Abstract
Purpose
The purpose of this study was to investigate the two-dimensional peripheral refraction in fellow eyes of patients with isomyopia and anisomyopia.
Methods
Sixty-eight young adults were recruited, including 25 isomyopes with interocular differences (IODs) of foveal refraction < 1.00 D and 43 anisomyopes with IOD > 1.50 D. Peripheral refraction across an area of the visual field of 60° × 36° with a resolution of 1° was measured using a custom-made Hartmann-Shack wavefront sensor. The retinal area was divided into 3 × 3 zones for comparison between the fellow eyes.
Results
There was no difference of refraction in all corresponding zones between the fellow eyes in the isomyopic group (all P > 0.05). The IODs between more myopic (MM) eyes and less myopic (LM) eyes in the anisomyopic group ranged from −1.40 to approximately −2.46 D (all P <0.001), which was flagged in the center and attenuated in peripheral zones by varied magnitudes. In the stratification analysis for different levels of anisomyopia, the nasal retina first presented significant relative hyperopic shifts compared to the center, followed by the temporal retina. In contrast, the superior and inferior periphery only differed from the center when the central IOD was greater than 3.00 D.
Conclusions
The two-dimensional peripheral refraction patterns showed a mirror symmetry between the fellow eyes of a patient with isomyopia. However, in the anisomyopic group, peripheral refraction showed significantly relative hyperopic shift when compared with the center and developed with a varied rate in different areas. These findings may indicate an asymmetrical variation in the peripheral refraction patterns during myopia progression.
Keywords: myopia, anisomyopia, peripheral refraction, optics
Peripheral defocus has been suggested by numerous studies to play a role during myopia onset and progression. For instance, ocular growth accelerates (i.e. myopia development and progression) if the focal point shifts behind the retina by the minus lens, and slows down if the focal point shifts in front of the retina by the plus lens in different species, such as chickens,1 tree shrews,2 guinea pigs,3 and rhesus monkeys.4 The effects of optical defocus are mediated by mechanisms that integrate visual signals in a locally selective manner.5–7 In primates particularly, studies show that the peripheral retina is able to modulate ocular growth regardless of whether the central retina is intact8 or impaired by laser photoablation.9 Several optical devices claiming to manipulate peripheral focal properties, including spectacles10 and contact lenses,11,12 were reported to show some success in inhibiting the progression of human myopia, albeit with different levels. However, the causal effect of peripheral defocus on myopia progression has been challenged by evidence present in humans. Specifically, a longitudinal study of different ethnicities found that relative peripheral hyperopia exerted little consistent influence on the risk of onset myopia and future myopia progression.13 The baseline relative peripheral refraction (RPR) and the changes in RPR, at least in the horizontal meridian, were unable to predict the development and progression of myopia in children.14,15 These findings suggest that peripheral optics are more likely a consequence, rather than the cause of myopia development. Thus, the actual role of peripheral defocus in human myopia still needs further investigation.
Obviously, a prerequisite step in answering this critical question is to depict the change of peripheral defocus with myopia progression in a detailed and comprehensive manner, and actually there were several studies aimed at answering this question. Along the horizontal visual field, most of the studies agreed that the hyperopic and emmetropic eyes have relative myopic peripheral refractions, whereas myopic eyes were relative hyperopic in peripheral areas.16–19 Studies also reported a strong correlation between peripheral refraction and retinal steepness20,21 the myopic eyes with hyperopic RPR have more prolate/less oblate retinal shapes. Recently, several studies have demonstrated that the myopia eye growth were not simply an axial elongation, but also accompanied by irregular changes especially in high myopia, which indicated that the peripheral refraction may also change in a regional way. However, the RPR investigations along vertical or oblique meridians were much fewer and hardly able to draw a consistent conclusion.22–24 In addition, previous studies were usually restricted with only several measurement points on the meridians with at least 5° to 10° intervals, due to the limitation of measuring range and intensity achieved by available techniques, and, therefore, were difficult to provide a full picture of peripheral refraction profile. Recently, we adopted a tailor-made fast scanning peripheral wavefront sensor,25 and successfully provided a high resolution 2-dimentional (2D) peripheral refraction profile in emmetropic children.26 This technique was able to measure the peripheral refraction covering a visual field of 60° × 36° (horizontal × vertical), every 1° in a relatively short time duration, which offered a useful approach to investigate the peripheral refraction profile with unprecedented detail.
Previous studies were mostly cross-sectional, probably for reasons of time efficiency. Given that the fellow eye of an individual shares an identical genetic background and environmental exposure, the comparison of possible biometric traits between the fellow eye of an individual is a convenient and efficient approach to avoid unknown interindividual confounding variables.27,28 In this context, anisometropia (dissimilar refraction in fellow eyes) provides an ideal model to investigate the role of peripheral defocus during myopia progression by comparing the different peripheral refractive error patterns in subjects with different levels of anisometropia.
The purpose of this study was to investigate the peripheral refraction in the fellow eyes of a group of anisomyopic adults, along with another group of isomyopic adults that served as control.
Methods
Subjects
The study was approved by the Committee of Research Ethics of the Aier School of Ophthalmology, Central South University (ID: AIER 20191RB16), and all examinations involved and the data collection procedures followed the tenets of the Declaration of Helsinki. All participants signed the written informed consent form after an explanation of the nature and possible consequences of the study. Students with myopia in both eyes from Central South University were invited to participate in the study. All participants were required to have a best-corrected visual acuity of 20/20 or better at distance for each eye. Participants with astigmatism > 2.00 D, a history of ocular diseases, refractive surgeries, and those who wear contact lenses were excluded from the study. A total of 68 participants ranging from 19 to 38 years of age were recruited and completed the necessary measurements.
Measurement's Procedure
Peripheral refraction was measured using an open-view Hartmann-Shack wavefront sensor (Voptica Peripheral Refractor, [VPR]; Voptica SL, Murcia, Spain). The details of the VPR instrument and the measurement process were previously published.25,26 In brief, the instrument measures the wavefront aberration at the central 60° horizontal visual field every 1° in 1.3 seconds for each scan, from series of 61 Hartmann-Shack images. To obtain the images at different vertical locations, participants were asked to fixate on 10 different targets placed vertically 2.5 meters away. The vertical range measurement was limited to 20° on the superior side of the retina to 16° on the inferior side of the retina, within intervals of 4° due to the limitation of ocular vertical mobility. Both eyes of each participant were measured in a dark room, and the fellow eye was covered during each trial. The obtained images were analyzed within a pupil diameter of 4 mm to estimate the aberrations that were expressed as a Zernike polynomial expansion. For each retinal location, the mean of the four measurements was obtained and the refraction was calculated from the second order terms, with the expression form of spherical equivalent refractive error (SER; spherical power + 1/2 cylindrical power). Although the data of the high and low order aberrations was given, only the mean of the SER was considered for the current analysis.
Data Analysis and Statistics
Two-dimensional (2D) maps of every single eye were obtained from the results of 10 horizontal sections (610 points in the retina) using customized scripts in MATLAB (MathWorks, Natick, MA, USA). For the maps of each eye, a positive value indicated the nasal side, and a negative value indicated the temporal side in abscissas. In the ordinates, a positive value indicated the location of the superior retina, and a negative value indicated the location of the inferior retina. The center refraction of equatorial (parallel 0°) measurements was defined as foveal refraction, and this was adopted to help divide participants into the anisomyopic and isomyopic groups. The RPR maps were generated by subtracting the center value at the fovea. Maps of interocular refractive differences (IODs) were generated using each point of SER minus the fellow eye's corresponding point. The IOD of the foveal refraction was used to further divide the anisomyopic participants into different levels.
In order to describe regional characteristics, each 2D map was divided into 3 × 3 zones.26 The dividing points were set on superior 5.5°, inferior 5.5°, temporal 10.5°, and nasal 10.5°. The average value was calculated for each specific zone. In addition, the data around the optic nerve head (horizon: nasal 13.5° to 21.5°, vertical: superior 5.5° to inferior 3.5°) was excluded from the analysis.
A paired t-test was used to make zone-to-zone comparisons between the peripheral and central zones, or the corresponding zones between the two eyes. One-way analysis of variance (ANOVA) analysis was used to compare the interocular differences among categories in the isomyopic group was by using SPSS commercial software (version 20.00; IBM, Armonk, NY). A two-tailed P < 0.05 was set as a statistically significant level.
Results
According to the IOD of the foveal refraction, 25 participants were isomyopes (IOD < 1.00 D), and 43 were anisomyopes (IOD > 1.50 D). Therefore, the isomyopic group had a mean foveal refraction of −2.53 ± 2.20 D (range = −0.02 to approximately −8.40) in both eyes, and an average IOD of 0.33 ± 0.25 D. In contrast, the anisomyopic group had a mean foveal refraction of −4.03 ± 2.61 D (range = 0.16 to approximately −9.00) in both eyes, and an average IOD of 2.93 ± 1.07 D. The demographic information of the participants is shown in Table 1.
Table 1.
Demographic Information of Participants in the Study
| Isomyopes | Anisomyopes | |||
|---|---|---|---|---|
| OD | OS | MM | LM | |
| No. participants (eyes) | 25 (50) | 43 (86) | ||
| Sex, no. participants (eyes) | ||||
| Male | 6 (12) | 9 (18) | ||
| Female | 19 (38) | 34 (69) | ||
| Age, y, mean ± SD [range] | 25.64 ± 4.64 [20, 38] | 24.35 ± 3.82 [19, 33] | ||
| Foveal SER, D, mean ± SD [range] | −2.56 ± 2.16 [−7.95, −0.18] | −2.51 ± 2.28 [−8.40, −0.02] | −5.50 ± 2.19 [−9.00, −1.64] | −2.56 ± 2.15 [−6.89, 0.16] |
D = diopters; SER = spherical equivalent refraction; MM = more myopic eyes; LM = less myopic eyes
.
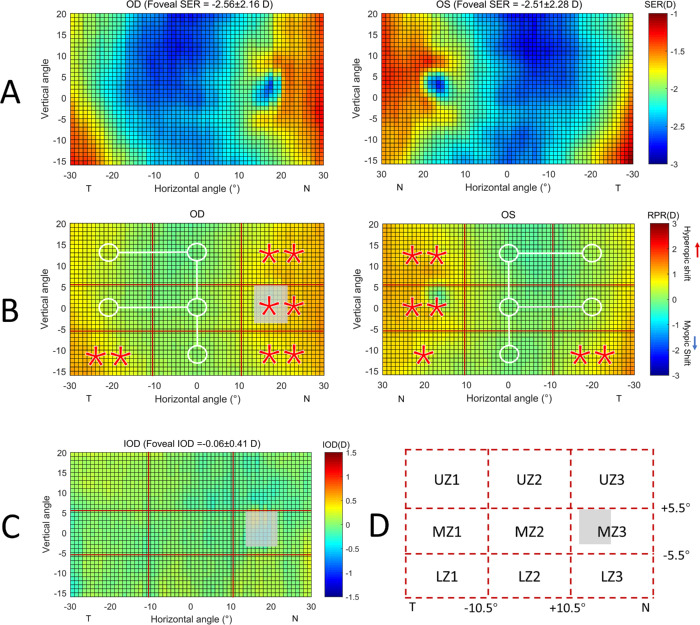
Figure 1A shows the average SER of both eyes across the visual field for the isomyopic participants. To facilitate a detailed comparison, the SER of each retinal location was subtracted by the foveal SER to obtain the RPR map (Fig. 1B), which was divided into 3 × 3 zones, as illustrated in Figure 1D. The mean SER value of each zone is shown in Table 2. Compared to the central zone (MZ2), the nasal and temporal retina demonstrated different levels of hyperopic shifting, especially for UZ3, MZ3, LZ3, and LZ1 (all P < 0.05). Nevertheless, the mean SER of the central superior and inferior retina were very similar to the center (OD:superior −2.51 ± 1.91 D / inferior −2.39 ± 2.07 D versus center −2.40 ± 2.07 D, P = 0.346 / 0.871; and OS:−2.48 ± 2.01 D / −2.42 ± 2.20 D versus −2.36 ± 2.23 D, P=0.254 / 0.274). In addition, the IOD for each zone was found to be very close to zero (Table 2; all P > 0.05) and there was no significant difference across all nine zones (F = 0.353, P = 0.944), suggesting the RPR pattern between the fellow eyes are mirror-symmetrical (Fig. 1C).
Figure 1.
(A) Averaged 2D maps of SER in the isomyopic group. Values in the x-axis indicate the nasal retina (“N”) and the temporal retina (“T”), respectively. For the y-axis, positive values represent the superior retina and negative values represent the inferior retina. The color-code is in diopters. (B) Statistical analysis of the changes of relative peripheral refraction (RPR) in eight zones with respect to the center. A zone with a white circle represents no statistical difference with the center, whereas a red star represents a statistically significant hyperopic difference (*P < 0.05, **P < 0.01). (C) Averaged 2D map of the interocular differences (IODs) generated by SER-maps of right eyes minus the corresponding points on left eyes. No significant differences among the nine zones were observed (F = 0.535, P = 0.829). (D) Abbreviations were used to represent the different zones: L: low, M: middle, and U: up. The grey area in each map represents the optics disc area, which were not included in the analysis.
Table 2.
Comparison of Spherical Equivalent Refraction (D; mean ± SD) in Nine Zones Between Eyes
| Isomyopes (n = 25) | Anisomyopes (n = 43) | |||||||
|---|---|---|---|---|---|---|---|---|
| Zones | OD | OS | d (OD-OS) | P Value | MM | LM | d (MM-LM) | P Value |
| UZ1 | −2.21 ± 1.86 | −2.29 ± 1.9 | +0.08 ± 0.47 | 0.41 | −3.81 ± 2.18 | −2.15 ± 1.99 | −1.67 ± 1.03 | <0.001 |
| UZ2 | −2.51 ± 1.91 | −2.48 ± 2.01 | −0.03 ± 0.54 | 0.76 | −4.79 ± 2.07 | −2.40 ± 2.13 | −2.40 ± 0.98 | <0.001 |
| UZ3 | −1.71 ± 1.39 | −1.64 ± 1.52 | −0.07 ± 0.45 | 0.45 | −3.45 ± 2.13 | −2.05 ± 2.38 | −1.40 ± 0.99 | <0.001 |
| MZ1 | −2.19 ± 1.93 | −2.17 ± 2.09 | −0.02 ± 0.43 | 0.83 | −4.08 ± 2.13 | −2.22 ± 1.94 | −1.86 ± 1.02 | <0.001 |
| MZ2 | −2.40 ± 2.07 | −2.36 ± 2.23 | −0.04 ± 0.42 | 0.68 | −5.25 ± 2.16 | −2.46 ± 2.10 | −2.79 ± 0.93 | <0.001 |
| MZ3 | −1.59 ± 1.55 | −1.61 ± 1.67 | +0.02 ± 0.45 | 0.84 | −3.86 ± 2.34 | −2.19 ± 2.24 | −1.67 ± 0.93 | <0.001 |
| LZ1 | −1.89 ± 1.89 | −1.88 ± 2.11 | −0.01 ± 0.50 | 0.95 | −3.87 ± 2.19 | −2.28 ± 2.03 | −1.59 ± 1.19 | <0.001 |
| LZ2 | −2.39 ± 2.07 | −2.42 ± 2.20 | +0.03 ± 0.39 | 0.71 | −5.38 ± 2.20 | −2.92 ± 2.28 | −2.46 ± 1.02 | <0.001 |
| LZ3 | −1.8 ± 1.66 | −1.89 ± 1.79 | +0.09 ± 0.45 | 0.31 | −4.40 ± 2.49 | −2.68 ± 2.31 | −1.72 ± 1.08 | <0.001 |
UZ = upper zone, MZ = middle zone, LZ = lower zone, details in Figure 1D; MM = more myopic eyes; LM = less myopic eyes.
Pair t-test was performed to analysis the differences (d) between eyes. The row with grey background indicates the central zone of the map.
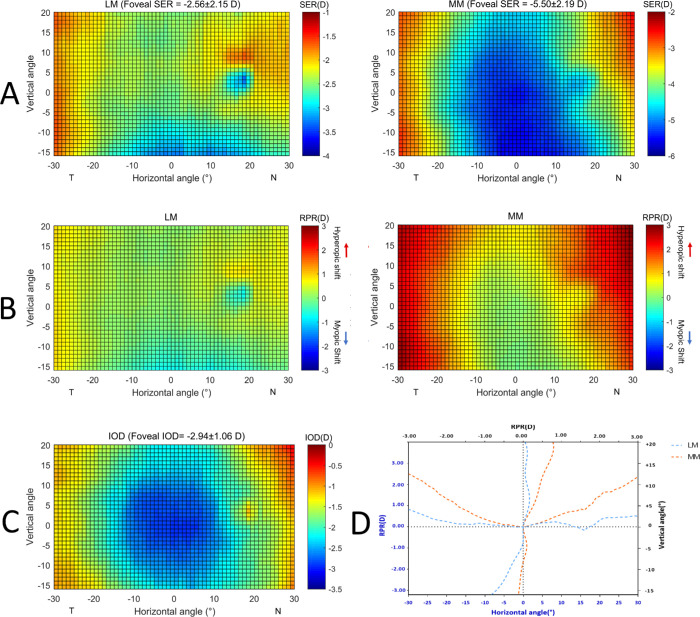
In the anisomyopic group, the right eye with a relatively high degree of myopia were seen in 74.4% (32/43) of participants. To facilitate the comparison, we summed up the eyes with less degrees of myopia (less myopic [LM]) and the eyes with higher degrees of myopia (more myopic [MM]), respectively, for all participants and produced the averaged map of SER in Figure 2A. Accordingly, the foveal SER of the LM eyes and the MM eyes were −2.56 ± 2.15 and −5.50 ± 2.19, respectively (P < 0.001). Using the RPR map (Fig. 2B), it is clear that the average RPR of MM in all peripheral zones became more myopic compared with the LM eyes (Table 2; all P < 0.001), indicating that with myopia progression the peripheral retina demonstrated centrifugally less progression compared to the center (Fig. 2C). Figure 2D shows the RPR value in the two principle meridians. It tends to be more hyperopic (i.e. less myopic) in MM eyes than in LM eyes for both horizontal and vertical directions, but the magnitude of the inferior vertical meridian was less compared to other directions.
Figure 2.
(A) Averaged 2D map of SER of less myopic eyes (LM) and more myopic eyes (MM) in the anisomyope group (n = 43). Values in the x-axis indicate the nasal retina (“N”) and temporal retina (“T”), respectively. For the y-axis, positive values represent the superior retina and negative values represent the inferior retina. The color-code is in diopters. (B) Averaged 2D map of relative peripheral refraction (RPR) of the LM and MM eyes. (C) Averaged 2D map of interocular differences (IOD) generated by using SER maps of the MM eyes minus the LM eyes. (D) The horizontal and vertical section of the average peripheral refraction in the LM and MM eyes has been depicted in panel C.
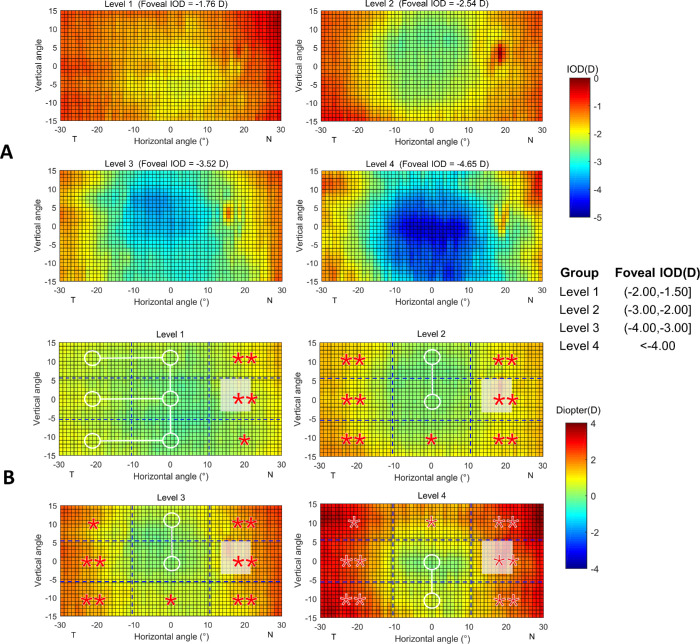
The subjects in the anisomyopic group were further stratified into four groups according to different levels of foveal IOD: level 1 with foveal IOD within 1.50 to 2.00 D; level 2 within 2.00 to 3.00 D; level 3 within 3.00 to 4.00 D; and level 4 within more than 4.00 D. Accordingly, the average foveal IOD for these four groups was 1.76 ± 0.15 D (n = 11), 2.54 ± 0.29 D (n = 15), 3.52 ± 0.29 D (n = 9), and 4.65 ± 0.45 D (n = 8), respectively. Given that the measurement was greater in the superior area (approximately 20°) than the inferior area (approximately 16°), the data of the superior and inferior retina beyond 15° was excluded in the subsequent analysis in order to achieve the comparability between the upper (U) and lower (L) zones.
The results of the statistical analysis of peripheral IOD in different levels of anisomyopia are presented in Table 3 and Figure 3. In the smallest level of anisomyopia (level 1), only the nasal zones (UZ3, MZ3, and LZ3) were statistically less myopic compared to the central zone (all P < 0.05). However, with an increase in the central IOD (level 2 to approximately level 4), the temporal periphery (UZ1, MZ1, and LZ1) also demonstrated a statistically significant hyperopic shift (all P < 0.05). Additionally, the magnitude of the center-periphery difference became greater with the increase of the foveal IOD. For instance, in the mean foveal IOD of 4.65 D (level 4), the center-periphery difference reached 2.26 D in UZ3. In contrast, the superior and inferior peripheries (UZ2 and LZ2) differed from the center only when the central IOD was greater than 3.00 D.
Table 3.
Comparison of IOD Between Peripheral Zones with the Central Zone (MZ2) in Different Degree of Anisomyopia
| Level 1 (n = 11) Center IOD (−2, −1.5] | Level 2 (n = 15) Center IOD (−3, −2] | Level 3 (n = 9) Center IOD (−4, −3] | Level 4 (n = 8) Center IOD < −4.00 | |||||
|---|---|---|---|---|---|---|---|---|
| IOD (D) | d (D) | IOD (D) | d (D) | IOD (D) | d (D) | IOD (D) | d (D) | |
| MZ2 | −1.83 ± 0.30 | – | −2.42 ± 0.33 | – | −3.29 ± 0.38 | – | −4.21 ± 0.53 | – |
| UZ1 | −1.27 ± 1.17 | 0.57 ± 1.16 | −1.55 ± 0.66 | 0.87 ± 0.58** | −2.24 ± 1.06 | 1.06 ± 1.07* | −2.13 ± 1.08 | 2.08 ± 0.85** |
| UZ2 | −1.53 ± 0.67 | 0.31 ± 0.66 | −2.41 ± 0.69 | 0.00 ± 0.65 | −3.15 ± 0.59 | 0.15 ± 0.52 | −3.30 ± 0.87 | 0.91 ± 0.89* |
| UZ3 | −0.90 ± 0.65 | 0.94 ± 0.72** | −1.51 ± 0.91 | 0.91 ± 1.00** | −1.79 ± 0.79 | 1.50 ± 0.77** | −1.95 ± 1.26 | 2.26 ± 1.19** |
| MZ1 | −1.31 ± 1.13 | 0.52 ± 1.08 | −1.64 ± 0.81 | 0.78 ± 0.61** | −2.25 ± 0.81 | 1.05 ± 0.82** | −2.59 ± 0.98 | 1.62 ± 0.70** |
| MZ3 | −1.11 ± 0.58 | 0.72 ± 0.60** | −1.52 ± 0.83 | 0.90 ± 0.87** | −1.86 ± 0.88 | 1.44 ± 0.69** | v2.50 ± 1.02 | 1.71 ± 0.79** |
| LZ1 | −1.22 ± 1.18 | 0.61 ± 1.11 | −1.28 ± 1.02 | 1.14 ± 0.85** | −2.1 ± 1.15 | 1.20 ± 1.03** | −2.24 ± 1.22 | 1.97 ± 0.93** |
| LZ2 | −1.77 ± 0.55 | 0.06 ± 0.49 | −2.03 ± 0.66 | 0.39 ± 0.51* | −2.91 ± 0.74 | 0.38 ± 0.49* | −3.86 ± 0.77 | 0.35 ± 0.50 |
| LZ3 | −1.27 ± 0.78 | 0.57 ± 0.78* | −1.42 ± 0.87 | 1.00 ± 0.88** | −2.07 ± 1.14 | 1.23 ± 0.86** | −2.58 ± 1.25 | 1.63 ± 1.01** |
IOD = interocular difference (MM - LM); D = diopters; Pair t-test was performed to analysis the differences (d) between eyes each peripheral zone with the central zone (MZ2), *P < 0.05/**P < 0.01.
Figure 3.
Averaged maps of interocular differences (IOD) in different levels of anisomyopes using absolute (A) and relative values (B) with respect to the central IOD for each map. The actual value of interocular SER difference was presented on the top of the map. The positive and negative values in the x-axis indicate the nasal retina (“N”) and temporal retina (“T”), respectively. For the y-axis, positive values represent the superior retina and negative values represent the inferior retina. Statistical analysis of the changes of relative IOD in eight zones with respect to the center were shown: A zone with a white circle represents no statistical difference with the center, and a zone with red star represents a statistical reduction compared to the center (*P < 0.05, **P < 0.01). Given that the measurement range was greater in the superior area than in the inferior area, the data of the superior retina beyond 15° was excluded to achieve the comparability between the upper and lower zones.
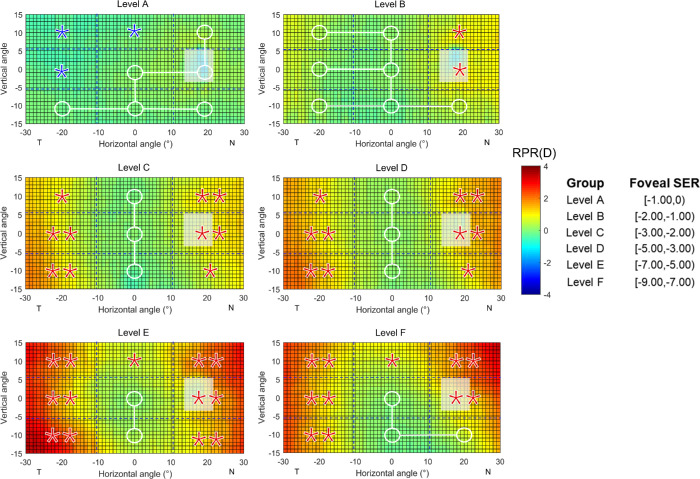
Because the RPR pattern between the fellow eyes in the isomyopic participants were found to be mirror-symmetrical, it is logical to pool the data from all 68 participants in the study and observe the change of the RPR pattern with the increase of the foveal SER from 0 to −9.00 D. We stratified the foveal SER into different levels with an interval of 1.00 D (Fig. 4). In very mild myopic eyes, the RPR was overall homogeneous across the visual field, except in the temporal and superior retina, where there were statistically significant but no clinically significant peripheral-central differences (UZ1: −0.38 ± 0.46 D, P = 0.019; UZ2: −0.12 ± 0.14 D, P = 0.016; MZ1: −0.33 ± 0.36 D, P = 0.011). With the increase of the foveal SER, first the nasal and then the temporal retina demonstrated a statistically significant hyperopic shift compared to the center (all P < 0.05), whereas the inferior retina was still on the same level with the center in the highest level of the study.
Figure 4.
Averaged 2D maps of relative peripheral refraction (RPR) in different level of myopia (data from right eyes of 68 subjects). The positive and negative values in the x-axis indicate the nasal retina (“N”) and temporal retina (“T”), respectively. For the y-axis, positive values represent the superior retina and negative values represent the inferior retina. The color-code is in diopters. A zone with a white circle represents no statistical difference with the center, with a red star a statistically significant hyperopic difference, with a blue star a statistically significant myopic difference (*P < 0.05, **P < 0.01).
Discussion
The present study shows the results of high-resolution 2D peripheral refraction maps in groups of both isomyopes and anisomyopes. Through comparison of the fellow eyes, we found that the RPR pattern was mirror symmetrical in isomyopes. However, with the development of anisomyopia, the peripheral refraction evolved with a varied rate in different areas.
Previously, most studies that investigated peripheral refraction only measured one eye of an individual. Osuagwu et al.29 and Lundström et al.30 performed an interocular comparation and reported mirror symmetrical RPR patterns in fellow eyes in isomyopic subjects. Here, by applying a higher measuring resolution across a wider visual field, we confirmed this finding in isomyopic subjects who presented foveal SER up to −8.40 D. Taken together, it is justified for the design of measuring only one eye of a person to improve time efficiency, especially for studies using traditional instruments.
A previous study conducted on anisomyopia found that the RPR of the more myopic eye of anisomyopia was shifted hyperopically, as occurs in isomyopia with similar central refraction. But less myopic eyes were much less hyperopically shifted in RPR than the corresponding isomyopic eyes.31 They considered such an RPR pattern in LM eyes might be a factor responsible for slowing down the progression of myopia. Similarly, in our study, the LM eyes in anisomyopia have comparable foveal refraction with isomyopic group, but exhibited a significant myopia shifts in inferior retina area, which was absent in isomyopia in the current study or even in emmetropia in the previous study.
In our earlier report,26 we observed a relatively homogeneous RPR pattern across all emmetropic children participants. More than 70% showed a nearly flat horizontal refraction in the fovea, with a slightly myopic shift in the superior retina. Here, in myopic eyes, we observed a completely different RPR pattern. The pattern featured an overall trend of hyperopic shifting in the periphery, especially in the nasal retina. As far as we know, there is only one previous study that reported 2D peripheral refraction results in adults.18 Both studies reported myopes had more positive RPR than hyperopes and emmetropes. But different with our findings, the previous study found the RPR along the vertical meridian was still myopic in myopes, even in the cases with similar foveal refraction. It also found the RPR along the horizontal meridian were also less hyperopic compared with the current study. Thus, both studies suggest that with the progression of myopia, ocular elongation occurs primarily in the posterior pole, whereas the periphery lags behind during the process. The inconsistence between the two studies might be due to the ethnic differences of the participants. Verkicharla et al.32 have reported that east Asians had steeper retinas than Caucasians, which might be related to more positive RPR. Lim et al.33 also found that Chinese eyes have fewer oblate shapes than Malay and Indian eyes, especially in non-myopic eyes.
By comparing the IOD among different levels of anisomyopia, one could predict the change in peripheral refraction along with myopia progression in more detail. Here, we found that the nasal side first began to have a significant relative hyperopic shift compared to the central area. In higher degrees of anisomyopia, it was observed that not only the magnitude of the difference between the center and the nasal retina increased, but also the temporal and then the superior-center and inferior-center began to differ from the central area. This trend was also observed when the data from both the isomyopic and anisomyopic participants were pooled. These findings suggest that peripheral refraction develops with a varied rate in different areas with central myopia progression, or, more directly, that myopia has more effect on peripheral refraction along the horizontal visual field, rather than along the vertical visual field.22 The Supplementary Video demonstrates the process.
Rather than using axial length alone, the overall eye/retinal shape containing more morphological information has received increasing attention as a biomarker for myopia development. Several studies conducted in subjects with different ages or races have evaluated the eye/retinal shape change in myopia progression.34–38 The most common pattern was reportedly the “less oblate - more prolate” pattern, along with a positive shift (“less myopic - more hyperopic”) in peripheral refraction.20,21,38 The peripheral variations we found in the refraction match the change in eye shape during myopia progression very well. Atchison et al.39 measured the axial, vertical, and horizontal eye dimensions in myopic and emmetropic eyes using magnetic resonance imaging (MRI). They found that myopic eyes became much larger in all three dimensions, more so in length (i.e. posterior or center) than in height (i.e. superior/inferior side), and even less so in width (i.e. nasal/temporal side). Our findings confirmed the speculation that “this change of eye shape may result in smaller relative hyperopic shifts along the vertical meridian than along the horizontal meridian.”39
High myopia is usually accompanied by irregular eye growth and the formation of abnormal structures. In the classification of the position for posterior staphyloma in high myopia, both Moriyama et al.40 and Ohno-Matsui et al.41 summarized that the inferior retina, in regard to the central axis, is the second most common position following the visual axis. Compared to the posterior staphyloma, tessellation fundus was a more sensitive sign regarding the change in eye shape.42,43 A longitudinal population-based study also revealed that the progression of fundus tessellation varied significantly in different retinal regions.44 The greatest progression occurred in the temporal parapapillary region (corresponding to the central zones in the present study), followed by the inferior, the nasal, and then the superior parapapillary region. The corresponding zone in the present study to these regions is close to the central zone, the inferior zone (LZ3), the horizon nasal zone (MZ3), and the superior nasal zone (UZ3), respectively. Thus, their findings align with our findings and speculation regarding the varied progression rate across the peripheral retina in myopia progression.
Our findings may have several clinical implications. First, in clinical examinations, which measure the visual function across the retina, such as multifocal electroretinography (mfERG) and perimetry tests, it is known that refraction correction is required prior to examination to avoid the confounding impact of the optical blur.45,46 Because the routine procedure corrects the refraction simply based on the foveal refraction, corresponding adjustments for the peripheral visual field might be necessary, especially for highly myopic patients.47 Meanwhile, with the popularization of refractive surgery, including cornea-based and crystal-lens-based approaches, this surgery is challenged by increasing expectations for the postoperational visual quality of patients. The current findings might provide important information in the optimization of the refractive surgery procedures, as well as the design of implanted artificial intraocular lenses.
The strengths of the study include the provision of wide-field high-resolution 2D maps, a relatively large sample size compared to previous studies (see the summary by Osuagwu et al.18), as well as varying levels of anisometropia. However, the sample size for the stratified analysis might be relatively small when providing sufficient power to detect the possible difference among different retinal zones. In addition, the results of the present study need further investigation in subjects with a greater range of myopia in order to validate the generality of these findings. A database of 2D peripheral refractions containing subjects with different types and degrees of refractive error is currently being established, and the number of samples continues to increase. Another limitation of the study is the cross-sectional nature of the design. Further evidence is necessary to confirm whether the regional variation of refraction compared with the central retina is the cause, or simply the consequence of myopia development. Thus, a large group of emmetropic children are currently waiting for follow-up procedures through a longitudinal study, in the hopes of better understanding this critical question.26
In summary, wide-field, high-resolution 2D data of peripheral refraction in isomyopic and anisomyopic young adults were obtained. It was found that the fellow eyes of an individual demonstrated mirror-symmetrical peripheral refraction pattern when they had a comparable foveal refraction. But with the development of anisomyopia, the progression of peripheral refraction shows a relative hyperopic shift compared with the center retina, resulting in asymmetrical peripheral refraction pattern between the fellow eyes. In addition, it was noted that the peripheral refraction progressed with varied rates in the different retina regions.
Supplementary Material
Acknowledgments
Supported by grants of Key Research and Development Project (2019SK2051) and the Science Fund for Distinguished Young Scientists (2019JJ20034) from the Hunan Provincial Science and Technology Ministry, Hunan, China. The authors alone are responsible for the content and writing of the paper.
Disclosure: S. Wang, None; Z. Lin, None; X. Xi, None; Y. Lu, None; L. Pan, None; X. Li, None; P. Artal, None; W. Lan, None; Z. Yang, None
References
- 1. Schaeffel F, Howland HC.. Mathematical model of emmetropization in the chicken. J Opt Soc Am A. 1988; 5: 2080–2086. [DOI] [PubMed] [Google Scholar]
- 2. Metlapally S, McBrien NA.. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008; 8(3): 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Howlett MH, McFadden SA.. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009; 49: 219–227. [DOI] [PubMed] [Google Scholar]
- 4. Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995; 1: 761–765. [DOI] [PubMed] [Google Scholar]
- 5. Smith EL 3rd, Hung LF, Huang J, Arumugam B. Effects of local myopic defocus on refractive development in monkeys. Optom Vis Sci. 2013; 90: 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith EL 3rd, Hung LF, Huang J, et al.. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010; 51: 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tepelus TC, Vazquez D, Seidemann A, et al.. Effects of lenses with different power profiles on eye shape in chickens. Vision Research. 2012; 54: 12–19. [DOI] [PubMed] [Google Scholar]
- 8. Smith EL 3rd, Kee CS, Ramamirtham R, et al.. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005; 46: 3965–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith EL 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009; 49: 2386–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lam CSY, Tang WC, Tse DY, et al.. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. 2020; 104: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam CS, Tang WC, Tse DY, et al.. Defocus incorporated soft contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol. 2014; 98: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sankaridurg P, Holden B, Smith E 3rd, et al.. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci. 2011; 52: 9362–9367. [DOI] [PubMed] [Google Scholar]
- 13. Mutti DO, Sinnott LT, Mitchell GL, et al.. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Invest Ophthalmol Vis Sci. 2011; 52: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atchison DA, Li SM, Li H, et al.. Relative peripheral hyperopia does not predict development and progression of myopia in children. Invest Ophthalmol Vis Sci. 2015; 56: 6162–6170. [DOI] [PubMed] [Google Scholar]
- 15. Lee TT, Cho P.. Relative peripheral refraction in children: twelve-month changes in eyes with different ametropias. Ophthalmic Physiol Opt. 2013; 33: 283–293. [DOI] [PubMed] [Google Scholar]
- 16. Lundstrom L, Gustafsson J, Unsbo P. Population distribution of wavefront aberrations in the peripheral human eye. J Opt Soc Am A Opt Image Sci Vis. 2009; 26: 2192–2198. [DOI] [PubMed] [Google Scholar]
- 17. Lundstrom L, Mira-Agudelo A, Artal P. Peripheral optical errors and their change with accommodation differ between emmetropic and myopic eyes. J Vis. 2009; 9(6): 17.1–1. [DOI] [PubMed] [Google Scholar]
- 18. Osuagwu UL, Suheimat M, Atchison DA. Peripheral aberrations in adult hyperopes, emmetropes and myopes. Ophthalmic Physiol Opt. 2017; 37: 151–159. [DOI] [PubMed] [Google Scholar]
- 19. Rosen R, Lundstrom L, Unsbo P. Sign-dependent sensitivity to peripheral defocus for myopes due to aberrations. Invest Ophthalmol Vis Sci. 2012; 53: 7176–7182. [DOI] [PubMed] [Google Scholar]
- 20. Schmid GF. Variability of retinal steepness at the posterior pole in children 7-15 years of age. Curr Eye Res. 2003; 27: 61–68. [DOI] [PubMed] [Google Scholar]
- 21. Schmid GF. Association between retinal steepness and central myopic shift in children. Optom Vis Sci. 2011; 88: 684–690. [DOI] [PubMed] [Google Scholar]
- 22. Atchison DA, Pritchard N, Schmid KL. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res. 2006; 46: 1450–1458. [DOI] [PubMed] [Google Scholar]
- 23. Ehsaei A, Mallen EA, Chisholm CM, Pacey IE. Cross-sectional sample of peripheral refraction in four meridians in myopes and emmetropes. Invest Ophthalmol Vis Sci. 2011; 52: 7574–7585. [DOI] [PubMed] [Google Scholar]
- 24. Shen J, Spors F, Egan D, Liu C. Peripheral refraction and image blur in four meridians in emmetropes and myopes. Clin Ophthalmol. 2018; 12: 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaeken B, Lundstrom L, Artal P. Fast scanning peripheral wave-front sensor for the human eye. Opt Express. 2011; 19: 7903–7913. [DOI] [PubMed] [Google Scholar]
- 26. Lan W, Lin Z, Yang Z, Artal P. Two-dimensional peripheral refraction and retinal image quality in emmetropic children. Sci Rep. 2019; 9: 16203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parssinen O, Kauppinen M.. Anisometropia of spherical equivalent and astigmatism among myopes: a 23-year follow-up study of prevalence and changes from childhood to adulthood. Acta Ophthalmol. 2017; 95: 518–524. [DOI] [PubMed] [Google Scholar]
- 28. Tian Y, Tarrant J, Ophthalmic CFWJ, Optics P. Optical and biometric characteristics of anisomyopia in human adults. Ophthalmic Physiol Opt. 2011; 31: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osuagwu UL, Suheimat M, Atchison DA. Mirror symmetry of peripheral monochromatic aberrations in fellow eyes of isomyopes and anisomyopes. Invest Ophthalmol Vis Sci. 2016; 57: 3422–3428. [DOI] [PubMed] [Google Scholar]
- 30. Lundström L, Rosén R, Baskaran K, et al.. Symmetries in peripheral ocular aberrations. J Mod Opt. 2011; 58: 1690–1695. [Google Scholar]
- 31. Chen J, He JC, Chen Y, et al.. Interocular difference of peripheral refraction in anisomyopic eyes of schoolchildren. PLoS One. 2016; 11: e0149110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verkicharla PK, Suheimat M, Schmid KL, Atchison DA. Differences in retinal shape between East Asian and Caucasian eyes. Ophthalmic Physiol Opt. 2017; 37: 275–283. [DOI] [PubMed] [Google Scholar]
- 33. Lim LS, Matsumura S, Htoon HM, et al.. MRI of posterior eye shape and its associations with myopia and ethnicity. Br J Ophthalmol. 10.1136/bjophthalmol-2019-315020. [DOI] [PubMed] [Google Scholar]
- 34. Ehsaei A, Chisholm CM, Pacey IE, Mallen EA. Off-axis partial coherence interferometry in myopes and emmetropes. Ophthalmic Physiol Opt. 2013; 33: 26–34. [DOI] [PubMed] [Google Scholar]
- 35. Ding X, Wang D, Huang Q, et al.. Distribution and heritability of peripheral eye length in Chinese children and adolescents: the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci. 2013; 54: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 36. Ishii K, Iwata H, Oshika T. Quantitative evaluation of changes in eyeball shape in emmetropization and myopic changes based on elliptic fourier descriptors. Invest Ophthalmol Vis Sci. 2011; 52): 8585–8591. [DOI] [PubMed] [Google Scholar]
- 37. Pope JM, Verkicharla PK, Sepehrband F, et al.. Three-dimensional MRI study of the relationship between eye dimensions, retinal shape and myopia. Biomed Opt Express. 2017; 8: 2386–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verkicharla PK, Suheimat M, Schmid KL, Atchison DA. Peripheral refraction, peripheral eye length, and retinal shape in myopia. Optom Vis Sci. 2016; 93: 1072–1078. [DOI] [PubMed] [Google Scholar]
- 39. Atchison DA, Jones CE, Schmid KL, et al.. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004; 45: 3380–3386. [DOI] [PubMed] [Google Scholar]
- 40. Moriyama M, Ohno-Matsui K, Hayashi K, et al.. Topographic analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology. 2011; 118: 1626–1637. [DOI] [PubMed] [Google Scholar]
- 41. Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014; 121: 1798–1809. [DOI] [PubMed] [Google Scholar]
- 42. Chen H, Wen F, Li H, et al.. The types and severity of high myopic maculopathy in Chinese patients. Ophthalmic Physiol Opt. 2012; 32: 60–67. [DOI] [PubMed] [Google Scholar]
- 43. Hayashi K, Ohno-Matsui K, Shimada N, et al.. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010; 117: 1595–1611, 611.e1–4. [DOI] [PubMed] [Google Scholar]
- 44. Yan YN, Wang YX, Yang Y, et al.. Long-term progression and risk factors of fundus tessellation in the Beijing Eye Study. Sci Rep. 2018; 8: 10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hood DC, Bach M, Brigell M, et al.. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol. 2012; 124: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson RS, McDowell DR, Ennis FA. Effect of localized defocus on detection thresholds for different sized targets in the fovea and periphery. Acta Ophthalmol Scand. 2001; 79: 60–63. [DOI] [PubMed] [Google Scholar]
- 47. Ho WC, Wong OY, Chan YC, et al.. Sign-dependent changes in retinal electrical activity with positive and negative defocus in the human eye. Vision Res. 2012; 52: 47–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.