Abstract
Purpose
We hypothesize that patients with type 1 diabetes (T1D) may have abnormal retinal vascular responses before diabetic retinopathy (DR) is clinically evident. Optical coherence tomography angiography (OCTA) was used to dynamically assess the retinal microvasculature of diabetic patients with no clinically visible retinopathy.
Methods
Controlled nonrandomized interventional study. The studied population included 48 eyes of 24 T1D patients and 24 demographically similar healthy volunteers. A commercial OCTA device (AngioVue) was used, and two tests were applied: (1) the hypoxia challenge test (HCT) and (2) the handgrip test to induce a vasodilatory or vasoconstrictive response, respectively. The HCT is a standardized test that creates a mild hypoxic environment equivalent to a flight cabin. The handgrip test (i.e., isometric exercise) induces a sympathetic autonomic response. Changes in the parafoveal superficial and deep capillary plexuses in both tests were compared in each group. Systemic cardiovascular responses were also comparatively evaluated.
Results
In the control cohort, the vessel density of the median parafoveal superficial and deep plexuses increased during hypoxia (F1,23 = 15.69, P < 0.001 and F1,23 = 16.26, P < 0.001, respectively). In the T1D group, this physiological response was not observed in either the superficial or the deep retinal plexuses. Isometric exercise elicited a significant decrease in vessel density in both superficial and deep plexuses in the control group (F1,23 = 27.37, P < 0.0001 and F1,23 = 27.90, P < 0.0001, respectively). In the T1D group, this response was noted only in the deep plexus (F1,23 = 11.04, P < 0.01).
Conclusions
Our work suggests there is an early impairment of the physiological retinal vascular response in patients with T1D without clinical diabetic retinopathy.
Keywords: optical coherence tomography angiography, handgrip test, hypoxia challenge test, retinal vascular reactivity, subclinical retinopathy
Diabetes mellitus and diabetic retinopathy (DR) in particular are major public health challenges and a leading cause of blindness in the working age population worldwide.1,2 Before the first typical signs of DR are detected on retinal examination, it is believed that substantial neural retinal damage and subclinical microvascular changes have already developed.3,4 In fact, there is cumulative evidence of an altered neurovascular coupling early in the pathophysiology of the disease. Previous studies using the laser Doppler flowmeter, functional magnetic resonance imaging, the dynamic vessel analyzer, and flicker electroretinography suggest that this abnormal retinal vessels’ autoregulation is associated with an increased risk of DR progression.4–9
Optical coherence tomography (OCT) angiography (OCTA) is an extension of structural OCT with increasing applications in both clinical and research settings. OCTA technology uses infrared wavelengths to provide noninvasive high-contrast imaging of the retinal microvasculature with unprecedented resolution. It does so by detecting motion contrast produced by moving red blood cells in retinal vessels over sequential B-scans without any need for a contrast injection. By examining serial images over time, the generated final image clearly defines retinal vascular plexuses.10–14
Recently, a few studies using OCTA have been reporting structural quantitative changes (i.e., reduced vessel density and increased foveal avascular zone area) in patients with diabetes before clinically evident DR.15–20 However, little is known about the possibility of using OCTA to evaluate individual functional retinal vascular changes. Our group recently published a safe, reproducible and inexpensive protocol to assess retinal microvasculature reactivity in healthy subjects, detailing how OCTA technology is able to detect retinal vasodilation and vasoconstriction in response to two physiological conditions—mild hypoxia and isometric exercise, respectively.21
We hypothesize that patients with diabetes may have altered physiological retinal vascular responses early in the natural history of the disease. Therefore, OCTA was used to dynamically study the retinal microvasculature of diabetic patients with no visible signs of retinopathy, thus contributing to the understanding of the earliest processes of DR development.
Material and Methods
Ethics and Informed Consent
Our research protocol follows the tenets of the Declaration of Helsinki22 and was submitted and approved by the Ethics Committee of Lisbon Academic Medical Center. Written, informed consent was obtained from all participants after detailing the aims, procedures and risks of the study. Two standardized tests were applied: (1) the hypoxia challenge test (HCT)23 and (2) the handgrip test.24 As recommended by the Ethics Committee, to minimize ethical concerns regarding the HCT, patients and volunteers recruited must have had the intention to fly in the future. All safety recommendations regarding the handgrip test were also followed and the test was discontinued if necessary.24 Medical confidentiality was assured. At any time, subjects could anonymously withdraw from the study. The study protocol has been registered in the ISRCTN clinical studies online platform with the number 98388473, available online at http://www.isrctn.com/ISRCTN98388473.
Study Design, Participants, and Inclusion/Exclusion Criteria
A controlled nonrandomized interventional study was conducted, including one group of patients with type 1 diabetes (T1D) without clinical signs of DR, and a demographically similar control group of healthy subjects.
Patients with T1D were recruited from an adult diabetes outpatient clinic, and a demographically (age and sex) similar sample of healthy volunteers was selected as a control group. An anonymous questionnaire was carried out, including the following questions: age, sex, smoking-pack years, known diseases and current chronic medication, previous intraocular surgery or trauma, symptoms during previous flights, and intention to fly in the future. Clinical data available from the electronic health records included demographic characteristics, time from T1D diagnosis, glycated hemoglobin (HbA1c) level, current medication, comorbidities, and presence of microalbuminuria. Subjects were also asked to abstain from alcohol and caffeine for at least six hours before the study to reduce the possible autonomic effects and measurement bias.25 Patients with T1D were treated with rapid acting insulin analogues (continuous subcutaneous insulin infusion [CSII]), or long- and rapid-acting insulin analogues (multiple daily injections [MDI]). All volunteers were asked not to eat or take insulin (MDI) or insulin boluses (CSII) in the two hours preceding the study to minimize its vasodilatory effects in the observed vascular response.26 It was also confirmed before the start of the experimental protocol that no diabetic patient had hypoglycemia (<70 mg/dL) or level 1 hyperglycemia (>180 mg/dL). Last, to minimize the effect of diurnal variations in the systemic and ocular measurements, the individuals of each group were evenly distributed among the scheduled morning and afternoon study sessions.
Ophthalmic exclusion criteria for both groups were as follows: the presence of significant lens opacities (Lens Opacities Classification System III equal to or more stage 2), diabetic retinopathy, high refractive error (spherical equivalent below −6.50 or above +4.00 diopters), history of glaucoma or ocular hypertension, neuro-ophthalmic disease, and previous intraocular surgery. Systemic exclusion criteria included the following: hypertension (defined as systolic blood pressure higher than 140 mm Hg or diastolic blood pressure higher than 90 mm Hg), medically treated hypertension, nephropathy or other documented microvascular complications, local or systemic inflammatory diseases, and smokers of more than 20 cigarettes a day. Pregnant women were also excluded.
Study Protocol
First, the study protocol was explained individually to every subject, written consent was obtained, and the health questionnaire was completed. Then, all subjects underwent a complete ophthalmological examination including best-corrected visual acuity, slit-lamp biomicroscopy with fundoscopy, autorefraction (RK-5; Canon, Inc., Tokyo, Japan), fundus photography (CR-2; Canon, Inc.), intraocular pressure measurement, and ocular biometry (Lenstar; Haag-Streit Diagnostics, Köniz, Switzerland). Other baseline measurements performed included arterial blood pressure and pulse oximetry (Carescape V100; GE Healthcare, Chicago, IL, USA). Room temperature was maintained at 22°C, and consistent mesopic conditions were maintained throughout the study.
A commercial OCTA device was used (Avanti XR, version 2017.1.0.151; Optovue, Fremont, CA, USA), with an A-scan rate of 70,000 A-Scan/s with 5-mm axial resolution and using a split-spectrum amplitude-decorrelation angiography algorithm, thus giving an enhanced signal-to-noise ratio of flow detection. The device used also included the latest projection artefact removal software, allowing for a more-precise analysis of the deep plexus. All examinations were performed by an experienced technician at the determined timepoints using the 6- × 6-mm standard protocol for macular OCTA examination. Two repeated scans were performed—one at baseline and another during the stress test. Vessel density of the superficial and deep plexuses were assessed from the en face angiograms by analyzing a predefined annulus with an outer diameter of 3 mm and an inner diameter of 1 mm, corresponding to the parafoveal region. This vessel density value was automatically generated using built-in AngioAnalytics. Only high-quality images (signal strength > 8/10, focused, and without movement artefacts) were included in the analysis. No subjects were excluded as a result of poor imaging quality. However, and of note for future studies, it is worth mentioning that because of the sustained isometric effort required during the handgrip test, a minority of the volunteers found it difficult to keep a completely steady position in the OCTA chinrest. This specific situation affected the imaging mostly with movement artefacts, and the examinations needed to be repeated once in four of the 48 subjects (two in each group).
Vasodilatory Response–HCT
The physiological response to hypoxia has been previously reported. Similarly to the cerebral vasculature, retinal vessels respond to a decrease in PaO2 with vasodilation and increase in blood flow. This local autoregulatory adaptation contributes to keep a rather stable oxygen pressure in the inner retina until PaO2 levels are as low as 40 mm Hg.27–31
The following protocol has been described in detail in a previous publication.21 Briefly, the HCT is a standard test23 performed at sea level to create a normobaric hypoxic environment by reducing the FiO2 and making it equivalent to that of a flight cabin. The parameters monitored during HCT include oxygen peripheral saturation, arterial pressure, and continuous electrocardiography. As established by the British Thoracic Society, the recommended HCT duration to obtain stable conditions is 20 minutes. Accordingly, OCTA was performed at baseline and then again 30 minutes after HCT start (i.e., in plateau hypoxic conditions). All symptoms were recorded, and the test was stopped if medically necessary.
Vasoconstrictive Response–Handgrip Test
It is known that isometric exercise is used to evaluate sympathetic autonomic response causing steady and safe increases in heart rate and arterial blood pressure, along with physiological peripheral vasoconstriction.24 In the retina, the blood flow remains relatively unchanged until the mean ocular perfusion pressure increases by 35% to 60% above baseline. This is achieved through a local autoregulatory increase in vascular resistance—i.e., retinal vasoconstriction.32–34
The following protocol has been described in detail in a previous publication.21 In brief, subjects sit in front of the OCTA device, with the forearm in neutral position, the elbow flexed at approximately 90°, and the wrist with the thumb facing upward. Using a Jamar hydraulic dynamometer, the participants are asked to keep a steady contraction of at least one third of the maximal calculated force. The OCTA acquisition starts in the plateau phase, i.e., after 90 seconds, being completed for both eyes within the 3- to 5-minute test period. The arterial pressure in the contralateral arm is measured every 90 seconds. According to the test recommendations, if a diastolic blood pressure reaches values higher than 120 mm Hg or any adverse symptom is registered, the test is immediately interrupted.
Primary and Secondary Outcomes
A comparative analysis for each group—T1D patients and healthy controls—was undertaken for the following outcomes: (1) parafoveal vessel density evaluated using OCTA at baseline and during HCT; (2) parafoveal vessel density evaluated using OCTA at baseline and during the handgrip test; and (3) systemic cardiovascular response in both scenarios.
Statistics
Sample size was calculated considering a 5% clinically significant difference in mean vessel density between groups and a standard deviation of 5%. Considering a power of 90%, an alpha value of 0.05, a minimum of 17 T1D patients and controls should be included. To account for the attrition rate and to maintain the power of the study, a total of 24 patients were included in each group.
Statistical analysis was performed using STATA v14.1. A repeated-measures analysis of variance (ANOVA) model was used to assess differences between the baseline and stress tests’ measurements. The skewness-kurtosis test was used to assess the normal distribution of the variables considered, and the inexistence of significant outlier values was also confirmed. Equality of variances was investigated, and the results were reported accordingly, applying the Greenhouse-Geisser correction when variables’ variances were not equal.35 A P value <0.05 was considered for statistical significance. To guarantee independent observations, only the right eye of each patient was considered for analysis.
Results
Demographics and Baseline Data
Forty-eight eyes of 24 healthy subjects and 24 T1D patients without evidence of diabetic retinopathy were studied. In the T1D group, the mean HbA1c value was 7.9% ± 1.4% (range 6.2%–11.5%) and the mean time from the diagnosis was 14.8 ± 9.7 years (range 2–36 years). Both groups were similar with respect to the demographic and baseline characteristics, including age, gender, arterial blood pressure, heart rate, body mass index, ocular axial length, intraocular pressure and best-corrected visual acuity (Table 1). Of note, and as previously reported elsewhere,36,37 a baseline rarefaction of the superficial and deep parafoveal plexuses was noted in the group of patients with diabetes, even before clinically evident retinopathy (Table 1).
Table 1.
Demographic and Baseline Data
| Control | Type 1 Diabetes | P Value | |
|---|---|---|---|
| Age, mean (SD), years | 31.8 (8.2) | 36.9 (10.4) | 0.07 |
| Male/Female, n | 10/14 | 10/14 | 1.00 |
| SAP, mean (SD), mm Hg | 117 (12) | 117 (9) | 0.85 |
| DAP, mean (SD), mm Hg | 78 (9) | 77 (6) | 0.13 |
| Heart rate, median (IQR), beats/min | 62 (58–67) | 62 (56–71) | 0.63 |
| Body mass index, mean (SD), kg/m2 | 22.6 (3.0) | 25.1 (3.9) | 0.06 |
| Axial length, mean (SD), mm | 24.1 (0.9) | 23.5 (1.0) | 0.07 |
| Intraocular pressure, mean (SD), mm Hg | 13.3 (2.1) | 14.6 (3.0) | 0.14 |
| Visual acuity, median (IQR), logMAR | 0 (0–0) | 0 (0–0) | 0.45 |
| Parafoveal vessel density, median (IQR) | |||
| Superficial plexus | 55.1 (53.1–56.4) | 53.1 (48.9–55.1) | 0.016 |
| Deep plexus | 60.4 (59.3–61.8) | 57.2 (53.3–60.0) | < 0.001 |
| HbA1c, mean (SD), % | — | 7.9 (1.4) | — |
| Time since diagnosis, mean (SD) | — | 14.8 ± 9.7 | — |
P values obtained with Student's t test, Wilcoxon rank-sum (Mann-Whitney) test or Pearson's χ2 test, as appropriate. DAP, diastolic arterial blood pressure; HbA1c, glycated hemoglobin, pressure; SAP, systolic arterial blood pressure; SD, standard deviation; IQR, interquartile range.
Systemic Response
Hypoxia Challenge Test
The median peripheral oxygen hemoglobin saturation decreased from 97% to 88% in both groups (Table 2). Also, as expected, an increase in the heart rate was noted in hypoxic conditions in both groups to increase the cardiac output. The arterial blood pressure changes were less notable, with a mild decrease in mean arterial pressure noted in the control group and no significant differences in T1D patients (Table 2).
Table 2.
Systemic Response to the Hypoxia Challenge Test
| Control | P Value | Type 1 Diabetes | P Value | |
|---|---|---|---|---|
| O2 Hb saturation, median (IQR), % | ||||
| Baseline | 97 (97–98) | — | 97 (97–98) | — |
| Hypoxia | 88 (85–89) | <0.0001 | 88 (86–89) | <0.001 |
| SAP, mean (SD), mm Hg | ||||
| Baseline | 117 (12) | — | 117 (9) | — |
| Hypoxia | 114 (10) | 0.05 | 118 (11) | 0.25 |
| DAP, mean (SD), mm Hg | ||||
| Baseline | 78 (9) | — | 77 (6) | — |
| Hypoxia | 75 (10) | 0.045 | 75 (7) | 0.10 |
| MAP, mean (SD), mm Hg | ||||
| Baseline | 91 (10) | — | 90 (6) | — |
| Hypoxia | 88 (9) | 0.02 | 89 (8) | 0.40 |
| Heart rate, median (IQR), beats/min | ||||
| Baseline | 62 (58–67) | — | 62 (56–71) | — |
| Hypoxia | 74 (71–80) | <0.001 | 79 (63–86) | <0.001 |
P values (versus baseline) obtained with Student's t test and Wilcoxon rank-sum (Mann-Whitney) test, as appropriate. DAP, diastolic arterial pressure; Hb, hemoglobin; MAP, mean arterial blood pressure; SAP, systolic arterial pressure; SD, standard deviation; IQR, interquartile range.
Handgrip Test
As shown in Table 3, the handgrip test was associated with a significant increase of the heart rate and systolic and diastolic arterial blood pressure in both groups.
Table 3.
Systemic Response to the Handgrip Test
| Control | P Value | Type 1 Diabetes | P Value | |
|---|---|---|---|---|
| SAP, mean (SD), mm Hg | ||||
| Baseline | 118 (11) | — | 123 (12) | — |
| Handgrip | 150 (18) | <0.0001 | 155 (24) | <0.0001 |
| DAP, mean (SD), mm Hg | ||||
| Baseline | 75 (9) | — | 79 (9) | — |
| Handgrip | 102 (14) | <0.0001 | 98 (13) | <0.0001 |
| MAP, mean (SD), mm Hg | ||||
| Baseline | 89 (9) | — | 94 (9) | — |
| Handgrip | 123 (13) | <0.0001 | 122 (17) | <0.0001 |
| Heart rate, median (IQR), beats/min | ||||
| Baseline | 67 (64–73) | — | 62 (67–78) | — |
| Handgrip | 77 (71–85) | <0.01 | 79 (68–87) | <0.01 |
P values (versus baseline) obtained with Student's t test and Wilcoxon rank-sum (Mann-Whitney) test, as appropriate.
DAP, diastolic arterial pressure; MAP, mean arterial blood pressure; SAP, systolic arterial pressure; SD, standard deviation; IQR, interquartile range.
Retinal Vascular Response
Hypoxia Challenge Test (Fig. 1)
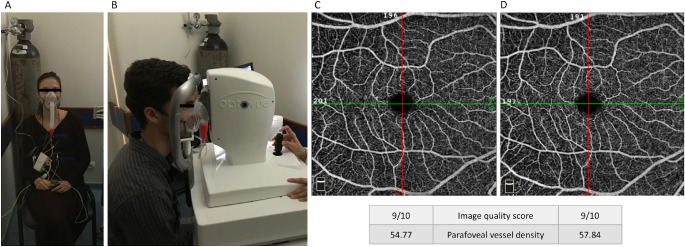
Figure 1.
Exemplar of the setup during the hypoxia challenge test and OCT-angiography examination (A, B) and macular en-face 6- × 6-mm angiograms obtained in baseline conditions (C) and during the hypoxic test (D). The angiograms belong to the healthy volunteer depicted in B. Vessel density increased in hypoxic conditions as expected. Image quality score and parafoveal vessel density are provided according to the built-in angioanalytics software, as described in the Methods section.
In the healthy cohort, the median parafoveal vessel density in the superficial plexus increased from 55.1 (53.1–56.4) in baseline conditions to 56.5 (54.0–57.6) in hypoxia (F1,23 = 15.69, P < 0.001). The median parafoveal vessel density in the deep plexuses also increased, from 60.4 (59.3–61.8) at baseline to 62.0 (59.9–62.8) during hypoxia (F1,23 = 16.26, P < 0.001; Table 4).
Table 4.
Retinal Vascular Response to the Hypoxia Challenge Test
| Parafoveal Vessel Density, Median (IQR) | Control | P Value | Type 1 Diabetes | P Value |
|---|---|---|---|---|
| Superficial plexus | ||||
| Baseline | 55.1 (53.1–56.4) | — | 53.1 (48.9–55.1) | — |
| Hypoxia | 56.5 (54.0–57.6) | <0.001 | 52.0 (50.0 - 54.2) | 0.81 |
| Deep plexus | ||||
| Baseline | 60.4 (59.3 - 61.8) | — | 57.2 (53.3 - 60.0) | — |
| Hypoxia | 62.0 (59.9 - 62.8) | < 0.001 | 56.2 (54.4 - 57.9) | 0.31 |
P values (versus baseline) obtained with ANOVA repeated-measures. IQR, interquartile range.
In the T1D group, there were no statistically significant differences during hypoxia for the superficial (F1,23 = 0.06, P = 0.81) or deep (F1,23 = 1.08, P = 0.31) parafoveal plexuses.
Handgrip Test (Fig. 2)
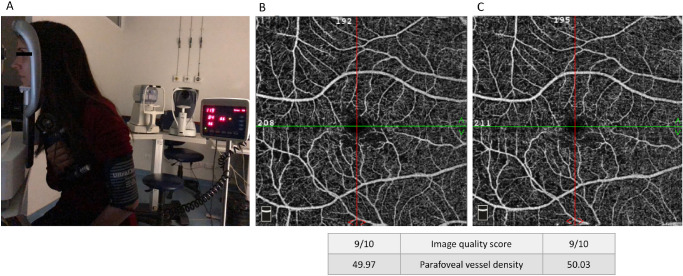
Figure 2.
Exemplar of the setup during the handgrip test and OCT-angiography examination (A) and macular en-face 6- × 6-mm angiograms obtained in baseline conditions (B) and during the handgrip test (C). The angiograms belong to a volunteer with type 1 diabetes with no clinical evidence of diabetic retinopathy. The physiological decrease in vessel density during the handgrip test was not observed. Image quality score and parafoveal vessel density are provided according to the built-in angioanalytics software, as described in the Methods section.
In the control group, isometric exercise elicited a significant decrease in vessel density in both superficial (55.8 [53.5–56.9] to 54.1 [52.2–54.5], F1,23 = 27.37, P < 0.0001) and deep (60.4 [58.7–61.2] to 57.1 [53.7–58.6], F1,23 = 27.90, P < 0.0001) parafoveal plexuses.
In the T1D group, the expected vasoconstrictive response with decrease in vessel density during the test was not observed in the superficial plexus (F1,23 = 3.86, P = 0.06), being noticed only in the deep (F1,23 = 11.04, P < 0.01) parafoveal plexus (Table 5).
Table 5.
Retinal Vascular Response to the Handgrip Test
| Parafoveal Vessel Density, Median (IQR) | Control | P Value | Type 1 Diabetes | P Value |
|---|---|---|---|---|
| Superficial plexus | ||||
| Baseline | 55.8 (53.5–-56.9) | — | 53.5 (51.1–55.4) | — |
| Handgrip | 54.1 (52.2–-54.5) | <0.0001 | 52.8 (48.7–54.8) | 0.06 |
| Deep plexus | ||||
| Baseline | 60.4 (58.7–61.2) | — | 58.5 (54.1–60.3) | |
| Handgrip | 57.1 (53.7–58.6) | <0.0001 | 55.9 (52.5–60.3) | <0.01 |
P values (versus baseline) obtained with ANOVA repeated-measures. IQR, interquartile range.
Discussion
Our study used OCTA to dynamically study the retinal microvasculature functional responses to mild hypoxia and isometric exercise using two standardized tests—the HCT and the handgrip test, respectively.
A protocol for this functional analysis using OCTA has been previously reported in healthy subjects,21 and this work replicated the same results in terms of retinal responses to isometric exercise and mild hypoxia. Importantly, this study is the first to use OCTA to document the impairment of two physiological retinal vascular responses in patients with diabetes before any clinical features of DR are evident. Our sample of young patients with T1D lacked other vascular comorbidities, such as hypertension and atherosclerosis that are likely to influence OCTA measurements. Thus the changes identified are most likely due to diabetes-specific factors influencing retinal vascular behavior.38
The retina is one of the most metabolically active tissues in the body, and for its normal functioning an effective autoregulation is crucial.12 The lack of the expected vascular response pattern to both stimuli—mild hypoxia and isometric exercise—suggests there is an early impairment of the retinal autoregulatory function in the diabetic group. Our results are supported by previous findings of preclinical structural and functional changes in patients with diabetes.16–19,37,39 The altered retinal vascular response that we observed also corroborates previous evidence on the importance of neurovascular coupling dysfunction very early in DR development. These studies used the laser Doppler blood flowmeter, functional magnetic resonance imaging, flicker electroretinography and the dynamic vessel analyzer as devices to assess retinal vascular function.5–9,40 However, these tools are generally used for research-only purposes and therefore not widely accessible in ophthalmology clinics. In our study we used OCTA technology, which is increasingly available in clinical practices worldwide and may be further optimized for this purpose. The ability to evaluate individual functional responses has multiple potential advantages. First, it overcomes the limitations of interpreting single structural exams that may vary among individuals, including predetermined OCTA metrics, such as the foveal avascular zone.41 Also, the retinal functional response may be a more sensitive marker to detect earlier changes, when compared with a structurally normal OCT scan or fluorescein angiography. This may well have potential implications when considering monitoring and managing the metabolic, systemic and ophthalmic manifestations of the disease.42 We found a significantly altered vascular response in subjects with type 1 diabetes before any clinical evidence of diabetic retinopathy. This abnormal retinal microvascular response may correspond to an earlier marker of endothelial dysfunction or changes in the signaling between the retina and the vessels (i.e., neurovascular coupling),27,43,44 and we provide a novel way of studying it noninvasively using modern OCTA technology.
Another interesting finding of our study is the notion that the cardiovascular systemic response does not appear to be significantly different between the groups. The circumstantial finding of a statistically significant decrease in the arterial blood pressure of the healthy cohort during mild hypoxia (which was not clearly observed in the diabetic group) seems to have limited clinical meaning. However, this may be worth clarifying in future studies. The identified abnormal regional retinal response suggests there is an increased ability of OCTA to sensitively identify vascular changes. As an innovative, noninvasive, and safe technology, able to study a central nervous system (i.e., retinal) microvasculature, OCTA is also becoming a useful tool for the study of nonophthalmic conditions. Multiple reports have been published with OCTA applications mainly in neurodegenerative conditions (e.g., Parkinson, Alzheimer's, multiple sclerosis), but also in other diseases.45–52 Therefore studying a patient's retinal vascular responses can be a useful adjunct to routine structural anatomical evaluation with interesting application also in other medical specialties. This study highlights the potential to adapt the available OCTA technology to combine this form of functional analysis to the currently available structural angiogram.
With this study, we have demonstrated an attenuated retinal vascular response in patients with type 1 diabetes with no clinical evidence of ocular disease. However, these findings should be interpreted along with the limitations of our study. Although well-powered for the main outcomes, the young age group, Caucasian population and relatively small sample size may limit the external validity of the study and also the possibility of multivariate analysis with certain demographics and subgroup features. Also, the diurnal changes of the systemic and ocular variable analyzed were minimized as possible but should not be excluded as a potential source of bias. Despite careful recruitment, the wide range of diabetes’ duration in our sample may suggest the underestimation of the diabetic retinopathy status, because some patients would be above the usual time for the early manifestations of DR (such as peripheral hemorrhages, microaneurysms, or both),53 that could have been underreported with our methodology. Larger studies, including patients with a range of disease severity through the various ETDRS levels would be useful to add weight to our findings. Second, although individuals with type 1 diabetes were selected to minimize the influence of any co-pathologies and systemic medication in the vascular analysis, a small number of subjects were under systemic medication (other than insulin): four patients were on levothyroxine, two were on simvastatin, one patient was taking sertraline, and one patient was on mexazolam and levothyroxine. The patients taking levothyroxine had normal thyroid function test results. Although unlikely to affect the observed patterns of retinal vascular response, we acknowledge the potential vascular effects of these drugs.54–57 Also, insulin is inherently vasoactive.26 Despite being asked not to administer insulin boluses in the two hours preceding the OCTA measurements to reduce its influence on our observations, we should not exclude the potential for some degree of measurement bias introduced by its cardiovascular effects. Likewise, current blood glucose concentration itself may have a significant vascular effect.58,59 Because this parameter was not systematically evaluated in both groups, it is also a limitation of our study protocol that we were not able to rigorously control the observed vascular response for the current glycemia. Third, although widely used in clinics and research, the OCTA technology is not yet optimized for these functional analyses, and it should be remembered that this technology measures perfused vessel densities, not absolute blood flows. Last, to ensure that the protocol for dynamic retinal microvasculature analysis is as reproducible as possible, we used the manufacturer's default software for superficial and deep capillary plexuses analysis. Thus the inherent bias related to segmentation and differences to other devices and models should be considered.11 We are certain that this study acts as a valuable contributor to the development of the OCTA technology as a realistic tool in functional retinal analysis.
In conclusion, we used OCTA technology in conjunction with standardized stress tests and observed an early impairment in the physiological retinal vascular response in patients with type 1 diabetes before any clinical evidence of retinopathy. Further work is required to better delineate the process of DR development and to develop tools that optimize its clinical significance.
Acknowledgments
The authors thank the Faculty of Medicine and the University of Lisbon, as well as the technicians involved in the study: Diana Francisco, Sofia Silva, and Telma Gala. The authors also thank J. L. Ducla-Soares and the Autonomic Nervous System Department of Lisbon Academic Medical Center for all the support, as well as José Cotta Ems Lda for providing the necessary equipment and logistical backing for the study. And last, the authors appreciate the help with spelling and grammar revision provided by Andrew Walkden (University of Manchester, Manchester, UK).
Disclosure: D.C. Sousa, None; I. Leal, None; S. Moreira, None; S. do Vale, None; A.S. Silva-Herdade, None; P. Aguiar, None; P. Dionísio, None; L. Abegão Pinto, None; M.A.R.B. Castanho, None; C. Marques-Neves, None
References
- 1. Nentwich MM, Ulbig MW. Diabetic retinopathy—ocular complications of diabetes mellitus. World J Diabetes. 2015; 6: 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leasher JL, Bourne RRA, Flaxman SR, et al.. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016; 39: 1643–1649. [DOI] [PubMed] [Google Scholar]
- 3. Tavares Ferreira J, Proença R, Alves M, et al.. Retina and Choroid of Diabetic Patients Without Observed Retinal Vascular Changes: A Longitudinal Study. Am J Ophthalmol. 2017; 176: 15–25. [DOI] [PubMed] [Google Scholar]
- 4. Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H. Early detection of diabetic retinopathy. Surv Ophthalmol. 2018; 63: 601–608. [DOI] [PubMed] [Google Scholar]
- 5. Lasta M, Pemp B, Schmidl D, et al.. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Investig Ophthalmol Vis Sci. 2013; 54: 842–847. [DOI] [PubMed] [Google Scholar]
- 6. Trick GL, Edwards P, Desai U, Berkowitz BA. Early supernormal retinal oxygenation response in patients with diabetes. Investig Ophthalmol Vis Sci. 2006; 47: 1612–1619. [DOI] [PubMed] [Google Scholar]
- 7. Tecilazich F, Feke GT, Mazzantini S, Sobrin L, Lorenzi M. Defective myogenic response of retinal vessels is associated with accelerated onset of retinopathy in type 1 diabetic individuals. Investig Ophthalmol Vis Sci. 2016; 57: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 8. Lim LS, Ling LH, Ong PG, Foulds W, Shyong Tai E, Wong TY. Dynamic responses in retinal vessel caliber with flicker light stimulation and risk of diabetic retinopathy and its progression. Investig Ophthalmol Vis Sci. 2017; 58: 2449–2455. [DOI] [PubMed] [Google Scholar]
- 9. Zeng Y, Cao D, Yu H, et al.. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 2019; 103: 1747–1752. [DOI] [PubMed] [Google Scholar]
- 10. Koustenis A, Harris A, Gross J, Januleviciene I, Shah A, Siesky B. Optical coherence tomography angiography: An overview of the technology and an assessment of applications for clinical research. Br J Ophthalmol. 2017; 101: 16–20. [DOI] [PubMed] [Google Scholar]
- 11. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018; 64: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei X, Balne PK, Meissner KE, Barathi VA, Schmetterer L, Agrawal R. Assessment of flow dynamics in retinal and choroidal microcirculation. Surv Ophthalmol. 2018; 63: 646–664. [DOI] [PubMed] [Google Scholar]
- 13. Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017; 58: 5548–5555. [DOI] [PubMed] [Google Scholar]
- 14. Sadda SVR. Defining the role of OCT angiography in clinical practice. Ophthalmol Retin. 2017; 1: 261–262. [DOI] [PubMed] [Google Scholar]
- 15. Sousa DC, Leal I, Moreira S, et al.. Optical coherence tomography angiography study of the retinal vascular plexuses in type 1 diabetes without retinopathy. Eye. 2020; 34: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carnevali A, Sacconi R, Corbelli E, et al.. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017; 54: 695–702. [DOI] [PubMed] [Google Scholar]
- 17. Sacconi R, Casaluci M, Borrelli E, et al.. Multimodal imaging assessment of vascular and neurodegenerative retinal alterations in type 1 diabetic patients without fundoscopic signs of diabetic retinopathy. J Clin Med. 2019; 8: 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Carlo TE, Chin AT, Bonini Filho MA, et al.. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015; 35: 2364–2370. [DOI] [PubMed] [Google Scholar]
- 19. Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig Ophthalmol Vis Sci. 2017; 58: 190–196. [DOI] [PubMed] [Google Scholar]
- 20. Durbin MK, An L, Shemonski ND, et al.. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017; 135: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sousa DC, Leal I, Moreira S, et al.. A protocol to evaluate retinal vascular response using opticalcoherence tomography angiography. Front Neurosci. 2019; 13: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carlson R V, Boyd KM, Webb DJ.. The revision of the Declaration of Helsinki: Past, present and future. Br J Clin Pharmacol. 2004; 57: 695–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vohra KP, Klocke RA.. Detection and correction of hypoxemia associated with air travel. Am Rev Respir Dis. 1993; 148: 1215–1219. [DOI] [PubMed] [Google Scholar]
- 24. Ewing D, et al.. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Ewing D, Martyn C al. 1985; 8: 491–498. [DOI] [PubMed] [Google Scholar]
- 25. Vinader-Caerols C, Monleón S, Carrasco C, Parra A. Effects of alcohol, coffee, and tobacco, alone or in combination, on physiological parameters and anxiety in a young population. J Caffeine Res. 2012; 2: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007; 28: 463–491. [DOI] [PubMed] [Google Scholar]
- 27. Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008; 27: 284–330. [DOI] [PubMed] [Google Scholar]
- 28. Cheng RW, Yusof F, Tsui E, et al.. Relationship between retinal blood flow and arterial oxygen. J Physiol. 2016; 594: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sousa DC, Leal I, Moreira S, Dionísio P, Abegão Pinto L, Marques-Neves C. Hypoxia challenge test and retinal circulation changes—a study using ocular coherence tomography angiography. Acta Ophthalmol. 2018; 96:e315–e319. [DOI] [PubMed] [Google Scholar]
- 30. Ahmed J, Pulfer MK, Linsenmeier RA. Measurement of blood flow through the retinal circulation of the cat during normoxia and hypoxemia using fluorescent microspheres. Microvasc Res. 2001; 62: 143–153. [DOI] [PubMed] [Google Scholar]
- 31. Stefánsson E, Olafsdottir OB, Eliasdottir TS, et al.. Retinal oximetry: Metabolic imaging for diseases of the retina and brain. Prog Retin Eye Res. 2019; 70: 1–22. [DOI] [PubMed] [Google Scholar]
- 32. Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986; 27: 722–726. [PubMed] [Google Scholar]
- 33. Blum M, Bachmann K, Wintzer D, Riemer T, Vilser W, Strobel J. Noninvasive measurement of the Bayliss effect in retinal autoregulation. Graefe's Arch Clin Exp Ophthalmol. 1999; 237: 296–300. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Emeterio Nateras OS, Peng Q, Rosende CA, Duong TQ. Blood flow MRI of the human retina/choroid during rest and isometric exercise. Investig Ophthalmol Vis Sci. 2012; 53: 4299–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdi H. The greenhouse-geisser correction. Encycl Res Des Sage Publ. 2010; 95: e751–e755. [Google Scholar]
- 36. Li T, Jia Y, Wang S, et al.. Retinal Microvascular Abnormalities in Children with Type 1 Diabetes Mellitus Without Visual Impairment or Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2019; 60: 990–998. [DOI] [PubMed] [Google Scholar]
- 37. Simonett JM, Scarinci F, Picconi F, et al.. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017; 95: e751–e755. [DOI] [PubMed] [Google Scholar]
- 38. Song SH. Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res Care. 2015; 3: e000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao D, Yang D, Huang Z, et al.. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018; 55: 469–477. [DOI] [PubMed] [Google Scholar]
- 40. Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H. Early detection of diabetic retinopathy. Surv Ophthalmol. 2018; 63: 601–608. [DOI] [PubMed] [Google Scholar]
- 41. Kaizu Y, Nakao S, Arima M, et al.. Flow density in optical coherence tomography angiography is useful for retinopathy diagnosis in diabetic patients. Sci Rep. 2019; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun Z, Tang F, Wong R, et al.. OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: a prospective study. Ophthalmology. 2019; 126: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 43. Justesen BL, Mistry P, Chaturvedi N, et al.. Retinal arterioles have impaired reactivity to hyperoxia in type 1 diabetes. Acta Ophthalmol. 2010; 88: 453–457. [DOI] [PubMed] [Google Scholar]
- 44. Ashimatey BS, Green KM, Chu Z, Wang RK, Kashani AH. Impaired retinal vascular reactivity in diabetic retinopathy as assessed by optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2019; 60: 2468–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kwapong WR, Ye H, Peng C, et al.. Retinal microvascular impairment in the early stages of Parkinson's disease. Investig Ophthalmol Vis Sci. 2018; 59: 4115–4122. [DOI] [PubMed] [Google Scholar]
- 46. Kim SV, Semoun O, Pedinielli A, Jung C, Miere A, Souied EH. Optical coherence tomography angiography quantitative assessment of exercise-induced variations in retinal vascular plexa of healthy subjects. Investig Ophthalmol Vis Sci. 2019; 60: 1412–1419. [DOI] [PubMed] [Google Scholar]
- 47. O'Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol. 2018; 136: 1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feucht N, Maier M, Lepennetier G, et al.. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult Scler J. 2019; 25: 224–234. [DOI] [PubMed] [Google Scholar]
- 49. DeBuc DC, Somfai GM, Koller A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am J Physiol Heart Circ Physiol. 2017; 312: H201–H212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vasudevan S, Vo J, Shafer B, Nam AS, Vakoc BJ, Hammer DX. Toward optical coherence tomography angiography-based biomarkers to assess the safety of peripheral nerve electrostimulation. J Neural Eng. 2019; 16: 036024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang YS, Zhou N, Knoll BM, et al.. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer's Disease on optical coherence tomography angiography. PLoS One. 2019; 14: e0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang L, Li M, Liu Y, Zhou Q. Combining optical coherence tomography with magnetic resonance angiography and Doppler ultrasonography for clinical detection of scleroderma. Anat Rec. 2019, doi: 10.1002/ar.24340. [DOI] [PubMed] [Google Scholar]
- 53. Thomas RL, Dunstan FD, Luzio SD, et al.. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015; 99: 64–68. [DOI] [PubMed] [Google Scholar]
- 54. Colussi GL, Di Fabio A, Catena C, Chiuch A, Sechi LA. Involvement of endothelium-dependent and-independent mechanisms in midazolam-induced vasodilation. Hypertens Res. 2011; 34: 929–934. [DOI] [PubMed] [Google Scholar]
- 55. Lefer AM, Scalia R, Lefer DJ. Vascular effects of HMG CoA-reductase inhibitors (statins) unrelated to cholesterol lowering: New concepts for cardiovascular disease. Cardiovasc Res. 2001; 49: 281–287. [DOI] [PubMed] [Google Scholar]
- 56. Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009; 86: 527–532. [DOI] [PubMed] [Google Scholar]
- 57. Carrillo-Sepúlveda MA, Ceravolo GS, Fortes ZB, et al.. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc Res. 2010; 85: 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nowaczewska M, Kamińska A, Kukulska-Pawluczuk B, Junik R, Pawlak-Osińska K. Effect of hyperglycemia on cerebral blood flow in patients with diabetes. Diabetes Res Clin Pract. 2019; 153: 1–5. [DOI] [PubMed] [Google Scholar]
- 59. Luksch A, Lasta M, Polak K, et al.. Twelve-hour reproducibility of retinal and optic nerve blood flow parameters in healthy individuals. Acta Ophthalmol. 2009; 87: 875–880. [DOI] [PubMed] [Google Scholar]