Abstract
Background
Poly (ADP-ribose) polymerase-1 (PARP-1) inhibitor has therapeutic potential for acute ischemic stroke by suppressing microglial activation and facilitating neuroprotection. In this first-in-human study, we investigate the safety, tolerability and pharmacokinetics (PK) of JPI-289 in healthy male volunteers.
Subjects and Methods
In single ascending dose (SAD) study, 35, 75, 150, 300, 600 mg JPI-289 or placebo was infused intravenously over 30 minutes to 40 subjects. In multiple ascending dose (MAD) study, 150, 300, 450 mg JPI-289 or placebo was infused over 1 hour every 12 hours to each of 24 subjects for 3.5 days (7 times). The plasma and urine concentrations of JPI-289 and its metabolites were determined.
Results
In the SAD study, AUClast and Cmax tended to increase supra-proportionally especially at higher doses in SAD study. However, Cmax showed dose-proportionality in the range of 75–600mg. JPI-289 reached a mean Tmax within 0.50 hour after dosing and a mean elimination half-life (t1/2) was 2.18 to 3.21 hours. In the MAD study, observed accumulation index ranged from 1.52 to 1.76. The effective half-life of JPI-289 was 1.88 to 3.05 hours, indicating that the plasma JPI-289 concentration rapidly reaches steady state. % recovered of JPI-289 measured in urine was 1.59–9.05%. In both studies, concentration of metabolites was less than 10% of JPI-289. Adverse events reported in the study were all mild in intensity and resolved without any sequelae.
Conclusion
The tolerable dose ranges and pharmacokinetic characteristics of JPI-289 evaluated in these studies will be useful in further clinical development of JPI-289.
Keywords: stroke, pharmacokinetics, pharmacodynamics, healthy subject, PARP-1 inhibitor
Introduction
Acute ischemic stroke (AIS) is characterized by sudden loss of blood circulation to a brain area, resulting in loss of neurologic function. The only available FDA-approved drug is intravenous recombinant tissue plasminogen activator (r-tPA), which can be initiated within 3 h (Grade 1A) or 4.5 h (Grade 2C) of symptom onset.1–3 However, r-tPA is prone to inducing life-threatening complications such as intracerebral hemorrhage4 and angioedema.5 Currently, there is no viable treatment option for AIS beyond 4.5 h of symptom onset, and there is a need for development of a new class of drug for AIS-affected patients. Neuronal damage induced by AIS leads to permanent disability of affected patients, but only a limited number of therapeutic options are available to protect neuronal damages. Physiologically, PARP-1 activation is a part of DNA damage repair mechanism; however, excessive PARP-1 activation is neurotoxic, and it is observed in the brain after acute ischemic stroke. This is regarded as a main process leading to irreversible neuronal damage by compromising the integrity of the neurovascular unit, increasing blood-brain barrier permeability, and releasing proinflammatory mediators.6
PARP-1 inhibition has a distinct mechanism of a therapeutic effect by directly protecting neurons7,8 as well as blood-brain barrier,9 and is thus expected to show high efficacy in clinical trials on AIS patients. Inhibition of PARP-1 activation has been reported as a potential therapeutic option for providing neuroprotection by diminishing the infarct size, shrinking edema volume, and attenuating neurovascular unit damage after acute ischemic stroke.10–12 PARP-1 inhibitor is expected to be especially useful for many patients who missed the therapeutic window of 3 h for reperfusion therapy by r-tPA. Animal studies have shown that that combination therapy with PARP-1 inhibitor reduces the incidence of tPA-induced serious adverse events.11
Effective PARP-1 inhibition and the mechanism of action by a PARP-1 inhibitor, MP-124 have been demonstrated in a non-human primate transient middle cerebral artery occlusion (tMCAO) stroke model;10 particularly, the results of this study are in compliance with the recommendations laid by Stroke Therapy Academic Industry Roundtable (STAIR),10,13 which specifies selection of stroke patients for entry in clinical trials, clarification of trial outcome measures, and informed consent issues.
JPI-289 is a PARP-1 inhibitor with therapeutic potential for AIS by suppressing microglial activation and facilitating neuroprotection.11 In vitro treatment of JPI-289 for oxygen glucose deprived rat cortical neuron showed neuroprotective effects by restoring ATP and NAD+ levels and reducing apoptosis-associated molecules such as apoptosis inducing factor (AIF), cytochrome C and cleaved caspase-3.11 JPI-289 treatment showed efficacy more than 10 h after stroke onset in an animal model, and is thus considered as one of the most promising agents for the treatment of stroke. (unpublished data on file, Jeil Pharma, Seoul, Korea) JPI-289 is one of few PARP-1 inhibitors currently being investigated for the treatment of acute ischemic stroke. In a tMCAO stroke model using monkeys, JPI-289 showed 53% decrease in infarction volume,14 which is much higher than the 21% decrease in infarction volume by MP-124.10 The immunological mechanism of action how PARP-1 inhibition by JPI-289 yields neuroprotection is not fully understood but a recent study reported that proportions of regulatory T cells (Tregs) are reduced in peripheral blood mononuclear cells (PBMCs) of ischemic stroke patients and can be increased when PBMCs are incubated for 24 h with high-dose JPI-289.15
Thus, it is necessary to investigate JPI-289 through a first-in-human trial. The principal aim of our current study was to explore the safety, tolerability, and pharmacokinetics of JPI-289 in healthy volunteers.
Methods
Subjects
Healthy male volunteers aged 19–55 years with body mass index ranging from 20 to 27 kg/m2 were included for this study. Within 4 weeks prior to the first administration of the study drug, the volunteers were examined if they were in favorable physical condition based on vital sign measurements (blood pressure, heart rate, and body temperature), medical history, physical examinations, 12-lead electrocardiograms, clinical laboratory tests (hematology, chemistry, and urinalysis), serology (hepatitis B surface antigen, hepatitis C virus antibody, and HIV antigen/antibody) and urine drug screening (cocaine, opiate, amphetamine, barbiturate, tetrahydrocannabinol, benzodiazepine, methadone and methamphetamine).
Volunteers were excluded for the following reasons: exposure to any investigational drug or placebo within 60 days of the first study medication dose; any illness within 14 days of the first study medication dose; aspartate aminotransferase or alanine aminotransferase levels 1.25× the upper normal limit; total bilirubin level 1.5× the upper normal limit; a platelet count 170,000 or 360,000; prothrombin time (PT) or activated partial thromboplastin time (aPTT) 1.25× the upper normal limit; and bleeding time 0.8 minutes.
Study Design
The first trial was Phase I, randomized, double-blind, and placebo-controlled single ascending dose (SAD) trial. In the SAD study, single dose of JPI-289 (manufactured and packaged by Avrio Biopharmaceuticals, LLC [CA, USA] and Jeil Pharmaceutical Co., Ltd. [Seoul, Republic of Korea]) was diluted in normal saline and infused over 0.5 h at 35, 75, 150, 300, or 600 mg (JPI-289: placebo = 6: 2 in each dose group) in 40 subjects. In the following multiple ascending dose (MAD) study, JPI-289 was diluted in normal saline and infused over 1 h every 12 h for seven times during a course of 3.5 days at 150 mg, 300 mg, and 450 mg (JPI-289: placebo = 6: 2 in each dose group) in 24 subjects.
The starting dose in the first in human SAD study was based on 4-week good laboratory practice study toxicology study results in rats, which was the most sensitive animal; in this study, 40 mg/kg was identified as the no observed adverse event level. 0.65 mg/kg, which is 39.0 mg in humans weighing 60 kg, was determined as a maximum safe starting dose by multiplying the human equivalent dose factor of 16. The starting dose adopted for this study was 35 mg.16
In the SAD study, the subjects were admitted to the Clinical Trial Center (CTC) at Asan Medical Center (AMC) from day 1 through day 2. On day 3, subjects re-visited the CTC for safety, tolerability and pharmacokinetics assessment. Follow-up visit was performed seven days after the drug administration. In the MAD study, subjects were admitted to the AMC CTC from day 1 through day 5. Follow-up visit was performed eight days after the last drug administration. Dose escalation was strictly based on the decision by Data and Safety Monitoring Board (DSMB).
The study protocol was approved by the Ministry of Food and Drug Safety and the institutional review board of AMC, Seoul, Republic of Korea (ClinicalTrials.gov identifier: SAD, NCT01983358; MAD, NCT02396069). The study was conducted at the CTC of AMC from April 2012 to August 2015. All subjects provided written informed consent prior to screening. The trial is conducted according to the principles of the Declaration of Helsinki (64th version, October 2013)
Determination of JPI-289 Concentrations in Plasma and Urine
Plasma and urine concentrations of JPI-289 were determined using a validated high performance liquid chromatography coupled with tandem mass spectrometry method (HPLC-MS/MS). The internal standard was JPI-289-d8 (dihydrochloride dihydrate). The sample extracts were analyzed using high performance liquid chromatography (Shiseido nanospace SI-2; Shiseido, Tokyo, Japan) and an XBridge™ C18 column (3.5 μm, 50 mm × 2.1 mm; Waters, Milford, MA, USA) with mobile phase consisting of methanol and 10 mM ammonium acetate (65:35, v/v).
The mass spectrometry system (API4000; AB Sciex, Framingham, MA, USA) was operated in positive ion electrospray mode with multiple reaction monitoring. The precursor-to-production reactions monitored were m/z 344.3 → 201.2 for JPI-289 and 352.3 → 201.2 for internal standard. In the SAD study, the assay for plasma samples was validated over a range of 1–3000 ng/mL for the 35 mg, 75 mg, and 150 mg groups and 10–30,000 ng/mL for the 300 mg and 600 mg groups (R2 >0.995). The assay for urine samples was validated over a range of 5–15,000 ng/mL for the 35 mg, 75 mg, and 150 mg groups, and 50–150,000 ng/mL for the 300 mg and 600 mg groups (R2 >0.995). In the MAD study, the assay for plasma samples was validated over a range of 10–30,000 ng/mL and the assay for urine samples was validated over a range of 50–150,000 ng/mL. For all assays, accuracy and precision of calibration standard curve were within 85–115% and <10% in both plasma and urine.
Pharmacokinetic Assessment
Serial blood samples (8 mL each) were taken at 0 h (predose), 5 min, 10 min, 20 min, 30 min, 45 min, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 24 h, and 48 h after the beginning of drug infusion in the SAD study. In the MAD study, blood was drawn at 0 h (one day 0 h, predose), 0.25 h, 0.5 h, 0.75 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h (one day 12 h, predose), 60 h (three days 12 h, predose), 72 h (four days 0 h, predose), 72.25 h, 72.5 h, 72.75 h, 73 h, 73.5 h, 74 h, 75 h, 76 h, 78 h, 80 h, 84 h, and 96 h (five days, 0 h). The blood samples were separated by centrifugation at 1800 g for eight minutes at 4°C and stored at −70°C until analysis. Urine samples of 6 mL were collected and urine volumes were measured at predefined intervals of 0 h (day one, spot urine), 0–4 h, 4–8 h, 8–12 h, and 12–24 h (spot urine was collected, and urine volume was measured) after the start of the drug infusion in the SAD study. In the MAD study, urine samples were collected and urine volumes were measured at 0 h (day one, spot urine only), 0–4 h, 4–8 h, 8–12 h, 72 h (day four, spot urine only), 72–76 h, 76–80 h, 80–84 h, and 84–96 h after the beginning of the first drug infusion. Urine samples were stored at −70°C before analysis.
The plasma and urine concentration-time profiles of JPI-289 were analyzed by a noncompartmental method (Phoenix® WinNonlin® 7.0; Pharsight Corporation, Sunnyvale, CA, USA). All analyses were performed using the actual sampling times. The concentration values below the lower limit of quantification were considered as zero (predose) or handled as missing values (postdose). The measured maximum plasma concentration (Cmax) and time at Cmax (Tmax) were determined from the observed values. The terminal elimination rate constant (λz) was estimated by linear regression of the terminal log-linear portion of the plasma concentration-time curves. The area under the plasma concentration-time curve (AUC) from time 0 to the last measurable time (AUClast) and the area under the plasma concentration-time curve within a dosing interval (AUCτ) were calculated by the trapezoidal rule; the AUC extrapolated to infinity (AUCinf) was obtained by AUClast + Clast/λz (Clast: the last quantifiable concentration). The terminal elimination half-life (t1/2z) was calculated for each participant as ln(2)/λz. Renal clearance (CLR) was calculated as the cumulative amount of drug excreted in urine in time t (Ae[t0,t]) divided by the AUC calculated from time 0 to the latest time point at which the urine concentration is quantifiable (AUClast). Urinary recovery (%) was calculated as the excreted amount unchanged in urine divided by the total administered amount of drug.
Safety and Tolerability Assessment
Tolerability was assessed using vital sign measurements, 12-lead electrocardiograms, clinical laboratory tests (hematology, blood chemistry, and urinalysis), physical examinations, and monitoring of adverse events (AEs). AEs were recorded in terms of symptoms and signs, intensity, duration, relationship to the study drug, action taken, outcome, and seriousness.
Statistical Analysis
All subjects who received the protocol-specified dose of JPI-289 or placebo and had adequate pharmacokinetic samples collected for noncompartmental analysis were included in the respective analyses. All subjects who received any dose of JPI-289 or placebo were included in the safety population. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and R version 3.3.1. Demographic data and pharmacokinetic parameters were summarized using descriptive statistics. In the SAD study, the dose proportionality was investigated by evaluating the dose-normalized Cmax and AUC values using the Kruskal–Wallis test.
Results
Study Participants
Forty and 24 healthy Korean male subjects participated and received the study drugs in the SAD and MAD study, respectively. All subjects completed the study, and were included in safety and pharmacokinetic assessments.
The mean (SD) age, height, and weight of the SAD study participants were 25.35 (3.73) years, 174.75 (6.26) cm, and 70.72 (6.55) kg, respectively. In the MAD study, the mean (SD) age, height, and weight of the study participants was 28.46 (4.24) years, 173.30 (5.46) cm, and 67.94 (6.27) kg, respectively. (Table 1) The age, height, and weight did not show statistically significant difference between the participants of SAD and MAD studies, and among the dose groups within each study as well.
Table 1.
Demographics of Subjects
| Parameters | Single Intravenous Dose n=40 | Multiple Intravenous Doses n=24 | Total n=60 |
|---|---|---|---|
| Age (years old) | 25.35 (3.73) [19.0–40.0] | 28.46 (4.24) [23.0–38.0] | 26.52 (4.18) [19.0–40.0] |
| Height (cm) | 174.75 (6.26) [163.4–186.9] | 173.30 (5.46) [161.3–182.6] | 174.20 (5.97) [161.3–186.9] |
| Weight (kg) | 70.02 (6.55) [58.1–90.5] | 67.94 (6.27) [56.2–85.2] | 69.24 (6.48) [56.2–90.5] |
Note: Data are expressed as mean (SD) [minimum–maximum].
Pharmacokinetic Analysis
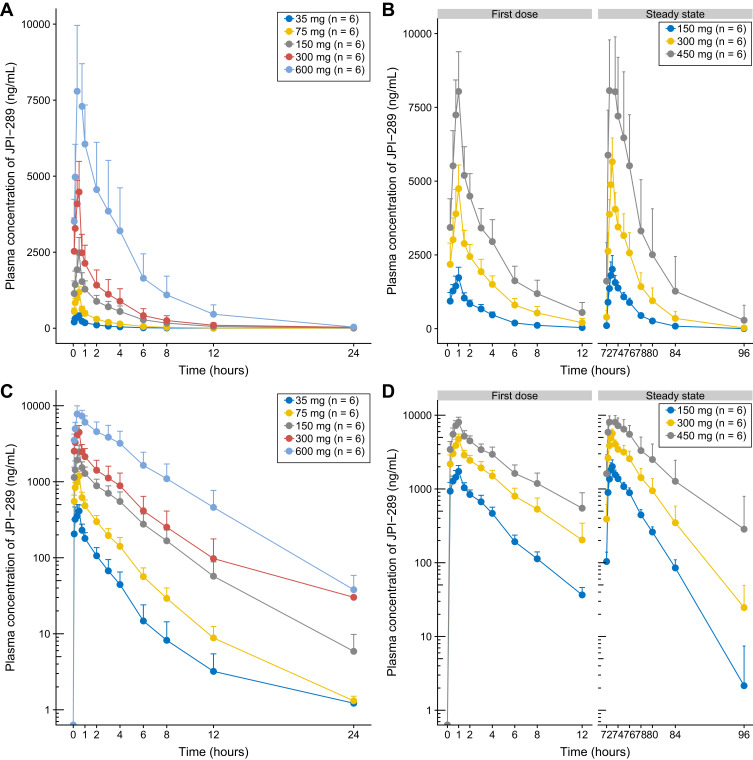
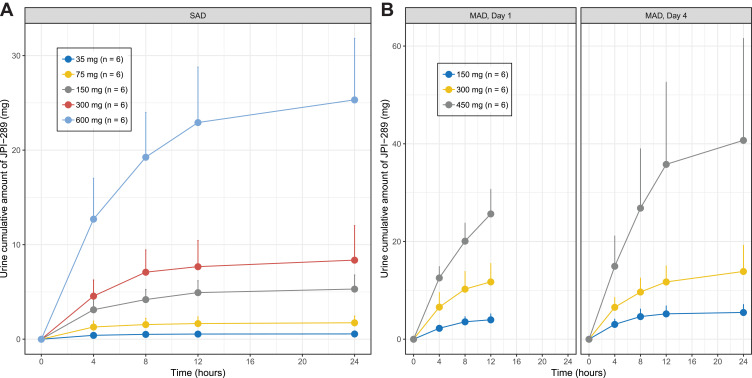
The mean plasma concentration-time profiles of JPI-289 following single or multiple intravenous infusions are shown in Figure 1. The mean urine concentration-time profiles of JPI-289 following single or multiple intravenous infusions are shown in Figure 2.
Figure 1.
Mean (SD) plasma concentration time curves of JPI-289 after (A) a single intravenous administration in linear y axis, (B) multiple intravenous administration in linear y axis, (C) a single intravenous administration in log y axis, and (D) multiple intravenous administration in log y axis (each group, n=6).
Figure 2.
Mean (SD) urine cumulative amount of JPI-289 after (A) a single intravenous administration, and (B) multiple intravenous administration (each group, n=6).
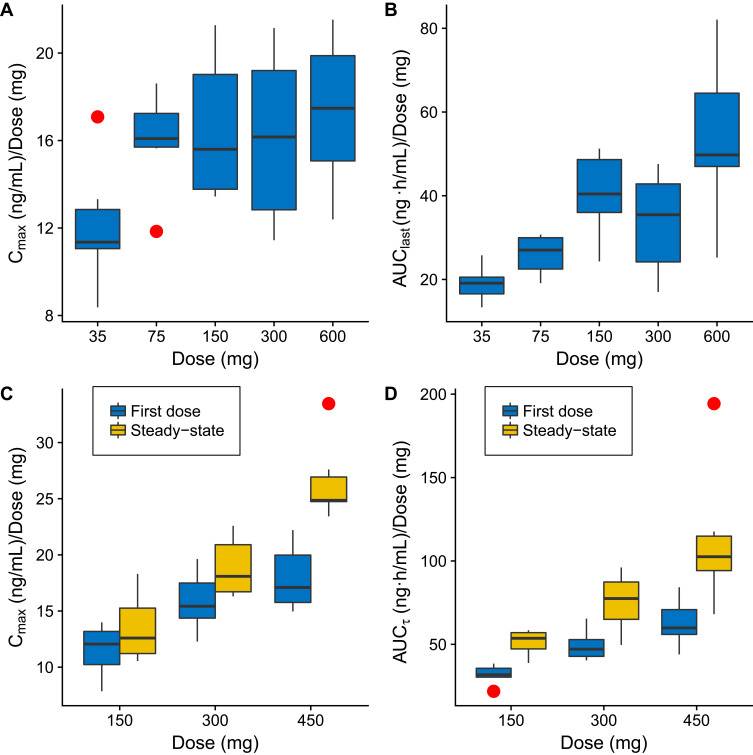
In the SAD study, JPI-289 reached a mean Tmax at 0.47 to 0.50 h after the beginning of 30-min infusion and then declined, with a mean elimination half-life (t1/2z) of 2.18 to 3.21 h (Table 2A). AUClast (658.82 to 32,066.88 ng·h/mL); Cmax (422.72 to 10,381.25 ng/mL) tended to increase supra-proportionally, especially at higher doses. In linear regression, the 95%CI of the slope for log-transformed Cmax and AUC0-∞ of the 35–600 mg single-dose group were 1.023–1.185 and 1.185–1.436, respectively. However, log-transformed Cmax in the range of 75–600 mg showed a slope of 0.917–1.135, suggesting dose-proportionality. Although dose-normalized AUClast values were different across doses in the rage of 35–600 mg with statistical significance (p-value <0.001), dose-normalized Cmax values were not significantly different (p-value = 0.0831) in the SAD study (Figure 3). The mean urinary recovery ratio was 1.59 to 4.22%.
Table 2.
Pharmacokinetic Parameters of JPI-289 After (A) a Single Intravenous Dose and (B) Multiple Intravenous Doses
| (A) | ||||||
|---|---|---|---|---|---|---|
| Parameter | Single Intravenous Dose of JPI-289 | |||||
| 35 mg (n=6) | 75 mg (n=6) | 150 mg (n=6) | 300 mg (n=6) | 600 mg (n=6) | Total (n=30) | |
| AUClast (ng·h/mL) | 658.82 (148.39) | 1940.45 (367.40) | 6029.78 (1520.32) | 9786.58 (3534.51) | 32,066.88 (11,725.80) | 10,096.50 (12,719.54) |
| AUCinf (ng·h/mL) | 665.50 (149.62) | 1947.57 (366.92) | 6057.90 (1533.04) | 10,061.82 (3690.15) | 32,228.35 (11,796.05) | 10,192.23 (12,791.37) |
| AUCinf/dose (ng·h/mL/mg) | 19.01 (4.28) | 25.97 (4.89) | 40.39 (10.22) | 33.54 (12.30) | 53.71 (19.66) | 34.52 (16.32) |
| Cmax (ng/mL) | 422.72 (102.10) | 1197.77 (173.37) | 2481.05 (502.31) | 4842.02 (1202.57) | 10,381.25 (2097.17) | 3864.96 (3789.52) |
| Cmax/dose (ng/mL/mg) | 12.08 (2.92) | 15.97 (2.31) | 16.54 (3.35) | 16.14 (4.01) | 17.30 (3.50) | 15.61 (3.55) |
| Tmax (h) | 0.47 [0.33, 0.50] | 0.48 [0.33, 0.52] | 0.48 [0.43, 0.50] | 0.48 [0.17, 0.50] | 0.50 [0.48, 0.52] | 0.48 [0.17, 0.52] |
| Vz (L) | 160.73 (42.91) | 183.32 (61.92) | 118.12 (34.25) | 124.50 (30.65) | 81.46 (16.60) | 133.63 (51.80) |
| Vd,ss (L) | 101.33 (26.74) | 91.18 (17.49) | 85.17 (15.31) | 92.52 (15.20) | 73.01 (13.20) | 88.64 (19.42) |
| CL (L/h) | 54.88 (12.54) | 39.77 (8.07) | 26.41 (8.03) | 34.28 (15.06) | 21.34 (9.69) | 35.33 (15.66) |
| t1/2z (h) | 2.18 (1.01) | 3.21 (0.81) | 3.12 (0.40) | 2.73 (0.76) | 2.88 (0.72) | 2.83 (0.80) |
| Ae (mg) | 0.56 (0.22) | 1.74 (0.71) | 5.31 (1.49) | 8.37 (3.65) | 25.32 (6.50) | 8.26 (9.65) |
| CLR (L/h) | 0.84 (0.28) | 0.88 (0.26) | 0.89 (0.21) | 0.91 (0.45) | 0.84 (0.20) | 0.87 (0.27) |
| (B) | ||||||
| Parameter | Multiple Intravenous Doses of JPI-289 | |||||
| 150 mg qd (n=6) | 300 mg qd (n=6) | 450 mg qd (n=6) | Total (n=18) | |||
| AUCτ,1st dose (ng·h/mL) | 4766.06 (878.28) | 14,809.79 (2793.29) | 28,278.07 (6412.76) | 15,951.31 (10,623.66) | ||
| AUCτ,ss (ng·h/mL) | 7696.21 (1157.59) | 22,608.80 (5198.68) | 50,840.74 (19,417.91) | 27,048.58 (21,404.87) | ||
| Cmax,1st dose (ng/mL) | 1728.17 (353.61) | 4747.25 (798.21) | 8070.98 (1320.10) | 4848.80 (2800.32) | ||
| Cmax,ss (ng/mL) | 2023.47 (460.41) | 5658.20 (804.71) | 11,921.28 (1655.56) | 6534.32 (4330.54) | ||
| Cav,ss (ng/mL) | 641.35 (96.47) | 1884.07 (433.23) | 4236.73 (1618.16) | 2254.05 (1783.74) | ||
| CLss (L/h) | 19.91 (3.37) | 13.95 (3.63) | 9.78 (3.11) | 14.54 (5.33) | ||
| Vz (L) | 85.65 (17.01) | 67.95 (11.04) | 63.02 (8.23) | 72.21 (15.52) | ||
| Vd,ss (L) | 112.20 (31.78) | 74.92 (11.18) | 72.06 (8.72) | 86.39 (26.65) | ||
| t1/2z (L) | 2.44 (0.28) | 2.74 (0.80) | 3.50 (1.18) | 2.89 (0.91) | ||
| t1/2,eff (L) | 1.88 (0.13) | 2.46 (0.67) | 3.05 (0.97) | 2.46 (0.81) | ||
| Observed AI | 1.64 (0.21) | 1.52 (0.22) | 1.76 (0.28) | 1.64 (0.24) | ||
| Ae,1st dose (mg) | 3.98 (1.21) | 11.74 (3.77) | 25.66 (5.03) | 13.79 (9.86) | ||
| Ae,last dose (mg) | 5.48 (1.64) | 13.87 (5.36) | 40.71 (20.87) | 20.02 (19.40) | ||
| CLR,1st dose (L/h) | 0.84 (0.21) | 0.80 (0.25) | 0.92 (0.16) | 0.85 (0.20) | ||
| CLR,last dose (L/h) | 0.73 (0.24) | 0.61 (0.14) | 0.77 (0.22) | 0.70 (0.21) | ||
Note: Data are expressed as mean (SD) except for Tmax, for which median (min, max) is shown.
Abbreviations: Ae, amount of unchanged drug in urine from the time of dosing to the last measurable concentration; AI, accumulation index; AUC0-∞, area under the plasma concentration-time curve from time 0 to infinity; AUClast, area under the plasma concentration-time curve from time 0 to last measurable time point; AUCτ,ss, area under the plasma concentration-time curve for dosing interval (τ); CL, clearance; CLR, renal clearance; CLss, clearance at steady-state; Cmax, measured maximum plasma concentration; t1/2z, terminal elimination half-life; t1/2, eff, effective half-life; Tmax, time to reach peak concentration; SAD, single ascending dose; Vd,ss, volume of distribution at steady state; Vz, volume of distribution during terminal phase.
Figure 3.
Box-whisker plots of dose-normalized Cmax (A), dose-normalized AUClast (B) in the single ascending dose study, dose-normalized Cmax (C), dose-normalized AUCτ (D) in the multiple ascending dose study. Red dots beyond the whiskers are outliers, defined as any number outside 1.5 times the interquartile range.
Abbreviations: AUClast, area under the plasma concentration-time curve from time 0 to last measurable time point; AUCτ, area under the plasma concentration-time curve for dosing interval (τ); Cmax, measured maximum plasma concentration.
In MAD study, Tmax were 0.95–0.98 h during one-hour infusion, and t1/2z was 2.44–3.50 (Table 2B). The effective half-life of JPI-289 was 1.88–3.05 h. The mean observed accumulation index was 1.52–1.76 after seven repeated doses every 12 h. The mean urinary recovery ratio was 2.65–9.05%.
There was a dose-proportional increase in Cmax of JPI-289 in the selected dose range (75–600 mg, slope of 0.917–1.135), and nonlinearity of Cmax in the range was not observed in the SAD study. However, there was a supra-proportional increase in exposure as shown by AUClast (slope of 1.185–1.436), and dose-normalized AUClast were different across doses in the range of 35–600 mg with statistical significance (p-value <0.001), which is suspected to be mainly due to the increased exposure in the higher dose. (Table 3)
Table 3.
Summary of Results from Dose Proportionality Test for Two Pharmacokinetic Parameters
| Parameter | Dose Range Studied (mg) | Slope (95%CI for Slope) |
|---|---|---|
| AUClast (ng·h/mL) | 35–600 | 1.310 (1.185–1.436) |
| Cmax (ng/mL) | 35–600 | 1.104 (1.023–1.185) |
| 75–600 | 1.026 (0.917–1.135) |
Abbreviations: AUClast, area under the plasma concentration-time curve from time 0 to last measurable time point; Cmax, measured maximum plasma concentration.
We also measured the major metabolites in the samples obtained in the highest dose groups (600 mg in SAD and 450 mg in MAD). Twenty-two metabolites were detected, among which JPI-289 had the highest proportion, followed by M15 which has modified morpholine ring, and M21 which does not have morpholine ring. In both studies, the concentration of each metabolite were less than 10% of JPI-289.
Safety and Tolerability
Seven AEs were observed in six subjects of 40 in the SAD study. Two AEs—increased total bilirubin and dizziness—were determined to have possible relationship to the study drug. Four cases of dizziness were observed in the dose group of 600 mg, and DSMB decided that these cases suggest dose-limiting toxicity and the maximum tolerated dose was determined to be 600 mg in the SAD study. Other AEs included diarrhea and increased heart rate. All AEs were mild in intensity, and spontaneously resolved with no observable clinical sequelae.
In the MAD study, seven subjects experienced a total of 12 AEs—out of those, 10 AEs (2 headache, 2 dizziness, 2 urticaria, 1 diarrhea, 1 abdominal discomfort, 1 pyrexia, 1 sinus tachycardia, 1 stomatitis, 1 bilirubinemia) in six subjects were possibly related to the study drug (Table 4). Eight drug-related AEs are observed in the highest dose, 450 mg. All AEs were spontaneously resolved without any treatment, except for two cases of urticaria which were cured with appropriate medication. All AEs were mild in intensity and no clinical sequelae were observed.
Table 4.
Summary of Adverse Events (AEs) After Single or Multiple Administration of JPI-289
| (A) | ||||||
|---|---|---|---|---|---|---|
| Single Intravenous Dose of JPI-289 (n=40) | Multiple Intravenous Doses of JPI-289 (n=24) | |||||
| Adverse Events | Not Related | Drug-Related | Total | Not Related | Drug-Related | Total |
| Diarrhea | 1 [1] | 0 [0] | 1 [1] | 1 [1] | 0 [0] | 1 [1] |
| Bilirubin total increased | 0 [0] | 1 [1] | 1 [1] | 0 [0] | 1 [1] | 1 [1] |
| Heart rate increased | 1 [1] | 0 [0] | 1 [1] | 0 [0] | 0 [0] | 0 [0] |
| Dizziness | 0 [0] | 3 [4] | 3 [4] | 0 [0] | 2 [2] | 2 [2] |
| Headache | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 2 [2] | 2 [2] |
| Urticaria | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 2 [2] | 2 [2] |
| Abdominal discomfort | 0 [0] | 0 [0] | 0 [0] | 1 [1] | 0 [0] | 1 [1] |
| Stomatitis | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 1 [1] | 1 [1] |
| Fever | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 1 [1] | 1 [1] |
| Sinus tachycardia | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 1 [1] | 1 [1] |
| (B) | ||||||
| Adverse Events | Number of Subjects | Number of Subjects with AEs (%) | Number of Subjects with Drug Related AEs (% total) | |||
| Single intravenous dose | 35 mg | 6 | 3 (50.0) | 1 (16.7) | ||
| 75 mg | 6 | 0 (0.0) | 0 (0.0) | |||
| 150 mg | 6 | 0 (0.0) | 0 (0.0) | |||
| 300 mg | 6 | 0 (0.0) | 0 (0.0) | |||
| 600 mg | 6 | 3 (50.0) | 3 (50.0) | |||
| Multiple intravenous doses | 150 mg | 6 | 0 (0.0) | 0 (0.0) | ||
| 300 mg | 6 | 1 (16.7) | 1 (16.7) | |||
| 450 mg | 6 | 5 (83.3) | 4 (66.7) | |||
Notes: (A) Data are expressed as the number of subjects [cases]. (B) Data are expressed as the number of subjects (% of the number of subjects of each dose group).
No serious AEs occurred during the entire course of the study. No clinically significant abnormalities in laboratory tests, physical examinations, vital signs, or electrocardiograms were observed.
Discussion
Since discovery of the PARP-1 inhibition on neuroprotection, efforts have been made over the last few years to develop a therapeutic intervention for ischemic stroke patients. This first-in-human study of JPI-289, PARP-1 inhibitor for investigating the safety, tolerability, and pharmacokinetics is an important initial step to the development of a potential treatment option for AIS patients. The current study evaluated PK characteristics and tolerability of ivabradine in healthy Korean males after single intravenous administration at doses of 35, 75, 150, 300, or 600 mg and repeated qd oral administration at doses of 150, 300, or 400 mg.
All observed AEs were mild in intensity, and spontaneously resolved with no observable clinical sequelae. The most frequent AE after JPI-289 administration was dizziness, whose relationship of the study drug was “unlikely related” or “definitely not related”. Although AEs were increased in the 450 mg multiple dosing group, considering the short-term treatment duration of JPI-289 infusion for acute ischemic stroke patients, JPI-289 intravenous infusion at the multiple dose of up to 450 mg over one hour every 12 h is generally safe in human. Orally administered PARP-1 inhibitors have been recently approved for the treatments for several types of malignancy with satisfactory safety,17–19 Although we investigated 450 mg dose in the MAD study, the same dose was not evaluated in the SAD study. However, we believe that multiple dosing of 450 mg would be more appropriate for clinical use, and pharmacometric modeling and simulation were performed to assess multiple dosing pharmacokinetic profiles of intravenous infusion of 450 mg for one hour. Based on the simulation data (unpublished data on file, Jeil Pharmaceutical, Seoul, Korea), the Ministry of Food and Drug Safety of Korea allowed the multiple-dosing protocol of 450 mg.
The AUC ratio of JPI-289 and M15 ranged from 0.08 to 0.10 based on the average of each dose, and the concentration of M21 was extremely low compared to that of JPI-289, and therefore the blood concentration of the parent drug JPI-289 was much higher than that of M15 and M21. In both SAD and MAD studies, the concentrations of metabolites were less than 10% of JPI-289. Obviously, there are no human studies using M15 and M21, but considering their low exposure, the minor or insignificant pharmacological effects are expected.
The effective half-life of JPI-289 was 1.88–3.05 h, indicating that JPI-289 rapidly reaches steady state and the blood concentration rapidly decreases when the infusion is completed. The mean urinary recovery ratio was 1.59 to 4.22% in the SAD study, and 2.65–9.05% in the MAD study and tend to increase in the higher JPI-289 dosing groups. This suggests that the relative proportion of nonrenal clearance of the total clearance decreases and the proportion of renal clearance increases in the higher JPI-289 doses and needs to be explored whether this has a clinical implication in the renally impaired special population.
It should be noted that this study has several limitations and additional clinical studies are required. First, our results were obtained from healthy male volunteers, and the pharmacokinetics of JPI-289 may differ in acute stroke patient populations in relation to many factors such as age and sex. Therefore, future studies on different populations are needed. Second, all subjects in this study were Korean, and pharmacokinetics in the different races (ie Caucasian patients) should be explored. Third, PARP-1 inhibitor might be used as an add-on therapy or combination therapy with tPA, the drug–drug interaction should be explored in the future. Lastly, the recommended phase 2 dose (RP2D) of JPI-289 can be determined in the study in the acute ischemic stroke patients and RP2D cannot be properly estimated or projected in this study with the healthy subjects.
In summary, JPI-289 was well tolerated in normal healthy subjects in dose ranges of 35–600 mg in the SAD study, and 150–450 mg in the MAD study. This is the first study to show the tolerable dose ranges of JPI-289 in humans. Based on these findings, we suggest that the treatment efficacy and safety of JPI-289 should be further evaluated in clinical studies involving patients.
Conclusion
In conclusion, two first-in-human, phase I, randomized, double-blind, placebo-controlled dose escalation clinical trials of intravenous JPI-289 (single dose (n=40) and multiple dose (n=24)) were conducted in 64 healthy volunteers. The results provide data on the safety, tolerability, and pharmacokinetics of JPI-289 for application in humans.
Data Sharing Statement
Upon request, and subject to certain criteria, conditions and exceptions, the authors will provide access to individual de-identified participant data. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply. The proposals should be directed to the corresponding author.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, revising the manuscript critically, read and approve the final draft of the manuscript for submission, gave final approval of the manuscript version to be published and agreed to be accountable for every step of the work.
Disclosure
This study was presented at the 13th Congress of the European Association for Clinical Pharmacology and Therapeutics. This research was supported by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (KDDF.org identifier: SAD, KDDF-201306-04, Republic of Korea; MAD, KDDF-201410-08, Republic of Korea) and Jeil Pharmaceutical Co., Ltd, Seoul, Republic of Korea, the manufacturer of JPI-289. DJS, JK, JN, and JWK were employees of Jeil Pharmaceutical Co., Ltd at the time of the study. There are no financial relationships in the previous three years with any organization that might have an interest in the submitted work, and there are no other relationships or activities that could have influenced the results and interpretation of the submitted work.
References
- 1.Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 2.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 3.Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: american college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e601S–e636S. doi: 10.1378/chest.11-2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute of Neurological D. Stroke rt PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 5.Myslimi F, Caparros F, Dequatre-Ponchelle N, et al. Orolingual angioedema during or after thrombolysis for cerebral ischemia. Stroke. 2016;47(7):1825–1830. doi: 10.1161/STROKEAHA.116.013334 [DOI] [PubMed] [Google Scholar]
- 6.Moroni F, Chiarugi A. Post-ischemic brain damage: targeting PARP-1 within the ischemic neurovascular units as a realistic avenue to stroke treatment. FEBS J. 2009;276(1):36–45. doi: 10.1111/j.1742-4658.2008.06768.x [DOI] [PubMed] [Google Scholar]
- 7.Kauppinen TM. Multiple roles for poly(ADP-ribose)polymerase-1 in neurological disease. Neurochem Int. 2007;50(7–8):954–958. doi: 10.1016/j.neuint.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 8.Stoica BA, Loane DJ, Zhao Z, et al. PARP-1 inhibition attenuates neuronal loss, microglia activation and neurological deficits after traumatic brain injury. J Neurotrauma. 2014;31(8):758–772. doi: 10.1089/neu.2013.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rom S, Zuluaga-Ramirez V, Dykstra H, Reichenbach NL, Ramirez SH, Persidsky Y. Poly(ADP-ribose) polymerase-1 inhibition in brain endothelium protects the blood-brain barrier under physiologic and neuroinflammatory conditions. J Cereb Blood Flow Metab. 2015;35(1):28–36. doi: 10.1038/jcbfm.2014.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura S, Egi Y, Yuki S, Horikawa T, Satoh H, Akira T. MP-124, a novel poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor, ameliorates ischemic brain damage in a non-human primate model. Brain Res. 2011;1410:122–131. doi: 10.1016/j.brainres.2011.05.069 [DOI] [PubMed] [Google Scholar]
- 11.Kim Y, Kim YS, Noh MY, et al. Neuroprotective effects of a novel poly (ADP-ribose) polymerase-1 inhibitor, JPI-289, in hypoxic rat cortical neurons. Clin Exp Pharmacol Physiol. 2017;44(6):671–679. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu T, Macey TA, Quillinan N, et al. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J Cereb Blood Flow Metab. 2013;33(10):1549–1555. doi: 10.1038/jcbfm.2013.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford AL, Lee JM. Climbing STAIRs towards clinical trials with a novel PARP-1 inhibitor for the treatment of ischemic stroke. Brain Res. 2011;1410:120–121. doi: 10.1016/j.brainres.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Kim YS, Kim HY, et al. Early treatment with poly(ADP-Ribose) polymerase-1 Inhibitor (JPI-289) reduces infarct volume and improves long-term behavior in an animal model of ischemic stroke. Mol Neurobiol. 2018;55(9):7153–7163. doi: 10.1007/s12035-018-0910-6 [DOI] [PubMed] [Google Scholar]
- 15.Noh MY, Lee WM, Lee SJ, Kim HY, Kim SH, Kim YS. Regulatory T cells increase after treatment with poly (ADP-ribose) polymerase-1 inhibitor in ischemic stroke patients. Int Immunopharmacol. 2018;60:104–110. doi: 10.1016/j.intimp.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, MD: USFDA; 2005. [Google Scholar]
- 17.Lin KY, Kraus WL. PARP inhibitors for cancer therapy. Cell. 2017;169(2):183. doi: 10.1016/j.cell.2017.03.034 [DOI] [PubMed] [Google Scholar]
- 18.Chan EM, Shibue T, McFarland JM, et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature. 2019;568(7753):551–556. doi: 10.1038/s41586-019-1102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuli R, Shiao SL, Nissen N, et al. A Phase 1 study of veliparib, a PARP-1/2 inhibitor, with gemcitabine and radiotherapy in locally advanced pancreatic cancer. EBioMedicine. 2019;40:375–381. doi: 10.1016/j.ebiom.2018.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]