Summary
Biological nitrogen fixation in rhizobia occurs primarily in root or stem nodules and is induced by the bacteria present in legume plants. This symbiotic process has fascinated researchers for over a century, and the positive effects of legumes on soils and their food and feed value have been recognized for thousands of years. Symbiotic nitrogen fixation uses solar energy to reduce the inert N2 gas to ammonia at normal temperature and pressure, and is thus today, especially, important for sustainable food production. Increased productivity through improved effectiveness of the process is seen as a major research and development goal. The interaction between rhizobia and their legume hosts has thus been dissected at agronomic, plant physiological, microbiological and molecular levels to produce ample information about processes involved, but identification of major bottlenecks regarding efficiency of nitrogen fixation has proven to be complex. We review processes and results that contributed to the current understanding of this fascinating system, with focus on effectiveness of nitrogen fixation in rhizobia.
The effectiveness of rhizobia in symbiosis with legumes is reviewed, with references from older and most recent literature.
Efficiency of rhizobia–legume symbiosis
Nitrogen is an essential component of urea and amino acids (proteins), nucleic acids (DNA and RNA), adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD) in all living cells (Burén and Rubio, 2017). Nitrogen is a major component of chlorophyll, the most important pigment required for photosynthesis, and plays a critical role for plant growth and production (Wagner, 2012). Dinitrogen (N2) is the most available gas and the major constituent of the atmosphere. However, only the reactive types of nitrogen like oxidized (e.g. NOx) or reduced (e.g. NH3 and amines) nitrogen types can be assimilated by plants (Burén and Rubio, 2017). The conversion of N2 to the biologically available form of nitrogen (NH3) could be performed either by the industrial Haber‐Bosch process or via biological nitrogen fixation by certain bacteria and archaea (diazotrophic prokaryotes).
Rhizobia is a generic name for a certain Gram‐negative group of Alphaproteobacteria and Betaproteobacteria that can form nodules on the root, or in some cases on the stems, of their hosts and fix nitrogen in symbiosis with legumes as their host plants (Garrity et al., 2005; Sprent, 2008). The genus Rhizobium was the first described group of these bacteria, and that is why this name has been frequently used for the nitrogen‐fixing bacteria of legumes. Today, this group of bacteria are scattered in 18 genera in the families Rhizobiaceae (Rhizobium, Ensifer (syn. Sinorhizobium), Allorhizobium, Pararhizobium, Neorhizobium, Shinella), Phyllobacteriaceae (Mesorhizobium, Aminobacter, Phyllobacterium), Brucellaceae (Ochrobactrum), Methylobacteriaceae (Methylobacterium, Microvirga), Bradyrhizobiaceae (Bradyrhizobium), Xanthobacteraceae (Azorhizobium), Hyphomicrobiaceae (Devosia) and Burkholderiaceae (Paraburkholderia, Cupriavidus). The genus Rhizobium, accommodating 112 species, is the largest genus of rhizobia (Mousavi, 2016; de Lajudie et al., 2019; http://www.bacterio.net/).
The recent history of the symbiotic nitrogen fixation process offers a fascinating tour of the development of this branch of biological research. The so‐called ‘energy crisis’ in the late 1970s, a consequence of raising oil prices on the world market, sparked a renewed interest in the mechanisms behind the process, which uses solar energy to drive the production of combined nitrogen for plant growth. In this review, we start at that point and proceed until the current climate and environmental crises. We search for factors contributing to the efficiency or effectiveness of the process, a goal that is as important today as in the 1970s, when trying to feed the world population in a sustainable way.
We also chose to include work on which current advances build upon. Before the molecular and technical advances that we now take for given, careful and sophisticated experiments were designed, performed and reported, building the foundations for today’s knowledge step by step. For this part, we mainly rely on review articles written by experts of their time.
The words efficiency, efficacy and effectiveness are used in rhizobium literature almost without distinction. However, since they do mean different things, a distinction might be useful when discussing symbiotic nitrogen fixation (SNF) in associations between rhizobia and legumes. According to encyclopaedia (e.g. Wikipedia, www.wikipedia.org and Diffen, https://www.diffen.com), efficiency means how well something is done. In biological systems, efficiency is defined as the effectiveness with which energy is used for a particular process. Energy efficiency is calculated by relating energy use to minimum energy cost under defined conditions (Phillips, 1980). For rhizobial SNF, this would mean the function of the nitrogenase enzyme: energy use in SNF and the outcome of the process in terms of N2 fixed. Efficacy defines how well a system or product functions under ideal conditions; in rhizobium terms, this could mean how much nitrogen is fixed, for example, in axenic culture in pouches, test tubes or enclosed jars, taking the whole system into account. However, this term is seldom used in the context. Effectiveness again indicates how useful the system is in practice. For the legume–rhizobium symbiosis, this term could mean how productive the system is for our purposes, i.e. dry matter and protein production.
In SNF research, efficiency and effectiveness of nitrogen fixation are often used in parallel. Strictly speaking, efficiency should be used when we take an energy perspective, taking into account the function of the nitrogenase enzyme as well as energy provision by the plant to support the reaction. Effectiveness would again mean the outcome of the whole system, including in addition to energy use, all other factors contributing to a successful symbiosis.
Community‐level efficiency was introduced by Phillips (1980) to stand for the amount of N2 reduced per unit of land area. This expression is hardly longer in use. However, it shows how much emphasis was put on the energy aspect in the 1970s and 1980s when the so‐called energy crisis prompted the society to look for alternative, sustainable means of energy production to replace fossil fuels. The attention was turned to biological nitrogen fixation, which uses biological processes for transformation of atmospheric N2 gas to biologically useful ammonia, in contrast to the Haber‐Bosch method, which relies on fossil energy.
The research on SNF has through the years taken diverse routes to reach the common aim of replacing synthetic N fertilizer with N reduced by the enzyme nitrogenase. Today, one line of research aims at applying synthetic biology and biotechnology to engineer a biocatalyst for fertilizer production. Another main direction is to take on the challenge of engineering non‐legumes to either harbour nitrogenase without rhizobial infection or to become nodulated by rhizobia. Studies with these aims result in a great deal of basic research on biological mechanisms of SNF, some of which could be applied when looking for efficiency and trying to understand effectiveness in rhizobia. Two different concepts should be kept in mind when dealing with this topic, namely nodulation efficiency and N2‐fixation efficiency. This minireview mainly deals with the latter, though it is not wise to draw sharp borderlines between concepts defined to help us keep track on complex biological processes.
Rhizobia and SNF
The best‐known group of symbiotic nitrogen‐fixing bacteria are the rhizobia. However, two other groups of bacteria including Frankia and Cyanobacteria can also fix nitrogen in symbiosis with plants. Rhizobia fix nitrogen in plant species of the family Leguminosae, and species of another family, e.g. Parasponia. Frankia, can nodulate actinorhizal plants and Cyanobacteria can enter a symbiotic relation with a wide range of plants (Dawson, 2008; Normand and Fernandez, 2009; Lindström and Mousavi, 2010; Op den Camp, et al., 2011; Mousavi, 2016), according to the definition ‘Symbiosis is the acquisition of an organism(s) by another unlike organism(s), and through subsequent long‐term integration, new structures and metabolism(s) emerge’ (Zook, 2015). The SNF interaction between rhizobia and legumes is a mutual symbiosis in which both plants and bacteria are benefited. In this symbiotic relationship, rhizobia are hosted and supplied with carbon sources by legumes and in return legumes receive ammonia provided by rhizobia (Fig. 1).
Figure 1.
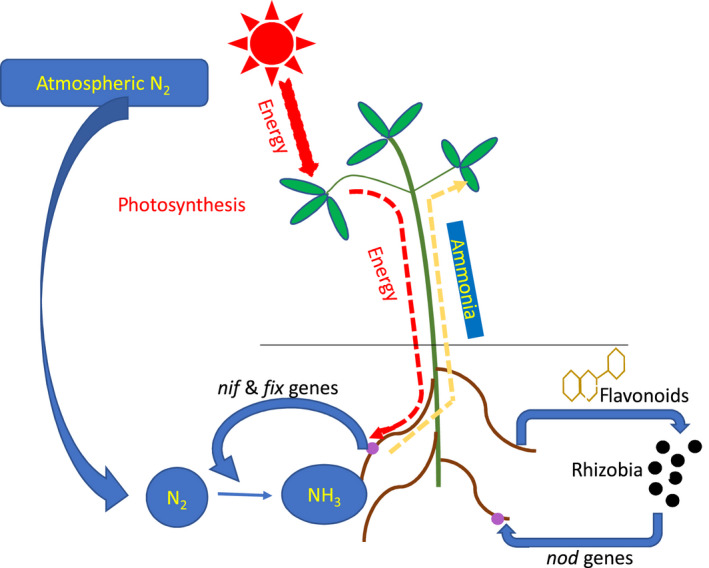
Summarized model for symbiotic nitrogen fixation in legumes by rhizobia.
Symbiotic nitrogen fixation in rhizobia is mostly controlled by nod, nif and fix genes (Table 1). Nod and Nif proteins are encoded by accessory genes of bacteria that are placed in transmissible genetic elements like plasmids, symbiotic islands and chromids. Thus, these sets can be transferred horizontally in high frequencies within the species of a bacterial genus and infrequently between genera (Remigi et al., 2016). The rhizobial lipo‐chito‐oligosaccharide signal molecules called Nod factors are encoded by a unique set of genes of rhizobia called nod genes. They are important genes for bacterial invasion and induction of nodules in the early stages of nodulation by inducing host responses such as root hair deformation and cortical cell division (Moulin et al., 2004). Recently, the role of rhizobial NFs has been revisited, as their interaction with NF receptors and chitinases point to a putative role in the balance between symbiosis and defence (Kelly et al., 2017). The interaction between legume roots and rhizobia is initiated by the exudation of flavonoids by legumes that is recognized by rhizobial NodD, which in turn regulates the transcription of other nodulation genes (nod, nol, and noe) in rhizobia (Bonaldi et al., 2010; Rogel et al., 2011). The three nod genes, nodA, nodB and nodC, exist as single copy genes in most rhizobia. They are involved in synthesis of the Nod factor backbone (Moulin et al., 2004). According to Bonaldi et al., (2010), three nod genes are essential for the synthesis of the NF core structure: i) nodC encodes an N‐acetyl‐glucosaminyltransferase that polymerizes UDP‐N‐acetyl‐D‐glucosamine into oligosaccharide chains, ii) nodB encodes a deacetylase that removes the N‐acetyl moiety from the non‐reducing terminus of these oligosaccharides, and iii) nodA encodes an acyltransferase that links an acyl chain to the non‐reducing end of the oligosaccharides. Moreover, the different other nodulation genes (nod, nol or noe) can be found in different rhizobial species, being more or less essential for the host specificity of each species, by decoration of the basic Nod factor backbone, leading to different substitutions, such as acetylation, glycosylation, methylation and sulfation (Bonaldi et al., 2010; Andrews and Andrews, 2017). It is worth noting that Giraud et al. (2007) reported that two symbiotic, photosynthetic, Bradyrhizobium strains, BTAi1 and ORS278, can establish nodules on stem and roots of Aeschynomene indica and A. sensitiva in the absence of nodABC and lipochito‐oligosaccharidic Nod factors (NF). This phenomenon was also found in a symbiotic relationship between a NF‐independent mutant of B. elkanii and Glycine max cv. Enrei, as well as the symbiosis between the model plant Lotus japonicus and NF mutants of Mesorhizobium loti (Madsen et al., 2010; Okazaki et al., 2013). For NF‐independent symbiosis, Okazaki et al. (2016) proposed a process dependent on the type III secretion system (T3SS) by which rhizobial effectors are translocated to certain legume species to activate symbiosis in host legumes. Recognition of NFs by legumes is mediated by Nod factor receptors (NFRs) that are plasma membrane localized serine/threonine receptor kinases that contain sugar‐binding LysM domains. NFR1 and NFR5, and NFP and LYK3 are the reported NFRs in the case of the model legumes Lotus japonicus and Medicago truncatula, respectively (Madsen et al., 2003; Andrews and Andrews, 2017).
Table 1.
A list of the most common rhizobial nod, nif and fix genes (after Laranjo et al., 2014).
| Genes | Function of gene product |
|---|---|
| Nodulation genes | |
| nodA | Acyltransferase |
| nodB | Chitooligosaccharide deacetylase |
| nodC | N‐acetylglucosaminyltransferase |
| nodD | Transcriptional regulator of common nod genes |
| nodIJ | Nod factor transport |
| nodPQ | Synthesis of Nod factor substituents |
| nodX | Synthesis of Nod factor substituents |
| nofEF | Synthesis of Nod factor substituents |
| Other nod genes | Several functions in synthesis of Nod factors |
| nol genes | Several functions in synthesis of Nod factor substituents and secretion |
| noe genes | Synthesis of Nod factos substituents |
| Nitrogen fixation genes | |
| nifH | Dinitrogenase reductase (Fe protein) |
| nifD | α subunits of dinitrogenase (MoFe protein) |
| nifK | β subunits of dinitrogenase (MoFe protein) |
| nifA | Transcriptional regulator of the other nif genes |
| nifBEN | Biosynthesis of the Fe‐Mo cofactor |
| fixABCX | Electron transport chain to nitrogenase |
| fixNOPQ | Cytochrome oxidase |
| fixLJ | Transcriptional regulators |
| fixK | Transcriptional regulator |
| fixGHIS | Copper uptake and metabolism |
| fdxN | Ferredoxin |
Colonization of legume roots by rhizobia results in the deformation and curling of root hairs, and several genes, e.g. early nodulation (ENOD) genes and Msrip1, are expressed in the epidermis of legumes (Franssen et al., 1995; McAdam et al., 2018). Several recent reviews deal with the complex biological interactions leading to infection and nodulation by rhizobia, such as how the legume controls the massive infection caused by rhizobia (Berrabah et al., 2018; Buhian and Bensmihen, 2018; Poole et al., 2018). The two major types of nodules formed are classified as indeterminate or determinate. Indeterminate nodules are characterized by maintaining meristematic tissue, whereas determinate nodules are spherical and have a transient meristem. The type nodule is determined by the host plant and in rare cases legumes may form both nodule types (Andrews and Andrews, 2017).
The Nif family of rhizobia plays a crucial role in SNF by encoding the nitrogenase complex as well as a number of regulatory proteins involved in nitrogen fixation. Nif genes can be found in other groups of bacteria than rhizobia as well. Besides nif genes, regulation of nitrogen fixation is dependent on the so‐called fix genes. The two main regulatory cascades that can be found in rhizobia are the RpoN‐NifA and the oxygen‐responsive two‐component FixL‐FixJ system, together with FixK. NifA is the master regulator of nitrogen fixation and belongs to the enhancer‐binding protein family of transcriptional regulators that interact with RNA polymerase sigma factor, σ54 (RpoN). The group of Hauke Henneke greatly contributed to the early exploration of nif and fix genes in Bradyrhizobium japonicum (e.g. Fischer and Hennecke, 1984; Fischer et al., 1986; Fischer, 1994). In the case of Rhizobium etli CFN42, rpoN is expressed in two copies: RpoN1 and RpoN2 (Girard et al., 2000; Dixon and Kahn, 2004; Salazar et al., 2010). Rpon1 is encoded in the chromosome in free‐living conditions, whereas rpoN2 is located in the symbiotic plasmid and is expressed independently of rpoN1 in bacteroids (Michiels et al., 1998; Salazar et al., 2010). Österman et al. (2015) showed that Neorhizobium galegae sv. orientalis required rpoN2 for nitrogen fixation. Interestingly, rpoN2 could be found only in symbiovar orientalis but not in the other symbiovars, N. galegae sv. officinalis. The other regulator cascade (FixL‐FixJ‐FixK) is one of the two regulators of transcription of nifA and fix genes in SNF (Dixon and Kahn, 2004).
Energy aspects of nitrogenase
The nitrogenase enzyme complex is the central unit of nitrogen fixation in all known diazotrophic organisms, which are all prokaryotes. The development of biochemistry opened up avenues to characterize and understand nitrogenase and SNF. Non‐symbiotic N2‐fixing bacteria were better amenable to studies of the structure and function of nitrogenase. The first report on the activity of nitrogenase enzyme preparations from Clostridium pasteurianum was by Carnahan et al. (1960). Bulen and LeComte (1966) in their paper The nitrogenase system from Azotobacter: two‐enzyme requirement for N2 reduction, ATP‐dependent H2 evolution, and ATP hydrolysis, described the function of dinitrogenase of Azotobacter vinelandii, Clostridium pasteurianum and Rhodospirillum rubrum, paving the path for further study into this complex system. Mortenson and Thorneley (1979) in their review described in detail the advances of the knowledge of the two‐component nitrogenase enzyme system–the Fe protein, a dimer, and the tetrameric FeMo‐Co protein with its cofactor.
Nitrogenase from the rhizobium–legume symbiosis was isolated by Whiting and Dilworth (1974), while there was still uncertainty about the role of the bacteria vs. the host plant regarding the location of the nitrogenase‐encoding nif genes. However, based on genetic mapping of Klebsiella and Rhizobium, already Streicher et al., (1972) speculated about the construction of rhizobial strains with high effectiveness and broad host range, based on transformation of strains, and Hardy and Havelka (1975) outlined a scheme for the transfer of nif genes to plants. The transfer to other bacteria became a realistic alternative with the detection of nif gene carrying plasmids in Rhizobium leguminosarum (Nuti et al., 1979), but so far, no transfer of functional nif genes to plants has been reported. The construction of the broad host range cloning system by Ditta et al. (1980) opened up the door for more precise characterization of nif and other symbiotic genes. This line of molecular research, making use of the new powerful tools, soon became mainstream.
Before the molecular revolution, however, research on efficiency went through interesting and important stages. Minchin and Pate (1973) performed detailed studies on the carbon balance of the legume and the functional economy of its root nodules, using the garden pea (Pisum sativum) inoculated with Rhizobium strain V 200 as their model legume. Their studies relied on thorough studies on legume physiology reported in a series of papers from Dr Pate’s group, by adding the perspective of energy economy to the functioning of the symbiotic system. They found that the respiratory efficiency of a nodulated root in terms of nitrogen fixation was very similar to that of an uninoculated root assimilated nitrate, i.e. 5.9 vs 6.2 mg C/mg N fixed or assimilated. A mean value of 6 mg C/mg N was obtained by Vance and Heichel (1991) over 35 determinations. Phillips (1980) in his extensive review compiled information from a range of studies on the whole‐plant energy costs of symbiotic N2 fixation in nodulated legumes. The estimated costs expressed as gC/gN varied from below 1 to 20, depending on the method used for the estimation of N2 fixation. Values around 6 gC/gN were obtained when the acetylene reduction method was used to measure nitrogenase activity. Other methods used were the N difference method, obtained by comparison of dry matter and N yields in nitrogen‐fixing and a non‐nodulated crop, or CO2 evolution in relation to N content of nodules or nodulated roots or alternatively, 15N gas incorporated into these.
Estimation of the amounts of N fixed is crucial in all kinds of N2 fixation research. It is also a question of much debate. Herridge et al. (2008) discussed several methods, which were further listed in Lindström and Mousavi (2010): (1) Acetylene reduction, which is an indirect method mainly useful for nitrogenase activity comparisons; (2) total nitrogen balance or determination of nitrogen inputs and outputs in soil and crops; (3) nitrogen difference, obtained by comparing a nitrogen‐fixing crop with a suitable non‐fixing crop; (4) 15N isotope accumulation (incubation with heavy isotope‐enriched nitrogen gas); (5) 15N isotope dilution (application of 15N enriched N fertilizer, which is taken up by mainly non‐fixing plants) and (6) natural 15N isotope abundance. The nitrogen difference method is the simplest and most straightforward, but relies on suitable controls, as does the isotope dilution method. The natural abundance method makes use of the observation that the nitrogenase enzyme discriminates between the nitrogen isotopes, favouring 14N over 15N. Thus, nitrogen‐fixing systems are enriched in 14N. It should be emphasized that all methods and thus all estimates are prone to numerous errors. Phillips (1980) further collected data from literature concerning the community efficiency of symbiotic N2 fixation by temperate legumes in the field. The results varied depending on cropping system and methods used, and ranged from 52 to over 300 kg N2 fixed per ha and year, comparable to the figures (55–140 kg/ha/year) reported by Hardy and Havelka (1975) for corresponding systems and also reported by Herridge et al. (2008). The annual production of industrial nitrogen is 30 Tg, whilst the nitrogen that can be fixed naturally by diazotrophic prokaryotes amounts to 100–122 Tg per year (Herridge et al., 2008).
Hardy and Havelka (1975) suggested that energy was the main factor limiting N2 fixation in legumes. The nitrogenase system can break the very strong triple bond in the dinitrogen molecule. The reaction is energy demanding, as is the industrial reduction by the Haber‐Bosch method, which requires 400–450°C and a pressure of 200 atm to proceed (https://www.chemguide.co.uk/physical/equilibria/haber.html). Seefeldt et al. (2009) estimated that at least 8 moles of electrons and the hydrolysis of 16 moles of ATP are required to reduce one mole of dinitrogen. However, the reaction in legume root nodules is driven by solar energy and it can take place even at temperatures near freezing (Lindström, 1984a), as demonstrated by employing the acetylene reduction method. The nitrogenase reaction formula reveals that for one mole of dinitrogen reduced, one mole of hydrogen gas is evolved:
N2 + 8e− + 8 H+ + 16 ATP → 2NH3 + H2 + 16 ADP + 16Pi (Hoffman et al., 2014).
The evolution of hydrogen gas from soybean nodules in the absence of N2 was already observed by Hoch et al. (1957), suggesting that H2 evolution in the absence of N2 could be used to measure nitrogenase activity. If acetylene is added, nitrogenase prefers this substrate over N2 and at 5% acetylene, N2 reduction is abandoned and replaced by acetylene reduction. Since the affinity for acetylene by nitrogenase is greater than for N2, all hydrogen produced in the presence of enough acetylene is used to produce ethylene (C2H4). The ethylene evolved is easy to quantify by gas chromatography, and the method has provided a handy tool for nitrogenase activity measurements. It is, however, not advisable to use the method for quantification of amounts of N2 fixed over time, but for activity comparisons between rhizobial strains and activity measurements in diverse field conditions it is excellent (see e.g. Lindström, 1984a, 2010,1984a, 2010). A simple conversion factor between ethylene produced and N2 fixed is not advisable, because the acetylene reduction method is indirect, inhibits N2 provision to the plant by nitrogenase, and consumes all H2 produced. Nevertheless, acetylene reduction meant a great leap forward for research needing nitrogenase activity measurements and for practice. Hardy et al. (1968) describe the development of the method and its applications in detail. Hunt and Layzell (1993) gave a list of factors to be aware of when using the method, which had then been debated for almost a decade.
The role of hydrogen in symbiotic nitrogen fixation
The evolution of H2 from the nitrogenase reaction was for a long time considered as energy wasted. Schubert and Evans (1976) paid attention to the evaluation of the magnitude of energy loss in terms of efficiency of electron transfer to nitrogen via nitrogenase. They measured H2 evolution from nodules in air and in argon, and in addition acetylene reduction activity for 19 inoculated rhizobium–legume combinations and five actinorhizal symbionts. Based on the results, they calculated the relative efficiency (RE) of the nodules as 1‐ H2(air)/H2(Ar) and 1‐ H2(air)/C2H2. The values obtained showed some variation for the individual systems depending on the method. The greatest differences were however observed between the symbiotic combinations with cowpea and mung bean inoculated with strain Rhizobium 32H1 (Bradyrhizobium according to current classification) and legumes inoculated with other strains. The measurements with soybean were repeated with shoots exposed to light and only roots incubated in acetylene, to ensure optimal energy charge for the symbiosis. Cowpea and the non‐legumes Alnus rubra, Purshia tridentata, Elaeagnus angustifolia, Caenothus velutinus and Myrica californica had superior REs, indicating that these plants have mechanisms for dealing with H2 loss. The REs of the other symbiotic reactions were generally around 0.50 (0.20–0.69), whereas that of cowpea was 0.99 and of 32H1‐inoculated mung bean was 0.73–0.82. Since the REs below 1.0 imply energy loss, the study prompted a renewed interest in hydrogenases in nodules.
According to the brief review by Dixon (1978), already in 1941, Phelps and Wilson (1941) reported the presence of hydrogenase in pea root nodules induced by Rhizobium leguminosarum ONA 311. This could not be confirmed, but the report on H2 evolution by soybean nodules led to investigations of pea nodules. The discovery that pea nodules did not evolve hydrogen was followed by the finding that hydrogenase was located in pea bacteroids formed by strain ONA 311. Dixon (1978) described several properties of rhizobial uptake hydrogenase and speculated about its role, suggesting that it can recycle electrons and in respiration contribute to the energy supply to nitrogenase. Further studies on RE in different rhizobial strains and symbioses showed that hydrogenases are widespread but their efficiencies vary, being one component of the RE of legume root nodules. Dr Ruiz‐Argueso contributed to work on rhizobial hydrogenases already early on (e.g. Emerich et al., 1979) and went on with his group to study hydrogenases of R. leguminosarum (e.g. Leyva et al., 1987). They were able to unravel regulation of the hup operon by NifA protein (Brito et al., 1997) and detected the presence of at least 17 common genes (hupSLCDFGHIJKhypABFCDEX) arranged in at least three operons with conserved gene composition and organization (Baginsky et al., 2002). These hup genes were also identified in species of Rhizobium, Bradyrhizobium and Azorhizobium. However, whole‐genome sequencing of the model strain Rlv UPM791 revealed that the presence of hup genes is rather strain specific and that the genes might have different evolutionary origin in different species.
Schubert et al. (1978) studied the effect of hydrogenase possessing (Hup+) and non‐possessing (Hup−) strains in symbiosis with soybean and cowpea. They concluded that the Hup+ strains produced more biomass than the Hup− ones, probably due to hydrogen recycling. The experiments were performed in axenic culture in the greenhouse. Later studies have shown that hydrogen excreted from legume nodules might serve as an energy source for rhizosphere‐dwelling bacteria (Dong et al., 2003), which was already speculated about by Schubert and Evans (1976). Thus, experiments in axenic culture cannot be directly extrapolated to field conditions.
It is still not understood why nitrogenase wastes 25% of the energy obtained from ATP in the reaction, as recently noted by Hoffman et al. (2014) in their extensive review. The role of the excreted H2 has, however, been clarified in some symbiotic systems. La Favre and Focht (1983) studied the rhizosphere of Cajanus cajan inoculated with rhizobia possessing varying degrees of Hup+ activity. They concluded that all H2 emitted from the nodules would, independent of the Hup status, be consumed by rhizosphere bacteria within a 3‐4.5 cm radius of the nodule surface. Uratsu et al. (1982) collected soybean nodules from the major soybean growing areas and concluded that a majority (> 75%) of the Rhizobium japonicum strains isolated from those areas were lacking H2 uptake activity. Dong et al. (2003) found that pre‐treatment of soil with H2 increased yields of non‐legume crops and uninoculated soybean over controls, giving an indirect explanation to traditional knowledge of pre‐crop effects of legumes and benefits of legume intercropping. During the succeeding decades, the role of plant growth‐promoting bacteria in the rhizosphere of legumes and other crops has been detected (for a review see Glick, 2012). It is close at hand to conclude that the H2 emitted from nodules serves as an energy source for those. Recently, Li et al. (2018) reported that hydrogen treatment of rhizosphere soil samples of Medicago sativa plots influenced the composition of microbial communities as determined by amplicon sequencing of 16S rRNA genes, in comparison with untreated soil. Taken together, the role of uptake of hydrogenases for effectiveness in rhizobia remains obscure.
Hydrogen gas plays a major role in the nodule during SNF. Hunt and Layzell (1993) put great emphasis on techniques used to measure gas exchange and nitrogenase activity in nodules, especially hydrogen and oxygen, and reviewed results from several studies. Their article offers a thorough view on multiple aspects of gas exchange and is still an excellent source of information on the topic. Though H2 production theoretically accounts for 25% of the electron flux to nitrogenase, in practice only 40%–70% is used for SNF. H2 inhibition of nitrogenase might thus be an important factor in reducing nitrogenase efficiency and legume yields.
Energy supply to nitrogenase: the O2 paradox
Rhizobia are obligate aerobes, thus needing oxygen for their energy metabolism, also in SNF. On the other hand, both nitrogenase proteins and the protein‐free FeMo‐Co are sensitive to oxygen with the sensitivity in the order FeMo‐co»>Fe protein» MoFe protein (Mortenson and Thorneley, 1979). The protection of nitrogenase is mediated by several mechanisms. Vance and Heichel (1991) summarized the importance of oxygen and carbon for nodule physiology and uncover several factors/processes with importance for efficiency. The nodule inner cortex, which surrounds the central zone of infected cells, serves as a diffusion barrier which limits the flux of O2 to the bacteroids. Together with leghaemoglobin, this barrier regulates the flux of O2 to nitrogenase. Further, they list plant redirection of glycolysis to malate with subsequent reductive formation of succinate under microaerobic conditions and bacteroid ATP formation coupled to a high‐O2‐affinity terminal oxidase as factors regulating the O2 flux.
Leghaemoglobin is invariably associated with nitrogen‐fixing nodules in legumes and has been used as an index of fixation potential (Virtanen et al., 1955). This is due to the pigment being present in fairly constant cellular concentration within the nitrogen‐fixing cells of nodules. Its nodular concentration is therefore an index of the amount of nitrogen‐fixing tissue which is present (Bergersen, 1961). Appleby (1984), being a pioneer in nodule physiological research, summarized knowledge on leghaemoglobin and rhizobial respiration accumulated thus far, confirming the role of leghaemoglobin as a facilitator of O2 flux to the vigorously respiring, phosphorylating, N2‐fixing rhizobium bacteroids, albeit at a stabilized O2 tension (10 nm in soybean nodules). However, not until 2005 was it demonstrated that leghaemoglobins are required for efficient nitrogenase activity. Plant reverse genetics employing RNAi was then used by Ott et al. (2005) in the model legume Lotus japonicus to create plants carrying nodules abolished in leghaemoglobin synthesis, causing an increase in nodule free oxygen, loss of nitrogenase and absence of SNF, whereas growth on combined nitrogen was not affected. The non‐legume Parasponia seemed to be the only exception to N2‐fixing nodules having leghaemoglobin, but van Velzen et al. (2018) in a comprehensive study of this plant showed that this non‐legume has indeed recruited the class I leghaemoglobin HB1 for balancing oxygen levels in the nodules, whereas legumes and the actinorhizal Casuarina use class 2 haemoglobins for this purpose.
Following the demonstration of the bacteroid as the nitrogen‐fixing organelle (Bergersen and Turner, 1967), the energy provision for nitrogenase has been a long‐standing and complicated issue that has received the attention of scientists over the years (e.g. Bergersen, 1971; Appleby, 1984; Kaminski et al., 1996; Marchal and Vanderleyden, 2000). The respiratory machineries in different rhizobia are diverse and complex. Fischer (1994) gives a thorough account of nif and fix genes in S. meliloti, B. japonicum and A. caulinodans identified by then, showing gene arrangements and regulatory networks. Genes, mutations in which caused loss of N2 fixation, were named fix genes. Important fix genes are the microaerobically induced fixNOQP, which encode a membrane‐bound cytochrome oxidase. fixGHIS genes, which map immediately downstream of the fixNOQP operon, encode the symbiotically essential cbb3‐type haem‐copper oxidase complex (Preisig et al., 1996). The fixABCX operon, also required for nitrogenase activity, was later shown to encode an electron‐transferring‐flavoprotein (ETF) dehydrogenase (fixABC), whereas FixX shows similarity to ferredoxins. Delgado et al. (1998) reviewed genes involved in the formation and assembly of rhizobial cytochromes and their role in symbiotic nitrogen fixation. However, still many features of rhizobial respiration are unknown. The genome of B. japonicum encodes as many as eight different terminal oxidases for respiration in oxic conditions (Youard et al., 2015). Thus, even though respiration is crucial for efficiency, no definite answers to whether respiratory activity or nitrogenase function limits efficiency of N2 fixation have so far been identified in this complex system.
Modern molecular approaches now enable genome comparisons and phylogenetic approaches to be employed for renewed studies on symbiotic processes with a wider scope. Degli Esposti and Martinez Romero (2016) present an interesting advance regarding the respiratory chain of symbiotic rhizobia. They depict a current view on rhizobial energy provision to nitrogenase (Fig. 2). The respiratory complex I is a NADH: ubiquinone oxidoreductase (Nuo), and one of the largest membrane protein assemblies known. Complex I has a central role in energy production in mitochondria. Whereas the mitochondrial complex I consists of 45 subunits, the prokaryotic enzyme consists of 14 ‘core’ subunits, conserved from bacteria to humans (Efremov et al., 2010; Degli Esposti, 2015). Degli Esposti and Martinez Romero (2016) compiled data from literature into a figure with enzyme expression or protein data of 16 enzymes (nitrogenase and respiratory) from Frankia and seven rhizobial species representing five genera. They proposed that in rhizobia the NUO14 complex 1 was downregulated in symbiosis in comparison with non‐symbiotic conditions, and the so‐called green complex I, an ancient form of the enzyme, was upregulated in Rhizobium and Sinorhizobium, but absent from the other genera. They also studied the evolution of respiratory complex I which showed that about 70 Alphaproteobacteria, predominantly Rhizobiaceae, possessed two different operons for respiratory complex I, namely the standard NUO14 and another called the green complex I (Degli Esposti and Martinez Romero, 2016). The peculiar rearrangement of gene order of green complex I was discovered in about half of the Rhizobium and Sinorhizobium strains studied by Degli Esposti and Martinez Romero (2016). The complex was always upregulated in the symbiosis of strains that possessed it. The authors thus proposed that the function of the hypothetical green complex I is to increase the efficiency of N2 fixation in symbiotic nodules.
Figure 2.
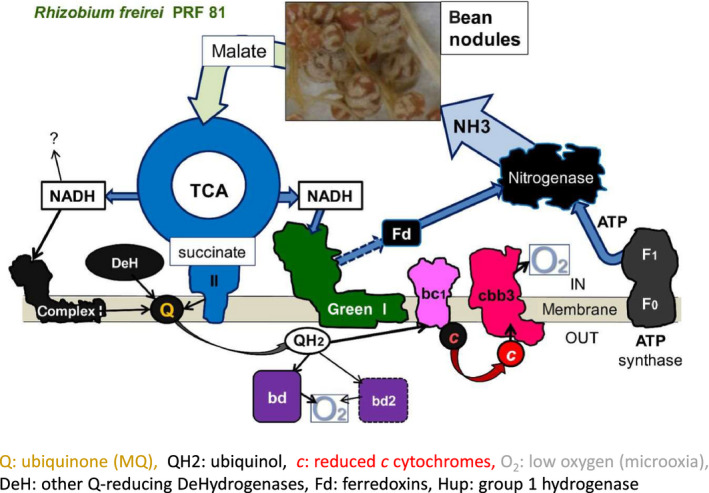
Deduced energy metabolism in nodulating symbionts of Rhizobium freirei and hypothetical role of green complex. Reprinted from Degli Esposti and Martinez Romero (2016) with the permission of Oxford University Press.
Supply of carbon and other elements
Transport and metabolism of an infected nodule cell are depicted in Fig. 3 (Liu et al., 2018). Since the energy used for SNF is provided by the plant, delivery of photosynthate is crucial for the SNF process. Sucrose from the shoots is cleaved by sucrose synthase in the plant cytoplasm, and hexoses are formed and further metabolized to malate. Sucrose metabolism influences the efficiency of SNF, as sucrose synthase was proven to be essential for SNF in pea nodules, demonstrated in a plant mutant, unable to form effective nodules (Gordon et al., 1999). By transcriptomics it was shown in the model plant Lotus japonicus that several metabolic pathways appeared to be coordinately upregulated in nodules, among them are glycolysis, CO2 fixation, amino acid biosynthesis, purine, haem and redox metabolism (Colebatch et al., 2004).
Figure 3.
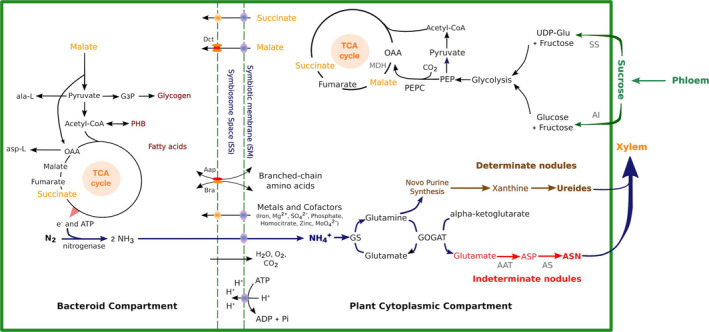
Schematics of carbon and nitrogen metabolic pathways with key enzymes, metabolites and transporters in determinate nodules and indeterminate nodules. Reprinted from Liu et al. (2018) with the permission of Frontiers.
Ronson et al. (1981) showed that a mutant of R. leguminosarum sv. trifolii defective in dicarboxylate was Fix− on its white clover host. Now we know that it is malate that is transported across the symbiosome membrane and further to the bacteroid by a dicarboxylic acid transporter, DctA. The gene dctA is regulated by dctB/dctC, which is one of the first sensor‐regulator systems to be characterized (Ronson et al., 1984, 1987). Malate is further processed for the TCA cycle, which is central for energy and carbon provision to nitrogenase. Malic enzyme alone or in conjunction with PEP carboxykinase in combination with pyruvate kinase converts malate to pyruvate, needed for acetyl‐CoA, which condensed with oxaloacetate to feed the TCA cycle. Since the rhizobial bacteroid is sometimes limited in carbon skeletons for biosynthesis, gluconeogenesis might also occur in bacteroids (Udvardi and Poole, 2013).
The role of storage polymers in the bacteroid, such as PHB, seems to vary depending on the plant and bacterial species. Bacteroids from determinate nodules store large amounts of PHB, whereas in indeterminate nodules it might serve as storage for excess reductant, thereby influencing efficiency. Other such compounds are glycogen or lipid or even starch (Udvardi and Poole, 2013). The photosynthetic Bradyrhizobium sp. ORS278 has an operating Calvin cycle (Bonaldi et al., 2010) and the CO2‐fixing enzyme RuBisCO (Gourion et al., 2011), which were both shown to be important for SNF in the Bradyrhizobium‐Aeschynomene system and might serve a similar purpose as the storage compound PHB.
Transport of other elements has received less attention. Liu et al. (2018) in their review pay attention to phosphate, concluding that phosphorous deficiency affects the carbon and nitrogen metabolism in nodules and might thus affect the efficiency of N2 fixation. P deficiency depends on soil and rhizosphere conditions. Vance (2001) discusses the role of legumes, N and P in agriculture and emphasizes the role of mycorrhizal symbioses for P uptake. The need for Mg, Fe and Mo in the bacteroid is obvious, but very little is known about their transport. A curiosity in most rhizobia studied, is the lack of homocitrate synthase, encoded by nifV, which is present in free‐living N2 fixers. In legume nodules, homocitrate, needed for the synthesis of the FeMo‐Co, is synthesized by the plant enzyme FEN1 and transported through the symbiosome membrane. In some bradyrhizobia nodulating Aeschynomene species, a bacterial nifV gene is, however, needed for effective symbiosis (Nouwen et al., 2017), and a copy of this gene was detected in the genome of Sinorhizobium americanum (Peralta et al., 2016).
Transport of fixed N
Ammonia formed by the nitrogenase system is most probably transferred from bacteroids via simple diffusion or via unspecific protein channels through the symbiotic membrane or through channels for NH3 or cations or aquaporin‐like channels. Bacteroids can thus be considered ammonium‐secreting ammoniaplasts. Once in the symbiotic plant cell, ammonia is incorporated in amino acids, mainly glutamine and asparagine, whereas in soybean fixed ammonia is transported in ureides (Udvardi and Poole, 2013). Already Herridge (1982) devised a method for estimation of SNF in ureide transporting plants, by measuring the relative abundance of ureides and nitrate in plant tissues. Amino acids present in bacteroids were for a long time confusing scientists. Their role is now thought to be provision of compounds needed before the onset of SNF and transport systems for branched amino acids have been identified for them, but they are still under study (Udvardi and Poole, 2013).
Unkovich and Layzell in 2016 (http://www.ini2016.com/1360) speculated that the nitrogenase enzyme might be N2 limited. Tegeder and Perchlik (2018) stress the importance of amino acid and ureide transporters localized to critical positions, where they control nitrogen partitioning, which influences nitrogen use efficiency (NUE) in peas and soybeans. Nitrogen translocation to seeds in pulse legumes contributes to seed yield, which is an estimate of the effectiveness of nitrogen fixation in pulse legumes. Optimal source–sink relationships should be achieved by matching rhizobial and plant germplasm with prevailing environmental conditions. Lindström (1984b) reported for red clover inoculated with different rhizobial strains that symbioses in which acetylene reduction activity and plant growth rate were well correlated gave the best yields. Strains aggressive at early stages of plant development might have allocated excessive resources for SNF, at the cost of other plant functions. Considering the environmental impact of crops, NUE is an important breeding and cultivation target, since it includes N yields but also losses from soil by leaching and emissions of nitrous oxide (Zhang et al., 2015).
Regulation of N2 fixation
NifA was demonstrated to act as a positive regulator of nif genes in Klebsiella pneumoniae around 1980 (Buchanan‐Wollaston et al., 1981), and NifL was found to be a repressor. The nifLA operon was found to respond to combined N and O2. The S. meliloti NifA was identified by Szeto et al. (1984), and experiments by Ditta et al. (1987) with S. meliloti indicated that reduced oxygen tension but not combined N was potentially a major trigger for symbiotic activation of nitrogen fixation in Rhizobium species. Fischer (1994) in his review compared the nif–fix operons of S. meliloti (Rm), B. japonicum (Bj) and A. caulinodans (Ac). In contrast to Klebsiella, most rhizobia studied have the fixLJ oxygen‐responsive two‐component regulatory system, which is involved in positive control of fixK (Rm, Bj, Ac) and nifA (Rm). FixK and FixK2 are positive regulators of fixNOQP (Rm, Bj, Ac), nifA (Ac), rpoN1 and ‘nitrate respiration’(Bj), and also a negative regulator of nifA and fixK (Rm).
The regulatory cascades around the master regulator NifA depicted in the paper by Fischer (1994) are revisited and refined by Terpolilli et al. (2012) and extended to Rhizobium etli, R. leguminosarum and R. phaseoli. B. japonicum displays some unique features: fixK2 in microaerobic conditions controls fixNOPQ and fixGHIS, genes involved in haeme biosynthesis, denitrification genes, phbC, rpoN and fixK1. NifA is autoregulated but also dependent on regRS. The three R. leguminosarum strains studied have different regulatory mechanisms, one strain (VF39) possessing a hybrid regulatory sensor fixL, for example, autoregulates nifA, contrary to the other strains. Likewise, two bean‐nodulating strains now classified as R. etli and R. phaseoli show different regulatory networks. It is obvious that diverse regulatory patterns have evolved even among strains representing the same species and even symbiovar. Based on these studies, one can conclude that several variations on the theme of regulation have evolved. This diversity might tell us something about adaptation.
NCR peptides
A new era in BNF research was opened up with the discovery of the NCR (nodule‐specific cystein‐rich) peptides in nodules of Medicago truncatula (Mergaert et al., 2003). By transcriptome analysis, over 300 members of this peptide family were discovered. They were characterized by a signal peptide and a conserved cysteine motif, but otherwise showed great divergence. Interestingly, they were discovered only in galegoid legumes, now renamed IRLC (inverted repeat‐lacking clade) (Wojciechowski et al., 2004). Since their discovery, reports about the biological functions of NCR peptides and their occurrence have been frequent, as illustrated by several reviews (e.g. Kondorosi et al., 2013; Pan and Wang, 2017; Kereszt et al., 2018).
The NCR peptides are part of a larger group of peptides with biological functions involved in a wide range of processes, loosely defined as gene‐encoded small proteins of about 2 to 100 amino acids (Kereszt et al., 2018). They are very diverse, illustrated, e.g. by their very different isoelectrical points. The cationic NCR peptides seem to have the greatest influence in nodules. The nodule development in IRLC legumes is special in that bacteroid maturation inside the indeterminate nodules of legumes from this clade shows remarkable similarity to host cell differentiation. Since the effects of NCR peptides resemble antibacterial peptides (AMPs) or plant defensins, causing cell death, Van de Velde et al. (2010) investigated the role of these peptides in the morphological changes of rhizobia observed in IRLC legumes. Using M. truncatula as a model, they found that the NCR peptides indeed were involved in the terminal differentiation (endoreduplication, cell enlargement, loss of cell division capacity, and increased membrane permeability) in IRLC legumes. These bacteroids are not able to convert to a free‐living state, whereas bacteria inside determinate nodules with no differentiation can revert back to a free‐living state. In vitro, NCR peptides display antimicrobial activity against a broad range of bacterial species; however, only a few NCR peptides have been studied in detail and the precise in planta activity of NCR peptides remains uncertain.
The process of terminal differentiation, repeated DNA replication (endoreduplication) without cell divisions, results in extensive amplification of the entire bacterial genome and elongation of bacteria, revealing a positive correlation between DNA content and cell size, similar to that in eukaryotes (Mergaert et al., 2006). This endoreduplication results in up to a 128‐fold increase in cell DNA. By comparing different legumes, Montiel et al. (2016, 2017) were able to describe different nodule morphotypes: (S) standing for swollen bacteroids, found in five out of six major papilionid subclades; (EB) displaying elongated and branched bacteroids, in Medicago, Vicia and Galega belonging to the vicioid subclade; (E) bacteroids meaning elongated; (SP) standing for spherical bacteroids; and (U) for undifferentiated ones. A correlation between the number of NCR peptides and bacteroid morphology was found in all the studied IRLC legumes, including the Vicioid Cicer, Ononis, Trifolium and Pisum, in addition to Medicago, Vicia and Galega, as well as in Astragalean Astragalus, Hedysaroid Onobrychis and Glycyrrhiza. The highest number of NCR peptides predicted from nodule transcriptomes was around 600 in Medicago truncatula, whereas in Glycyrrhiza uralensis only seven were detected. EB bacteroids were characteristic of plants with high NCR numbers. NCR peptides or their genes were so far found only in plants forming indeterminate nodules, and were absent from at least Lotus japonicus, Glycine max, Glycine sojae, Phaseolus vulgaris and Cajanus cajan with U‐type bacteroids. The terminal differentiation of bacteroids has, however, only been properly studied in the IRLC legumes and in Aeschynomene in the Dalbergoid clade, in which NCR‐like peptides have been detected (Czernic et al., 2015; Montiel et al., 2017). In the Dalbergoid Aeschynomene, bacteroids are in some species of the elongated type, whereas in other species in this genus and in the likewise Dalbergoid Arachis hypogaea the bacteroids are large spheres (SP type), just like those of the IRLC Cicer arietinum and Ononis spinosa. It has been assumed that the host determines the bacteroid and nodule types (e.g. Bakkou, 2011). However, Crespo‐Rivas et al. (2016) showed that Sinorhizobium fredii HH103 bacteroids were not terminally differentiated in the nodules of the IRLC legume Glycyrrhiza uralensis, an exception from the terminally differentiated nodules harbouring Mesorhizobium tianshanense HAMBI 3372, as observed by Montiel et al., (2016, 2017). Lamouche et al. (2019b) reported a relationship between bacteroid type and effectiveness. A transcriptomic analysis on E‐ and S‐type bacteroids formed by Aeschynomene afraspera and A. indica nodules identified bacterial functions activated in bacteroids specific to each bacteroid type. More studies are needed to further investigate the role of bacterial strains for this process.
Kereszt et al. (2018) give an update on the current knowledge of plant peptides in rhizobium–legume symbioses. Already in response to Nod factors, plant peptides are involved in processes leading to nodule formation and SNF. Two systemic regulation processes are especially prominent. Peptides of the CEP family exert a positive effect on nodulation in response to N starvation, whereas CLE is a negative regulator of nodule numbers. Other peptides contribute to nodule formation. The RALF and DLV peptides negatively affect the infection process in early stages of nodule development. During later stages, RALF has a negative but DLV a positive effect. ENOD40 and miPEP172c affect primordium formation, uORFp1 participates in the control of meristem maintenance, and apart from NCR peptides, GRP and SNARP peptides probably control bacteroid differentiation and functioning. All of these have their own roles in nodule formation and SNF. The NCR peptides, however, are more relevant for the topic of this minireview.
The cationic NCR247 is involved in a complex interaction network, which affects bacterial cell division machinery by binding to FtsZ, preventing septum formation, and also translation, and protein folding (Farkas et al., 2014). Pan and Wang (2017) reviewed recent developments regarding molecular events guiding bacteroid development in indeterminate nodules. These nodules can be divided into 4‐5 zones, as first described by Vasse et al. (1990): nodule meristem, infection zone, interzone, fixation zone, and senescence zone. Determinate nodules lack a persistent apical meristem and do not possess the respective zones, just the fixation zone. NCR peptides all have in common that the signal peptide is cleaved by the DNF1 signal peptidase complex, ensuring excretion of the peptides into the bacteroid (Wang et al., 2010). According to their working model, thioredoxin s1 acts on disulfide bridges of cysteines producing oxidized peptides, mainly in the infection zone. NCR peptides NCR211 and NCR169 were identified as essential for N2 fixation (Horvath et al., 2015; Kim et al., 2015), after two ineffective plant mutants dnf4 and dnf7 were found to be defective in genes encoding these peptides. NCR211 acts in the infection zone and the interzone, NCR169 mainly in the interzone and fixation zone (Pan and Wang, 2017).
Rhizobia can defend themselves by producing the HrrP metallopeptidase, expressed in all nodule zones in bacteria carrying the hrrP gene (Price et al., 2015). Two accessions of Medicago truncatula, A20 and A17, were compared. Strain S. meliloti B800 was Fix+ on A17 but Fix− on A20. Fix+ was restored on A20 by curing strain B800 of a 199‐kb plasmid. Further studies demonstrated that this plasmid carried the hrrP gene. HrrP expression caused the rhizobia to escape terminal differentiation and contributed to maintain living cells inside the nodule. It seems that the NCR peptides contribute to the host specificity of nitrogen fixation and that their role is to optimize nitrogen supply to the plant by specifically stimulating bacteroids to grow and fix N2 and not become parasitic, the so‐called ‘cheaters’ (Oono et al., 2009; Van de Velde et al., 2010).
NCR peptides can also be involved in the selection of the bacterial partner, since incompatibility between M. truncatula ecotype Jemalong and S. meliloti strains Rm41 and A145, which form effective symbioses with other Medicago partners, is eliminated from nodules by allelic forms of two peptides, NFS1 and NFS2 (Wang et al., 2017). In IRLC legumes, especially in the vicioid clade, the symbiotic interaction is especially fine‐tuned. It has thus been further speculated that NCR peptides contribute to efficiency of N2 fixation by stimulating bacteroids to grow, prolong the lifetime of nodules, and reduce senescence in the senescence zone, especially in strains lacking HrrP. Oono and Denisonet al. (2010) give an overview of the phylogeny of 40 Papillionid species with nodule and bacteroid types indicated. The tree can guide in the selection of symbioses for comparative studies. It shows that swollen bacteroids have multiple origins and might confer some host benefits, such as optimization of N2 fixation efficiency, as further elaborated by Lamouche et al. (2019b) for Aeschynomenae species.
The rhizobial bacA gene, encoding an ABC transporter, was identified in Sinorhizobium meliloti by Glazebrook et al. (1993) as being essential for bacteroid development. Its role was further manifested by Haag et al. (2011) as critical for the legume symbiosis, by protecting S. meliloti against the bactericidal effects of NCR AMPs. In Bradyrhizobium, a corresponding gene bclA was identified as being necessary for bacteroid differentiation in Aeschynomene. Since the NCR peptides in IRLC and Dalbergoid legumes seem to have evolved independently, phylogenetically diverse legumes seem to have evolved convergently. A productive N2‐fixing symbiosis can be seen as the result of a carefully maintained balance between the effect of NCR peptides and the ability of rhizobia to resist them. The result of this interplay might contribute to host specificity.
NO in symbioses
Nitric oxide (NO) is a gaseous reactive oxygen species (ROS) that has evolved as a gaseous intracellular and intercellular signalling molecule. NO has a diverse range of regulatory functions (signalling hormone) in many physiological processes in mammals. Beside animals, NO functions as a signalling molecule involved in plant growth and development, as well as in plant response to abiotic and biotic stresses (Boscari et al., 2013a; Domingos et al., 2015). In plants, NO can induce the plant defence system, and thus evolution of pathogens armed them with the mechanism of NO detection and modulation and adaptive response to this signal (Meilhoc et al., 2010). NO per se, at high concentration, can become very toxic, and Meilhoc et al. (2010) showed that the presence of NO in culture could inhibit the growth of Sinorhizobium meliloti. Regarding SNF, NO can potentially act as a nitrogenase inhibitor (Boscari et al., 2013b). Cueto et al. (1996), however, reported a putative NO synthase in lupine nodules, by which arginine is converted to NO. In 1998, Mathieu et al. demonstrated that a complex of NO and leghaemoglobin (Lb) was detected in the nodules formed by Bradyrhizobium japonicum strain Bj110 in Glycine max. In the 2000s, production of NO was observed in bacteroid‐containing cells of nodules of Medicago truncatula and M. sativa and in mature nodules of Lotus japonicus (Baudouin et al., 2006; Pii et al., 2007; Shimoda et al., 2009). Since 2010, many hypotheses were proposed to explain the sources of NO and the effects of NO on the molecular dialogue between legumes and rhizobia, some of which are presented here (for reviews, see Meilhoc et al., 2011; Boscari et al., 2013a; Hichri et al., 2016).
The origin of NO and the responses of plant and rhizobia to NO at different steps of the SNF is diverse. The production of NO in the early stage of rhizobium–legume interaction can occur as a result of plant‐defence activity (Boscari et al., 2013a). Studying the SNF interaction between Mesorhizobium loti and Lotus japonicus showed an accumulation of NO in the root surface in the first four hours after inoculation (Nagata et al., 2008). The lipopolysaccharide (LPS) molecules produced by M. loti induced NO production in the roots of L. japonicus (Murakami et al., 2011). In mature nodules, both plant and bacteroid partners are involved in the production of NO (Hichri et al., 2016). The studied sources of NO and NO scavengers in SNF interactions are listed in a recent article by Berger et al. (2018). In summary, the specific NO‐generating systems in SNF interactions can be listed as follows: the mitochondrial electron transport chain, nitrate reductase, nitric oxide synthase and nitrate‐independent nitrate reductase in the plant, and in the bacteria nitrate reductase, periplasmic nitrate reductase (Nap) and nitric oxide synthase (Berger et al., 2018). In nodules, the nitric oxide synthase (NOS)‐like NO generating system (O2 consuming process) occurs in normoxic tissues, while in hypoxic or microoxic conditions, nitrate reductase (NR) and mitochondrial electron transport chain (ETc) mostly occur (Meakin et al., 2007; Sánchez et al., 2010; Horchani et al., 2011). Performance of transcriptomic experiments in the past two decades identified thousands of plant genes (Ferrarini et al., 2008; Meilhoc et al., 2011; Boscari et al., 2013b; Hichri et al., 2016) and hundreds of rhizobial genes (Hyduke et al., 2007; Meilhoc et al., 2010; Hichri et al., 2016) as upregulated by NO. Ferrarini et al. (2008) showed that among 999 genes upregulated as a response to NO in roots of M. truncatula, 290 (278 induced and 12 repressed) genes were detected during nodule development. This finding supported the hypothesis of involvement of NO in nodule development. Furthermore, NO upregulates various genes participating in flavonoid biosynthesis (e.g. chalcone synthase), development of root hairs at the nodulation stage (kinases, receptor‐like kinases, transcription factors), in carbon metabolism (sucrose transport, sucrose synthase, isocitrate dehydrogenase, glyceraldehyde‐3‐phosphate dehydrogenase or malate dehydrogenase), in nitrogen metabolism (glutamine), and in proteasome‐dependent proteolysis (Meilhoc et al., 2011). Moreover, NO in concert with reactive oxygen species (ROS) are key regulatory players of the redox reactions of symbiosis (Puppo et al., 2013). Shimoda et al. (2005) demonstrated that NO upregulates the expression of LjHb1 that encodes nsHb1 protein. The expression of Hb1 modulates the level of NO (downregulating) to reduce plant defence response and permitting reception of symbiont in the roots (Murakami et al., 2011). The other regulatory role of NO on nodule development occurs via plant genes involved in nodule development, such as MtCRE1 and MtCCS52A (Ferrarini et al., 2008; del Giudice et al., 2011).
The other scenario of response to NO, a toxic molecule for bacteria, occurs in rhizobia (Fig. 4). The majority of rhizobial genes upregulated in response to NO are regulated through either FixLJ or NnrR (Meilhoc et al., 2010). The signal of low oxygen leads to autophosphorylation of FixL (hem‐based sensor kinase) that transfers the phosphoryl group to FixJ (response regulator). Phosphorylated FixJ triggers the transcription of FixK and NifA genes (Tuckerman et al, 2001; Meilhoc et al., 2011). NnrR, a member of FNR/CRP family, is a NO‐dedicated sensor. Tosques et al. (1996) reported that the products of NnrR were responsible for production (by nitrate reductase, nir) and reduction (by NO reductase, nor) of NO in a denitrifying strain of Rhodobacter sphaeroides. NnrR could be considered as the regulator that can tune the level of nitrate and NO in nodules, and protect nitrogenase against inhibition by these molecules. Furthermore, flavohaemoglobin (Hmp) and two proteins of the NnrS family (NnrS1 and NnrS2) are involved in NO detoxification (Meilhoc et al., 2010; Cam et al., 2012; Blanquet et al., 2015; Hichri et al., 2016). Interestingly, in one of our studies (Österman et al., 2015), we found that the superior Neorhizobium galegae nitrogen fixers harboured nnrRS genes that could not be identified among the other strains.
Figure 4.
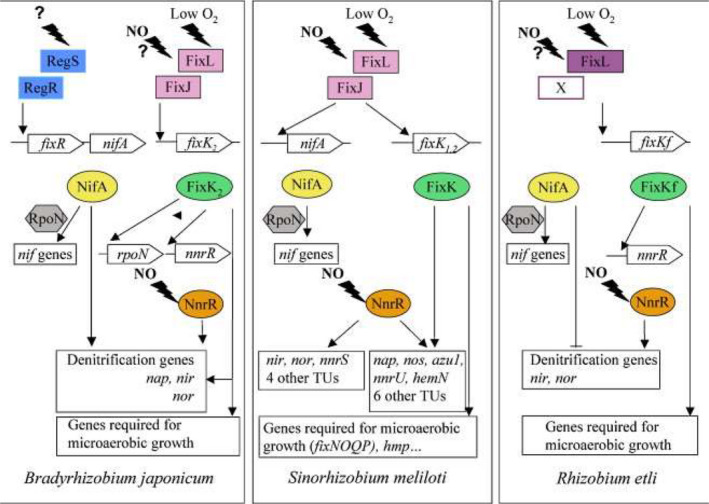
Comparison of NO signalling cascades in the symbiotic bacteria Bradyrhizobium japonicum, Sinorhizobium meliloti and Rhizobium etli. TU: transcription unit. Reprinted from Meilhoc et al. (2011) with the permission of Elsevier.
A diversity and evolutionary perspective
Enormous development regarding the symbiotic interactions has been made during the last decades. Medicago truncatula with its microsymbiont Sinorhizobium meliloti has been used as a model system for symbioses with indeterminate nodules induced, whereas Lotus pedunculatus– Mesorhizobium loti has served as the model for symbioses with determinate nodules. Great advances were also made concerning rhizobium phylogeny and taxonomy. However, including both the bacterial and the legume symbionts in experiments rapidly increases the amount of experiments needed, which thus increases and becomes difficult to handle. Looking for determinants governing symbiotic efficiency becomes like looking for a needle in a haystack. Traditional screening for effective symbioses, i.e. inoculation of selected rhizobia on plants, can reveal superior combinations useful in agriculture. But the mechanisms behind the results are seldom revealed.
The recent studies on the role of NCR peptides, bacA and hrrP for symbiosis, referred to above, evoked a renewed interest in molecular mechanisms behind efficient symbioses and host specificity. Comparisons of N2 fixation in diverse hosts producing different bacteroid types showed that bacteroid morphology makes a difference. Peas and peanuts with EB and SP bacteroids, respectively, had greater fixation efficiency than beans and cowpeas with U bacteroids inoculated with the same rhizobial strain (Oono and Denison, 2010). Investigating bacteroid differentiation may reveal mechanisms behind these observations, useful for further research into the topic of swollen bacteroids. Are they symbiotically more efficient than non‐swollen ones, and can we this way modify other host species to have higher nitrogen fixation efficiency as well?
Price et al. (2015) screened 150 Sinorhizobium isolates and noted that about 20% of natural isolates harboured hrrP homologs, all of which appeared to be encoded on large accessory plasmids. Further, diCenza et al. (2017) in their study on definition of a minimum rhizobium symbiotic genome, using the model S. meliloti–M. sativa symbiosis, performed studies with S. meliloti, S. fredii and R. leguminosarum to explore the role of bacA for specificity. Using bacA protein sequences from 82 sequenced genomes, they found that the S. meliloti BacA has evolved towards a specific interaction with Medicago, more related to some Gammaproteobacteria than to those of other rhizobia. They concluded that their results highlight the limitations of using a single model system for the study of complex biological topics.
Matching compatible bacterial and plant accessions for improved performance is important. Our own work with N. galegae and its host plants Galega orientalis and G. officinalis has shown that symbiotic bacteria and host plants co‐evolved (Suominen et al., 2001). The bacterial species N. galegae can be divided into two symbiovars, orientalis and officinalis. Both symbiovars nodulate both host plant species, but depending on with which host plant an effective symbiosis is formed, we distinguish the symbiovars. Other legume species are not nodulated by these bacteria. By studying a collection of bacterial root nodule isolates representing either symbiovar or plant corresponding accessions from the Caucasus, we demonstrated that the diversity of N. galegae sv. orientalis bacteria as well their host G. orientalis was greatest in their gene centre Caucasus, in comparison with sv. officinalis and its host, which are thought to originate in eastern Mediterranean (Österman et al., 2011).
A comparison of ten whole‐genome‐sequenced N. galegae strains, five of each symbiovar, displayed similarities but also interesting differences. Both symbiovars possessed a nifQ gene, demonstrated to be indispensable for N2 fixation by gene displacement. However, the N. galegae nifQ was very divergent from model gene sequences from Klebsiella pneumoniae, Azotobacter vinelandii, Rhizobium tropici and Sinorhizobium fredii. All sv. orientalis strains tested carried an additional sigma factor gene, rpoN2, which was shown to be necessary for effective N2 fixation on G. orientalis. New genes that were identified as specific for strains of one symbiovar may be involved in determining host specificity. Many additional genes involved in transcriptional regulation or in metabolic functions of strains with similar growth promoting properties were identified and might play a role in the fine‐tuning of effectiveness (Österman et al., 2014, 2015).
Li et al. (2012) investigated 159 endophytic bacteria isolated from surface‐sterilized root nodules of wild perennial Glycyrrhiza legumes growing on 40 sites in central and northwestern China. Amplified fragment length polymorphism (AFLP) genomic fingerprinting and sequencing of partial 16S rRNA genes revealed that the collection mainly consisted of Mesorhizobium, Rhizobium, Sinorhizobium, Agrobacterium and Paenibacillus species. Based on symbiotic properties with the legume hosts Glycyrrhiza uralensis and Glycyrrhiza glabra, the nodulating species were divided into true and sporadic symbionts. Five distinct Mesorhizobium groups represented true symbionts of the host plants, the majority of strains inducing N2‐fixing nodules. Sporadic symbionts consisted of either species with infrequent occurrence or species with weak or no N2 fixation ability.
The studies by Degli Esposti and Martinez Romero (2016) point in a similar direction. The studies of respiratory complex I revealed a hypothetical role for this complex and provide new perspectives also to understanding the physiological and biochemical links between the respiratory chain and N2 fixation in rhizobia. The green complex I operon has been lost in several nodulating organisms but retained in rhizobia showing superior N2 fixation capacity. Possibly, the function of green complex I increases the efficiency of N2 fixation in the nodules.
Future perspectives – can we define effectiveness?
One aim of SNF research is the sustainable production of food and feed. For optimal production, efficient symbioses are desirable. To ensure nodulation and N2 fixation, farmers inoculate legume seed with bacterial preparations. Selection of inoculant strains is mainly done from bacterial collections created by isolation of strains from host nodules often sampled in diverse environments. Strains are first tested for efficacy in controlled environments and selected bacteria are taken to the field. An important property of the inoculant strains is their competitiveness, but there is no general rule for selection of competitive strains other than plant testing. An ideal strain should perform well and be competitive in the respective environment, but not be too persistent. This issue has been known for decades and is thoroughly reviewed by, e.g. Vlassak et al. (1997). Martínez et al. (2016) argued that rhizobial strains are continuously evolving in plants and therefore recommend an approach inspired on experimental evolution studies where strains adapted to particular conditions may be selected. The selection and detection of efficient rhizobial strains should take place under local field conditions in order to obtain superior nitrogen‐fixing symbionts.
Can we then make use of recent findings to improve yields? Terpolilli et al. (2012) present a comprehensive list of metabolic mutations which alter symbiotic N2 fixation. Can that information be useful? The vast knowledge of gas exchange, regulation, respiration, NCR peptides and so on has brought an insight into intricate mechanisms which do not per se reveal an answer to the big question of efficiency, but help us understand the systems.
One reason for the boost of research into SNF in the 1970s was the wish to replace fossil energy with solar power for the production of combined N. This is still a very good, though often neglected, reason to boost symbiotic legume use in agriculture. Steffen et al. (2015) in their paper on planetary boundaries regarding several human‐induced disturbances on globally critical functions estimated regarded reactive N to be close to its planetary boundary. By growing N2‐fixing legumes, one can contribute to soil conservation and health and to sustainable food production. N2O release from agricultural land contributes to global warming. Rhizobial inoculants with complete sets of denitrification genes might in the future mitigate N2O emissions from legume fields (Mania et al., 2019).
We have reviewed some of the putative bottlenecks and listed old and new discoveries, many at the molecular level. However, it might be wise to pay more attention to the diversity of natural systems and look at how SNF has evolved to cope with constraints and yet survived to be productive in varying environments. Vance and Heichel (1991) wrote that the evolution of exquisite adaptations that facilitate the N2 fixation process is extremely efficient in biological terms. Udvardi and Poole (2013) concluded that knowledge of how transport and metabolism are integrated to achieve effective SNF and how they vary in different symbioses may help us to identify bottlenecks in SNF in specific legume–rhizobia systems. Degli Esposti and Martinez Romero (2016) put the attention to the respiratory chain, and recent discoveries regarding NCR peptides add to the factors to be taken into account. The regulatory roles of NO during symbiosis reveal yet one interesting player in this field.
Plant endophytes and bacteria inhabiting the rhizosphere have been reported to enhance nodule formation and tolerance of biotic and abiotic stress in controlled conditions (e.g. Egamberdieva et al., 2010, 2013, 2016, 2017a, 2017b,2017a, 2017b). These plant growth‐promoting rhizobacteria (PGPR) represent diverse taxa and have sometimes been successfully used as biofertilizers. Hydrogen excreted from N2‐fixing root nodules might help feed the PGPR (Schuler and Conrad, 1991). Co‐inoculation with Azospirillum + Bradyrhizobium on soybean seed was evaluated by Hungria et al. (2015), who found beneficial plant effects under different soil and climate conditions in Brazil. For practical purposes, carefully selected and tested, high‐quality plant growth‐promoting biofertilizer formulations could replace synthetic N fertilizer applied to legumes worldwide. By locally adapting crop rotations and mixed cropping, more sustainable food production could be achieved in the near future. This is the immediate aim of SNF in the near future.
Conflict of interest
There is no conflict of interest.
Acknowledgements
The support to SAM by the Finnish Society of Sciences and Letters is gratefully acknowledged.
Microbial Biotechnology (2020) 13(5), 1314–1335
Funding Information
No funding information provided.
References
- Adv Bot ResHoch, G. , Little, H.N. , and Burris, R. (1957) Hydrogen evolution from soy‐bean root nodules. Nature 179: 430. [Google Scholar]
- Andrews, M. , and Andrews, M.E. (2017) Specificity in legume‐rhizobia symbioses. Int J Mol Sci 18: 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby, C.A. (1984) Leghemoglobin and Rhizobium respiration. Annu Rev Plant Physiol 35: 443–478. [Google Scholar]
- Atkins, C. (1984) Efficiencies and inefficiencies in the legume/Rhizobium symbiosis—A review. Plant Soil 82: 273–284. [Google Scholar]
- Baginsky, C. , Brito, B. , Imperial, J. , Palacios, J.M. , and Ruiz‐Argueso, T. (2002) Diversity and evolution of hydrogenase systems in rhizobia. Appl Environ Microbiol 68: 4915–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkou, N. (2011) Characterization of the endosymbiotic forms of Sinorhizobium sp.Strain NGR234. PhD. Dissertation, Geneva, Switzerland: University of Geneva. [Google Scholar]
- Barbosa, L.P. , Costa, P.F. , Ribeiro, P.R.A. , Rufini, M. , Guimarães, A.A. , and Moreira, F.M.S. (2017) Symbiotic efficiency and genotypic characterization of variants of Bradyrhizobium spp. in commercial inoculants for soybeans. Rev Bras Ciênc Solo 41. [Google Scholar]
- Baudouin, E. , Pieuchot, L. , Engler, G. , Pauly, N. , and Puppo, A. (2006) Nitric oxide is formed in Medicago truncatula‐Sinorhizobium meliloti functional nodules. Mol Plant Microbe Interact 19: 970–975. [DOI] [PubMed] [Google Scholar]
- Berger, A. , Brouquisse, R. , Pathak, P.K. , Hichri, I. , Bhatia, S. , Boscari, A. , et al (2018) Pathways of nitric oxide metabolism and operation of phytoglobins in legume nodules: missing links and future directions. Plant Cell Environ 41: 2057–2068. [DOI] [PubMed] [Google Scholar]
- Bergersen, F. (1961) Haemoglobin content of legume root nodules. Biochim Biophys Acta 50: 576–578. [Google Scholar]
- Bergersen, F.J. (1969) Nitrogen fixation in legume root nodules: biochemical studies with soybean. Proc R Soc Lond B Biol Sci 172: 401–416. [DOI] [PubMed] [Google Scholar]
- Bergersen, F. (1971) Biochemistry of symbiotic nitrogen fixation in legumes. Annu Rev Plant Physiol 22: 121–140. [Google Scholar]
- Bergersen, F. , and Turner, G. (1967) Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta Gen Subj 141: 507–515. [DOI] [PubMed] [Google Scholar]
- Berrabah, F. , Ratet, P. , and Gourion, B. (2018) Legume nodules: massive infection in the absence of defense induction. Mol Plant Microbe Interact 32: 35–44. [DOI] [PubMed] [Google Scholar]
- Blanquet, P. , Silva, L. , Catrice, O. , Bruand, C. , Carvalho, H. , and Meilhoc, E. (2015) Sinorhizobium meliloti controls nitric oxide–mediated post‐translational modification of a Medicago truncatula nodule protein. Mol Plant Microbe Interact 28: 1353–1363. [DOI] [PubMed] [Google Scholar]
- Bonaldi, K. , Gourion, B. , Fardoux, J. , Hannibal, L. , Cartieaux, F. , Boursot, M. , et al (2010) Large‐scale transposon mutagenesis of photosynthetic Bradyrhizobium sp. strain ORS278 reveals new genetic loci putatively important for nod‐independent symbiosis with Aeschynomene indica . Mol Plant Microbe Interact 23: 760–770. [DOI] [PubMed] [Google Scholar]
- Boscari, A. , Meilhoc, E. , Castella, C. , Bruand, C. , Puppo, A. , and Brouquisse, R. (2013a) Which role for nitric oxide in symbiotic N2‐fixing nodules: toxic by‐product or useful signaling/metabolic intermediate? Front Plant Sci 4: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscari, A. , Del Giudice, J. , Ferrarini, A. , Venturini, L. , Zaffini, A. L. , Delledonne, M. , and Puppo, A. (2013b) Expression dynamics of the Medicago truncatula transcriptome during the symbiotic interaction with Sinorhizobium meliloti: which role for nitric oxide? Plant Physiol 161: 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhmouch, I. , Souad‐Mouhsine, B. , Brhada, F. , and Aurag, J. (2005) Influence of host cultivars and Rhizobium species on the growth and symbiotic performance of Phaseolus vulgaris under salt stress. J Plant Physiol 162: 1103–1113. [DOI] [PubMed] [Google Scholar]
- Bourion, V. , Heulin‐Gotty, K. , Aubert, V. , Tisseyre, P. , Chabert‐Martinello, M. , Pervent, M. , et al (2018) Co‐inoculation of a pea core‐collection with diverse rhizobial strains shows competitiveness for nodulation and efficiency of nitrogen fixation are distinct traits in the interaction. Front Plant Sci 8: 2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito, B. , Martínez, M. , Fernández, D. , Rey, L. , Cabrera, E. , Palacios, J.M. , et al (1997) Hydrogenase genes from Rhizobium leguminosarum bv. viciae are controlled by the nitrogen fixation regulatory protein NifA. Proc Natl Acad Sci USA 94: 6019–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan‐Wollaston, V. , Cannon, M. , Beynon, J. , and Cannon, F. (1981) Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae . Nature 294: 776. [DOI] [PubMed] [Google Scholar]
- Buhian, W.P. , and Bensmihen, S. (2018) Mini‐review: nod factor regulation of phytohormone signaling and homeostasis during rhizobia‐legume symbiosis. Front Plant Sci 9: 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen, W.A. , and LeComte, J.R. (1966) The nitrogenase system from Azotobacter: two‐enzyme requirement for N2 reduction, ATP‐dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci USA 56: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burén, S. , and Rubio, L.M. (2017) State of the art in eukaryotic nitrogenase engineering. FEMS Microbiol Lett 365: fnx274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris, R.H. (1972) Nitrogen fixation—Assay methods and techniques. Methods Enzymol 24: 415–431. [DOI] [PubMed] [Google Scholar]
- Cam, Y. , Pierre, O. , Boncompagni, E. , Hérouart, D. , Meilhoc, E. , and Bruand, C. (2012) Nitric oxide (NO): a key player in the senescence of Medicago truncatula root nodules. New Phytol 196: 548–560. [DOI] [PubMed] [Google Scholar]
- Carnahan, J.E. , Mortenson, L.E. , Mower, H.F. , and Castle, J.E. (1960) Nitrogen fixation in cell‐free extracts of Clostridium pasteurianum . Biochim Biophys Acta 44: 520–535. [DOI] [PubMed] [Google Scholar]
- Checcucci, A. , DiCenzo, G.C. , Bazzicalupo, M. , and Mengoni, A. (2017) Trade, diplomacy, and warfare: the quest for elite rhizobia inoculant strains. Front microbiol 8: 2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasov, N. , Ibhadon, A. , and Fitzpatrick, P. (2015) A review of the existing and alternative methods for greener nitrogen fixation. Chem Eng Process 90: 24–33. [Google Scholar]
- Colebatch, G. , Desbrosses, G. , Ott, T. , Krusell, L. , Montanari, O. , Kloska, S. , et al (2004) Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus . Plant J 39: 487–512. [DOI] [PubMed] [Google Scholar]
- Crespo‐Rivas, J.C. , Guefrachi, I. , Mok, K.C. , Villaécija‐Aguilar, J.A. , Acosta‐Jurado, S. , Pierre, O. , et al (2016) Sinorhizobium fredii HH 103 bacteroids are not terminally differentiated and show altered O‐antigen in nodules of the Inverted Repeat‐Lacking Clade legume Glycyrrhiza uralensis. Environ Microbiol 18: 2392–2404. [DOI] [PubMed] [Google Scholar]
- Cueto, M. , Hernández‐Perera, O. , Martín, R. , Bentura, M.L. , Rodrigo, J. , Lamas, S. , and Golvano, M.P. (1996) Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus . FEBS Lett 398: 159–164. [DOI] [PubMed] [Google Scholar]
- Czernic, P. , Gully, D. , Cartieaux, F. , Moulin, L. , Guefrachi, I. , Patrel, D. , et al (2015) Convergent evolution of endosymbiont differentiation in dalbergioid and inverted repeat‐lacking clade legumes mediated by nodule‐specific cysteine‐rich peptides. Plant Physiol 169: 1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, J.O. (2008) Ecology of Actinorhizal plants In Nitrogen‐fixing Actinorhizal Symbioses. Nitrogen Fixation: Origins, Applications, and Research Progress. Pawlowski, K., and Newton, W. (eds). Dordrecht, the Netherlands: Springer Netherlands, pp. 199–234. [Google Scholar]
- Degli Esposti, M. (2015) Genome analysis of structure–function relationships in respiratory complex I, an ancient bioenergetic enzyme. Genome Biol Evol 8: 126–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli Esposti, M. , and Martinez Romero, E. (2016) A survey of the energy metabolism of nodulating symbionts reveals a new form of respiratory complex I. FEMS Microbiol Ecol 92: fiw084. [DOI] [PubMed] [Google Scholar]
- Del Giudice, J. , Cam, Y. , Damiani, I. , Fung‐Chat, F. , Meilhoc, E. , Bruand, C. , et al (2011) Nitric oxide is required for an optimal establishment of the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytol 191: 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado, M.J. , Bedmar, E.J. , and Downie, J.A. (1998) Genes involved in the formation and assembly of rhizobial cytochromes and their role in symbiotic nitrogen fixation. Adv Microb Physiol 40: 191–231. [DOI] [PubMed] [Google Scholar]
- Demtröder, L. , Narberhaus, F. , and Masepohl, B. (2019) Coordinated regulation of nitrogen fixation and molybdate transport by molybdenum. Mol Microbiol 111: 17–30. [DOI] [PubMed] [Google Scholar]
- Denison, R.F. (2000) Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am Nat 156: 567–576. [DOI] [PubMed] [Google Scholar]
- diCenzo, G.C. , Zamani, M. , Ludwig, H.N. , and Finan, T.M. (2017) Heterologous complementation reveals a specialized activity for BacA in the Medicago‐Sinorhizobium meliloti symbiosis. Mol Plant Microbe Interact 30: 312–324. [DOI] [PubMed] [Google Scholar]
- diCenzo, G.C. , Zamani, M. , Checcucci, A. , Fondi, M. , Griffitts, J.S. , Finan, T.M. , and Mengoni, A. (2018) Multidisciplinary approaches for studying rhizobium–legume symbioses. Can J Microbiol 65: 1–33. [DOI] [PubMed] [Google Scholar]
- Ditta, G. , Stanfield, S. , Corbin, D. , and Helinski, D.R. (1980) Broad host range DNA cloning system for gram‐negative bacteria: construction of a gene bank of Rhizobium meliloti . Proc Natl Acad Sci USA 77: 7347–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta, G. , Virts, E. , Palomares, A. , and Kim, C.H. (1987) The nifA gene of Rhizobium meliloti is oxygen regulated. J Bacteriol 169: 3217–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, R. (1978) Nitrogenase—hydrogenase interrelationships in Rhizobia. Biochimie 60: 233–236. [DOI] [PubMed] [Google Scholar]
- Dixon, R. , and Kahn, D. (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2: 621. [DOI] [PubMed] [Google Scholar]
- Domingos, P. , Prado, A. , Wong, A. , Gehring, C. , and Feijo, J. (2015) Nitric oxide: a multitasked signaling gas in plants. Molecular Plant 8: 506–520. [DOI] [PubMed] [Google Scholar]
- Dong, Z. , Wu, L. , Kettlewell, B. , Caldwell, C. , and Layzell, D. (2003) Hydrogen fertilization of soils–is this a benefit of legumes in rotation? Plant Cell Environ 26: 1875–1879. [Google Scholar]
- Efremov, R.G. , Baradaran, R. , and Sazanov, L.A. (2010) The architecture of respiratory complex I. Nature 465: 441. [DOI] [PubMed] [Google Scholar]
- Egamberdieva, D. , Berg, G. , Lindström, K. , and Räsänen, L. (2010) Co‐inoculation of Pseudomonas spp. with Rhizobium improves growth and symbiotic performance of fodder galega (Galega orientalis Lam.). Eur J Soil Biol 46: 269–272. [Google Scholar]
- Egamberdieva, D. , Berg, G. , Lindström, K. , and Räsänen, L.A. (2013) Alleviation of salt stress of symbiotic Galega officinalis L. (goat's rue) by co‐inoculation of Rhizobium with root‐colonizing Pseudomonas. Plant Soil 369: 453–465. [Google Scholar]
- Egamberdieva, D. , Li, L. , Lindström, K. , and Räsänen, L.A. (2016) A synergistic interaction between salt‐tolerant Pseudomonas and Mesorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl Microbiol Biotechnol 100: 2829–2841. [DOI] [PubMed] [Google Scholar]
- Egamberdieva, D. , Wirth, S.J. , Shurigin, V.V. , Hashem, A. , and Abd_Allah, E.F. (2017a) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front microbiol 8: 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva, D. , Wirth, S. , Li, L. , Abd‐Allah, E.F. , and Lindström, K. (2017b) Microbial cooperation in the rhizosphere improves liquorice growth under salt stress. Bioengineered 8: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich, D.W. , Ruiz‐Argueso, T. , Ching, T.M. , and Evans, H.J. (1979) Hydrogen‐dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol 137: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas, A. , Maroti, G. , Durgo, H. , Gyorgypal, Z. , Lima, R.M. , Medzihradszky, K.F. , et al (2014) Medicago truncatula symbiotic peptide NCR247 contributes to bacteroid differentiation through multiple mechanisms. Proc Natl Acad Sci USA 111: 5183–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini, A. , De Stefano, M. , Baudouin, E. , Pucciariello, C. , Polverari, A. , Puppo, A. , and Delledonne, M. (2008) Expression of Medicago truncatula genes responsive to nitric oxide in pathogenic and symbiotic conditions. Mol Plant Microbe Interact 21: 781–790. [DOI] [PubMed] [Google Scholar]
- Fischer, H.M. (1994) Genetic regulation of nitrogen fixation in rhizobia. Microbiol Rev 58: 352–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, H. , and Hennecke, H. (1984) Linkage map of the Rhizobium japonicum nifH and nifDK operons encoding the polypeptides of the nitrogenase enzyme complex. Mol Gen Genet 196: 537–540. [Google Scholar]
- Fischer, H. , Alvarez‐Morales, A. , and Hennecke, H. (1986) The pleiotropic nature of symbiotic regulatory mutants: Bradyrhizobium japonicum nifA gene is involved in control of nif gene expression and formation of determinate symbiosis. EMBO J 5: 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen, H. , Mylona, P. , Pawlowski, K. , Van de Sande, K. , Heidstra, R. , Geurts, R. , et al (1995) Plant genes involved in root‐nodule development on legumes. Philos Trans R Soc Lond B Biol Sci 350: 101–107. [Google Scholar]
- Galibert, F. , Finan, T.M. , Long, S.R. , Puhler, A. , Abola, P. , Ampe, F. , et al (2001) The composite genome of the legume symbiont Sinorhizobium meliloti . Science 293: 668–672. [DOI] [PubMed] [Google Scholar]
- Garrity, G. , Bell, J. , and Lilburn, T. (2005)Class I. Alphaproteobacteria class. nov In Bergey's Manual® of Systematic Bacteriology. Brenner, D., Krieg, N., and Staley, J. (eds). New York, NY: Springer US, pp. 1–574. [Google Scholar]
- Girard, L. , Brom, S. , Dávalos, A. , López, O. , Soberón, M. , and Romero, D. (2000) Differential regulation of fixN‐reiterated genes in Rhizobium etli by a novel fixL—fixK cascade. Mol Plant Microbe Interact 13: 1283–1292. [DOI] [PubMed] [Google Scholar]
- Giraud, E. , Moulin, L. , Vallenet, D. , Barbe, V. , Cytryn, E. , Avarre, J.C. , et al (2007) Legumes symbioses: absence of nod genes in photosynthetic bradyrhizobia. Science 316: 1307–1312. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J. , Ichige, A. , and Walker, G. C. (1993) A Rhizobium meliloti homolog of the Escherichia coli peptide‐antibiotic transport protein SbmA is essential for bacteroid development. Genes Dev 7: 1485–1497. [DOI] [PubMed] [Google Scholar]
- Glick, B. R. (2012) Plant growth‐promoting bacteria: mechanisms and applications. Scientifica 2012: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, A.J. , Minchin, F.R. , James, C.L. , and Komina, O. (1999) Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol 120: 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion, B. , Delmotte, N. , Bonaldi, K. , Nouwen, N. , Vorholt, J.A. , and Giraud, E. (2011) Bacterial RuBisCO is required for efficient Bradyrhizobium/Aeschynomene symbiosis. PLoS ONE 6: e21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion, B. , Berrabah, F. , Ratet, P. , and Stacey, G. (2015) Rhizobium–legume symbioses: the crucial role of plant immunity. Trends Plant Sci 20: 186–194. [DOI] [PubMed] [Google Scholar]
- Gussin, G.N. , Ronson, C.W. , and Ausubel, F.M. (1986) Regulation of nitrogen fixation genes. Annu Rev Genet 20: 567–591. [DOI] [PubMed] [Google Scholar]
- Haag, A.F. , Baloban, M. , Sani, M. , Kerscher, B. , Pierre, O. , Farkas, A. , et al (2011) Protection of Sinorhizobium against host cysteine‐rich antimicrobial peptides is critical for symbiosis. PLoS Biol 9: e1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoyama, T. , Niimi, K. , Watanabe, H. , Tabata, R. , Matsubara, J. , Sato, S. , et al (2009) Host plant genome overcomes the lack of a bacterial gene for symbiotic nitrogen fixation. Nature 462: 514. [DOI] [PubMed] [Google Scholar]
- Hardy, W. , and Burns, R. (1968) Biological nitrogen fixation. Annu Rev Biochem 37: 331–358. [Google Scholar]
- Hardy, R. , and Havelka, U. (1975) Nitrogen fixation research: a key to world food? Science 188: 633–643. [DOI] [PubMed] [Google Scholar]
- Hardy, R.W. , Holsten, R.D. , Jackson, E.K. , and Burns, R.C. (1968) The acetylene‐ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43: 1185–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando, Y. , Palacios, J.M. , Imperial, J. , and Ruiz‐Argueso, T. (1995) The hypBFCDE operon from Rhizobium leguminosarum biovar viciae is expressed from an Fnr‐type promoter that escapes mutagenesis of the fnrN gene. J Bacteriol 177: 5661–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge, D.F. (1982) Relative abundance of ureides and nitrate in plant tissues of soybean as a quantitative assay of nitrogen fixation. Plant Physiol 70: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge, D.F. , Peoples, M.B. , and Boddey, R.M. (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311: 1–18. [Google Scholar]
- Hichri, I. , Boscari, A. , Castella, C. , Rovere, M. , Puppo, A. , and Brouquisse, R. (2015) Nitric oxide: a multifaceted regulator of the nitrogen‐fixing symbiosis. J Exp Bot 66: 2877–2887. [DOI] [PubMed] [Google Scholar]
- Hichri, I. , Meilhoc, E. , Boscari, A. , Bruand, C. , Frendo, P. , and Brouquisse, R. (2016) Chapter Ten – Nitric oxide: Jack‐of‐all‐trades of the nitrogen‐fixing symbiosis? Adv Bot Res 77: 193–218. [Google Scholar]
- Hoch, G. , Little, H.N. , and Burris, R. (1957) Hydrogen evolution from soy‐bean root nodules. Nature 179: 430. [Google Scholar]
- Hoch, G. , Schneider, K. , and Burris, R. (1960) Hydrogen evolution and exchange, and conversion of N2O to N2 by soybean root nodules. Biochim Biophys Acta 37: 273–279. [DOI] [PubMed] [Google Scholar]
- Hoffman, B. M. , Lukoyanov, D. , Yang, Z. , Dean, D.R. , and Seefeldt, L.C. (2014) Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem Rev 114: 4041–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horchani, F. , Prevot, M. , Boscari, A. , Evangelisti, E. , Meilhoc, E. , Bruand, C. , et al (2011) Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen‐fixing nodules. Plant Physiol 155: 1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, B. , Domonkos, A. , Kereszt, A. , Szucs, A. , Abraham, E. , Ayaydin, F. , et al (2015) Loss of the nodule‐specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc Natl Acad Sci USA 112: 15232–15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungria, M. , Nogueira, M.A. , and Araujo, R.S. (2015) Soybean seed co‐inoculation with Bradyrhizobium spp. and Azospirillum brasilense: a new biotechnological tool to improve yield and sustainability. American Journal of Plant Scis 6: 811. [Google Scholar]
- Hunt, S. , and Layzell, D.B. (1993) Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu Rev Plant Biol 44: 483–511. [Google Scholar]
- Hyduke, D.R. , Jarboe, L.R. , Tran, L.M. , Chou, K.J. , and Liao, J.C. (2007) Integrated network analysis identifies nitric oxide response networks and dihydroxyacid dehydratase as a crucial target in Escherichia coli . Proc Natl Acad Sci USA 104: 8484–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura, M. , Uchida, Y. , Akiyama, H. , Hoshino, Y.T. , Shimomura, Y. , Morimoto, S. , et al (2013) Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat Clim Chang 3: 208. [Google Scholar]
- Kaminski, P.A. , Kitts, C.L. , Zimmerman, Z. , and Ludwig, R.A. (1996) Azorhizobium caulinodans uses both cytochrome bd (quinol) and cytochrome cbb3 (cytochrome c) terminal oxidases for symbiotic N2 fixation. J Bacteriol 178: 5989–5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S. , Radutoiu, S. , and Stougaard, J. (2017) Legume LysM receptors mediate symbiotic and pathogenic signalling. Curr Opin Plant Biol 39: 152–158. [DOI] [PubMed] [Google Scholar]
- Kereszt, A. , Mergaert, P. , Montiel, J. , Endre, G. , and Kondorosi, É. (2018) Impact of plant peptides on symbiotic nodule development and functioning. Front Plant Sci 9: 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers, E.T. , Rousseau, R.A. , West, S.A. , and Denison, R.F. (2003) Host sanctions and the legume–rhizobium mutualism. Nature 425: 78. [DOI] [PubMed] [Google Scholar]
- Kim, M. , Chen, Y. , Xi, J. , Waters, C. , Chen, R. , and Wang, D. (2015) An antimicrobial peptide essential for bacterial survival in the nitrogen‐fixing symbiosis. Proc Natl Acad Sci USA 112: 15238–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi, E. , Mergaert, P. , and Kereszt, A. (2013) A paradigm for endosymbiotic life: cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu Rev Microbiol 67: 611–628. [DOI] [PubMed] [Google Scholar]
- Koskey, G. , Mburu, S.W. , Njeru, E.M. , Kimiti, J.M. , Ombori, O. , and Maingi, J.M. (2017) Potential of native rhizobia in enhancing nitrogen fixation and yields of climbing beans (Phaseolus vulgaris L.) in contrasting environments of Eastern Kenya. Front Plant Sci 8: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurchak, O. , Provorov, N. , and Simarov, B. (2001) Plasmid pSym1‐32 of Rhizobium leguminosarum bv. viceae controlling nitrogen fixation activity, effectiveness of symbiosis, competitiveness, and acid tolerance. Russ J Genet 37: 1025–1031. [PubMed] [Google Scholar]
- Kuzma, M.M. , Hunt, S. , and Layzell, D.B. (1993) Role of oxygen in the limitation and inhibition of nitrogenase activity and respiration rate in individual soybean nodules. Plant Physiol 101: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Favre, J.S. , and Focht, D.D. (1983) Conservation in soil of h2 liberated from n2 fixation by hup ‐ nodules. Appl Environ Microbiol 46: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lajudie, P.M. , Andrews, M. , Ardley, J. , Eardly, B. , Jumas‐Bilak, E. , Kuzmanović, N. , et al (2019) Minimal standards for the description of new genera and species of rhizobia and agrobacteria. Int J Syst Evol Microbiol. [DOI] [PubMed] [Google Scholar]
- Lamouche, F. , Bonade‐Bottino, N. , Mergaert, P. , and Alunni, B. (2019a) Symbiotic efficiency of spherical and elongated bacteroids in the Aeschynomene‐Bradyrhizobium symbiosis. Front Plant Sci 10: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouche, F. , Gully, D. , Chaumeret, A. , Nouwen, N. , Verly, C. , Pierre, O. , et al (2019b) Transcriptomic dissection of Bradyrhizobium sp. strain ORS285 in symbiosis with Aeschynomene spp. inducing different bacteroid morphotypes with contrasted symbiotic efficiency. Environ Microbiol 21: 3244–3258. [DOI] [PubMed] [Google Scholar]
- Laranjo, M. , Alexandre, A. , and Oliveira, S. (2014) Legume growth‐promoting rhizobia: an overview on the Mesorhizobium genus. Microbiol Res 169: 2–17. [DOI] [PubMed] [Google Scholar]
- Leyva, A. , Palacios, J.M. , and Ruiz‐Argueso, T. (1987) Conserved plasmid hydrogen‐uptake (hup)‐specific sequences within Hup+ Rhizobium leguminosarum strains. Appl Environ Microbiol 53: 2539–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Sinkko, H. , Montonen, L. , Wei, G. , Lindstrom, K. , and Rasanen, L.A. (2012) Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiol Ecol 79: 46–68. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Liu, X. , Liu, R. , Li, L. , Wang, L. , and Wang, W. (2018) Insight into bacterial community diversity and monthly fluctuations of Medicago sativa rhizosphere soil in response to hydrogen gas using Illumina high‐throughput sequencing. Curr Microbiol 75: 1626–1633. [DOI] [PubMed] [Google Scholar]
- Lindström, K. (1984a) Analysis of factors affecting in situ nitrogenase (C2H2) activity of Galega orientalis, Trifolium pratense and Medicago sativa in temperate conditions. Plant Soil 79: 329–341. [Google Scholar]
- Lindström, K. , and Mousavi, S.A. (2010) Rhizobium and other N‐fixing symbioses. Encyclopaedia of Life Science (ELS). Chichester, UK: John Wiley & Sons. [Google Scholar]
- Lindström, K. , Aserse, A.A. , and Mousavi, S.A. (2015) Evolution and taxonomy of nitrogen‐fixing organisms with emphasis on rhizobia In Biological Nitrogen Fixation. de Bruijn F.J. (ed.). Hoboken, NJ: Wiley, pp. 21–38. [Google Scholar]
- Lindstrom, K. (1984b) Effect of various Rhizobium trifolii strains on nitrogenase (C2H2) activity profiles of red clover (Trifolium pratensecv. Venla). Plant Soil 80: 79–89. [Google Scholar]
- Liu, A. , Contador, C.A. , Fan, K. , and Lam, H. (2018) Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front Plant Sci 9: 1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodwig, E. , Leonard, M. , Marroqui, S. , Wheeler, T. , Findlay, K. , Downie, J. , and Poole, P. (2005) Role of polyhydroxybutyrate and glycogen as carbon storage compounds in pea and bean bacteroids. Mol Plant Microbe Interact 18: 67–74. [DOI] [PubMed] [Google Scholar]
- Lucy, M. , Reed, E. , and Glick, B.R. (2004) Applications of free living plant growth‐promoting rhizobacteria. Antonie Van Leeuwenhoek 86: 1–25. [DOI] [PubMed] [Google Scholar]
- Madsen, E.B. , Madsen, L.H. , Radutoiu, S. , Olbryt, M. , Rakwalska, M. , Szczyglowski, K. , et al (2003) A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425: 637. [DOI] [PubMed] [Google Scholar]
- Madsen, L.H. , Tirichine, L. , Jurkiewicz, A. , Sullivan, J.T. , Heckmann, A. B. , Bek, A. S. , et al (2010) The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus . Nat Commun 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mania, D. , Woliy, K. , Degefu, T. , and Frostegård, Å. (2019) A common mechanism for efficient N2O reduction in diverse isolates of nodule‐forming bradyrhizobia. Environ Microbiol n/a. [DOI] [PubMed] [Google Scholar]
- Marchal, K. , and Vanderleyden, J. (2000) The “oxygen paradox” of dinitrogen‐fixing bacteria. Biol Fertility Soils 30: 363–373. [Google Scholar]
- Martínez, J. , Negrete‐Yankelevich, S. , Godinez, L.G. , Reyes, J. , Degli Esposti, M. , and Romero, E.M. (2016) Short‐term evolution of rhizobial strains toward sustainability in agriculture In Microbial Models: From Environmental to Industrial Sustainability. Castro‐Sowinski S. (ed.). Singapore: Springer, pp. 277–292. [Google Scholar]
- Mathieu, C. , Moreau, S. , Frendo, P. , Puppo, A. , and Davies, M.J. (1998) Direct detection of radicals in intact soybean nodules: presence of nitric oxide‐Leghemoglobin complexes. Free Radic Biol Med 24: 1242–1249. [DOI] [PubMed] [Google Scholar]
- McAdam, E.L. , Reid, J.B. , and Foo, E. (2018) Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. J Exp Bot 69: 2117–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, S. (2018) Rhizobial strain‐dependent restriction of nitrogen fixation in a legume‐Rhizobium symbiosis. Plant J 93: 3–4. [DOI] [PubMed] [Google Scholar]
- Meakin, G.E. , Bueno, E. , Jepson, B. , Bedmar, E.J. , Richardson, D.J. , and Delgado, M.J. (2007) The contribution of bacteroidal nitrate and nitrite reduction to the formation of nitrosylleghaemoglobin complexes in soybean root nodules. Microbiology 153: 411–419. [DOI] [PubMed] [Google Scholar]
- Meilhoc, E. , Cam, Y. , Skapski, A. , and Bruand, C. (2010) The response to nitric oxide of the nitrogen‐fixing symbiont Sinorhizobium meliloti . Mol Plant Microbe Interact 23: 748–759. [DOI] [PubMed] [Google Scholar]
- Meilhoc, E. , Boscari, A. , Bruand, C. , Puppo, A. , and Brouquisse, R. (2011) Nitric oxide in legume–rhizobium symbiosis. Plant Sci 181: 573–581. [DOI] [PubMed] [Google Scholar]
- Mergaert, P. , Nikovics, K. , Kelemen, Z. , Maunoury, N. , Vaubert, D. , Kondorosi, A. , and Kondorosi, E. (2003) A novel family in Medicago truncatula consisting of more than 300 nodule‐specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol 132: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergaert, P. , Uchiumi, T. , Alunni, B. , Evanno, G. , Cheron, A. , Catrice, O. , et al (2006) Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium‐legume symbiosis. Proc Natl Acad Sci USA 103: 5230–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels, J. , Moris, M. , Dombrecht, B. , Verreth, C. , and Vanderleyden, J. (1998) Differential regulation of Rhizobium etli rpoN2 gene expression during symbiosis and free‐living growth. J Bacteriol 180: 3620–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin, F. , and Pate, J. (1973) The carbon balance of a legume and the functional economy of its root nodules. J Exp Bot 24: 259–271. [Google Scholar]
- Minchin, F.R. , Witty, J.F. , and Mytton, L.R. (1994) Reply to ‘Measurement of nitrogenase activity in legume root nodules: in defense of the acetylene reduction assay’ by JK Vessey. Plant Soil 158: 163–167. [Google Scholar]
- Montiel, J. , Szűcs, A. , Boboescu, I.Z. , Gherman, V.D. , Kondorosi, É. , and Kereszt, A. (2016) Terminal bacteroid differentiation is associated with variable morphological changes in legume species belonging to the inverted repeat‐lacking clade. Mol Plant Microbe Interact 29: 210–219. [DOI] [PubMed] [Google Scholar]
- Montiel, J. , Downie, J.A. , Farkas, A. , Bihari, P. , Herczeg, R. , Balint, B. , et al (2017) Morphotype of bacteroids in different legumes correlates with the number and type of symbiotic NCR peptides. Proc Natl Acad Sci USA 114: 5041–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson, L.E. , and Thorneley, R.N. (1979) Structure and function of nitrogenase. Annu Rev Biochem 48: 387–418. [DOI] [PubMed] [Google Scholar]
- Moulin, L. , Béna, G. , Boivin‐Masson, C. , and Stępkowski, T. (2004) Phylogenetic analyses of symbiotic nodulation genes support vertical and lateral gene co‐transfer within the Bradyrhizobium genus. Mol Phylogenet Evol 30: 720–732. [DOI] [PubMed] [Google Scholar]
- Mousavi, S.A. (2016) Revised taxonomy of the family Rhizobiaceae, and phylogeny of mesorhizobia nodulating Glycyrrhiza spp. Dissertationes Schola Doctoralis Scientiae Circumiectalis, Alimentariae, Biologicae. [Google Scholar]
- Murakami, E. , Nagata, M. , Shimoda, Y. , Kucho, K. , Higashi, S. , Abe, M. , et al (2011) Nitric oxide production induced in roots of Lotus japonicus by lipopolysaccharide from Mesorhizobium loti . Plant Cell Physiol 52: 610–617. [DOI] [PubMed] [Google Scholar]
- Nagata, M. , Murakami, E. , Shimoda, Y. , Shimoda‐Sasakura, F. , Kucho, K. , Suzuki, A. , et al (2008) Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant Microbe Interact 21: 1175–1183. [DOI] [PubMed] [Google Scholar]
- Normand, P. , and Fernandez, M.P. (2009) Evolution and diversity of Frankia In Prokaryotic symbionts in plants. Pawlowski K. (ed.). Berlin & Heidelberg, Germany: Springer, pp. 103–125. [Google Scholar]
- Nouwen, N. , Arrighi, J. , Cartieaux, F. , Chaintreuil, C. , Gully, D. , Klopp, C. , and Giraud, E. (2017) The role of rhizobial (NifV) and plant (FEN1) homocitrate synthases in Aeschynomene/photosynthetic Bradyrhizobium symbiosis. Sci Rep 7: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuti, M. , Lepidi, A. , Prakash, R. , Schilperoort, R. , and Cannon, F. (1979) Evidence for nitrogen fixation (nif) genes on indigenous Rhizobium plasmids. Nature 282: 533. [Google Scholar]
- O'Connell, M. , Noel, T.C. , Yeung, E.C. , Hynes, M. , and Hynes, M.F. (1998) Decreased symbiotic effectiveness of Rhizobium leguminosarum strains carrying plasmid RP4. FEMS Microbiol Lett 161: 275–283. [DOI] [PubMed] [Google Scholar]
- Okazaki, S. , Kaneko, T. , Sato, S. , and Saeki, K. (2013) Hijacking of leguminous nodulation signalling by the rhizobial type III secretion system. Proc Natl Acad Sci USA 110: 17131–17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki, S. , Tittabutr, P. , Teulet, A. , Thouin, J. , Fardoux, J. , Chaintreuil, C. , et al (2016) Rhizobium–legume symbiosis in the absence of Nod factors: two possible scenarios with or without the T3SS. ISME J 10: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono, R. , and Denison, R.F. (2010) Comparing symbiotic efficiency between swollen versus nonswollen rhizobial bacteroids. Plant Physiol 154: 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono, R. , Denison, R.F. , and Kiers, E.T. (2009) Controlling the reproductive fate of rhizobia: how universal are legume sanctions? New Phytol 183: 967–979. [DOI] [PubMed] [Google Scholar]
- Op den Camp, R. , Streng, A. , De Mita, S. , Cao, Q. , Polone, E. , Liu, W. , et al (2011) LysM‐type mycorrhizal receptor recruited for rhizobium symbiosis in nonlegume Parasponia . Science 331: 909–912. [DOI] [PubMed] [Google Scholar]
- Österman, J. , Chizhevskaja, E. , Andronov, E. , Fewer, D.P. , Terefework, Z. , Roumiantseva, M. , et al (2011) Galega orientalis is more diverse than Galega officinalis in Caucasus—whole‐genome AFLP analysis and phylogenetics of symbiosis‐related genes. Mol Ecol 20: 4808–4821. [DOI] [PubMed] [Google Scholar]
- Österman, J. , Marsh, J. , Laine, P.K. , Zeng, Z. , Alatalo, E. , Sullivan, J.T. , et al (2014) Genome sequencing of two Neorhizobium galegae strains reveals a noeT gene responsible for the unusual acetylation of the nodulation factors. BMC Genom 15: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österman, J. , Mousavi, S. A. , Koskinen, P. , Paulin, L. , and Lindström, K. (2015) Genomic features separating ten strains of Neorhizobium galegae with different symbiotic phenotypes. BMC Genom 16: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott, T. , van Dongen, J.T. , Gu, C. , Krusell, L. , Desbrosses, G. , Vigeolas, H. , et al (2005) Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr Biol 15: 531–535. [DOI] [PubMed] [Google Scholar]
- Pan, H. , and Wang, D. (2017) Nodule cysteine‐rich peptides maintain a working balance during nitrogen‐fixing symbiosis. Nat Plants 3: 17048. [DOI] [PubMed] [Google Scholar]
- Peralta, H. , Aguilar, A. , Díaz, R. , Mora, Y. , Martínez‐Batallar, G. , Salazar, E. , et al (2016) Genomic studies of nitrogen‐fixing rhizobial strains from Phaseolus vulgaris seeds and nodules. BMC Genom 17: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, A. , and Wilson, P. (1941) Occurrence of hydrogenase in nitrogen-fixing organisms. Proc Soc Exp Biol Med 47: 473–476. [Google Scholar]
- Phillips, D.A. (1980) Efficiency of symbiotic nitrogen fixation in legumes. Annu Rev Plant Physiol 31: 29–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pii, Y. , Crimi, M. , Cremonese, G. , Spena, A. , and Pandolfini, T. (2007) Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, P. , Ramachandran, V. , and Terpolilli, J. (2018) Rhizobia: from saprophytes to endosymbionts. Nat Rev Microbiol 16: 291. [DOI] [PubMed] [Google Scholar]
- Preisig, O. , Zufferey, R. , and Hennecke, H. (1996) The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high‐affinity cbb3‐type cytochrome oxidase. Arch Microbiol 165: 297–305. [DOI] [PubMed] [Google Scholar]
- Price, P.A. , Tanner, H.R. , Dillon, B.A. , Shabab, M. , Walker, G.C. , and Griffitts, J. S. (2015) Rhizobial peptidase HrrP cleaves host‐encoded signaling peptides and mediates symbiotic compatibility. Proc Natl Acad Sci USA 112: 15244–15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo, A. , Pauly, N. , Boscari, A. , Mandon, K. , and Brouquisse, R. (2013) Hydrogen peroxide and nitric oxide: key regulators of the legume—rhizobium and mycorrhizal symbioses. Antioxid Redox Signal 18: 2202–2219. [DOI] [PubMed] [Google Scholar]
- Reckling, M. , Bergkvist, G. , Watson, C.A. , Stoddard, F.L. , Zander, P.M. , Walker, R.L. , et al (2016) Trade‐offs between economic and environmental impacts of introducing legumes into cropping systems. Front Plant Sci 7: 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remigi, P. , Zhu, J. , Young, J.P.W. , and Masson‐Boivin, C. (2016) Symbiosis within symbiosis: evolving nitrogen‐fixing legume symbionts. Trends Microbiol 24: 63–75. [DOI] [PubMed] [Google Scholar]
- Rivera‐Ortiz, J.M. , and Burris, R.H. (1975) Interactions among substrates and inhibitors of nitrogenase. J Bacteriol 123: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel, M.A. , Ormeno‐Orrillo, E. , and Romero, E.M. (2011) Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst Appl Microbiol 34: 96–104. [DOI] [PubMed] [Google Scholar]
- Ronson, C.W. , Lyttleton, P. , and Robertson, J.G. (1981) C4‐dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens . Proc Natl Acad Sci USA 78: 4284–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson, C.W. , Astwood, P.M. , and Downie, J.A. (1984) Molecular cloning and genetic organization of C4‐dicarboxylate transport genes from Rhizobium leguminosarum . J Bacteriol 160: 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson, C.W. , Nixon, B.T. , and Ausubel, F.M. (1987) Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell 49: 579–581. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Argüeso, T. , Emerich, D.W. , and Evans, H.J. (1979) Hydrogenase system in legume nodules: a mechanism of providing nitrogenase with energy and protection from oxygen damage. Biochem Biophys Res Commun 86: 259–264. [DOI] [PubMed] [Google Scholar]
- Salazar, E. , Diaz‐Mejia, J.J. , Moreno‐Hagelsieb, G. , Martinez‐Batallar, G. , Mora, Y. , Mora, J. , and Encarnacion, S. (2010) Characterization of the NifA‐RpoN regulon in Rhizobium etli in free life and in symbiosis with Phaseolus vulgaris . Appl Environ Microbiol 76: 4510–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, C. , Gates, A.J. , Meakin, G.E. , Uchiumi, T. , Girard, L. , Richardson, D.J. , et al (2010) Production of nitric oxide and nitrosylleghemoglobin complexes in soybean nodules in response to flooding. Mol Plant-Microbe Interact 23: 702–711. [DOI] [PubMed] [Google Scholar]
- Schubert, K.R. , and Evans, H.J. (1976) Hydrogen evolution: a major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci USA 73: 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, K.R. , Jennings, N.T. , and Evans, H.J. (1978) Hydrogen reactions of nodulated leguminous plants: II. Effects on dry matter accumulation and nitrogen fixation. Plant Physiol 61: 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler, S. , and Conrad, R. (1991) Hydrogen oxidation in soil following rhizobial H2 production due to N2 fixation by a Vicia faba‐Rhizobium leguminosarum symbiosis. Biol Fertility Soils 11: 190–195. [Google Scholar]
- Seefeldt, L.C. , Hoffman, B.M. , and Dean, D.R. (2009) Mechanism of Mo‐dependent nitrogenase. Annu Rev Biochem 78: 701–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseldin, A. (2013) The role of different genes involved in symbiotic nitrogen fixation—Review. Global Journal of Biotechnology & Biochemistry 8: 84–94. [Google Scholar]
- Shimoda, Y. , Nagata, M. , Suzuki, A. , Abe, M. , Sato, S. , Kato, T. , et al (2005) Symbiotic rhizobium and nitric oxide induce gene expression of non‐symbiotic hemoglobin in Lotus japonicus . Plant Cell Physiol 46: 99–107. [DOI] [PubMed] [Google Scholar]
- Shimoda, Y. , Shimoda‐Sasakura, F. , Kucho, K. , Kanamori, N. , Nagata, M. , Suzuki, A. , et al (2009) Overexpression of class 1 plant hemoglobin genes enhances symbiotic nitrogen fixation activity between Mesorhizobium loti and Lotus japonicus . Plant J 57: 254–263. [DOI] [PubMed] [Google Scholar]
- Sprent, J.I. (2008) 60Ma of legume nodulation. What's new? What's changing? J Exp Bot 59: 1081–1084. [DOI] [PubMed] [Google Scholar]
- Steffen, W. , Richardson, K. , Rockstrom, J. , Cornell, S.E. , Fetzer, I. , Bennett, E.M. , et al (2015) Sustainability. Planetary boundaries: guiding human development on a changing planet. Science 347: 1259855. [DOI] [PubMed] [Google Scholar]
- Streicher, S.L. , Gurney, E.G. , and Valentine, R.C. (1972) The nitrogen fixation genes. Nature 239: 495. [DOI] [PubMed] [Google Scholar]
- Suominen, L. , Roos, C. , Lortet, G. , Paulin, L. , and Lindström, K. (2001) Identification and structure of the Rhizobium galegae common nodulation genes: evidence for horizontal gene transfer. Mol Biol Evol 18: 907–916. [DOI] [PubMed] [Google Scholar]
- Szeto, W. W. , Zimmerman, J.L. , Sundaresan, V. , and Ausubel, F.M. (1984) A Rhizobium meliloti symbiotic regulatory gene. Cell 36: 1035–1043. [DOI] [PubMed] [Google Scholar]
- Tegeder, M. , and Perchlik, M. (2018) The importance of organic nitrogen transport processes for plant productivity and nitrogen use efficiency In Engineering Nitrogen Utilization in Crop Plants. Shrawat A., Zayed A., and Lightfoot D. (eds). Cham, Switzerland: Springer, pp. 233–253. [Google Scholar]
- Terpolilli, J.J. , Hood, G.A. , and Poole, P.S. (2012) What determines the efficiency of N2‐fixing Rhizobium‐legume symbioses? Adv Microb Physiol 60: 325–389. [DOI] [PubMed] [Google Scholar]
- Thilakarathna, M.S. , and Raizada, M.N. (2017) A meta‐analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol Biochem 105: 177–196. [Google Scholar]
- Tosques, I.E. , Shi, J. , and Shapleigh, J.P. (1996) Cloning and characterization of nnrR, whose product is required for the expression of proteins involved in nitric oxide metabolism in Rhodobacter sphaeroides 2.4.3. J Bacteriol 178: 4958–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckerman, J.R. , Gonzalez, G. , and Gilles‐Gonzalez, M. (2001) Complexation precedes phosphorylation for two‐component regulatory system FixL/FixJ of Sinorhizobium meliloti . J Mol Biol 308: 449–455. [DOI] [PubMed] [Google Scholar]
- Turan, M. , Kitir, N. , Elkoca, E. , Uras, D. , Ünek, C. , Nikerel, E. , et al (2017) Nonsymbiotic and symbiotic bacteria efficiency for legume growth under different stress conditions In Microbes for Legume Improvement. Zaidi A., Khan M., and Musarrat J. (eds). Cham, Switzerland: Springer, pp. 387–404. [Google Scholar]
- Udvardi, M. , and Poole, P. S. (2013) Transport and metabolism in legume‐rhizobia symbioses. Annu Rev Plant Biol 64: 781–805. [DOI] [PubMed] [Google Scholar]
- Uratsu, S. , Keyser, H. , Weber, D. , and Lim, S. (1982) Hydrogen uptake (HUP) activity of Rhizobium japonicum from major US soybean production areas. Crop Sci 22: 600–602. [Google Scholar]
- Van de Velde, W. , Zehirov, G. , Szatmari, A. , Debreczeny, M. , Ishihara, H. , Kevei, Z. , et al (2010) Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327: 1122–1126. [DOI] [PubMed] [Google Scholar]
- Vance, C. P. (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127: 390–397. [PMC free article] [PubMed] [Google Scholar]
- Vance, C. , and Heichel, G. (1991) Carbon in N2 fixation: limitation or exquisite adaptation. Annu Rev Plant Biol 42: 373–390. [Google Scholar]
- Vasse, J. , de Billy, F. , Camut, S. , and Truchet, G. (1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172: 4295–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen, R. , Holmer, R. , Bu, F. , Rutten, L. , van Zeijl, A. , Liu, W. , et al (2018) Comparative genomics of the nonlegume Parasponia reveals insights into evolution of nitrogen‐fixing rhizobium symbioses. Proc Natl Acad Sci USA 115: E4700–E4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey, J.K. (1994) Measurement of nitrogenase activity in legume root nodules: in defense of the acetylene reduction assay. Plant Soil 158: 151–162. [Google Scholar]
- Virtanen, A. , Moisio, T. , and Burris, R. (1955) Fixation of nitrogen by nodules excised from illuminated and darkened pea plants. Acta Chem Scand 9: 184–186. [Google Scholar]
- Vlassak, K.M. , Vanderleyden, J. , and Graham, P. (1997) Factors influencing nodule occupancy by inoculant rhizobia. Crit Rev Plant Sci 16: 163–229. [Google Scholar]
- Wagner, S.C. (2012) Biological nitrogen fixation. Nature Education Knowledge 3: 15. [Google Scholar]
- Wang, D. , Griffitts, J. , Starker, C. , Fedorova, E. , Limpens, E. , Ivanov, S. , et al (2010) A nodule‐specific protein secretory pathway required for nitrogen‐fixing symbiosis. Science 327: 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Liu, J. , Li, H. , Yang, S. , Körmöczi, P. , Kereszt, A. , and Zhu, H. (2017) Nodule‐specific cysteine‐rich peptides negatively regulate nitrogen‐fixing symbiosis in a strain‐specific manner in Medicago truncatula . Mol Plant Microbe Interact 31: 240–248. [DOI] [PubMed] [Google Scholar]
- Whiting, M. , and Dilworth, M. (1974) Legume root nodule nitrogenase: purification, properties, and studies on its genetic control. Biochim Biophys Acta Prot Struct 371: 337–351. [PubMed] [Google Scholar]
- Wilson, P.W. , and Burris, R.H. (1947) The mechanism of biological nitrogen fixation. Bacteriol Rev 11: 41–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski, M.F. , Lavin, M. , and Sanderson, M.J. (2004) A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well‐supported subclades within the family. Am J Bot 91: 1846–1862. [DOI] [PubMed] [Google Scholar]
- Yamaya‐Ito, H. , Shimoda, Y. , Hakoyama, T. , Sato, S. , Kaneko, T. , Hossain, M.S. , et al (2018) Loss‐of‐function of ASPARTIC PEPTIDASE NODULE‐INDUCED 1 (APN 1) in Lotus japonicus restricts efficient nitrogen‐fixing symbiosis with specific Mesorhizobium loti strains. Plant J 93: 5–16. [DOI] [PubMed] [Google Scholar]
- Youard, Z.A. , Abicht, H.K. , Mohorko, E. , Serventi, F. , Rigozzi, E. , Ledermann, R. , et al (2015) A plethora of terminal oxidases and their biogenesis factors in Bradyrhizobium japonicum . Biological Nitrogen Fixation 1: 293–305. [Google Scholar]
- Zhang, X. , Davidson, E.A. , Mauzerall, D.L. , Searchinger, T.D. , Dumas, P. , and Shen, Y. (2015) Managing nitrogen for sustainable development. Nature 528: 51. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , and Oldroyd, G.E. (2017) Plant signalling in symbiosis and immunity. Nature 543: 328. [DOI] [PubMed] [Google Scholar]
- Zook, D. (2015) Symbiosis—Evolution’s co‐author In Reticulate Evolution. Gontier N. (ed.). Cham, Switzerland: Springer, pp. 41–80. [Google Scholar]


