Pesticides and fertilizers are applied to agricultural crops to reduce losses by pathogens, and enhance crop biomass, although their production and application can also incur several economic and environmental penalties. Microbial Volatile Organic Compounds (mVOCs) could provide more sustainable alternatives to these inputs, due to their ability to inhibit plant pathogens, induce plant resistance against pathogens, and enhance plant growth promotion. This review will highlight recent advances in our understanding of the biological activities of mVOCs, highlighting their potential suitability as a more sustainable alternative to pesticides and fertilizers.

Summary
Global agricultural systems are under increasing pressure to deliver sufficient, healthy food for a growing population. Seasonal inputs, including synthetic pesticides and fertilizers, are applied to crops to reduce losses by pathogens, and enhance crop biomass, although their production and application can also incur several economic and environmental penalties. New solutions are therefore urgently required to enhance crop yield whilst reducing dependence on these seasonal inputs. Volatile Organic Compounds (VOCs) produced by soil microorganisms may provide alternative, sustainable solutions, due to their ability to inhibit plant pathogens, induce plant resistance against pathogens and enhance plant growth promotion. This review will highlight recent advances in our understanding of the biological activities of microbial VOCs (mVOCs), providing perspectives on research required to develop them into viable alternatives to current unsustainable seasonal inputs. This can identify potential new avenues for mVOC research and stimulate discussion across the academic community and agri‐business sector.
Introduction
By 2100, the United Nations projects that the global population will increase by around 4 billion, which may require agricultural production to double or triple to keep pace with population growth (United Nations Department of Economic and Social Affairs Population Division, 2017; Rohr et al., 2019). To date, agricultural practice has relied on the application of synthetic chemical inputs to optimize crop yields, including synthetic pesticides, which reduce crop losses by targeting plant pathogens, and synthetic fertilizers, applied to increase crop biomass. Synthetic pesticides play a critical role in mitigating crop damage by pathogens, which are responsible for annual crop losses of 17–30% for the five major crops (Savary et al., 2019). However, the development of synthetic pesticides is in itself unsustainable, estimated to cost approximately $250 million to bring a single active ingredient to market, with an estimated success rate of 1 in 140 000 synthesized compounds (Lamberth et al., 2013). Moreover, the over‐application of pesticides can lead to the development of pesticide resistance, rendering them less effective. The production and application of inorganic nitrogen fertilizer has resulted in crop production being the largest cause of human alteration to the global nitrogen cycle (Smil, 1999). The Haber–Bosch process is used to produce inorganic nitrogen fertilizer, through the conversion of hydrogen and nitrogen into ammonia. However, this process is energy intensive, occurring at high temperatures and pressure and generating a carbon footprint contributing ~1.2% of overall global anthropogenic CO2 emissions (Nørskov and Chen, 2016). Furthermore, the application of inorganic nitrogen to soils leads to enhanced microbial production of nitrous oxide (N2O), the potent greenhouse gas, through soil microbial nitrification and de‐nitrification. As such, concentrations of N2O have substantially increased in the atmosphere since 1960 as a direct result of fertilizer applications (Davidson, 2009). With projected increases in crop demand, agricultural expansion could result in approximately 10‐fold increases in pesticide use, and 2.7‐fold increases in fertilizer application (Rohr et al., 2019). Concerted efforts should therefore be made to develop more sustainable control methods to reduce over‐reliance on synthetic fertilizer and pesticides, through shifts in agronomic practice (Tester and Langridge, 2010; Fisher et al., 2018). Whilst genetically modified crops demonstrating enhanced disease resistance show potential to reduce pathogen damage and could potentially reduce the requirement for pesticide inputs, the regulatory frameworks required to commercialize the crops are lengthy (Kanchiswamy et al., 2015a). Therefore, it is an opportune time to explore alternative control strategies to chemical inputs or genetic modification.
One alternative solution to chemical inputs is through the addition of antagonistic, beneficial, microorganisms, due to their ability to antagonize pathogenic soil microbes, and enhances plant biomass. Soil microorganisms produce a wide spectrum of secondary metabolites enabling them to compete with neighbouring microorganisms, which they have likely evolved to compete for the same resources within soil (Brakhage and Schroeckh, 2011; Garbeva and Weisskopf, 2020). For example, bacteria from the genus of soil‐dwelling Streptomyces spp. produce a diverse range of secondary metabolites, which have been exploited for human medicine, with approximately 80% of antibiotics currently being sourced from the genus (de Lima Procópio et al., 2012). The structural diversity of secondary metabolites explains their broad spectrum of activities, including mediating communication intra‐ and inter‐specifically, defence against competitors, nutrient acquisition and symbiotic interactions (Spiteller, 2015; Macheleidt et al., 2016). Whilst most research on microbial secondary metabolites focusses on non‐volatile compounds, increasing attention is being paid to microbial volatile organic compounds (mVOCs). VOCs are a class of secondary metabolites with a low molecular weight (< 300 Da), high vapour pressure and low boiling points, which tend to be lipophilic in nature (Schulz‐Bohm et al., 2017). Their ability to diffuse through gas and water‐filled pores within the heterogenous soil matrix make them suitable for both short‐ and long‐distance signalling (Maffei et al., 2011; Kanchiswamy et al., 2015b; Schulz‐Bohm et al., 2017). Under competitive soil conditions, due to the presence of other competing organisms, VOCs are important for antibiosis and signalling for symbiotic interactions (Effmert et al., 2012). The capability of mVOCs to suppress neighbouring pathogens and signal to plants demonstrates their potential to be exploited as alternatives to chemical fertilizers and pesticides, which could provide a more sustainable solution, as well as having negligible hazardous effects on animals and the environment (Tilocca et al., 2020). This review focuses on the role of mVOCs in maintaining plant health, through the direct suppression of plant pathogens, the induction of plant resistance against pathogens and the promotion of plant growth (Fig. 1), highlighting their potential as alternative solutions to synthetic pesticides and fertilizers.
Fig. 1.
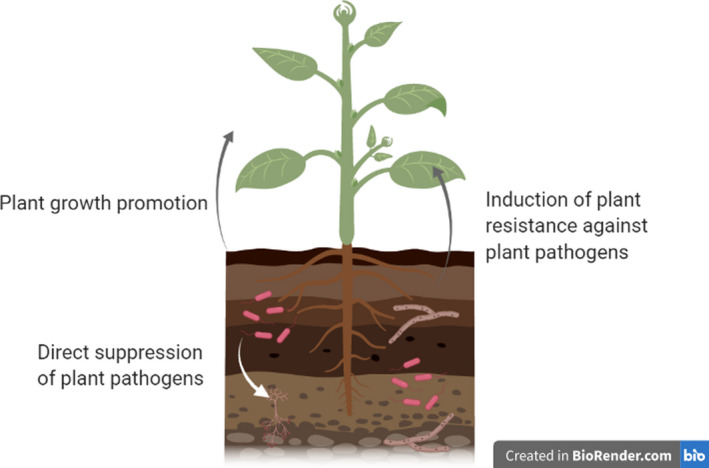
Overview of the biological activities of microbial Volatile Organic Compounds (mVOCs).
Role of volatiles in the suppression of plant pathogens
Several studies demonstrate mVOCs can inhibit a range of plant pathogens, highlighting their suitability as a potential sustainable alternative to pesticides. One of the first examples demonstrating an inhibitory role for mVOCs against plant pathogens were those produced by Pseudomonas species isolated from soybean and canola, in the inhibition of Sclerotinia sclerotiorum; a fungal pathogen with a broad host range of over 400 plant species (Fernando et al., 2005). Of 23 VOCs identified from Pseudomonas species, six significantly reduced mycelial growth of S. sclerotiorum. Similarly, VOC production by two strains of Bacillus endophytes significantly reduced the weight and number of the vegetative, long‐term survival structures (sclerotia) of S. sclerotiorum (Massawe et al., 2018). VOCs from Burkholderia ambifaria (Groenhagen et al., 2013) and a range of other rhizobacterial isolates (Velivelli et al., 2015) have also demonstrated the ability to inhibit growth of the ubiquitous soil‐borne pathogen Rhizoctonia solani. MVOCs can also display inhibitory activity against bacterial pathogens. Exposure of Clavibacter michiganensis, the causal agent of bacterial ring rot of potato, to VOCs from Bacillus subtilis led to significant inhibition of pathogen growth, with benzaldehyde, nonanal, benzothiazole and acetophenone specifically demonstrating inhibitory activities (Rajer et al., 2017). Bacillus VOCs also inhibited the growth of Xanthomonas oryzae, the causal agent of bacterial leaf blight of rice, with decyl alcohol and 3,5,5‐trimethylhexanol specifically inhibiting pathogen growth (Xie et al., 2018). As well as inhibition against fungal and bacterial pathogens, mVOCs can display inhibitory activity against pathogenic oomycetes. Exposure of Phytophthora capsici to Bacillus and Acinetobacter VOCs significantly reduced mycelial growth of the oomycete, with 3‐methyl‐1‐butanol, isovaleraldehyde, isovaleric acid, 2‐ethylhexanol and 2‐heptanone showing specific inhibitory roles (Syed‐Ab‐Rahman et al., 2019). VOCs produced by Nodulisporium also demonstrated anti‐oomycete activity against several Pythium species, although the causal VOCs involved in this inhibition were not individually assayed (Sánchez‐Fernández et al., 2016). These studies highlight mVOCs can display inhibitory activity against a range of fungal, bacterial and oomycete pathogens, which could have biotechnological potential as alternatives to pesticides. A summary of the individual VOCs involved in pathogen suppression from the studies discussed is presented in Table 1.
Table 1.
Summary of mVOC producing stains, their active VOCs and their target pathogens.
| VOC producing strain | Active VOCs | Target pathogen | Reference |
|---|---|---|---|
| Bacteria | |||
| Bacillus amyloliquefaciens FZB42, | Benzaldehyde | Ralstonia solanacearum | Tahir and colleagues (2017a) |
| Bacillus artrophaeus LSSC22 | 1,2‐Benzisothiazol‐3(2H)‐one | ||
| 1,3‐Butadiene | |||
| Bacillus subtilis FA26 | Benzaldehyde | Clavibacter michiganensis | Rajer and colleagues (2017) |
| Nonanal | |||
| Benzothiazole | |||
| Acetophenone | |||
| Bacillus spp. (VM10, VM11, VM42) | 2‐Undecanone | Sclerotinia sclerotiorum | Massawe and colleagues (2018) |
| 1,3‐Butadiene | |||
| Benzothiazole | |||
| N,N‐Dimethyldodecylamine | |||
| Bacillus strain D13 | Decyl alcohol | Xanthomonas oryzae | Xie and colleagues (2018) |
| 3,5,5‐Trimethylhexanol | |||
| Bacillus amyloliquefaciens UQ154 | 3‐Methyl‐1‐butanol | Phytophthora capsici | Syed‐Ab‐Rahman and colleagues (2019) |
| Bacillus velezensis UQ156 | Isovaleric acid | ||
| Acinetobacter spp. UQ202 | 2‐Ethylhexanol | ||
| 2‐Heptanone | |||
| Isovaleraldehyde | |||
| Burkholderia ambifaria | 2‐Undecanone | Rhizoctonia solani | Groenhagen and colleagues (2013) |
| 4‐Octanone | Alternaria alternata | ||
| Dimethyl trisulfide | |||
| S‐Methyl methanethiosulfonate | |||
| 2‐Propylacetophenone | |||
| Dimethyl disulfide | |||
| Penicillium expansum | (R)‐(−)‐1‐Octen‐3‐ol | Penicillium chrysogenum | Yin et al., 2019 |
| Penicillium solitum | |||
| Penicillium paneum | |||
| Pseudomonas fluorescens | Benzothiazole | Sclerotinia sclerotiorum | Fernando and colleagues (2005) |
| Pseudomonas chloroaphis | Cyclohexanol | ||
| Pseudomonas aurantiaca | n‐Decanal | ||
| Dimethyl trisulfide | |||
| 2‐Ethyl 1‐hexanol | |||
| Nonanal | |||
| Rhizobacterial isolates | 2,4‐Di‐tert‐butylphenol | Rhizoctonia solani | Velivelli and colleagues (2015) |
| 2‐Hexen‐1‐ol | |||
| Streptomyces spp. | Caryolan‐1‐ol | Botrytis cinerea | Cho and colleagues (2017) |
| Streptomyces alboflavus | Not identified | Aspergillus flavus | Yang and colleagues (2019) |
| Fungi | |||
| Nodulisporium sp. GS4dII1a | Not identified | Pythium aphanidermatum | Sánchez‐Fernández and colleagues (2016) |
| Saccharomyces cerevisiae | 3‐Methyl‐1‐butanol | Colletotrichum gloeosporoides | Rezende and colleagues (2015) |
| 2‐Methyl‐1‐butanol | Colletotrichum acutatum | ||
| Trichoderma spp. | Not identified | Sclerotinia sclerotiorum | Ojaghian and colleagues (2019) |
Due to the presence of a chiral centre, 1‐octen‐3‐ol has two optical isomers: (R)‐(−)‐1‐octen‐3‐ol and (S)‐(+)‐1‐octen‐3‐ol. Interestingly, when these optical isomers were investigated for inhibitory roles against the fruit spoilage pathogen Penicillium chrysogenum, (R)‐(−)‐1‐octen‐3‐ol inhibited spore germination of five out of seven isolates, whereas (S)‐(+)‐1‐octen‐3‐ol inhibited spore germination of only two isolates, suggesting the different enantiomers display differences in inhibitory activities (Yin et al., 2019). Furthermore, (R)‐(−)‐1‐octen‐3‐ol modulated the transcription of a greater number of genes in Penicillium chrysogenum. This highlights an important consideration in the specificity of mVOCs for target pathogens, providing a potential avenue for future research in the investigation of the bioactivity of chiral VOCs, as well as providing chemical structural information for the development of active substances to replace pesticides.
Whilst the role of mVOCs in the suppression of plant pathogens is well established, the molecular mechanisms involved in their inhibitory activities are receiving increasing attention. When exposed to Bacillus VOCs, the tomato wilt pathogen Ralstonia solanacearum showed a reduction in the expression of a range of virulence factor genes, including those related to chemotaxis, type 3 and type 4 secretion systems, and extracellular polysaccharides, as well as increasing the expression of a global virulence factor (Tahir et al., 2017a). Specifically, benzaldehyde, 1,2‐benzisothiazol‐3(2H)‐one and 1,3‐butadiene produced by Bacillus were involved in the modulation of virulence factor expression of the pathogen. Similarly, expression of genes involved in virulence and biofilm formation in Xanthomonas oryzae were also downregulated upon exposure to Bacillus VOCs (Xie et al., 2018). VOCs produced by Streptomyces spp. inhibited the production of aflatoxins from the fungal pathogen Aspergillus flavus, through the downregulation of several genes involved in aflatoxin biosynthesis (Yang et al., 2019; Lyu et al., Lyu2020). Exposure of Sclerotinia sclerotiorum to VOCs produced by Trichoderma species led to the upregulation of four glutathione S‐transferase genes, which are involved in the detoxification of antifungal secondary metabolites, which may contribute to the virulence of Sclerotinia sclerotiorum (Ojaghian et al., 2019). Sphingolipid metabolic processes, vesicle formation and trafficking, and membrane localization were all disrupted upon exposure of Botrytis cinerea to the Streptomyces‐derived VOC caryolan‐1‐ol (Cho et al., 2017). Plasma membrane disruption of pathogens has also been observed upon exposure of Colleotrichum species to the yeast derived VOCs 3‐methyl‐1‐butanol and 2‐methyl‐1‐butanol, leading to increased electrolyte loss (Rezende et al., 2015). Whilst the modes of action underpinning pathogen suppression by mVOCs are receiving increasing attention, a greater understanding of their molecular targets across a broader range of pathogenic microorganisms is critical prior to their deployment into open fields.
Role of volatiles in induced resistance
As well as directly suppressing plant pathogens, mVOCs can induce plant resistance to pathogens, where plant defences are preconditioned by prior treatment, resulting in enhanced resistance and reducing susceptibility to plant diseases. This was first observed by Ryu and colleagues (2004), who exposed Arabidopsis thaliana seedlings to Bacillius VOCs, which reduced the severity of symptoms by the soft‐rot causing bacterial pathogen Erwinia carotovora. Seedlings exposed to VOCs produced by strains deficient in 2,3‐butanediol and acetoin biosynthesis developed greater disease symptoms relative to wild‐type strain VOCs, suggesting a specific role for these VOCs in induced systemic resistance. These findings have been extended under greenhouse conditions, where exposure of cucumber to 2,3‐butanediol led to enhanced resistance against the bacterial pathogen Pseudomonas syringae (Song et al., 2019b). Interestingly, specificity in the ability of the different isomers of 2,3‐butanediol to induce plant resistance have also been observed, with (2R, 3R)‐butanediol inducing resistance of tobacco against Erwinia carotovorus, whereas (2S, 3S)‐butanediol was ineffective (Han et al., 2006). Whilst most work on mVOCs in induced resistance has focussed on 2,3‐butanediol and acetoin, 3‐pentanol and 2‐butanone have also been shown to induce resistance of cucumber against Pseudomonas syringae, and albuterol and 1,3‐butadiene play a role in the induction of resistance of tobacco against Ralstonia solanacearum (Song and Ryu, 2013; Tahir et al., 2017b). As stomata can act as entry points for bacterial invasion, mVOCs may induce stomatal closure to reduce pathogen internalization. This was investigated by Wu and colleagues (2018), who demonstrated that exposure of A. thaliana and tobacco to 2,3‐butanediol and acetoin induced stomatal closure, although the influence of stomatal closure on pathogen populations was not determined.
Fungal VOCs have also demonstrated a role in inducing plant resistance against pathogens. A. thaliana seedlings exposed to Trichoderma virens VOCs demonstrated significantly reduced disease symptoms when inoculated with Botrytis cinerea, and symptoms were greater in seedlings exposed to a Trichoderma virens mutant deficient in sesquiterpene production, suggesting a role for sesquiterpenes in induced resistance (Contreras‐Cornejo et al., 2014). Exposure of A. thaliana seedlings to 6‐pentyl‐2H‐pyran‐2‐one, a VOC commonly produced by a range of Trichoderma species (Jeleń et al., 2014), demonstrated significant reductions in disease symptoms when inoculated with the fungal pathogens Botrytis cinerea and Alternaria brassicicola (Kottb et al., 2015). 1‐Octen‐3‐ol, another commonly reported fungal‐derived VOC, elicited A. thaliana defence responses against Botrytis cinerea (Kishimoto et al., 2007), although as this was tested as a racemic mixture, the role of the two optical isomers of 1‐octen‐3‐ol in induced resistance cannot be discerned. More recently, VOC production from archaea (Nitrosocosmicus oleophilus), which have received little attention relative to bacteria and fungi, have also been shown to induce systemic resistance of A. thaliana against Pseudomonas syringae and Pectobacterium carotovorum; a necrotrophic bacterium responsible for soft‐rot of a range of vegetables (Song et al., 2019a). This suggests the biotechnological potential for mVOCs in sustainable agriculture is not limited to bacteria and fungi, and archaea may provide a new avenue for future research. A summary of the individual VOCs involved in induced resistance from the studies discussed is presented in Table 2.
Table 2.
Summary of mVOC producing stains, their active VOCs, the plants displaying induced resistance upon VOC exposure and the target pathogens.
| VOC producing strain | Active VOCs | Target pathogen/plant species | Reference |
|---|---|---|---|
| Bacteria | |||
| Ampleomyces sp. F‐a‐3 | Methyl benzoate | Pseudomonas syringae/A. thaliana | Naznin and colleagues (2014) |
| Bacillus subtilis GB03 | 2,3‐Butanediol | Erwinia carotovora/A. thaliana | Ryu and colleagues (2004) |
| Bacillus amyloliquefaciens IN937a | Acetoin | ||
| Bacillus spp. | 3‐Pentanol | Pseudomonas syringae/Cucumis sativus | Song and Ryu (2013) |
| 2‐Butanone | Song and colleagues (2015) | ||
| Bacillus amyloliquefaciens FZB42 | Benzaldehyde | Ralstonia solanacearum/Nicotiana benthamiana | Tahir and colleagues (2017a) |
| Bacillus artrophaeus LSSC22 | 1,2‐Benzisothiazol‐3(2H)‐one | ||
| 1,3‐Butadiene | |||
| Bacillus subtilis SYST2 | Albuterol | Ralstonia solanacearum/Nicotiana benthamiana | Tahir and colleagues (2017b) |
| 1,3‐Propanediol | |||
| Bacillus amyloliquefaciens FZB42 | 2,3‐Butanediol | A. thaliana/Nicotiana benthamiana | Wu and colleagues (2018) |
| Acetoin | |||
| Bacillus subtilis GB03 | 2,3‐Butanediol | Pseudomonas syringae/Cucumis sativa | Song and colleagues (2019b) |
| Acetoin | |||
| Paenibacillus polymyxa E681 | Tridecane | Pseudomonas syringae/A. thaliana | Lee and colleagues (2012) |
| Pseudomonas chlororaphis O6 | (2R, 3R)‐Butanediol | Erwinia carotovora/Nicotiana benthamiana | Han and colleagues (2006) |
| Fungi | |||
| Cladosporium sp. D‐c‐4 | M‐Cresol | Pseudomonas syringae/A. thaliana | Naznin and colleagues (2014) |
| Talaromyces wortmannii FS2 | β‐Caryophyllene | Colletotrichum higginsianum/Brassica campestris | Yamagiwa and colleagues (2011) |
| Trichoderma virens | Terpenes | Botrytis cinerea/A. thaliana | Contreras‐Cornejo and colleagues (2014) |
| Trichoderma asperellum | 6‐Pentyl‐2H‐pyran‐2‐one | Botrytis cinerea, Alternaria brassicicola/A. thaliana | Kottb and colleagues (2015) |
| Archaea | |||
| Nitrosocosmicus oleophilus MY3 | Not identified | Pectobacterium carotovorum, Pseudomonas syringae/A. thaliana | Song and colleagues (2019a) |
| Exogenous application | |||
| N.A. | 1‐Octen‐3‐ol | Botrytis cinerea/A. thaliana | Kishimoto and colleagues (2007) |
| N.A. | Dimethyl disulfide | Sclerotinia minor/Tomato | Tyagi and colleagues (2020) |
Several studies indicate mVOCs induce resistance of plants against pathogens through the regulation of plant hormones, which can be specifically elicited by different mVOCs. Plant defences are modulated by two main resistance pathways. Induced systemic resistance is mediated by jasmonic acid and ethylene, and commonly associated with beneficial microbes (Pieterse et al., 2014), and systemic acquired resistance, mediated by salicylic acid and commonly induced by pathogens (Shine et al., 2019). However, in some cases, beneficial microbes can trigger salicylic acid dependent induced systemic resistance (Pieterse et al., 2014). Induced systemic resistance of A. thaliana against Erwinia carotovorans by Bacillus subtilis GB03 VOCs was dependent on ethylene biosynthesis, although induced resistance by Bacillus amyloliquefaciens IN937A was independent of ethylene signalling, suggesting different VOCs present in the blends may utilize alternative pathways to induce resistance (Ryu et al., 2004). Contrastingly, resistance of cucumber to Pseudomonas syringae exposed to Bacillus subtilis GB03 VOCs involved jasmonic acid, but not ethylene signalling (Song et al., 2019b). Discrepancies in these findings may relate to differences in plant species under investigation, which may utilize different defence pathways in VOC perception, or redundancy in salicylic acid, jasmonic acid and ethylene signalling pathways in induced resistance (Ryu et al., 2004). A role for jasmonic acid signalling has also been observed in 3‐pentanol and 2‐butanone induced resistance, although expression of salicylic acid and ethylene marker genes were not induced (Song and Ryu, 2013). Similarly, A. thaliana mutants exposed to 3‐pentanol confirmed 3‐pentanol mediated immune response involved jasmonic acid and salicylic acid signalling pathways, as well as the non‐pathogenesis related 1 (NPR‐1) gene, but that ethylene signalling genes were not involved (Song et al., 2015). Tridecane, produced by Paenibacillus polymyxa E681, was involved in the enhanced resistance of A. thaliana after pathogen challenge with Pseudomonas syringae, through the modulation of salicylic acid and jasmonic acid marker genes (Lee et al., 2012). The Bacillus VOCs albuterol and 1,3‐propanediol enhanced defences of tobacco against Ralstonia solanacearum by inducing expression of resistance (RRS1) and pathogenesis‐related proteins (Pr1a and Pr1b), which act as markers for salicylic acid signalling (Tahir et al., 2017b). Interestingly, 1,3‐propanediol induced greater expression of the RRS1 gene relative to albuterol, whereas albuterol induced greater expression of pathogenesis‐related (PR) genes, suggesting specificity in the mechanisms of induced resistance for the VOCs. Similar specificity in VOC induction was observed by Naznin and colleagues (2014), who demonstrated M‐cresol, the dominant VOC from Cladosporium, induced salicylic acid and jasmonic acid signalling pathways in A. thaliana when challenge inoculated with Pseudomonas syringae, whereas methyl benzoate, the dominant VOC from Ampleomyces, induced jasmonic acid signalling with partial salicylic acid signals. Expression of genes involved in salicylic acid signalling is also induced in tomato plants exposed to dimethyl disulfide, enhancing defence against Sclerotinia minor (Tyagi et al., 2020). Interestingly, as well as directly suppressing growth of Ralstonia solanacearum, benzaldehyde, 1,2‐benzisothiazol‐3(2H)‐one and 1,3‐butadiene elicited induced systemic resistance in tobacco, through induction in the transcriptional expression of defence related genes, demonstrating potential multi‐functional roles of mVOCs (Tahir et al., 2017a).
Role of volatiles in plant growth promotion
Microbial VOCs also have the potential to enhance plant growth, enabling them to potentially be exploited as a new category of fertilizer, previously described as ‘gaseous fertilizer’ (Sharifi and Ryu, 2018). The role of mVOCs in promoting plant growth has been recognized for over a decade and was first reported by Ryu and colleagues (2003). A. thaliana seedlings exposed to VOCs of Bacillus subtilis GB03 and Bacillus amyloliquefaciens IN937a showed enhancements in leaf area, for which 2,3‐butanediol and acetoin demonstrated a role when applied exogenously. Since this, VOCs from several species of Bacillus have shown a role in plant growth promotion. VOCs from a different strain of Bacillus subtilis (SYS2) also promoted growth of tomato, for which albuterol and 1,3‐propanediol played a specific role (Tahir et al., 2017c), suggesting different strains of the same species of Bacillus can deploy different VOCs to enhance plant growth. 2‐Pentylfuran, produced by cultures of Bacillus megaterium, demonstrated dose‐dependent growth promotion of A. thaliana, with an approximate 1.5‐fold increase in plant biomass observed at a 10 µg dose (Zou et al., 2010). As well as Bacillus spp., VOCs produced by other rhizobacteria can enhance plant growth, including Proteus vulgaris, which enhanced plant growth of Chinese cabbage, for which indole demonstrated a role (Yu and Lee, 2013). Groenhagen and colleagues (2013) also observed significant increases in A. thaliana biomass when exposed to a range of VOCs, with dimethyl disulfide, the most abundantly produced VOC across a range of Burkholderia ambifaria strains, demonstrating the greatest plant growth promoting effects between doses of 1 ng and 1 mg.
Several fungal VOCs have also demonstrated a role in plant growth promotion, with 6‐pentyl‐2H‐pyran‐2‐one from Trichoderma spp. shown specifically to influence plant growth. A. thaliana seedlings exposed to 6‐pentyl‐2H‐pyran‐2‐one demonstrated a reduction in fresh plant weight, but also a reduction in disease symptoms when inoculated with certain fungal pathogens (Kottb et al., 2015). Contrastingly, Garnica‐Vergara and colleagues (2016) showed the application of 6‐pentyl‐2H‐pyran‐2‐one led to increased biomass and root branching of A. thaliana between 50 and 175 µM, although at the highest tested doses, a phytotoxic effect was observed. Discrepancies in the findings between these studies are likely due to differences in the doses and methods of application of 6‐pentyl‐2H‐pyran‐2‐one used in each study. Whilst 6‐pentyl‐2H‐pyran‐2‐one is the most well‐studied Trichoderma VOC, evidence suggests other VOCs may also be involved in plant growth promotion. Exposure of A. thaliana to VOCs from a range of Trichoderma species showed 6‐pentyl‐2H‐pyran‐2‐one production was reported from certain strains which did not promote plant growth and was not produced by certain strains which did, suggesting other VOCs could contribute to the growth promotion observed. (Lee et al., 2016). This is supported by findings from Estrada‐Rivera and colleagues (2019), who showed that 2‐heptanol, 3‐octanol and 2‐heptanone produced by Trichoderma atroviride can also promote plant growth of A. thaliana. VOCs from other fungal species have also demonstrated roles in plant growth promotion, including Fusarium oxysporum, which significantly enhanced lettuce biomass, with β‐caryophyllene demonstrating a specific role in growth promotion (Minerdi et al., 2011). Interestingly, β‐caryophyllene enhanced the biomass of Brassica campestris, as well as inducing resistance against Colletotrichum higginsianum (Yamagiwa et al., 2011). A summary of the individual VOCs involved in plant growth promotion from the studies discussed is presented in Table 3.
Table 3.
Summary of mVOC producing stains, their active VOCs and the plants displaying enhanced growth promotion upon VOC exposure.
| VOC producing strain | Active VOCs | Plant species | Reference |
|---|---|---|---|
| Bacteria | |||
| Bacillus subtilis GB03 | 2,3‐Butanediol | A. thaliana | Ryu and colleagues (2003) |
| Bacillus amyloliquefaciens IN937a | Acetoin | ||
| Bacillus megaterium XTBG34 | 2‐Pentylfuran | A. thaliana | Zou and colleagues (2010) |
| Bacillus subtilis SYST2 | Albuterol | Tomato (Solanum lycopersicum) | Tahir and colleagues (2017c) |
| 1,3‐Propanediol | |||
| Burkholderia ambifaria | Dimethyl disulfide | A. thaliana | Groenhagen and colleagues (2013) |
| Acetophenone | |||
| 3‐Hexanone | |||
| Fungi | |||
| Fusarium oxysporum | β‐Caryophyllene | Lettuce (Lactuca sativa) | Minerdi and colleagues (2011) |
| Proteus vulgaris | Indole | Chinese cabbage (Brassica rapa) | Yu and Lee (2013) |
| Trichoderma virens | 6‐Pentyl‐2H‐pyran‐2‐one | A. thaliana | Garnica‐Vergara and colleagues (2016) |
| Trichoderma spp. | 1‐Decene | A. thaliana | Lee and colleagues (2019) |
| Trichoderma atroviride | 6‐Pentyl‐2H‐pyran‐2‐one | A. thaliana | Estrada‐Rivera and colleagues (2019) |
| 2‐Heptanol | |||
| 3‐Octanol | |||
| 2‐Heptanone | |||
| Exogenous application | |||
| N.A. | Dimethyl disulfide | A. thaliana | Tyagi and colleagues (2019) |
Several studies indicate mVOCs may promote plant growth through modulating plant hormone responses. The cytokinin‐ and ethylene‐insensitive 2 (ein‐2) and Arabidopsis cytokinin receptor‐deficient 1 (cre‐1) mutants exposed to Bacillus subtilis GB03 VOCs did not display increases in plant biomass, suggesting a role for cytokinin signalling pathways plant growth promotion (Ryu et al., 2003). ein‐2 also demonstrated a role in the growth promotion of A. thaliana by the VOC 6‐pentyl‐2H‐pyran‐2‐one, as well as auxin transport proteins (Garnica‐Vergara et al., 2016). Exposure of A. thaliana to 1‐decene, a plant growth promoting Trichoderma VOC, led to the differential expression of 123 genes, 17 of which were upregulated and several of which were auxin related (Lee et al., 2019). Similarly, dimethyl disulfide altered the root system architecture of A. thaliana, which were dependent on canonical auxin signalling pathways, with mutants deficient in auxin responsive genes and transcription factors not exhibiting lateral root development or growth enhancement (Tyagi et al., 2019).
Field applications of VOCs
For mVOCs to serve as an alternative to synthetic pesticides and fertilizers, it is important to determine the efficacy of active VOCs under open‐field conditions. Dimethyl disulfide is a VOC produced by bacteria including Bacillus cereus, which can suppress soil‐borne pathogens and nematodes, and elicit systemic resistance against Botrytis cinerea and Cochliobolus hetereostrophus (Huang et al., 2012). Dimethyl disulfide has been successfully commercialized as an alternative to pesticides as the soil fumigant PALADIN®, which has been patented (Paladin Technical EPA Reg. No. 55050‐3), highlighting the potential of mVOCs to serve as alternatives to chemical inputs (de Boer et al., 2019). Performance of other VOCs demonstrating a role in induced plant resistance under laboratory conditions, which commonly occur in Petri dish environments, are also demonstrating promise in the field and under soil conditions. Field trials with 2,3‐butanediol induced resistance of cucumber to viruses (Kong et al., 2018) and maize to the northern corn leaf blight fungus Setosphaeria turcica under a soil context (D’Alessandro et al., 2014). As well as 2,3‐butanediol, cucumber plants exposed to 3‐pentanol and 2‐butanone showed reduced disease symptoms against the Pseudomonas syringae under open‐field conditions (Song and Ryu, 2013). These studies demonstrate promise in the performance of mVOCs in the field, and future work should investigate the efficacy of bioactive VOCs identified from laboratory‐based studies under field conditions.
Conclusions and future outlook
The biological activities of mVOCs highlight their potential to act as alternatives to unsustainable agricultural chemical inputs, to feed a growing population. So far, much work investigating mVOCs focusses on the model plant species A. thaliana and Nicotiana benthamiana (Tables 2 and 3), and therefore, future research should focus on the protective and growth stimulating effects of mVOCs on crop and vegetable species. Similarly, characterization of mVOCs has been performed on limited range of microbial species. In terms of bacteria, Bacillus spp., in particular 2,3‐butanediol and acetoin, have been the focus of several studies, and for fungi, Trichoderma species has attracted the most attention, specifically 6‐pentyl‐2H‐pyran‐2‐one (Tables 2 and 3). Current estimates indicate that < 10% of mVOCs have been ascribed a function (Lemfack et al., 2018), suggesting enormous potential for identifying other mVOCs with biotechnological applications. Moreover, most studies reported here investigate VOC production from axenic cultures of microbes, although growing bodies of evidence suggest interspecific interactions between microorganisms can enhance production of VOCs which have demonstrated inhibitory activity against pathogens (Tyc et al., 2014, 2017). This could enable identification of biologically relevant VOCs involved in the suppression of pathogenic microorganisms. Whilst several studies also investigate the role of mVOCs on a single biological activity, there are likely overlaps in the roles of these VOCs. For example, 6‐pentyl‐2H‐pyran‐2‐one has demonstrated roles in pathogen suppression (e.g. Jeleń et al., 2014), plant growth promotion (Garnica‐Vergara et al., 2016), and induced resistance (Kottb et al., 2015), suggesting biological activities should not be considered in isolation. Moreover, whilst many studies demonstrate VOCs have suppressive effects on plant pathogens, it is important to determine the effect of these inhibitory VOCs on plant development. For example, inhibitory mVOCs produced by Streptomyces yanglinensis 3–10 against Aspergillus were tested to determine their effects on plant development and showed that VOCs did not inhibit peanut seedling germination, suggesting promise for use under field conditions (Lyu et al., 2020). The modes of action of VOCs in the suppression of target pathogens (Dalilla et al., 2015; Cho et al., 2017; Tahir et al., 2017a; Xie et al., 2018; Yang et al., 2019; Ojaghian et al., 2019), enhanced disease resistance of plants (Ryu et al., 2004; Lee et al., 2012; Song and Ryu, 2013; Tahir et al., 2017a; Tahir et al., 2017b; Song et al., 2019b; Tyagi et al., 2020) and plant growth promotion (Ryu et al., 2003; Garnica‐Vergara et al., 2016; Lee et al., 2019; Tyagi et al., 2019) are receiving increasing attention, future research priority should focus on understanding the mode of action of biologically active VOCs on target plants and pathogens. Whilst investigation of the efficacy of VOCs under field conditions has demonstrated promise, a wider range of VOCs require testing at this scale. More research on methods of application of mVOCs onto fields is also required, for example, the effectiveness of drench versus spraying application (Garbeva and Weisskopf, 2020). The potential for plant production of active VOCs for the biological control of fungal pathogens through companion cropping systems is another potential form of delivery. Bean cultivars resistant to Colletotrichum lindemuthianum, the causal agent of black spot disease, enhanced resistance of susceptible cultivars to the pathogen when exposed to VOCs from resistant cultivars (Quintana‐Rodriguez et al., 2015). These findings could be translated in the field for the control of plant pathogens, through companion cropping systems, using VOCs from disease‐resistant cultivars to deliver VOCs to neighbouring crops to enhance disease resistance against fungal pathogens. In conclusion, studies reviewed here demonstrate mVOCs can be exploited to serve as sustainable alternatives to agricultural chemical inputs, which can potentially reduce our overreliance on the current unsustainable methods at a time when population growth, and food demand, is likely to substantially increase.
Funding information
Rothamsted International (Grant/Award Number: BBS/OS/CP/000001); Biotechnology and Biological Sciences Research Council (Grant/Award Number: 1622285).
Conflict of interest
None declared.
Acknowledgements
GT’s PhD studentship was funded by a Biotechnology and Biological Sciences Research Council (BBSRC) South West doctoral training partnership award (project no. 1622285). The work formed part of the Rothamsted Smart Crop Protection (SCP) strategic programme (BBS/OS/CP/000001) funded through Biotechnology and Biological Sciences Research Council’s Industrial Strategy Challenge Fund.
Microbial Biotechnology (2020) 13(5), 1366–1376
Funding Information
Rothamsted International: BBS/OS/CP/000001
Biotechnology and Biological Sciences Research Council: 1622285
References
- Brakhage, A.A. , and Schroeckh, V. (2011) Fungal secondary metabolites – strategies to activate silent gene clusters. Fungal Genet Biol 48: 15–22. [DOI] [PubMed] [Google Scholar]
- Cho, G. , Kim, J. , Park, C.G. , Nislow, C. , Weller, D.M. , and Kwak, Y.S. (2017) Caryolan‐1‐ol, an antifungal volatile produced by Streptomyces spp., inhibits the endomembrane system of fungi. Open Biol 7: e170075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras‐Cornejo, H.A. , Macías‐Rodríguez, L. , Herrera‐Estrella, A. , and López‐Bucio, J. (2014) The 4‐phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant Soil 379: 261–274. [Google Scholar]
- D’Alessandro, M. , Erb, M. , Ton, J. , Brandenburg, A. , Karlen, D. , Zopfi, J. , and Turlings, T.C.J. (2014) Volatiles produced by soil‐borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant, Cell Environ 37: 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, E.A. (2009) The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat Geosci 2: 659–662. [Google Scholar]
- De Boer, W. , Li, X. , Meisner, A. , and Garbeva, P. (2019) Pathogen suppression by microbial volatile organic compounds in soils. FEMS Microbiol Ecol 95: fiz105. [DOI] [PubMed] [Google Scholar]
- Effmert, U. , Kalderás, J. , Warnke, R. , and Piechulla, B. (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38: 665–703. [DOI] [PubMed] [Google Scholar]
- Estrada‐Rivera, M. , Rebolledo‐Prudencio, O.G. , Pérez‐Robles, D.A. , Rocha‐Medina, M.D.C. , González‐López, M.D.C. , and Casas‐Flores, S. (2019) Trichoderma histone deacetylase HDA‐2 modulates multiple responses in arabidopsis. Plant Physiol 179: 1343–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando, W.G.D. , Ramarathnam, R. , Krishnamoorthy, A.S. , and Savchuk, S.C. (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37: 955–964. [Google Scholar]
- Fisher, M.C. , Hawkins, N.J. , Sanglard, D. , and Gurr, S.J. (2018) Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360: 739–742. [DOI] [PubMed] [Google Scholar]
- Garbeva, P. , and Weisskopf, L. (2020) Airborne medicine: bacterial volatiles and their influence on plant health. New Phytol 226: 32–43. [DOI] [PubMed] [Google Scholar]
- Garnica‐Vergara, A. , Barrera‐Ortiz, S. , Muñoz‐Parra, E. , Raya‐González, J. , Méndez‐Bravo, A. , Macías‐Rodríguez, L. , et al (2016) The volatile 6‐pentyl‐2H‐pyran‐2‐one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol 209: 1496–1512. [DOI] [PubMed] [Google Scholar]
- Groenhagen, U. , Baumgartner, R. , Bailly, A. , Gardiner, A. , Eberl, L. , Schulz, S. , and Weisskopf, L. (2013) Production of bioactive volatiles by different Burkholderia ambifaria strains. J Chem Ecol 39: 892–906. [DOI] [PubMed] [Google Scholar]
- Han, S.H. , Lee, S.J. , Moon, J.H. , Park, K.H. , Yang, K.Y. , Cho, B.H. , et al (2006) GacS‐dependent production of 2R, 3R‐butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol Plant‐Microbe Interact 19: 924–930. [DOI] [PubMed] [Google Scholar]
- Huang, C.J. , Tsay, J.F. , Chang, S.Y. , Yang, H.P. , Wu, W.S. , and Chen, C.Y. (2012) Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag Sci 68: 1306–1310. [DOI] [PubMed] [Google Scholar]
- Jeleń, H. , Błaszczyk, L. , Chełkowski, J. , Rogowicz, K. , and Strakowska, J. (2014) Formation of 6‐n‐pentyl‐2H‐pyran‐2‐one (6‐PAP) and other volatiles by different Trichoderma species. Mycol Prog 13: 589–600. [Google Scholar]
- Kanchiswamy, C.N. , Malnoy, M. , and Maffei, M.E. (2015a) Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci 20: 206–211. [DOI] [PubMed] [Google Scholar]
- Kanchiswamy, C.N. , Malnoy, M , and Maffei, M.E. (2015b) Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front Plant Sci 6: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto, K. , Matsui, K. , Ozawa, R. , and Takabayashi, J. (2007) Volatile 1‐octen‐3‐ol induces a defensive response in Arabidopsis thaliana . J Gen Plant Pathol 73: 35–37. [Google Scholar]
- Kong, H.G. , Shin, T.S. , Kim, T.H. , and Ryu, C.M. (2018) Stereoisomers of the bacterial volatile compound 2,3‐butanediol differently elicit systemic defense responses of pepper against multiple viruses in the field. Front Plant Sci 9: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottb, M. , Gigolashvili, T. , Großkinsky, D.K. , and Piechulla, B. (2015) Trichoderma volatiles effecting Arabidopsis: from inhibition to protection against phytopathogenic fungi. Front Microbiol 6: 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberth, C. , Jeanmart, S. , Luksch, T. , and Plant, A. (2013) Current challenges and trends in the discovery of agrochemicals. Science 341: 742–746. [DOI] [PubMed] [Google Scholar]
- Lee, B. , Farag, M.A. , Park, H.B. , Kloepper, J.W. , Lee, S.H. , and Ryu, C.M. (2012) Induced resistance by a long‐chain bacterial volatile: elicitation of plant systemic defense by a C13 Volatile produced by Paenibacillus polymyxa . PLoS One 7: e48744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Yap, M. , Behringer, G. , Hung, R. , and Bennett, J.W. (2016) Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol Biotechnol 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Behringer, G. , Hung, R. , and Bennett, J. (2019) Effects of fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression. Fungal Ecol 37: 1–9. [Google Scholar]
- Lemfack, M.C. , Gohlke, B.O. , Toguem, S.M.T. , Preissner, S. , Piechulla, B. , and Preissner, R. (2018) MVOC 2.0: A database of microbial volatiles. Nucleic Acids Res 46: D1261–D1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima Procópio, R.E. , da Silva, I.R. , Martins, M.K. , de Azevedo, J.L. , and de Araújo, J.M. (2012) Antibiotics produced by Streptomyces. Brazilian J Infect Dis 16: 466–471. [DOI] [PubMed] [Google Scholar]
- Lyu, A. , Yang, L. , Wu, M. , Zhang, J. , and Li, G. (2020) High efficacy of the volatile organic compounds of Streptomyces yanglinensis 3–10 in suppression of aspergillus contamination on peanut kernels. Front Microbiol 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheleidt, J. , Mattern, D.J. , Fischer, J. , Netzker, T. , Weber, J. , Schroeckh, V. , et al (2016) Regulation and role of fungal secondary metabolites. Annu Rev Genet 50: 371–392. [DOI] [PubMed] [Google Scholar]
- Maffei, M.E. , Gertsch, J. , and Appendino, G. (2011) Plant volatiles: production, function and pharmacology. Nat Prod Rep 28: 1359–1380. [DOI] [PubMed] [Google Scholar]
- Massawe, V.C. , Hanif, A. , Farzand, A. , Mburu, D.K. , Ochola, S.O. , Wu, L. , et al (2018) Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum . Phytopathology 108: 1373–1385. [DOI] [PubMed] [Google Scholar]
- Minerdi, D. , Bossi, S. , Maffei, M.E. , Gullino, M.L. , and Garibaldi, A. (2011) Fusarium oxysporum and its bacterial consortium promote lettuce growth and expansin A5 gene expression through microbial volatile organic compound (MVOC) emission. FEMS Microbiol Ecol 76: 342–351. [DOI] [PubMed] [Google Scholar]
- Naznin, H.A. , Kiyohara, D. , Kimura, M. , Miyazawa, M. , Shimizu, M. , and Hyakumachi, M. (2014) Systemic resistance induced by volatile organic compounds emitted by plant growth‐promoting fungi in Arabidopsis thaliana . PLoS One 9: e86882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørskov, J. , and Chen, J. (2016) Sustainable Ammonia Synthesis: Roundtable Discussion. US Department of Energy. [Google Scholar]
- Ojaghian, S. , Wang, L. , Xie, G.L. , and Zhang, J.Z. (2019) Effect of volatiles produced by Trichoderma spp. on expression of glutathione transferase genes in Sclerotinia sclerotiorum . Biol Control 136: 103999. [Google Scholar]
- Pieterse, C.M.J. , Zamioudis, C. , Berendsen, R.L. , Weller, D.M. , Van Wees, S.C.M. , and Bakker, P.A.H.M. (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52: 347–375. [DOI] [PubMed] [Google Scholar]
- Quintana‐Rodriguez, E. , Morales‐Vargas, A.T. , Molina‐Torres, J. , Ádame‐Alvarez, R.M. , Acosta‐Gallegos, J.A. , and Heil, M. (2015) Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum . J Ecol 103: 250–260. [Google Scholar]
- Rajer, F.U. , Wu, H. , Xie, Y. , Xie, S. , Raza, W. , Tahir, H.A.S. , and Gao, X. (2017) Volatile organic compounds produced by a soil‐isolate, Bacillus subtilis FA26 induce adverse ultra‐structural changes to the cells of Clavibacter michiganensis ssp. Sepedonicus, the causal agent of bacterial ring rot of potato. Microbiology 163: 523–530. [DOI] [PubMed] [Google Scholar]
- Rezende, D.C. , Mauricio, B.F. , Simone, C.B. , Silvia, B. , and Sergio, F.P. (2015) Antimicrobial activity of volatile organic compounds and their effect on lipid peroxidation and electrolyte loss in Colletotrichum gloeosporioides and Colletotrichum acutatum mycelia. African J Microbiol Res 9: 1527–1535. [Google Scholar]
- Rohr, J.R. , Barrett, C.B. , Civitello, D.J. , Craft, M.E. , Delius, B. , DeLeo, G.A. , et al (2019) Emerging human infectious diseases and the links to global food production. Nat Sustain 2: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, C.M. , Faragt, M.A. , Hu, C.H. , Reddy, M.S. , Wei, H.X. , Paré, P.W. , and Kloepper, J.W. (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100: 4927–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, C.M. , Farag, M.A. , Hu, C.H. , Reddy, M.S. , Kloepper, J.W. , and Paré, P.W. (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134: 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Fernández, R.E. , Diaz, D. , Duarte, G. , Lappe‐Oliveras, P. , Sánchez, S. , and Macías‐Rubalcava, M.L. (2016) Antifungal volatile organic compounds from the Endophyte nodulisporium sp. Strain GS4d2II1a: a qualitative change in the intraspecific and interspecific interactions with Pythium aphanidermatum . Microb Ecol 71: 347–364. [DOI] [PubMed] [Google Scholar]
- Savary, S. , Willocquet, L. , Pethybridge, S.J. , Esker, P. , McRoberts, N. , and Nelson, A. (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3: 430–439. [DOI] [PubMed] [Google Scholar]
- Schulz‐Bohm, K. , Martín‐Sánchez, L. , and Garbeva, P. (2017) Microbial volatiles: small molecules with an important role in intra‐ and inter‐kingdom interactions. Front Microbiol 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi, R. , and Ryu, C.M. (2018) Revisiting bacterial volatile‐mediated plant growth promotion: lessons from the past and objectives for the future. Ann Bot 122: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine, M.B. , Xiao, X. , Kachroo, P. , and Kachroo, A. (2019) Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci 279: 81–86. [DOI] [PubMed] [Google Scholar]
- Smil, V. (1999) Nitrogen in crop production: an account of global flows. Global Biogeochem Cycles 13: 647–662. [Google Scholar]
- Song, G.C. , and Ryu, C.M. (2013) Two volatile organic compounds trigger plant self‐defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int J Mol Sci 14: 9803–9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, G.C. , Choi, H.K. , and Ryu, C.M. (2015) Gaseous 3‐pentanol primes plant immunity against a bacterial speck pathogen, Pseudomonas syringae pv. Tomato via salicylic acid and jasmonic acid‐dependent signaling pathways in Arabidopsis. Front Plant Sci 6: 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, G.C. , Im, H. , Jung, J. , Lee, S. , Jung, M.Y. , Rhee, S.K. , and Ryu, C.M. (2019a) Plant growth‐promoting archaea trigger induced systemic resistance in Arabidopsis thaliana against Pectobacterium carotovorum and Pseudomonas syringae . Environ Microbiol 21: 940–948. [DOI] [PubMed] [Google Scholar]
- Song, G.C. , Riu, M. , and Ryu, C.M. (2019b) Beyond the two compartments Petri‐dish: optimising growth promotion and induced resistance in cucumber exposed to gaseous bacterial volatiles in a miniature greenhouse system. Plant Methods 15: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteller, P. (2015) Chemical ecology of fungi. Nat Prod Rep 32: 971–993. [DOI] [PubMed] [Google Scholar]
- Syed‐Ab‐Rahman, S.F. , Xiao, Y. , Carvalhais, L.C. , Ferguson, B.J. , and Schenk, P.M. (2019) Suppression of Phytophthora capsici infection and promotion of tomato growth by soil bacteria. Rhizosphere 9: 72–75. [Google Scholar]
- Tahir, H.A.S. , Gu, Q. , Wu, H. , Niu, Y. , Huo, R. , and Gao, X. (2017a) Bacillus volatiles adversely affect the physiology and ultra‐structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci Rep 7: 40481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir, H.A.S. , Gu, Q. , Wu, H. , Raza, W. , Safdar, A. , Huang, Z. , et al (2017b) Effect of volatile compounds produced by Ralstonia solanacearum on plant growth promoting and systemic resistance inducing potential of Bacillus volatiles. BMC Plant Biol 17: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir, H.A.S. , Gu, Q. , Wu, H. , Raza, W. , Hanif, A. , Wu, L. , et al (2017c) Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front Microbiol 8: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester, M. , and Langridge, P. (2010) Breeding technologies to increase crop production in a changing world. Science 327: 818–822. [DOI] [PubMed] [Google Scholar]
- Tilocca, B. , Cao, A. , and Migheli, Q. (2020) Scent of a killer: microbial volatilome and its role in the biological control of plant pathogens. Front Microbiol 11: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi, S. , Kim, K. , Cho, M. , and Lee, K.J. (2019) Volatile dimethyl disulfide affects root system architecture of Arabidopsis via modulation of canonical auxin signaling pathways. Environ Sustain 2: 211–216. [Google Scholar]
- Tyagi, S. , Lee, K.J. , Shukla, P. , and Chae, J.C. (2020) Dimethyl disulfide exerts antifungal activity against Sclerotinia minor by damaging its membrane and induces systemic resistance in host plants. Sci Rep 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc, O. , van den Berg, M. , Gerards, S. , van Veen, J.A. , Raaijmakers, J.M. , de Boer, W. , and Garbeva, P. (2014) Impact of interspecific interactions on antimicrobial activity among soil bacteria. Front Microbiol 5: 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc, O. , de Jager, V.C.L. , van den Berg, M. , Gerards, S. , Janssens, T.K.S. , Zaagman, N. , et al (2017) Exploring bacterial interspecific interactions for discovery of novel antimicrobial compounds. Microb Biotechnol 10: 910–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs Population Division (2017) World population prospects the 2017 revision. World Popul Prospect 2017: 1–146. [Google Scholar]
- Velivelli, S.L.S. , Kromann, P. , Lojan, P. , Rojas, M. , Franco, J. , Suarez, J.P. , and Prestwich, B.D. (2015) Identification of mVOCs from Andean rhizobacteria and field evaluation of bacterial and mycorrhizal inoculants on growth of potato in its center of origin. Microb Ecol 69: 652–667. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Li, X. , Ma, L. , Borriss, R. , Wu, Z. , and Gao, X. (2018) Acetoin and 2,3‐butanediol from Bacillus amyloliquefaciens induce stomatal closure in Arabidopsis thaliana and Nicotiana benthamiana . J Exp Bot 69: 5625–5635. [DOI] [PubMed] [Google Scholar]
- Xie, S. , Zang, H. , Jun Wu, H. , Uddin Rajer, F. and Gao, X. (2018) Antibacterial effects of volatiles produced by bacillus strain D13 against Xanthomonas oryzae pv. oryzae. Mol Plant Pathol 19: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagiwa, Y. , Inagaki, Y. , Ichinose, Y. , Toyoda, K. , Hyakumachi, M. , and Shiraishi, T. (2011) Talaromyces wortmannii FS2 emits β‐caryphyllene, which promotes plant growth and induces resistance. J Gen Plant Pathol 77: 336–341. [Google Scholar]
- Yang, M. , Lu, L. , Pang, J. , Hu, Y. , Guo, Q. , Li, Z. , et al (2019) Biocontrol activity of volatile organic compounds from Streptomyces alboflavus TD‐1 against Aspergillus flavus growth and aflatoxin production. J Microbiol 57: 396–404. [DOI] [PubMed] [Google Scholar]
- Yin, G. , Zhang, Y. , Fu, M. , Hua, S.S.T. , Huang, Q. , Pennerman, K.K. , et al (2019) Influence of R and S enantiomers of 1‐octen‐3‐ol on gene expression of Penicillium chrysogenum . J Ind Microbiol Biotechnol 46: 977–991. [DOI] [PubMed] [Google Scholar]
- Yu, S.M. , and Lee, Y.H. (2013) Plant growth promoting rhizobacterium Proteus vulgaris JBLS202 stimulates the seedling growth of Chinese cabbage through indole emission. Plant Soil 370: 485–495. [Google Scholar]
- Zou, C. , Li, Z. , and Yu, D. (2010) Bacillus megaterium strain XTBG34 promotes plant growth by producing 2‐pentylfuran. J Microbiol 48: 460–466. [DOI] [PubMed] [Google Scholar]


