Abstract
Individuals with opioid use disorder (OUD) have high prevalence of smoking and poor cessation outcomes. Data suggest that smokers with OUD may experience heightened nicotine reinforcement and more severe tobacco withdrawal compared to smokers without OUD. FDA is currently considering reducing the nicotine content of cigarettes to reduce smoking prevalence and smoking-related disease. It is critical to understand the effects of reduced nicotine content cigarettes (RNCCs) on tobacco withdrawal in this subgroup. In this secondary analysis, we investigated the ability of RNCCs to attenuate acute tobacco withdrawal and craving severity in smokers with OUD vs. without SUDs.
Smokers maintained on methadone or buprenorphine (opioid-maintained, OM; n=65) vs. without other substance use disorders (Non-SUD; n=135) completed 5 laboratory sessions wherein they smoked their usual brand (UB) or a research cigarette varying in nicotine content (0.4, 2.4, 5.2, 15.8 mg/g of tobacco) under double-blind, acute abstinence conditions. Participants completed the Minnesota Tobacco Withdrawal Scale, including a Desire to Smoke (craving) item, before and every 15 minutes for one hour following smoking each cigarette.
Tobacco withdrawal and craving did not differ significantly by OM status in response to UB or RNCCs. In addition to the dose x time interaction, greater depression and cigarette dependence consistently predicted withdrawal and craving (p ’s<.05).
Across all cigarettes, tobacco withdrawal and craving did not significantly differ by OM status, suggesting that smokers receiving opioid agonist treatment may respond favorably to RNCCs. Additional studies with larger and more diverse samples are needed to address this question more definitively.
Keywords: Reduced nicotine content cigarettes, addiction potential, tobacco regulatory science, vulnerable populations, tobacco withdrawal, opioid use disorder, methadone, buprenorphine, depression
INTRODUCTION
While the prevalence of smoking among the general US adult population has declined over the past several decades, it remains entrenched among individuals with co-morbid substance use disorders (SUDs), non-SUD psychiatric disorders and socioeconomic disadvantage (Hiscock, Bauld, Amos, Fidler, & Munafò, 2012; Lasser et al., 2000). Individuals with opioid use disorder (OUD) represent a population that is particularly vulnerable to cigarette smoking and its adverse health consequences. Prevalence of smoking in this group is up to six-fold higher than the general population (84–94% vs. 14%, respectively)(Guydish et al., 2016; U.S. Department of Health and Human Services, 2014). In addition, response to smoking cessation interventions among smokers with OUD is notoriously poor, with abstinence outcomes one-fourth that of non-SUD smokers and standard first-line pharmacotherapies largely ineffective (Miller & Sigmon, 2015).
One potential reason for the high rates of smoking, cigarette dependence and poor cessation outcomes in this population is that smokers with concurrent OUD may experience more severe abstinence effects upon discontinuation of smoking, including elevated withdrawal severity. Tobacco withdrawal is a hallmark feature of cigarette dependence and is associated with relapse to smoking in the general smoker population (American Psychiatric Association, 2013; Hughes, 2007). However, there is a paucity of empirical data characterizing tobacco withdrawal among smokers with OUD, and the limited studies available offer mixed results. Of the three studies that have examined withdrawal among smokers with OUD, one suggested that opioid agonists may be associated with more severe withdrawal (Story & Stark, 1991), one suggested that opioids may attenuate withdrawal (Elkader, Brands, Selby, & Sproule, 2009), and one found no difference in withdrawal between OUD and non-SUD smokers (Streck, Ochalek, Badger, & Sigmon, 2018). Methodological differences across studies may account for these mixed findings, as they employed a variety of scientific designs and analytic approaches (e.g., within-subject vs. cross-sectional designs, evaluating withdrawal only among abstinent vs. all smokers, evaluating smoking during stable vs. acute changes in opioid dose). Further, none of the prior studies utilized double-blind cigarette administration or examined multiple cigarette doses.
There is an urgent need to understand the severity of withdrawal experienced by vulnerable smokers under conditions of reduced nicotine intake, as a national policy is currently under consideration to reduce the nicotine content in cigarettes to minimal or non-addictive levels to reduce smoking prevalence and smoking-related disease in the US (Benowitz & Henningfield, 1994; Gottlieb & Zeller, 2017). The 2009 Family Smoking Prevention and Tobacco Control Act granted the US Food and Drug Administration (FDA) regulatory authority over cigarettes and other tobacco products (111th Congress, 2009). Reducing the nicotine content of cigarettes below the threshold necessary to establish and sustain nicotine dependence may in turn reduce smoking prevalence and related disease by disrupting initiation of smoking by new users and increasing cessation rates among current smokers. Well-controlled studies have demonstrated that use of reduced nicotine content cigarettes (RNCCs) in the general smoker population is associated with reductions in smoking rates, nicotine exposure, dependence and toxicant exposure, as well as increases in smoking abstinence (e.g., Benowitz et al., 2007; Donny et al., 2015; Hatsukami et al., 2013, 2010). In those studies, RNCCs reduced withdrawal symptoms and craving, possibly because RNCCs provide sensory stimuli that have acquired conditioned reinforcing effects through repeated pairings with nicotine delivery (Rose & Levin, 1991).
However, most of those studies excluded individuals with SUDs and non-SUD psychiatric disorders and instead focused on stable, generally “healthy” smokers. Considering their poor response to standard smoking-cessation treatments and substantial burden experienced from smoking, it is important to understand whether smokers with concurrent SUDs or other vulnerabilities may respond differently to RNCCs. Given the scientific evidence that opioid agonist medications may increase the reinforcing effects of nicotine administration (Chait & Griffiths, 1984; Mello, Lukas, & Mendelson, 1985), and the increasing numbers of individuals with OUD receiving methadone or buprenorphine maintenance treatment (Alderks, 2017; Wen, Hockenberry, & Pollack, 2018), it is critical to understand whether these smokers will experience a unique profile of subjective effects following use of RNCCs or compensate for reduced nicotine levels by increasing their smoking rates. Towards this end, the focus of the present study was whether cigarette craving or withdrawal symptom severity in response to RNCCs may be distinct among smokers with vs. without concurrent OUD.
We recently completed a double-blind, randomized controlled laboratory study examining the acute relative reinforcing and subjective effects of research cigarettes varying in nicotine content following acute smoking abstinence in three vulnerable populations (Higgins et al., 2017). The present study is a secondary analysis of that prior experimental study and focuses on the ability of RNCCs to attenuate acute tobacco withdrawal and craving severity in individuals receiving methadone or buprenorphine maintenance treatment for OUD (i.e., opioid-maintained smokers) vs. smokers without SUDs. We also examined the contribution of additional characteristics that frequently co-occur with OUD and have been associated with smoking vulnerability (e.g., depression, education level) to evaluate their effects on tobacco withdrawal and craving over and above OM status. Given the absence of existing data on withdrawal and craving in response to RNCCs among smokers with OUD and other vulnerabilities, this laboratory study provided a unique opportunity to examine the early time course of withdrawal and craving relief following acute exposure to RNCCs under highly-controlled experimental conditions.
METHODS
Participants
Participants were 200 adult daily smokers enrolled in a multi-site, double-blind, within-subject clinical-laboratory study. Details of study methodology have been reported previously (Higgins et al., 2017). Briefly, participants were recruited though advertisements placed on Facebook, bulletin boards throughout the community, buses and local newspapers at the University of Vermont, Johns Hopkins University and Brown University. Participants were recruited as exemplars of three different vulnerable populations: Individuals with opioid use disorder (OUD) as an exemplar of those with smokers with SUDs (n=65), individuals with affective disorders, as an exemplar of smokers with mental illness (n=64), and women of reproductive age (18–44 years old) with limited educational attainment as an exemplar of smokers with socioeconomic disadvantage (n=71), with the latter two groups not having a current other SUD. Participants in the OUD group were currently receiving methadone or buprenorphine maintenance via opioid treatment programs or office-based providers in the community. They were required to be stable in methadone or buprenorphine treatment (defined as no change in opioid dose in the past 30 days and <30% urine toxicology samples testing positive for illicit drug use in the month preceding study intake). Confirmation of participants’ past-month dose and urinalysis results were obtained from their treatment provider. Given our participants were currently receiving opioid treatment and stable in treatment, we refer to this group herein as “opioid-maintained (OM).” The study was approved by the respective universities’ Institutional Review Boards and all participants provided written informed consent.
Research Cigarettes
Cigarettes for this study were obtained from the National Institute on Drug Abuse and manufactured by the 22nd Century Group (Clarence, NY). Participants were each exposed to four nicotine content levels: 0.4, 2.4, 5.2, and 15.8 milligrams of nicotine per gram (mg/g) of tobacco (Donny et al., 2015; Higgins et al., 2017). The 15.8 mg/g cigarette was designed to have a nicotine content similar to commercially-available cigarettes and served as a control condition. Cigarettes were available in menthol or non-menthol flavor based on participants’ usual brand cigarette preference. All sessions involving research cigarettes took place under double-blind conditions with each cigarette dose being represented by arbitrary letter codes.
Measures
Demographic and Smoking Characteristics
Demographic variables included education level, gender, age, race, marital status and employment status. Smoking characteristics assessed included the average number of cigarettes smoked per day, menthol smoking status, and the Fagerstrom Test of Cigarette Dependence (FTCD; Fagerström, 2012). Breath carbon monoxide (CO) level was also collected as a biochemical measure of smoking level. We also collected data on opioid treatment characteristics including the opioid agonist medication participants were maintained on (methadone vs. buprenorphine).
Tobacco Withdrawal and Craving
The Minnesota Tobacco Withdrawal Scale (MTWS; Hughes & Hatsukami, 1986) was used to assess tobacco withdrawal and desire to smoke. The MTWS is a 15-item self-report measure of tobacco withdrawal. Each item is rated on a 5-point ordinal scale (0=none, 1=slight, 2=mild, 3=moderate, 4=severe). The seven DSM-5 withdrawal symptoms (anger/irritability/ frustration, anxiety/nervousness, difficulty concentrating, impatience/restlessness, increased appetite/hunger, insomnia/awakening at night, depressed mood/sad) are averaged to construct a single withdrawal severity score (i.e., MTWS Total score) and the Desire to Smoke single item of the measure is analyzed separately as a measure of cigarette craving (Hughes & Hatsukami, 1998).
Psychiatric Screening Measures
Psychiatric screening measures included the Overall Anxiety Severity and Impairment Scale (OASIS; Norman, Cissell, Means-Christensen, & Stein, 2006) and the Beck Depression inventory (BDI; Beck, 1996). The OASIS is a 5-item screening measure of anxiety severity in the past week with items rated on a 0–4 scale with total scores ranging from 0 to 20. The BDI is a 21-item measure which assesses depression severity in the past two weeks with items rated on a 0–3 scale with total scores ranging from 0 to 63. We chose to use a dichotomous variable for BDI in our analyses for ease of interpretation using a commonly established cut-off of 17 to distinguish those with clinically meaningful depression levels (Beck, 1996; Sprinkle et al., 2002).
Procedures
Participants presented to an intake screening visit where we assessed demographic, smoking, opioid treatment, and psychiatric screening measures and determined study eligibility. Eligible participants completed 14 experimental sessions across three study phases. All sessions were conducted under conditions of acute smoking abstinence with participants abstaining for 6–8 hours prior to each session (operationalized as breath CO≤50% of intake level). Evidence suggests that this time frame of abstinence is sufficient to capture withdrawal symptoms (Hendricks, Ditre, Drobes, & Brandon, 2006). At the start of each session, participants took two puffs of their own brand cigarette to equate the time since last cigarette across all participants and sessions (Henningfield & Griffiths, 1981). Following a 30-minute break, participants smoked the assigned cigarette for that session ad libitum. Session 1 functioned as a baseline session where participants smoked their own brand cigarette. Phase 1 (Sessions 2–5) assessed subjective responses to a single cigarette smoked in the laboratory, Phase 2 (Sessions 6–11) assessed choices between cigarette puffs differing in nicotine content when all were available for an equal response cost, and Phase 3 (Sessions 12–14), assessed choices between the highest and lowest dose cigarettes when the response requirement for the low dose was fixed and the response requirement for the high dose increased progressively. As this report focuses on data from the baseline session (Session 1) and Phase 1 (Sessions 2–5) only, we briefly provide more detailed information on those sessions here.
In Session 1, participants smoked their usual brand cigarette ad libitum. Before smoking (i.e., under conditions of abstinence) and every 15 minutes for an hour after smoking, participants completed the MTWS. During Sessions 2–5, participants sampled one dose research cigarette per session in a random order. In other words, as this was a within-subject study, participants were exposed to all dose cigarettes, with one dose cigarette smoked per session. Participants similarly smoked cigarettes ad libitum during these sessions. Before and every 15 minutes for an hour after smoking, participants completed the MTWS.
Statistical Analyses
We examined data from participants who completed Phase 1 of the parent study (N=202). Two participants were excluded from analyses for missing BDI and OASIS data at intake (N=200). As the primary aim of this secondary analysis was to investigate the role of opioid maintenance status on tobacco withdrawal, participants were dichotomized as opioid-maintained (OM; n=65) or non-SUD (n=135) smokers.
Demographic, psychiatric and smoking characteristics were examined by OM status using Fisher’s Exact Tests for categorical variables and Wilcoxon Rank Sum tests for continuous variables. When examining response to the usual brand cigarette in Session 1, we used mixed-model repeated-measures (RM) analyses with MTWS Total and Desire to Smoke scores as our dependent measures. Fixed effects included time (i.e., pre-smoking baseline and 15, 30, 45, and 60 minutes post-smoking the cigarette), as a within-subject factor, and OM status as an across-subject factor. Participants and study site were included as random effects.
When examining MTWS Total and Desire to Smoke scores across Phase 1 in response to the cigarettes varying in nicotine content, we similarly performed mixed-model RM analyses. Fixed effects included time (i.e., pre-smoking baseline and 15, 30, 45, and 60 minutes after smoking the cigarette), research cigarette dose (0.4, 2.4, 5.2, 15.8 mg/g), and session (2–5) as within-subject factors and OM status as an across-subject factor. Random effects included participants, study site and sequence of presentation of cigarette doses. For both Session 1 and Phase 1, additional mixed models were conducted to examine the effects of opioid treatment medication (methadone vs. buprenorphine) on withdrawal and craving outcomes.
Finally, to examine the effects of other vulnerabilities to smoking on withdrawal and craving above and beyond OM status, we tested multivariate mixed RM models with demographic, smoking and psychiatric characteristics as additional predictors. Time and nicotine dose were within-subject factors and OM status was an across-subject factor. Additional variable selection for construction of the models was based on both univariate testing and the empirical literature. Age, race, gender, employment status, marital status, FTCD total score, cigarettes smoked per day, BDI and OASIS scores were included in preliminary models as there was evidence of differences by OM status at intake (Table 1). Education level, age started smoking regularly and screening CO level were included in preliminary models based on the literature. Since the correlation between the BDI categorical variable and OASIS total score was high (Spearman ρ = 0.73, p < 0.001), only the BDI categorical variable was used in subsequent modeling. Models included the same random effects described above. All post-hoc testing used a Bonferroni adjustment for multiple comparisons. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). For all analyses, other than post-hoc comparisons with Bonferroni adjustment, statistical significance was defined as p<.05.
Table 1.
Baseline Demographic and Smoking Characteristics by Opioid Status
| All | Opioid-Maintained (OM) | No Substance Use Disorder (Non-SUD) | p value | |
|---|---|---|---|---|
| N | 200 | 65 | 135 | |
| Demographics | ||||
| Age | 35±12 | 41±11 | 32±10 | <.001 |
| Female (%) | 72 | 60 | 78 | .01 |
| Race (%) | .07 | |||
| White | 76 | 72 | 77 | |
| Black | 11 | 19 | 8 | |
| Other | 13 | 9 | 15 | |
| Education (%) | .57 | |||
| <High school | 14 | 18 | 12 | |
| High school | ||||
| degree/equivalent | 35 | 35 | 34 | |
| Some college | 40 | 37 | 41 | |
| Associate degree or higher | 12 | 9 | 13 | |
| Employment (%) | <.001 | |||
| Full-time work | 25 | 15 | 30 | |
| Part-time work | 16 | 9 | 19 | |
| Casual employment | 8 | 8 | 8 | |
| Unemployed | 27 | 23 | 28 | |
| Other | 25 | 45 | 16 | |
| Marital Status (%) | <.01 | |||
| Never married | 61 | 52 | 65 | |
| Married | 16 | 9 | 19 | |
| Divorced or separated | 21 | 34 | 14 | |
| Widowed | 3 | 5 | 1 | |
| Smoking Characteristics | ||||
| Cigarettes/day | 16±7 | 16±6 | 15±8 | .06 |
| Intake CO level | 22±11 | 23±12 | 21±11 | .33 |
| Age started smoking regularly | 16±4 | 16±5 | 16±3 | .29 |
| FTCD total score | 4.9±2 | 5.3±2 | 4.7±2 | .03 |
| Menthol smoker (%) | 35 | 35 | 34 | .87 |
| Psychiatric Characteristics | ||||
| BDI total score ≥ 17 (%) | 31 | 14 | 39 | <.01 |
| OASIS total score | 6±5 | 3±3 | 7±6 | <.001 |
| Opioid Treatment Characteristics | ||||
| Methadone maintained (%) | 58 | |||
| Methadone dose, mg | 97±30 | |||
| Buprenorphine maintained (%) | 42 | |||
| Buprenorphine dose, mg | 14±9 |
Note. Mean ± standard deviation unless otherwise noted; Bolded values represent p<.05; BDI, Beck Depression Inventory with scores ≥ 17 representing clinically meaningful levels of depression (Beck, 1996); OASIS, Overall Anxiety Severity and Impairment Scale (Norman et al., 2006); FTCD, Fagerström Test for Cigarette Dependence (Fagerström, 2012).
RESULTS
Participant Characteristics
Baseline demographic and smoking characteristics by OM status are presented in Table 1. Briefly, OM smokers were on average 41 years of age, smoked 16 cigarettes per day and presented with an FTCD score of 5, suggesting moderate cigarette dependence. OM smokers were older, less likely to be female, more likely to be cigarette dependent, and had lower levels of baseline anxiety and depression than non-SUD smokers.
Effects of OM Status on Response to Usual Brand Cigarette
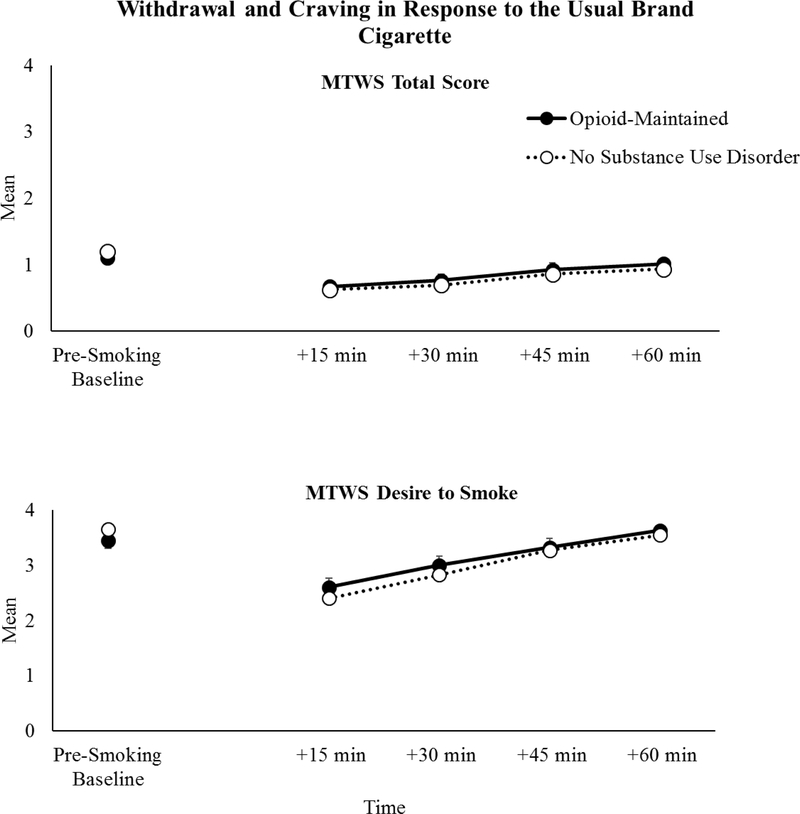
There was a significant main effect of time on mean tobacco withdrawal severity scores (i.e., MTWS Total scores) (F(4, 795)=52.80, p<.001) with scores decreasing 15 minutes after smoking the usual brand cigarette and then gradually increasing over time (i.e., across the remaining 45 minutes of the hour following smoking). Mean tobacco withdrawal severity scores during the usual brand cigarette baseline (Session 1) did not differ as a function of OM status (F(1, 185)=0.69, p=.41; Figure 1, upper panel). There also was no evidence of an interaction between OM status and time on withdrawal during the baseline session (F(4, 791)=1.48, p=.21). There was a significant main effect of opioid treatment medication type (methadone vs. buprenorphine) on withdrawal scores in response the usual brand cigarette, with higher levels of withdrawal observed across time in buprenorphine- vs. methadone-maintained participants (F(1,63)=4.74, p=03).
FIGURE 1.
Minnesota Tobacco Withdrawal Scale (MTWS) Total (upper panel) and Desire to Smoke (lower panel) mean scores at the baseline usual brand cigarette session across time in opioid-maintained (solid lines) smokers vs. smokers without other substance use disorders (dashed lines). Error bars represent standard error of the mean. The pre-smoking baseline timepoint represents conditions of acute abstinence prior to smoking the usual brand cigarette and +15, +30, +45, +60 min represent assessment timepoints after smoking the usual brand cigarette.
For cigarette craving (i.e., the Desire to Smoke item of the MTWS), there was evidence of a significant main effect of time (F(4, 795)=62.82, p<.001) with scores decreasing 15 minutes after smoking the usual brand cigarette and then gradually increasing over time. Craving severity following smoking the usual brand cigarette did not differ as a function of OM status (F(1, 198)=0.19, p=.66; Figure 1, lower panel), nor was there a significant interaction between OM status and time on reports of cigarette craving (F(4, 791)= 1.53, p=.19). There was no effect of opioid treatment medication type on craving scores in response to the usual brand cigarette (F(1, 55)=0.76, p=.39).
Effects of OM Status on Response to Research Cigarettes Varying in Nicotine Content
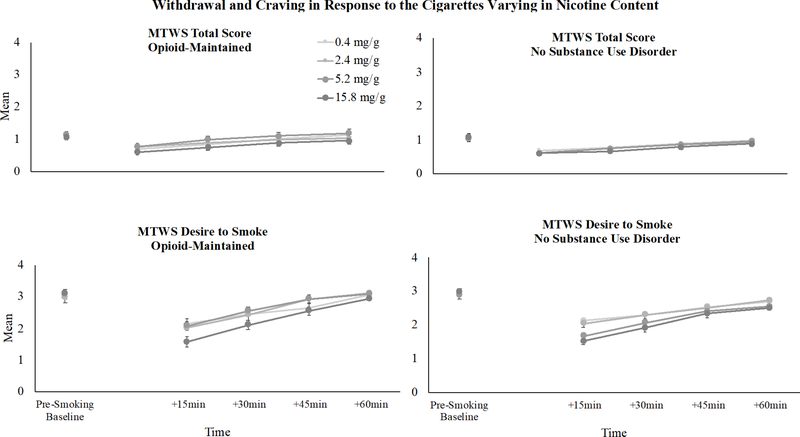
In response to the cigarettes varying in nicotine content, there was a significant dose x time interaction on mean withdrawal scores (F(12, 2386)=3.00, p<.001) with scores decreasing over time following smoking each of the research cigarettes and then gradually returning to baseline levels, with larger magnitude reductions seen at higher dose cigarettes. Withdrawal severity in response to cigarettes varying in nicotine content (Sessions 2–5) did not differ as a function of OM status (F(1, 175)=1.65, p=.20; Figure 2, upper panels). There also was no significant interaction between OM status and nicotine dose on withdrawal (F(3, 591 )=2.15, p=.09) or among OM status, time and nicotine dose (F(19, 3146)=1.35, p=.14). There was no significant main effect of opioid treatment medication type on withdrawal scores in response to the cigarettes varying in nicotine content (F(1, 51)=3.69, p=.06).
FIGURE 2.
Minnesota Tobacco Withdrawal Scale (MTWS) Total (upper panels) and Desire to Smoke (lower panels) mean scores across the research cigarette doses and across time in opioid-maintained (left panels) smokers vs. smokers without other substance use disorders (right panels). Error bars represent standard error of the mean. The pre-smoking baseline timepoint represents conditions of acute abstinence prior to smoking the research cigarettes and +15, +30, +45, +60 min represent assessment timepoints after smoking each research cigarette.
Similarly, there was a significant dose x time interaction on craving scores in response to the cigarettes varying in nicotine content (F(12, 2385)=6.42, p<.001), with scores decreasing over time following smoking the research cigarettes and then gradually returning to baseline levels, with larger magnitude reductions seen at higher dose cigarettes. Craving severity following exposure to research cigarettes did not differ as a function of OM status (F(1, 198)=2.76, p=.10). There was also no OM status x nicotine dose interaction (F(3, 591)=2.34, p=.07) nor OM status x time x nicotine dose interaction (F(19, 3146)=1.57, p=.06) on craving (Figure 2, lower panels). There was no effect of opioid treatment medication type on craving scores in response to the cigarettes varying in nicotine content (F(1, 63)=3.32, p=.07).
Effects of Other Vulnerabilities
During the sessions evaluating research cigarettes varying in nicotine content, multivariate models resulted in the following significant predictors of withdrawal: BDI (F(1, 149)=28.23, p<.0001), FTCD total score (F(1, 189)=9.34, p<.01) and education level (F(3, 193)=3.08, p=.03), in addition to the dose x time interaction (F(12, 2386)=3.00, p<.0001). Higher baseline levels of depression (mean difference between high vs. low depressed=0.64, p<.001) and cigarette dependence severity (0.07 per unit increase in FTCD, p<.01) were predictive of greater tobacco withdrawal. Lower educational attainment (i.e., high school education vs. some college: mean difference=0.29 and some college vs. associate’s degree or higher: mean difference=0.33; p’s<.05) also predicted greater withdrawal in response to the cigarettes varying in nicotine content.
In multivariate models predicting craving, in addition to the dose x time interaction (F(12, 2386)=6.42, p<.0001), FTCD total score was the only significant predictor of craving in response to research cigarettes varying in nicotine content (F(1, 171)=32.09, p<.0001), with greater cigarette dependence severity predicting higher craving across research cigarettes (0.16 per unit increase in FTCD, p<.01).
DISCUSSION
Smokers with concurrent opioid use disorder have an extremely high prevalence of smoking, experience poor cessation outcomes, and bear a disproportionate burden of smoking-related adverse health consequences. A promising national policy is currently under consideration by the FDA to decrease the nicotine content of cigarettes (111th Congress, 2009; Gottlieb & Zeller, 2017) and it is critical to understand the effects of reduced nicotine cigarettes on tobacco withdrawal and cigarette craving severity in this vulnerable smoker group.
Across usual brand and reduced nicotine cigarettes, tobacco withdrawal and craving did not significantly differ as a function of OM status in the present study. These results are consistent with our recent study examining tobacco withdrawal severity in a different sample of OM vs. non-SUD smokers in which no differences in withdrawal or craving severity were observed between groups across a 2-week period of biochemically-verified smoking abstinence (Streck, Heil, Higgins, Bunn, & Sigmon, 2018). Whereas Streck et al. (2018) examined individuals who quit smoking their usual brand cigarettes for a 2-week study period, the current study further extends that work by including an evaluation of the effects of multiple cigarettes varying in nicotine content using rigorous double-blind conditions. The finding that OM smokers did not experience greater tobacco withdrawal or craving relative to other smokers selected from vulnerable populations without OUD (smokers with affective disorders or socioeconomic disadvantage) are promising, though additional research is needed to thoroughly understand the effects of this proposed policy in OM smokers. Further, when examining effects of opioid treatment medication type, we found modest evidence that buprenorphine (vs. methadone) may be associated with higher levels of withdrawal in response to the usual brand cigarette, though additional research with larger sample sizes is needed.
We also examined several other characteristics reflective of smoking vulnerability that frequently co-occur with opioid use (e.g., depression, cigarette dependence, educational attainment) and their associations with tobacco withdrawal and craving severity in these vulnerable groups. Once again, OM status was not associated with withdrawal or craving severity after accounting for other potential explanatory variables. Clinically meaningful depressive symptoms at study intake was associated with increased withdrawal across all nicotine doses. This is generally consistent with prior research showing elevated incidence and severity of tobacco withdrawal among smokers with affective disorders, particularly depression (Smith, Mazure, & McKee, 2014; Weinberger, Desai, & McKee, 2010). Overall, given the high rates of concomitant depression and other psychiatric disorders among OM patients (Barry et al., 2016; Strain, 2002), these findings may hold clinical significance for efforts to tackle smoking cessation among OM patients with co-occurring psychiatric distress. However, also important to note is that we did not see additive effects of OM status and depression on withdrawal severity in this study. This is consistent with a prior report that used nationally-representative data to examine the effects of having a co-morbid psychiatric disorder and SUD compared to a psychiatric disorder alone on tobacco withdrawal (Weinberger et al., 2010). In that study, while the presence of a SUD and a non-SUD psychiatric disorder were each independently associated with increased presence and severity of withdrawal symptoms, having both disorders did not act additively to increase withdrawal symptoms. That study did not examine the effects of OUD specifically. Also worth noting is that we did not examine the potential role of concomitant physical (vs. psychiatric) symptoms in withdrawal and craving, though a growing body of work suggests that individuals’ ability to tolerate withdrawal-related discomfort in the context of smoking abstinence among smokers with OUD and other SUDs may play a role in their subsequent cessation outcomes (Martin, Rohsenow, & Tidey, 2019; Rohsenow et al., 2015).
Lower educational attainment was also associated with more severe tobacco withdrawal across nicotine doses. Limited educational attainment has been identified as an important proxy for socioeconomic disadvantage (Shavers, 2007) and is associated with increased prevalence of smoking and smoking-related adverse consequences (Agaku, King, Dube, & Centers for Disease Control and Prevention (CDC), 2014; Jamal, 2016). Several prior reports have hypothesized that withdrawal may be greater among those with socioeconomic disadvantage more generally (Harwood, Salsberry, Ferketich, & Wewers, 2007; Hiscock et al., 2012; Marmot & Wilkinson, 2005), though we are aware of only two empirical investigations on this topic. In the first study, Breslau and colleagues examined epidemiological data from young adults in one state and found no effects of education level on tobacco withdrawal (Breslau, Kilbey, & Andreski, 1992). In the second, which was conducted in Syria, the authors reported that higher educational attainment was associated with lower withdrawal scores among patients enrolled in a smoking cessation trial (Ben Taleb et al., 2016). To our knowledge, the present study is the first to report on the potential role of educational attainment on tobacco withdrawal in response to RNCCs.
Potential limitations of this study are important to note. First, we utilized an acute exposure paradigm wherein participants abstained from smoking for 6–8 hours (versus abstaining for 12-hours or longer), sampled each dose research cigarette during one laboratory session, and rated their withdrawal across one hour post-smoking (versus days or weeks). As such, we did not examine the full time-course of tobacco withdrawal during extended exposure but rather abstinence effects and, more specifically, the extent to which RNCCs attenuate tobacco withdrawal severity under conditions of acute abstinence. Additional extended exposure studies are needed to determine if our results generalize to a longer time-course of withdrawal under conditions of extended abstinence and prolonged exposure to these reduced nicotine cigarettes. However, acute laboratory models are a well-validated and safe approach to begin examining cigarettes with reduced nicotine content in medically and socially unstable populations. The present study utilized a sufficient duration of abstinence to examine withdrawal based on previous literature (Hendricks et al., 2006), and results from prior studies of acute response to cigarettes with reduced nicotine content in laboratory settings in the general population of smokers used similar methods, and results align closely with those seen during chronic exposure in naturalistic settings. Second, to be eligible for the present study, participants were required to be stable in their opioid treatment, with limited opioid medication dose changes or illicit drug use. It is possible that smokers with OUD not currently receiving treatment, or in treatment but not clinically stable, may respond differently to the RNCCs and that question merits further investigation. A large number of individuals with OUD are not currently enrolled in opioid treatment (Saloner & Karthikeyan, 2015), and one study has reported higher levels of nicotine dependence and less motivation to quit among smokers not in opioid maintenance treatment (and actively using intravenous opioids) compared to those receiving treatment (Clarke, Stein, McGarry, & Gogineni, 2001). Third, this was a secondary analysis of data from a study that was not originally designed, intended or powered to examine outcomes as a function of OM status; that is, the samples recruited for the parent study did not involve equal numbers of OM and Non-SUD smokers or comparable sociodemographic characteristics (e.g., gender) across OM groups. Although we controlled for various sociodemographic characteristics that differed by OM status in multivariable analyses, it does not rule out the presence of other potential confounders and additional studies with larger sample sizes and more diverse demographic and smoking characteristics (e.g., race, gender, menthol status) are warranted to examine these questions more definitively. Finally, as the parent trial was entirely focused on understanding RNCC response among smokers with concomitant vulnerabilities (e.g., anxiety/depression, socioeconomic disadvantage), there was no control group of ‘healthy’ smokers without these co-occurring factors in the present analyses.
This study also had several important strengths. It is the only study to date, to our knowledge, to rigorously evaluate tobacco withdrawal in response to RNCCs in OM smokers. The study methodology included a rigorous, double-blind, highly controlled design, multiple nicotine doses, availability of multiple empirically-supported measures reflecting vulnerability to smoking, and minimal missing data or attrition. Finally, it also is the first study to investigate the separate and combined effects of multiple co-occurring vulnerabilities (e.g., OUD, depression, socioeconomic disadvantage) and their impact on tobacco withdrawal and craving severity in response to RNCCs.
In summary, the FDA is actively considering reducing the nicotine levels of cigarettes. Scientific efforts are urgently needed to understand the impact of such a policy on the populations of smokers with co-occurring vulnerabilities that smoke the majority of the cigarettes in the US (Lasser et al., 2000). Prior data suggesting that OM smokers may respond differently to nicotine and experience more severe withdrawal during reductions in nicotine intake. In our study, OM smokers responded similarly to other vulnerable subgroups to reduced nicotine content cigarettes. Additional research is needed to determine the beneficial effects of a national nicotine reduction policy among individuals with OUD.
Public Significance Statement:
Being maintained on opioid agonist treatment for opioid use disorder did not influence self-reported ratings of tobacco withdrawal or desire to smoke (craving) in response to usual brand or reduced nicotine content cigarettes. Opioid-maintained smokers may respond favorably to a national nicotine reduction policy for reducing smoking-related consequences.
ACKNOWLEDGEMENTS
We thank Drs. Julie A. Dumas and Rex L. Forehand for their invaluable feedback on this dissertation project. Portions of this manuscript have been previously presented at the Society for Research on Nicotine and Tobacco 2019 Annual Meeting.
FUNDING
This project was supported by Tobacco Centers of Regulatory Science award P50DA036114/U54DA036114 from the National Institute on Drug Abuse (NIDA) and Food and Drug Administration (FDA), Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences (NIGMS), and NIDA Institutional Training Grant T32DA007242. Support for JWT was also provided by U54DA031659. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, FDA, or NIGMS.
Footnotes
DECLARATION OF INTERESTS
JRH has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several non-profit organizations that promote tobacco control. He has recently received consulting fees from Swedish Match, Altria and Philip Morris International to assist their efforts to develop lower risk tobacco products. All other authors have nothing to declare.
REFERENCES
- 111th Congress. (2009, 2011). Family Smoking Prevention and Tobacco Control Act. HR 1256. [Google Scholar]
- Agaku IT, King BA, Dube SR, & Centers for Disease Control and Prevention (CDC). (2014). Current cigarette smoking among adults—United States, 2005–2012. MMWR. Morbidity and Mortality Weekly Report, 63(2), 29–34. [PMC free article] [PubMed] [Google Scholar]
- Alderks CE (2013). Trends in the Use of Methadone, Buprenorphine, and Extended-Release Naltrexone at Substance Abuse Treatment Facilities: 2003–2015 (Update). In The CBHSQ Report. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK469748/ [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Retrieved from http://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596
- Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, & Schottenfeld RS (2016). Psychiatric Disorders Among Patients Seeking Treatment for Co-Occurring Chronic Pain and Opioid Use Disorder. The Journal of Clinical Psychiatry, 77(10), 1413–1419. 10.4088/JCP.15m09963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Ben Taleb Z, Ward KD, Asfar T, Jaber R, Auf R, & Maziak W (2016). Predictors of nicotine withdrawal symptoms: Findings from the first randomized smoking cessation trial in a low-income country setting. International Journal of Public Health, 61(6), 701–708. 10.1007/s00038-016-0818-8 [DOI] [PubMed] [Google Scholar]
- Benowitz, & Henningfield. (1994). Establishing a nicotine threshold for addiction. The implications for tobacco regulation. The New England Journal of Medicine, 331(2), 123–125. 10.1056/NEJM199407143310212 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, & Jacob P (2007). Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 16(11), 2479–2485. 10.1158/1055-9965.EPI-07-0393 [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, & Andreski P (1992). Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemiologic study of young adults. The American Journal of Psychiatry, 149(4), 464–469. 10.1176/ajp.149A464 [DOI] [PubMed] [Google Scholar]
- Chait LD, & Griffiths RR (1984). Effects of methadone on human cigarette smoking and subjective ratings. The Journal of Pharmacology and Experimental Therapeutics, 229(3), 636–640. [PubMed] [Google Scholar]
- Clarke JG, Stein MD, McGarry KA, & Gogineni A (2001). Interest in smoking cessation among inj ection drug users. The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions, 10(2), 159–166. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, … Hatsukami DK (2015). Randomized Trial of Reduced-Nicotine Standards for Cigarettes. The New England Journal of Medicine, 373(14), 1340–1349. 10.1056/NEJMsa1502403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkader AK, Brands B, Selby P, & Sproule BA (2009). Methadone-nicotine interactions in methadone maintenance treatment patients. Journal of Clinical Psychopharmacology, 29(3), 231–238. 10.1097/JCP.0b013e3181a39113 [DOI] [PubMed] [Google Scholar]
- Fagerström K (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 14(1), 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Gottlieb S, & Zeller M (2017). A Nicotine-Focused Framework for Public Health. The New England Journal of Medicine. 10.1056/NEJMp1707409 [DOI] [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Pagano A, Martínez C, Le T, Chun J, … Delucchi K (2016). An international systematic review of smoking prevalence in addiction treatment: Smoking prevalence in addiction treatment. Addiction, 111(2), 220–230. 10.1111/add.13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood GA, Salsberry P, Ferketich AK, & Wewers ME (2007). Cigarette smoking, socioeconomic status, and psychosocial factors: Examining a conceptual framework. Public Health Nursing (Boston, Mass.), 24(4), 361–371. 10.1111/j.1525-1446.2007.00645.x [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, … Allen SS (2013). Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 22(6), 1015–1024. 10.1158/1055-9965.EPI-12-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, … Hecht SS (2010). Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction (Abingdon, England), 105(2), 343–355. 10.1111/j.1360-0443.2009.02780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, & Brandon TH (2006). The early time course of smoking withdrawal effects. Psychopharmacology, 187(3), 385–396. 10.1007/s00213-006-0429-9 [DOI] [PubMed] [Google Scholar]
- Henningfield JE, & Griffiths RR (1981). Cigarette smoking and subjective response: Effects of d-amphetamine. Clinical Pharmacology and Therapeutics, 30(4), 497–505. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Sigmon SC, Tidey JW, Gaalema DE, Hughes JR, … Tursi L (2017). Addiction Potential of Cigarettes With Reduced Nicotine Content in Populations With Psychiatric Disorders and Other Vulnerabilities to Tobacco Addiction. JAMA Psychiatry, 74(10), 1056–1064. 10.1001/jamapsychiatry.2017.2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock R, Bauld L, Amos A, Fidler JA, & Munafò M (2012). Socioeconomic status and smoking: A review. Annals of the New York Academy of Sciences, 1248, 107–123. 10.1111/j.1749-6632.2011.06202.x [DOI] [PubMed] [Google Scholar]
- Hughes J, & Hatsukami DK (1998). Errors in using tobacco withdrawal scale. Tobacco Control, 7(1), 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289–294. [DOI] [PubMed] [Google Scholar]
- Hughes John R. (2007). Effects of abstinence from tobacco: Etiology, animal models, epidemiology, and significance: a subjective review. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 9(3), 329–339. 10.1080/14622200701188927 [DOI] [PubMed] [Google Scholar]
- Jamal A (2016). Current Cigarette Smoking Among Adults—United States, 2005–2015. MMWR. Morbidity and Mortality Weekly Report, 65 10.15585/mmwr.mm6544a2 [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, & Bor DH (2000a). Smoking and mental illness: A population-based prevalence study. JAMA, 284(20), 2606–2610. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, & Bor DH (2000b). Smoking and mental illness: A population-based prevalence study. JAMA, 284(20), 2606–2610. [DOI] [PubMed] [Google Scholar]
- Marmot M, & Wilkinson RG (2005). Social patterning of individual health behaviours: The case of cigarette smoking. In Marmot M & Wilkinson R (Eds.), Social Determinants of Health (pp. 224–237). 10.1093/acprof:oso/9780198565895.003.11 [DOI] [Google Scholar]
- Martin RA, Rohsenow DJ, & Tidey JW (2019). Smokers with opioid use disorder may have worse drug use outcomes after varenicline than nicotine replacement. Journal of Substance Abuse Treatment, 104, 22–27. 10.1016/jjsat.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, & Mendelson JH (1985). Buprenorphine effects on cigarette smoking. Psychopharmacology, 86(4), 417–425. [DOI] [PubMed] [Google Scholar]
- Miller ME, & Sigmon SC (2015). Are Pharmacotherapies Ineffective in Opioid-Dependent Smokers? Reflections on the Scientific Literature and Future Directions. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 17(8), 955–959. 10.1093/ntr/ntv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Cissell SH, Means-Christensen AJ, & Stein MB (2006). Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS). Depression and Anxiety, 23(4), 245–249. 10.1002/da.20182 [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Kahler CW, Martin RA, Colby SM, & Sirota AD (2015). Intolerance for Withdrawal Discomfort and Motivation Predict Voucher-Based Smoking Treatment Outcomes for Smokers with Substance Use Disorders. Addictive Behaviors, 43, 18–24. https://doi.org/10.1016Zj.addbeh.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, & Levin ED (1991). Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. British Journal of Addiction, 86(5), 605–609. [DOI] [PubMed] [Google Scholar]
- Saloner B, & Karthikeyan S (2015). Changes in Substance Abuse Treatment Use Among Individuals With Opioid Use Disorders in the United States, 2004–2013. JAMA, 314(14), 1515–1517. 10.1001/jama.2015.10345 [DOI] [PubMed] [Google Scholar]
- Shavers VL (2007). Measurement of socioeconomic status in health disparities research. Journal of the National Medical Association, 99(9), 1013–1023. [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Mazure CM, & McKee SA (2014). Smoking and mental illness in the U.S. population. Tobacco Control, 23(e2), e147–153. 10.1136/tobaccocontrol-2013-051466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinkle SD, Lurie D, Insko SL, Atkinson G, Jones GL, Logan AR, & Bissada NN (2002). Criterion validity, severity cut scores, and test-retest reliability of the Beck Depression Inventory-II in a university counseling center sample. Journal of Counseling Psychology, 49(3), 381–385. 10.1037/0022-0167.49.3381 [DOI] [Google Scholar]
- Story J, & Stark MJ (1991). Treating cigarette smoking in methadone maintenance clients. Journal of Psychoactive Drugs, 23(2), 203–215. 10.1080/02791072.1991.10472237 [DOI] [PubMed] [Google Scholar]
- Strain EC (2002). Assessment and treatment of comorbid psychiatric disorders in opioid-dependent patients. The Clinical Journal of Pain, 18(4 Suppl), S14–27. [DOI] [PubMed] [Google Scholar]
- Streck JM, Heil SH, Higgins ST, Bunn JY, & Sigmon SC (2018). Tobacco withdrawal among opioid-dependent smokers. Experimental and Clinical Psychopharmacology, 26(2), 119–124. 10.1037/pha0000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck JM, Ochalek TA, Badger GJ, & Sigmon SC (2018). Interim buprenorphine treatment during delays to comprehensive treatment: Changes in psychiatric symptoms. Experimental and Clinical Psychopharmacology, 26(4), 403–409. 10.1037/pha0000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014 | SurgeonGeneral.gov (p. 944) [A Report of the Surgeon General]. Retrieved from Centers for Disease Control and Prevention; website: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html [Google Scholar]
- Weinberger AH, Desai RA, & McKee SA (2010). Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug and Alcohol Dependence, 108(1–2), 7–12. 10.1016/j.drugalcdep.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Hockenberry JM, & Pollack HA (2018). Association of Buprenorphine-Waivered Physician Supply With Buprenorphine Treatment Use and Prescription Opioid Use in Medicaid Enrollees. JAMA Network Open, 1(5), e182943–e182943. 10.1001/jamanetworkopen.2018.2943 [DOI] [PMC free article] [PubMed] [Google Scholar]