Abstract
Study objective
To synthesize the evidence regarding the infection risk associated with different modalities of oxygen therapy used in treating patients with severe acute respiratory infection. Health care workers face significant risk of infection when treating patients with a viral severe acute respiratory infection. To ensure health care worker safety and limit nosocomial transmission of such infection, it is crucial to synthesize the evidence regarding the infection risk associated with different modalities of oxygen therapy used in treating patients with severe acute respiratory infection.
Methods
MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials were searched from January 1, 2000, to April 1, 2020, for studies describing the risk of infection associated with the modalities of oxygen therapy used for patients with severe acute respiratory infection. The study selection, data extraction, and quality assessment were performed by independent reviewers. The primary outcome measure was the infection of health care workers with a severe acute respiratory infection. Random-effect models were used to synthesize the extracted data.
Results
Of 22,123 citations, 50 studies were eligible for qualitative synthesis and 16 for meta-analysis. Globally, the quality of the included studies provided a very low certainty of evidence. Being exposed or performing an intubation (odds ratio 6.48; 95% confidence interval 2.90 to 14.44), bag-valve-mask ventilation (odds ratio 2.70; 95% confidence interval 1.31 to 5.36), and noninvasive ventilation (odds ratio 3.96; 95% confidence interval 2.12 to 7.40) were associated with an increased risk of infection. All modalities of oxygen therapy generate air dispersion.
Conclusion
Most modalities of oxygen therapy are associated with an increased risk of infection and none have been demonstrated as safe. The lowest flow of oxygen should be used to maintain an adequate oxygen saturation for patients with severe acute respiratory infection, and manipulation of oxygen delivery equipment should be minimized.
Introduction
Background
Viral severe acute respiratory infections are infectious transmittable diseases that have pandemic potential.1 The World Health Organization declared the coronavirus disease 2019 (COVID-19) outbreak a public health emergency of international concern on February 11, 2020, and a pandemic on March 11, 2020.2 As of June 7, 2020, COVID-19 had been diagnosed in approximately 7 million patients worldwide, with the number of new cases continually increasing.3
Editor’s Capsule Summary.
What is already known on this topic
The delivery of oxygen may create fomites and aerosols that can spread pathogens to health care workers.
What question this study addressed
Which oxygen delivery methods elevate the risk of respiratory pathogen transmission to bedside health care workers?
What this study adds to our knowledge
From a meta-analysis of 50 trials, most with bias threats, intubation carried the highest risk of potential transmission, but other methods also likely elevated risk compared with nonuse.
How this is relevant to clinical practice
Carefully weigh the need for oxygen and the delivery method, especially when a potentially transmissible severe viral respiratory infection is suspected.
Severe acute respiratory infections often present with acute respiratory distress.4 , 5 Consequently, the initial treatment most often provided is oxygen therapy.4 , 5 Although some cases require early mechanical ventilation, others can be managed with supplemental oxygen alone or noninvasive ventilation.4 , 6 Also, before intubation for mechanical ventilation, patients often need supplemental oxygen or noninvasive ventilation, and these may be the only treatments available for some patients in the midst of a pandemic, given the surge of patients in respiratory distress.6 , 7 Some modalities of oxygen therapy have been shown to generate aerosols, which can increase severe acute respiratory infection transmission.8
Importance
Although all health care workers face a significant risk of infection when treating patients with severe acute respiratory infection, the modality of oxygen therapy used might modify that risk.8, 9, 10, 11 Ideally, respiratory protection should be maximized for all health care workers in contact with patients, but this might not be possible during a pandemic.12 A better understanding of the risk involved in providing different modalities of oxygen therapy to patients with severe acute respiratory infection would assist clinicians in selecting the most suitable approach for patients, improve the allocation of respiratory protective equipment, improve health care workers’ confidence when caring for these patients, and decrease the overall burden of these diseases.
Goals of This Investigation
To maximize health care worker safety and limit nosocomial transmission of severe acute respiratory infections, it is crucial to synthesize the evidence regarding the health care workers’ risk of infection when caring for patients with severe acute respiratory infection requiring oxygen therapy. Therefore, this review’s main objective was to describe the rate of health care worker severe acute respiratory infection according to the modality used to provide oxygen.
Materials and Methods
The present systematic review and meta-analysis was registered before its initiation. Its results are presented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (Table E1, available online at http://www.annemergmed.com).13
Study Design
The search strategy aimed to find both published and unpublished studies. MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials were searched from January 1, 2000, to April 1, 2020 (Appendix E1, available online at http://www.annemergmed.com). Gray literature was searched with Google Scholar. The references provided in the guidelines or the care of severe acute respiratory infection patients of major free open-access medical education blogs, global, American, and European health organizations, as well as in all previously identified articles and main review articles, were reviewed in search of additional studies. The authors of included articles were also contacted to assess whether they had access to pertinent unpublished data.
A 3-stage selection process was used. In the first stage, after automatic removal of duplicates, each citation title was screened to exclude obviously unrelated studies. In the second stage, the titles and abstracts of the remaining citations were screened for potential relevance by pairs of independant reviewers (S.G.M. and V.M., J.L. and V.C., J.-M.C. and A.-L.F.-P., M.M. and E.P., A.F. and R.-X.L., Z.G.-T. and J.P., or A.-S.T. and R.D.). In the final stage, the full text of remaining citations was evaluated against the following inclusion and exclusion criteria by pairs of independent reviewers (A.C. and S.G.M., J.L., J.-M.C., V.C., M.M., A.F., V.H., or A.-S.T.). Discrepancies were resolved by consensus with a third reviewer (A.C. for the second stage and R.D. for the final stage).
Inclusion criteria were original studies of all designs describing the risk (rate and total number) of infection for health care workers caring for adult patients with severe acute respiratory infection (COVID-19, severe acute respiratory syndrome, Middle East respiratory syndrome, and emerging or pandemic influenza) according to the modality of oxygen therapy provided (intubation, noninvasive ventilation [bilevel positive airway pressure {BiPAP} or continuous positive airway pressure], high-flow nasal cannula, bag-valve-mask ventilation, and face mask with or without reservoir and nasal cannula). Because it was anticipated that limited clinical data would be available for some modalities of oxygen therapy, studies on aerosol generation and droplet dispersion were also considered for inclusion. Studies describing only patients already receiving mechanical ventilation were excluded as outside the scope of this review, which focused on the oxygen therapy initially provided and also because the nature of care these patients frequently receive (eg, tracheal suctioning) is often different. Animal studies were also excluded. There were no language restrictions, but studies published before January 1, 2000, were excluded because they were published before the first modern-day severe acute respiratory infection pandemic (severe acute respiratory syndrome 2002 to 2003).14
Data Collection and Processing
The data (summary estimates) for all pertinent variables (eg, first author, publication year, study design, disease treated, number of health care workers exposed, number of patients treated, modality of oxygen therapy evaluated, health care worker infection) were extracted independently by 2 reviewers (A.C. and S.G.M.) using a standardized electronic form, with conflicts resolved through consensus. For each modality of oxygen therapy, an exposed health care worker had to have been in the room in which the oxygen therapy was provided. An unexposed health care worker had to have cared for patients with severe acute respiratory infection but must not have been present in the room while the studied modality of oxygen therapy was administered. An attempt was made to contact the authors of the included articles to ensure that the abstraction and interpretation of their data were accurate and to certify that there were no duplicate data.
Outcome Measures
The primary outcome measure was the development of a severe acute respiratory infection for health care workers. The preferred timing of measurement was at 14 days postexposure, given the incubation period of the diseases of interest.2 , 15 The secondary outcome measure, used for aerosol-generation models, was aerosol or exhaled air dispersion during oxygen therapy. When multiple results were presented for the same modality of oxygen therapy, the maximal dispersion distance was reported. Adjusted odds ratio (OR) was the effect measure used whenever available. If no adjusted OR was provided, unadjusted OR was used or calculated from the available data. For case reports and case series, the proportion or number of health care workers infected was described separately.
The quality assessment of all retained articles was performed by 2 independent reviewers (A.C. and S.G.M.), with conflicts resolved through consensus. The risk of bias was evaluated with a modified Newcastle-Ottawa Scale.16 Articles with a score of 8 or more were considered at low risk of bias, 6 or 7 at moderate risk, and 5 or less at high risk. Abstracts, case reports, case series, and models were considered at high risk of bias.
Primary Data Analysis
For outcomes reported in at least 3 clinical studies, results were pooled in a meta-analysis. Heterogeneity was assessed statistically with I 2. If the I 2 was greater than 75%, the results were described only qualitatively, without a meta-analysis. A random-effect model was used to better account for the expected differences in design among the included studies. The results are presented according to the modality of oxygen therapy provided. Results from case series, case reports, and models were not meta-analyzed and are presented after clinical results, in the appropriate subgroup of oxygen therapy. Studies in which risks for different modalities of oxygen therapy or another high-risk intervention were combined were evaluated separately (mixed exposure). All results are presented with their 95% confidence intervals (CIs).
For each analysis in which more than 10 articles would be included, a funnel plot was constructed to assess for a publication bias.17 When fewer than 10 articles were available, the reporting bias was assessed qualitatively.
In addition, 2 sets of sensitivity analyses were performed: 1 excluding articles at high risk of bias and 1 excluding studies with an n of less than 50.
All analyses were performed with RevMan (version 5.3; Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark).
Results
Characteristics of Study Subjects
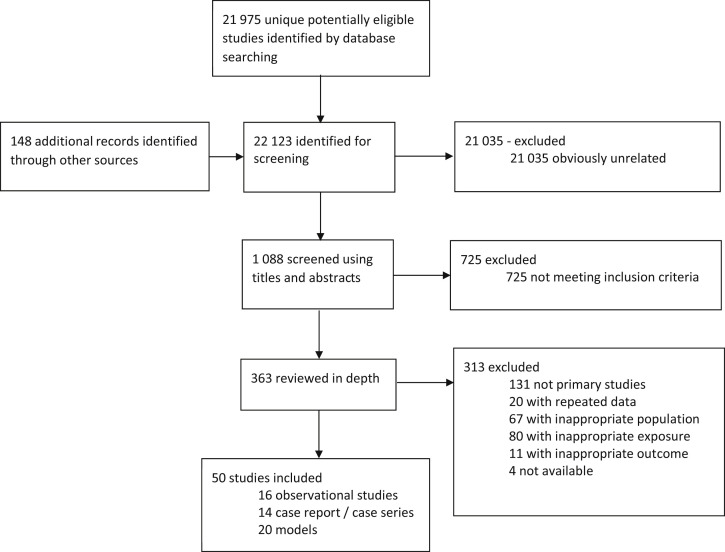
Of 22,123 unique citations, 50 studies were included (Figure 1 ). A total of 16 observational studies (either cohort studies or case-control studies) reported clinical outcomes and were included in the meta-analysis (cohort studies 8; case-control studies 8). An additional 14 case reports or series and 20 studies reporting on aerosol or droplet dispersion were included in the systematic review. Most of the 30 clinical studies described the risk of transmission of severe acute respiratory syndrome (n=18; 60%) or influenza virus (n=7; 23%). Given the recent emergence of COVID-19, only 3 studies (10%) evaluated the infection of health care workers by the virus. A total of 16 studies presented results regarding intubation, 5 for bag-valve-mask manual ventilation, 22 for noninvasive ventilation, 9 for high-flow nasal cannula, 11 for face mask with or without reservoir, and 4 for nasal cannula. Three studies reported outcomes with the use of more than one modality of oxygen therapy or in combination with another high-risk intervention. The individual characteristics of the 50 studies included are presented in the Table . All included studies were considered at moderate (n=4) or high (n=46) risk of bias and globally provided a very low certainty of evidence (Table E2, available online at http://www.annemergmed.com). One article described the odds of having a superspreading event (3 nosocomial cases or more) in a hospital.18 Twelve authors provided a reply and validated the extraction of their data.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30
Figure 1.
Study flow chart.
Table.
Demographics and study characteristics.
| Study | Study Design | Risk of Bias | Disease Treated | No. of Patients Treated | No. of HCWs Exposed | Modality of Oxygen Therapy Assessed | HCWs Infected, %, 95% CI | HCWs Who Always Wore an N95 Respirator, Equivalent, or Greater Protection While in Patient’s Room, % |
|---|---|---|---|---|---|---|---|---|
| Belenguer-Muncharaz, 201156 | Case series | High | Influenza | 5 | NA | CPAP, BiPAP | 0, NA | NA |
| Cai, 202057 | Case series | High | COVID-19 | 12 | 9 | Bronchoscope-guided intubation | 0, 0–37 | 100 |
| Caputo, 200629 | Case series | High | SARS | 35 | 33 | Intubation | 9, 2–25 | 91 |
| Chan, 201358 | Model | High | — | — | — | Bag-valve-mask ventilation | — | — |
| Chan, 201859 | Model | High | — | — | — | Bag-valve-mask ventilation | — | — |
| Chen, 200640 | Case control | High | SARS | 98 | NA | Oxygen therapy (undefined) | NA | NA |
| Chen, 200931 | Case control | Moderate | SARS | NA | 758 | Intubation | 12, 10–15 | NA |
| Cheng, 201532∗ | Retrospective cohort | High | Influenza | 1 | 82 | BiPAP, intubation | 0, 0–5 | 6 |
| Cheung, 200460 | Case series | High | SARS | 20 | 105 | BiPAP | 0, 0–4 | NA |
| Christian, 200442 | Case series | High | SARS | 1 | 9 | Mixed exposure | 22, 6–55 | 100 |
| Fowler, 200428 | Retrospective cohort | High | SARS | 7 | 122 | Intubation, BiPAP | 10, 5–14 | NA |
| Ha, 200439 | Retrospective cohort | High | SARS | NA | 62 | BiPAP | 0, 0–7 | 31 |
| Han, 200419 | Case series | High | SARS | 30 | NA | BiPAP | 0, NA | NA |
| Heinzerling, 202033 | Retrospective cohort | High | COVID-19 | 1 | 43 | High-flow oxygen (undefined), face mask, NIV (undefined), bag-valve-mask ventilation, intubation | 7, 2–20 | 0 |
| Hui, 200661 | Model | High | — | — | — | Face mask | — | — |
| Hui, 200662 | Model | High | — | — | — | BiPAP | — | — |
| Hui, 201146 | Model | High | — | — | — | Nasal cannula | — | — |
| Hui, 201444 | Model | High | — | — | — | Nasal cannula, face mask, BiPAP | — | — |
| Hui, 201563 | Model | High | — | — | — | BiPAP | — | — |
| Hui, 201964 | Model | High | — | — | — | High-flow nasal cannula, CPAP | — | — |
| Ip, 200747 | Model | High | — | — | — | Face mask | — | — |
| Iwashyna, 202025 | Model | High | — | — | — | Nasal cannula, face mask, high-flow nasal cannula | — | — |
| Kotoda, 202065 | Model | High | — | — | — | High-flow nasal cannula | — | — |
| Leonard, 202022 | Model | High | — | — | — | Nasal cannula, high-flow nasal cannula | — | — |
| Leung, 201966 | Model | High | — | — | — | High-flow nasal cannula | — | — |
| Liu, 200934 | Case control | Moderate | SARS | NA | 477 | Intubation | 11, 8–14 | 7 |
| Loeb, 200427 | Retrospective cohort | Moderate | SARS | 3 | 32 | Face mask, BiPAP, bag-valve-mask ventilation, intubation | 25, 13–42 | 50 |
| Loh, 202020 | Model | High | — | — | — | High-flow nasal cannula | — | — |
| Luo, 201545 | Case report | High | MERS | 1 | NA | High-flow nasal cannula | 0, NA | NA |
| Mardimae, 200626 | Model | High | — | — | — | Face mask | — | — |
| Nam, 201743 | Case report | High | MERS | 1 | 6 | Mixed exposure | 17, 3–56 | 100 |
| Ng, 202035 | Retrospective cohort | High | COVID-19 | 1 | 41 | NIV (undefined), intubation | 0, 0–9 | 15 |
| Nishiyama, 200841 | Retrospective cohort | High | SARS | NA | 146 | Oxygen therapy (undefined) | 29, 22–38 | NA |
| O’Neil, 201767 | Model | High | — | — | — | BiPAP | — | — |
| Park, 200430 | Retrospective cohort | High | SARS | 6 | 110 | Mixed exposure | 0, 0–5 | 52 |
| Pei, 200636 | Case control | Moderate | SARS | NA | 443 | Intubation | 33, 29–38 | NA |
| Raboud, 201024 | Case control | High | SARS | 45 | 624 | High-flow oxygen (undefined), face mask, BiPAP, bag-valve-mask ventilation, intubation | 4, 3–6 | 87 |
| Rello, 201221 | Case series | High | Influenza | 20 | NA | High-flow nasal cannula | 0, NA | NA |
| Roberts, 201548∗ | Model | High | — | — | — | High-flow nasal cannula | — | — |
| Scales, 200337 | Case control | High | SARS | 1 | 31 | NIV (undefined), intubation | 19, 8–38 | 19 |
| Simonds, 201050 | Model | High | — | — | — | Face mask, BiPAP | — | — |
| Somogyi, 200468 | Model | High | — | — | — | Face mask | — | — |
| Teleman, 200438 | Case control | High | SARS | 3 | 86 | Oxygen therapy (undefined), intubation | 47, 37–57 | 30 |
| Thompson, 201369 | Model | High | Influenza | 5 | — | Intubation | — | — |
| Tonveronachi, 201170∗ | Case series | High | Influenza | 25 | NA | BiPAP | 0, NA | NA |
| Vivarelli, 201371 | Case series | High | Influenza | 14 | NA | CPAP, BiPAP | 0, NA | NA |
| Wong, 201023 | Case series | High | Influenza | 1 | 29 | BiPAP | 0, 0–15 | NA |
| Wong, 201172∗ | Case series | High | Influenza | 1 | 3 | NIV (undefined) | 100, 44–100 | NA |
| Yu, 200718 | Case control | High | SARS | NA | NA | Oxygen therapy (undefined), face mask, BiPAP, intubation | NA | NA |
| Zhao, 200373 | Case series | High | SARS | 1 | 4 | Intubation | 100, 51–100 | NA |
HCW, Health care worker; NA, not available, CPAP, continuous positive airway pressure; SARS, severe acute respiratory syndrome; NIV, noninvasive ventilation; MERS, Middle East respiratory syndrome, -, not relevant.
Abstract only.
Main Results
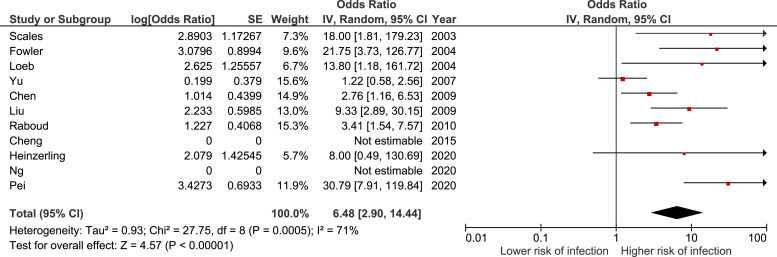
A total of 2,675 health care workers (10% exposed, 14% infected) were included in the 12 observational studies in the meta-analysis assessing the risk of intubation (Table E3, available online at http://www.annemergmed.com).18 , 24 , 27 , 28 , 31, 32, 33, 34, 35, 36, 37, 38 In these studies, there was an association between being present at the intubation and the risk of infection among health care workers. The summary estimate for these studies yielded an OR of 5.34 (95% CI 2.44 to 11.68), with high statistical heterogeneity (I 2 = 71%) (Figure E1, available online at http://www.annemergmed.com). The results presented in the study by Teleman et al38 were discordant with the results from the other studies. The OR calculated from their results (0.68 [95% CI 0.12 to 3.91]) is very different from the OR presented in the study itself (1.5 [95% CI 0.4 to 5.4]), and no answer was received from the authors to explain that difference. For these reasons, it was decided to exclude that study from the main model. The summary estimate for the 11 remaining studies yielded an OR of 6.48 (95% CI 2.90 to 14.44), with high statistical heterogeneity (I 2 = 71%) (Figure 2 ). The results of one aerosol dispersion model pertaining to the performance of an intubation are presented in Appendix E2 and Table E3, available online at http://www.annemergmed.com.
Figure 2.
Forest plot describing the infection risk during intubation.
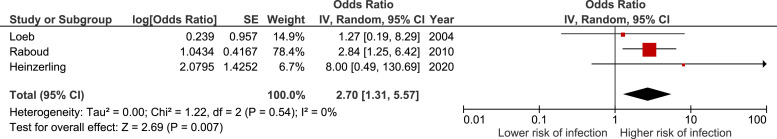
A total of 693 health care workers (18% exposed, 5% infected) were included in the 3 observational studies in the meta-analysis assessing the risk of bag-valve-mask ventilation (Table E4, available online at http://www.annemergmed.com).24 , 27 , 33 In these studies, there was an association between bag-valve-mask ventilation and the risk of infection among health care workers. The summary estimate for these studies yielded an OR of 2.70 (95% CI 1.31 to 5.56), with no statistical heterogeneity (I 2 = 0%) (Figure 3 ). The results of 2 aerosol dispersion models pertaining to the use of bag-valve-mask ventilation are presented in Appendix E2 and Table E4, available online at http://www.annemergmed.com.
Figure 3.
Forest plot describing the infection risk during bag-valve-mask ventilation.
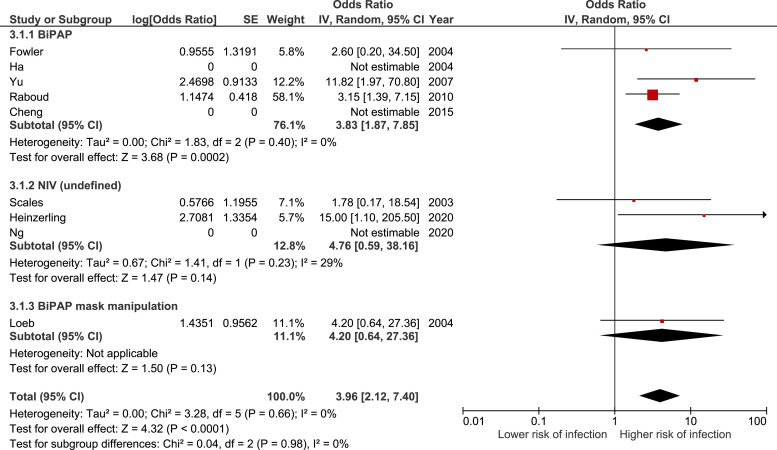
A total of 942 health care workers (25% exposed, 5% infected) were included in the 9 observational studies in the meta-analysis assessing the risk of being exposed to noninvasive ventilation (Table E5, available online at http://www.annemergmed.com).18 , 24 , 27 , 28 , 32 , 33 , 35 , 37 , 39 Subgroups were created depending on the specific exposure of health care workers (BiPAP, noninvasive ventilation [undefined], and BiPAP mask manipulation). Overall, there was an association between being exposed to noninvasive ventilation and the infection risk among health care workers. The summary estimate for these studies yielded an OR of 3.96 (95% CI 2.12 to 7.40), with no statistical heterogeneity (I 2 = 0%) (Figure 4 ). The results of 6 case series and 6 aerosol or droplet dispersion models pertaining to the use of noninvasive ventilation are presented in Appendix E2 and Table E5, available online at http://www.annemergmed.com.
Figure 4.
Forest plot describing the infection risk during noninvasive ventilation.
No observational studies reported on the use of high-flow nasal cannula. The results of 2 case series and 7 aerosol or droplet dispersion models pertaining to the use of high-flow nasal cannula are presented in Appendix E2 and Table E6, available online at http://www.annemergmed.com.
Seven observational studies reported on the use of conventional oxygen therapy (Table E7, available online at http://www.annemergmed.com).18 , 24 , 27 , 33 , 38 , 40 , 41 It was decided not to perform a meta-analysis because of the uncertainty of the specific exposure for most of these studies and the overlapping data. In one study, Yu et al18 observed an increased risk of a superspreading event in a ward when oxygen was administered with a face mask at more than 6 L/min (OR=7.08 [95% CI 1.30 to 38.42]). In the studies by Heinzerling et al33 and Raboud et al,24 there was no statistically significant association between being exposed to high-flow oxygen and infection among health care workers (OR=1.39 [95% CI 0.11 to 17.24] and OR=0.39 [95% CI 0.09 to 1.66], respectively). In studies in which oxygen therapy was not defined, Chen et al40 and Yu et al18 reported an increased risk of infection (OR=4.60 [95% CI 1.40 to 15.08] and OR=10.97 [95% CI 1.73 to 69.39], respectively), whereas Nishiyama et al41 and Teleman et al38 did not (OR=2.65 [95% CI 0.66 to 10.70] and OR=0.97 [95% CI 0.33 to 2.84], respectively). Three studies reported on the risk associated with manipulation of the oxygen mask.24 , 27 , 33 One of these studies reported an increased risk of infection with such an exposure (OR=17.00 [95% CI 1.75 to 165.00]),27 whereas the results of the others did not reach statistical significance (OR=11.60 [95% CI 0.88 to 153.29]33 and OR=2.14 [95% CI 0.94 to 4.89]).24 The results of 9 aerosol dispersion models pertaining to the administration of conventional oxygen therapy are presented in Appendix E2 and Table E7, available online at http://www.annemergmed.com.
No observational studies reported on the use of high-flow nasal cannula. The results of 3 case series in which a mixed exposure was observed are presented in Appendix E2 and Table E8, available online at http://www.annemergmed.com.
Sensitivity Analyses
Sensitivity analyses yielded no additional information. The exclusion of articles at high risk of bias did not significantly influence the results regarding the exposure to intubation (Figure E2, available online at http://www.annemergmed.com). Only one article remained available for the exposure to bag-valve-mask ventilation and noninvasive ventilation (Figures E3 and E4, available online at http://www.annemergmed.com). A publication bias might have prevented small studies without significant results regarding the exposure to intubation from being published (Figure E5, available online at http://www.annemergmed.com). A publication bias might also have prevented case series with an intermediate rate of infection from being published because only 3 of the 14 case reports and series included did not report a risk of infection of either 0% or 100%. No other evidence of a publication bias was found.
Limitations
The main limitation of the present review is the quality of the studies included. Most studies had significant limitations in their design and included only a small number of health care workers, of whom only a few were infected. However, the results were consistent in the sensitivity analyses in which articles at high risk of bias were excluded. Some studies did not report any infection, which prevented the calculation of an OR. Most of the clinical studies that were included described the risk of severe acute respiratory syndrome transmission. Other severe acute respiratory infections might have a different predisposition of transmission and this limits the generalizability of the presented results to the current COVID-19 pandemic. In addition, it is possible that improvement in technical aspects of oxygen therapy (eg, video-assisted rapid sequence intubation, double-limb circuit noninvasive ventilation) could decrease the risk of contamination. Although every author was contacted to validate that there were no repeated data, it remains possible that some health care workers were included in multiple studies that were conducted at the same site. There were no clinical data for some modalities of oxygen therapy, which prevented the realization of a meta-analysis and left some conclusions relying on indirect data. Finally, it is probable that the presented results were confounded to some extent by the increased disease severity and contagiousness of the patients requiring oxygen therapy, the type of personal protective equipment used by health care workers, and the infection control training they received.
Discussion
In this systematic review and meta-analysis, it was observed that exposure to intubation, bag-valve-mask ventilation, and noninvasive ventilation was associated with an increased risk of severe acute respiratory infection for health care workers. No clinical studies assessed the risk associated with the use of high-flow nasal cannula. The provision of conventional oxygen therapy was generally associated with an increased risk of infection even though no meta-analysis was performed, given the uncertainty of the specific exposure, the overlapping data between some studies, and the various study designs. Most models described significant air or droplet dispersion for all modalities of oxygen therapy. However, most models measuring specifically the quantity of aerosol generated did not observe a significant increase.
The greatest risk factor for contracting a severe acute respiratory infection is probably performing or being exposed to an intubation. This had already been observed in a previous systematic review.8 Despite the high heterogeneity in the analysis, the consistency of this finding throughout studies that observed at least some infections, with the exception of the study by Teleman et al,38 adds some strength to that observation. As described earlier, it is possible that there was a statistical error in that study, given the discrepancy in the OR that was presented by the authors and the OR calculated from their results. The observed association is likely caused by the fact that intubation requires some proximity to the patient’s airway. Other interventions putting health care workers at risk (high-flow oxygen, airway suctioning, bag-valve-mask ventilation, chest compressions, etc) are also often performed in the context of intubation and might not have been reported while still contributing to the burden of infection associated with this procedure.42 , 43 Intubation is also frequently provided urgently for acutely ill patients, who might have higher contagiousness than their counterparts with milder symptoms. Likewise, the mental burden and stress associated with performing the intubation could increase the odds of self-contaminating during or after the procedure.
Bag-valve-mask ventilation or noninvasive ventilation was also associated with a significantly higher risk of contagion. There was less evidence to support these findings than for intubation. The same factors as those involved in intubation support these associations. In addition, for noninvasive ventilation, the high flow and pressure of the oxygen delivered can generate jets of air and droplets, which could easily facilitate transmission of the disease.44
No clinical evidence was available for the use of high-flow nasal cannula. One case report and one case series reported no health care worker infection with the use of this modality while patients with severe acute respiratory infection were treated.21 , 45 However, the air and droplet dispersion observed in some studies was similar to that observed for BiPAP, which is generally accepted as an aerosol-generating procedure.20 , 22 , 44 Given the observed results for other oxygenation modalities, it remains possible that contamination risk is significant when patients with severe acute respiratory infection are treated with high-flow nasal cannula.
There was also no clinical evidence, except for higher flows of oxygen, for infection with the use of conventional oxygen therapy by face mask or nasal cannula. Air dispersion distance observed for nasal cannula at 5 L/min was, on some occasions, as high as the distance observed for BiPAP.22 , 44 , 46 The various air dispersion distances observed at the same flow were likely caused by a complex interaction between the patient’s physiognomy, the precise positioning of the nasal cannula, and the room configuration and ventilation.22 , 44 , 46 Nasal cannulae, especially at higher flows, have the potential to at least disperse naturally occurring aerosols and could even generate some aerosols in particular settings. At a similar flow, air dispersion distances were generally lower when a face mask was used rather than a nasal cannula. At a similar oxygen flow, these distances also seemed to be higher with venturi masks in comparison with simple or nonrebreather masks.44 , 47 This is likely explained by the air entrainment that increase the total air flow for venturi masks. The air dispersion distances observed for all types of face masks increased along with the oxygen flow.44 , 47 It is difficult to identify a precise cutoff that would cause aerosol generation. Yu et al18 identified an increased risk of superspreading events when flows higher than 6 L/min were used. In addition, in some circumstances, with oxygen flows of 8 to 10 L/min air dispersion distances were in the range of those observed with some BiPAP settings.44 , 47 Because air dispersion distances are also likely affected by complex mask-patient-room interactions, oxygen flows higher than 6 L/min should be used with more caution by health care workers. Oxygen delivery with a face mask could also be preferred to the use of a nasal cannula.
It remains hypothetical that any of the increased risk observed was caused by “aerosol generation.”25 , 48 Although some studies have reported probable aerosol transmission in wards, the main route of transmission for severe acute respiratory infection might be droplets and fomites, which are spread out when these modalities of oxygen therapy are used.20 , 22 , 23 , 49 , 50 It is also possible that naturally occurring aerosols can be dispersed by the flow of oxygen and contaminate health care workers more easily.51, 52, 53, 54, 55 The precautionary principle would suggest maximizing health care worker training and protection to the extent possible when patients with severe acute respiratory infection are treated, keeping in mind the limited quantities of such specialized equipment and the hierarchy of risk described previously. The present review can contribute to the complex decision facing clinicians regarding the optimal modality of oxygen therapy for patients with severe acute respiratory infection by providing a better understanding of the risk involved, which can improve health care worker safety and contribute to preserving health care system capacity, thus reducing the global morbidity associated with severe acute respiratory infection. In general, the lowest flow of oxygen should be used to maintain an adequate oxygen saturation for patients with severe acute respiratory infection, and manipulation of oxygen delivery equipment should be minimized to limit the risk of infection among health care workers.
In summary, most modalities of oxygen therapy are associated with an increased risk of infection in health care workers and none are demonstrated as safe. Better-designed studies would improve the certainty of these observations, particularly for the modalities for which clinical data were lacking. Future studies should also evaluate whether adequate protection and training can mitigate the increased risks of transmission described in the present review.
Acknowledgments
The authors acknowledge Monique Clar, BSc, for her help in designing the search strategy and Massimiliano Iseppon, MD, Marie-Claude Béland, MA, and Wes Martin, BSc, for their revision of the article. This project received funding from the Fonds des Urgentistes de l’Hôpital du Sacré-Cœur de Montréal, Canada.
Footnotes
Please see page 20 for the Editor’s Capsule Summary of this article.
Supervising editor: David Barlas, MD. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
Author contributions: AC, A-ML, SC, and RD designed the study. AC designed the search strategy. AC, SGM, JL, J-MC, VC, MM, AF, VH, ZG-T, A-ST, EP, JP, SC, A-LF-P, R-XL, VM, and RD assessed study eligibility. AC and SGM assessed the included studies’ quality and extracted the data. AC performed the statistical analyses and wrote the first draft of the article. All authors contributed to the interpretation and subsequent edits of the article. AC takes responsibility for the paper as a whole.
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. This project received funding from the Fonds des Urgentistes de l’Hôpital du Sacré-Cœur de Montréal.
Trial registration number: CRD42020175256
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Continuing Medical Education exam for this article is available at http://ecme.acep.org/diweb/catalog/t/51820.
Supplementary Data
Search Terms
Appendix E2. Supplementary results
Table E1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
Figure E1. Forest plot describing the infection risk during endotracheal intubation, including the study by Teleman et al
Figure E2. Forest plot describing the infection risk during endotracheal intubation, excluding articles at high risk of bias
Figure E3. Forest plot describing the infection risk during bag-valve-mask ventilation, excluding articles at high risk of bias
Figure E4. Forest plot describing the infection risk during non-invasive ventilation, excluding articles at high risk of bias
Figure E5. Funnel plot for the evaluation of publication bias for endotracheal intubation
Table E2. Quality Assessment and Risk of Bias
Table E3. Infection risk during endotracheal intubation
Table E4. Infection risk during bag-valve-mask ventilation
Table E5. Infection risk during non-invasive ventilation
Table E6: Infection risk with high-flow nasal cannula
Table E7. Infection risk during conventional oxygen therapy
Table E8: Infection risk during mixed exposure
References
- 1.McCloskey B., Dar O., Zumla A., et al. Emerging infectious diseases and pandemic potential: status quo and reducing risk of global spread. Lancet Infect Dis. 2014;14:1001–1010. doi: 10.1016/S1473-3099(14)70846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Shih T.P., Ko W.C., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease 2019 (COVID-19) Situation Report – 1392020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200607-covid-19-sitrep-139.pdf?sfvrsn=79dc6d08_2. Accessed July 26, 2020.
- 4.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui D.S., Memish Z.A., Zumla A. Severe acute respiratory syndrome vs the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 6.Rello J., Rodriguez A., Ibanez P., et al. Intensive care adult patients with severe respiratory failure caused by influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran K., Cimon K., Severn M., et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald L.C., Simor A.E., Su I.J., et al. SARS in healthcare facilities, Toronto and Taiwan. Emerg Infect Dis. 2004;10:777–781. doi: 10.3201/eid1005.030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- 11.Esquinas A.M., Egbert Pravinkumar S., Scala R., et al. Noninvasive mechanical ventilation in high-risk pulmonary infections: a clinical review. Eur Respir Rev. 2014;23:427–438. doi: 10.1183/09059180.00009413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel A., Lee L., Pillai S.K., et al. Approach to prioritizing respiratory protection when demand exceeds supplies during an influenza pandemic: a call to action. Health Secur. 2019;17:152–155. doi: 10.1089/hs.2019.0027. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., et al. Preferred Reporting Items for Systematic Reviews and Meta-analyses Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong N.S., Zheng B.J., Li Y.M., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuk A.Y.C., Ma S. The estimation of SARS incubation distribution from serial interval data using a convolution likelihood. Stat Med. 2005;24:2525–2537. doi: 10.1002/sim.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 26, 2020.
- 17.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011.
- 18.Yu I.T., Xie Z.H., Tsoi K.K., et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han F., Jiang Y.Y., Zheng J.H., Gao Z.C., He Q.Y. Noninvasive positive pressure ventilation treatment for acute respiratory failure in SARS. Sleep Breath. 2004;8:97–106. doi: 10.1007/s11325-004-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh N.W., Tan Y., Taculod J., et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 2020 doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rello J., Perez M., Roca O., et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care. 2012;27:434–439. doi: 10.1016/j.jcrc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Leonard S., Atwood C.W., Jr., Walsh B.K., et al. Preliminary findings of control of dispersion of aerosols and droplets during high velocity nasal insufflation therapy using a simple surgical mask: implications for high flow nasal cannula. Chest. 2020 doi: 10.1016/j.chest.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong B.C., Lee N., Li Y., et al. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin Infect Dis. 2010;51:1176–1183. doi: 10.1086/656743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raboud J., Shigayeva A., McGeer A., et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwashyna T.J., Boehman A., Capelcelatro J., et al. 2020. Variation in aerosol production across oxygen delivery devices in spontaneously breathing human subjects. [DOI] [Google Scholar]
- 26.Mardimae A., Slessarev M., Han J., et al. Modified N95 mask delivers high inspired oxygen concentrations while effectively filtering aerosolized microparticles. Ann Emerg Med. 2006;48:391–399. doi: 10.1016/j.annemergmed.2006.06.039. 399.e391-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loeb M., McGeer A., Henry B., et al. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler R.A., Guest C.B., Lapinsky S.E., et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1198–1202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]
- 29.Caputo K.M., Byrick R., Chapman M.G., et al. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53:122–129. doi: 10.1007/BF03021815. [DOI] [PubMed] [Google Scholar]
- 30.Park B.J., Peck A.J., Kuehnert M.J., et al. Lack of SARS transmission among healthcare workers, United States. Emerg Infect Dis. 2004;10:244–248. doi: 10.3201/eid1002.030793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W.Q., Ling W.H., Lu C.Y., et al. Which preventive measures might protect health care workers from SARS? BMC Public Health. 2009, 81;9 doi: 10.1186/1471-2458-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng V.C.C., Lee W.M., Sridhar S., et al. Prevention of nosocomial transmission of influenza A (H7N9) in Hong Kong. J Hosp Infect. 2015;90:355–356. doi: 10.1016/j.jhin.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Heinzerling A., Stuckey M.J., Scheuer T., et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient: Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W., Tang F., Fang L.Q., et al. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Trop Med Int Health. 2009;14:52–59. [Google Scholar]
- 35.Ng K., Poon B.H., Kiat Puar T.H., et al. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020;172:766-767 doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei L.Y., Gao Z.C., Yang Z., et al. Investigation of the influencing factors on severe acute respiratory syndrome among health care workers. Beijing Da Xue Xue Bao Yi Xue Ban. 2006;38:271–275. [PubMed] [Google Scholar]
- 37.Scales D.C., Green K., Chan A.K., et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205–1210. doi: 10.3201/eid0910.030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teleman M.D., Boudville I.C., Heng B.H., et al. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol Infect. 2004;132:797–803. doi: 10.1017/s0950268804002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha L.D., Bloom S.A., Nguyen Q.H., et al. Lack of SARS transmission among public hospital workers, Vietnam. Emerg Infect Dis. 2004;10:265–268. doi: 10.3201/eid1002.030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen M.I., Chow A.L., Earnest A., et al. Clinical and epidemiological predictors of transmission in severe acute respiratory syndrome (SARS) BMC Infect Dis. 2006;6:151. doi: 10.1186/1471-2334-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiyama A., Wakasugi N., Kirikae T., et al. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn J Infect Dis. 2008;61:388–390. [PubMed] [Google Scholar]
- 42.Christian M.D., Loutfy M., McDonald L.C., et al. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis. 2004;10:287–293. doi: 10.3201/eid1002.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nam H.S., Yeon M.Y., Park J.W., et al. Healthcare worker infected with Middle East respiratory syndrome during cardiopulmonary resuscitation in Korea, 2015. Epidemiol Health. 2017;39 doi: 10.4178/epih.e2017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui D.S.C., Chan M.T.V., Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20:9–13. [PubMed] [Google Scholar]
- 45.Luo Y., Ou R., Ling Y., et al. [The therapeutic effect of high flow nasal cannula oxygen therapy for the first imported case of Middle East respiratory syndrome to China] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27:841–844. [PubMed] [Google Scholar]
- 46.Hui D.S., Chow B.K., Chu L., et al. Exhaled air dispersion and removal is influenced by isolation room size and ventilation settings during oxygen delivery via nasal cannula. Respirology. 2011;16:1005–1013. doi: 10.1111/j.1440-1843.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 47.Ip M., Tang J.W., Hui D.S.C., et al. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control. 2007;35:684–689. doi: 10.1016/j.ajic.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts S., Kabaliuk N., Spence C.J.T., et al. Nasal high-flow therapy and dispersion of nasal aerosols in an experimental setting. J Crit Care. 2015;30:842. [Google Scholar]
- 49.Yu I.T., Wong T.W., Chiu Y.L., et al. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin Infect Dis. 2005;40:1237–1243. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonds A.K., Hanak A., Chatwin M., et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14:131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 51.Lindsley W.G., Blachere F.M., Thewlis R.E., et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindsley W.G., Blachere F.M., Davis K.A., et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50:693–698. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- 53.Guo Z.D., Wang Z.Y., Zhang S.F., et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020:26. doi: 10.3201/eid2607.200885. 1586-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blachere F.M., Lindsley W.G., Pearce T.A., et al. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 55.Milton D.K., Fabian M.P., Cowling B.J., et al. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belenguer-Muncharaz A., Reig-Valero R., Altaba-Tena S., et al. [Noninvasive mechanical ventilation in severe pneumonia due to H1N1 virus] Med Intensiva. 2011;35:470–477. doi: 10.1016/j.medin.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Cai S.J., Wu L.L., Chen D.F., et al. [Analysis of bronchoscope-guided tracheal intubation in 12 cases with COVID-19 under the personal protective equipment with positive pressure protective hood] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E033. doi: 10.3760/cma.j.cn112147-20200222-00153. [DOI] [PubMed] [Google Scholar]
- 58.Chan M.T., Chow B.K., Chu L., et al. Mask ventilation and dispersion of exhaled air. Am J Respir Crit Care Med. 2013;187:e12–e14. doi: 10.1164/rccm.201201-0137im. [DOI] [PubMed] [Google Scholar]
- 59.Chan M.T.V., Chow B.K., Lo T., et al. Exhaled air dispersion during bag-mask ventilation and sputum suctioning: implications for infection control. Sci Rep. 2018;8:198. doi: 10.1038/s41598-017-18614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung T.M.T., Yam L.Y.C., So L.K.Y., et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845–850. doi: 10.1378/chest.126.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hui D.S., Ip M., Tang J.W., et al. Airflows around oxygen masks: a potential source of infection? Chest. 2006;130:822–826. doi: 10.1378/chest.130.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hui D.S., Hall S.D., Chan M.T., et al. Noninvasive positive-pressure ventilation: an experimental model to assess air and particle dispersion. Chest. 2006;130:730–740. doi: 10.1378/chest.130.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hui D.S., Chow B.K., Lo T., et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147:1336–1343. doi: 10.1378/chest.14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hui D.S., Chow B.K., Lo T., et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53 doi: 10.1183/13993003.02339-2018. P1802339. [DOI] [PubMed] [Google Scholar]
- 65.Kotoda M., Hishiyama S., Mitsui K., et al. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J Hosp Infect. 2020;104:534–537. doi: 10.1016/j.jhin.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leung C.C.H., Joynt G.M., Gomersall C.D., et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 67.O'Neil C.A., Li J., Leavey A., et al. Characterization of aerosols generated during patient care activities. Clin Infect Dis. 2017;65:1335–1341. doi: 10.1093/cid/cix535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Somogyi R., Vesely A.E., Azami T., et al. Dispersal of respiratory droplets with open vs closed oxygen delivery masks: implications for the transmission of severe acute respiratory syndrome. Chest. 2004;125:1155–1157. doi: 10.1378/chest.125.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson K.A., Pappachan J.V., Bennett A.M., et al. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic—the risk of aerosol generation during medical procedures. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tonveronachi E., Valentini I., Fabiani A., et al. Noninvasive mechanical ventilation in patients with acute respiratory failure due to H1N1 infection. Eur Respir J. 2011;38:2968. [Google Scholar]
- 71.Vivarelli M., Perazzo A., Gatto P., et al. Management of severe respiratory failure following influenza a H1N1 pneumonia. Ital J Med. 2013;7:293–299. [Google Scholar]
- 72.Wong B., Lai R., Chan P., et al. A hospital outbreak of seasonal influenza involving three health care workers—implications on the optimal choice of respiratory protection. BMC Proc. 2011;5:100. [Google Scholar]
- 73.Zhao Z., Zhang F., Xu M., et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Terms
Appendix E2. Supplementary results
Table E1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
Figure E1. Forest plot describing the infection risk during endotracheal intubation, including the study by Teleman et al
Figure E2. Forest plot describing the infection risk during endotracheal intubation, excluding articles at high risk of bias
Figure E3. Forest plot describing the infection risk during bag-valve-mask ventilation, excluding articles at high risk of bias
Figure E4. Forest plot describing the infection risk during non-invasive ventilation, excluding articles at high risk of bias
Figure E5. Funnel plot for the evaluation of publication bias for endotracheal intubation
Table E2. Quality Assessment and Risk of Bias
Table E3. Infection risk during endotracheal intubation
Table E4. Infection risk during bag-valve-mask ventilation
Table E5. Infection risk during non-invasive ventilation
Table E6: Infection risk with high-flow nasal cannula
Table E7. Infection risk during conventional oxygen therapy
Table E8: Infection risk during mixed exposure