Abstract
Background:
Neighborhood socioeconomic deprivation is associated with adverse health outcomes. We sought to determine if neighborhood socioeconomic deprivation was associated with adherence to immunosuppressive medications after liver transplantation.
Methods:
We conducted a secondary analysis of a multicenter, prospective cohort of children enrolled in the Medication Adherence in children who had a Liver Transplant study (enrollment 2010–2013). Participants (N=271) received a liver transplant ≥1 year prior to enrollment and were subsequently treated with tacrolimus. The primary exposure, connected to geocoded participant home addresses, was a neighborhood socioeconomic deprivation index (range 0–1, higher indicates more deprivation). The primary outcome was the Medication Level Variability Index (MLVI), a surrogate measure of adherence to immunosuppression in pediatric liver transplant recipients. Higher MVLI indicates worse adherence behavior; values ≥2.5 are predictive of late allograft rejection.
Findings:
There was a 5% increase in MLVI for each 0.1 increase in deprivation index (95%CI: −1%, 11%, p=0.08). Roughly 24% of participants from the most deprived quartile had an MLVI ≥2.5 compared to 12% in the remaining 3 quartiles (p=0.018). Black children were more likely to have high MLVI even after adjusting for deprivation (AOR 4.0 95%CI: 1.7, 10.6).
Conclusions:
This is the first study to evaluate associations between neighborhood socioeconomic deprivation and an objective surrogate measure of medication adherence in children posttransplant. These findings suggest that neighborhood context may be an important consideration when assessing adherence. Differential rates of medication adherence may partly explain links between neighborhood factors and adverse health outcomes following pediatric liver transplantation.
BACKGROUND
Neighborhood environment, including neighborhood socioeconomic deprivation, is associated with adverse health outcomes across multiple diseases.1–3 These links have informed changes to clinical practice by reorienting care toward community-focused, equity-driven interventions.4–6 The Chronic Care Model frames community as essential to self-management.7–9 While individual measures of psychosocial stress are associated with reduced medication adherence,10–15 the impact of neighborhood socioeconomic deprivation on medication adherence has been underexplored.6,16–24 Indeed, we found no studies in children or adults following solid organ transplantation that have evaluated the impact of neighborhood deprivation on a direct surrogate measure25 of medication adherence. If such an association emerged, it suggests that nonadherence may be a mechanism by which neighborhood deprivation leads to adverse health outcomes.
Strict adherence to immunosuppressive medications is necessary for pediatric liver transplant recipients to ensure long-term patient and allograft survival.26–28 To our knowledge, the landmark, multicenter Medication Adherence in children who had a Liver Transplant (MALT) study is the largest cohort of pediatric liver transplant recipients that includes an objective, direct surrogate measure of immunosuppressive medication adherence.29 This measure, the Medication Level Variability Index (MLVI), is a robust predictor of late allograft rejection (LAR)29–31 which itself is thought to be due to nonadherence in up to 90% of cases.32–36
Our primary objective was to determine if a validated composite measure of neighborhood socioeconomic deprivation was positively associated with MLVI. Secondarily, we sought to evaluate relationships between deprivation and family-reported medication barriers to more fully assess links between socioeconomic context and medication adherence in this clinically vulnerable population.
MATERIALS AND METHODS
Study Design and Data
We conducted a secondary analysis of data from the prospective, observational cohort of pediatric liver transplant recipients enrolled in the MALT29 study between 2010–2013 (NCT01154075). The present study utilized data from 4 of the 5 MALT sites and was approved by the Institutional Review Boards at all 4 institutions (Approval numbers: Cincinnati Children’s Hospital Medical Center 2017–2656; Lurie Children’s 2018–1718; University of Pittsburgh PRO18020604; and University of California Los Angeles 18–000032). We were unable to acquire data from the remaining site due to their IRB’s concerns regarding the collection and use of participant street addresses. Informed consent requirement was waived for these secondary analyses at each of the participating centers.
Participants
MALT participants were patients who underwent liver transplantation >1 year prior to enrollment and were on tacrolimus for immunosuppression. MALT inclusion/exclusion criteria, participant characteristics, and follow-up procedures are described elsewhere.29 For the present analyses, the most recently available participant street addresses were extracted from the electronic medical record (EMR) at each institution (obtained September 2017-May 2018). Participants whose addresses were unable to be collected or whose addresses were unsuccessfully geocoded (see below) were excluded from the analysis.
Outcomes
Our primary outcome was the MLVI.29 The MLVI is calculated as the standard deviation of ≥3 sequential tacrolimus trough levels; higher values indicate worse adherence.29,30,32,34–37 Tacrolimus trough levels are obtained at least quarterly at each of the participating institutions as standard of care immunosuppression management. We included all tacrolimus troughs collected over the 2-year study period. For patients who had an episode of LAR, trough levels after the episode of rejection were excluded because immunosuppression is often intensified in the treatment of LAR. The MLVI was found to be a predictor of LAR when used as a continuous variable or dichotomized.29 The a priori dichotomization in the primary MALT trial used <2.5 and ≥2.5.
Exposures
Within the broader MALT study, individual socioeconomic variables were collected, including: insurance type, caregiver’s highest educational attainment, and caregiver’s marital status.
An approximation of neighborhood-level socioeconomic deprivation was derived a compilation of unique measures available from the 2015 5-year American Community Survey completed by the US Census Bureau. We matched these measures to participants by geocoding their home street addresses to census tracts using DeGAUSS38 (SDC Methods). Census tracts, geographical units designated by the US Census Bureau, are “small, relatively permanent statistical subdivisions of a county…[they] generally have a population size between 1200 and 8000, with an optimum size of 4000.”39 They are intended to capture a relatively homogenous population for area-based study and therefore, are an ideal geographical unit to study neighborhood level effects.40
Given the lack of robust patient- or household-level socioeconomic data in MALT, beyond that introduced above, and given the hypothesis that context is relevant to child health in and of itself, we used an area-level variable to both approximate family/household experience and contextualize the patient’s environment. This area-level primary exposure variable was obtained via a validated index of neighborhood socioeconomic deprivation.41 The index has a range [0,1]. Values closer to 1 indicate more deprived census tracts. The components of the deprivation index (census tract-level fraction of households below federal poverty level, median household income, fraction of adults with high school education, fraction of individuals with health insurance, fraction of households receiving public assistance, and fraction of housing units that are vacant) are each associated with various health outcomes and can be extracted at the census tract level for every tract in the US.42 Methods for index development are described elsewhere.41
We also evaluated area-level covariables--urban/rural classification and distance to transplant center. For urban/rural classification, we coded participants’ counties of residence by the degree of urbanization using the Urban Influence Codes43 and dichotomized participants into “rural” or “urban” home environments based on previously published means of dichotomization.44 We calculated distance to transplant center using each participant’s address and the address of their transplant center. We defined distance using the great circle approach—the shortest distance between 2 points on the surface of a sphere (i.e. the shortest “as the crow flies” distance). We used the great circle distance instead of the driving distance or travel time because of the relative ease in calculating this distance across regions of the country.
Medication Barriers
We used the Parent Medication Barrier Scale (PMBS) and Adolescent Medication Barrier Scale (AMBS), both included in MALT-collected data.45 The PMBS/AMBS addresses 4 domains of medication adherence: Disease Frustration/Adolescent Issues, Regimen Adaptation/Cognition, Ingestion Issues, and Parent Reminder. The PMBS and AMBS are 16- and 17-question tools, respectively, with each question scored on a 5-point Likert scale. Scores are aggregated by domain. Higher scores are associated with more nonadherence45,46; thus, total scores were used in the present analyses.
Statistical Analysis
Participants with missing outcome data or whose addresses were unable to be geocoded precisely enough to assign a census tract were removed prior to analysis.
Descriptive statistics were then performed for baseline exposure variables. For our primary analytic strategy, we analyzed both the MLVI and deprivation index continuously and categorically. We evaluated these measures continuously to identify any potential nonlinear relationships; recognizing that, while this is statistically robust, it may not be clinically meaningful. Thus, we also evaluated these measures categorically as this could more seamlessly lead to the identification of at-risk subgroups. We used dichotomized MLVI (<2.5 and ≥2.5) because this could represent both a clinically meaningful cutoff for nonadherence and was predefined in the original MALT cohort.29 We classified participants by deprivation index quartiles to capture the effect of different levels of deprivation as done in previous adherence work.16 The PMBS/AMBS were kept as continuous variables in line with their previous use.
Chi-square testing was used to evaluate for associations among categorical variables. Cochran-Armitage test of trend was used for ordered categorical variables. The Cochran-Mantel-Haenszel statistic was used to test for differences in proportions across race groups for ordered categorical variables. To understand relationships between covariates and both primary exposure and outcome measures, Pearson’s or Spearman’s correlation coefficients were calculated for continuous data depending on whether underlying data distributions were parametric or nonparametric, respectively. Since the continuous MLVI was not normally distributed, it was log10-transformed for subsequent analyses. We used linear regression models for continuous log-transformed MLVI. Logistic regression was used for dichotomized MLVI. We adjusted for race because we conceptualized that race may affect neighborhood choice and outcomes separately, and thus, could confound the relationship between neighborhood deprivation and outcome.
Significance levels for all statistical analyses above were defined as p=0.05 a priori and hypothesis testing was 2-sided. Analyses were performed in SAS 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Study Sample
Of 320 available participants, 283 (88.4%) were geocoded to a range of street address (highest precision of software). Of the 283, MLVI data were missing from 12 leaving 271 participants (84.7%) for these analyses. Table 1 depicts participant demographic characteristics. About half were female; 72% identified as white and 11% as black. About 40% had state-funded insurance, and 83% came from 2-parent households. Table S1 depicts demographic characteristics of excluded participants. Compared to the included participants, the excluded center had more black participants, less participants with state-funded insurance or managed care health plans, and more participants from single-parent households.
Table 1.
Baseline characteristics by deprivation index and MLVI
| Overall N=271 | Deprivation Index | MLVI | |||
|---|---|---|---|---|---|
| Characteristic | Mean ± SD or n (%)1 | Mean ± SD or Correlation Coefficient2 | p-value3 | Median (Q1, Q3) or Spearman’s Correlation Coefficient4 | p-value5 |
| Age at MALT Enrollment (years) | 9.50 ± 4.499 | 0.0779 | 0.2009 | −0.1128 | 0.0636 |
| Age at Transplant (years) | 3.01 ± 3.554 | −0.0439 | 0.4713 | 0.1388 | 0.0223 |
| Distance to Transplant Center | 74.94 (27.050, 186.087) | −0.1717 | 0.0046 | −0.0226 | 0.7113 |
| Site | |||||
| Center A | 65 (23.99 %) | 0.34 ± 0.107 | 0.0013 | 1.45 (0.906, 2.084) | 0.1355 |
| Center B | 69 (25.46 %) | 0.36 ± 0.152 | 1.31 (0.821, 2.308) | ||
| Center C | 63 (23.25 %) | 0.34 ± 0.113 | 1.16 (0.826, 1.561) | ||
| Center D | 74 (27.31 %) | 0.42 ± 0.160 | 1.42 (0.891, 2.114) | ||
| Primary Diagnosis | |||||
| Acute Liver Failure | 28 (10.33 %) | 0.40 ± 0.141 | 0.6300 | 1.56 (1.093, 1.980) | 0.6687 |
| Biliary Atresia | 135 (49.82 %) | 0.37 ± 0.145 | 1.31 (0.839, 2.174) | ||
| Other Cholestatic diseases | 49 (18.08 %) | 0.36 ± 0.140 | 1.30 (0.861, 2.047) | ||
| Metabolic Diseases that primarily affect other organs | 21 (7.75 %) | 0.37 ± 0.126 | 1.40 (1.001, 1.663) | ||
| Liver Malignancies | 21 (7.75 %) | 0.40 ± 0.131 | 1.06 (0.699, 2.059) | ||
| Other | 17 (6.27 %) | 0.35 ± 0.121 | 1.23 (1.091, 1.529) | ||
| Donor Type | |||||
| Deceased | 218 (80.44 %) | 0.37 ± 0.143 | 0.2775 | 1.36 (0.882, 2.114) | 0.0680 |
| Living | 53 (19.56 %) | 0.35 ± 0.124 | 1.25 (0.742, 1.713) | ||
| Gender | |||||
| Male | 131 (48.34 %) | 0.36 ± 0.140 | 0.3276 | 1.33 (0.839, 2.035) | 0.9302 |
| Female | 140 (51.66 %) | 0.38 ± 0.139 | 1.35 (0.889, 2.019) | ||
| Race | |||||
| Asian | 13 (4.80 %) | 0.31 ± 0.117 | 0.0002 | 1.43 (1.278, 2.477) | 0.0215 |
| Black or African American | 30 (11.07 %) | 0.47 ± 0.143 | 1.67 (1.067, 3.336) | ||
| White or Caucasian | 195 (71.96 %) | 0.36 ± 0.137 | 1.30 (0.819, 1.860) | ||
| Other | 33 (12.18 %) | 0.37 ± 0.125 | 1.27 (0.844, 2.392) | ||
| Ethnicity | |||||
| Hispanic or Latino | 60 (22.14 %) | 0.48 ± 0.136 | <0.0001 | 1.34 (0.866, 2.154) | 0.6926 |
| Not Hispanic or Latino | 204 (75.28 %) | 0.33 ± 0.121 | 1.34 (0.856, 1.928) | ||
| Not reported | 7 (2.58 %) | 0.44 ± 0.140 | 1.15 (0.794, 2.047) | ||
| Primary Insurance | |||||
| Medicaid or equivalent and/or state funded children’s services | 114 (42.07 %) | 0.44 ± 0.142 | <0.0001 | 1.51 (0.943, 2.321) | 0.1259 |
| HMO/managed care | 77 (28.41 %) | 0.34 ± 0.113 | 1.24 (0.832, 1.752) | ||
| Traditional private insurance | 65 (23.99 %) | 0.29 ± 0.118 | 1.27 (0.811, 1.738) | ||
| Other | 15 (5.54 %) | 0.34 ± 0.084 | 1.17 (1.012, 1.523) | ||
| Primary Caregiver’s Marital Status | |||||
| Single-parent household | 45 (16.61 %) | 0.42 ± 0.138 | 0.0098 | 1.35 (0.821, 2.254) | 0.6653 |
| Two-parent household | 224 (82.66 %) | 0.36 ± 0.138 | 1.33 (0.869, 1.943) | ||
| Missing | 2 (0.74 %) | 0.43 ± 0.186 | 0.69 (0.560, 0.811) | ||
| Primary Caregiver’s Highest Education Level | |||||
| Some high school or less | 28 (10.33 %) | 0.53 ± 0.136 | <0.0001 | 1.43 (0.923, 2.336) | 0.1142 |
| High school degree/GED | 58 (21.40 %) | 0.43 ± 0.123 | 1.54 (1.019, 2.433) | ||
| Vocational school or some college | 56 (20.66 %) | 0.38 ± 0.109 | 1.32 (0.933, 2.072) | ||
| College degree | 79 (29.15 %) | 0.31 ± 0.117 | 1.34 (0.817, 1.860) | ||
| Professional or graduate degree | 34 (12.55 %) | 0.26 ± 0.099 | 1.25 (0.783, 1.663) | ||
| Missing | 16 (5.90 %) | 0.37 ± 0.153 | 1.11 (0.826, 1.746) | ||
| Rurality | |||||
| Metropolitan | 234 (86.35 %) | 0.36 ± 0.145 | 0.0727 | 1.32 (0.861, 1.936) | 0.5748 |
| Non-Metropolitan | 37 (13.65 %) | 0.41 ± 0.087 | 1.48 (0.851, 2.420) | ||
MLVI: Medication level variability index; SD: standard deviation; Q: quartile; MALT: Medication Adherence in children who had a Liver Transplant; HMO: Health maintenance organization; GED: General education development
Mean ± SD or Median (Q1, Q3) for continuous variables and frequency and percentage for categorical variables
Pearson’s/Spearman’s correlation coefficient for continuous variables and Mean ± SD in each of the category of a categorical variable
p-value to test statistical significance of correlation coefficient between continuous variables or to test significant difference in mean Deprivation Index across the categories of a categorical variable
Spearman’s correlation coefficient for continuous variables and Median (Q1, Q3) in each of the category of a categorical variable
p-value to test statistical significance of correlation coefficient between continuous variables or to test significant difference in median MLVI across the categories of a categorical variable
Baseline Characteristics, Exposure and Outcome Variables
The mean deprivation index was 0.37 (SD ±0.14). The median MLVI was 1.33 (IQR 0.85–2.01). Figure S1 depicts distributions of the deprivation index and MLVI. Baseline characteristics and the association of baseline characteristics with both the primary exposure and outcome are shown in Table 1. The deprivation index was associated with each individually-collected MALT socioeconomic variable: participants with state-funded health insurance, single-parent-headed households, and caregivers with less educational attainment lived in census tracts with higher deprivation scores. Black race was also associated with both more neighborhood socioeconomic deprivation and higher MLVIs. Rurality was not associated with socioeconomic deprivation or MVLI. There was no difference in the number of tacrolimus trough levels obtained per participant across deprivation index quartiles (p=0.53).
Continuous MLVI and deprivation index
There was a 5% increase (95%CI: −1%, 11%, p=0.08) in MLVI for each 0.1 increase in the deprivation index (Table 2). When race was included, there was a 3% (95%CI: −3%, 9%, p=0.29) increase in MLVI for each 0.1 increase in the deprivation index. The effect of black race remained significant in the adjusted model (β: 1.03; 95%CI: 1.01, 1.06, p=0.01).
Table 2.
Linear regression models with log-transformed MLVI as a continuous outcome variable
| Variable | Estimatesa | 95% CI | p-value | |
|---|---|---|---|---|
| Unadjusted | Deprivation Index | 1.05 | 0.99 – 1.11 | 0.08 |
| Adjusted | Deprivation Index | 1.03 | 0.97 – 1.09 | 0.29 |
| Race | ||||
| Black or AA | 1.03 | 1.01 – 1.06 | 0.01 | |
| All other races (reference) |
MLVI: Medication level variability index; AA: African American; CI: Confidence interval
Estimates reflect the expected fold change in MLVI for every 0.1 increase in the deprivation index.
Dichotomized MLVI and deprivation index
Because we did not find a linear relationship between the continuous measures of deprivation and MLVI, participants were then categorized into deprivation index quartiles and by the clinically meaningful MLVI cut-off of 2.5 (<2.5 and ≥2.5, hereafter referred to as “low variability” and ”high variability,” respectively).29 There did not appear to be a dose-response relationship (p=0.12); however, there did appear to be a threshold effect (Figure 1). That is, a total of 23.5% of participants from the most deprived quartile had an MLVI ≥2.5 compared to 11.8% for the remainder of the cohort (p=0.018). There were no differences in the proportion of black participants from the most deprived quartile that had high variability compared to black participants from the remaining quartiles of neighborhood deprivation (p=0.19).
Figure 1. Percent of participants with an MVLI ≥ 2.5 by deprivation index quartiles.
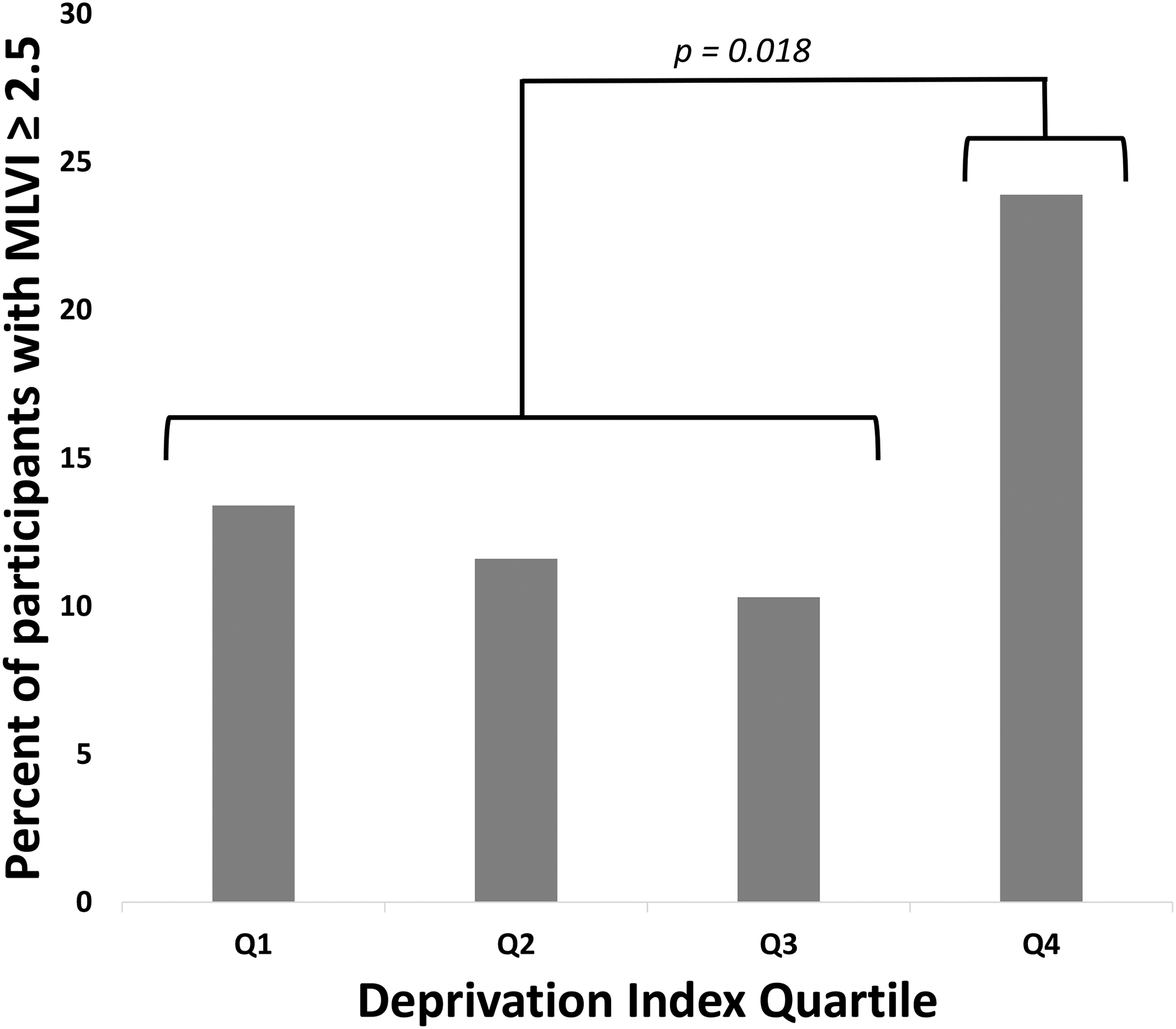
Q1: Quartile 1 (0 – 0.27, n = 67); Q2: Quartile 2 (0.27 – 0.34, n = 69); Q3: Quartile 3 (0.34 – 0.47, n = 68); Q4: Quartile 4 (0.47– 1, n = 67); MLVI: Medication Level Variability Index; OR: Odds ratio
When evaluating for a trend across quartiles, Cochran-Armitage test was p = 0.12. Chi-square comparing the highest quartile to the rest of the cohort (23.9% vs. 11.8%, respectively) yielded a p = 0.018.
That said, when we examined the entire cohort, black participants were more likely to have high variability compared to white participants (OR 4.23 95%CI: 1.83, 9.78, p<0.001) (Table 3). The OR for black participants to have high variability was 3.97, compared to white participants (95%CI: 1.65, 9.58, p=0.002) after adjusting for neighborhood deprivation.
Table 3.
Logistic regression models with dichotomized MLVI (≥2.5 or <2.5)
| Variable | Odds Ratioa | 95% CI | p-value | |
|---|---|---|---|---|
| Unadjusted | Race | |||
| Black | 4.23 | 1.83 – 9.78 | <0.001 | |
| All other races (reference) | ||||
| Adjusted | Deprivation index | 1.06 | 0.82 – 1.36 | 0.65 |
| Race | ||||
| Black race | 3.97 | 1.65 – 9.58 | 0.002 | |
| All other races (reference) |
MLVI: Medication level variability index; AA: African American; CI: Confidence interval.
The reported odds ratio represents the odds of having an MLVI ≥2.5 for each 0.1 increase in the deprivation index.
Parent & Adolescent Barriers to Medication Scales
Increased deprivation was correlated with a higher number of barriers on the PMBS (Figure S2; Pearson’s rho=0.17, p=0.004); however, the number of barriers on the PMBS was not correlated with MLVI. The deprivation index was not correlated with the number of barriers on the AMBS, but a greater number of barriers on the AMBS was correlated with increased MLVI (Pearson’s rho=0.2, p=0.04).
DISCUSSION
We sought to determine if neighborhood deprivation was associated with medication adherence using data from a multisite US cohort of pediatric liver transplant recipients. MLVI was not correlated with deprivation when it was assessed as a continuous variable. When we used deprivation index quartiles and dichotomized MLVI to reflect clinically meaningful cutoffs, those from the most deprived quartile were twice as likely to be nonadherent. There was no effect of race on adherence for participants from the most deprived quartile—further suggesting that this finding was due to neighborhood socioeconomic deprivation. This suggests a nonlinear relationship whereby deprivation is associated with nonadherence risk only among those who live in severely deprived areas. Certainly, past research has demonstrated a possible nonlinear relationship between neighborhood poverty and medication adherence at a population level.16 From a theoretical standpoint, it would make sense that there might be a point where neighborhood characteristics (e.g. violence, severe poverty, poor social cohesion) overwhelm and diminish one’s ability to adhere to strict medication regimens.
It is likely that the impact of neighborhood deprivation on outcomes following liver transplant is complex and may affect both adherence and graft survival47 which could further confound the assessment of nonadherence on long-term graft survival. Certainly, data across diseases and patient populations indicate that neighborhood contextual factors are important to one’s health48,49 and may be important contributors to effective self-management. We suggest that future studies are needed to explore, both quantitatively and qualitatively, why children from the most deprived neighborhoods are at greatest risk. Such data may lead to the identification of novel interventions that effectively address nonadherence in this subset of patients.
Our study did not demonstrate an association between individual socioeconomic factors available for the MALT cohort and medication adherence—a finding that has been demonstrated in some reports but not others.50 It is possible that neighborhood socioeconomic deprivation is a better proxy measure for a more direct cause of nonadherence behavior (e.g. medical illiteracy, increased parental psychosocial stress); however, future studies are needed. We posit that a child’s individual socioeconomic background and their neighborhood context both have influences on adherence behavior following liver transplantation, that neighborhood deprivation might provide similar yet distinct contextual characteristics not captured with individual socioeconomic measures alone.
Our study demonstrated an association between black race and increased MLVI. This finding persisted in a multivariable regression model that included the neighborhood-level deprivation index and, thus, cannot be explained by our measures of deprivation alone. While there are known racial and genotypic differences in tacrolimus metabolism,51,52 the MLVI reflects the intrapatient variability of tacrolimus; therefore, such differences cannot explain our findings since one’s metabolism would not be expected to change over time. We posit that our findings linking race to adverse outcomes stem primarily from race’s role as a social construct. Indeed, we see it as encompassing multiple dimensions that may not be adequately captured by our exposure variables.53 Such dimensions include adversity over time, institutional and interpersonal discrimination, bias, limited trust in the healthcare system, and elevated risk of exposure to psychosocial stressors; all of which might lead to medication nonadherence.53,54 This work provides further support that black children undergoing liver transplantation are systematically disadvantaged across the phases of transplant. Future work to identify how the healthcare system could improve care for black children is sorely needed to realize equitable outcomes.
The influence of neighborhood contextual characteristics on medication adherence has been minimally explored following solid organ transplantation.16–18 To our knowledge, there are no published studies that utilize an objective surrogate of adherence as the primary outcome. Census tracts are regarded as the ideal geographic unit to study the effects of place-based risk on health outcomes,49 yet their use is limited in multicenter studies38 due to the complexity of transmitting private health information under HIPAA. While ZIP codes (groups of streets defined for efficiency reasons by the US Postal Office) are often used, they have the greatest potential for bias.40 Through the use of a specialized and open source software tool called DeGAUSS, we utilized census tract-level measures without requiring study sites to share private health information. The resulting increased precision and decreased measurement error is an obvious strength of this study.
We acknowledge the following limitations. First, most studies on neighborhood risk use much larger samples49—our study had only 42% power to detect a 5% increase in MLVI for each 0.1 increase in the deprivation index in univariable analysis. Therefore, we were underpowered to determine the effect of neighborhood deprivation in excess of individual socioeconomic status. However, the MALT dataset29,30 is the largest pediatric liver transplant dataset to include an objective surrogate measure of nonadherence. Most studies on medication adherence use indirect measures of adherence.25 The MALT data is further strengthened by having a well-defined cohort and rigorously collected data. Second, we were limited by our use of most recent address rather than enrollment address. This likely lead to a small, nondifferential misclassification error for participants who moved after enrollment in MALT that could bias our results towards the null. Furthermore, residential mobility tends to be higher in lower income groups despite their moving to sociodemographically similar neighborhoods.55 Future studies could assess whether housing instability is associated with medication adherence. Third, although the deidentified MALT dataset was available to us in its entirety, 1 site was unable to reaccess medical charts because of confidentiality concerns. Given the slight differences in the demographics of this excluded site from the rest of the MALT cohort, the potential for bias, while minimal, does exist. Fourth, this study captured adherence behavior over 2 years, which is a relatively long period. However, adherence behavior does change over time30 and, therefore, there may be differences in adherence trajectory for children with different neighborhood-based exposures. Finally, this study only included pediatric liver transplant recipients. While, after the first year of transplant, pediatric liver transplant recipients are similar to other children with a transplant, there may be differences in provider engagement, perceived risk of nonadherence, or other factors that affect adherence. Therefore, generalizability beyond pediatric liver transplant recipients is limited; however, we suspect that the etiologies of nonadherence are similar across pediatric solid organ transplantation.
Neighborhood-level ‘geomarkers’5 are increasingly seen as tools that could help stratify patients and inform the targeted deployment of relevant interventions.4–6 In recognition of the role of community/neighborhood on health and disease, the National Academy of Medicine now recommends the routine use of geocoded area-based measures in clinical care in addition to individual psychosocial measures.56,57 While such screening is being implemented in primary care,3–6,58–60 use in pediatric transplant medicine is limited. A necessary first step is to identify the mechanism by which place-based risk affects outcomes in pediatric liver transplantation; medication adherence may be a key component as nonadherence remains a major challenge for the medical community.61 As we seek to personalize care for our patients,62 a better understanding of our patients’ place-based context will allow for a more comprehensive understanding of their health risks and could lead to targeted strategies to improve their health.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Estella M. Alonso, MD; Stephanie Farabaugh, BSN, RN; Susan Feist, RN; Andre Hawkins, MA; George Mazariegos, MD; Katie Neighbors, MPH and Robert Venick, MD for their help in acquiring the additional data. The authors would like to acknowledge Lee (Ted) Denson, MD; James E. Heubi, MD; Bin Huang, PhD; Scott Pentiuk, MD and Patrick Ryan, PhD for their feedback on study design and analytic plans.
Financial Disclosure: NIH / NIDDK R01DK080740 (PI: Eyal Shemesh); NIH T32DK007727-24 (PI: Lee Denson; support for S.I.W.); 1K23AI112916 (PI: Andrew Beck). The research reported was partially supported by the Sherrod Family Fund; The research reported was partially supported by the Arnold W. Strauss Fellow Award at Cincinnati Children’s Hospital Medical Center.
The funders had no role in the design or analysis of the study. Furthermore, the corresponding author had full access to the data and had final responsibility for decision to submit this manuscript.
Abbreviations
- ABM
area-based measures
- AMBS
Adolescent Medication Barriers Scale
- EMR
electronic medical record
- LAR
late acute cellular rejection
- MALT
Medication Adherence in children who had a Liver Transplant
- MLVI
medication level variability index
- PHI
Private Health Information
- PMBS
Parent Medication Barriers Scale
- ZIP
Zone Improvement Plan
Footnotes
Disclaimer: The authors declare no conflicts of interest.
Clinical Trial: This was a secondary analysis of data collected as part of ClinicalTrials.gov: NCT01154075.
REFERENCES
- 1.Schroeder SA. We can do better--improving the health of the American people. N Engl J Med. 2007;357(12):1221–1228. [DOI] [PubMed] [Google Scholar]
- 2.Heiman HJ, Artiga S. Beyond health care: the role of social determinants in promoting health and health equity. Kaiser Family Foundation website. 2015. Available at https://www.kff.org/disparities-policy/issue-brief/beyond-health-care-the-role-of-social-determinants-in-promoting-health-and-health-equity/. Accessed November 4, 2015. [Google Scholar]
- 3.Beck AF, Simmons JM, Huang B, et al. Geomedicine: area-based socioeconomic measures for assessing risk of hospital reutilization among children admitted for asthma. Am J Public Health. 2012;102(12):2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck AF, Klein MD, Schaffzin JK, et al. Identifying and treating a substandard housing cluster using a medical-legal partnership. Pediatrics. 2012;130(5):831–838. [DOI] [PubMed] [Google Scholar]
- 5.Beck AF, Sandel MT, Ryan PH, et al. Mapping neighborhood health geomarkers to clinical care decisions to promote equity in child health. Health Aff (Millwood). 2017;36(6):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kercsmar CM, Beck AF, Sauers-Ford H, et al. Association of an asthma improvement collaborative with health care utilization in Medicaid-insured pediatric patients in an urban community. JAMA Pediatr. 2017;171(11):1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman K, Austin BT, Brach C, et al. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood). 2009;28(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner EH. Managed care and chronic illness: health services research needs. Health Serv Res. 1997;32(5):702–714. [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4. [PubMed] [Google Scholar]
- 10.Killian MO. Psychosocial predictors of medication adherence in pediatric heart and lung organ transplantation. Pediatr Transplant. 2017;21(4). [DOI] [PubMed] [Google Scholar]
- 11.Dobbels F, Van Damme-Lombaert R, Vanhaecke J, et al. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant. 2005;9(3):381–390. [DOI] [PubMed] [Google Scholar]
- 12.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahon S, Lahmek P, Saas C, et al. Socioeconomic and psychological factors associated with nonadherence to treatment in inflammatory bowel disease patients: results of the ISSEO survey. Inflamm Bowel Dis. 2011;17(6):1270–1276. [DOI] [PubMed] [Google Scholar]
- 14.Cousino MK, Rea KE, Schumacher KR, et al. A systematic review of parent and family functioning in pediatric solid organ transplant populations. Pediatr Transplant. 2017;21(3). [DOI] [PubMed] [Google Scholar]
- 15.Fredericks EM, Lopez MJ, Magee JC, et al. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. Am J Transplant. 2007;7(8):1974–1983. [DOI] [PubMed] [Google Scholar]
- 16.Hensley C, Heaton PC, Kahn RS, et al. Poverty, transportation access, and medication nonadherence. Pediatrics. 2018;141(4):e20173402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billimek J, August KJ. Costs and beliefs: understanding individual- and neighborhood-level correlates of medication nonadherence among Mexican Americans with type 2 diabetes. Health Psychol. 2014;33(12):1602–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.August KJ, Billimek J. A theoretical model of how neighborhood factors contribute to medication nonadherence among disadvantaged chronically ill adults. J Health Psychol. 2016;21(12):2923–2933. [DOI] [PubMed] [Google Scholar]
- 19.Hoang C, Kolenic G, Kline-Rogers E, et al. Mapping geographic areas of high and low drug adherence in patients prescribed continuing treatment for acute coronary syndrome after discharge. Pharmacotherapy. 2011;31(10):927–933. [DOI] [PubMed] [Google Scholar]
- 20.Denhaerynck K, Berben L, Dobbels F, et al. Multilevel factors are associated with immunosuppressant nonadherence in heart transplant recipients: the international BRIGHT study. Am J Transplant. 2018;18(6):1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbass I, Revere L, Mitchell J, et al. Medication nonadherence: the role of cost, community, and individual factors. Health Serv Res. 2017;52(4):1511–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surratt HL, Kurtz SP, Levi-Minzi MA, et al. Environmental influences on HIV medication adherence: the role of neighborhood disorder. Am J Public Health. 2015;105(8):1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pablos-Méndez A, Knirsch CA, Barr RG, et al. Nonadherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med. 1997;102(2):164–170. [DOI] [PubMed] [Google Scholar]
- 24.McQuaid EL, Everhart RS, Seifer R, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics. 2012;129(6):e1404–e1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. [DOI] [PubMed] [Google Scholar]
- 26.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 2009;88(5):736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshizawa A, Kaneshiro M, Uebayashi E, et al. Adherence of immunosuppression therapy and donor-specific anti-HLA antibodies in the pediatric liver transplant recipients. Transplantation. 2018;102:S879. [Google Scholar]
- 28.Hugon A, Roustit M, Lehmann A, et al. Influence of intention to adhere, beliefs and satisfaction about medicines on adherence in solid organ transplant recipients. Transplantation. 2014;98(2):222–228. [DOI] [PubMed] [Google Scholar]
- 29.Shemesh E, Bucuvalas JC, Anand R, et al. The medication level variability index (MLVI) predicts poor liver transplant outcomes: a prospective multi-site study. Am J Transplant. 2017;17(10):2668–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shemesh E, Duncan S, Anand R, et al. Trajectory of adherence behavior in pediatric and adolescent liver transplant recipients: the medication adherence in children who had a liver transplant cohort. Liver Transpl. 2018;24(1):80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayar M, Tron C, Jézéquel C, et al. High intrapatient variability of tacrolimus exposure in the early period after liver transplantation is associated with poorer outcomes. Transplantation. 2018;102(3):e108–e114. [DOI] [PubMed] [Google Scholar]
- 32.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant. 2010;14(8):940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christina S, Annunziato RA, Schiano TD, et al. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20(10):1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shemesh E, Annunziato RA, Shneider BL, et al. Improving adherence to medications in pediatric liver transplant recipients. Pediatr Transplant. 2008;12(3):316–323. [DOI] [PubMed] [Google Scholar]
- 35.Stuber ML, Shemesh E, Seacord D, et al. Evaluating non-adherence to immunosuppressant medications in pediatric liver transplant recipients. Pediatr Transplant. 2008;12(3):284–288. [DOI] [PubMed] [Google Scholar]
- 36.Shemesh E, Shneider BL, Savitzky JK, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113(4):825–832. [DOI] [PubMed] [Google Scholar]
- 37.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14(8):968–975. [DOI] [PubMed] [Google Scholar]
- 38.Brokamp C, Wolfe C, Lingren T, et al. Decentralized and reproducible geocoding and characterization of community and environmental exposures for multisite studies. J Am Med Inform Assoc. 2018;25(3):309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossiter K. Understanding geographic relationships: counties, places, tracts and more. United States Census Bureau Random Samplings website. 2014. Available at https://www.census.gov/newsroom/blogs/random-samplings/2014/07/understanding-geographic-relationships-counties-places-tracts-and-more.html. Accessed January 2020. [Google Scholar]
- 40.Krieger N, Waterman P, Chen JT, et al. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas--the Public Health Disparities Geocoding Project. Am J Public Health. 2002;92(7):1100–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brokamp C, Beck AF, Goyal NK, et al. Material community deprivation and hospital utilization during the first year of life: an urban population–based cohort study. Ann Epidemiol. 2019;30:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuurman N, Bell N, Dunn JR, et al. Deprivation indices, population health and geography: an evaluation of the spatial effectiveness of indices at multiple scales. J Urban Health. 2007;84(4):591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urban Influence Codes Documentation. United States Department of Agriculture: Economic Research Service website. 2017. Available at https://www.ers.usda.gov/data-products/urban-influence-codes/documentation/. Updated November 27, 2017. Accessed February 5, 2019.
- 44.Park KT, Bensen R, Lu B, et al. Geographical rural status and health outcomes in pediatric liver transplantation: an analysis of 6 years of national United Network of Organ Sharing Data. J Pediatr. 2013;162(2):313–318.e1. [DOI] [PubMed] [Google Scholar]
- 45.Simons LE, Blount RL. Identifying barriers to medication adherence in adolescent transplant recipients. J Pediatr Psychol. 2007;32(7):831–844. [DOI] [PubMed] [Google Scholar]
- 46.Simons LE, McCormick ML, Devine K, et al. Medication barriers predict adolescent transplant recipients’ adherence and clinical outcomes at 18-month follow-up. J Pediatr Psychol. 2010;35(9):1038–1048. [DOI] [PubMed] [Google Scholar]
- 47.Driollet B, Bayer F, Chatelet V, et al. Social deprivation is associated with poor kidney transplantation outcome in children. Kidney Int. 2019;96(3):769–776. [DOI] [PubMed] [Google Scholar]
- 48.Krieger N, Chen JT, Waterman PD, et al. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krieger N, Chen JT, Waterman PD, et al. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Killian MO, Schuman DL, Mayersohn GS, et al. Psychosocial predictors of medication non-adherence in pediatric organ transplantation: a systematic review. Pediatr Transplant. 2018;22(4):e13188. [DOI] [PubMed] [Google Scholar]
- 51.Andrews PA, Sen M, Chang RW. Racial variation in dosage requirements of tacrolimus. Lancet. 1996;348(9039):1446. [DOI] [PubMed] [Google Scholar]
- 52.Khaled SK, Palmer JM, Herzog J, et al. Influence of absorption, distribution, metabolism, and excretion genomic variants on tacrolimus/sirolimus blood levels and graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(2):268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. [DOI] [PubMed] [Google Scholar]
- 55.Brokamp C, LeMasters GK, Ryan PH. Residential mobility impacts exposure assessment and community socioeconomic characteristics in longitudinal epidemiology studies. J Expo Sci Environ Epidemiol. 2016;26(4):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Io Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 57.Adler NE, Stead WW. Patients in context--EHR capture of social and behavioral determinants of health. N Engl J Med. 2015;372(8):698–701. [DOI] [PubMed] [Google Scholar]
- 58.Beck AF, Klein MD, Kahn RS. Identifying social risk via a clinical social history embedded in the electronic health record. Clin Pediatr (Phila). 2012;51(10):972–977. [DOI] [PubMed] [Google Scholar]
- 59.Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children--outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063–1073. [DOI] [PubMed] [Google Scholar]
- 60.Shah AN, Simmons J, Beck AF. Adding a vital sign: considering the utility of place-based measures in health care settings. Hosp Pediatr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cutler DM, Everett W. Thinking outside the pillbox--medication adherence as a priority for health care reform. N Engl J Med. 2010;362(17):1553–1555. [DOI] [PubMed] [Google Scholar]
- 62.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


