc perimeter. Participants were positioned such that the center of their eyes was visible for them in the mirror at the center of the arc perimeter, and the fixation was monitored during testing. VF assessments were performed by the examiner following the standardized procedure using a Goldmann IV target.30 The same trained clinical investigator (A.S.) conducted the VF assessments for all 39 participants.31,32
Statistical Analysis
All data analysis was conducted using SPSS for Windows (version 25.0, SPSS, Inc., Cary, NC). The Shapiro-Wilk (“W” statistic) normality test was used to determine if the visual function parameters were normally distributed.33 Nonparametric, Spearman correlation coefficients were computed to determine relationships between CS and other visual function assessments measures for all participants (P < 0.05 were considered significant). The relationships between the qCSF and SVA, DVA, MP, and VF were also modeled using a linear regression model. Assumptions of normality of residuals and homoscedasticity were assessed using P-P plots and residual-predicted plots, respectively. Variation inflation factor values were less than 6, suggesting very low levels of multicollinearity.
The estimate of the variability of the qCSF measurements could be obtained by calculating the half-width of the 68.2% credible intervals (HWCI).34,35 Finally, to compare the variability of the measured sensitivities of our low vision population with that of a control population, HWCI for single qCSF run of the study population were compared with the HWCI width of individuals with normal vision measured using the same device, but at a different viewing distance (4 m instead of 1 m) (n = 17) (Lesmes LA, et al. IOVS 2016;57(12):ARVO E-Abstract 5161). Calculating the width of the credible interval of the posterior distribution pt(τ) from a single qCSF run is an alternative method to estimate the variability of the measured sensitivity (the 68.2% HWCI for a Gaussian distribution is equal to the standard deviation of the distribution). A 68.2% credible interval represents the shortest interval that contains the actual value with 68.2% probability.36 The HWCI is in units of decimal log sensitivity.
Results
Fifty-three (19 females) individuals from 21 countries participated in the study. The mean age of the participants was 25.79 ± 8.46 (range, 10–58 years).
Feasibility
SVA was quantifiable in 42 participants. Six participants had visual acuities of no light perception, and five had light perception only. Because the other visual function assessments were not measurable in these 11 participants, only participants with measurable static visual acuities were included in the remainder of the analysis. Descriptive statistics for all measures of visual function conducted are summarized in Table 1. The study population included individuals with a wide range of quantifiable static visual acuities (0.04–2.68 logMAR). Considering the highly heterogeneous nature of the population in the study, qCSF is a feasible tool for a low vision population as CSF was not measurable in only 24% of the study participants with measurable SVA.
Table 1.
Descriptive Statistics of the Visual Function Parameters Assessed in Individuals With Quantifiable Static Visual Acuities
| Characteristic | Quantifiable in (n) | Value | Range |
|---|---|---|---|
| Mean SVA (logMAR) | 42 | 1.51 ± 0.47 logMAR | 0.04–2.68 |
| Mean DVA (logMAR) | 35 (83%) | 1.67 ± 0.45 logMAR | 0.50–2.40 |
| Mean AULCSF | 32 (76%) | 0.41 ± 0.42 log CS | 0.03–1.90 |
| Mean PeakCS | 32 (76%) | 0.73 ± 0.33 log CS | 0.15–1.46 |
| Mean TMP | 26 (62%) | 47.8 ± 19.4% | 9.3–79.8 |
| Mean RMP | 25 (60%) | 48.7 ± 20.9% | 12.5–83.0 |
| Mean VF | 41 (98%) | 55.7 ± 26.7% | 1.7–100.0 |
Values are mean ± SD and unless otherwise noted. PeakCS, peak CS; TMP, TMP threshold; RMP, RMP threshold; VF, Esterman's functional VF scoring.
Of note, the study population also had a wide range of VF defects, varying from full VFs without defects (n = 1), to VF constrictions with central sparing, with the horizontal diameter of the VF varying from 165⁰ to 10⁰ (n = 30), peripheral field constrictions with central scotoma (n = 6), peripheral islands of vision (n = 2), peripheral constrictions with peripheral scotoma (n = 2), and ring scotomas (n = 1).
The 24% of athletes who had nonmeasurable AULCSF had poorer visual acuities and smaller VFs compared with the 76% of athletes with measurable AULCSF (Table 2). The types of VF defects were similar in both groups. Considering both visual acuity and VFs of athletes in both groups, visual acuity seems to be the factor affecting the feasibility of AULCSF measurement in our study group.
Table 2.
Descriptive Statistics of the Visual Acuity and VF in Individuals With Measurable AULCSF and Nonmeasurable AULCSF
| Characteristic | Measurable AULCSF (76%) | Nonmeasurable AULCSF (24%) |
|---|---|---|
| Mean SVA (min–max) | 1.39 ± 0.43 logMAR (0.04–2.20) | 1.90 ± 0.40 logMAR (1.48–2.68) |
| Mean VF (min–max) | 58.9 ± 26.4% (0.0–100.0) | 39.8 ± 28.3% (1.7–84.2) |
| Types of VF defects (n) | Peripheral islands of VF (2), central scotoma (6), peripheral constriction of VF (24) | Peripheral islands of VF (2), central scotoma (1), peripheral constriction of VF (7) |
Values are mean ± SD (range).
CS and Visual Functions
There are many parameters that can be used to describe the human CSF, such as peak sensitivity (peak CS), peak frequency, bandwidth, AULCSF, and low spatial frequency truncation.37 AULCSF is a simple, broad metric for the total gain in CS, including the gains in sensitivity as well as spatial frequencies and is calculated as the AULCSF curve, which quantifies the entire range of contrast visibility.29 The AULCSF has been used as a summary measure of spatial vision in the literature.29,38 It has also been reported that the predictive power and the test–retest precision of the qCSF assessment is better when AULCSF is used as a summary statistic instead of peak CS, using fractional rank precision analysis.28,39 To confirm that AULCSF was an appropriate measure to use for this analysis, the correlation between AULCSF and peak CS was checked and found to be r = 0.87; P < 0.001. Thus, for subsequent correlational analyses, the AULCSF was chosen for use as the measure used to describe CS.
There was a significant association between SVA and AULCSF (r = −0.79; P < 0.001), which shows that the AULCSF was significantly better in participants with better SVA. Linear regression analysis confirmed this significant association as SVA was found to a significant predictor of AULCSF (R2 = 0.76; P < 0.001) (Fig. 1).
Figure 1.
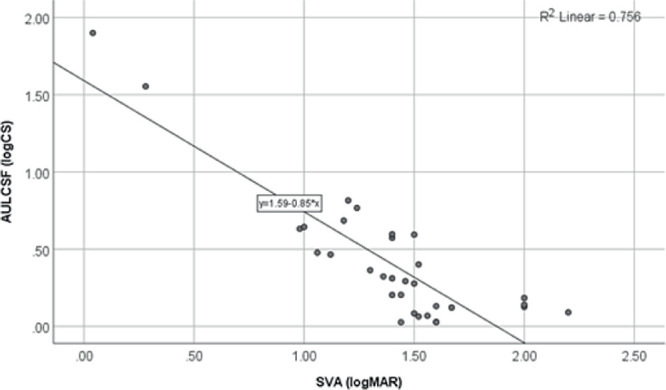
Scatterplot and linear regression line for the comparison of AULCSF and SVA.
Comparison of the AULCSF and DVA (Fig. 2), shows that DVA was also significantly associated with AULCSF (r = −0.63; P < 0.001) and that the AULCSF was significantly better in participants with a better DVA. Again, linear regression analysis confirmed this significant association as DVA was found to be a significant predictor of AULCSF (R2 = 0.51; P < 0.001).
Figure 2.
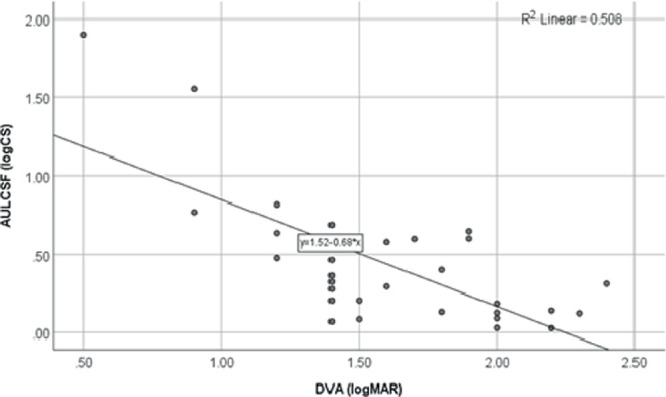
Scatterplot and linear regression line for the comparison of AULCSF and DVA.
Figure 3 shows the relationship between CS and TMP thresholds. TMP thresholds were not associated with AULCSF as demonstrated by the nonsignificant correlation coefficient (r = −0.16; P = 0.465). This finding was confirmed with the linear regression analysis (R2 = 0.18; P = 0.163).
Figure 3.
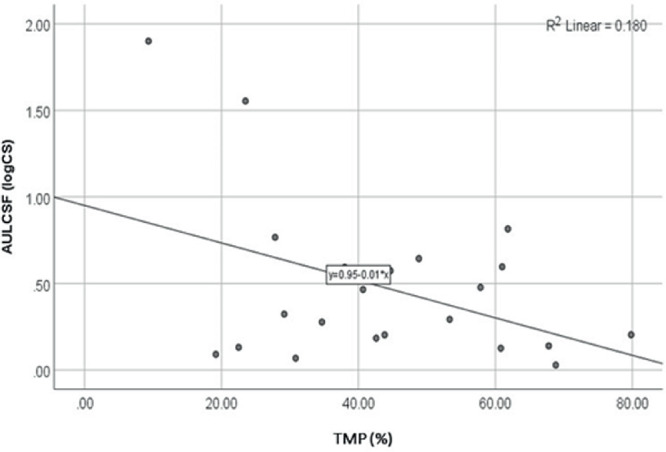
Scatterplot and linear regression line for the comparison of AULCSF and TMP thresholds.
The relationship between CS and RMP thresholds is shown in Figure 4. RMP thresholds were also nonsignificantly associated with AULCSF (r = −0.33; P = 0.138), which was confirmed with linear regression analysis (R2 = 0.16; P = 0.141).
Figure 4.
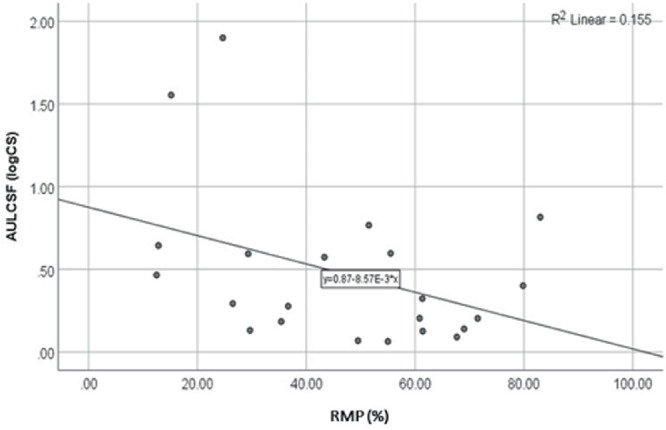
Scatterplot and linear regression line for the comparison of AULCSF and RMP thresholds.
Figure 5 shows the relationship between CS and Esterman's VF scoring. The AULCSF was not significantly correlated with the percentages of Esterman's VF scores of participants (r = 0.15; P = 0.415). Linear regression analysis confirmed this finding (R2 = 0.001; P = 0.896).
Figure 5.
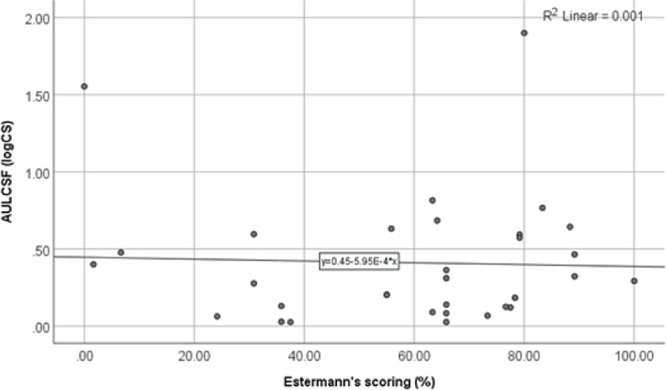
Scatterplot and linear regression line for the comparison of AULCSF and Esterman's VF scoring.
Finally, a multivariate linear regression analysis was performed with AULCSF as the dependent variable and SVA, DVA, and age as independent variables. TMP, RMP, and VF were not included in the analysis because they were not significantly correlated with AULCSF. Based on this analysis, SVA was a significant predictor for AULCSF, F(3,26) = 34.98; P < .001; R2 = .76 (76% of the variability accounted for by the variable). DVA and age were found not to be significant predictors of AULCSF based on the multivariate linear regression analysis.
The correlations between peak CS and other visual functions were similar to the correlations between AULCSF and the visual functions. There was a significant association between SVA and peak CS (r = −0.68; P < 0.001) and DVA and peak CS (r = −0.46; P = 0.01). The AULCSF was not significantly correlated with the TMP thresholds (r = −0.20; P = 0.371) or the percentages of Esterman's VF scores of participants (r = 0.26; P = 0.149). However, the RMP thresholds were significantly associated with the Peak CS (r = −0.44; P = 0.040).
Comparison of HWCIs
We compared the HWCI of the athletes with the HWCI of individuals with normal vision measured using the same device (All the HWCI and AULCSF values in this article were adjusted for the change in viewing distance, because of which the control data have a very different numeric range from all other qCSF publications), but at a different viewing distance (4 m instead of 1 m) (n = 17). Figure 6 presents the 68.2% HWCI of the CSF measured by the qCSF procedure in athletes (n = 32; mean, 0.15 ± 0.10; range, 0.03–0.46) and controls (n = 17; mean, 0.04 ± 0.02; range, 0.02–0.09). The distribution and mean value of the athletes was significantly different compared with that of the controls (independent sample t-test, F = 21.48; t = 4.32; df = 47; P = 0.000). However, the distributions were not significantly different when the control population was compared with athletes who had greater than 0.6 AULCSF (n = 8; mean, 0.06 ± 0.01; range, 0.03–0.07; F = 0.695; t = 1.97; df = 23; P = 0.061). The AULCSF of the controls was higher compared with that of athletes (2.42 ± 0.21 log CS; range, 2.08–2.68 log CS).
Figure 6.
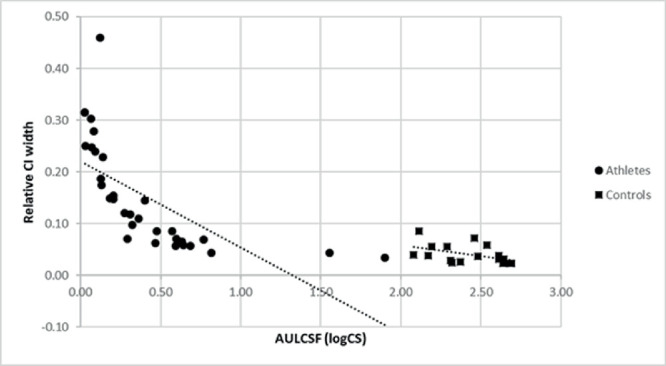
Scatterplot for the 68.2% HWCI of the CSF measured by the qCSF procedure in athletes (circle) and controls (square). The relative CI widths plotted were obtained by dividing the CI width by the AULCSF.
Discussion
This study investigated the feasibility of measuring the CS in individuals with varying levels of visual impairment, including a large number of individuals (95% of the population) with moderate to profound low vision using the qCSF. Using this method, CSF could be measured across the spatial frequency range of 0.35 to 9 cpd in 32 out of the 42 participants (76%) who had quantifiable SVAs. Thus, the qCSF can be used to measure CSF in a low vision population with severe and profound visual impairments, although measuring the AULCSF is more difficult in individuals with severe to profound visual impairments compared with individuals with moderate to severe vision impairments. The feasibility of the other tests conducted was also good, ranging from 65% for radial global MP to 98% for VFs.
CS is measured in terms of the ability to discern luminous differences between adjacent areas and measuring the CSF can help to explain an individual's ability to perceive objects of various sizes and shades. It can also explain subtle changes in functional vision that other static measures cannot.4,8,9,15,40–42 Because CSF has the potential to provide more information about the functional vision of an individual, it should be included as a part of routine eye examinations. To facilitate the inclusion of CSF measurements in routine eye exams, this study also examined the relationships between CSF (AULCSF as the summary statistic) with other visual functions in individuals with low vision using nonparametric correlations and linear regressions.
The high spatial frequency cut-off of the CSF can be indicative of the SVA of an individual. Even though SVA has been shown to be a poor predictor of an individual's CS at middle and low spatial frequencies, we found a strong significant correlation (r = −0.79; P < 0.001) between SVA and AULCSF. This result is similar to the strong correlations previously reported using the qCSF method. Lesmes et al. (2012) reported a significant correlation between CSF acuity obtained using qCSF and SVA (r = −0.69) and nonsignificant correlations between Pelli-Robson CS and SVA (r = −0.14). It is interesting to note that, when a Pelli-Robson chart is used for measurement, the magnitudes of association between SVA and CS vary from 0.27 to 0.79.43,44 Similar variations have been reported in studies using Vistech charts (correlations ranging from 0.25 to 0.88).45,46 Previous research has demonstrated that the strongest correlations between SVA and CS are usually found in heterogeneous populations.47 This study is the first study completed to our knowledge that looks at the relationship between binocular AULCSF and SVA in a broad population that includes individuals with moderate to severe visual impairment.
An individual's daily life involves activities that require the visualization of a target in the presence of relative motion between the stimulus and observer, yet all routine visual assessments are static. DVA is measured based on the ability of the visual system to track and identify the smallest optotypes moving randomly and is more predictive of real-world task performance than the static measures of vision.7,23,24 Previous research reported extremely low, and often nonsignificant correlations between CS and DVA in a homogenous population of individuals with binocular SVA better than or equal to 6/12.48 In our study, the correlation between AULCSF and DVA (r = −0.657; P < 0.001) was significant, unlike Long and May (1992).48 The population examined in this study was highly heterogeneous, which may explain the significant correlation found here between CS and DVA.47,48
In this study, motion coherence thresholds for both TMP and RMP were not significantly associated with AULCSF. This finding is in agreement with the previous literature on translational motion coherence thresholds measured with high-contrast RDKs compared with contrast thresholds obtained with same stimuli.49 Although radial motion coherence thresholds have not been compared with AULCSF previously, RMP is driven by similar mechanisms as TMP; therefore, it would have been unlikely that radial motion coherence thresholds would have had a different relationship with CS than translational motion coherence thresholds.
Binocular Esterman's VF scoring and AULCSF did not demonstrate a significant association in this study, suggesting that functional VF may have a weak relationship with CS. This finding does not agree with the previously reported significant correlations between monocular Humphrey mean depression scores and Pelli-Robson CS.50–52 Although there are no reports on the relationship between CSF and functional VF scoring, there are many descriptions of how CS varies over the VF. For all spatial frequencies, previous authors have reported the highest CS in the fovea and that CS decreases progressively with eccentricity.53–56 The majority of participants (n = 24) with measurable AULCSF in this study had peripheral field losses and preserved central VF, which means that this population is relatively homogenous, and the homogeneity of this population may account for the lower correlation between functional VF scoring and CS. That being said, the findings of this study suggest that the relationship between VF and CS may depend on how VF is quantified (functional Esterman scoring vs. mean depression on a Humphrey VF). The current study was not designed to investigate the relationships between different types of field defects or different VF measures; therefore, more research is needed to be able to make conclusions about the relationship between functional VF scoring and the CSF summary statistic.
Because it is uncommon to repeat the same measurement multiple times in clinical practice, the credible interval of the posterior distribution is a valuable tool to gauge the precision of a test in a single run. Comparison of the 68.2% half width credible intervals of the AULCSF of participants (with a wide range of low vision) with that of controls suggested that, even though the distributions were significantly different, there was a systematic decrease of means and the range of HWCI with an increase in AULCSF. Even though the mean AULCSFs of both were different by about 2.00 log CS, the log CS values of HWCIs were around 0.1, and this decreased to an insignificant level when the analysis included only athletes above 0.6 logCS. This finding suggests that, even though the viewing distance was decreased for participants with low vision, the qCSF measurements obtained are valid. In addition, the intrinsic relative variability seems to be no worse for those with low-vision compared with controls, except for those with extremely poor vision.
This study was unique in that it examined the relationship between CS and other visual functions that have been suggested to be essential for functional vision in a heterogeneous population of individuals with visual impairment, including individuals with moderate to severe vision loss. Because this was the first study of its kind, supporting research is needed to further examine and understand some of the findings of this study, for example, the nonsignificant relationship of CSF with functional VF scoring.
Conclusions
The qCSF is a feasible tool to measure CSF in low vision populations with moderate to profound visual impairments. Consistent with previous literature and the study hypotheses, the AULCSF (our summary statistic) was significantly associated with SVA and not significantly associated with motion coherence thresholds. In this study, DVA was found to be significantly associated with the AULCSF, which was contrary to the previous literature. Finally, the AULCSF did not demonstrate a significant relationship with VF scoring, which was contrary to the previous literature. Further studies with larger sample sizes in similar heterogeneous populations are needed to clearly understand the relationships between CS and other visual functions predictive of functional vision. Using the qCSF method in these studies would be recommended, because the qCSF is the first assessment method to have demonstrated the capacity for accurately measuring CS in patients with both moderate and severe vision loss.
Acknowledgments
The Adaptive Sensory Technology (AST) group provided the CS test used. The authors thank World Para Snow Sport for their help conducting these studies, AST, and Dr. Michael Dorr (CTO, Adaptive Sensory Technology) for providing us with the test equipment and assistance with some aspects of data analysis.
Supported by an Agitos Foundation grant in collaboration with World Para Snow Sport and the International Paralympic Committee.
Disclosure: A. Stalin, None; K. Dalton, None
References
- 1. Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982; 94: 91–96. [PubMed] [Google Scholar]
- 2. Jindra LF, Zemon V. Contrast sensitivity testing: a more complete assessment of vision. J Cataract Refract Surg. 1989; 15: 141–148. doi: 10.1016/S0886-3350(89)80002-1 [DOI] [PubMed] [Google Scholar]
- 3. Hess RF, Howell ER.. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 1977; 17: 1049–1055. doi: 10.1016/0042-6989(77)90009-8 [DOI] [PubMed] [Google Scholar]
- 4. Onal S, Yenice O, Cakir S, Temel A. FACT contrast sensitivity as a diagnostic tool in glaucoma. Int Ophthalmol. 2008; 28: 407–412. doi: 10.1007/s10792-007-9169-z [DOI] [PubMed] [Google Scholar]
- 5. Owsley C, McGwin G. Vision and driving. Vision Res. 2010; 50: 2348–2361. doi: 10.1016/j.visres.2010.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. West SK, Rubin GS, Broman AT, Muñoz B, Bandeen-Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol. 2002; 120: 774–780. doi: 10.1097/00132578-200210000-00023 [DOI] [PubMed] [Google Scholar]
- 7. Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of “real-world” targets. Br J Ophthalmol. 1987; 71: 791–796. doi: 10.1136/bjo.71.10.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pelli DG, Bex P. Measuring contrast sensitivity. Vision Res. 2013; 90: 10–14. doi: 10.1016/j.visres.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thayaparan K, Crossland MD, Rubin GS. Clinical assessment of two new contrast sensitivity charts. Br J Ophthalmol. 2007; 91: 749–752. doi: 10.1136/bjo.2006.109280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedman RD, Thibos LN.. Contrast sensitivity in humans with abnormal visual experience. J Physiol. 1975; 247: 687–710. doi: 10.1113/jphysiol.1975.sp010952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrahamsson M, Sjostrand J.. Impairment of contrast sensitivity function (CSF) as a measure of disability glare. Investig Ophthalmol Vis Sci. 1986; 27: 1131–1136. [PubMed] [Google Scholar]
- 12. Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR. Visual dysfunction in Parkinson's disease. Brain. 2016; 139: 2827–2843. doi: 10.1093/brain/aww175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson TL, Ong GL, Ripley LG. Orientational contrast sensitivity and chromatic contrast thresholds in multiple sclerosis. Am J Ophthalmol. 2004; 137: 283–286. doi: 10.1016/j.ajo.2003.08.032 [DOI] [PubMed] [Google Scholar]
- 14. Kelly DH, Savoie RE.. A study of sine-wave contrast sensitivity by two psychophysical methods. Percept Psychophys. 1973; 14: 313–318. doi: 10.3758/BF03212397 [DOI] [Google Scholar]
- 15. Pesudovs K, Hazel CA, Doran RML, Elliott DB. The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research. Br J Ophthalmol. 2004; 88: 11–16. doi: 10.1136/bjo.88.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lesmes LA, Lu Z-L, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. J Vis. 2010; 10: 1–21. doi: 10.1167/10.3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou F, Lesmes L, Bex P, Dorr M, Lu Z-L. Using 10AFC to further improve the efficiency of the quick CSF method. J Vis. 2015; 15: 2. doi: 10.1167/15.9.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hou F, Huang CB, Lesmes L, et al.. qCSF in clinical application: efficient characterization and classification of contrast sensitivity functions in amblyopia. Investig Ophthalmol Vis Sci. 2010; 51: 5365–5377. doi: 10.1167/iovs.10-5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou F, Lesmes L, Kim W, et al.. Evaluating the performance of the quick CSF method in detecting CSF changes: an assay calibration study. J Vis. 2016; 16: 1–19. doi: 10.1167/16.6.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung STL, Legge GE.. Comparing the shape of contrast sensitivity functions for normal and low vision. Investig Ophthalmol Vis Sci. 2016; 57: 198–207. doi: 10.1167/iovs.15-18084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bourne RRA, Flaxman SR, Braithwaite T, et al.. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Heal. 2017; 5: e888–e897. doi: 10.1016/S2214-109X(17)30293-0 [DOI] [PubMed] [Google Scholar]
- 22. Committee on Vision National Research Council. Emergent techniques for the assessment of visual performance. Washington, DC: National Academy Press; 1985: 66. [PubMed] [Google Scholar]
- 23. Burg A. Vision and driving: a report on research. Hum Factors J Hum Factors Ergon Soc. 1971; 13: 79–87. doi: 10.1177/001872087101300110 [DOI] [PubMed] [Google Scholar]
- 24. deKlerk LFW, Eernest JT, Hoogerheide J. The dynamic visual acuity of 30 selected pilots. Aeromed Acta. 1964; 9: 129–136. [PubMed] [Google Scholar]
- 25. Muiños M, Ballesteros S.. Sports can protect dynamic visual acuity from aging: a study with young and older judo and karate martial arts athletes. Atten Percept Psychophys. 2015; 77: 2061–2073. doi: 10.3758/s13414-015-0901-x [DOI] [PubMed] [Google Scholar]
- 26. Bailey IL, Jackson AJ, Minto H, Greer RB, Chu MA. The Berkeley rudimentary vision test. Optom Vis Sci. 2012; 89: 1257–1264. doi: 10.1097/OPX.0b013e318264e85a [DOI] [PubMed] [Google Scholar]
- 27. Hirano M, Hutchings N, Simpson T, Dalton K. Validity and repeatability of a novel dynamic visual acuity system. Optom Vis Sci. 2017; 94: 616–625. doi: 10.1097/OPX.0000000000001065 [DOI] [PubMed] [Google Scholar]
- 28. Dorr M, Lesmes LA, Elze T, Wang H, Lu Z-L, Bex PJ. Evaluation of the precision of contrast sensitivity function assessment on a tablet device. Sci Rep. 2017; 7: 46706. doi: 10.1038/srep46706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Applegate RA, Howland HC, Sharp RP, Cottingham AJ, Yee RW. Corneal aberrations and visual performance after radial keratotomy. J Refract Surg. 1998; 14: 397–407. doi: 10.3928/1081-597X-19980701-05 [DOI] [PubMed] [Google Scholar]
- 30. Grosvendor T. Primary care optometry. 5th ed. Hong Kong: Butterworth Heinemann Elsevier; 2007. [Google Scholar]
- 31. Esterman B. Functional scoring of the binocular field. Ophthalmology. 1982; 89: 1226–1234. doi: 10.1016/S0161-6420(82)34647-3 [DOI] [PubMed] [Google Scholar]
- 32. Chisholm C. Visual requirements for driving. Optom Today. 2008; 48: 40–44. [Google Scholar]
- 33. Shapiro SS, Wilk MB.. An analysis of variance test for normality (complete samples). Biometrika. 1965; 52(3/4): 591. doi: 10.2307/2333709 [DOI] [Google Scholar]
- 34. Dewey M, Clayton D, Hills M. Statistical models in epidemiology. J R Stat Soc Ser A (Statistics Soc). 1995; 157: 441–55. doi: 10.2307/2983301 [DOI] [Google Scholar]
- 35. Zheng H, Wang C, Cui R, et al.. Measuring the contrast sensitivity function using the qCSF method with 10 digits. Transl Vis Sci Technol. 2018; 7: 9. doi: 10.1167/tvst.7.6.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hughes AO. Statistical models in epidemiology. David Clayton and Michael Hills, Oxford University Press, Oxford, 1993. No. of Pages: vii + 367. Price: £30. ISBN: 0-19-852221-5. Stat Med. 1995; 14: 104–105. doi: 10.1002/sim.4780140113 [DOI] [Google Scholar]
- 37. Watson AB, Ahumada AJ.. A standard model for foveal detection of spatial contrast. J Vis. 2005; 5: 6. doi: 10.1167/5.9.6 [DOI] [PubMed] [Google Scholar]
- 38. Oshika T, Okamoto C, Samejima T, Tokunaga T, Miyata K. Contrast sensitivity function and ocular higher-order wavefront aberrations in normal human eyes. Ophthalmology. 2006; 113: 1807–1812. doi: 10.1016/j.ophtha.2006.03.061 [DOI] [PubMed] [Google Scholar]
- 39. Dorr M, Elze T, Wang H, Lu ZL, Bex PJ, Lesmes LA. New precision metrics for contrast sensitivity testing. IEEE J Biomed Heal Informatics. 2018; 22: 919–925. doi: 10.1109/JBHI.2017.2708745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Owsley C. Aging and vision. Vision Res. 2011; 51: 1610–1622. doi: 10.1016/j.visres.2010.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ridder A, Müller MLTM, Kotagal V, Frey KA, Albin RL, Bohnen NI. Impaired contrast sensitivity is associated with more severe cognitive impairment in Parkinson disease. Park Relat Disord. 2017; 34: 15–19. doi: 10.1016/j.parkreldis.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lalanne L, Ferrand-Devouge E, Kirchherr S, et al.. Impaired contrast sensitivity at low spatial frequency in cannabis users with early onset. Eur Neuropsychopharmacol. November 2017; 27: 1289–1297. doi: 10.1016/j.euroneuro.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 43. Elliott DB, Hurst MA.. Simple clinical techniques to evaluate visual function in patients with early cataract. Optom Vis Sci. 1990; 67: 822–825. doi: 10.1097/00006324-199011000-00006 [DOI] [PubMed] [Google Scholar]
- 44. Williamson TH, Strong NP, Sparrow J, Aggarwal RK, Harrad R. Contrast sensitivity and glare in cataract using the Pelli-Robson chart. Br J Ophthalmol. 1992; 76: 719–722. doi: 10.1136/bjo.76.12.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greene HA, Madden DJ. Adult age differences in visual acuity, stereopsis, and contrast sensitivity. Am J Optom Physiol Opt. 1987; 64: 749–753. http://www.ncbi.nlm.nih.gov/pubmed/3688177. [DOI] [PubMed] [Google Scholar]
- 46. Brown B, Lovie-Kitchin JE.. High and low contrast acuity and clinical contrast sensitivity tested in a normal population. Optom Vis Sci. 1989; 66: 467–473. doi: 10.1097/00006324-198907000-00010 [DOI] [PubMed] [Google Scholar]
- 47. Haegerstrom-Portnoy G, Schneck ME, Lott LA, Brabyn JA. The relation between visual acuity and other spatial vision Measures. Optom Vis Sci. 2000; 77: 653–662. doi: 10.1097/00006324-200012000-00012 [DOI] [PubMed] [Google Scholar]
- 48. Long GM, May PA.. Dynamic visual acuity and contrast sensitivity for static and flickered gratings in a college sample. Optom Vis Sci. 1992; 69: 915–922. doi: 10.1097/00006324-199212000-00001 [DOI] [PubMed] [Google Scholar]
- 49. Chakraborty A, Anstice NS, Jacobs RJ, et al.. Global motion perception is independent from contrast sensitivity for coherent motion direction discrimination and visual acuity in 4.5-year-old children. Vision Res. 2015; 115: 83–91. doi: 10.1016/j.visres.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. J Glaucoma. 2003; 12: 134–138. doi: 10.1097/00061198-200304000-00008 [DOI] [PubMed] [Google Scholar]
- 51. Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. J Glaucoma. 2003; 12: 134–138. doi: 10.1097/00061198-200304000-00008 [DOI] [PubMed] [Google Scholar]
- 52. Wilensky JT, Hawkins A.. Comparison of contrast sensitivity visual acuity, and Humphrey visual field testing in patients with glaucoma. Trans Am Ophthalmol Soc. 2001; 99: 213–218. [PMC free article] [PubMed] [Google Scholar]
- 53. Rijsdijk JP, Kroon JN, van der Wildt GJ. Contrast sensitivity as a function of position on the retina. Vision Res. 1980; 20: 235–241. doi: 10.1016/0042-6989(80)90108-X [DOI] [PubMed] [Google Scholar]
- 54. Regan D, Beverley KI.. Visual fields described by contrast sensitivity, by acuity, and by relative sensitivity to different orientations. Invest Ophthalmol Vis Sci. 1983; 24: 754–759. http://www.ncbi.nlm.nih.gov.ezproxy.bu.edu/pubmed/6853102. [PubMed] [Google Scholar]
- 55. Rosén R, Lundström L, Venkataraman AP, Winter S, Unsbo P. Quick contrast sensitivity measurements in the periphery. J Vis. 2014; 14: 3. doi: 10.1167/14.8.3 [DOI] [PubMed] [Google Scholar]
- 56. Bambo MP, Ferrandez B, Güerri N, et al.. Evaluation of contrast sensitivity, chromatic vision, and reading ability in patients with primary open angle glaucoma. J Ophthalmol. 2016; 2016; 7074016. doi: 10.1155/2016/7074016 [DOI] [PMC free article] [PubMed] [Google Scholar]


