Highlights
-
•
Rift Valley fever virus causes abortion, teratogenicity and mortality in domestic ruminants.
-
•
Safety and immunogenicity RVFV arMP-12ΔNSm21/384 vaccine was determined in pregnant ewes.
-
•
Vaccine was safe and immunogenic last stages of pregnancy, but may caused malformed lambs early stage.
-
•
Pregnant sheep should not be vaccinated with the RVFV vaccine during the first month of gestation.
Keywords: Rift Valley fever virus, Vaccine, Safety, Pregnant ewes, Lambs, Morocco
Abstract
Rift Valley fever (RVF) poses a threat to human and animal health as well as economic losses due to abortion, new-born teratogenic effect and mortality. Safe and effective vaccines are critically needed to prevent the disease in humans and livestock. The objective of this study was to assess safety and immunogenicity of the Rift Valley fever virus (RVFV) arMP-12DNSm21/384 attenuated vaccine in 32 pregnant ewes at different stages of pregnancy including 17 ewes vaccinated during the early stage (G1) of pregnancy (<35 days) and 15 ewes vaccinated during the last two stages (G2) of pregnancy (>35 days). Ewes were monitored for clinical observations, rectal temperature and abortions and lambs were monitored for general health and rectal temperature. Vaccinated ewes and lambs were periodically sampled for their neutralizing antibody response to RVFV vaccination. All ewes were positive for antibody two weeks post-vaccination and 79% of ewes were positive at delivery. None of the 32 ewes aborted during pregnancy and all ewes vaccinated during the G2 stages of pregnancy gave birth to healthy lambs. However, among the 17 ewes vaccinated during the G1 stage of pregnancy, 2 ewes gave birth to 2 lambs with fore limb malformations that died at 1-day of age. One ewe gave birth to 2 punny twins that died at 2 days of age. Another ewe, gave birth to one lamb with a deformed tail that died at 20 days of age. At post-mortem, tissues of dead lambs (spleen, lung, brain and long bone) were negative for RVFV by PCR assay. While the findings did not link the malformed lambs directly to infection by the vaccine virus, these results indicated that pregnant sheep should not be vaccinated with the RVFV arMP-12DNSm21/384 vaccine during the first month of gestation.
1. Introduction
Rift Valley fever (RVF) is a mosquito-borne disease of ruminants and human caused by Rift Valley fever virus (RVFV) of the order Bunyavirales, family Phenuiviridae, genus Phlebovirus [1], [2]. The development of effective prevention measures for RVF is a global priority because of the devastating impact to human and animal health in Africa and neighbouring countries as well as the threat of the spread and potential impact of RVFV beyond the enzootic region [3]. Several live-attenuated vaccines have been developed for livestock, with the RVFV Smithburn and Clone 13 being the more commonly used vaccines. The Smithburn vaccine has been reported to be abortigenic and teratogenic, and Clone 13 is temperature sensitive with a potential teratogenic effect among pregnant sheep based on experimental studies [4], [5], [6], [7].
The live-attenuated RVFV MP-12 candidate vaccine was developed by mutagenizing a pathogenic wildtype RVFV strain (ZH548) isolated from a patient in Egypt [8]. This candidate was shown to be safe and efficacious in ruminants and nonhuman primates and human volunteers [9], [10], [11], [12], [13], [14]. However, one report claimed that the MP-12 vaccine caused abortions in sheep and foetal malformation, but this alleged observation has not been confirmed [15]. The MP-12 parent vaccine virus was used to develop a recombinant arMP-12ΔNSm21/384 vaccine candidate with nucleotides deleted from the genes that encoded for the non-structural M protein [26]. Experimental studies including pregnant sheep and calves demonstrated that this recombinant vaccine was safe, efficacious and non-teratogenic [16], [17], [18]. Therefore, this vaccine candidate was selected for further studies as a more immunogenic vaccine candidate in target domestic ruminant animal species, including sheep, goats and cattle [19]. The objective of this experiment was to assess safety and immunogenicity of the live attenuated RVFV arMP-12ΔNSm21/384 vaccine in 32 pregnant ewes during the early (G1) stage (<35 days) and the last (G2) two stages (>35 days) of pregnancy.
2. Material and methods
2.1. Animals
Sardi breed sheep (2 to 3 years old) were purchased from local vendors and housed in the MCI Animal Biosafety Level 3 (ABSL 3) facility throughout the study. Among 20 pregnant ewes randomly selected in their 2 last (G2) stages of pregnancy (>2 months) based on ultrasound, 15 were vaccinated and 5 were used as control animals. Also, twenty-five ewes were housed together with 4 males for 4 weeks and then the males were removed. Of these 25 ewes, 18 in their early (G1) stage (>35 days) of pregnancy were vaccinated and 7 were used as control animals. One month later, ultrasound was used to select 17 pregnant ewes for vaccination and 5 for use as control animals. Animal experiments involving the reproductive safety evaluation of the RVFV arMP-12ΔNSm21/384 vaccine were carried out in accordance with international guidelines for the care and handling of experimental animals as described in chapter 7.8 of the Terrestrial Animal Health Code and Directive 2010/63/UE of the European commission (EU Commission, 2010; OIE and Terrestrial Animal Health Code, 2016). The animal use protocol, #2019-MCI-014 was approved on 16/06/19 by the internal ‘‘Ethic Committee for Animal Experiment in MCI Santé Animale”.
2.2. Vaccine production process
The pre-master RVFV arMP-12ΔNSm21/384 vaccine candidate virus seed stock was produced in Vero cells propagated in Dulbeccos Modified Eagles media (DMEM) supplemented with 10% fetal bovine serum (FBS) (19). The Vero cells were propagated in roller bottles and inoculated at a concentration of 0.01 multiplicity of infection (MOI). The virus yield was mixed at a concentration of 40% with a stabilizer consisting of 4% peptone, 8% sucrose and 2% glutamate. The vaccine was freeze-dried in a lyophilizer according to a cycle of 46 h and then reconstituted with Phosphate Buffered Saline (PBS) and the identity of the vaccine was confirmed and the vaccine was shown to be sterile and free of adventitious agents [20], [21].
2.3. Vaccination monitoring
Before vaccination, blood samples were collected from each ewe to verify the serological status using both an enzyme-linked immuno-adsorbent assay (ELISA) and virus neutralization test (VNT) to test for RVFV antibody as described below. A group of 17 ewes (G1) were vaccinated subcutaneously with 105 Tissue Culture Infectious Dose 50% endpoint (TCID50) of the RVFV arMP-12ΔNSm21/384 vaccine diluted in PBS during the early (G1) stage of gestation (<35 days), when foetal sensitivity to RVFV vaccine-induced teratogenesis was likely to be the highest (Table 1). The use of a 105 TCID was used to assess safety of the vaccine and was consistent with an overdose of the vaccine as described previously (Boumart et al, 2019). A group of 15 ewes were vaccinated with the same dose during the last (G2) 2 stages of pregnancy (>35 days) (Table 2). Ten pregnant ewes (5 for each stage) were used as controls and vaccinated with PBS. Vaccinated ewes were examined daily by an attending veterinarian for general health, signs of abortion and any other possible health complications throughout the study. Rectal temperatures were recorded daily during 14 days post-vaccination (pv). After delivery, all lambs were monitored weekly during the first month for general health and temperatures. Tissues and body fluid from lambs that died were collected for testing using polymerase chain reaction (PCR) assay for RVFV and an examination for macroscopic lesions.
Table 1.
Estimated gestation days that ewes were vaccinated with RVFV arMP-12DNSm21/384 vaccine during the early stage of pregnancy and general health of new born lambs.
| Ewes N° | Groups | Day of vaccination/152 days* | Lambs N° | Lambs general health |
|---|---|---|---|---|
| 687 | Vaccinated | 34 | 799 | Good |
| 611 | 34 | 800 | Good | |
| 631 | 33 | 757 | Good | |
| 170 | 28 | 521 522 |
Good | |
| 176 | 28 | 524 525 526 |
Lambs 524 and 526: healthy. Lamb 525: tail deformity died at 20 days old |
|
| 629 | 27 | 527 | Good | |
| 630 | 26 | 528 | Good | |
| 625 | 25 | 507 | Good | |
| 173 | 25 | 504 | Good | |
| 628 | 24 | 501 | Good | |
| 175 | 22 |
520 523 |
Puny lambs, died at 2 days old | |
| 182 | 22 | 791 | Good | |
| 181 | 22 | 798 | Good | |
| 183 | 21 | 594 | Good | |
| 634 | 20 | 583 | Good | |
| 178 | 19 | 588 | Fore limb malformation, died at one day old | |
| 141 | 14 | 508 | ||
| 652 | Control | 28 | 987 | Good |
| 134 | 24 | 724 | Good | |
| 129 | 18 | 670 | Good | |
| 128 | 10 | 665 | Good | |
| 132 | 7 | 9262 | Good |
* Duration of gestation (152 days).
Table 2.
Estimated gestation days that ewes were vaccinated with RVFV arMP-12DNSm21/384 vaccine during the last 2 stages of pregnancy and general health of new born lambs.
| Ewes N° | Groups | Day of vaccination/ 152 days* | Delivery time pv | Lambs N° | Lambs general health |
|---|---|---|---|---|---|
| 139 | Vaccinated | 150 | 2 | 150 | Good |
| 142 | 149 | 3 | 149 | Good | |
| 136 | 147 | 5 | 81 | Good | |
| 148 | 130 | 22 | 87 | Good | |
| 140 | 129 | 23 | 88 | Good | |
| 145 | 111 | 41 | 821 | Good | |
| 671 | 96 | 56 | 866 | Good | |
| 147 | 89 | 63 | 165 | Good | |
| 672 | 81 | 71 | 829 | Good | |
| 673 | 68 | 84 | 753 | Good | |
| 918 | 59 | 93 | 666 | Good | |
| 917 | 50 | 102 | 9257 | Good | |
| 144 | 45 | 107 | 089 | Good | |
| 970 | 45 | 107 | 090 | Good | |
| 248 | 43 | 109 | 828 | Good | |
| 617/615 | Control | 147 | 5 | 513 | Good |
| 613 | 145 | 7 | 510 | Good | |
| 679 | 145 | 7 | 509 | Good | |
| 653 | 131 | 21 | 516 | Good | |
| 670 | 129 | 23 | 56 | Good |
*Duration of gestation (152 days).
As described below, whole blood of the RVFV arMP-12DNSm21/384 vaccinated ewes was collected at 2 days intervals until day 14 pv for testing for virus by a PCR assay and sera samples were test for neutralizing antibody at 7 day interval until day 35 and at monthly interval thereafter until the delivery of the newborn lambs using a VNT. After delivery, serum was collected from lambs at day 0 and at 7 days intervals during one month for neutralizing antibody testing.
2.4. Virus neutralization test
The immune response of ewes and lambs to the RVFV arMP-12ΔNSm21/384 vaccine virus was determined by testing sera samples collected from the animal pv using a VNT. The cut-off value was 1.02 logs corresponding to a 1:10 dilution of serum in accordance to previously described methods [22]. Serial 1:3 dilutions of heat inactivated sera samples were mixed with a constant dose of the arMP-12ΔNSm21/384 vaccine virus (100 TCID50), and then incubated for one hour and inoculated onto Vero cells and observed for cytopathic effect (CPE) on day 5 of the incubation period. A mixture of equal volumes of the virus dose and Dulbecco’s Modified Eagle’s Medium (DMEM) was incubated for one hour and tested in Vero cells to verify the dose of virus used in the VNT. The neutralizing antibodytiter was calculated in accordance with the Reed and Muench method [22]
2.5. Enzyme linked immunosorbent assay
Sera samples from sheep were tested at a 1:2 dilution for RVFV IgG antibody by an enzyme linked immunosorbent assay (ELISA) using a commercial kit ID Rift Valley Fever Competition Multi-species (IDvet Innovative Diagnostics), according to the manufacturer instructions. Briefly, diluted sera samples were added to 96 well plates, coated with a recombinant RVFV nucleoprotein (NP) and incubated for 1 h at 37 °C. After washing the wells of the plates with PBS, 100 ml of an anti-NP peroxidase conjugate was added to fix the remaining free NP epitopes. After 30 min of incubation at room temperature, 100 ml of Tetramethylbenzidine (TMB) substrate solution were added to each well. The reaction was stopped after 15 min at room temperature by the addition of 100 ml of 0.16 M sulfuric acid, and then the reactivity results were read at 450 nm. Antibody positive and negative cut-off values were calculated as recommended by manufacturer with the sera samples being negative, if a percentage of competition was S/N > 50%, doubtful if a percentage of competition was 40% < S/N 50% and positive if a percentage of competition was S/N 40%.
2.6. PCR
The blood of vaccinated ewes collected during the first 14 days pv and tissues of lambs that died were tested for RVF viral RNA by two qPCR assays that targeted the L and M viral RNA segments of the virus [23], [24]. RVF viral RNA extracted from the arMP-12DNSm21/384 vaccine virus were included in each test run as controls to verify that the qPCR assay performed properly. The Cut-off of the RT-qPCR assay was Ct 39.
3. Results
3.1. Clinical observation
The ewes vaccinated with the RVFV arMP-12ΔNSm21/384 vaccine and the unvaccinated ewes that received PBS remained healthy and did not show any sign of abortion or any other complication. Body temperature of vaccinated and unvaccinated animals remained within normal limits. The average temperature of each group of ewes is reported in Fig. 1, Fig. 2. Very low levels of RVF viral RNA (Cycle Threshold values from 37 to 39 among a total of 40 cycles) were detected in the blood of the vaccinated animals. Seventeen ewes vaccinated during the G1 stage of pregnancy, or first 35 days of gestation gave birth to 21 lambs. These lambs included 16 healthy lambs from 14 ewes, and 2 ewes (141 & 178) gave birth to 2 lambs with fore limb malformations that died at 1-day of age. Another ewe (175) gave birth to 2 puny lambs that dies at 2 days of age for unknown reasons, Another ewe (176), gave birth to one lamb with a deformed tail that appeared to be healthy, but died at 20 days of age (table 1). At post-mortem, tissues of the 5 dead lambs (spleen, lung, brain and long bone) were negative for RVFV by PCR assay (CT > 39). All of the 3 malformed lambs were born by 3 ewes vaccinated at D14, D19 and D28 of pregnancy, corresponding to 9% (3/32) of all vaccinated ewes and 18% (3/17) of ewes vaccinated during the G1 stage of gestation. Five control unvaccinated ewes during the G1 stage of pregnancy gave birth to 5 healthy lambs. Fifteen vaccinated and 5 control unvaccinated ewes during the G2 stages of gestation gave birth to 15 and 5 healthy lambs, respectively (table 2).
Fig. 1.
Average temperature for RVFV arMP-12DNSm21/384 vaccinated and unvaccinated ewes during the early stage of pregnancy for 14 days post-vaccination.
Fig. 2.
Average temperature for RVFV arMP-12DNSm21/384 vaccinated and unvaccinated ewes during the last 2 stages of pregnancy for 14 days post-vaccination.
3.2. Serological monitoring
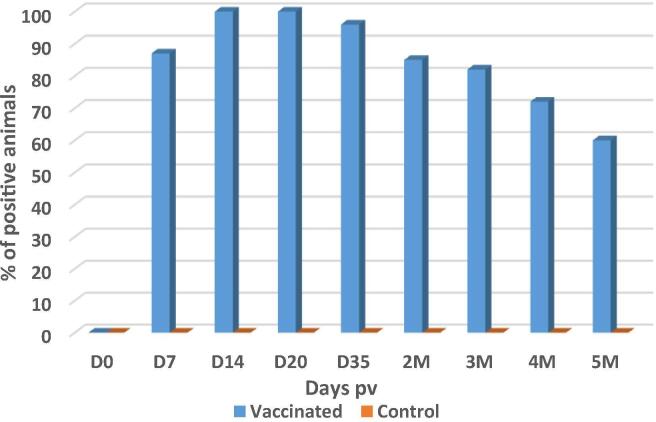
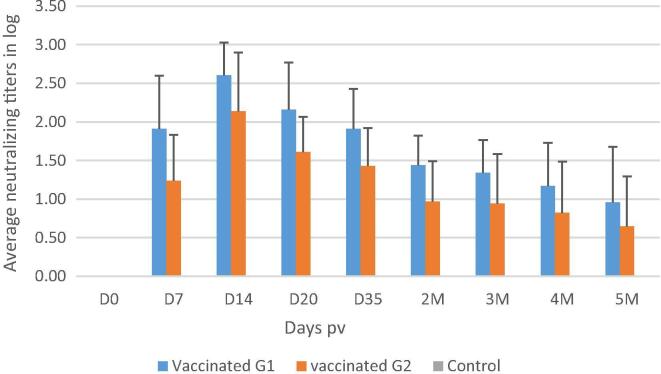
Sera samples obtain from all of the ewes prior to vaccination were negative for ELISA IgG antibody and for neutralizing IgG antibody (data not shown). Among the vaccinated ewes, RVFV neutralizing antibody was detected as early as D7 pv for 87% of animals. At D14, all the animals seroconverted (100%) but antibody was not detectable in 15% at 2 month and 40% were negative at 5 months pv (Fig. 3). Regarding immunogenicity, at D14, the maximum antibody titer value was 2.6 log and 2.14 log for animals in the G1 stage of pregnancy and G2 stage of pregnancy respectively. Both groups showed a decrease in neutralizing antibody weeks later through 5 months pv with a value of 0.96 log and 0.65 log for G1 and G2, respectively. In unvaccinated control ewes, RVFV antibody were not detected during the pregnancy period (Fig. 4). In new born lambs, RVFV neutralizing antibody were detected in the blood at D0 in 55% of lambs, all from ewes that were positive for RVFV antibody at delivery. Since the new born lambs may have consumed antibody in colostrum before blood samples were taken for antibody testing, it was not possible to understand whether or not the antibody were elicited in foetuses or acquired from ingesting the colostrum. Overall, antibody was detected in 87% of the G1 off-springs and 66% of G2 off-springs one month after birth.
Fig. 3.
Percentage of RVFV arMP-12DNSm21/384 vaccine neutralizing antibody positive for all vaccinated ewes during 5 months post-vaccination.
Fig. 4.
Average RVFV arMP-12DNSm21/384 vaccine neutralizing antibody titers for sheep vaccinated with arMP-12DNSm21/384 and unvaccinated ewes at the early and the last 2 stages of pregnancy.
4. Discussion
The most notable and economically devastating outcome of RVF among domestic pregnant ruminants is abortion, foetal malformation and neonatal mortality. Although there are many causes of fetal anomalies in domestic ruminants, RVFV has a special predilection for developing foetal central nervous system and may induce various congenital malformations and inflammatory lesions in the immature foetal brain. The most obvious clinical finding in new born lambs is their inability to stand, as their front legs are flexed and crooked, and, in some cases, convulsions and circling movements. These malformations are consistent with the consequences of transplacental virus infection, especially during the first trimester of pregnancy [25]. RVFV virus replicates efficiently in maternal placental epithelial cells before the virus infects foetal trophoblasts. The virus has also been shown to bypass the maternal epithelial cell layer by directly targeting foetal trophoblasts in the haemophagous zone, a region of the ovine placenta where maternal blood is in direct contact with foetal cells [26]. Thus, the two lambs with fore limb malformations and one lamb with a deformed tail born during the first trimester of pregnancy in this study were consistent with transplacental virus infection, suggesting that the malformation could be attributed to infection by the RVFV arMP-12ΔNSm21/384 vaccine virus. Vaccination is the most efficient tool to control the spread of RVFV in enzootic/endemic countries since the virus is transmitted by mosquitoes, making a virus erradication based strategy not realistic. In Africa, the huge ruminant population with poor infrastructures, warrants the use of live vaccines for RVF prevention, because inducing life lasting immunity with only one injection would afford effective prevention of this disease. Live vaccines are based on attenuated viruses with a variable level of residual virulence. The ovine foetus is the most susceptible species to RVFV infection. Consequently, inoculation of RVFV susceptible pregnant animals is a stringent test of the degree of attenuation of RVFV vaccine candidates.
Several RVFV vaccines have been tested in pregnant sheep but only a few animals have been vaccinated during the early stage of pregnancy which is the high-risk stage of infection. The well-known modified live RVFV Smithburn vaccine strain has been reported to cause abortions and malformations in ovine foetus between 42 and 74 days of gestation [25]. The RVFV Clone 13 vaccine, a naturally live attenuated strain has been shown to be safe in pregnant cattle and ewes when vaccinated during the last two stages of pregnancy, but vaccination during the early stage of pregnancy caused 25% abortion and 7 malformations among new born lambs. [5], [6].
In the present study, the RVFV arMP-12ΔNSm21/384 vaccine was tested for the first time on a sufficient number of pregnant sheep, to assess safety at different stages of pregnancy. All ewes were positive for RVFV neutralizing antibody two weeks after vaccination, thus providing evidence of arMP-12ΔNSm21/384 virus replication in the vaccinated ewes. We used a vaccine dose of 105 TCID50 as an overdose that has been shown to produce virus replication and to confer immunity in sheep, goats and cattle (19). The RVFV neutralizing antibody titers were higher in animals vaccinated during the G1 stage of pregnancy as compared to those vaccinated during the G2 stages of pregnancy and the rate of seroconversion of new born lambs during the G1 stage was also higher than G2 stage, suggesting that susceptibility to the virus was higher in ewes during the G1 stage of pregnancy.
In our study, RVFV arMP-12ΔNSm21/384 vaccine was shown to be safe for pregnant ewes vaccinated between 35 and 152 days of gestation as supported by no abortion and no teratogenic effects. These findings were consistent with observations that showed a RVFV arMP-12ΔNSm21/384 vaccine to be safe following the vaccination of 29 pregnant ewes at 42 days of pregnancy [27]. Also, another study showed that the RVFV arMP-12ΔNSm21/384 was safe after vaccination of 4–10 females between 30 and 50 days of pregnancy [17], [27]. However, in our study, ewes vaccinated at G1 stage of pregnancy gave birth to 3 malformed new born lambs, including 2 lambs with fore limb malformation and one with a deformed tail. From ewes vaccinated at D14, D19 and D28 of pregnancy, respectively. Thus, malformed newborn lambs were observed among 9% (3/32) of all vaccinated ewes and 18% (3/17) of ewes vaccinated at G1 stage of gestation. The malformations were likely to be related to possible infection by RVF arMP-12ΔNSm21/384 virus even though the three lambs were negative for RVFV by PCR assay at post-mortem. We are not aware of any evaluation of the RVFV arMP-12ΔNSm21/384 vaccine in pregnant ewes before 30 days of gestation in order to confirm our observations. Therefore, our reproductive safety study is the first to be conducted in African domestic sheep and the first study to include pregnant animals in all stages of pregnancy. As such, these are the first findings to suggest that the vaccine caused teratogenic effects during the first month of gestation, thus demonstrating that safety precautions need to be considered for using the RVFV arMP-12ΔNSm21/384 vaccine among sheep during the early stage of pregnancy in Africa.
Another study in Africa involving the parent strain RVFV MP12 vaccine of the arMP-12ΔNSm21/384 vaccine claimed that 10% of pregnant sheep aborted at 35 and 42 days of pregnancy and that 15–23% had teratogenic effects between 35 days and 56 days of pregnancy [15]. However, others studies in the United States demonstrated that the RVFV MP-12 was immunogenic, non-abortogenic in sheep and cattle vaccinated at the second or late stage of pregnancy and afforded protection to their foetuses against experimental challenge with virulent RVFV [9], [10], [12]. Also, no abortions or lesions in the placenta or teratogenic effect were observed in lambs, when vaccinated during the second stage of pregnancy, which is consistent with our observations for the RVFV arMP-12ΔNSm21/384 vaccine [28].
The teratogenic effects observed during the G1 stage of pregnancy in this study could possibly be explained by the affinity the RVFV arMP-12ΔNSm21/384 virus for rapidly dividing cells of the nervous tissue, which in sheep foetuses occurs approximately in the first trimester of pregnancy [15], [25]. With other attenuated viruses such as bluetongue, observations showed that when sheep were infected at 50–58 days of pregnancy, infection caused hydranencephaly, but only caused mild encephalitis when infected at 100 days of pregnancy [29].
In conclusion, in spite of few malformed lambs observed during the G1 stage of pregnancy, the RVFV arMP-12ΔNSm21/384 vaccine is one of the safest available live attenuated vaccine that has the potential to be used for the prevention of RVF in enzootic/endemic zones. However, the findings of this study suggested that the RVFV arMP-12ΔNSm21/384 vaccine virus infection could have caused the malformed lambs born to ewes vaccinated during the G1 stage of pregnancy. As a result, the vaccine is not recommended for use in pregnant ewes during the G1 stage of pregnancy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
All authors have seen and approved the content of this report and have contributed significantly to the work. We gratefully acknowledge the support for this study by MCI (Multi-Chemical Industry) Santé Animale and University of Texas at El Paso (UTEP), Texas, the recipient of a Cooperative Agreement (AID-OAA-A-13-00084) from the United Stated Agency for International Development (USAID).
Support
This study was funded under a subcontract from the University of Texas at El Paso (UTEP), Texas, the recipient of a Cooperative Agreement (AID-OAA-A-13-00084) from the USAID.
Disclaimer
The author’s views expressed in this publication do not necessarily reflect the views of the USAID or the United States Government. The contents are the responsibility of MCI Sante Animale and do not necessarily reflect the views of UTEP, USAID or the United States Government. The funders did not have any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
The following authors, Z. Boumart, Z. Bamouh, J. Hamdi, N. Safini and M.EL Harrak contributed substantially to the conception and design of the study, the collection and analysis and interpretation of data and the preparation of the manuscript, and D.M. Watts and G.E. Bettinger contributed to the preparation of the manuscript and interpretation of the data for the work, and all authors participated in the revision of the manuscript, and agreed to be accountable for all aspects of the work and approved the final version of this manuscript. All authors reviewed and complied with the journal policies detailed in the guide for authors.
Contributor Information
Z. Boumart, Email: Z.boumart@mci-santeanimale.com.
Z. Bamouh, Email: Z.bamouh@mci-santeanimale.com.
J. Hamdi, Email: J.hamdi@mci-santeanimale.com.
N. Safini, Email: N.safini@mci-santeanimale.com.
K.O. Tadlaoui, Email: K.tadlaou@mci-santeanimale.com.
D.M. Watts, Email: dwatts2@utep.edu.
M. Elharrak, Email: M.Elharrak@mci-santeanimale.com.
References
- 1.Rima B., Collins C., Easton A., Fouchier R., Kurath G., Lamb R. ICTV virus taxonomy profile: pneumoviridae. J Gen Virol. 2017;98:2912–2913. doi: 10.1099/jgv.0.000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ICTV Virus Taxonomy. Genus: Pegivirus – Flaviviridae – Positive-sense RNA Viruses – International Committee on Taxonomy of Viruses (ICTV). 10th Rep Int Comm Taxon Viruses; 2017.
- 3.Pepin M., Bouloy M., Bird B.H., Kemp A., Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41 doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Teichman B., Engelbrecht A., Zulu G., Dungu B., Pardini A., Bouloy M. Safety and efficacy of Rift Valley fever Smithburn and Clone 13 vaccines in calves. Vaccine. 2011;29:5771–5777. doi: 10.1016/j.vaccine.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Dungu B., Louw I., Lubisi A., Hunter P., von Teichman B.F., Bouloy M. Evaluation of the efficacy and safety of the Rift Valley Fever Clone 13 vaccine in sheep. Vaccine. 2010;28:4581–4587. doi: 10.1016/j.vaccine.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 6.Makoschey B., van Kilsdonk E., Hubers W.R., Vrijenhoek M.P., Smit M., Wichgers Schreur P.J. Rift valley fever vaccine virus clone 13 is able to cross the ovine placental barrier associated with foetal infections, malformations, and stillbirths. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botros B., Omar A., Elian K., Mohamed G., Soliman A., Salib A. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J Med Virol. 2006;78:787–791. doi: 10.1002/jmv.20624. [DOI] [PubMed] [Google Scholar]
- 8.Caplen H., Peters C.J., Bishop D.H.L. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66:2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- 9.Morrill J.C., Carpenter L., Taylor D., Ramsburg H.H., Quance J., Peters C.J. Further evaluation of a mutagen-attenuated Rift Valley fever vaccine in sheep. Vaccine. 1991;9:35–41. doi: 10.1016/0264-410X(91)90314-V. [DOI] [PubMed] [Google Scholar]
- 10.Morrill J.C., Jennings G.B., Caplen H., Turell M.J., Johnson A.J., Peters C.J. Pathogenicity and immunogenicity of a mutagen-attenuated Rift Valley fever virus immunogen in pregnant ewes. Am J Vet Res. 1987;48:1042–1047. [PubMed] [Google Scholar]
- 11.Morrill J.C., Mebus C.A., Peters C.J. Safety of a mutagen-attenuated Rift valley fever virus vaccine in fetal and neonatal bovids. Am J Vet Res. 1997;58:1110–1114. [PubMed] [Google Scholar]
- 12.Morrill J.C., Mebus C.A., Peters C.J. Safety and efficacy of a mutagen-attenuated Rift valley fever virus vaccine in cattle. Am J Vet Res. 1997;58:1104–1109. [PubMed] [Google Scholar]
- 13.Pittman P.R., McClain D., Quinn X., Coonan K.M., Mangiafico J., Makuch R.S. Safety and immunogenicity of a mutagenized, live attenuated Rift Valley fever vaccine, MP-12, in a Phase 1 dose escalation and route comparison study in humans. Vaccine. 2016;34:424–429. doi: 10.1016/j.vaccine.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Pittman P.R., Norris S.L., Brown E.S., Ranadive M.V., Schibly B.A., Bettinger G.E. Rift Valley fever MP-12 vaccine Phase 2 clinical trial: Safety, immunogenicity, and genetic characterization of virus isolates. Vaccine. 2016;34:523–530. doi: 10.1016/j.vaccine.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter P., Erasmus B.J., Vorster J.H. Teratogenicity of a mutagenised Rift Valley fever virus (MVP 12) in sheep. Onderstepoort J Vet Res. 2002;69:95–98. [PubMed] [Google Scholar]
- 16.Weingartl H.M., Nfon C.K., Zhang S., Marszal P., Wilson W.C., Morrill J.C. Efficacy of a recombinant rift valley fever virus MP-12 with NSm deletion as a vaccine candidate in sheep. Vaccine. 2014;32:2345–2349. doi: 10.1016/j.vaccine.2013.12.064. [DOI] [PubMed] [Google Scholar]
- 17.Morrill J.C., Laughlin R.C., Lokugamage N., Pugh R., Sbrana E., Weise W.J. Safety and immunogenicity of recombinant Rift Valley fever MP-12 vaccine candidates in sheep. Vaccine. 2013;31:559–565. doi: 10.1016/j.vaccine.2012.10.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrill J.C., Laughlin R.C., Lokugamage N., Wu J., Pugh R., Kanani P. Immunogenicity of a recombinant Rift Valley fever MP-12-NSm deletion vaccine candidate in calves. Vaccine. 2013;31:4988–4994. doi: 10.1016/j.vaccine.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boumart Z., Daouam S., Bamouh Z., Jazouli M., Tadlaoui K.O., Dungu B. Safety and immunogenicity of a live attenuated Rift Valley Fever recombinant arMP-12ΔNSm21/384 vaccine candidate for sheep, goats and calves. Vaccine. 2019;37:1642–1650. doi: 10.1016/j.vaccine.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 20.European Pharmacopeia OIE 2008 n.d.;1.1.
- 21.Authenticated U.S government information; animal and plant health inspection service, USDA n.d. n.d.;113:701–2.
- 22.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 23.Nguku P.M., Sharif S.K., Mutonga D., Amwayi S., Omolo J., Mohammed O. An investigation of a major outbreak of rift valley fever in Kenya: 2006–2007. Am J Trop Med Hyg. 2010;83:5–13. doi: 10.4269/ajtmh.2010.09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busquets N., Xavier F., Martín-Folgar R., Lorenzo G., Galindo-Cardiel I., Del Val B.P. Experimental infection of young adult European breed sheep with Rift Valley fever virus field isolates. Vector-Borne Zoonotic Dis. 2010;10:689–696. doi: 10.1089/vbz.2009.0205. [DOI] [PubMed] [Google Scholar]
- 25.Coetzer J.A., Barnard B.J. Hydrops amnii in sheep associated with hydranencephaly and arthrogryposis with wesselsbron disease and rift valley fever viruses as aetiological agents. Onderstepoort J Vet Res. 1977;44:119–126. [PubMed] [Google Scholar]
- 26.Oymans J., Wichgers Schreur P.J., van Keulen L., Kant J., Kortekaas J. Rift Valley fever virus targets the maternal-foetal interface in ovine and human placentas. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird B.H., Maartens L.H., Campbell S., Erasmus B.J., Erickson B.R., Dodd K.A. Rift valley fever virus vaccine lacking the NSs and NSm genes is safe, nonteratogenic, and confers protection from viremia, pyrexia, and abortion following challenge in adult and pregnant sheep. J Virol. 2011;85:12901–12909. doi: 10.1128/jvi.06046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baskerville A., Hubbard K.A., Stephenson J.R. Comparison of the pathogenicity for pregnant sheep of Rift Valley fever virus and a live attenuated vaccine. Res Vet Sci. 1992;52:307–311. doi: 10.1016/0034-5288(92)90029-2. [DOI] [PubMed] [Google Scholar]
- 29.Coetzer JAW, Thomson GR, Tustin RC. Infectious diseases of livestock with special reference to Southern Africa. In: Coetzer JAW, Thomson GR, R.C. Tustin. Cape T. Oxford Univ. Press; 1994. p. 1539–48.