Abstract
Background
Radical cystectomy is a standard treatment for muscle-invasive bladder cancer but frequently entails postoperative pulmonary complications (PPCs). Nutrition is closely associated with postoperative outcomes. Therefore, we evaluated the impact of preoperative prognostic nutritional index (PNI) on PPCs in radical cystectomy.
Methods
PNI was calculated as 10 × (serum albumin) + 0.005 × (total lymphocyte count). The risk factors for PPCs were evaluated using multivariate logistic regression analysis. A receiver operating characteristic curve analysis of PNI was performed, and an optimal cut-off value was identified. Propensity score-matched analysis was used to determine the impact of PNI on PPCs. Postoperative outcomes were also evaluated.
Results
PPCs occurred in 112 (13.6%) of 822 patients. Multivariate logistic regression analysis identified PNI, age, and serum creatinine level as risk factors. The area under the receiver operating characteristic curve of PNI for predicting PPCs was 0.714 (optimal cut-off value: 45). After propensity score matching, the incidence of PPCs in the PNI ≤ 45 group was significantly higher compared with the PNI > 45 group (20.8% vs. 6.8%; p < 0.001), and PNI ≤ 45 was associated with a higher incidence of PPCs (odds ratio 3.308, 95% confidence interval 1.779–6.151; p < 0.001). The rates of intensive care unit admission and prolonged (> 2 days) stay thereof were higher in patients who developed PPCs.
Conclusions
Preoperative PNI ≤ 45 was associated with a higher incidence of PPCs in radical cystectomy, suggesting that PNI provides useful information regarding pulmonary complications after radical cystectomy.
Radical cystectomy is the gold-standard treatment for non-metastatic muscle-invasive bladder cancer;1 however, it is one of the most technically demanding urological procedures, with 30–70% of patients undergoing the procedure showing various postoperative complications (e.g. pulmonary, cardiovascular, neurological, genitourinary, thromboembolic, and septic complications).2–5 In particular, postoperative pulmonary complications (PPCs) are associated with prolonged hospital stays and increased cost.6–8 Therefore, careful management is necessary to prevent PPCs in radical cystectomy.
Nutrition is associated with oncological survival, disease progression, and postoperative outcomes.9–12 In particular, malnutrition leads to a higher likelihood of postoperative complications, and as many as 40–80% of cancer patients may be affected by malnutrition.13,14 Therefore, evaluating the preoperative nutrition of cancer patients is crucial. The prognostic nutritional index (PNI) is widely utilized and may predict postoperative complications and outcomes in cancer patients.15–17 By using serum albumin concentration and total lymphocyte count, PNI allows for routine and easy assessment of patients’ nutritional status before surgery.18 However, to our knowledge, there are no studies on the association between PNI and pulmonary complications in patients undergoing radical cystectomy.
Thus, we evaluated the impact of preoperative PNI on PPCs in radical cystectomy. First, we identified the risk factors for PPCs using multivariate logistic regression analysis in patients who underwent radical cystectomy. Second, using propensity score-matched analysis, we evaluated the capability of PNI for predicting the occurrence of PPCs. Additionally, we compared the postoperative outcomes, including intensive care unit (ICU) admission rate, prolonged (more than 2 days) ICU stay rate, and hospital stay duration between patients who developed PPCs (PPC group) and those who did not (non-PPC group).
Materials and Methods
Patients
The Institutional Review Board of Asan Medical Center (Seoul, Republic of Korea) approved this study (approval no. 2019-1279). Patients who underwent radical cystectomy due to bladder cancer at Asan Medical Center between January 2007 and February 2019 were retrospectively reviewed and included in the analysis. Patients with incomplete medical records or those who underwent radical cystectomy combined with other surgery were excluded. Medical records were reviewed and PPCs were noted.
Intraoperative Protocols
Before the induction of anesthesia, all patients were monitored with electrocardiography, pulse oximetry, non-invasive blood pressure, end-tidal carbon dioxide concentration, and the bispectral index. General anesthesia was induced with 4–5 mg/kg thiopental sodium or 1.5–2 mg/kg propofol and 0.5–0.8 mg/kg rocuronium, and maintained with 1–4 vol% sevoflurane and 50% oxygen. Arterial catheterization was performed to continuously monitor the arterial blood pressure, and central venous catheterization was performed through the internal jugular vein. Tidal volume was adjusted to 8–10 mL per ideal body weight, and the respiratory rate was adjusted to maintain an end-tidal carbon dioxide concentration of 35–40 cmH2O. Positive end-expiratory pressure and the recruitment maneuver were not applied in any patient. The concentration of sevoflurane was adjusted to maintain a bispectral index of 40–60. Mean arterial blood pressure was maintained above 65 mmHg, with fluid administration and the intermittent use of inotropic agents or vasopressors (e.g. ephedrine, phenylephrine, or norepinephrine). For fluid administration, both crystalloids such as lactated Ringer’s solution or plasma solution A (CJ Pharmaceutical, Seoul, Republic of Korea) and colloids such as 6% hydroxyethyl starch or 5% albumin were used. Transfusion of red blood cells was performed when hemoglobin concentration was < 8 g/dL. Neuromuscular blockade was reversed with a neostigmine-glycopyrrolate mixture or sugammadex at the discretion of the anesthesiologist. Intravenous patient-controlled analgesia with fentanyl was used for postoperative pain management.
Radical cystectomy and pelvic lymphadenectomy were performed according to the standard technique used at our center.19,20 Standard or extended pelvic lymph node dissection was performed at the discretion of urologic surgeons. Standard pelvic lymph node dissection included the hypogastric, distal common iliac, external iliac, obturator, and perivesical lymph nodes. Extended lymph node dissection included the lymph node to the extent of the inferior vena cava, distal aorta, and proximal common iliac artery. A subsequent urinary diversion with an ileal neobladder or ileal conduit was performed at the discretion of urologic surgeons. Five highly experienced urologic surgeons performed all the operations.
Definition of Prognostic Nutritional Index and Postoperative Pulmonary Complications
The following formula was used to calculate the PNI: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per mm3).18 The existence of PPCs was determined if any of the following occurred within 7 days of radical cystectomy:21 atelectasis, pleural effusion, pneumothorax, bronchospasm, respiratory infection, respiratory failure, or aspiration pneumonitis. Clinicians diagnosed atelectasis, pleural effusion, and pneumothorax with chest X-rays. Bronchospasm was defined as newly detected expiratory wheezing that required treatment with bronchodilators. Respiratory infection was defined as the presence of one or more symptoms (i.e. new or changed lung opacities, new or changed sputum, leukocyte count > 12,000/mm3, or fever) while being treated with antibiotics for a suspected respiratory infection. Respiratory failure was defined as a partial arterial oxygen pressure < 60 mmHg in room air, partial arterial oxygen pressure/fractional inspired oxygen concentration < 300, or arterial oxygen saturation measured with pulse oximeter < 90%, requiring oxygen therapy. Aspiration pneumonitis was defined as an acute lung injury based on the aspiration of gastric contents.
Data Collection and Definitions
We collected demographic and preoperative data, including sex, age, body mass index, American Society of Anesthesiologists Physical Status, comorbidities (i.e. diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, and chronic obstructive pulmonary disease), smoking history, tumor stage, tumor grade, neoadjuvant chemotherapy, and preoperative laboratory tests (i.e. white blood cell count, lymphocyte count, hemoglobin concentration, platelet count, serum albumin concentration, PNI, and serum creatinine level). Coronary artery disease included a history of myocardial infarction, angina, heart attack, interventional angioplasty, or coronary artery bypass graft surgery; cerebrovascular disease included cerebrovascular accident, transient ischemic accident, stroke, or mini-stroke; and chronic obstructive pulmonary disease included chronic obstructive pulmonary disease, emphysema, or chronic bronchitis. Tumor stage was classified according to the 2010 American Joint Committee on Cancer tumor-node-metastasis staging system,22 and tumor grade was classified according to the 2016 World Health Organization grading system.23 Neoadjuvant chemotherapy was performed using one of the following combinations: gemcitabine and cisplatin; methotrexate and vinblastine; gemcitabine and carboplatin; or sulfate, cisplatin, and doxorubicin. Intraoperative data included operation duration, anesthesia duration, crystalloid and colloid amounts, red blood cell transfusion, reversal agent of neuromuscular blockade, and urinary diversion type. Postoperative outcomes included ICU admission rate, prolonged (more than 2 days) ICU stay,24 and hospital stay duration (number of days from surgery to discharge).
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as number (%). Continuous variables were compared using the Student’s t test or Mann–Whitney U-test, and categorical variables were compared using the χ2 test or Fisher’s exact test, between the PPC and non-PPC groups in all cohorts. Univariate and multivariate logistic regression analyses were performed to identify independent risk factors for PPCs. All covariates with a p-value < 0.05 in the univariate logistic regression analysis were entered into the multivariate logistic regression analysis. The ability of preoperative PNI for predicting PPCs in patients who underwent radical cystectomy was determined by calculating the area under the receiver operating characteristic (ROC) curve, which was calculated using the trapezoid rule. The optimal cut-off value was determined as the value with the highest sensitivity and specificity. The variance inflation factor was examined to check the multicollinearity. Variables with a variance inflation factor of more than 10 were considered as highly multicollinear and were eliminated from analyses. The Hosmer–Lemeshow goodness-of-fit statistic was used to measure the calibration of the logistic regression model, and the C-statistic was used for measuring the discrimination of the logistic regression model.
A 1:1 propensity score-matched analysis was performed using the nearest-neighbor method, with a caliper size of 0.2 to identify the impact of PNI on PPCs. To reduce selection bias and confounding factors, the propensity score was calculated using logistic regression analysis by including the following variables: sex, age, body mass index, American Society of Anesthesiologists Physical Status, diabetes mellitus, hypertension, coronary artery disease, cerebrovascular accident, chronic obstructive pulmonary disease, smoking history, tumor stage, tumor grade, neoadjuvant chemotherapy, hemoglobin concentration, serum creatinine concentration, operation duration, crystalloid and colloid amounts, red blood cell transfusion, reversal agent of neuromuscular blockade, and urinary diversion type. The standardized mean difference (SMD) was measured to determine the balance between the two groups before and after propensity score matching. The SMD cut-off value was < 0.2, which was regarded as indicating a sufficient balance between the two groups. After 1:1 propensity score matching, continuous variables were compared using the paired t-test, and categorical variables were compared using the McNemar’s test. The predictive value of PNI for the occurrence of PPCs in the propensity score-matched cohort was evaluated by conditional logistic regression analysis. All statistical analyses were carried out using STATA version 13.1 (StataCorp LLC, College Station, TX, USA) and SPSS® version 21 software (IBM Corporation, Armonk, NY, USA), with significance set at p < 0.05.
Results
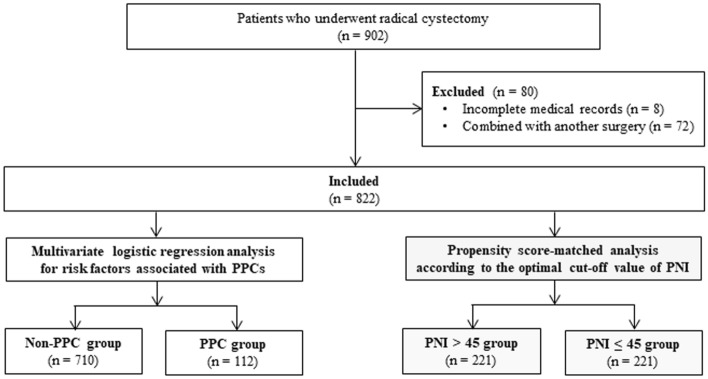
A total of 902 patients underwent radical cystectomy between January 2007 and February 2019. Eighty patients were excluded (8 due to incomplete medical records and 72 due to having other surgical procedures combined with radical cystectomy). Thus, 822 patients were included, of whom PPCs occurred in 112 patients (13.6%) (Fig. 1).
Fig. 1.
Study patients. A total of 902 patients who underwent radical cystectomy were evaluated, 822 of whom were included in this study. Patients were categorized according to the occurrence of PPCs in radical cystectomy, and multivariate logistic regression analysis was performed. The patients were then dichotomized according to the optimal cut-off value (i.e. 45) of PNI for predicting PPCs in radical cystectomy and a propensity score-matched analysis was performed. PPC postoperative pulmonary complication, PNI prognostic nutritional index
The demographic, preoperative, and intraoperative data of the patients are shown in Table 1. Age, hypertension, neoadjuvant chemotherapy, lymphocyte count, hemoglobin concentration, serum albumin concentration, PNI, serum creatinine level, operation duration, anesthesia duration, red blood cell transfusion, reversal agent of neuromuscular blockade, and urinary diversion type were all significantly different between the PPC and non-PPC groups. Univariate logistic regression analysis demonstrated that PNI, age, hypertension, neoadjuvant chemotherapy, hemoglobin concentration, serum creatinine level, operation duration, red blood cell transfusion, reversal agent of neuromuscular blockade, and urinary diversion type were associated with PPCs in patients who underwent radical cystectomy. Multivariate logistic regression analysis revealed that PNI (odds ratio [OR] 0.883, 95% confidence interval [CI] 0.854–0.914; p < 0.001), age (OR 1.027, 95% CI 1.004–1.050; p = 0.020), and serum creatinine level (OR 1.265, 95% CI 1.041–1.537; p = 0.018) were all significantly associated with PPCs in radical cystectomy (Table 2). ROC curve analysis revealed that the area under the curve of PNI was 0.714; the optimal cut-off value was 45, with a sensitivity of 66.2% and specificity of 66.1%. The highest variance inflation factor was 4.122, ensuring a lack of multicollinearity. The Hosmer–Lemeshow goodness-of-fit probability was 0.311, and the C-statistic for the model was 0.734, indicating good calibration and discrimination.
Table 1.
Demographic, preoperative, and intraoperative data
| Variables | All patients [n = 822] | Non-PPC group [n = 710] | PPC group [n = 112] | p-Valuea |
|---|---|---|---|---|
| Female sex | 131 (15.9) | 107 (15.1) | 24 (21.4) | 0.088 |
| Age, years | 64.2 ± 10.1 | 63.7 ± 10.0 | 67.2 ± 10.1 | < 0.001 |
| Body mass index, kg/m2 | 24.1 ± 3.2 | 24.1 ± 3.1 | 24.2 ± 3.8 | 0.845 |
| ASA Physical Status | 0.070 | |||
| ≤ 2 | 731 (88.9) | 637 (89.7) | 94 (83.9) | |
| 3 | 91 (11.1) | 73 (10.3) | 18 (16.1) | |
| Diabetes mellitus | 152 (18.5) | 126 (17.7) | 26 (23.2) | 0.166 |
| Hypertension | 331 (40.3) | 275 (38.7) | 56 (50.0) | 0.024 |
| Coronary artery disease | 40 (4.9) | 33 (4.6) | 7 (6.3) | 0.464 |
| Cerebrovascular disease | 30 (3.6) | 23 (3.2) | 7 (6.3) | 0.114 |
| COPD | 29 (3.5) | 24 (3.4) | 5 (4.5) | 0.563 |
| Smoking history | 0.169 | |||
| Non-smoker | 344 (41.8) | 288 (40.6) | 56 (50.0) | |
| Ex-smoker | 381 (46.4) | 336 (47.3) | 45 (40.2) | |
| Current smoker | 97 (11.8) | 86 (12.1) | 11 (11.3) | |
| Tumor stage | 0.863 | |||
| 1 | 51 (6.2) | 43 (6.1) | 8 (7.1) | |
| 2 | 541 (65.8) | 469 (66.1) | 72 (64.3) | |
| 3 | 138 (16.8) | 117 (16.5) | 21 (18.8) | |
| 4 | 92 (11.2) | 81 (11.4) | 11 (9.8) | |
| Tumor grade | 0.070 | |||
| 2 | 33 (4.0) | 25 (3.5) | 8 (7.1) | |
| 3 | 789 (96.0) | 685 (96.5) | 104 (92.9) | |
| Neoadjuvant chemotherapy | 163 (19.8) | 129 (18.2) | 34 (30.4) | 0.003 |
| Preoperative laboratory tests | ||||
| White blood cells, /mm3 | 6869.4 ± 2742.0 | 6885.2 ± 2747.5 | 6769.6 ± 2716.7 | 0.679 |
| Lymphocyte, /mm3 | 1918.6 ± 793.8 | 1968.1 ± 797.5 | 1604.4 ± 694.6 | < 0.001 |
| Hemoglobin, g/dL | 12.3 ± 2.0 | 12.4 ± 1.9 | 11.4 ± 2.2 | < 0.001 |
| Platelets, 103/µL | 245.4 ± 85.7 | 245.8 ± 84.0 | 242.8 ± 96.0 | 0.730 |
| Albumin, g/dL | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.3 ± 0.6 | < 0.001 |
| PNI | 46.6 ± 6.7 | 47.4 ± 6.1 | 41.4 ± 8.1 | < 0.001 |
| Creatinine, mg/dL | 1.1 ± 0.9 | 1.0 ± 0.7 | 1.4 ± 1.4 | < 0.001 |
| Operation duration, min | 430.0 ± 103.0 | 433.3 ± 100.4 | 409.6 ± 116.5 | 0.024 |
| Anesthesia duration, min | 451.1 ± 99.9 | 454.0 ± 97.3 | 432.9 ± 114.0 | 0.038 |
| Crystalloid amount, mL | 3421.5 ± 1368.5 | 3431.3 ± 1342.7 | 3359.4 ± 1527.2 | 0.606 |
| Colloid amount, mL | 570.2 ± 463.9 | 577.4 ± 468.9 | 524.7 ± 430.0 | 0.265 |
| Red blood cell transfusion | 482 (58.6) | 404 (56.9) | 78 (69.6) | 0.011 |
| Reversal agent of NMB | 0.001 | |||
| Neostigmine-glycopyrrolate | 730 (88.8) | 641 (90.3) | 89 (79.5) | |
| Sugammadex | 92 (11.2) | 69 (9.7) | 23 (20.5) | |
| Urinary diversion type | < 0.001 | |||
| Ileal conduit | 310 (37.7) | 247 (34.8) | 463 (56.3) | |
| Ileal neobladder | 512 (62.3) | 463 (65.2) | 49 (43.8) | |
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as number (%)
PPC postoperative pulmonary complication, ASA American Society of Anesthesiologists, COPD chronic obstructive pulmonary disease, PNI prognostic nutritional index, NMB neuromuscular blockade
aFor comparisons between the PPC and non-PPC groups
Table 2.
Univariate and multivariate logistic regression analyses of risk factors for postoperative pulmonary complications in radical cystectomy
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Sex (female) | 1.537 (0.936–2.524) | 0.089 | ||
| Age | 1.039 (1.017–1.062) | 0.001 | 1.027 (1.004–1.050) | 0.020 |
| Body mass index | 1.006 (0.945–1.071) | 0.845 | ||
| ASA Physical Status | ||||
| ≤ 2 | 1.000 | |||
| 3 | 1.671 (0.955–2.924) | 0.072 | ||
| Diabetes mellitus | 1.401 (0.868–2.262) | 0.167 | ||
| Hypertension | 1.582 (1.060–2.360) | 0.025 | ||
| Coronary artery disease | 1.368 (0.590–3.172) | 0.466 | ||
| Cerebrovascular accident | 1.991 (0.834–4.756) | 0.121 | ||
| COPD | 1.336 (0.499–3.567) | 0.565 | ||
| Smoking history | ||||
| Non-smoker | 1.000 | |||
| Ex-smoker | 0.689 (0.451–1.051) | 0.084 | ||
| Current-smoker | 0.658 (0.330–1.311) | 0.234 | ||
| Tumor stage | ||||
| 1 | 1.000 | |||
| 2 | 0.825 (0.373–1.826) | 0.635 | ||
| 3 | 0.965 (0.398–2.340) | 0.937 | ||
| 4 | 0.730 (0.273–1.951) | 0.530 | ||
| Tumor grade | ||||
| 2 | 1.000 | |||
| 3 | 0.474 (0.208–1.080) | 0.076 | ||
| Neoadjuvant chemotherapy | 1.963 (1.257–3.066) | 0.003 | ||
| Hemoglobin | 0.769 (0.692–0.855) | < 0.001 | ||
| PNI | 0.872 (0.844–0.901) | < 0.001 | 0.883 (0.854–0.914) | < 0.001 |
| Creatinine | 1.308 (1.104–1.550) | 0.002 | 1.265 (1.041–1.537) | 0.018 |
| Operation duration | 0.998 (0.996–1.000) | 0.024 | ||
| Crystalloid amount | 1.000 (1.000–1.000) | 0.605 | ||
| Colloid amount | 1.000 (0.999–1.000) | 0.264 | ||
| Red blood cell transfusion | 1.738 (1.131–2.669) | 0.012 | ||
| Reversal agent of NMB | ||||
| Neostigmine-glycopyrrolate | 1.000 | |||
| Sugammadex | 2.401 (1.425–4.044) | 0.001 | ||
| Urinary diversion type | ||||
| Ileal conduit | 1.000 | |||
| Ileal neobladder | 0.415 (0.277–0.621) | < 0.001 | ||
OR odds ratio, CI confidence interval, ASA American Society of Anesthesiologists, COPD chronic obstructive pulmonary disease, PNI prognostic nutritional index, NMB neuromuscular blockade
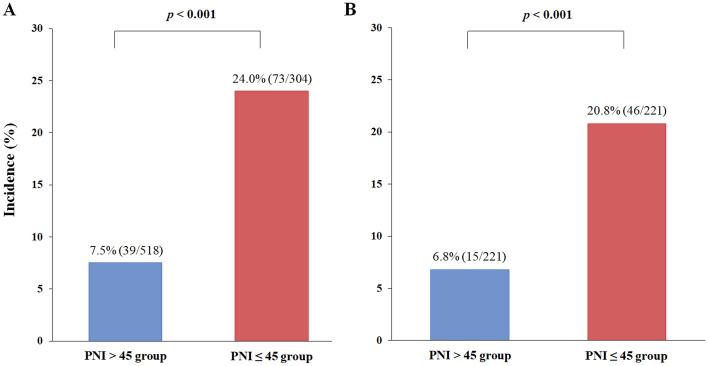
Of the 822 patients, 518 (63.0%) had PNI > 45 and 304 (37.0%) had PNI ≤ 45 (Table 3). After 1:1 propensity score matching, we generated 221 matched pairs and divided the patients into PNI > 45 (n = 221) and PNI ≤ 45 (n = 221) groups (Table 3). All covariates were well-balanced, with an SMD < 0.2, and no significant differences were found between the PNI > 45 and PNI ≤ 45 groups (Table 3). The incidence of PPCs in the PNI ≤ 45 group was significantly higher than in the PNI > 45 group, before (24.0% vs. 7.5%; p < 0.001) (Fig. 2a) and after (20.8% vs. 6.8%; p < 0.001) (Fig. 2b) propensity score matching. The predictive value of the PNI ≤ 45 group for the occurrence of PPCs is shown in Fig. 3. Compared with PNI > 45, PNI ≤ 45 was associated with higher incidences of PPCs in unadjusted (OR 3.881, 95% CI 2.552–5.903; p < 0.001), multivariate-adjusted (OR 3.542, 95% CI 2.224–5.643; p < 0.001), and propensity score-matched analyses (OR 3.308, 95% CI 1.779–6.151; p < 0.001) (Fig. 3).
Table 3.
Demographic, preoperative, and intraoperative data of patients dichotomized according to the optimal cut-off value of PNI before and after propensity score matching
| Variable | Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|---|
| PNI > 45 [n = 518] | PNI ≤ 45 [n = 304] | SMD | p-Value | PNI > 45 [n = 221] | PNI ≤ 45 [n = 221] | SMD | p-Value | |
| Female sex | 66 (12.7) | 65 (21.4) | 0.210 | 0.002 | 40 (18.1) | 45 (20.4) | 0.055 | 0.625 |
| Age, years | 62.8 ± 10.1 | 66.5 ± 9.7 | 0.389 | < 0.001 | 66.0 ± 10.0 | 66.0 ± 10.0 | 0.004 | 0.964 |
| Body mass index, kg/m2 | 24.7 ± 3.1 | 23.2 ± 3.1 | −0.492 | < 0.001 | 23.8 ± 2.7 | 23.6 ± 3.0 | −0.065 | 0.435 |
| ASA Physical Status | 0.126 | 0.065 | 0.013 | > 0.999 | ||||
| ≤ 2 | 469 (90.5) | 262 (86.2) | 194 (87.8) | 193 (87.3) | ||||
| 3 | 49 (9.5) | 42 (13.8) | 27 (12.2) | 28 (12.7) | ||||
| Diabetes mellitus | 96 (18.5) | 56 (18.4) | −0.003 | > 0.999 | 47 (21.3) | 45 (20.4) | −0.023 | 0.905 |
| Hypertension | 205 (39.6) | 126 (41.4) | 0.038 | 0.607 | 94 (42.5) | 94 (42.5) | < 0.001 | > 0.999 |
| Coronary artery disease | 24 (4.6) | 16 (5.3) | 0.028 | 0.738 | 12 (5.4) | 12 (5.4) | < 0.001 | > 0.999 |
| Cerebrovascular disease | 16 (3.1) | 14 (4.6) | 0.072 | 0.335 | 10 (4.5) | 8 (3.6) | −0.043 | 0.804 |
| COPD | 20 (3.9) | 9 (3.0) | −0.053 | 0.562 | 6 (2.7) | 5 (2.3) | −0.027 | > 0.999 |
| Smoking | −0.154 | 0.034 | 0.054 | 0.651 | ||||
| Non/Ex-smoker | 316 (61.0) | 162 (53.3) | 105 (47.5) | 99 (44.8) | ||||
| Current smoker | 202 (39.0) | 142 (46.7) | 116 (52.5) | 122 (55.2) | ||||
| Tumor stage | 0.101 | 0.171 | 0.098 | 0.368 | ||||
| <3 | 382 (73.7) | 210 (69.1) | 158 (71.5) | 148 (67.0) | ||||
| ≥3 | 136 (26.3) | 94 (30.9) | 63 (28.5) | 73 (33.0) | ||||
| Tumor grade | 0.295 | 0.009 | −0.071 | 0.727 | ||||
| 2 | 28 (5.4) | 5 (1.6) | 3 (1.4) | 5 (2.3) | ||||
| 3 | 490 (94.6) | 299 (98.4) | 218 (98.6) | 216 (97.7) | ||||
| Neoadjuvant chemotherapy | 76 (14.7) | 87 (28.6) | 0.308 | < 0.001 | 54 (24.4) | 63 (28.5) | 0.090 | 0.374 |
| Hemoglobin, g/dL | 13.0 ± 1.7 | 11.0 ± 1.7 | − 1.157 | < 0.001 | 11.7 ± 1.5 | 11.5 ± 1.6 | −0.123 | 0.210 |
| Creatinine, mg/dL | 1.0 ± 0.8 | 1.2 ± 1.0 | 0.110 | 0.090 | 1.1 ± 0.9 | 1.0 ± 0.4 | −0.087 | 0.188 |
| Operation duration, min | 440.0 ± 103.3 | 413.1 ± 100.5 | −0.268 | < 0.001 | 423.1 ± 103.2 | 419.8 ± 97.6 | −0.033 | 0.735 |
| Crystalloid amount, mL | 3491.8 ± 1290.9 | 3301.7 ± 1486.0 | −0.128 | 0.055 | 3370.6 ± 1294.3 | 3397.6 ± 1397.4 | 0.018 | 0.832 |
| Colloid amount, mL | 600.2 ± 460.4 | 519.1 ± 466.0 | −0.174 | 0.016 | 549.0 ± 455.0 | 514.4 ± 476.9 | −0.074 | 0.431 |
| Red blood cell transfusion | 276 (53.3) | 206 (67.8) | 0.309 | < 0.001 | 141 (63.8) | 138 (62.4) | −0.029 | 0.830 |
| Reversal agent of NMB | 0.249 | < 0.001 | 0.024 | 0.890 | ||||
| Neostigmine-glycopyrrolate | 478 (92.3) | 252 (82.9) | 191 (86.4) | 189 (85.5) | ||||
| Sugammadex | 40 (7.7) | 52 (17.1) | 30 (13.6) | 32 (14.5) | ||||
| Urinary diversion type | 0.484 | < 0.001 | −0.027 | 0.847 | ||||
| Ileal conduit | 149 (28.8) | 161 (53.0) | 100 (45.2) | 103 (46.6) | ||||
| Ileal neobladder | 369 (71.2) | 143 (47.0) | 121 (54.8) | 118 (53.4) | ||||
Data are expressed as mean ± standard deviation or number of patients (%), as appropriate
PNI prognostic nutritional index, SMD standardized mean difference, ASA American Society of Anesthesiologists, COPD chronic obstructive pulmonary disease, NMB neuromuscular blockade
Fig. 2.
Comparison of the incidence of postoperative pulmonary complications between the PNI > 45 and PNI ≤ 45 groups (a) before and (b) after propensity score matching in radical cystectomy. The incidences of postoperative pulmonary complications in the PNI ≤ 45 group were significantly higher than those in the PNI > 45 group. PNI prognostic nutritional index
Fig. 3.
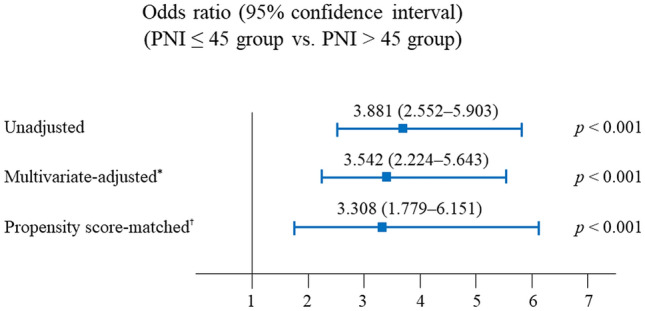
Predictive value of the PNI ≤ 45 group for the occurrence of postoperative pulmonary complications in radical cystectomy. *The multivariate-adjusted odds ratio was adjusted by using the variables shown in Table 2. †Propensity score matching was performed by using the variables shown in Table 3. PNI prognostic nutritional index
The ICU admission rate and prolonged (more than 2 days) ICU stay rate were significantly higher in the PPC group than in the non-PPC group (37/112 [33.0%] vs. 165/710 [23.2%], p = 0.025; 7/112 [2.7%] vs. 6/710 [0.8%], p < 0.001, respectively) (Table 4). However, there was no significant difference in hospital stay duration between the PPC and non-PPC groups.
Table 4.
Postoperative outcomes after radical cystectomy in 822 patients
| Variables | Non-PPC group [n = 710] | PPC group [n = 112] | p-Value |
|---|---|---|---|
| ICU admission rate | 165 (23.2) | 37 (33.0) | 0.025 |
| Prolonged (> 2 days) ICU stay rate | 6 (0.8) | 7 (2.7) | < 0.001 |
Variables are presented as number (%)
PPC postoperative pulmonary complication, ICU intensive care unit
Discussion
The main findings of the present study are that the incidence of PPCs in 822 patients who underwent radical cystectomy was 13.6%, and that PNI, age, and serum creatinine level were independent risk factors for PPCs. The optimal cut-off value of preoperative PNI for predicting PPCs in radical cystectomy was 45. After propensity score matching, the incidence of PPCs in the PNI ≤ 45 group was significantly higher than in the PNI > 45 group, and preoperative PNI ≤ 45 was significantly associated with an increased incidence of PPCs in radical cystectomy. Furthermore, ICU admission rate and a prolonged (more than 2 days) ICU stay were significantly higher in the PPC group than in the non-PPC group. To our knowledge, the present study is the first to demonstrate that preoperative PNI is significantly associated with PPCs in patients undergoing radical cystectomy.
Radical cystectomy is one of the most complex urological procedures for muscle-invasive bladder cancer and provides good local cancer control and long-term survival; however, radical cystectomy is associated with high likelihoods for postoperative morbidity and mortality.25,26 Pulmonary complications, in particular, are one of the most common postoperative complications.4,27 In previous studies, the incidence of PPCs varied between 5 and 80% depending on the type of surgery, patient characteristics, and the definition of PPCs.21,28 Xia et al. reported that the incidence of PPCs was 5.6% in radical cystectomy when PPCs were defined as pneumonia, unplanned reintubation, and ventilator support > 48 h within 30 days of radical cystectomy.29 The present study demonstrated that the incidence of PPCs was 13.6%, and the discrepancies in the incidence rates of PPCs might have resulted from the different definitions of PPCs used in the two studies. In contrast, Mossanen et al.30 reported that the incidence of PPCs was 13.4% in radical cystectomy. In their study, PPCs were defined as pleural effusion, respiratory failure, atelectasis, aspiration, and pneumothorax, which is similar to the definition used in this study. Therefore, the incidence of PPCs in the present study might be comparable with that reported by Mossanen et al.30
We found that preoperative PNI was an independent risk factor for PPCs in radical cystectomy. Nutritional status in cancer patients is an essential factor in determining postoperative prognosis, morbidity, and mortality.31–33 In particular, malnutrition might weaken respiratory muscles, decrease ventilatory drive, and damage the defense mechanism against infection.34,35 Malnutrition can also impair the immune system by suppressing and inhibiting lymphocyte transformation.36 PNI was initially proposed by Onodera et al.18 and was calculated using the serum albumin concentration to assess nutritional status and the lymphocyte count to reflect the aspects of immunity. Moreover, PNI has been used as a useful predictor of postoperative prognoses such as complications and mortality by estimating the nutritional status of various oncologic patients preoperatively.37,38 Previous studies reported that oncologic patients with low PNI had low survival rates and high rates of postoperative complications.39,40 Hypoalbuminemia delays tissue healing, reduces collagen synthesis, and impairs the immune response.36,41 A low lymphocyte count reflects an increase in the degree of systemic inflammation and impairment of the immune status of patients.42,43 Therefore, we assume that preoperative PNI, which can be easily calculated using routine laboratory testing, may be used to minimize PPCs in radical cystectomy.
Our propensity score-matched analysis showed that the optimal cut-off value of the preoperative PNI for predicting PPCs in radical cystectomy was 45. This cut-off value of the PNI is in line with the results of other studies and is related to poor postoperative prognosis in various cancers.38,44–46 Mohri et al.44 reported that in patients with colorectal cancer, PNI < 45 is significantly associated with serious complications, including anastomotic leakage, severe infection, bowel obstruction requiring additional surgery, cardiopulmonary failure, and pulmonary embolism after colorectal surgery. Chan et al.45 also reported that in patients with early-stage hepatocellular carcinoma, overall postoperative survival and disease-free survival were worse when the PNI was < 45. Additionally, several studies have reported that PNI < 45 indicates moderate-to-severe malnutrition and is associated with poor survival and surgical complications such as fistula, leakage, bleeding, and surgical site infection.38,46 However, the optimal cut-off value of the PNI to predict PPCs in radical cystectomy has not yet been evaluated, and our study is the first to report that bladder cancer patients with PNI ≤ 45 are at a higher risk of PPCs in radical cystectomy. Therefore, radical cystectomy patients with a preoperative PNI ≤ 45 should be given special attention to minimize the risk of PPCs.
The present study also found that PPCs were more common in older patients. Advanced age was consistently identified in several studies as a risk factor for PPCs.21,47 With aging, the thorax and lung parenchyma stiffen, ciliary function decreases, and dead space increases. The net pulmonary function overall declines, leading to an increased risk of PPCs.48 These PPCs account for approximately 40% of the perioperative mortality in patients over 65 years of age.49 Moreover, in previous studies, there were significant associations between age and overall complications in radical cystectomy.50,51 The incidence of bladder cancer increases with age and occurs frequently in people over 70 years of age.50 As life expectancy increases, a greater number of older patients are undergoing radical cystectomy. Therefore, meticulous perioperative management in older patients might be a key factor for improving postoperative outcomes in radical cystectomy.
Similar to previous studies,52–54 this study demonstrated that the preoperative serum creatinine level was associated with PPCs in radical cystectomy. Radical cystectomy requires copious fluid administration because of the extended operation time and bleeding. Patients with high serum creatinine levels have impaired kidney function, which might make it difficult to handle excessive perioperative fluid administration.55 Thus, these factors could increase the risk of postoperative pulmonary edema or effusion, which might explain why preoperative increased serum creatinine level is one of the risk factors for PPCs in radical cystectomy. Therefore, our results suggest that careful fluid management is an essential intervention for reducing PPCs in radical cystectomy patients with high serum creatinine levels.
In the present study, we found that low PNI, old age, and high serum creatinine level were associated with PPCs in bladder cancer patients undergoing radical cystectomy. However, our previous studies demonstrated that male sex, older age, and high body mass index were independent risk factors of PPCs in robot-assisted laparoscopic prostatectomy, percutaneous nephrolithotomy, and laparoscopic pylorus-preserving pancreaticoduodenectomy.7,8,56 These differences in independent risk factors for PPCs may be, at least in part, explained by differences in the patient characteristics and the type of surgery.
This study demonstrated that the ICU admission rate and prolonged (more than 2 days) ICU stay rate were significantly higher in the PPC group than in the non-PPC group. Nilsson et al. defined prolonged (more than 2 days) ICU stay as a poor outcome because it could reliably predict mortality and hospital costs in open-heart surgery.57 Michalopoulos et al. also reported that prolonged (more than 2 days) stay in the ICU increased overall hospital costs for patients undergoing elective coronary artery bypass graft surgery, and limited the number of other operations.24 As with other studies, we consider that a prolonged ICU stay, as well as the ICU admission rate, represent a meaningfully poor postoperative outcome.
The present study has several limitations. First, because this study had a retrospective design, we could not evaluate all covariates that may have affected the analysis. Therefore, our study may be, at least in part, influenced by inevitable selection bias. However, we included almost all covariate factors related to PPCs in patients undergoing radical cystectomy. Moreover, we performed a propensity score-matched analysis to minimize the bias. Second, because all surgery was performed by highly specialized surgeons at a single, large-sized center, our results might not be readily applicable in other types of facilities. Therefore, the results should be generalized with caution. Third, because the relationship between PNI and PPCs in other surgeries has not been specifically evaluated, further studies are needed to clarify it.
Conclusion
PPCs occurred in 13.6% of patients undergoing radical cystectomy. PPCs were associated with PNI, age, and serum creatinine level. PNI ≤ 45 was significantly associated with a higher likelihood of PPCs in patients undergoing radical cystectomy. Moreover, the rates of ICU admission and prolonged ICU stay were higher in radical cystectomy patients with PPCs than those without. These results suggest that preoperative PNI provides useful information about pulmonary complications that lead to poor postoperative outcomes in radical cystectomy. Additionally, preoperative PNI evaluation can be recommended in radical cystectomy patients at risk of PPCs.
Acknowledgment
None.
Disclosure
Jihion Yu, Bumsik Hong, Jun-Young Park, Jai-Hyun Hwang, and Young-Kug Kim have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–825. doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Chang SS, Cookson MS, Baumgartner RG, Wells N, Smith JA., Jr Analysis of early complications after radical cystectomy: results of a collaborative care pathway. J Urol. 2002;167:2012–2016. [PubMed] [Google Scholar]
- 3.Cookson MS, Chang SS, Wells N, Parekh DJ, Smith JA., Jr Complications of radical cystectomy for nonmuscle invasive disease: comparison with muscle invasive disease. J Urol. 2003;169:101–104. doi: 10.1016/S0022-5347(05)64045-1. [DOI] [PubMed] [Google Scholar]
- 4.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–174. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Joung KW, Choi SS, Kong YG, et al. Incidence and risk factors of acute kidney injury after radical cystectomy: importance of preoperative serum uric acid level. Int J Med Sci. 2015;12:599–604. doi: 10.7150/ijms.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology. 2000;92:1467–1472. doi: 10.1097/00000542-200005000-00037. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Park JY, Kim DH, et al. Incidence and risk factors of pulmonary complications after robot-assisted laparoscopic prostatectomy: a retrospective observational analysis of 2208 patients at a large single center. J Clin Med. 2019;8:1509. doi: 10.3390/jcm8101509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Choi JM, Lee J, et al. Risk factors for pulmonary complications after percutaneous nephrolithotomy: a retrospective observational analysis. Medicine. 2016;95:e4513. doi: 10.1097/MD.0000000000004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerantola Y, Grass F, Cristaudi A, Demartines N, Schafer M, Hubner M. Perioperative nutrition in abdominal surgery: recommendations and reality. Gastroenterol Res Pract. 2011;2011:739347. doi: 10.1155/2011/739347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh CA, Kim DH, Oh SJ, et al. Nutritional risk index as a predictor of postoperative wound complications after gastrectomy. World J Gastroenterol. 2012;18:673–678. doi: 10.3748/wjg.v18.i7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa-Rodriguez M, Pocovi-Gerardino G, Callejas-Rubio JL, et al. The prognostic nutritional index and nutritional risk index are associated with disease activity in patients with systemic lupus erythematosus. Nutrients. 2019;11:638. doi: 10.3390/nu11030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–785. doi: 10.1038/sj.ejcn.1601412. [DOI] [PubMed] [Google Scholar]
- 13.Ollenschlager G, Viell B, Thomas W, Konkol K, Burger B. Tumor anorexia: causes, assessment, treatment. Recent Results Cancer Res. 1991;121:249–259. doi: 10.1007/978-3-642-84138-5_28. [DOI] [PubMed] [Google Scholar]
- 14.Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12:S15–S19. doi: 10.1016/0899-9007(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 15.Takagi K, Domagala P, Polak WG, Buettner S, Ijzermans JNM. The controlling nutritional status score and postoperative complication risk in gastrointestinal and hepatopancreatobiliary surgical oncology: a systematic review and meta-analysis. Ann Nutr Metab. 2019;74:303–312. doi: 10.1159/000500233. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Kono Y, Murakami Y, et al. Influence of prognostic nutritional index and tumor markers on survival in gastric cancer surgery patients. Langenbecks Arch Surg. 2017;402:501–507. doi: 10.1007/s00423-017-1572-y. [DOI] [PubMed] [Google Scholar]
- 17.Okada S, Shimada J, Teramukai S, et al. Risk stratification according to the prognostic nutritional index for predicting postoperative complications after lung cancer surgery. Ann Surg Oncol. 2018;25:1254–1261. doi: 10.1245/s10434-018-6368-y. [DOI] [PubMed] [Google Scholar]
- 18.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 19.Jeong IG, You D, Kim JW, et al. Outcomes of single lymph node positive urothelial carcinoma after radical cystectomy. J Urol. 2011;185:2085–2090. doi: 10.1016/j.juro.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 20.Jeong IG, You D, Kim J, et al. Factors associated with non-orthotopic urinary diversion after radical cystectomy. World J Urol. 2012;30:815–820. doi: 10.1007/s00345-012-0846-9. [DOI] [PubMed] [Google Scholar]
- 21.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 23.Comperat EM, Burger M, Gontero P, et al. Grading of urothelial carcinoma and the new “World Health Organisation classification of tumours of the urinary system and male genital organs 2016”. Eur Urol Focus. 2019;5:457–466. doi: 10.1016/j.euf.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Michalopoulos A, Tzelepis G, Pavlides G, Kriaras J, Dafni U, Geroulanos S. Determinants of duration of ICU stay after coronary artery bypass graft surgery. Br J Anaesth. 1996;77:208–212. doi: 10.1093/bja/77.2.208. [DOI] [PubMed] [Google Scholar]
- 25.Hollenbeck BK, Miller DC, Taub D, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005;174:1231–1237. doi: 10.1097/01.ju.0000173923.35338.99. [DOI] [PubMed] [Google Scholar]
- 26.Jun IJ, Kim J, Kim HG, Koh GH, Hwang JH, Kim YK. Risk factors of postoperative major adverse cardiac events after radical cystectomy: implication of diastolic dysfunction. Sci Rep. 2019;9:14096. doi: 10.1038/s41598-019-50582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novara G, De Marco V, Aragona M, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol. 2009;182:914–921. doi: 10.1016/j.juro.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Ferreyra GP, Baussano I, Squadrone V, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg. 2008;247:617–626. doi: 10.1097/SLA.0b013e3181675829. [DOI] [PubMed] [Google Scholar]
- 29.Xia L, Taylor BL, Guzzo TJ. Characteristics and associated factors of postoperative pulmonary complications in patients undergoing radical cystectomy for bladder cancer: a national surgical quality improvement program study. Clin Genitourin Cancer. 2017;15:661–669. doi: 10.1016/j.clgc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Mossanen M, Krasnow RE, Lipsitz SR, et al. Associations of specific postoperative complications with costs after radical cystectomy. BJU Int. 2018;121:428–436. doi: 10.1111/bju.14064. [DOI] [PubMed] [Google Scholar]
- 31.van Bokhorst-de van der S, van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86:519–527. [PubMed] [Google Scholar]
- 32.Warren S. The immediate causes of death in cancer. Am J Med Sci. 1932;184:610–615. [Google Scholar]
- 33.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI) Br J Cancer. 2012;106:1439–1445. doi: 10.1038/bjc.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezzell L, Jensen GL. Malnutrition in chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;72:1415–1416. doi: 10.1093/ajcn/72.6.1415. [DOI] [PubMed] [Google Scholar]
- 35.Rochester DF, Esau SA. Malnutrition and the respiratory system. Chest. 1984;85:411–415. doi: 10.1378/chest.85.3.411. [DOI] [PubMed] [Google Scholar]
- 36.Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343–1346. doi: 10.1016/0016-5085(86)90185-x. [DOI] [PubMed] [Google Scholar]
- 37.Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28:396–400. doi: 10.1053/ejso.2002.1257. [DOI] [PubMed] [Google Scholar]
- 38.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe M, Iwatsuki M, Iwagami S, Ishimoto T, Baba Y, Baba H. Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg. 2012;36:1632–1639. doi: 10.1007/s00268-012-1526-z. [DOI] [PubMed] [Google Scholar]
- 40.Migita K, Takayama T, Saeki K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647–2654. doi: 10.1245/s10434-013-2926-5. [DOI] [PubMed] [Google Scholar]
- 41.Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998;53:789–803. doi: 10.1046/j.1365-2044.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 42.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 43.Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945–950. doi: 10.7314/apjcp.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 44.Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–2692. doi: 10.1007/s00268-013-2156-9. [DOI] [PubMed] [Google Scholar]
- 45.Chan AW, Chan SL, Wong GL, et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol. 2015;22:4138–4148. doi: 10.1245/s10434-015-4516-1. [DOI] [PubMed] [Google Scholar]
- 46.Nakatani M, Migita K, Matsumoto S, et al. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus. 2017;30:1–7. doi: 10.1093/dote/dox020. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. 2017;152:157–166. doi: 10.1001/jamasurg.2016.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beliveau MM, Multach M. Perioperative care for the elderly patient. Med Clin North Am. 2003;87:273–289. doi: 10.1016/s0025-7125(02)00155-4. [DOI] [PubMed] [Google Scholar]
- 49.Zaugg M, Lucchinetti E. Respiratory function in the elderly. Anesthesiol Clin North Am. 2000;18:47–58. doi: 10.1016/s0889-8537(05)70148-6. [DOI] [PubMed] [Google Scholar]
- 50.Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. 2009;56:443–454. doi: 10.1016/j.eururo.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Knap MM, Lundbeck F, Overgaard J. Early and late treatment-related morbidity following radical cystectomy. Scand J Urol Nephrol. 2004;38:153–160. doi: 10.1080/00365590310020060. [DOI] [PubMed] [Google Scholar]
- 52.Johnson RG, Arozullah AM, Neumayer L, Henderson WG, Hosokawa P, Khuri SF. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1188–1198. doi: 10.1016/j.jamcollsurg.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien MM, Gonzales R, Shroyer AL, et al. Modest serum creatinine elevation affects adverse outcome after general surgery. Kidney Int. 2002;62:585–592. doi: 10.1046/j.1523-1755.2002.00486.x. [DOI] [PubMed] [Google Scholar]
- 54.Money SR, Rice K, Crockett D, et al. Risk of respiratory failure after repair of thoracoabdominal aortic aneurysms. Am J Surg. 1994;168:152–155. doi: 10.1016/s0002-9610(94)80057-x. [DOI] [PubMed] [Google Scholar]
- 55.Etz CD, Di Luozzo G, Bello R, et al. Pulmonary complications after descending thoracic and thoracoabdominal aortic aneurysm repair: predictors, prevention, and treatment. Ann Thorac Surg. 2007;83:870–876. doi: 10.1016/j.athoracsur.2006.10.099. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Seo H, Kim HK, Kim SC, Kim YK. Risk Factors for Pulmonary Complications After Laparoscopic Pylorus-preserving Pancreaticoduodenectomy: a Retrospective Observational Analysis. Surg Laparosc Endosc Percutan Tech. 2018;28:128–132. doi: 10.1097/SLE.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson J, Algotsson L, Hoglund P, Luhrs C, Brandt J. EuroSCORE predicts intensive care unit stay and costs of open heart surgery. Ann Thorac Surg. 2004;78:1528–1534. doi: 10.1016/j.athoracsur.2004.04.060. [DOI] [PubMed] [Google Scholar]