Abstract
Background
Dioxygenase 12-lipoxygenase (12-LOX) plays an important role in tumorigenesis and promotes angiogenesis and proliferation in several tumors, including prostate and breast tumors. Radiotherapy enhances the expression of 12-LOX in esophageal squamous cell carcinoma (ESCC). Two types of macrophages can be found in the tumor microenvironment. The M2 subtype accelerates tumor progression; however, the relationship between 12-LOX and macrophages is not well established. Here, we explore this interaction and its effect on ESCC to induce tumor progression.
Methods and Results
RT-qPCR and Western blot analyses were used to evaluate the mRNA and protein expression levels of 12-LOX and chemokine (C-C motif) ligand 5 (CCL5) in ESCC after radiotherapy. CCL5 expression was increased by 12-LOX upregulation but was suppressed by the well-established 12-LOX inhibitor, baicalein. Furthermore, CCL5 attracted and repolarized human myeloid leukemia mononuclear cells (THP-1)-derived macrophages. Finally, ESCC co-culture with THP-1-derived macrophages led to a strong cancer migratory capacity.
Conclusion
Radiation-induced 12-LOX overexpression in ESCC upregulates CCL5 expression, thereby attracting THP-1-derived macrophages and promoting their polarization to the M2 subtype, which enhances cellular metastasis.
Keywords: radiotherapy, 12-LOX, CCL5, macrophage
Introduction
Esophageal cancer (EC) is one of the most common cancers and is the sixth cause of cancer-related death worldwide, with an overall 5-year survival rate between 15–25%.1,2 In China, EC is amongst the top five leading causes of cancer-related death in both men and women.3 Esophageal squamous cell carcinoma (ESCC) is the most common type of EC, and radiotherapy is its standard treatment.4,5 However, radiotherapy resistance results in poor outcomes in nearly half of all the cases.6 Therefore, overcoming this challenge is crucial.
Dioxygenase 12-lipoxygenase (12-LOX) is mostly expressed in human platelets and metabolizes arachidonic acid into 12-hydroxyeicosatetraenoic acid (12-HETE).7 However, increased levels of this enzyme are associated with many types of cancer, including prostate, colon, and lung cancers, and contribute to tumor promotion, progression, and metastasis.8,9 A 12-LOX inhibitor, baicalein, can inhibit the growth of H460 cells, a human lung non–small carcinoma cell line, in a dose-dependent manner.10 In prostate cancer cells, 12-LOX inhibition has been shown to increase radiation sensitivity, and the combined administration of 12-LOX inhibitor and radiation suppresses colony formation.11,12
The tumor microenvironment interacts with tumor tissues to promote tumor progression and has recently received research attention. The M2 macrophage subtype is the predominantly found in the tumor microenvironment, where it stimulates cancer cell metastasis and angiogenesis.13,14 Patients with high macrophage levels have a poor prognosis after surgery. In gastric cancer, a high risk of macrophage infiltration results in a poor outcome.15,16 Furthermore, radiotherapy has been shown to facilitate myeloid recruitment and polarity, thus potentially triggering radiotherapy resistance and tumor regrowth.17,18 Therefore, macrophages are a novel potential target for tumor therapy.
Macrophages induce tumor growth by altering polarity and recruiting to cancer tissues. The levels of chemokines that attract macrophages to the tumor microenvironment, including chemokine ligand (CCL) 2 and 5, increase after radiotherapy,19,20 Additionally, it has been shown that the conditioned media (CM) from 12-HETE-treated pneumocytes contains elevated levels of IL-4 and IL-13 cytokines, which trigger the polarization of macrophages to the M2 subtype.21 Therefore, we hypothesized that the CM from esophageal cancer cells with elevated 12-LOX expression could also regulate macrophage polarity. Here, we explored the effect of 12-LOX on macrophage chemotaxis and polarization and the relationship between 12-LOX and CCL5 in two ESCC lines, Kyse150 and Eca109.
Materials and Methods
Cell Culture
The Eca109 (Procell Life Science & Technology Co., Ltd., China) and Kyse150 (Cell Bank, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) cell lines were purchased and selected as representative human ESCC cells. The human leukemia monocytic cell line THP-1 was also purchased from Cell Bank. All cells were maintained in RPMI 1640 medium (Gibco, Waltham, MA, USA) with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) at 37°C in a humidified atmosphere with 5% CO2.
Baicalein (Selleckchem, Houston, TX, USA) is a well-recognized 12-LOX inhibitor. Eca109 cells were treated with various concentrations (0, 20, 40, or 80 µmol/L) of baicalein for 24 h. Then Western blot was used to evaluate the 12-LOX expression. For the next step, 0 or 40 µmol/L baicalein was added to Eca109 cells. After 24 h, the medium was removed and replaced with fresh medium. The groups were then subjected to radiation.
We used viral transfection to upregulate the expression of 12-LOX in Kyse150 cells (Kyse150lv cells). A blank vector was used as a negative control (Kyse150con cells) (Shanghai Genechem Co, Ltd., Shanghai, China). Then their expression levels were verified at both the mRNA and protein levels.
In the mono-culture experiment, 1 × 106 cancer cells were planted in the 6-well plates and allowed to adhere. Then the wells in 6-well plates were replaced with fresh medium and cultured for 24 hrs.
Irradiation
Cells were irradiated with different X-ray doses (0, 3, and 6 Gy) at room temperature using an X-ray irradiator (Varian23EX; VARIAN, USA) with a 6 MV photo beam at a dose rate of 4 Gy/min.
Conditioned Medium
Cells were seeded into 6-well plates (Corning, NY, USA) and allowed to adhere overnight. When the cells reached 80% confluence in each well, the supernatant was removed and replaced with 2 mL fresh medium. After 24 h, the supernatant was again removed, centrifuged at 1000 rpm for 10 min, and collected as conditioned medium (CM).
The CM of Kyse150 with or without radiation was collected after 24 h and added to the macrophages. After another 24 h, total RNA was extracted from the macrophages. RT-qPCR was then performed to assess the changes in the expression of M1-related (IL1β, IL12p40, and IFN-γ) and M2-related (IL10, MMP9, CCL5, and CCL22) genes after different treatments.
Differentiation Assay and Co-Culture Experiment
THP-1 cells (1 × 106) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. Then THP-1 cells were differentiated to macrophages with 100 nmol/mL Phorbol-12-myristate 13-acetate (PMA, Sigma-Aldrich, St. Louis, MO, USA) treatment for 48 h. An optical microscope (LV150N, Nikon, Japan) was used to verify cell differentiation by evaluating cell adhesion.
The macrophages (5 × 105) were seeded into the upper well (0.4-μm pores; Corning, NY, USA) of 6-well plates, and the cancer cells were seeded into the lower wells of the 6-well plates. The ratio of macrophages and cancer cells was 1:2. Both groups of cells were allowed to adhere. Afterward, the upper wells and lower wells in the 6-well plates were replaced with fresh medium. Then, macrophages and cancer cells were co-cultured for 24 h. The migratory abilities of the co-cultured and mono-cultured cancer cells were determined using the transwell assay. The co-cultured macrophages (macrophage CO) and Kyse150 cells (Kyse150 CO) were cultured separately for an additional 24 hrs to investigate whether the cancer cells can self-stimulate. The resulting CM (Kyse150 CO CM and macrophage CO CM) was added to untreated Kyse150 cells.
Transwell Assay
THP-1 cells (1 × 105) were planted into the upper transwell chamber (8-µm pore size, 6.5-mm diameter; Corning, NY, USA), and the recombinant human CCL5 (0 or 100 ng/mL; Affinity Biosciences, Cincinnati, OH, USA) was added into the lower chamber. After 4 h, the lower chamber medium was collected to count the cells. The macrophages or cancer cells (5 × 104–1 × 105) were planted into the transwell inserts, and the recombinant human CCL5 (0 or 100 ng/mL) or CM was added into the lower chamber. After 24 h, the inserts were rinsed with phosphate-buffered saline (PBS, Gibco, Waltham, MA, USA) twice and then soaked in methanol for 20 min. Finally, the inserts were stained with 0.1% crystal violet (Solarbio, Beijing, China) for 30 min and then observed under the microscope.
Western Blot Analysis
Cell lysates were prepared with RIPA (Solarbio, Beijing, China) using Phenylmethylsulfonyl fluoride (PMSF, Solarbio, Beijing, China), which is 1% RIPA. The extracts were centrifuged at 15,000 rpm for 15 min at 4°C. The lysates were boiled at 99°C for 10 min in the presence of SDS-PAGE loading buffer (Solarbio, Beijing, China) and were then resolved by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel (Solarbio, Beijing, China) electrophoresis. The proteins were electrophoretically transferred to polyvinylidene fluoride membranes (Millipore SAS, Molsheim Cedex, France). The blots were blocked with 5% nonfat milk dissolved in Tris-buffered saline Tween (TBST, Solarbio, Beijing, China) for 1 h at room temperature. The primary CCL5 antibody was purchased from Abcam (Cambridge, MA, USA), and antibodies against GAPDH, p-AKT, and 12-LOX were purchased from Affinity Biosciences (Cincinnati, OH, USA), Cell Signaling Technology (Danvers, MA, USA), and Novus Biologicals (Littleton, Colorado, USA), respectively. Antibodies against p65, psp65, AKT, integrinβ1, and E-cadherin were purchased from Servicebio (Wuhan, China). The blots were probed with the primary antibodies at 4°C overnight and were then incubated with secondary antibody (dilution: 1:5000; Affinity Biosciences, Cincinnati, OH, USA) for 1 h at room temperature. A chemiluminescence detection system (EMD Millipore, Billerica, MA, USA) was used to visualize the blots.
Quantification Real-Time PCR
RNA was isolated by RNA Fast 2000 (Fastagen, Shanghai, China). First strand cDNA synthesis was performed by a cDNA reverse transcription kit (All-in-One First Strand Synthesis Kit, Genecopeia, Rockville, MD, USA). Next, qRT-PCR was conducted using the qPCR mix (All-in-One qPCR Mix, Genecopeia, Rockville, MD, USA). A qRT-PCR system (Light Cylcer 480II, ROCHE, Switzerland) was employed to determine the mRNA expression of target genes. The following primers were purchased from Beijing Dingguo Changsheng Biotechnology Co., LTD (Beijing, China): GAPDH, ALOX12, VEGFA, CCL2, IL4, CCL5, IL10, IL12p40, IL1β, MMP9, CCL22, CCR4, CCR5, E-cadherin, integrinβ1, and IFN-Gamma, The forward and reverse primer sequences are listed in Table 1.
Table 1.
Primers used for qRT-PCR
| Gene | Forward Primer Sequences (5ʹ–3ʹ) | Reverse Primer Sequences (5ʹ–3ʹ) |
|---|---|---|
| CCL2 | CTCTCGCCTCCAGCATGAAA | TTTGCTTGTCCAGGTGGTCC |
| MMP9 | TCTATGGTCCTCGCCCTGAA | CATCGTCCACCGGACTCAAA |
| CCL5 | CAGTCGTCTTTGTCACCCGA | CGGGTGGGGTAGGATAGTGA |
| integrin-beta1 | CCGCGCGGAAAAGATGAAT | ATGTCATCTGGAGGGCAACC |
| E-cadherin | GCTGGACCGAGAGAGTTTCC | CAAAATCCAAGCCCGTGGTG |
| GAPDH | GAAGACGGGCGGAGAGAAAC | TGGAATTTGCCATGGGTGGA |
| IL-10 | TACGGCGCTGTCATCGATTT | TAGAGTCGCCACCCTGATGT |
| IL-4 | TCTTTGCTGCCTCCAAGAACA | GTTCCTGTCGAGCCGTTTCA |
| VEGFA | AGGGCAGAATCATCACGAAGT | AGGGTCTCGATTGGATGGCA |
| ALOX12 | CCATCTCACTGACCATTGTGG | CAGGCGGATGATGATGAGC |
| CCL22 | ATCGCCTACAGACTGCACTC | GACGGTAACGGACGTAATCAC |
| IL12p40 | AAAATAGATGCGTGCAAGAGAGG | GGGGAAGACCTGTGACTTGAG |
| CCR1 | ACTATGACACGACCACAGAGT | CCAACCAGGCCAATGACAAATA |
| CCR3 | TGGCATGTGTAAGCTCCTCTC | CCTGTCGATTGTCAGCAGGATTA |
| CCR4 | TCTCGCCAAGACACTGAACAG | GGCCCTGCATTCCTCAAGAAG |
| CCR5 | TTCTGGGCTCCCTACAACATT | TTGGTCCAACCTGTTAGAGCTA |
| IFN-Gamma | GGCCTCTACCACTATCTTCTCTC | ACACTGCTGAATTGACAAGGTTT |
| TGF-beta1 | GTAGCTCTGATGAGTGCAATGAC | CAGATATGGCAACTCCCAGTG |
| CSF1 | TGGCGAGCAGGAGTATCAC | AGGTCTCCATCTGACTGTCAAT |
| IL1beta | GA GCCCCAAAAGCAAGAGGAA | TGCGGGCATA CGGTTTCATC |
Immunohistochemistry
Forty human ESCC specimens were purchased from the Department of Pathology, Qilu Hospital (Jinan, China). All data, including age, sex, smoking and drinking habits, histologic grade, T stage, N stage, TNM stage, tumor location, and number of dissected lymph nodes, were obtained from clinical or pathological records. The protocol was approved by the Institutional Ethic Committee of Qilu Hospital and Shandong University (Jinan, China). Data were collected from 2008 to 2013.
The specimens were incubated with primary antibodies for 12-LOX, CCL5, and CD68 (1:200, 1:200, and 1:100, respectively) at 4°C overnight. The antibody against 12-LOX, CCL5, CD68 was purchased from Novus Biologicals, Abcam and Servicebio, respectively. Afterward, the specimens were incubated with secondary antibodies (ZSGB-BIO, Beijing, China) and then stained with 3,3ʹ-diaminobenzidine (DAB, ZSGB-BIO, Beijing, China), and nuclei were counter-stained with hematoxylin (Solarbio, Beijing, China).
The stained specimens were analyzed using ImageJ software (US National Institutes of Health, Bethesda, MD, USA). Analysis classified 12-LOX and CCL5 expression into four grades (negative, low positive, positive, and high positive) ranking from 0–3 points. The final score of each specimen was the sum of points × percentage. The average of the highest and lowest score was calculated. The specimens were categorized into two groups: above average (ie, high-expression group) and below average (ie, low-expression group).
For CD68 evaluation, the number of CD68-positive cells in five fields was randomly counted for each section, and the average value per field was calculated. The mean of maximum and minimum values was also calculated. Cases with less CD68-positive cells than the mean number were considered to be part of the low-expression group, and the rest were considered to be part of the high-expression group.
Ethical Statement
The research protocol was approved by the Institutional Ethics Committee of Qilu Hospital and Shandong University (Jinan, China). All enrolled patients signed informed consents.
Statistical Analysis
GraphPad Prism version 6 software (GraphPad Software Inc., La Jolla, CA) and R software (version 3.0.1) were used for data analyses. Prior to statistical analysis, qPCR data were log-transformed. For comparisons of two groups, statistical significance was estimated using the Student’s t-test. The Kaplan-Meier method was used for survival analyses. The Log rank test was used for survival curve comparisons and univariate survival analyses. The Cox regression model was applied for multivariable survival analyses. Pearson correlation analysis was used to determine the relationship between two factors. P-values less than 0.05 were considered statistically significant.
Results
Radiotherapy Increases 12-LOX Levels in EC Cells
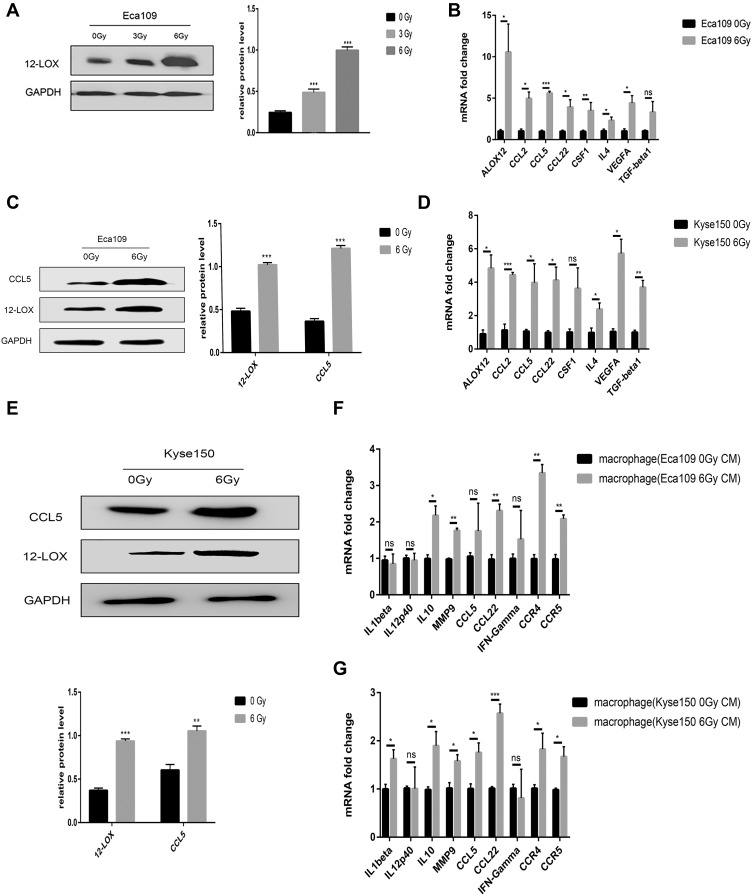
RT-qPCR and Western blot analyses were performed to assess the post-radiotherapy protein levels of 12-LOX in Eca109 cells. A remarkable increase in 12-LOX expression occurred at the dose of 6 Gy (Figure 1A and C). Therefore, later experiments were conducted with this fixed dose. In addition, the mRNA expression of 12-LOX increased (Figure 1B). Furthermore, increased mRNA levels were shown in some cytokines (VEGF, TGF-β1, and IL-4) and chemotactic factors (CCL2, CCL5, CCL22, and CSF1). These results are consistent with those of the Kyse150 cell line (Figure 1D and E).
Figure 1.
Radiotherapy increases 12-LOX levels in esophageal cancer cells. (A) 12-LOX expression in Eca109 cells irradiated with different X-ray doses (0, 3, and 6 Gy). (B and D) Cytokines (12-LOX, VEGF, TGF-beta1, and IL-4) and chemotactic factors (CCL2, CCL5, CCL22, and CSF1) showed increased mRNA levels after radiotherapy in Eca109 and Kyse150 cells. (C and E) CCL5 and 12-LOX showed increased protein levels after radiotherapy in Eca109 cells. (F and G) Changes in the mRNA levels of the M1- and M2-related genes of THP-1-derived macrophages with CM from irradiated cells. Experimental data were obtained from three independent tests. *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations: CM, conditioned media; ns, no significance.
The CM of Kyse150 with or without radiation was collected after 24 h and added to the macrophages. The RT-qPCR results indicated macrophage polarization to the M2 subtype after treatment with CM from irradiated cells. In addition, the CCL5 receptors, CCR4 and CCR5, increased dramatically (Figure 1G). Consistent results were obtained in Eca109 cells (Figure 1F).
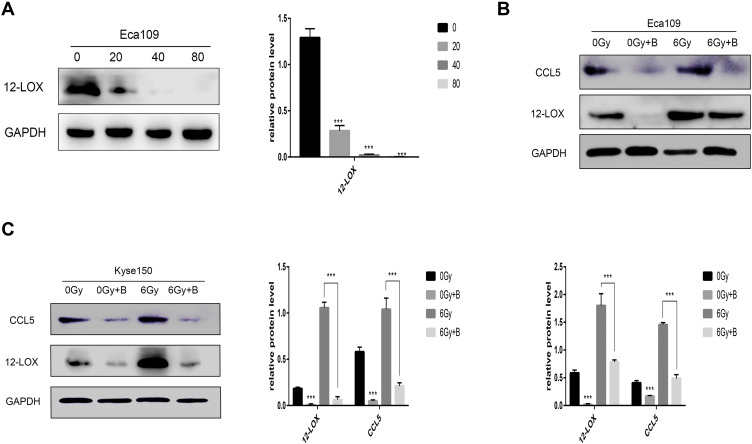
Baicalein Inhibits Increased 12-LOX Levels in Irradiated Cells
When Eca109 cells were treated with various concentrations of baicalein, 12-LOX protein levels decreased in a dose-dependent manner. Additionally, 12-LOX expression was significantly inhibited at 40 µmol/L (Figure 2A). Next, the Eca109 cells were treated with 0 or 40 µmol/L baicalein, and the groups were then subjected to radiation. In the inhibitor-treated group, the radiation-induced increase in 12-LOX expression was inhibited (Figure 2B). Consistent results were obtained in Kyse150 cells (Figure 2C).
Figure 2.
Baicalein can inhibit increased 12-LOX levels in irradiated cells. (A) 12-LOX protein levels decreased in a dose-dependent manner after 24-h baicalein treatment (0, 20, 40, or 80 µmol/L) in Eca109 cells. (B and C) Increased expression of 12-LOX after radiation was inhibited in the baicalein-treated group in Eca109 and Kyse150 cells. Experimental data were obtained from three independent tests.***P < 0.001.
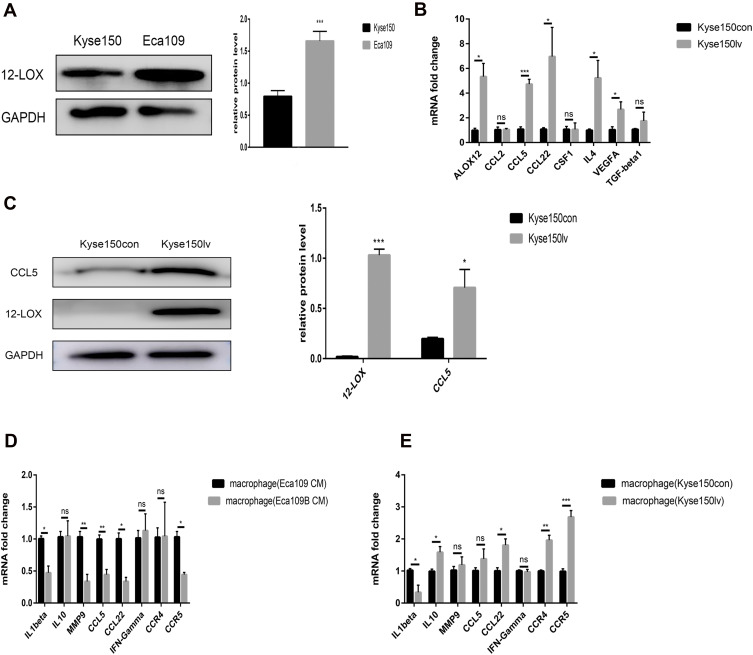
12-LOX Expression in Cancer Cells Regulates Macrophage Polarity
Compared with the Kyse150 cell line, Eca109 cells expressed higher 12-LOX levels (Figure 3A), so we used viral transfection to upregulate the 12-LOX expression in Kyse150. Kyse150 lv cells showed higher 12-LOX expression at both the mRNA and protein levels compared with Kyse150con cells. (Figure 3B and C). Next, the CM was collected from Kyse150con and Kyse150lv cells and used to treat macrophages for 24 h. The results showed that many M2-related genes (CCL5, CCL22, IL10, and MMP9), CCR4, and CCR5 were expressed in the high 12-LOX expression group (Figure 3E).
Figure 3.
12-LOX expression in cancer cells can regulate macrophage polarization. (A) Eca109 cells expressed higher levels of 12-LOX than Kyse150 cells. (B) Significantly higher expression of ALOX12, CCL5, CCL22, IL4, and VEGFA was observed in Kyse150lv cells than in Kyse150con cells. (C) Higher CCL5 and 12-LOX protein levels were detected in Kyse150lv than in Kyse150con cells. (D and E) Changes in the mRNA levels of the M1- and M2-related genes of macrophages with CM from Eca109B and Kyse150lv cells compared with those with CM from Eca109 and Kyse150con cells. Experimental data were obtained from three independent tests. *P < 0.05; **P < 0.01;***P < 0.001.
Abbreviations: CM, conditioned media; ns, no significance.
In addition, baicalein was added to Eca109 cells (Eca109B cells). After 24 h, the medium was replaced with fresh medium. After another 24 h, CM was collected and added to the THP-1-derived macrophages. Our results showed that the expression of M2-related genes decreased in the low 12-LOX expression group (Figure 3D).
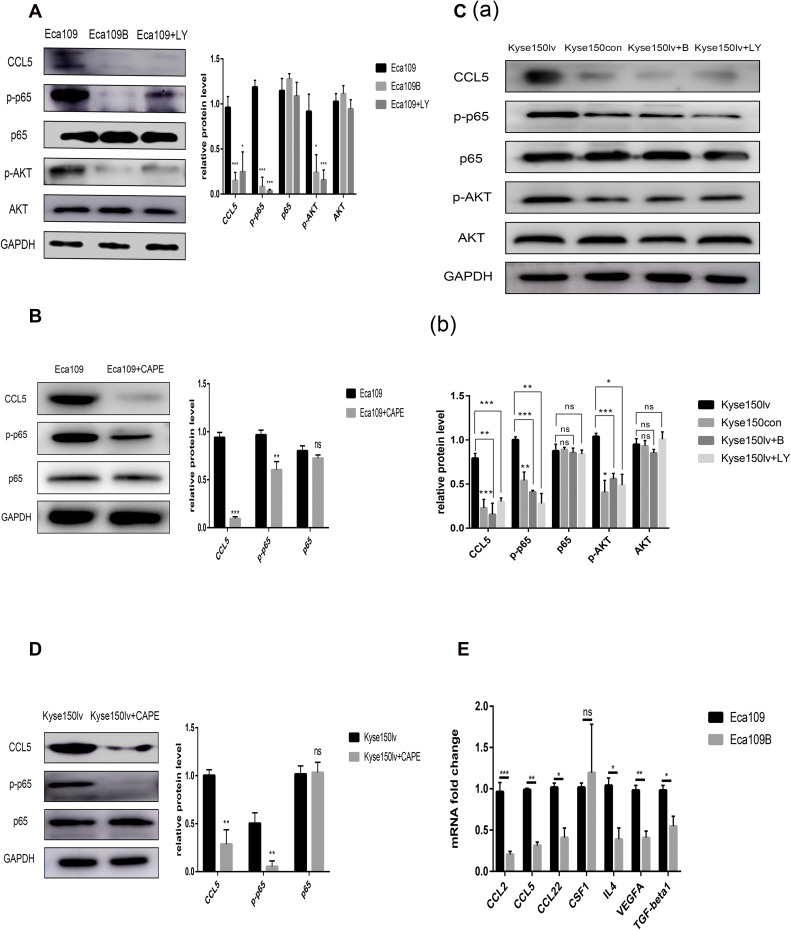
12-LOX Modulates CCL5 Expression in Cancer Cells Through AKT/NF-κB Pathway
The post-radiotherapy protein levels of CCL5 increased in Eca109 and Kyse150 cells but were inhibited by baicalein (Figure 2B and C).
RT-qPCR was conducted to identify the changes in the related genes of Kyse150lv cells. Compared with Kyse150con cells, Kyse150lv cells exhibited increased mRNA levels of some chemotactic factors (CCL5 and CCL22) and cytokines (VEGF and IL-4) (Figure 3B). A similar experiment was conducted in Eca109 and Eca109B cells. The results show decreased expression of CCL2, CCL5, CCL22, CSF1, VEGF, TGF-β1, and IL-4 (Figure 4E). IL-4 could bind to ILR4α to induce an M2-like phenotype in macrophages, and CCL2 and CCL5 were chemokines for monocytes. Both 12-LOX upregulation and inhibition changed the mRNA level of CCL5. Western blot analysis was conducted to assess CCL5 expression, and the results were consistent with those of RT-qPCR (Figures 2B and 3B).
Figure 4.
12-LOX modulates CCL5 expression in cancer cells through the AKT/NF-κB pathway. (A and B) Inhibition of 12-LOX, p-AKT, or p-p65 decreased CCL5 expression in Eca109 cells. (C and D) 12-LOX overexpression increased CCL5 levels, which were inhibited by treatment with baicalein, LY294002, or CAPE in Kyse150lv cells. (E) Lower mRNA levels for CCL2, CCL5, CCL22, CSF1, VEGF, TGF-β1, and IL-4 in Eca109B cells compared with Eca109 cells. Experimental data were obtained from three independent tests. *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviation: ns, no significance.
The pathway in which 12-LOX modulates CCL5 expression was also examined. Because 12-LOX can enhance the expression of AKT and NF-κB, which are related to CCL5, we speculated that 12-LOX could modulate CCL5 expression through the AKT/NF-κB pathway. LY294002 (Selleckchem) and Caffeic Acid Phenethyl Ester (CAPE, Selleckchem), which are recognized as selective inhibitors of AKT and p-p65, were added to the Kyse150lv cells. Western blot results suggested that the increase in CCL5 levels by 12-LOX overexpression was inhibited by LY294002 or CAPE (Figure 4C and D). These experiments were repeated in Eca109 cells, and the results revealed that 12-LOX regulates CCL5 expression through the AKT/NF-κB pathway (Figure 4A and B).
CCL5 Attracts THP-1-Derived Macrophages and Induces Their Polarity
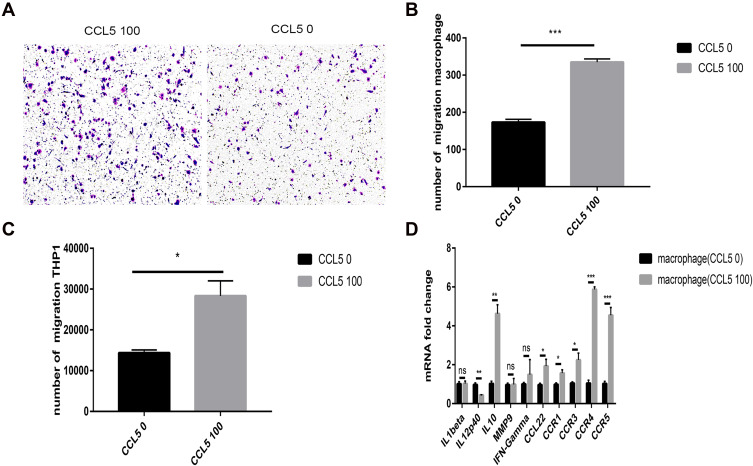
Human THP-1 cells were added to the upper chamber of the transwell plate. After 4 h, the THP-1 cell count in the CCL5-treated group increased dramatically (Figure 5C). In addition, The transwell assay was conducted by adding macrophages to the upper chamber. After 24 h, The number of macrophages in the CCL5-treated group increased significantly (Figure 5A and B), indicating that CCL5 can recruit macrophages derived from THP1 cells. In addition, we investigated whether CCL5 could induce the repolarization of macrophages. Macrophages incubated with CCL5 exhibited increased expression of M2-related genes and low expression of M1-related genes. These findings are indicative of polarization to the M2 subtype (Figure 5D).
Figure 5.
CCL5 attracts macrophages and induces their polarity into the M2 subtype. (A and B) The number of migratory macrophages in the CCL5 100 group increased significantly. (C) The number of migratory THP-1 cells in the CCL5 100 group increased significantly. (D) M1- and M2-related gene expression levels of macrophages changed at the mRNA level with exogenous CCL5 expression. Experimental data were obtained from three independent tests. *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviation: ns, no significance.
THP-1-Derived Macrophages Attracted by CCL5 Can Increase Cancer Cell Migration and Regulate Associated Protein Expression
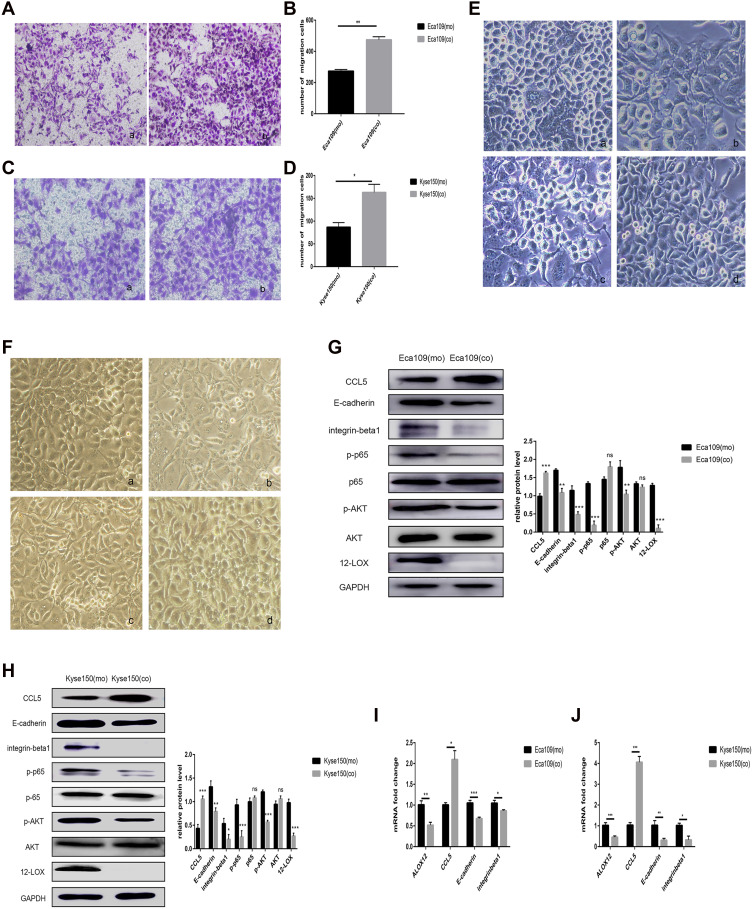
Macrophages and Kyse150 cells were co-cultured at a 1:2 ratio to determine the effect of the macrophages on the EC cells. After 24 h, the Kyse150 cells had a changed morphology with increased pseudopodia (Figure 6F and B) compared with mono-cultured Kyse150 cells (Figure 6F and A). After co-culture, the Kyse150 cells also exhibited an enhanced migratory ability (Figure 6C and D). Then, the Kyse150 CO CM or macrophage CO CM was added to untreated Kyse150 cells. After 24 h, the morphology of these mono-cultured Kyse150 cells with Kyse150 CO CM was similar to that of the co-cultured Kyse150 cells (Figure 6F and C). However, the mono-cultured Kyse150 cells with macrophage CO CM did not exhibit drastic morphological changes (Figure 6F and D).
Figure 6.
Macrophages attracted by CCL5 can increase cancer cell migration and regulate associated protein expression. (A–D) Co-cultured cancer cells showed enhanced migratory ability in Eca109 (A and B) and Kyse150 cells (C and D), as revealed by the transwell assays: (a) mono-cultured cancer cells (b) co-cultured cancer cells. (E and F) Morphology of Eca109 (E) and Kyse150 cancer cells (F) with different treatments: (a) mono-cultured cancer cells, (b) co-cultured cancer cells, (c) monocultured cancer cells with co-cultured Eca109 cell CM or co-cultured Kyse150 cell CM, and (d) monocultured cancer cells with co-cultured macrophage CM. (G and H) Western blot analysis showed that CCL5 expression increased after culture with macrophages, but 12-LOX, E-cadherin, integrin-β1, p-AKT, and p-p65 expression levels decreased in Eca109 and Kyse150 cells. (I and J) Co-culture with macrophages increased CCL5 expression but decreased ALOX12, E-cadherin, and integrin-β1 expression at the mRNA level in cancer cells. Experimental data were obtained from three independent tests. *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations: CM, conditioned media; ns, no significance.
RT-qPCR and Western blot analyses showed low expression of E-cadherin and integrin-β1 and high expression of CCL5 (Figure 6H and J), both of which are modulated by 12-LOX.22 Thus, RT-qPCR and Western blot analyses were used to assess the expression of 12-LOX. These experiments revealed low expression levels of 12-LOX, p-AKT, and p-p65 (Figure 6H and J). These results suggest that the co-cultured cancer cells used different pathways for modulating CCL5 expression. However, this hypothesis requires further exploration. All discussed experiments were also conducted in Eca109 cells, and similar results were obtained (Figure 6A, B, E, G, and I).
Increased Levels of 12-LOX in ESCC Patients with Poor Prognosis
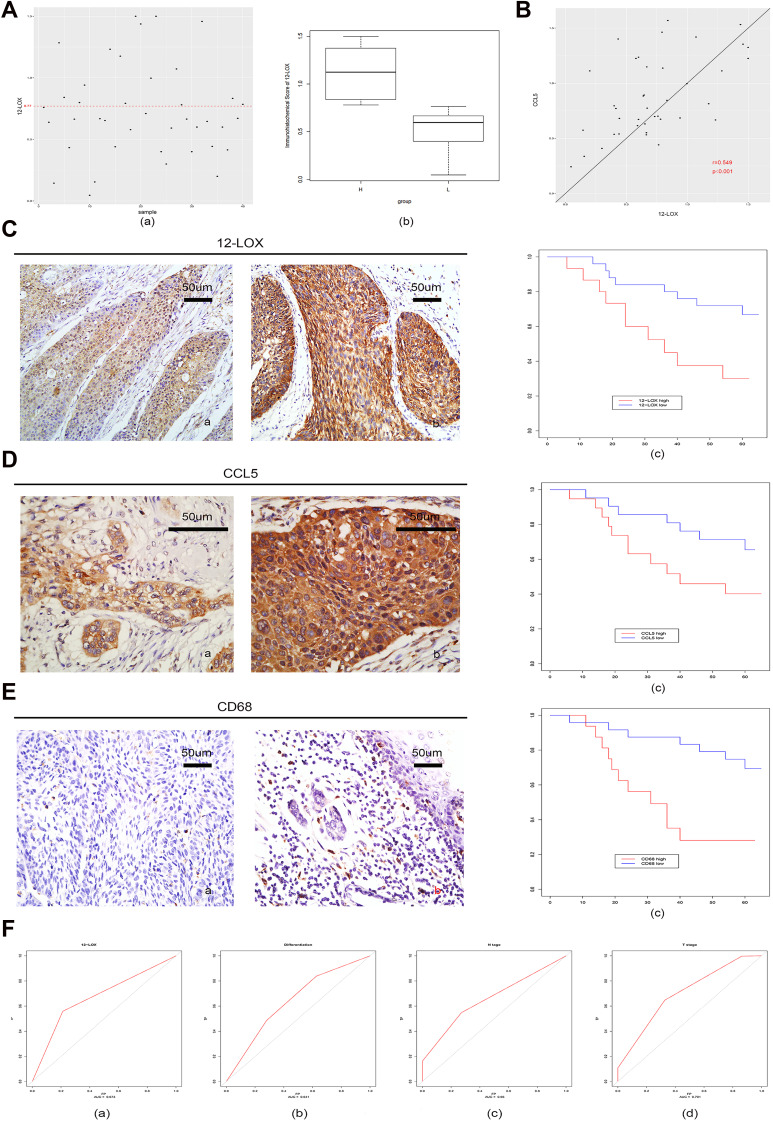
Expression of 12-LOX in cancer cells can modulate CCL5 expression and attract macrophages into the tumor microenvironment to facilitate tumorigenesis. Next, the expression levels of 12-LOX, CCL5, and CD68 were assessed in ESCC tissue sections. Basic information is shown in Table 2. Immunohistochemical 12-LOX scores of all samples are shown in Figure 7A. Analysis revealed high expression levels of 12-LOX, CCL5, and CD68, which were associated with poor survival prognoses (Figure 7C–E). Immunohistochemistry followed by Pearson correlation analysis in ESCC tissues showed that 12-LOX protein levels were positively correlated with CCL5 levels in ESCC tissues (r = 0.549; P < 0.001; Figure 7B). Univariate survival analysis indicated that high 12-LOX expression was correlated with decreased overall survival (hazard ratio [HR] = 5.70; P < 0.001, Table 3). Multivariate analysis identified 12-LOX expression as an independent prognostic factor for overall survival (HR = 5.895; P = 0.006, Table 4). The receiver operating characteristic curves of 12-LOX, T stage, N stage, and differentiation were also plotted, and their AUCs were calculated (Figure 7F).
Table 2.
The Correlation of ESCC Clinicopathologic Variables with 12-LOX in Cancertissue Samples
| Clinicopathological Features | Low Expression Level (n=24) | High Expression Level (n=16) | Pa-Value |
|---|---|---|---|
| Age(years) <60 ≥60 |
11 13 |
11 5 |
0.270 |
| Gender Male Female |
17 7 |
13 3 |
0.711 |
| Smoking Never or light Heavy |
13 11 |
10 6 |
0.747 |
| Drinking Never or light Heavy |
17 7 |
13 3 |
0.711 |
| Tumor location Upper Middle Lower |
1 17 6 |
1 9 6 |
0.647 |
| Differentiation Well Moderate Poor |
10 8 6 |
1 6 9 |
0.030* |
| T stage T1 T2 T3 T4 |
3 13 8 0 |
0 5 9 2 |
0.059 |
| N stage N0 N1 N2 N3 |
17 7 0 0 |
7 6 2 1 |
0.088 |
| TNM stage I II III IV |
8 8 8 0 |
0 7 7 2 |
0.022* |
| CCL5 Low expression High expression |
18 6 |
4 12 |
0.003* |
| CD68 Low expression High expression |
18 6 |
6 10 |
0.025* |
| Adjuvant therapy No Yes |
14 10 |
9 7 |
1.00 |
Note: *P<0.05.
Abbreviations: ESCC, oesophageal squamous cell cancer; Pa, Chi-square test; TNM, tumour node metastasis.
Figure 7.
Increased levels of 12-LOX in ESCC patients are associated with poor prognoses. (A) (a) Immunohistochemical scores of 12-LOX of all samples. The samples above the red line (score = 0.77) were in the high-expression group, and the rest were in the low-expression group; (b) box plots of the high-expression and low-expression groups. (B) Pearson correlation analysis in ESCC tissues showed that 12-LOX protein levels positively correlated with CCL5 in ESCC tissues (r = 0.549; P < 0.001). Immunohistochemical scores of 12-LOX are on the x-axis, and immunohistochemical scores of CCL5 are on the y-axis. (C–E) Expression of 12-LOX (C), CCL5 (D), and CD68 (E) in the low-expression group (a) and high-expression group (b). Their high expression levels were all associated with poor survival prognoses in the K-M curve (c) (12-LOX, P < 0.001; CCL5, P = 0.03; CD68, P < 0.001). (F) ROC curve and AUC of 12-LOX, differentiation, N stage, and T stage: (a) 12-LOX, AUC = 0.673; (b) differentiation, AUC = 0.641; (c) N stage, AUC = 0.660; and (d) T stage, AUC = 0.701.
Abbreviations: AUC, area under the curve; ESCC, esophageal squamous cell carcinoma; ROC, receiver operating characteristic.
Table 3.
Univariate Analyses of Prognostic Variables
| variable | P-value | HR (95% CI) |
|---|---|---|
| Gender | 0.733 | 1.213(0.399–3.688) |
| Age | 0.423 | 1.022(0.969–1.079) |
| Smoking | 0.875 | 1.077(0.425–2.731) |
| Drinking | 0.795 | 1.147(0.409–3.218) |
| T stage | 0.009* | 3.048(1.313–7.072) |
| N stage | <0.001* | 3.357(1.805–6.241) |
| Differentiation | 0.078 | 1.777(0.938,3.369) |
| 12-LOX | <0.001* | 5.700(2.102–15.46) |
| Tumor location | 0.538 | 1.322(0.544–3.213) |
Note: *P<0.05.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
Table 4.
Multivariate Analyses of Prognostic Variables
| Variable | P-value | HR (95% CI) |
|---|---|---|
| Gender | 0.104 | 0.211(0.032–1.379) |
| Age | 0.347 | 1.030(0.968–1.097) |
| Smoking | 0.595 | 1.398(0.406–4.813) |
| Drinking | 0.364 | 2.028(0.440–9.346) |
| T stage | 0.054 | 3.067(0.979–9.611) |
| N stage | 0.006* | 2.586(1.310–5.107) |
| Differentiation | 0.768 | 1.118(0.533–2.341) |
| 12-LOX | 0.006* | 5.895(1.645–21.118) |
| Tumor location | 0.653 | 1.234(0.494–3.081) |
Note: *P<0.05.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
Discussion
After radiotherapy, 12-LOX expression increases and thus promotes CCL5 expression through the AKT/NF-κB pathway. These elevated CCL5 levels can recruit macrophages to tumor tissues to participate in tumor progression. To the best of our knowledge, this study is the first to investigate the relationship of 12-LOX with CCL5 expression and macrophages.
The tumor microenvironment is essential for driving tumor evolvement by promoting cancer cell survival and migration.23 Macrophages represent the main immune cell population in the tumor microenvironment that promote tumor growth, metastasis, and angiogenesis.24 It has been previously shown that 5-LOX can recruit THP-1-derived macrophages to tumor tissues.25 Furthermore, 5-LOX and 12-LOX are subtypes of lipoxygenases, and the latter is also crucial for tumor promotion, progression, and metastasis.8,26 However, the effect of 12-LOX on macrophages is unclear, so the underlying mechanisms remain unknown. In this study, we aimed to identify the effects of increased 12-LOX expression after radiotherapy as well as the underlying mechanisms through various in vitro experiments.
Dioxygenase 12-LOX catalyzes the conversion of arachidonic acid into 12-HETE. Unfortunately, the recruitment of macrophages by 12-HETE (Cayman Chemical, Ann Arbor, Michigan, USA) was not observed in this study (data not shown). CCL2 and CCL5 are macrophage chemokines. However, few experiments have found that CCL5 recruits macrophages that are differentiated from THP-1 cells. In our results, we confirmed that CCL5 and CCL2 levels increased after radiotherapy, which was consistent with other reports.27 However, whether 12-LOX regulates CCL2 and CCL5 must be further explored in the Eca109 cell line. Baicalein, a recognized inhibitor of 12-LOX, successfully inhibited CCL2 and CCL5 expression, which was verified by RT-qPCR. However, only CLL5 expression increased in Kyse150 cells with elevated 12-LOX levels. These results were further verified by Western blot analysis, which also showed high levels of CCL5. Given that elevated 12-LOX expression activates the AKT/NF-κB pathway and that AKT can modulate CCL5 expression,28,29 this study investigated whether 12-LOX modulates CCL5 levels in an AKT/NF-κB pathway-dependent manner. Nonetheless, our results confirmed this speculation.
In addition to chemotaxis, macrophage polarization into either the M1 or M2 subtype is also essential for tumor progression. The two resulting subtypes have antagonistic effects: the M1 subtype has tumor-suppressive effects, whereas the M2 subtype is known to promote tumorigenesis.17 We observed increased levels of M2 markers (IL10, MMP9, and CCL22) after co-culture with CCL5, which was consistent with the findings of other reports.30 Furthermore, CM was collected from cancer cells with or without radiation, Kyse150 cells with or without 12-LOX upregulation, and Eca109 cells with or without baicalein incubation. Our transwell assay results showed that macrophages were likely to polarize to the M2 subtype when the CM originated from irradiated cells or from cancer cells with high 12-LOX expression.
Our immunohistochemistry results of ESCC tissue sections showed high expression of 12-LOX, CCL5, and CD68, which was associated with poor survival prognosis. In addition, 12-LOX was confirmed as an independent prognostic factor for overall survival, thus reflecting its value for prognosis research.
Conclusions
The radiation-induced overexpression of 12-LOX upregulates CCL5 expression via the AKT/NF-κB pathway in ESCC. As a result, THP-1-derived macrophages are recruited to the tumor microenvironment, where their repolarization into the M2 subtype is promoted to enhance the metastatic ability of the cancer cells and accelerate tumor progression. Furthermore, the high expression levels of 12-LOX, CCL5, and CD68 are associated with poor survival prognoses, and 12-LOX is an independent prognostic factor for overall survival.
Funding
This study was funded by National Natural Science Foundation of China (81572958) and Special Funds for Taishan Scholar Project (No. ts20190973).
Abbreviations
12-HETE, 12-hydroxyeicosatetraenoic acid; 12-LOX, 12-lipoxygenase; CCL5, chemokine ligand 5; CM, conditioned media; EC, esophageal cancer; SCC, esophageal squamous cell carcinoma; PMA, phorbol-12-myristate 13-acetate.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe M, Otake R, Kozuki R, et al. Recent Progress in Multidisciplinary Treatment for Patients with Esophageal Cancer. Surg Today; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Berger B, Belka C. Evidence-based radiation oncology: oesophagus. Radiother Oncol. 2009;92(2):276–290. doi: 10.1016/j.radonc.2009.02.019 [DOI] [PubMed] [Google Scholar]
- 5.Nakajima M, Kato H. Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother. 2013;14(10):1345–1354. doi: 10.1517/14656566.2013.801454 [DOI] [PubMed] [Google Scholar]
- 6.Borghesi S, Hawkins MA, Tait D, et al. Oesophagectomy after definitive chemoradiation in patients with locally advanced oesophageal cancer. Clin Oncol. 2008;20(3):221–226. doi: 10.1016/j.clon.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Porro B, Songia P, Squellerio I, et al. Analysis, physiological and clinical significance of 12-HETE: a neglected platelet-derived 12-lipoxygenase product. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:26–40. doi: 10.1016/j.jchromb.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 8.Ding XZ, Tong W-G, Adrian TE, et al. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatology. 2001;1(4):291–299. doi: 10.1159/000055827 [DOI] [PubMed] [Google Scholar]
- 9.Nie D, Nemeth J, Qiao Y, et al. Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clin Exp Metastasis. 2003;20(7):657–663. doi: 10.1023/A:1027302408187 [DOI] [PubMed] [Google Scholar]
- 10.Leung HW, Yang WH, Lai MY, et al. Inhibition of 12-lipoxygenase during baicalein-induced human lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol. 2007;45(3):403–411. doi: 10.1016/j.fct.2006.08.021 [DOI] [PubMed] [Google Scholar]
- 11.Dilly AK, Ekambaram P, Guo Y, et al. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-κB. Int J Cancer. 2013;133(8):1784–1791. doi: 10.1002/ijc.28165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovey J, Nie D, Tóvári J, et al. Radiosensitivity of human prostate cancer cells can be modulated by inhibition of 12-lipoxygenase. Cancer Lett. 2013;335(2):495–501. doi: 10.1016/j.canlet.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Zheng P, Luo Q, Wang W, et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9(4):434. doi: 10.1038/s41419-018-0465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212(4):435–445. doi: 10.1084/jem.20150295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishigami S, Natsugoe S, Tokuda K, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23(5a):4079–4083. [PubMed] [Google Scholar]
- 16.Wang HC, Chen C-W, Yang C-L, et al. Tumor-associated macrophages promote epigenetic silencing of gelsolin through DNA methyltransferase 1 in gastric cancer cells. Cancer Immunol Res. 2017;5(10):885–897. doi: 10.1158/2326-6066.CIR-16-0295 [DOI] [PubMed] [Google Scholar]
- 17.Genard G, Lucas S, Michiels C, et al. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017;8:828. doi: 10.3389/fimmu.2017.00828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafford JH, Hirai T, Deng L, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18(6):797–806. doi: 10.1093/neuonc/nov272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKelvey KJ, Hudson AL, Back M, et al. Radiation, inflammation and the immune response in cancer. Mamm Genome. 2018;29(11–12):843–865. doi: 10.1007/s00335-018-9777-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabe D, Hagenkort A, Page BDG, et al. Abstract PR02: metastasis suppressors regulate the tumor microenvironment by blocking recruitment of pro-metastatic tumor-associated macrophages. Cancer Res. 2016;76:PR02. doi: 10.1158/0008-5472.CAN-16-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung EJ, Reedy JL, Kwon S, et al. 12-lipoxygenase is a critical mediator of type II pneumocyte senescence, macrophage polarization and pulmonary fibrosis after irradiation. Radiat Res. 2019;192(4):367–379. doi: 10.1667/RR15356.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klampfl T, Bogner E, Bednar W, et al. Up-regulation of 12(S)-lipoxygenase induces a migratory phenotype in colorectal cancer cells. Exp Cell Res. 2012;318(6):768–778. doi: 10.1016/j.yexcr.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laplagne C, Domagala M, Le Naour A, et al. Latest Advances in Targeting the Tumor Microenvironment for Tumor Suppression. Int J Mol Sci. 2019;20(19):4719. doi: 10.3390/ijms20194719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laviron M, Boissonnas A. Ontogeny of tumor-associated macrophages. Front Immunol. 2019;10. doi: 10.3389/fimmu.2019.01799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen Z, Liu H, Li M, et al. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene. 2015;34(10):1241–1252. doi: 10.1038/onc.2014.85 [DOI] [PubMed] [Google Scholar]
- 26.Krishnamoorthy S, Jin R, Cai Y, et al. 12-Lipoxygenase and the regulation of hypoxia-inducible factor in prostate cancer cells. Exp Cell Res. 2010;316(10):1706–1715. doi: 10.1016/j.yexcr.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillon MT, Bergerhoff KF, Pedersen M, et al. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment. Clin Cancer Res. 2019;25(11):3392–3403. doi: 10.1158/1078-0432.CCR-18-1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandouz M, Nie D, Pidgeon GP, et al. Platelet-type 12-lipoxygenase activates NF-kappaB in prostate cancer cells. Prostaglandins Other Lipid Mediat. 2003;71(3–4):189–204. doi: 10.1016/S1098-8823(03)00042-X [DOI] [PubMed] [Google Scholar]
- 29.Pan Y, Smithson LJ, Ma Y, et al. Ccl5 establishes an autocrine high-grade glioma growth regulatory circuit critical for mesenchymal glioblastoma survival. Oncotarget. 2017;8(20):32977–32989. doi: 10.18632/oncotarget.16516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie Y, Huang H, Guo M, et al. Breast phyllodes tumors recruit and repolarize tumor-associated macrophages via secreting CCL5 to promote malignant progression, which can be inhibited by CCR5 inhibition therapy. Clin Cancer Res. 2019;25(13):3873–3886. doi: 10.1158/1078-0432.CCR-18-3421 [DOI] [PubMed] [Google Scholar]