Abstract
Purpose
Visual acuity (VA) and contrast sensitivity (CS) characterize different aspects of visual function. Whereas VA is a standard test in routine eye exams and clinical trials, CS is often not included. We investigated the pathology-specific dissociation between VA and CS by quantifying and comparing the relationship between these two measures in common ocular pathologies.
Methods
VA and CS data were assembled from 1113 subjects, including groups with cataract (n = 450), age-related macular degeneration (AMD; n = 232), glaucoma (n = 100), retinitis pigmentosa (RP; n = 87), and normal ocular health (n = 244). VA and CS were measured by the Early Treatment Diabetic Retinopathy Study chart and Pelli–Robson chart, respectively.
Results
Even when VA was relatively normal (<0.3 logMAR), the four ocular pathology groups showed quantitatively different mean CS deficits relative to normal controls, ranging from –0.01 log units for cataract to 0.23 log units for RP. When the entire range of VA was considered, the corresponding deficits in CS were noticeably different across these four groups, being least for cataract and progressively more severe for glaucoma, AMD, and RP. For every 1.0 logMAR loss of VA, the corresponding deficit in CS ranged from 0.22 logCS for cataract to 0.97 logCS for RP.
Conclusions
The quantitative relationship between VA and CS depends on the ocular pathology. CS appears to provide valuable complementary information to VA in the early detection of eye disease and when evaluating visual impairment.
Keywords: visual acuity, contrast sensitivity, eye disease
Visual acuity (VA) and contrast sensitivity (CS) characterize different aspects of visual function.1 In everyday activities, the ability to resolve fine details, often measured by VA, is critical to pattern recognition, such as reading small print,2,3 whereas the ability to distinguish an object against its background, often measured by CS, is crucial for mobility,4 posture stability,5,6 safe driving,7 and perceived ability in daily tasks.8 Although both VA and CS are frequently measured for functional evaluation in low-vision rehabilitation clinics, CS is rarely assessed in routine eye exams, when managing age-related macular degeneration (AMD) or glaucoma, or when making surgical decisions for patients with cataract whose VA is relatively normal.9 Furthermore, VA is usually included as an outcome measure in clinical trials, whereas CS is much less frequently included.10–12 Thus, the current study aimed to investigate the degree of dissociation between these two clinically important measures by quantifying and comparing their relationship in four common ocular pathologies—AMD, glaucoma, retinitis pigmentosa (RP), and cataract.
When VA remains relatively normal, particularly in the early stages of eye diseases, it is not clear whether CS deficits are likely to develop quickly for specific ocular diagnoses, and if so, what the magnitude of such CS deficits might be. Patients with ocular pathologies often complain about poor vision despite relatively normal VA. These complaints may be related to CS deficits. Hawkins et al.13 found that glaucoma patients whose VA was better than 0.3 logMAR showed CS deficits that were significantly associated with their visual field loss. CS deficits were also reported in patients with early cataract or AMD who had relatively normal VA.14–16 CS deficits despite relatively normal VA have also been documented in patients with diseases in later visual pathways (e.g., optic neuritis,17 visual pathway lesions18), but these are beyond the scope of the current study. It is plausible that CS might be a more sensitive measure of visual function loss in early stages of ocular pathologies. It is important to ascertain whether the extent of CS deficits is quantitatively different across ocular pathologies, which may be particularly important for the management of early functional loss in certain eye diseases and whether to include CS measures in clinical studies or trials depending on the ocular pathology of interest.
It is also of interest to elucidate the extent of CS deficits as VA declines. Correlations between VA and CS have been documented in subjects with normal ocular health but without corrected refractive errors19 and in large samples of older adults in population-based studies,20–22 as well as in patients with specific diagnoses, such as AMD,23 cataract,14 and RP.24,25 Thus, the question arises as to whether it is still useful to include CS measures in addition to the conventional VA measures when the two aspects of central visual function are correlated.14,19 However, it is possible that for similar levels of VA reduction, corresponding deficits in CS might differ across ocular diseases. For ocular conditions that exhibit larger changes in CS with respect to VA, it would be more critical to track the CS changes while monitoring the disease progression and provide appropriate interventions to maximize contrast enhancement during performance of activities of daily living.
Despite existing literature addressing the relationship between VA and CS, it is still challenging to draw definitive conclusions about the relationship between the two from previous studies. This is largely due to the fact that these studies utilized different methodologies to assess and perform statistical analyses of VA and CS, many had small sample sizes and did not consider the confounding effect of age-related changes in VA and CS. Therefore, it is not appropriate to make comparisons across existing published studies to form conclusions about VA and CS loss in various ocular diseases. For this reason, we have assembled VA and CS data from large samples of subjects in four ocular pathology groups and a normal control group measured with standard Early Treatment Diabetic Retinopathy Study (ETDRS) and Pelli–Robson charts.26,27 Both charts have robust design principles, standard testing and scoring protocols, and good test–retest reliability,28,29 which permit comparison across different studies and various ocular diagnoses. In the present study, we aggregated VA and CS data across studies to investigate the quantitative relationship between VA and CS for four eye diseases when VA was either within or outside of a relatively normal range (<0.3 logMAR), in order to answer unresolved questions about the relationships between VA and CS deficits.
Methods
Study Design
This study involved de-identified data analysis from cross-sectional studies or baseline data from clinical trials collected between 2001 to 2018.30–40 All studies received Institutional Review Board (IRB) approval and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects before data collection.
Data Sources
The data were collected from various studies conducted by the co-authors30–38 and from two population-based studies directed by Cynthia Owsley.39,40 All cited studies had other primary research purposes and had conducted VA and CS testing during the standard screening or baseline study procedures. Several studies reported binocular VA and CS in the cited publications due to their primary interest in binocular function (e.g., reading,32,33 driving30,39,40), but monocular data were provided for analysis in the current study, although they were not necessarily reported in the cited publications. The diagnoses of the subjects were confirmed via ophthalmic examination by optometrists or ophthalmologists during subject recruitment35–38 or were obtained from previous medical records after subject enrollment.30–34,39,40 The population-based studies included some patients with multiple comorbidities in the cited reports,39,40 but those patients were excluded from our current analysis. The other studies had excluded patients with ocular comorbidities in the original recruitment.30–38
Subjects
The ocular pathologies included in the current study were cataract, AMD, glaucoma, and RP. Subjects in the cited studies who had other or additional ocular diagnoses (e.g., diabetic retinopathy35) were not included in the present study. Control subjects with normal vision and no ocular disease were included for comparison. Table 1 provides the data sources, the number of subjects, and the pathology-specific inclusion criteria of the cited studies.30–40 We further excluded subjects with incomplete monocular VA or CS data, missing age, or age < 18 years. The exclusion details for each group are provided in Table 1. A final sample of 1113 subjects included 244 subjects (487 eyes) with normal or corrected to normal vision without ocular disease, 450 subjects (899 eyes) with cataract, 232 subjects (353 eyes) with AMD, 100 subjects (196 eyes) with glaucoma, and 87 subjects (153 eyes) with RP. The mean age and gender distributions are provided in Table 2. Detailed age distributions for each group are shown in Figure 1.
Table 1.
Data Sources and Subject Numbers for Each Subject Group
| Ocular Pathology | Data Sources | Subjects Excluded | Final Total | Pathology-Specific Inclusion Criteria |
|---|---|---|---|---|
| Normal | Kwon et al.30 (n = 179) Kwon et al.31 (n = 30) Kwon et al.32 (n = 21) Liu et al.33 (n = 39) Chien et al.34 (n = 27) | Age < 18 (n = 1) Incomplete monocular CS (n = 51) | 244 | Best-corrected visual acuity ≥ 20/30 in each eye; no history of ocular or neurologic disease other than cataract surgery |
| Cataract | Owsley et al.39 (n = 274) Huisingh et al.40 (n = 176) | NA | 450 | Primary diagnosis of cataract in the medical record; no previous cataract surgery in either eye |
| AMD | Giacomelli et al.35 (n = 109) Huisingh et al.40 (n = 123) | NA | 232 | Primary diagnosis of AMD in the medical record40; clinically stable macular changes; no maculopathy due to other causes35 |
| Glaucoma | Kwon et al.30 (n = 81) Kwon et al.32 (n = 17) Chien et al.34 (n = 13) | Incomplete monocular CS (n = 11) | 100 | Primary diagnosis of primary open-angle glaucoma in the medical record: (1) glaucoma-specific changes of optic nerve or nerve fiber layer defect; (2) glaucoma-specific visual field defects; (3) no history of other ocular or neurologic disease or surgery that caused visual field loss; (4) not including ocular hypertension or glaucoma suspect |
| RP | Bittner et al.36 (n = 36) Bittner et al.37 (n = 12) Bittner et al.38 (n = 21) Unpublished (n = 25)* | Missing age (n = 2) Age < 18 (n = 5) | 87 | No vision loss due to ocular diseases other than RP; RP further confirmed by electroretinography and optical coherence tomography exams |
These were subjects in a study by Bittner et al. who completed the VA and CS measures at baseline but did not complete the longer term study that was reported in the previously published paper.36
Table 2.
Subject Statistic and Descriptive Summary of Vision Functions
| Characteristics | Normal | Cataract | AMD | Glaucoma | RP |
|---|---|---|---|---|---|
| Subjects, eyes (n) | 244, 487 | 450, 899 | 232, 353 | 100, 196 | 87, 153 |
| Females, males (n) | 116, 128 | 169, 281 | 124, 108 | 54, 46 | 47, 40 |
| Age (y), mean ± SD | 63 ± 22 | 76 ± 4 | 79 ± 7 | 76 ± 9 | 48 ± 15 |
| Visual acuity (logMAR), mean ± SD | –0.01 ± 0.10 | 0.15 ± 0.17 | 0.33 ± .33 | 0.17 ± 0.26 | 0.31 ± 0.36 |
| Contrast sensitivity (logCS), mean ± SD | 1.66 ± 0.15 | 1.57 ± 0.12 | 1.33 ± 0.33 | 1.48 ± 0.22 | 1.20 ± 0.50 |
Figure 1.
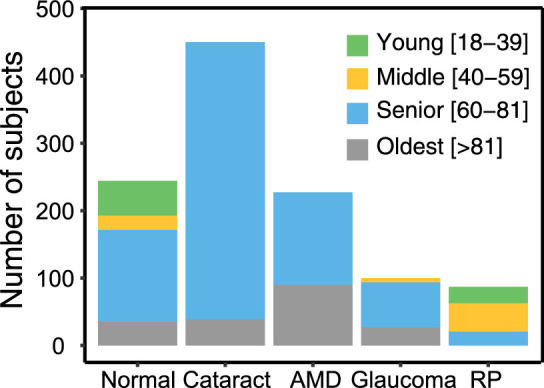
Distributions of age in the normal, cataract, AMD, glaucoma, and RP groups. Subjects were categorized into four age groups: young (18–39 y; green bars), middle (40–59 y; yellow bars), senior (60–81 y; blue bars), and oldest (>81 y; gray bars).
Clinical Tests
All subjects completed VA and CS tests at the same visit. Best-corrected VA was measured with the ETDRS chart.26 The VA test was conducted at 1 m (917 subjects), at 2 m (109 subjects), or at a distance adjusted for subjects with low vision as needed (87 subjects). The VA was scored on a letter-by-letter basis with the following formula: VA (logMAR) = smallest print size attempted + (total letter errors × 0.02). CS was measured by the Pelli–Robson chart at 1 m for all subjects.27 The CS was scored on a letter-by-letter basis and expressed in log units41 with the following formula: CS (logCS) = (total number of letters read correctly × 0.05) – 0.1. Across all studies, the test conditions were within the standard luminance range recommended for the ETDRS chart42 and Pelli-Robson chart43 and avoided sources of glare light. The measurements were terminated when no further letters were read on a set of letters with the same size or contrast. Subjects were, however, encouraged to provide their best guesses when they were uncertain.
Statistical Analysis
Data were analyzed using R software.44 In preliminary analyses, separate consideration of the better or worse eye (determined by VA) exhibited similar relationships between VA and CS. Thus, we pooled the data from each eye in the current analyses. The main statistical method was linear mixed-effects (LME) modeling using the lmer function in the lme4 package.45 The LME model allows inclusion of random effects to account for the correlation between the two eyes of the same subject,46,47 and it has been shown to have more statistical power in unbalanced designs,48 as in the current study in which the sample sizes of the five groups were unequal. To further account for the unbalanced design, the confidence intervals of the model coefficients were estimated by the bootstrap with resampling (n = 1000) method.49
In the first analysis, LME modeling was conducted on a subset of subjects who had VA better than 0.3 logMAR. The cutoff value of 0.3 logMAR was adopted in the current study based on the definition of vision impairment by the World Health Organization.50 This VA cutoff has been used in various population-based studies to calculate the prevalence of vision impairment (e.g., Chan et al.51). The cutoff value has also been used in previous clinical studies as the exclusion/inclusion criterion for the early stages of visual pathologies.10,13 The LME model compared CS across the five groups with CS as the dependent variable, groups as the independent variable, and VA and age as covariates to account for differences in the distributions of VA and age.
In the second analysis, LME modeling was conducted with the full dataset. The model compared the decrease in CS with respect to VA across the four ocular pathology groups with CS as the dependent variable, VA and groups as the independent variables, and age as the covariate. The significance of the independent variables was examined by the ANOVA function in the lme4 package, and the significance of the random effects was examined by the likelihood-ratio test. Insignificant variables were removed step-wise from the model. The final modeling was then followed by post hoc analysis with Bonferroni corrections.52
Results
Deficits in CS Despite Relatively Normal VA
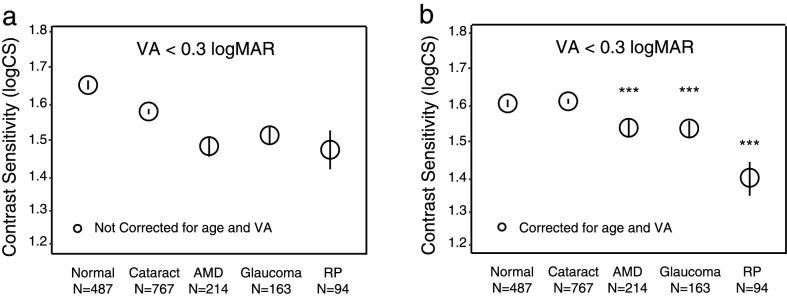
Figure 2a shows the average CS in the five groups when VA is better than 0.3 logMAR, both before (Fig. 2a) and after (Fig. 2b) correcting for age and VA. In Figure 2b, the plots are referenced to the average age (71 years) and mean VA (0.07 logMAR) in this subsample. The CS in the normal control group (i.e., no ocular pathology) had a mean of 1.66 log units (95% confidence interval [CI; 1.64, 1.68]). The group comparisons were conducted after adjusting for age and VA (Fig. 2b). Compared to the normal control group, CS was significantly lower on average in AMD by 0.08 log units (95% CI, [0.05, 0.11]), in glaucoma by 0.07 log units (95% CI, [0.04, 1.11]), and in RP by 0.23 log units (95% CI, [0.20, 0.28]) (all P < 0.001). The cataract group did not have a significantly different CS on average when compared to the normal group (–0.01; 95% CI [−0.03, 0.02]; P = 1.00). Among the three groups that showed significant CS deficits, the RP group had significantly greater CS deficits than the other pathology groups (all P < 0.001).
Figure 2.
The average CS values of the five groups when VA is relatively normal (VA < 0.3 logMAR). (a) Original CS values without correction for age and VA. (b) CS values after correction for age and VA. Each error bar represents 95% confidence intervals. Asterisks represent a significant difference from the normal group. ***P < 0.001.
Association Between VA and CS
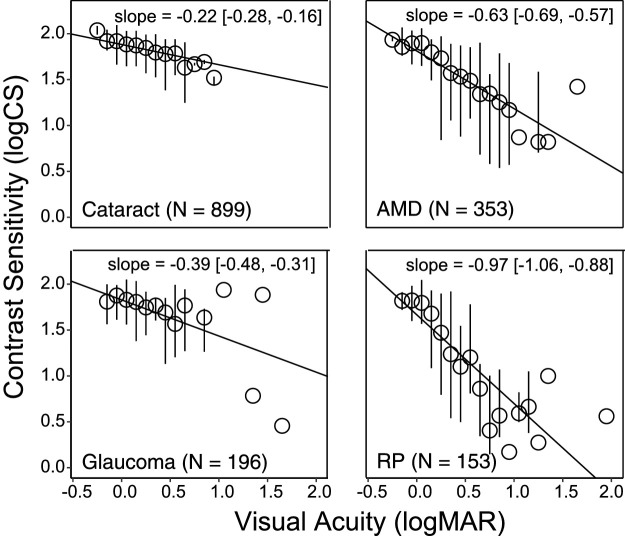
There was a significant correlation between VA and CS for each group (all P < 0.001): normal controls, r = –0.34; cataract, r = –0.43; AMD, r = –0.68; glaucoma, r = –0.50; and RP, r = –0.78. Figure 3 plots CS as a function of VA for the four ocular pathology groups, after adjusting for age. The decrease in CS with respect to VA is visualized by the slope indicated by a solid black line. Note that with a similar amount of VA decrease, a steeper slope represents a larger decrease in CS. The difference in the slopes (i.e., the CS decrease with respect to VA) was statistically significant across the four ocular pathology groups. The RP group had a steeper slope (–0.97; 95% CI [−1.06, −0.88]) than the other three ocular pathology groups (all P < 0.001). The slope value near –1 for the RP group means that, on average, for each 1 logMAR difference on the ETDRS letter acuity chart, we would expect almost 1 log unit difference on the Pelli–Robson CS chart. The cataract group had a smaller slope (–0.22; 95% CI [−0.28, −0.16]) than all of the other groups (all P < 0.001, except P = 0.010 when compared with the glaucoma group). The AMD group (–0.63; 95% CI [−0.69, −0.57]) and glaucoma group (–0.39; 95% CI [−0.48, −0.31]) had moderate slopes, but the AMD group had a significantly steeper slope than the glaucoma group (P < 0.001). A quantitative summary of the relationship between VA and CS is shown in Table 3, which provides the mathematical functions relating CS to age and VA derived from the LME model fitting.
Figure 3.
CS as a function of VA in the four ocular pathology groups, when adjusted by age. Median CS (circles) and the upper and lower quartiles (vertical lines) of each 0.1-logMAR VA bin are shown in the plots.
Table 3.
CS As a Function of Age and VA for Each Diagnosis
| Diagnosis | N | Regression Models* |
|---|---|---|
| Cataract | 899 | CS = 1.88 – 0.004 × Age – 0.22 × VA (R2 = 0.22) |
| AMD | 353 | CS = 1.81 – 0.004 × Age – 0.63 × VA (R2 = 0.47) |
| Glaucoma | 196 | CS = 1.83 – 0.004 × Age – 0.39 × VA (R2 = 0.29) |
| RP | 153 | CS = 1.67 – 0.004 × Age – 0.97 × VA (R2 = 0.60) |
Each equation is derived from the best model fit to the data.
Discussion
VA has long been regarded as a standard test in eye clinics and as a primary outcome measure in many clinical trials. Although CS is known to characterize aspects of visual functioning not captured by VA,1 CS is not often included in routine eye exams or clinical trials. The current study has quantified and compared the relationship between VA and CS in different ocular pathologies, based on a large dataset from subjects who had cataract, AMD, glaucoma, RP, or no ocular disease. Two key findings emerged from this study: (1) when VA is relatively normal (<0.3 logMAR), the extent of CS deficits is quantitatively different across ocular pathologies; and (2) the overall quantitative relationship between CS deficit and VA deficit varies across pathologies. The pathology-specific relationship between VA and CS indicates the importance of documenting both measures in clinical practice and trials for a more comprehensive visual assessment.
Previous studies have reported CS deficits in the early stages of various ocular pathologies.10–13 Consistent with previous findings, the current study showed that, despite relatively normal VA, the AMD, glaucoma, and RP groups all had significant CS deficits compared to the normal control group without ocular disease. It should be noted that, although the CS measured by the Pelli–Robson chart is a single value corresponding to an intermediate spatial frequency range on the contrast sensitivity curve,27 it provides a convenient assessment of the deficits near the peak CS. Importantly, these CS deficits existed even when the differences in age and VA were factored out as covariates in the comparisons. On the other hand, we did not find any CS deficits in the cataract group, which apparently contradicts the findings reported in previous studies. The CS deficits in early cataract have been reported when measured with letter or grating tests.14,15 This discrepancy might arise from the fact that, unlike previous analyses, our analyses included both age and VA as covariates, which may have eliminated the difference (if any) in CS between the cataract and normal control groups. To confirm this possibility, we performed an analysis of our data without the inclusion of the covariates and found significantly lower CS in the cataract group (P < 0.001; also see Fig. 2a) compared to the normal control group.
The presence of CS deficits with relatively normal VA justifies the inclusion of a measure of CS in the test battery when screening for visual function loss as a potentially helpful strategy to detect some forms of eye disease. Also, given that CS may be reduced while VA is relatively normal, it is important to refer any patient with functional complaints to vision rehabilitation services, regardless of the level of VA, as those patients may benefit from visual assistive aids, such as the use of bold print, task lighting, illuminated low-powered optical magnifiers, and/or video magnifiers, which can enhance contrast or provide reversed contrast (white-on-black text).
An important contribution of our approach is a direct comparison between pathologies while using the same statistical methodology for the analyses across groups. We found that the four ocular pathology groups had quantitatively different patterns of CS deficits, with the RP group exhibiting larger CS deficits with the same reductions in VA as the other groups. These differences may be helpful in functional evaluation and management of people with low vision. For example, both patients with RP and glaucoma might show peripheral visual field loss and reduced CS; however, for a VA of 1.0 logMAR, patients with RP in the current study tended to have lower CS than patients with glaucoma by 0.74 log units. In general, this means that it may be more important for RP patients than glaucoma patients to have visual assistive aids that include contrast enhancement, but, due to individual variation, clinical evaluation is necessary to determine patient preferences for contrast enhancement. The strong correlations between VA and CS, especially in the AMD and RP groups (Table 3), indicate that CS can be reasonably well estimated from VA and vice versa. The quantitative relationships between VA and CS may also be helpful in fine-tuning simulations of the effects of impaired vision on the visibility of text (Xiong YZ, et al. IOVS. 2017;58:ARVO E-Abstract 3276), faces, and other real-world objects.53,54
The differential effect of ocular pathology indicates that CS can be a valuable outcome measure to include in clinical trials, especially for pathologies with a larger rate of CS decline. Previous literature reviews of clinical trials for AMD treatments found that, although VA was often reported as the primary outcome, some treatments (e.g., verteporfin therapy) may provide additional benefits to CS.55,56 Butt et al.57 reported that CS was a more sensitive outcome measure than VA for demonstrating the cost-effectiveness of an anti-vascular endothelial growth factor (anti-VEGF) treatment. In a recent clinical trial for neovascular AMD, a treatment switch from ranibizumab to aflibercept induced significant improvement in CS, whereas VA remained stable over time, which led the researchers to conclude that CS as an independent outcome and endpoint may provide a more complete understanding of visual response in neovascular AMD treatment studies.58
When VA is relatively normal (<0.3 logMAR), early CS deficits are likely related to various aspects of the underlying retinal pathology. Even in the early stages of glaucoma, significant macular damage (i.e., loss of retinal ganglion cells and/or shrinkage of dendritic structures and cell bodies of the remaining cells) has been observed,34,59 which may explain the CS deficits observed in glaucoma despite relatively normal VA. In RP patients, the CS deficits despite relatively normal VA may be related to a uniform increase in inter-cone spacing in the fovea, enlargement of cone inner segments leading to increased optical bandwidth of cones, and/or light leakage between foveal cones.60 Future research is needed to investigate the influence of visual-field status (e.g., extremely restricted field or patchy central fields with paracentral scotoma) on VA and CS using microperimetry. The mechanisms underlying the different quantitative relationships between VA and CS for different ocular pathologies remain to be addressed in future studies.
We acknowledge three major limitations in our study. First, it is possible that relevant classifications of disease or gradings, such as cortical/nuclear type for cataract, neovascular or non-neovascular grading for AMD, or the multiple genetic inheritance forms of RP, would reveal additional distinctions in the relationship between VA and CS. But, because our datasets did not include such information, future studies are needed to explore these remaining questions. It would also be of interest to conduct similar analyses for other common ocular pathologies, such as diabetic retinopathy and high myopia. Second, we endeavored to exclude subjects who had comorbid visual disorders, but it is still possible that some subjects might have had secondary disorders that were not diagnosed at the time of testing. Third, we pooled data from convenient samples associated with prior studies, which may have led to the representation of subjects in our five groups that differed in some way from the broader spectrum of individuals with the ocular conditions studied. In spite of these limitations, our results revealed a quantitative relationship between VA and CS and the extent of the dissociation across eye diseases. These findings support the value of including CS in clinical visual test batteries, as an outcome measure in clinical trials and during functional evaluations of visual impairment.
Acknowledgments
Supported by Grants from the National Institutes of Health (R01 EY002934 to GEL; R01 EY027857 to MK), by the Envision Fellowship from the Envision Research Institute (Y-ZX), by a Research to Prevent Blindness/Lions Clubs International Foundation low vision research award (to MK), by grants from the National Eye Institute (K23 EY018356 to AKB; R21 EY023720 and P30 NR008995 to AKB), and by the Foundation Fighting Blindness. The authors thank Cynthia Owsley for sharing data (supported by National Institute on Aging grant P50 AG11684) and for commenting on our manuscript.
Disclosure: Y.-Z. Xiong, None; M. Kwon, None; A.K. Bittner, None; G. Virgili, None; G. Giacomelli, None; G.E. Legge, None
References
- 1. Owsley C. Vision and aging. Annu Rev Vis Sci. 2016; 2: 255–271. [DOI] [PubMed] [Google Scholar]
- 2. Whittaker SG, Lovie-Kitchin J. Visual requirements for reading. Optom Vis Sci. 1993; 70: 54–65. [DOI] [PubMed] [Google Scholar]
- 3. Rubin GS, Legge GE. Psychophysics of reading. VI–The role of contrast in low vision. Vision Res. 1989; 29: 79–91. [DOI] [PubMed] [Google Scholar]
- 4. Marron JA, Bailey IL. Visual factors and orientation-mobility performance. Am J Optom Physiol Opt. 1982; 59: 413–426. [DOI] [PubMed] [Google Scholar]
- 5. Lord SR, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc. 2001; 49: 508–515. [DOI] [PubMed] [Google Scholar]
- 6. Turano K, Rubin GS, Herdman SJ, et al.. Visual stabilization of posture in the elderly: fallers versus non-fallers. Optom Vis Sci. 1994; 70: 761–769. [DOI] [PubMed] [Google Scholar]
- 7. Owsley C, McGwin G Jr. Vision and driving. Vision Res. 2010; 50: 2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szlyk JP, Seiple W, Fishman GA, et al.. Perceived and actual performance of daily tasks: relationship to visual function tests in individuals with retinitis pigmentosa. Ophthalmology. 2001; 108: 65–75. [DOI] [PubMed] [Google Scholar]
- 9. Frost NA, Sparrow JM. Use of vision tests in clinical decision making about cataract surgery: results of a national survey. Br J Ophthalmol. 2000; 84: 432–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lichter PR, Musch DC, Gillespie BW, et al.. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001; 108: 1943–1953. [DOI] [PubMed] [Google Scholar]
- 11. Mahmud I, Kelley T, Stowell C, et al.. A proposed minimum standard set of outcome measures for cataract surgery. JAMA Ophthalmol. 2015; 133: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 12. Csaky K, Ferris F III, Chew EY, Nair P, Cheetham JK, Duncan JL. Report from the NEI/FDA endpoints workshop on age-related macular degeneration and inherited retinal diseases. Invest Ophthalmol Vis Sci. 2017; 58: 3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. J Glaucoma. 2003; 12: 134–138. [PubMed] [Google Scholar]
- 14. Elliott DB, Situ P. Visual acuity versus letter contrast sensitivity in early cataract. Vision Res. 1998; 38: 2047–2052. [DOI] [PubMed] [Google Scholar]
- 15. Shandiz JH, Derakhshan A, Daneshyar A, et al.. Effect of cataract type and severity on visual acuity and contrast sensitivity. J Ophthalmic Vis Res. 2011; 6: 26–31. [PMC free article] [PubMed] [Google Scholar]
- 16. Midena E, Degli Angeli C, Blarzino MC, Valenti M, Segato T. Macular function impairment in eyes with early age-related macular degeneration. Invest Ophthalmol Vis. Sci. 1997; 38,469–477. [PubMed] [Google Scholar]
- 17. Zimmern RL, Campbell FW, Wilkinson IMS. Subtle disturbances of vision after optic neuritis elicited by studying contrast sensitivity. J Neurol Neurosurg Psychiatry. 1979; 42: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kupersmith MJ, Siegel IM, Carr RE. Subtle disturbances of vision with compressive lesions of the anterior visual pathway measured by contrast sensitivity. Ophthalmology. 1982; 89: 68–72. [DOI] [PubMed] [Google Scholar]
- 19. Brown B, Lovie-Kitchin JE. High and low contrast acuity and clinical contrast sensitivity tested in a normal population. Optom Vis Sci. 1989; 66: 467–473. [DOI] [PubMed] [Google Scholar]
- 20. Rubin GS, Roche KB, Prasad-Rao P, Fried LP. Visual impairment and disability in older adults. Optom Vis Sci. 1994; 71: 750–760. [DOI] [PubMed] [Google Scholar]
- 21. Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Seeing into old age: vision function beyond acuity. Optom Vis Sci. 1999; 76: 141–158. [DOI] [PubMed] [Google Scholar]
- 22. West SK, Rubin GS, Broman AT, Munoz B, Bandeen-Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? Arch Ophthalmol. 2002; 3: 774–780. [DOI] [PubMed] [Google Scholar]
- 23. Bellmann C, Unnebrink K, Rubin GS, Miller D, Holz FG. Visual acuity and contrast sensitivity in patients with neovascular age-related macular degeneration. Results from the Radiation Therapy for Age-Related Macular Degeneration (RAD-) Study. Graefes Arch Clin Exp Ophthalmol. 2003; 241: 968–974. [DOI] [PubMed] [Google Scholar]
- 24. Alexander KR, Derlacki DJ, Fishman GA. Visual acuity vs letter contrast sensitivity in retinitis pigmentosa. Vision Res. 1995; 35: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 25. Kiser AK, Mladenovich D, Eshraghi F, Bourdeau D, Dagnelie G. Reliability and consistency of visual acuity and contrast sensitivity measures in advanced eye disease. Optom Vis Sci. 2005; 82: 946–954. [DOI] [PubMed] [Google Scholar]
- 26. Ferris FL III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982; 94: 91–96. [PubMed] [Google Scholar]
- 27. Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988; 2: 187–199. [Google Scholar]
- 28. Elliott DB, Sanderson K, Conkey A. The reliability of the Pelli-Robson contrast sensitivity chart. Ophthalmic Physiol Opt. 1990; 10: 21–24. [PubMed] [Google Scholar]
- 29. Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of Snellen versus ETDRS charts in clinical practice (an AOS thesis). Trans Am Ophthalmol Soc. 2009; 107: 311–324. [PMC free article] [PubMed] [Google Scholar]
- 30. Kwon M, Huisingh C, Rhodes LA, McGwin G Jr, Wood JM, Owsley C. Association between glaucoma and at-fault motor vehicle collision Involvement among older drivers: a population-based study. Ophthalmology. 2016; 123: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwon M, Liu R, Chien L. Compensation for blur requires increase in field of view and viewing time. PLoS One. 2016; 11: e0162711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwon M, Liu R, Patel BN, Girkin C. Slow reading in glaucoma: is it due to the shrinking visual span in central vision? Invest Ophthalmol Vis Sci. 2017; 58: 5810–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu R, Patel BN, Kwon M. Age-related changes in crowding and reading speed. Sci Rep. 2017; 7: 8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chien L, Liu R, Girkin C, Kwon M. Higher contrast requirement for letter recognition and macular RGC+ layer thinning in glaucoma patients and older adults. Invest Ophthalmol Vis Sci. 2017; 58: 6221–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giacomelli G, Virgili G, Giansanti F, et al.. Clinical and micro-perimetric predictors of reading speed in low vision patients: a structural equation modeling approach. Invest Ophthalmol Vis Sci. 2013; 54: 4403–4408. [DOI] [PubMed] [Google Scholar]
- 36. Bittner AK, Haythorthwaite JA, Diener-West M, Dagnelie G. Photopsias are related in part to perceived stress and positive mood in retinitis pigmentosa. Eye. 2012; 26: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bittner AK, Gould JM, Rosenfarb A, Rozanski C, Dagnelie G. A pilot study of an acupuncture protocol to improve visual function in retinitis pigmentosa patients. Clin Exp Optom. 2014; 97: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bittner AK, Seger K, Salveson R, et al.. Randomized controlled trial of electro-stimulation therapies to modulate retinal blood flow and visual function in retinitis pigmentosa. Acta Ophthalmol. 2018; 96: e366–e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Owsley C, Stalvey BT, Wells J, Sloane ME, McGwin G Jr. Visual risk factors for crash involvement in older drivers with cataract. Arch Ophthalmol. 2001; 119: 881–887. [DOI] [PubMed] [Google Scholar]
- 40. Huisingh C, McGwin G, Wood J, Owsley C. The driving visual field and a history of motor vehicle collision involvement in older drivers: a population-based examination. Invest Ophthalmol Vis Sci. 2015; 56: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli–Robson contrast sensitivity test. Ophthalmology. 1991; 6: 471–475. [Google Scholar]
- 42. International Council of Ophthalmology Visual Functions Committee. Visual acuity measurement standard. Italian J Ophthalmol. 1988; 2: 1–15. [Google Scholar]
- 43. Mantyjarvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg. 2001; 27: 261–266. [DOI] [PubMed] [Google Scholar]
- 44. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 45. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015; 67: 1–48. [Google Scholar]
- 46. Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2012; 33: 7–14. [DOI] [PubMed] [Google Scholar]
- 47. Glynn RJ, Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic Epidemiol. 2012; 19: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cudeck R, Harring JR. Analysis of nonlinear patterns of change with random coefficient models. Annu Rev Psychol. 2007; 58: 615–637. [DOI] [PubMed] [Google Scholar]
- 49. Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall; 1993. [Google Scholar]
- 50. WHO. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 51. Chan T, Friedman DS, Bradley C, Massof R. Estimates of incidence and prevalence of visual impairment, low vision, and blindness in the united states. JAMA Ophthalmol. 2018; 136: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Piepho HP. An algorithm for a letter-based representation of all pairwise comparisons. J Comput Graph Stat. 2004; 13: 456–466. [Google Scholar]
- 53. Peli E. Contrast in complex images. J Opt Soc Am A. 1990; A7: 2032–2040. [DOI] [PubMed] [Google Scholar]
- 54. Thompson WB, Legge GE, Kersten DJ, Shakespeare RA, Lei Q. Simulating visibility under reduced acuity and contrast sensitivity. J Opt Soc Am A Opt Image Sci Vis. 2017; 24: 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Monès J, Rubin GS. Contrast sensitivity as an outcome measure in patients with subfoveal choroidal neovascularisation due to age-related macular degeneration. Eye. 2005; 19: 1142–1150. [DOI] [PubMed] [Google Scholar]
- 56. Pauleikhoff D. Neovascular age-related macular degeneration: natural history and treatment outcomes. Retina. 2005; 25: 1065–1084. [DOI] [PubMed] [Google Scholar]
- 57. Butt T, Patel PJ, Tufail A, Rubin GS. Modelling cost effectiveness in neovascular age-related macular degeneration: the impact of using contrast sensitivity vs. visual acuity. Appl Health Econ Health Policy. 2014; 12: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nixon DR, Flinn NA. Evaluation of contrast sensitivity and other visual function outcomes in neovascular age-related macular degeneration patients after treatment switch to aflibercept from ranibizumab. Clin Ophthalmol. 2017; 11: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lahav K, Levkovitch-Verbin H, Belkin M, Glovinsky Y, Polat U. Reduced mesopic and photopic foveal contrast sensitivity in glaucoma. Arch Ophthalmol. 2011; 129: 16–22. [DOI] [PubMed] [Google Scholar]
- 60. Alexander KR, Derlacki DJ, Fishman GA. Contrast thresholds for letter identification in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1992; 33: 1846–1852. [PubMed] [Google Scholar]