Abstract
OBJECTIVE:
Opioid use disorder (OUD) is associated with chronic pain. We investigated the association between medication treatments for OUD and pain in a post-hoc secondary analysis of a randomized trial of methadone versus buprenorphine/naloxone.
METHODS:
1,241 individuals with OUD participated in an open label, pragmatic randomized trial of methadone versus buprenorphine/naloxone in nine treatment programs licensed to dispense agonist medication for OUD between 2006 to 2009. In this post-hoc analysis, pain was dichotomized (present or not present) using responses from the Short Form-36. Logistic regression models were fit to test the effect of (1) having baseline pain on week 24 retention, (2) treatment assignment on improvement in pain among those reporting pain at baseline, and (3) pain improvement at week 4 on week 24 retention among those reporting pain at baseline.
RESULTS:
Almost half (48.2%) of the sample reported pain at baseline. Participants with baseline pain did not significantly differ in week 24 retention compared to those without baseline pain. Among those reporting pain at baseline, there was no significant difference between treatment arms in improvement of pain at week 4, but improvement in pain at week 4 was associated with significantly greater odds of being retained at week 24 (OR [95% CI] = 1.76 [1.10, 2.82], p = .020).
CONCLUSION AND RELEVANCE:
In this large multisite randomized trial of medication treatments for OUD, nearly half of the participants reported pain at baseline, and improvement in pain early in treatment was associated with increased likelihood of retention in treatment.
Keywords: Buprenorphine, pain, opioid use disorder
Introduction
Comorbid pain and opioid use disorder (OUD) are common; about 1 in 3 individuals requiring chronic opioids for pain also meet criteria for OUD (Boscarino et al., 2011). However, few studies of people with OUD consider pain as a moderator of treatment success.
Of the trials that have been conducted, there are mixed findings with regards to the association between pain and OUD outcomes. Trials of comorbid pain and OUD show that individuals with persistent pain after detoxification are at increased risk of continued opioid use when compared to those without persistent pain (Larson et al., 2007; Potter et al., 2010). Several trials show that addressing chronic pain improves cravings and other OUD outcomes (Garland et al., 2014; Ilgen et al., 2011), others found no evidence that pain is associated with differential retention or illicit drug use outcomes (Barry et al. 2009; Weiss et al. 2011). Considering the potential for pain to result in poorer OUD outcomes, additional research is needed to understand the relationship and improve treatment.
FDA approved agonist treatments for OUD (i.e., methadone and buprenorphine) are also approved for the treatment of pain. Preliminary evidence suggests that buprenorphine and methadone share similar efficacy in treating pain among individuals with OUD (Neumann et al, 2013). However, buprenorphine/naloxone is a partial opioid agonist and has a ceiling effect on the opioid receptor at escalating doses, when compared with methadone (Walsh et al., 1994). For this reason, buprenorphine/naloxone is often assumed to be less efficacious than methadone at alleviating chronic pain in clinical practice (Bruce et al., 2017).
Thus, we undertook a secondary analysis of a large (N=1,241) randomized controlled trial of buprenorphine/naloxone versus methadone on the primary outcome of liver health among men and women with OUD (Saxon et al., 2013). This was a pragmatic trial in that it studied an intervention under real-world conditions to maximize external validity using a simple design, large sample size, and across diverse settings (Patsopoulos, 2011). Using this dataset, we considered three questions with regards to pain and opioid use disorder treatment: First, among all participants, does the presence of pain at baseline moderate the effect of medication assignment on retention? Second, is methadone, a full opioid agonist, more effective at decreasing pain compared to buprenorphine, a partial agonist? We hypothesized that among individuals reporting pain at baseline, the odds of reporting decreased pain after four weeks of treatment would be greater in the methadone versus buprenorphine/naloxone treatment arm. Third, among those with pain at the beginning of the trial, is improvement in pain at week 4 associated with treatment retention? We hypothesized that among those with pain, improvement in pain in the first 4 weeks of the trial would be associated with greater retention at the end of treatment (week 24).
Methods
Study Design
Data for this secondary analysis were drawn from a National Institute on Drug Abuse Clinical Trials Network randomized study of 24 weeks of open label buprenorphine/naloxone or methadone among individuals with OUD receiving treatment at nine outpatient addiction treatment programs. These programs were licensed to dispense methadone as a treatment for opioid use disorder from geographically distant cities throughout the United States. They were predominantly urban, private, non-profit clinics, serving a lower socioeconomic population. Clinics dispensed medications daily with observed dosing except for Sundays and holidays or when take home medications were permitted by local regulations.
A full description of the inclusion and exclusion criteria and study design has been published previously (Saxon et al., 2013). Briefly, eligible individuals were ≥18 years of age and met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition- Text Revision (DSM-IV-TR) criteria for opioid dependence. Individuals with medical, psychiatric, or substance use conditions that would make participation hazardous were excluded. Pain conditions were not considered exclusionary.
Potential participants signed a consent form and completed a baseline assessment, including the self-report survey, the Short Form 36 (SF-36), at the baseline study visit. Patients were asked to return to the clinic within the next few days in opioid withdrawal to prepare for buprenorphine induction. Participants were randomized to maintenance with open label buprenorphine/naloxone (up to 32 mg/day) or methadone (no predefined upper dose limit). Individual dosing schedules were determined based on weekly clinical evaluation; opioid withdrawal symptoms, continued cravings, or illicit opioid use were indications for dose increases. Participants remained on study medication for the 24 weeks and were then tapered off medication or referred for ongoing clinical treatment. The study received approval from the institutional review boards at all participating sites, and all participants provided written informed consent. Also, oversight was provided by the NIDA Clinical Trials Network Data Safety and Monitoring Board.
The initial randomization scheme was 1:1 but was switched midway through the trial to 2:1 (buprenorphine/naloxone:methadone) because of the need to have a minimum of 300 participants in each arm complete 24 weeks of treatment to adequately assess hepatic health outcomes. A total of 1,269 participants were randomized (Buprenorphine = 740, Methadone=529). The primary outcome paper demonstrated no difference in liver health by medication assignment, but that individuals receiving methadone were significantly more likely to remain in treatment (Saxon et al., 2013). For the present study aim 1, 28 participants missing baseline pain information were excluded from analyses leaving 1,241 subjects (Buprenorphine=723, Methadone=518).
Measurements and Outcome
Pain was assessed using a single item from the SF-36, a self-report instrument of health status (Ware and Sherbourne,1992), which was administered at baseline and weeks 4, 12, and 24. Pain at baseline was assessed at consent, before individuals were randomized, and therefore had not been instructed to begin detoxification and induction procedures. The item asked participants to rate bodily pain over the previous 4 weeks on a six-point Likert scale ranging from no pain to very severe pain. Participant responses were dichotomized as no or mild pain (no, very mild, or mild pain) or moderate to severe pain (moderate, severe, or very severe pain). This method of operationalizing the item was chosen based on previous work demonstrating predictive clinical value using the item as a dichotomized outcome (Caldeiro et al., 2008), as well as because of the lack of evidence for the validity of this measure as a continuous outcome. Pain improvement at week 4, among those with baseline pain, was also dichotomous: improvement (responding: no, very mild, or mild pain), or no improvement (responding moderate, severe, or very severe pain). We refer to the two pain categories as “no pain” and “pain” for the remainder of the document for convenience and ease of reading, but it should be noted that the “no pain” category also included individuals with very mild or mild pain.
Treatment retention at 24 weeks was dichotomous (retained in the trial or not retained in the trial) defined in the same way as the parent trial. Treatment retention at week 4 was defined similarly.
Data Analysis
Among all participants, a logistic regression model was fit to assess the association of baseline pain and treatment on retention at week 24 (aim 1). The model included the effect of baseline pain (pain versus no pain), treatment (methadone versus buprenorphine), and their 2-way interaction. If the 2-way interaction was not significant, it was omitted from the model and only the main effects were assessed.
Among all participants with pain at baseline (N=598), a second logistic model was fit to assess the effect of treatment on pain improvement at week 4 (aim 2). This model included the main effect of treatment (methadone versus buprenorphine). A third logistic model was fit to test if pain improvement at week 4 was associated with treatment retention at week 24 (aim 3). This model included the effect of pain improvement at week 4, treatment and their 2-way interaction. If the 2-way interaction was not significant, it was omitted from the model and only the main effects were assessed.
All models controlled for a random effect for site and adjusted for gender (male vs female), age (as continuous), Hispanic race (Hispanic vs non-Hispanic) and highest maximum medication dose (high vs low) (for methadone >60 mg per day and for buprenorphine >16 mg per day). These covariates were selected based on previous research demonstrating associations with retention in treatment (Hser et al., 2014).
In order to consider the possibility of withdrawal pain influencing patient’s responses to the SF-36 pain question we also performed an ANOVA analysis of the association of withdrawal severity at baseline on the clinical opioid withdrawal scale with pain status collected at the same time point. No other data was available on withdrawal severity throughout the trial.
All logistic model estimates are interpreted as log odds ratios and are exponentiated to obtain odds ratio (OR). All analyses were done using PROC GLIMMIX in SAS version 9.4, and all statistical tests were two-sided with a significance level of 5%.
Results
Sample Characteristics
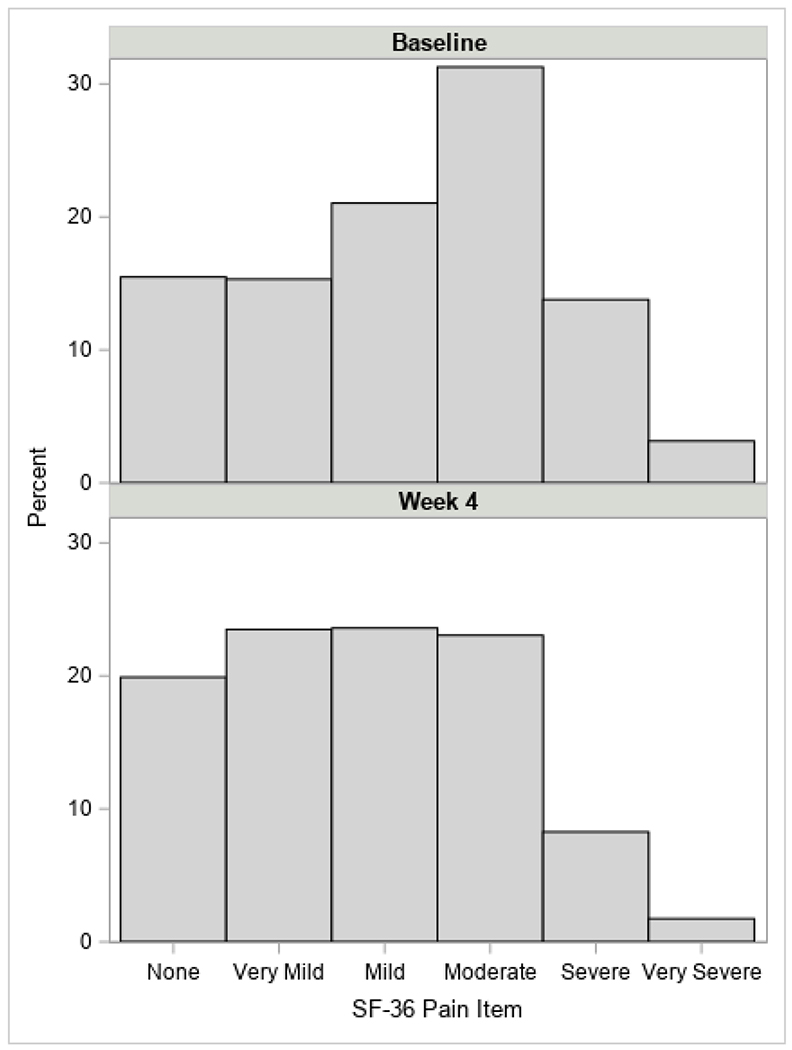
Figure 1 presents the distribution of the Likert-scale SF-36 pain item at baseline and week 4. Of the 1,241 participants enrolled, 598 (48.2%) reported experiencing pain at baseline. Table 1 presents demographic characteristics and retention rates for participants with and without pain at baseline by treatment assignment. Participants were on average 37 years old; age did not differ significantly between the pain and no pain group. Females were significantly more likely to report pain at baseline than males (52.9% vs 46.0%, p = .023).
Figure 1.
Distribution of Pain Scores at Baseline and Week 4
Table 1.
Baseline and week 24 measures by treatment (Buprenorphine=723, Methadone=518)* and baseline pain (total n=1,241)
| Pain at Baseline | Mild or No Pain at Baseline | |||||||
|---|---|---|---|---|---|---|---|---|
| Buprenorphine (N=359) | Methadone (N=239) | Buprenorphine (N=364) | Methadone (N=279) | |||||
| N | % or M (SD) | N | % or M (SD) | N | % or M (SD) | N | % or M (SD) | |
| Gender | ||||||||
| Female | 133 | 37.0 | 76 | 31.8 | 97 | 26.6 | 89 | 31.9 |
| Male | 226 | 63.0 | 163 | 68.2 | 267 | 73.4 | 190 | 68.1 |
| Age (years) | 359 | 37.8 (11.3) | 239 | 37.6 (11.0) | 364 | 37.0 (11.2) | 279 | 36.9 (10.7) |
| Hispanic | 60 | 16.7 | 35 | 14.6 | 62 | 17.0 | 46 | 16.5 |
| Race | ||||||||
| White (non-Hispanic) | 234 | 65.2 | 168 | 70.3 | 245 | 67.3 | 192 | 68.8 |
| Black (non-Hispanic) | 26 | 7.2 | 18 | 7.5 | 35 | 9.6 | 26 | 9.3 |
| Hispanic | 60 | 16.7 | 35 | 14.6 | 62 | 17.0 | 46 | 16.5 |
| Other/Unknown | 39 | 10.9 | 18 | 7.5 | 22 | 6.0 | 15 | 5.4 |
| Baseline clinical opioid withdrawal scale (COWS) | 359 | 13.1 (4.3) | 239 | 12.7 (4.7) | 364 | 12.4 (4.3) | 278 | 12.2 (4.1) |
| High Maximum Dose (Methadone: >60, Buprenorphine: >16) | 238 | 66.3 | 189 | 79.1 | 219 | 60.2 | 207 | 74.2 |
| Any positive urine (Visits 0-24) for amphetamine/opiate/cocaine/cannabis (%yes) | 146 | 40.7 | 146 | 61.1 | 173 | 47.5 | 154 | 55.2 |
| Baseline Pain (Mean) | 359 | 4.4 (0.6) | 239 | 4.4 (0.6) | 364 | 2.1 (0.8) | 279 | 2.2 (0.8) |
| Week 4 Pain (% yes) | 122 | 50.6 | 102 | 50.2 | 44 | 17.9 | 36 | 15.7 |
| Week 24 Pain (% yes) | 120 | 55.3 | 94 | 50.5 | 42 | 20.1 | 35 | 17.9 |
| Retained at Week 4 (% retained) | 283 | 78.8 | 231 | 96.7 | 300 | 82.4 | 255 | 91.4 |
| Retained at Week 24 (% retained) | 173 | 48.2 | 183 | 76.6 | 164 | 45.1 | 201 | 72.0 |
Randomization scheme was switched from 1:1 to 2:1 midway through the trial in order to meet the minimum number of participants per condition retained at 24 week causing unequal group sizes (buprenorphine/naloxone:methadone)
Patients with pain did not differ significantly from those without pain with regards to withdrawal scores rated on the clinical opioid withdrawal scale (ANOVA across groups: F3, 1236 = 2.43, p = .064), nor were these differences clinically relevant since differences between groups were on average less than one unit. The average maximum dose of buprenorphine in the study was 22.2 mg (SD = 8.1) while the average maximum dose of methadone was 93.5 (SD = 42.3). The median dose of buprenorphine was 24 mg (range = [2-32]; IQR = [16-32]) and the median dose of methadone was 90 (range = [5-397]; IQR = [70-110]). Dosing data did not include the 2 individuals whose medication was switched from buprenorphine to methadone during the trial due to pregnancy. For all other analysis these individuals were included in the buprenorphine arm in an intention-to-treat manner.
Of the 518 participants randomized to methadone, 74.1% were retained at week 24 (76.6% within those who reported baseline pain and 72.0% within those who do not report baseline pain). Of the 723 participants randomized to buprenorphine, 46.6% were retained at week 24 (48.2% within those who reported baseline pain and 45.1% within those who reported no baseline pain).
Aim 1: Baseline Pain on Retention at Week 24
The 2-way interaction between treatment assignment and baseline pain was not significant suggesting that baseline pain did not significantly moderate the relationship between treatment assignment and retention at week 24 (F1,1225 = 0.18, p = .668). Results for main effects model are presented in Table 2. Participants with pain at baseline did not have significantly different odds of being retained at week 24 compared to participants with no pain at baseline (p = .398). Participants randomized to methadone had about 3 times the odds of being retained at week 24 compared to those randomized to buprenorphine (OR [95% CI] = 3.17 [2.44, 4.11], p < .001). Older age, non-Hispanic ethnicity, and higher maximum dose were significantly associated with greater odds of being retained at week 24 among all randomized participants (all p < .05). Gender was not significantly associated with odds of retention at week 24 (p = .999).
Table 2.
Model estimated main effects from logistic regression models of aim 1
| Among all participants (n=1241) | |||||
|---|---|---|---|---|---|
| Aim 1 Outcome: Retained at Week 24 | |||||
| Effect | b | SE | p | OR | (95% CI) |
| Covariates: | |||||
| Age | 0.031 | 0.006 | <.001 | 1.032 | (1.019, 1.044) |
| Gender (ref=Female) | 0.000 | 0.137 | 0.999 | 1.000 | (0.764, 1.309) |
| Hispanic (ref=No) | −0.353 | 0.176 | 0.044 | 0.702 | (0.498, 0.991) |
| Maximum Dose (ref=Low) | 1.383 | 0.141 | <.001 | 3.988 | (3.023, 5.263) |
| Predictors: | |||||
| Treatment (ref=Buprenorphine) | 1.152 | 0.133 | <.001 | 3.166 | (2.438, 4.111) |
| Baseline Pain (ref=No Pain) | 0.109 | 0.128 | 0.398 | 1.115 | (0.866, 1.434) |
| Pain Improvement at week 4 (ref=No) | -- | -- | -- | -- | -- |
-- Refers to variables not relevant to the model
Aim 2: Treatment Assignment on Pain Improvement at Week 4
Among the 598 participants with pain at baseline, 514 (86.0%) participants were retained at week 4, 283 (78.8%) in the buprenorphine group and 231 (96.7%) in the methadone group. 444 of the individuals retained also completed the short form assessment form at that time point and were included in the analysis. Model results assessing treatment assignment on pain improvement at week 4 among those with baseline pain are presented in Table 3. There was no significant difference in the odds of pain improvement at week 4 between those randomized to methadone compared to those randomized to buprenorphine/naloxone (p = .862). Younger age and higher maximum dose were significantly associated with greater odds of pain improvement at week 4 (all p < .05). For each additional year of participant age, the odds of reporting no pain at four weeks decreased by 4% (OR [95% CI] = 0.96 [0.94, 0.98], p<.001). Participants with higher maximum dose had about half the odds of no pain at four weeks compared to those with lower maximum doses (OR [95% CI] =0.47 [0.29, 0.78], p=.003). Gender and Hispanic ethnicity were not significantly associated with pain improvement (p=.731 and p=.685, respectively).
Table 3.
Model estimated main effects from logistic regression models of aims 2 and 3
| Among participants with BL pain (n=444)* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aim 2 Pain Improved at Week 4 | Aim 3 Retained at Week 24 | |||||||||
| Effect | b | SE | p | OR | (95% CI) | b | SE | p | OR | (95% CI) |
| Covariates: | ||||||||||
| Age | −0.041 | 0.009 | <.001 | 0.959 | (0.943, 0.976) | 0.048 | 0.011 | <.001 | 1.049 | (1.026, 1.072) |
| Gender (ref=Female) | 0.072 | 0.211 | 0.731 | 1.075 | (0.710, 1.627) | −0.098 | 0.249 | 0.694 | 0.907 | (0.556, 1.478) |
| Hispanic (ref=No) | −0.108 | 0.266 | 0.685 | 0.898 | (0.533, 1.513) | 0.040 | 0.318 | 0.899 | 1.041 | (0.558, 1.944) |
| Maximum Dose (ref=Low) | −0.748 | 0.252 | 0.003 | 0.473 | (0.288, 0.777) | 0.307 | 0.284 | 0.280 | 1.359 | (0.779, 2.373) |
| Predictors: | ||||||||||
| Treatment (ref=Buprenorphine) | 0.034 | 0.198 | 0.862 | 1.035 | (0.701, 1.527) | 1.021 | 0.243 | <.001 | 2.777 | (1.721, 4.480) |
| Baseline Pain (ref=No Pain) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Pain Improvement at week 4 (ref=No) | -- | -- | -- | -- | -- | 0.564 | 0.241 | 0.020 | 1.758 | (1.095, 2.823) |
Sample Includes participants with pain at baseline who were retained at week 4 and have a non-missing week 4 pain score
-- Refers to variables not relevant to the model
Aim 3: Association Between Improvement in Pain at Week 4 and Retention at Week 24
As noted above, among the 598 patients who reported pain at baseline 444 of the individuals retained also completed the short form assessment form at that time point and were included in the analysis. The 2-way interaction between treatment and week 4 pain improvement was not significant, suggesting that the effect of week 4 pain improvement on week 24 retention did not significantly differ by treatment assignment (F1, 423 = 2.16, p=.143). Results for the main effects model are presented in Table 3. Among participants with pain at baseline, those with improved pain at week 4 had 1.76 times the odds of being retained at week 24 compared to those whose pain was not improved at week 4 (OR [95% CI] =1.76 [1.10, 2.82], p=.026). Additionally, participants randomized to methadone had 2.78 times the odds of being retained compared to those randomized to buprenorphine (OR [95% CI] = 2.78 [1.72, 4.48], p < .001). Among participants with pain at baseline, older age was significantly associated with greater odds of being retained at week 24 (OR [95% CI] = 1.05 [1.03, 1.07], p < .001), while gender, Hispanic ethnicity, and maximum dose were not significantly associated with retention at week 24 (all p > .05).
Discussion
This was a secondary analysis testing several hypotheses of the association between pain, medication treatment assignment, and retention in a randomized controlled trial of buprenorphine/naloxone versus methadone for OUD. Based on prior research, we hypothesized that baseline pain would lead to decreased retention, however, the analysis failed to show evidence of an association between baseline pain and retention in this trial. We also failed to confirm our hypothesis that individuals assigned to the methadone arm would experience lower levels of pain. We did find a positive association between improvement in pain and retention, as hypothesized.
The finding that baseline pain was not associated with differences in retention was consistent with secondary analysis of another large multisite trial of treatment-seeking individuals with OUD (Weiss et al., 2011). People with OUD and pain may be more motivated to continue in treatment in the hopes of both addressing their substance use disorder and pain.
Although no difference was found in the number of individuals who reported improvement in pain at week 4 by medication arm, caution should be taken in drawing conclusions. There was significant differential drop-out between the two treatment arms, with higher drop-out among individuals assigned to the buprenorphine arm. It is possible that individuals assigned to buprenorphine were more likely to experience continued pain compared to the methadone group and that this contributed to lower retention in the study. The dosing schedule used in the trial was once daily for both methadone and buprenorphine. This is the commonly used schedule for opioid maintenance treatment, but these medications may be more effective for treating pain when given in divided doses three or more times a day. This may have reduced effectiveness at treating pain and obscured effects on retention or differences between medications. Results are however consistent with recent findings that full opioid agonist medications do not provide superior outcomes in managing pain compared to partial agonists (Neumann et al., 2011) or other non-opioid approaches to pain management (Krebs et al., 2018; Chang et al., 2017). One reason for this may be that full opioid agonists like methadone induce tolerance and diminish analgesic effectiveness and may produce hyperalgesia in which chronic exposure actually increases sensitivity to pain (Brush 2012).
The finding that individuals with continued pain were significantly less likely to remain in treatment has important clinical implications and should be confirmed with further prospective studies that consider the mechanism of this relationship. Regardless, this finding coupled with the finding that half of the individuals included in the analysis continued to have moderate to severe pain highlights the need for screening, follow up, and expanded use of alternative interventions for pain in the OUD population. Current treatment infrastructure for OUD generally does not include resources to address pain early in treatment and this issue is difficult to address in the context of acute substance, psychiatric, psychosocial, and medical problems. The treatment and research community should explore feasible approaches to screen for and address pain in the first 4 weeks of treatment in light of the finding that early reduction in pain is predictive of long-term retention. Interventions may include psychosocial interventions and non-opioid medications or procedures. Psychosocial interventions like mindfulness based and cognitive behavioral psychosocial interventions for individuals with opioid use disorders and pain have shown promise in early trials (Garland et al., 2014; Zgierska et al., 2016; Guarino et al., 2018).
Several further limitations should also be considered. Differential drop out between the methadone and buprenorphine arms of the trial demand caution when interpreting results. The single pain item from the Short Form-36 has not been validated as an independent measure of clinically significant pain and is a self-reported subjective measure. In addition, the question did not differentiate between chronic, acute, or withdrawal pain. As individuals may have been experiencing significant withdrawal early in treatment this may have confounded our findings, although as noted above withdrawal scores did not significantly correlated with pain. In addition, the trials recruited a specific population of individuals with opioid use disorder, those seeking treatment at clinics dispensing opioids for agonist treatment (methadone clinics), and therefore lacks generalizability to all patients seeking treatment for opioid use disorders. Although these included clinics from a broad range of clinics throughout the country, this population likely does not represent other treatment seeking populations, for example those seeking treatment at outpatient primary care programs where many patients are treatment for opioid use disorder with buprenorphine.
Conclusion
Despite limitations, this study highlights the high prevalence of baseline pain in individuals with OUD and the significant portion of those individuals who continue to report pain despite treatment with agonist medication. Further research is needed to confirm the findings of this study, including the association between pain and retention and pain outcomes in individuals on medications for OUD. Future studies should be designed and powered to consider interventions addressing pain and OUD using comprehensive pain assessment, including pain-related disability.
Acknowledgments
Grant Funding: The study in this presentation was funded by the NIDA grant U10 DA013035 (PI: Nunes). Dr Shulman was funded by the NIDA grant T32 DA007294.
Footnotes
Disclosures/ Conflict of Interest: Dr. Nunes has received medication for research studies from Alkermes/Cephalon, Duramed Pharmaceuticals, and Reckitt-Benckiser. The remaining authors have no conflicts of interest to report.
References
- Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J Clin Psychiatry. 2009. September;70(9):1213–8. doi: 10.4088/JCP.08m04367. Epub 2009 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA, Rukstalis MR, Hoffman SN et al. Prevalence of prescription opioid-use disorder among chronic pain patients: comparison of the DSM-5 vs. DSM-4 diagnostic criteria. J Addict Dis. 2011. Jul-Sep;30(3):185–94. doi: 10.1080/10550887.2011.581961. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Merlin J, Lum PJ, et al. 2017 HIV Medicine Association of Infectious Diseases Society of America Clinical Practice Guideline for the Management of Chronic Pain in Patients Living With Human Immunodeficiency Virus. Clin Infect Dis. 2017. October 30;65(10):1601–1606. doi: 10.1093/cid/cix848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush DE. Complications of long-term opioid therapy for management of chronic pain: the paradox of opioid-induced hyperalgesia. J Med Toxicol. 2012. December;8(4):387–92. doi: 10.1007/s13181-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, Saxon AJ. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction. 2008. December;103(12):1996–2005. doi: 10.1111/j.1360-0443.2008.02358.x. Epub 2008 Oct 8. [DOI] [PubMed] [Google Scholar]
- Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a Single Dose of Oral Opioid and Nonopioid Analgesics on Acute Extremity Pain in the Emergency Department: A Randomized Clinical Trial. JAMA. 2017;318(17):1661–1667. doi: 10.1001/jama.2017.16190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014. June;82(3):448–459. doi: 10.1037/a0035798. Epub 2014 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino H, Fong C, Marsch LA, et al. Web-Based Cognitive Behavior Therapy for Chronic Pain Patients with Aberrant Drug-Related Behavior: Outcomes from a Randomized Controlled Trial. Pain Med. 2018. December 1;19(12):2423–2437. doi: 10.1093/pm/pnx334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. 2014. Addiction, 109(1), pp.79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen MA, Haas E, Czyz E, Webster L, Sorrell JT, Chermack S. 2011. Treating chronic pain in veterans presenting to an addictions treatment program. Cognitive and Behavioral Practice, 18(1), pp.149–160. [Google Scholar]
- Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, Kroenke K, Bair MJ, Noorbaloochi S. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain The SPACE Randomized Clinical Trial. JAMA. 2018;319(9):872–882. doi: 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction, 2007102(5), pp.752–760. [DOI] [PubMed] [Google Scholar]
- Neumann AM, Blondell RD, Jaanimägi U, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. J Addict Dis. 2013;32(1):68–78. doi: 10.1080/10550887.2012.759872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13(2):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Chakrabarti A, Domier CP, Hillhouse MP, Weiss RD, Ling W. Pain and continued opioid use in individuals receiving buprenorphine-naloxone for opioid detoxification: secondary analyses from the Clinical Trials Network. J Subst Abuse Treat. 2010;38 Suppl 1(Suppl 1):S80–S86. doi: 10.1016/j.jsat.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71–76. doi: 10.1016/j.drugalcdep.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994. 55(5), pp.569–580. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD, The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care, 1992. pp.473–483. [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive Counseling During Brief and Extended Buprenorphine-Naloxone Treatment for Prescription Opioid DependenceA 2-Phase Randomized Controlled Trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgierska AE, Burzinski CA, Cox J et al. Mindfulness meditation and cognitive behavioral therapy intervention reduces pain severity and sensitivity in opioid-treated chronic low back pain: pilot findings from a randomized controlled trial. Pain Medicine. 2016. 17(10), pp.1865–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]