Abstract
Introduction
Hospital wastewater contains highly resistant and virulent bacteria that can spread into the environment. This study was conducted to investigate the antimicrobial resistance of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) in raw and treated hospital wastewater.
Methods
During a three-month period, 40 sewage samples were collected from the hospital sewage (Kermanshah, Iran), and S. aureus were identified using culture and biochemical tests. MRSA was detected by resistance to cefoxitin. Antibiotic resistance (AR) was determined using disk diffusion according to the Clinical and Laboratory Standards Institute (CLSI) in 20 MSSA (10 raw and 10 treated sewage) and 40 MRSA isolates (20 raw and 20 treated sewage). The antimicrobial resistance genes (ARGs) were determined by PCR.
Results
Eleven and eight percent of the isolates were MRSA in raw and treated sewage samples, respectively. Out of 60 isolates, 59 (98%) were multidrug resistant (MDR). The most common ARGs were mecA (raw=100%, treated=100%), aacA-D (raw=100%, treated=85%) and tetK (raw =95%, treated =45%) in MRSA, while the tetM gene was the most abundant gene (raw=50%, treated=80%) in MSSA. None of isolates (n=60) was positive for the vanB gene. MSSR (n=20) had the highest level of resistance against penicillin (100%), clindamycin (raw=90%, treated=90%), azithromycin (raw=80%, treated=90%). All MRSA isolates (n=40,100%) in both raw and treated sewage samples were non-susceptible to penicillin, oxacillin and azithromycin. There was no significant difference in the frequency AR and ARGs between raw and treated sewage samples (p>0.05).
Conclusion
The results indicated a high frequency of MDR and ARGs in both raw and treated sewage isolates which could be released into the environment through sewage system and pose a serious threat to public health. Hospital wastewater treatment processes should be improved in order to prevent the dissemination of the most resistant strains of S. aureus.
Keywords: antibiotic resistance, antibiotic resistance genes, hospital wastewater, Staphylococcus aureus
Introduction
Staphylococcus aureus (S. aureus) is a Gram-positive bacterium that can act as both a commensal (in the upper respiratory tract and on the skin) and a major pathogen (causing infections in skin and various tissues as well as toxin-mediated diseases, eg, food poisoning and toxic shock syndrome).1 It can cause both community-acquired and hospital-acquired infections. S. aureus can easily acquire genes for resistance to antibiotics by horizontal gene transfer.2 Recently methicillin-resistant S. aureus (MRSA) has been reported as an important agent in the development of severe infections in health-care facilities, community, and farm environments.3 Methicillin resistance is due to the expression of the mecA gene, which encodes the penicillin-binding protein 2a (PBP2a), a protein that is essential to bacterial cell wall synthesis and confers resistance to most β-lactam antibiotics such as methicillin, nafcillin, oxacillin, and cephalosporins.4,5
The widespread use of antimicrobials for prophylaxis or treatment of human infections in hospitals results in the selection of resistant bacterial strains. About 10–90% of antibiotics used are excreted in the urine and/or feces of patients as unchanged or metabolites.6 The concentrations of antibiotics are much higher in the hospital wastewater than in the household effluent due to their widespread use in hospitals.7,8 Given the mixed population of various bacteria, antimicrobial agents, and nutrients in the sewage environment, it provides a selective pressure for the selection of antibiotic resistance strains or new resistant mutations in bacteria.9 Furthermore, the resistance genes can be transferred to other bacteria in the sewage. Despite advances in the wastewater treatment technology, resistant microorganisms are not completely removed from the hospital sewage during the wastewater treatment process.10,11 Thus, the hospital wastewater is a particular public health concern. Several studies have reported the presence of MRSA in hospital wastewater using both cultivation and molecular methods.12,13 There are also reports of the presence of Staphylococcus spp. in 80% of urban wastewater treatment plants in Spain.14
According to the Economic Co-operation and Development (OECD) report, Iran is among countries with high-level use of antibiotics.15 A study of the prevalence of MRSA strains in hospitalized patients in intensive care units (ICU) in Iran showed 93.3% MRSA in S. aureus isolates.3 Very few studies have evaluated the antibiotic susceptibility of S. aureus from hospital wastewater systems in Iran. It is pivotal to understand the prevalence of S. aureus in hospital wastewater to find appropriate strategies to prevent the dissemination of resistant strains in the environment. The aim of this study was to determine the antibiotic susceptibility of S. aureus isolated from the raw and treated hospital sewage.
Materials and Methods
Sample Collection
During 3 months from October 2017, sampling was done for 20 times and 40 samples were collected from the sewage treatment plant of Imam Reza Hospital, as the largest general and referral center in the west of Iran. With a few days interval between sampling, it was tried to cover any fluctuation in the bacterial load of the sewage. The pH and wastewater temperature were measured in situ. The samples were collected from the raw and treated sewage (20 samples for each sampling site). The samples were taken in 50 mL sterile bottles, transferred to the Microbiology Laboratory of Kermanshah University of Medical Sciences under cold conditions, and stored at 4 °C until tested. The wastewater treatment plant of this hospital used the extended aeration activated sludge system.
Bacterial Load of Sewage
To evaluate the bacterial load of the hospital sewage, six samples were randomly selected, three from raw and three from treated sewage samples, and tested within 3 hours after sampling. One mL of wastewater samples was added to the sterile test tube containing 9 mL of 0.85% NaCl solution and mixed properly. Three serial dilutions of wastewater samples, 0.001, 0.0001 and 0.00001 mL were then prepared; then, 15 mL of Muller-Hinton agar was poured in a plate containing 100 microliter of each sample and mixed thoroughly and uniformly with the agar medium. The plates were incubated at 37 °C for 24 hours. Colony count was performed and the average of three tests for each set was considered as the colony-forming unit per milliliter (CFU/mL) of hospital sewage.
Isolates Identification
The wastewater samples were cultured on Mannitol salt agar (Merck, Germany) plates. The plates were incubated at 37ºC for 48 h. Gram-positive cocci isolates that formed yellow colonies with a yellow halo on Mannitol salt agar were considered as presumptive S. aureus isolates. A few colonies per each wastewater sample with presumptive Staphylococci morphology were chosen and S. aureus isolates were identified using conventional microbiological methods (catalase, coagulase, deoxyribonuclease, hyaluronidase, hemolysis on sheep blood agar). To determine the MRSA frequency, 100 S. aureus colonies were picked up by toothpicks and cultured on Mannitol salt agar plates supplemented with cefoxitin (4 mg/L). Three sets of these cultures were curried out to determine the mean frequency of MRSA. Finally, 60 isolates included 20 MRSA and 10 MSSA isolates from raw sewage and the same sets from the treated sewage were randomly selected for further analysis.
Antibiotic Susceptibility Testing
The susceptibility of S. aureus isolates was tested against several antibiotic classes including β-lactam (penicillin, 10 μg and oxacillin, 1 µg), β-lactam+betalactamase inhibitor (amoxicillin+clavulanic, 30 µg), aminoglycosides (gentamicin, 10 μg), macrolide (azithromycin, 15 μg), tetracyclines (tetracycline, 30 μg), lincosamide (clindamycin, 2 μg) and ansamycins (rifampin, 5 μg) using the disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. After incubation at 37 °C for 16–18 h, the diameter of growth inhibition zones was measured. According to the inhibition zone diameter, the isolates were classified as resistant, intermediate and susceptible. S. aureus ATCC strain 25,923 was used for quality control. The isolates were defined as non-susceptible if they were resistant or intermediate to an antibiotic. Multidrug resistant (MDR) strains were determined by lack of susceptibility to at least three classes of antibiotics.16
DNA Extraction and Detection of Antibiotic Resistance Genes
Seven antimicrobial resistance genes including 1 β-lactam resistance gene (mecA), 2 tetracycline resistance genes (tetK, tetM), 3 glycopeptide resistance genes (vanA, vanB, vanC) and 1 aminoglycosides resistance gene (aacA-D) were detected by conventional PCR using specific primers (Table 1). The bacterial genome was extracted from the pure cultures using the High Pure PCR Template Preparation Kit (SinaClon, Iran) according to the manufacturer’s guidelines. The DNA concentration was assessed using a spectrophotometer. A number of strain collections from our laboratory were used as positive and negative controls for the genes in each PCR reaction. The PCR reactions were performed in a total volume of 25 μL. The PCR cycle was performed in a Thermal Cycler (Eppendorf, Germany) with an initial 5 min denaturation at 95 °C, followed by 30 amplification cycles (denaturation at 94 °C for 30 s, 30 s annealing at temperatures specified for each gene (Table 1) and elongation at 72 °C for 145 s) followed by a final extension step at 72 °C for 5 min.
Table 1.
Primers Used for PCR Amplification of Antibiotic Resistance Genes.17
| Gene Target | Sequence (5′→3′) | Amplicon Size (bp) | Annealing Temperature (ºC) |
|---|---|---|---|
| mecA-F | AAAATCGATGGTAAAGGTTGGC | 532 | 56 |
| mecA-R | AGTTCTGCAGTACCGGATTTGC | ||
| aacA-D -F | TAATCCAAGAGCAATAAGGGC | 227 | 56 |
| aacA-D -R | GCCACACTATCATAACCACTA | ||
| tetK-F | GTAGCGACAATAGGTAATAGT | 360 | 54 |
| tetK-R | GTAGTGACAATAAACCTCCTA | ||
| tetM-F | AGTGGAGCGATTACAGAA | 158 | 54 |
| tetM-R | CATATGTCCTGGCGTGTCTA | ||
| VatA-F | TGGTCCCGGAACAACATTTAT | 268 | 56 |
| VanA-R | TCCACCGACAATAGAATAGGG | ||
| VanB-F | GCTGCGAATTCAGTTGTTACA | 136 | 55 |
| VanB-R | CTGACCAATCCCACCATTTTA | ||
| VanC-F | AAGGCCCCAATCCAGAAGAA | 467 | 56 |
| VanC-R | TCAACGTTCTTTGTCACAACC |
Statistical Analysis
Statistical analysis was performed using the SPSS software (version 16, SPSS Inc., Chicago, IL, USA). For continuous variables, mean were calculated. Categorical variables were expressed as frequencies and percentages. The S. aureus load, pH and temperature in different sewage samples were compared using the independent-sample t-test. The relationship between antibiotic resistance and ARG of isolates in raw and treated sewage was analyzed using the chi-square or Pearson’s test. The p≤ 0.05 was considered significant.
Results
The mean bacterial load of raw and treated hospital sewage samples was 7.5×106 and 6.1×106, respectively (p=0.45). As shown in Table 2, there was no significant difference in pH and temperature between raw and treated sewage (p>0.05).
Table 2.
Values of pH, Temperature, and Bacterial Load in Raw and Treated Sewage Samples
| Parameter | Raw Sewage | Treated Sewage | p-value |
|---|---|---|---|
| Temperature, ºC (mean) | 21.8 | 19.6 | 0.002 |
| pH (mean) | 7.71 | 7.39 | 0.003 |
| Bacterial load, CFU/mL (mean) | 7.5×106 | 6.1×106 | 0.45 |
Frequency of MRSA Strains
The results showed that 11% and 8% of S. aureus isolates were MRSA in raw and treated hospital sewage on average, respectively. However, the difference was not statistically significant (p=0.411).
Antimicrobial Susceptibility
In order to determine the antibiotic resistance patterns, 20 MSSA (10 raw and 10 treated sewage) and 40 (20 raw and 20 treated sewage) MRSA isolates were evaluated.
The MSSA isolates (n=20) showed resistance to penicillin (100%), clindamycin (90%), azithromycin (85%), tetracycline (80%), amoxicillin/clavulanic acid (75%), gentamicin (70%), oxacillin (40%) and rifampin (20%). The frequency of MSSA isolates that were not susceptible to amoxicillin/clavulanic acid, azithromycin, gentamicin and rifampin was higher in treated sewage, but it was statistically significant only for rifampin (p=0.025). However, the frequency of MSSA isolates that were not susceptible to penicillin, tetracycline, oxacillin and clindamycin did not show marked changes after sewage treatment (Table 3).
Table 3.
Antimicrobial Susceptibility of MSSA and MRSA Isolates in Raw and Treated Sewage Samples
| Antibiotic | MSSA | P value | MRSA | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw sewage (n=10) | Treated Sewage (n=10) | Raw Sewage (n=20) | Treated Sewage (n=20) | |||||||
| S | N | S | N | S | N | S | N | |||
| Amoxicillin/Clavulanic acid | 3(30) | 7(70) | 2(20) | 8(80) | 0.606 | 4(20) | 16(80) | 4(20) | 16(80) | 1 |
| Azithromycin | 2(20) | 8(80) | 1(10) | 9(90) | 0.531 | 0 | 20(100) | 0 | 20(100) | – |
| Penicillin | 0 | 10(100) | 0 | 10(100) | – | 0 | 20(100) | 0 | 20(100) | – |
| Rifampin | 10(100) | 0 | 6(60) | 4(40) | 0.025 | 14(70) | 6(30) | 13(65) | 7(35) | 0.736 |
| Tetracycline | 2(20) | 8(80) | 2(20) | 8(80) | 1 | 8(40) | 12(60) | 14(70) | 6(30) | 0.057 |
| Gentamicin | 4(40) | 6(60) | 2(20) | 8(80) | 0.329 | 1(5) | 19(95) | 3(15) | 17(85) | 0.292 |
| Oxacillin | 6(60) | 4(40) | 6(60) | 4(40) | 1 | 0 | 20(100) | 0 | 20(100) | – |
| Clindamycin | 1(10) | 9(90) | 1(10) | 9(90) | 1 | 2(10) | 18(90) | 4(20) | 16(80) | 0.376 |
Note: Values have been expressed as number (%).
Abbreviations: N, non-susceptible; S, susceptible.
The MRSA isolates (n=40) showed resistance to penicillin (100%), oxacillin (100%), azithromycin (100%), gentamicin (90%), clindamycin (85%), amoxicillin/clavulanic acid (80%), tetracycline (45%) and rifampin (32.5%). For MRSA isolates, non-susceptibility to rifampin was higher after sewage treatment, non-susceptibility to amoxicillin/clavulanic acid, penicillin, azithromycin and oxacillin did not change following sewage treatment, and non-susceptibility to gentamicin, tetracycline and clindamycin was lower. The changes in the non-susceptibility of MRSA isolates to antibiotics were not statistically significant (p>0.05).
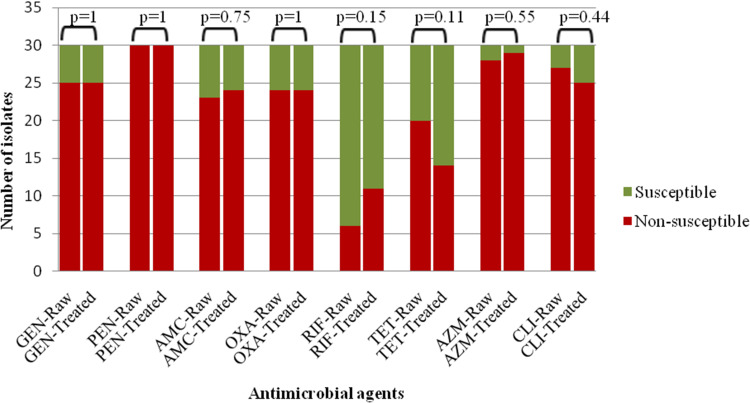
For the all S. aureus isolates, the highest rate of non-susceptibility was to penicillin (100% of raw and 100% of treated sewage isolates) followed by azithromycin (93% of raw and 96% of treated sewage isolates) and clindamycin (90% of raw and 83% of treated sewage isolates) (Figure 1).
Figure 1.
Comparison the antimicrobial susceptibility of S. aureus (MRSA and MSSA) isolates in raw (n=30) and treated (n=30) sewage samples.
Abbreviations: GEN, gentamicin; PEN, penicillin; AMC, amoxicillin/clavulanic acid; OXA, oxacillin; RIF, rifampicin; TET, tetracycline; AZM, azithromycin; CLI, clindamycin.
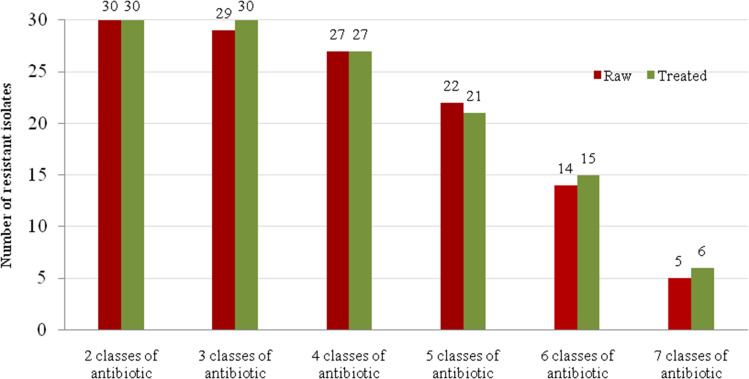
As shown in Figure 2, 11 (18.33%) isolates were resistant to all antibiotics tested, of which 5 (16.66%) and 6 (20%) were isolated from raw and treated hospital sewage, respectively. In addition, 98% (59/60) of the isolates were MDR (Figure 2).
Figure 2.
Frequency of multidrug-resistant S. aureus (MRSA and MSSA) isolates in raw (n=30) and treated (n=30) sewage samples.
Antibiotic Resistance Genes of Isolates
Table 4 presents the frequency of antibiotic resistance genes in MSSA and MRSA isolated from raw and treated sewage. Of the 40 MRSA isolates tested, the mecA gene was detected in 40 (100%), tetK in 28 (70%), tetM in 14 (35%), vanC in 8 (20%) and aacA-D gene in 37 (92.5%) isolates. None of these isolates was positive for vanB and vanA genes.
Table 4.
Antibiotic Resistance Genes of MSSA and MRSA Isolates in Raw and Treated Sewage Samples
| Resistant Genes | MSSA | MRSA | ||||
|---|---|---|---|---|---|---|
| Positive Number [n(%)] | Positive Number [n(%)] | |||||
| Raw Sewage (n= 10) | Treated Sewage (n=10) | p value | Raw Sewage (n= 20) | Treated Sewage (n=20) | p value | |
| mecA | 1(10) | 1(10) | 1 | 20(100) | 20(100) | 1 |
| vanA | 1(10) | 0 | 0.305 | 0 | 0 | – |
| vanB | 0 | 0 | – | 0 | 0 | – |
| vanC | 1(10) | 0 | 0.305 | 3(15) | 5(25) | 0.429 |
| aacA-D | 1(10) | 5(50) | 0.051 | 20(100) | 17(85) | 0.072 |
| tetK | 3(30) | 3(30) | 1 | 19(95) | 9(45) | 0.001 |
| tetM | 5(50) | 8(80) | 0.160 | 9(45) | 5(25) | 0.185 |
Regarding 20 MSSA isolates, mecA was detected in 2 (10%), tetK in 6 (30%), tetM in 13 (75%), vatA in 1 (5%), vatC in 6 (30%) and aacA-D gene in 6 (30%) isolates. None of isolates was positive for vatB gene. There was no significant difference in the frequency of antibiotic resistance genes between raw and treated samples (p>0.05).
Of the 60 MSSA and MRSA isolates examined, the most frequent ARGs were mecA (70% of raw and treated sewage isolates) followed by aacA-D (70% of raw and 73% of treated sewage isolates) and tetK (73% of raw and 40% of treated sewage isolates). There was no significant difference in the frequency of antibiotic resistance genes between raw and treated samples (p>0.05).
Discussion
Hospital sewage can be a dangerous source for spreading resistant bacterial strains including most pathogenic S. aureus. Research shows that the frequency of antibiotic resistant bacteria in the hospital wastewater could be an easy way to monitor the antibiotic resistance of the bacteria isolated from clinical specimens.18 It has been reported that the size of the hospitals could also contribute to the number of MRSA in the wastewater and wastewater treatment could reduce the number of MRSA isolates.13 Studies in Iran showed an increase in the prevalence of MRSA isolates among clinical specimens. For instance, 46.3%, 30.38%, and 25% of clinical isolates in Ardabil, Sanandaj, and Hamadan were MRSA.19–21 A study conducted in the USA found a decline in the MRSA isolates in treated wastewater.22 These data are consistent with the findings of the present study indicating a small decline in the MRSA frequency after hospital sewage treatment.
Rifampin, a semisynthetic antibiotic that inhibits DNA-dependent RNA polymerase, is used for treatment of S. aureus infections.23 In our study, the rate of isolates that were not susceptible to rifampin was low. It has been reported that S. aureus resistant to rifampin comprises 8–17% of the isolates in Iran.24 This low rate of resistance may be explained by the low prescription of this drug in Iran and regional distribution of resistant isolates.
The rate of non-susceptibility to penicillin (100%), clindamycin (90%), and azithromycin (85%) was higher in MSSA isolates (n=20). However, all MRSA isolates were non-susceptible to penicillin, oxacillin, and azithromycin, indicating higher resistance of MRSA to conventional antibiotics. In Iran, β-lactam and macrolides/lincosamides/streptogramins are the most frequently used antibiotic groups.25 The high prescription rate of these antibiotics can accelerate the development of resistance among bacterial population. In a study in South Africa in 2019, MRSA isolates from treated wastewater effluent and surface water showed a high frequency of resistance to oxacillin, ampicillin, and penicillin, which was consistent with our results.26
The genes that encode resistance to tetracyclines belong to four categories based on their resistance mechanisms (efflux genes, ribosomal protection genes, enzymatic and other genes).27 The ribosome protection (tetM) and efflux (tetK) genes were evaluated in our study. We found that the tetK gene was more prevalence than the tetM (56.6% vs 45%) gene in all MSSA and MRSA isolates (n=60). This is consistent with the results of a previous study that found that efflux genes were more common in the environmental isolates compared to the ribosomal protection genes.28 Moreover, in our study, only half of the isolates with tetracycline resistance genes showed a non-susceptible phenotype to tetracycline, which is similar to the findings of a previous study.27 These data suggest that some antibiotic resistance genes remain silent or inactive in the bacteria under certain circumstances. These silent ARGs may still be a threat since they can be expressed when they integrate into the appropriate genetic locations in the bacterial genome or mobile genetic elements.
In the present study, the aminoglycoside resistance gene,aacA-D, was found in 100% and 85% of MRSA isolates from raw and treated sewage, respectively. The low frequency of the aacA-D gene in the MRSA isolates of treated wastewater was previously reported.26 On the other hand, some authors found an increase in some antibiotic resistance genes after conventional wastewater treatment indicating that wastewater treatment may have different effects on various ARGs.29
Vancomycin is a glycopeptide usually used as the last choice for the treatment of MRSA infections. A systematic review showed that the overall prevalence of vancomycin intermediate S. aureus strains was 0.09% in Iran.30 We evaluated the presence of three genes (vanA, vanB and vanC) involved in resistance to vancomycin in S. aureus isolates. The overall frequency of these genes was low and none of the isolates harbored the vanB gene. This finding is supported with research results reporting lack of vanB in Staphylococcal isolates.31
The absolute majority of isolates in this study showed resistance to three different antibiotic classes and were designated as MDR as defined previously,32 which was similar to the results of a study investigating treated wastewater in South Africa in which 100% of the isolates were MDR.26 The present study found no significant difference in the frequency of MDR between raw and the treated sewage isolates. However, a study showed an increase in multidrug resistance after wastewater treatment.33 The treatment of infections caused by these MDR strains is challenging, particularly in vulnerable populations such as children, elderly subjects, and immunocompromised patients.
Conclusion
The findings of this study underlined an important public health concern regarding the high frequency of antibiotic resistant strains of S. aureus and related antibiotic resistance genes, not only in raw but also in treated hospital sewage. Given the fact that most of the antibiotic resistance genes are located on the mobile genetic elements in S. aureus, it is reasonable to assume that these genes can transfer to other pathogenic bacteria in the wastewater environment. It seems that hospital wastewater treatment should be improved to prevent dissemination of potential antibiotic resistant strains and their related genes.
Acknowledgments
We extend our thanks to clinical research development center of Imam Reza Hospital affiliated to Kermanshah University of Medical Sciences for their kind support. This study received financial support from Kermanshah University of Medical Sciences, Iran (Grant Number. 96339).
Data Sharing Statement
The data sets used and/or analyzed during this study are available from the corresponding author on reasonable request and were received permission for use by the Kermanshah University of Medical Sciences Ethics Committee.
Ethics Approval and Consent to Participate
The protocol was approved by the Kermanshah University of Medical Sciences Ethics Committee (IR.KUMS.REC.1396.338).
Disclosure
The authors declared no conflicts of interest in this work.
References
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. doi: 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haaber J, Penadés JR, Ingmer H. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol. 2017;25(11):893–905. doi: 10.1016/j.tim.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 3.Goudarzi M, Goudarzi H, Sá Figueiredo AM, et al. Molecular characterization of methicillin resistant Staphylococcus aureus strains isolated from intensive care units in Iran: ST22-SCCmec IV/t790 emerges as the major clone. PLoS One. 2016;11(5):e0155529. doi: 10.1371/journal.pone.0155529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ubukata K, Nonoguchi R, Matsuhashi M, Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989;171(5):2882–2885. doi: 10.1128/JB.171.5.2882-2885.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K, Bradford PA. β-lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med. 2016;6(8):a025247. doi: 10.1101/cshperspect.a025247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkner S, Konradi S, Schönfeld J. Antibiotic resistance and the environment—there and back again. EMBO Rep. 2014;15(7):740–744. doi: 10.15252/embr.201438978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amirsoleimani A, Brion GM, Diene SM, François P, Richard EM. Prevalence and characterization of Staphylococcus aureus in wastewater treatment plants by whole genomic sequencing. Water Res. 2019;158:193–202. doi: 10.1016/j.watres.2019.04.035 [DOI] [PubMed] [Google Scholar]
- 8.Bisseux M, Colombet J, Mirand A, et al. Surveillance of hepatitis A virus in urban sewages and comparison with cases notified in the course of an outbreak, Italy 2013. BMC Infect Dis. 2014;14(1):419. doi: 10.1186/1471-2334-14-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kümmerer K. Antibiotics in the aquatic environment–a review–part I. Chemosphere. 2009;75(4):417–434. doi: 10.1016/j.chemosphere.2008.11.086 [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Zhang G, Liu Y, et al. Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam. Chemosphere. 2008;72(6):968–973. doi: 10.1016/j.chemosphere.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Xu Y, Wang H, et al. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere. 2015;119:1379–1385. doi: 10.1016/j.chemosphere.2014.02.040 [DOI] [PubMed] [Google Scholar]
- 12.Abbasian H, Hajimolaali M, Yektadoost A, Zartab S. Antibiotic utilization in Iran 2000–2016: pattern analysis and benchmarking with organization for economic co-operation and development countries. J Res Pharm Pract. 2019;8(3):162–167. doi: 10.4103/jrpp.JRPP_19_42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boopathy R. Presence of methicillin resistant Staphylococcus aureus (MRSA) in sewage treatment plant. Bioresour Technol. 2017;240:144–148. doi: 10.1016/j.biortech.2017.02.093 [DOI] [PubMed] [Google Scholar]
- 14.Thompson J, Gündoğdu A, Stratton H, Katouli M. Antibiotic resistant Staphylococcus aureus in hospital wastewaters and sewage treatment plants with special reference to methicillin‐resistant Staphylococcus aureus (MRSA). J Appl Microbiol. 2013;114(1):44–54. doi: 10.1111/jam.12037 [DOI] [PubMed] [Google Scholar]
- 15.Gómez P, Lozano C, Benito D, et al. Characterization of staphylococci in urban wastewater treatment plants in Spain, with detection of methicillin resistant Staphylococcus aureus ST398. Environ Pollut. 2016;212:71–76. doi: 10.1016/j.envpol.2016.01.038 [DOI] [PubMed] [Google Scholar]
- 16.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 17.Chabi R, Momtaz H. Virulence factors and antibiotic resistance properties of the Staphylococcus epidermidis strains isolated from hospital infections in Ahvaz, Iran. Trop Med Health. 2019;47(1):56. doi: 10.1186/s41182-019-0180-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak Y-K, Colque P, Byfors S, Giske CG, Möllby R, Kühn I. Surveillance of antimicrobial resistance among Escherichia coli in wastewater in Stockholm during 1 year: does it reflect the resistance trends in the society? Int J Antimicrob Agents. 2015;45(1):25–32. doi: 10.1016/j.ijantimicag.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 19.Ahmadi E, Khojasteh M, Mortazavi SM, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus nasal carriage in the West of Iran: a population-based cross-sectional study. BMC Infect Dis. 2019;19(1):899. doi: 10.1186/s12879-019-4567-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dibah S, Arzanlou M, Jannati E, Shapouri R. Prevalence and antimicrobial resistance pattern of methicillin resistant Staphylococcus aureus (MRSA) strains isolated from clinical specimens in Ardabil, Iran. Iran J Microbiol. 2014;6(3):163. [PMC free article] [PubMed] [Google Scholar]
- 21.Effatpanah H, Mohammadi MJ, Safari N, et al. Determine antibiotic resistance model and identify methicillin-resistant Staphylococcus aureus (MRSA) in clinical isolates. Fresenius Environ Bull. 2018;27(1):622–626 [Google Scholar]
- 22.Rosenberg Goldstein RE, Micallef SA, Gibbs SG, et al. Methicillin-resistant Staphylococcus aureus (MRSA) detected at four US wastewater treatment plants. Environ Health Perspect. 2012;120(11):1551–1558. doi: 10.1289/ehp.1205436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrest GN, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev. 2010;23(1):14–34. doi: 10.1128/CMR.00034-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasani A, Sheikhalizadeh V, Hasani A, Naghili B, Valizadeh V, Nikoonijad AR. Methicillin resistant and susceptible Staphylococcus aureus: appraising therapeutic approaches in the Northwest of Iran. Iran J Microbiol. 2013;5(1):56–62. [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO report on surveillance of antibiotic consumption: 2016–2018 early implementation; 2018. Available from: https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf. Accessed July21, 2020.
- 26.Ramessar K, Olaniran AO. Antibiogram and molecular characterization of methicillin-resistant Staphylococcus aureus recovered from treated wastewater effluent and receiving surface water in Durban, South Africa. World J Microbiol Biotechnol. 2019;35(9):142. doi: 10.1007/s11274-019-2715-9 [DOI] [PubMed] [Google Scholar]
- 27.Roberts MC. Update on acquired tetracycline resistance genes. FEMS Microbiol Lett. 2005;245(2):195–203. doi: 10.1016/j.femsle.2005.02.034 [DOI] [PubMed] [Google Scholar]
- 28.Börjesson S, Mattsson A, Lindgren P-E. Genes encoding tetracycline resistance in a full-scale municipal wastewater treatment plant investigated during one year. J Water Health. 2010;8(2):247–256. doi: 10.2166/wh.2009.159 [DOI] [PubMed] [Google Scholar]
- 29.Pazda M, Kumirska J, Stepnowski P, Mulkiewicz E. Antibiotic resistance genes identified in wastewater treatment plant systems–A review. Sci Total Environ. 2019;697:134023. doi: 10.1016/j.scitotenv.2019.134023 [DOI] [PubMed] [Google Scholar]
- 30.Baseri N, Najar-Peerayeh S, Amiri FB. Prevalence of vancomycin-intermediate Staphylococcus aureus among clinical isolates in Iran: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2018;15:178–187. doi: 10.1016/j.jgar.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 31.Al-Amery K, Elhariri M, Elsayed A, et al. Vancomycin-resistant Staphylococcus aureus isolated from camel meat and slaughterhouse workers in Egypt. Antimicrob Resist Infect Control. 2019;8(1):129. doi: 10.1186/s13756-019-0585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55(12):1619–1629. doi: 10.1099/jmm.0.46747-0 [DOI] [PubMed] [Google Scholar]
- 33.Alouache S, Estepa V, Messai Y, Ruiz E, Torres C, Bakour R. Characterization of ESBLs and associated quinolone resistance in Escherichia coli and Klebsiella pneumoniae isolates from an urban wastewater treatment plant in Algeria. Microb Drug Resist. 2014;20(1):30–38. doi: 10.1089/mdr.2012.0264 [DOI] [PubMed] [Google Scholar]