Abstract
This study investigated predictors of overall and test-specific colorectal cancer screening (CRCS). Stool blood test (SBT) and/or colonoscopy screening were offered to primary care patients in two randomized controlled trials which assessed the impact of behavioral interventions on screening. Data were obtained through surveys and electronic medical records. Among 1942 participants, 646 (33%) screened. Exposure to interventions was associated with higher overall CRCS by twofold to threefold; older age, African American race, being married, and having a higher screening decision stage were also associated with higher overall CRCS (odds ratios = 1.30, 1.31, 1.34, and 5.59, respectively). Intervention, older age, female gender, and being married were associated with higher SBT adherence, while preference for colonoscopy was associated with lower SBT adherence. Intervention and higher decision stage were associated with higher colonoscopy adherence, while preference for SBT was associated with lower colonoscopy adherence. Among older individuals, African Americans had higher overall CRCS than whites, but this was not true among younger individuals (interaction p = 0.041). The higher screening adherence of African Americans over whites was due to stronger screening with a non-preferred test, i.e., higher SBT adherence only among individuals who preferred colonoscopy and higher colonoscopy adherence only among individuals who preferred SBT. Intervention exposure, sociodemographic background, and screening decision stage predicted overall CRCS adherence. Gender and test preference also affected test-specific screening adherence. Interactions involving race and test preference suggest that it is important to provide both colonoscopy and SBT screening options to patients, particularly African Americans.
INTRODUCTION
Healthy People 2020 has called for colorectal cancer screening (CRCS) rates of at least 70%,1 and the National Colorectal Cancer Roundtable has set the goal at 80%.2 Currently, about one in three Americans is not up-to-date with CRCS guidelines, and screening adherence among African Americans is lower than among whites.3,4 Colonoscopy is by far the most common screening modality (about six times more common than stool blood test).4
Previous studies have identified a number of sociodemographic characteristics (e.g., age, sex, race, and ethnicity, marital status, education, socioeconomic status and income),5–13 and behavioral factors (e.g., perceived susceptibility and self-efficacy)10,12–14 that may influence CRCS uptake. Provider and access factors (e.g., insurance status, provider recommendation, and frequency of health care visits) have been reported to play an equally strong role in primary care patients’ CRCS adherence.5–6,8–9,11–12,14–15 Screening test preference has also been identified as a potential predictor of whether an individual screens and through which modality.16–17
This paper aims to identify predictors of overall and test-specific CRCS adherence (i.e., stool blood test, SBT, and colonoscopy screening) among primary care patients, using data from two completed randomized controlled trials of CRCS behavioral interventions.16–17 We also aimed to determine whether the role of such predictors differs among whites and African Americans.
METHODS
Participants
Participants in two randomized controlled trials were between 50 and 79 years of age and were not up to date with American Cancer Society (ACS) and United States Preventive Services Task Force (USPSTF) CRCS guidelines. The first trial enrolled whites and non-whites at 10 primary care practices in Delaware, between 2007 and 2010.16 Participants were randomized to one of three groups: usual care (UC), mailed standard intervention (SI), or tailored navigation intervention (TNI). The second trial enrolled African Americans at 13 primary care practices in Philadelphia, Pennsylvania, between 2008 and 2011.17 Participants were randomized to either an SI or a TNI group. In addition, a linked sub-study included a non-randomized group of African American patients who received a mailed tailored intervention (TI). The trials were approved by the Institutional Review Boards of the participating sites and informed consent was obtained from all participants.
Intervention delivery and data collection procedures were similar in the two trials. The control group (UC) received the usual care at their practice, typically relying on providers to identify eligible patients, discuss CRCS, and recommend screening. When this occurred, the screening modality offered was almost invariably colonoscopy. SI participants were mailed both a stool blood test kit (SBT) and instructions on how to schedule a colonoscopy. TI participants received the mailed screening materials, but only for the screening test they preferred at enrollment. TNI participants were mailed similar tailored materials for their preferred test, and received telephone navigation to encourage adherence. Details about the rationale, design, and main outcomes of these studies have been reported elsewhere.16–17
Data Collection
Data on sociodemographic characteristics and perceptions related to CRCS were obtained via administration of a baseline and an endpoint survey. In accordance with the Preventive Health Model,18–19 we collected data that allowed us to compute a global CRCS perceptions scale score and perception scores on five component subscales: perceived colorectal cancer risk/susceptibility; perceived salience and coherence of screening; perceived response efficacy of screening; worries about screening; and perceived social support and influence related to screening. We also assessed each participant’s stage of decision making for overall CRCS, and stage of decision making separately for SBT screening and colonoscopy screening. These separate measures allowed for the determination of screening test preference.20 Each of these measures has been validated and has been used in previous studies.16–17
Screening adherence was assessed via an endpoint medical records review, supplemented by data from the endpoint survey. Adherence was defined as performance of any CRCS test recommended by guidelines that were in place when the trials were conducted (i.e., SBT annually or colonoscopy every 10 years). We counted any test performed within 12 months following trial enrollment.
Data Analyses
The objectives of this paper were to: 1) identify predictors of overall CRCS and test-specific screening adherence; and 2) determine if the effect of these factors differs for whites vs. African Americans. We pooled data from both trials, and analyzed overall CRCS adherence (yes/no) via binary logistic regression, and test-specific screening adherence (no screening, SBT screening, or colonoscopy screening) via multinomial logistic regression. The main model included practice, study group (exposure to behavioral intervention), five sociodemographic variables (age, sex, race, marital status, and education), the global screening attitudes score, the overall decision stage (decided to screen, undecided about screening, or decided not to screen), and test preference (prefer SBT, equal preference for SBT and colonoscopy, or prefer colonoscopy). Furthermore, we assessed interactions between race and other variables, one at a time. All statistical analyses were performed in SAS 9.4 in 2017. The study had 80% power to detect interaction odds ratios (ORs) of about 2.0 or higher, depending on the distribution of each candidate predictor in the two racial groups.
RESULTS
The two trials originally enrolled 1988 participants between 2007 and 2011 (see supplementary Figures S1 and S2). After excluding 28 participants who reported a race other than white or African American and 18 participants with incomplete data, we conducted analyses for 1942 individuals (732 whites and 1210 African Americans).
Table 1 shows that participants were predominantly female (65%) and between 50 and 59 years of age (71%), and overwhelmingly (90%) held favorable views on screening. The distribution of CRCS decision stage was: prefer to screen (82%), undecided about screening (15%), and prefer not to screen (3%). Finally, 32% of participants preferred colonoscopy screening, 50% had an equal preference for SBT and colonoscopy, and 18% preferred SBT.
Table 1.
Characteristics of study participants (N = 1,942).
| Study group/intervention, n (%) | ||
| Usual care (UC) | 299 | (15) |
| Standard intervention (SI) | 685 | (35) |
| Tailored intervention (TI) | 278 | (14) |
| Tailored navigation intervention (TNI) | 680 | (35) |
| Sex, n (%) | ||
| Male | 575 | (30) |
| Female | 1267 | (65) |
| Age (years), n (%) | ||
| 50–59 | 1375 | (71) |
| 60+ | 567 | (29) |
| Marital status, n (%) | ||
| Not married | 1061 | (55) |
| Married | 881 | (45) |
| Education, n (%) | ||
| High school or less | 1005 | (52) |
| Beyond high school | 937 | (48) |
| Screening attitudes (global score), n (%) | ||
| Low (≤3) | 195 | (10) |
| High (>3) | 1747 | (90) |
| Susceptibility,* n (%) | ||
| Low (≤3) | 1531 | (79) |
| High (>3) | 391 | (20) |
| Screening salience,* n (%) | ||
| Low (≤3) | 53 | (3) |
| High (>3) | 1887 | (97) |
| Screening response efficacy,* n (%) | ||
| Low (≤3) | 262 | (13) |
| High (>3) | 1629 | (84) |
| Screening worries,* n (%) | ||
| Low (≤3) | 1240 | (64) |
| High (>3) | 676 | (35) |
| Social influence/support* | ||
| Low (≤3) | 250 | (13) |
| High (>3) | 1670 | (86) |
| Decision stage | ||
| Prefer not to screen | 51 | (3) |
| Undecided about screening | 290 | (15) |
| Prefer to screen | 1601 | (82) |
| Preferred test | ||
| Stool blood test (SBT) | 351 | (18) |
| Equal preference | 975 | (50) |
| Colonoscopy (CX) | 616 | (32) |
Counts for the attitudes subscales may not sum to 1,942 because of occasional missing data.
Overall CRCS Adherence
A total of 646 (33%) participants adhered to screening within 12 months following enrollment. Table 2 summarizes the predictors of overall CRCS adherence. Exposure to behavioral interventions increased screening (twofold to fourfold, depending on the intensity of the intervention). Participants were also more likely to screen if they were 60 or more years of age (odds ratio, OR = 1.30; 95% confidence interval, CI: 1.04 to 1.63), were married (OR = 1.34, 95% CI: 1.08 to 1.68), or had a higher screening decision stage (undecided vs. prefer not to screen, OR = 2.33, 95% CI: 0.86 to 6.34; prefer to screen vs. prefer not to screen, OR = 5.59, 95% CI: 2.11 to 14.8). In additional analyses, no CRC screening perceptions were found to be associated with CRCS adherence (results not shown).
Table 2.
Predictors of overall screening adherence among study participants.
| Screened |
|||||
|---|---|---|---|---|---|
| N | n | (%) | OR | (95% CI) | |
| Study group/intervention | |||||
| Usual care (UC) | 299 | 52 | (17) | 1.00 | Ref |
| Standard (SI) | 685 | 233 | (34) | 2.50 | (1.72, 3.62) |
| Tailored (TI) | 278 | 67 | (24) | 1.70 | (1.05, 2.75) |
| Tailored navigation (TNI) | 680 | 294 | (43) | 3.76 | (2.60, 5.45) |
| Sex | |||||
| Male | 675 | 209 | (31) | 1.00 | Ref |
| Female | 1267 | 437 | (35) | 1.12 | (0.90, 1.40) |
| Age (years) | |||||
| 50–59 | 1375 | 445 | (32) | 1.00 | Ref |
| 60+ | 567 | 201 | (35) | 1.30 | (1.04, 1.63) |
| Race | |||||
| White | 732 | 237 | (32) | 1.00 | Ref |
| African American | 1210 | 409 | (34) | 1.31 | (1.04, 1.63) |
| Marital status | |||||
| Not Married | 1061 | 330 | (31) | 1.00 | Ref |
| Married | 881 | 316 | (36) | 1.34 | (1.08, 1.68) |
| Education | |||||
| High school or less | 1005 | 313 | (31) | 1.00 | Ref |
| Beyond high school | 937 | 333 | (36) | 1.19 | (0.96, 1.46) |
| Screening attitudes | |||||
| Low (<=3) | 195 | 60 | (31) | 1.00 | Ref |
| High (>3) | 1747 | 586 | (34) | 0.85 | (0.59, 1.23) |
| Decision stage | |||||
| Prefer not to screen | 51 | 5 | (10) | 1.00 | Ref |
| Undecided about screening | 290 | 58 | (20) | 2.33 | (0.86, 6.34) |
| Prefer to screen | 1601 | 583 | (36) | 5.59 | (2.11, 14.8) |
| Preferred test | |||||
| Stool blood test (SBT) | 351 | 130 | (37) | 1.08 | (0.82, 1.42) |
| Equal preference | 975 | 322 | (33) | 1.00 | Ref |
| Colonoscopy (CX) | 616 | 194 | (32) | 0.78 | (0.62, 0.99) |
OR: adjusted odds ratio (multivariable model included study practice and all variables shown in the table). CI: confidence interval.
SBT Screening Adherence
There were 329 (17%) participants who completed SBT screening. Table 3 shows that all interventions substantially increased SBT screening adherence, compared to usual care (ORs = 32.8, 7.84, and 42.6). SBT screening adherence was higher for individuals who were female (OR = 1.39), 60 years of age or older (OR = 1.53), married (OR = 1.44), and had a higher screening decision stage (OR = 2.17 for undecided vs. prefer not to screen and 4.14 for prefer to screen vs. prefer not to screen). Compared to an equal preference for SBT and colonoscopy, having a preference for SBT screening was also associated with higher SBT screening adherence (OR = 1.58), while having a preference for colonoscopy screening was associated with lower SBT screening adherence (OR = 0.41).
Table 3.
Predictors of test-specific screening adherence among study participants.
| SBT | SBT vs. No screening |
CX | CX vs. No screening |
|||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | OR | (95% CI) | n | (%) | OR | (95% CI) | |
| Study group | ||||||||
| Usual care (UC) | 3 | (1) | 1.00 | Ref | 49 | (16) | 1.00 | Ref |
| Standard (SI) | 150 | (22) | 32.8 | (10.2, 105) | 83 | (12) | 0.79 | (0.51, 1.24) |
| Tailored (TI) | 16 | (6) | 7.84 | (2.15, 28.5) | 51 | (18) | 1.10 | (0.63, 1.93) |
| Tailored navigation (TNI) | 160 | (24) | 42.6 | (13.2, 137) | 134 | (20) | 1.50 | (0.98, 2.31) |
| Sex | ||||||||
| Male | 93 | (14) | 1.00 | Ref | 116 | (17) | 1.00 | Ref |
| Female | 236 | (19) | 1.39 | (1.03, 1.87) | 201 | (16) | 0.93 | (0.70, 1.23) |
| Age (years) | ||||||||
| 50–59 | 207 | (15) | 1.00 | Ref | 238 | (17) | 1.00 | Ref |
| 60+ | 122 | (22) | 1.53 | (1.15, 2.03) | 79 | (14) | 1.10 | (0.81, 1.48) |
| Race | ||||||||
| White | 128 | (17) | 1.00 | Ref | 109 | (15) | 1.00 | Ref |
| African American | 201 | (17) | 1.07 | (0.61, 1.87) | 208 | (17) | 1.59 | (0.92, 2.74) |
| Marital status | ||||||||
| Not Married | 168 | (16) | 1.00 | Ref | 162 | (15) | 1.00 | Ref |
| Married | 161 | (18) | 1.44 | (1.07, 1.93) | 155 | (18) | 1.27 | (0.96, 1.69) |
| Education | ||||||||
| High school or less | 162 | (16) | 1.00 | Ref | 151 | (15) | 1.00 | Ref |
| Beyond high school | 167 | (18) | 1.17 | (0.89, 1.54) | 166 | (18) | 1.15 | (0.88, 1.50) |
| Screening attitudes | ||||||||
| Low (<=3) | 39 | (20) | 1.00 | Ref | 21 | (11) | 1.00 | Ref |
| High (>3) | 290 | (17) | 0.77 | (0.49, 1.20) | 296 | (17) | 0.92 | (0.55, 1.55) |
| Decision stage | ||||||||
| Prefer not to screen | 4 | (8) | 1.00 | Ref | 1 | (2) | 1.00 | Ref |
| Undecided about screening | 38 | (13) | 2.17 | (0.69, 6.79) | 20 | (7) | 3.61 | (0.46, 28.2) |
| Prefer to screen | 287 | (18) | 4.14 | (1.37, 12.5) | 296 | (18) | 11.7 | (1.55, 87.4) |
| Preferred test | ||||||||
| Stool blood test (SBT) | 102 | (29) | 1.58 | (1.14, 2.19) | 28 | (8) | 0.52 | (0.33, 0.82) |
| Equal preference | 169 | (17) | 1.00 | Ref | 153 | (16) | 1.00 | Ref |
| Colonoscopy (CX) | 58 | (9) | 0.41 | (0.29, 0.58) | 136 | (22) | 1.20 | (0.90, 1.60) |
SBT: stool blood test. CX: colonoscopy. OR: adjusted odds ratio (multivariable model included study practice and all variables shown in the table). CI: confidence interval.
Colonoscopy Screening Adherence
We found that 317 (16%) participants adhered to colonoscopy screening. Table 3 shows that exposure to the tailored navigation intervention was modestly associated with colonoscopy screening adherence (OR = 1.50). Higher screening decision stage was also associated with higher colonoscopy screening adherence (OR = 3.61 for undecided vs. prefer not to screen and 11.7 for prefer to screen vs. prefer not to screen). Compared to an equal preference for SBT and colonoscopy, having a preference for SBT was associated with lower colonoscopy screening adherence (OR = 0.52), while having a preference for colonoscopy screening was associated with slightly higher colonoscopy screening adherence (OR = 1.20).
Racial Differences in Overall and Test-Specific Adherence
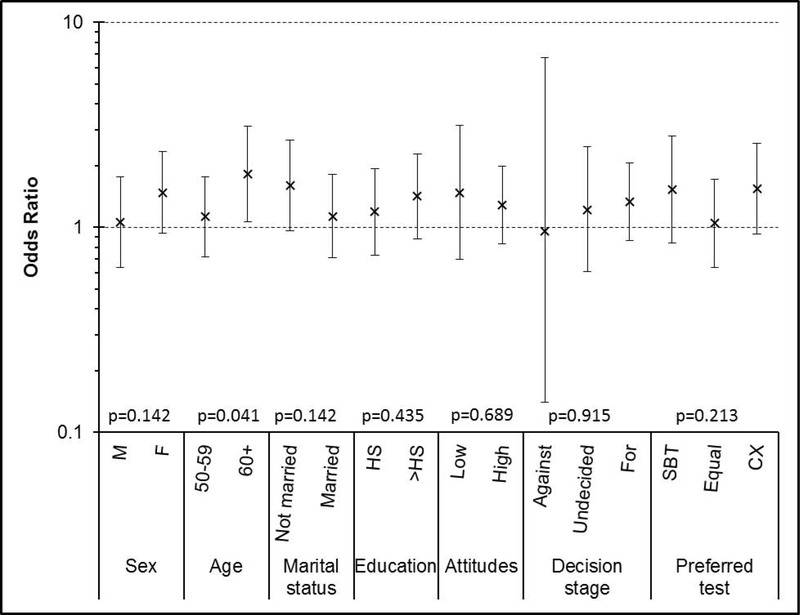
Figure 1 summarizes odds ratios for the interactions between race (African American versus white) and selected predictors. Only the interaction between race and age was statistically significant (p = 0.041). That is, older African Americans were more likely to screen than older whites (OR = 1.82, 95% CI: 1.07 to 3.11), while younger African Americans and younger whites had similar overall CRCS adherence (OR = 1.13, 95% CI: 0.72 to 1.77).
Figure 1.
Adjusted odds ratio for race (African Americans versus whites) with respect to overall screening adherence, within subgroups defined by sex, age, marital status, education, screening attitudes, screening decision stage, and preferred screening test.
P-values shown are for the interaction of race with each predictor.
HS: high school. SBT: stool blood test. CX: colonoscopy.
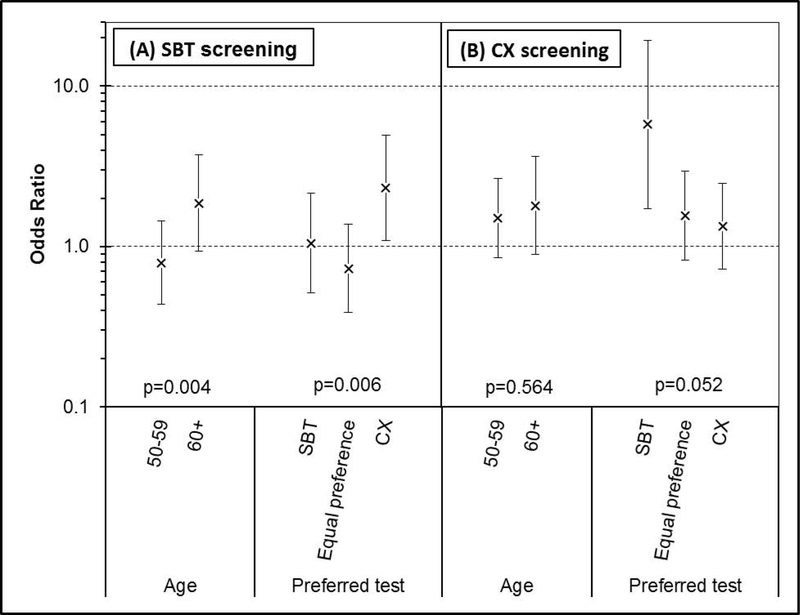
Figure 2 summarizes the odds ratios for African Americans versus whites with respect to test-specific screening adherence. Here, significant interaction effects for race again involved age, but also preferred screening test (global p-values for interaction = 0.017 and 0.002, respectively).
Figure 2.
Adjusted odds ratio for race (African Americans versus whites) with respect to test-specific screening adherence, within subgroups defined by age and preferred screening test.
(A) Results for stool blood test performance (vs. no screening).
(B) Results for colonoscopy completion (vs. no screening).
P-values shown are for the interaction of race with each predictor (age or preferred test).
SBT: stool blood test. CX: colonoscopy.
Racial differences with respect to SBT screening depended on both age and preferred screening test (interaction p = 0.004 and 0.006, respectively). Older African Americans were somewhat more likely to complete SBT screening than older whites (OR = 1.87, 95% CI: 0.94 to 3.72), while there was a small inverse association in the younger age group (OR = 0.79, 95% CI: 0.43 to 1.44). Among participants who preferred SBT, SBT screening adherence was similar for African Americans and whites (OR = 1.05, 95% CI: 0.51 to 2.14), and the same was true among participants who had and equal preference for SBT and colonoscopy screening (OR = 0.73, 95% CI: 0.39 to 1.38). However, among participants who preferred colonoscopy, African Americans were more likely than whites to complete SBT screening (OR = 2.32, 95% CI: 1.09 to 4.94).
With respect to colonoscopy screening adherence, the interaction between race and age was not statistically significant (p = 0.564), while that between race and preferred screening test was marginally significant (p = 0.052). Among participants who preferred SBT, African Americans were much more likely than whites to undergo colonoscopy (OR = 5.78, 95% CI: 1.73 to 19.4). In contrast, among participants who preferred colonoscopy, adherence to colonoscopy screening was not very different for African Americans vs. whites (OR = 1.33, 95% CI: 0.72 to 2.47), and the same was true for participants who had an equal preference for SBT and colonoscopy screening (OR = 1.56, 95% CI: 0.82 to 2.96).
DISCUSSION
In randomized controlled trials that we have conducted in diverse primary care patient populations,16–17,21 CRCS adherence has been shown to improve after exposure to behavioral interventions. The combination of mailing SBT kits and information for scheduling a colonoscopy, informed decision making, and navigation has had the greatest impact. Furthermore, individuals with a high baseline CRCS decision stage have shown greater overall screening adherence.
In our analyses, sociodemographic factors predicted overall CRCS adherence. These findings are consistent with other studies that have cited older age5,9,22–24 and being married22,24 as positive predictors of overall CRCS adherence. Regarding age, we found that higher CRCS adherence among older participants was due to higher SBT screening, but not colonoscopy screening. Again, this finding is consistent with prior studies that have reported higher SBT screening for older individuals,5,13,28 but comparable colonoscopy screening rates across different age groups.12 This phenomenon may reflect a higher sensitivity among older individuals to the inconvenience of test preparation and the need to arrange transportation and address other logistical issues involved in colonoscopy screening, compared to SBT screening. The higher level of CRCS adherence among married participants may reflect increased encouragement and support for screening offered by a significant other. It is unclear why SBT screening was more likely in this subgroup.
We did not find any effect of sex on overall CRCS adherence. Some prior studies have reported higher screening adherence among men,6,9,22–24,26 but others have reported higher adherence among women, or no gender differences.7,14,25 Perhaps this inconsistency is due to the type(s) of screening tests offered to study participants and the differential receptivity of women versus men to different screening modalities. Regarding test-specific screening adherence, women had higher SBT adherence than men, a finding that may reflect concern among women about the invasiveness of the colonoscopy procedure.26
The behavioral interventions used in our clinical trials involved offers of both SBT and colonoscopy screening to participants. Compared to individuals with an equal preference for the two tests, those who preferred SBT had higher SBT adherence and lower colonoscopy screening, while those who preferred colonoscopy had lower SBT adherence but only modestly higher colonoscopy adherence. This result probably reflects the fact that, compared to completing a SBT, actually undergoing a colonoscopy is more influenced by external factors (such as scheduling, transportation, and cost), beyond personal preferences.
A significant statistical interaction between race and age reflected the fact that older African Americans were more likely to have CRCS screening than older whites, while such a racial difference was not seen in younger individuals. It is possible that this effect may be due to a sense of greater vulnerability among older African Americans, compared to older whites, and may also reflect differential views related to challenges associated with performance of the screening tests.27–28 Nevertheless, our finding should be regarded with caution. One previous study found no interactions between race and any other patient characteristics, including age.29 Another previous study actually reported an interaction between race and age in the opposite direction: although there were no racial differences in the lower age groups, whites were more likely to screen than African Americans in older age groups.30
Our analyses produced some surprising findings related to race and screening test preference. Specifically, African Americans were more likely than whites to complete a non-preferred test (but had similar adherence with the preferred test). Among participants who preferred colonoscopy screening, African Americans had more than twice the odds of whites for completing SBT screening. And among participants who preferred SBT screening, African Americans had almost six times the odds of whites for undergoing colonoscopy screening. These results may suggest that factors other than test preference may be more likely to affect adherence among African Americans than among whites. These findings underscore the importance of providing diverse populations targeted for CRCS programs with access to both SBT and colonoscopy screening options, along with decision support and navigation contacts.31–32
Our data reflect the patient populations of 23 different primary care practices, serving urban, suburban, and rural areas in Pennsylvania and Delaware. However, the two trials that were the source of the data included only English-speaking patients and did not include any Hispanic/Latino or Asian patients. Therefore, our analyses cannot address screening disparities involving those populations. Generalizability is further limited by the fact that participants volunteered to join the randomized intervention trials, and that 85% of them were exposed to some type of intervention which provided no-cost access to CRCS modalities. Thus, our findings may not generalize to populations that would not normally participate in a research study with free access to screening, or populations that may have limited access to screening. A strength of our data is that they were based on primary care patient medical records, augmented by direct reporting from the laboratory that analyzed the stool blood test kits and participant surveys, an approach that allowed us near-complete ascertainment of screening. Finally, due to the large sample size, our analyses were powered to identify weak-to-modest predictors of CRCS adherence, as well as modest interactions.
Supplementary Material
Highlights.
Colorectal cancer screening is very effective, yet one in three Americans is not up-to-date with it
Older age, African American race, and being married are associated with higher likelihood of colorectal cancer screening
Both African Americans and whites are more likely to have colorectal cancer screening through the test they prefer
African Americans are more likely than whites to use a non-preferred test for colorectal cancer screening
Offering both a colonoscopy option and a stool blood test kit maximizes the likelihood of colorectal cancer screening
ACKNOWLEDGMENTS
We thank the participants, staff, and coinvestigators of the two trials, and especially acknowledge the contributions of Dr. Heather Bittner-Fagan and Dr. Randa Sifri. The study’s data were obtained in two clinical trials funded by NIH/NCI grant R01 CA116576 and ACS grant RSTG-08–017-01-CPPB (clinicaltrials.gov identifiers NCT00617071 and NCT00893295, respectively), with Dr. Ronald Myers as the PI for both. Stool blood tests were donated by Quest Diagnostics. The contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the views of NIH/NCI or ACS. CD and REM were responsible for the conceptual design of the manuscript. MD and REM oversaw implementation of the trials and data collection. CD and SH performed the data analyses. All authors contributed to the interpretation of the results. CD drafted the manuscript, and all authors worked on revisions and have approved the final version.
Footnotes
The authors report no financial disclosures.
Conflict of interest. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.U.S. Department of Health and Human Services (USDHHS), Office of Disease Prevention and Health Promotion. Healthy People 2020. http://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives (Accessed 11/20/2018) [Google Scholar]
- 2.National Colorectal Cancer Roundtable. Working toward the shared goal of 80% screened for colorectal cancer by 2018. http://nccrt.org/what-we-do/80-percent-by-2018/ (Accessed 11/20/2018)
- 3.Centers for Disease Control and Prevention (CDC). Cancer screening – United States, 2010. MMWR January 27, 2012;61(3):41–5. [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention (CDC). Vital Signs: Colorectal Cancer Screening Test Use – United States, 2012. MMWR November 8, 2013;62(44):881–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100(10):2093–103. [DOI] [PubMed] [Google Scholar]
- 6.Etzioni DA, Ponce NA, Babey SH, et al. A population-based study of colorectal cancer test use: results from the 2001 California Health Interview Survey. Cancer. 2004;101(11):2523–32. [DOI] [PubMed] [Google Scholar]
- 7.Moss SM, Campbell C, Melia J, Coleman D, Smith S, Parker R, Ramsell P, Patnick J, Weller DP. Performance measures in three rounds of the English bowel cancer screening pilot. Gut. 2012:61(1):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wee CC, McCarthy EP, Russell SP. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Preventative Medicine. 2005;41:23–9. [DOI] [PubMed] [Google Scholar]
- 9.Meissner HI, Breen N, Klabunde C, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–94. [DOI] [PubMed] [Google Scholar]
- 10.Myers RE, Hyslop T, Sifri R, Bittner-Fagan H, Katurakes NC, Corcroft J, DiCarlo M, Wolf T. Tailored navigation in colorectal cancer screening. Medical Care. 2008;46(9 Suppl 1):S123–31. [DOI] [PubMed] [Google Scholar]
- 11.Bittner-Fagan H, Myers RE, Daskalakis C, Sifri R, Mainous AG, Wender R. Race/Ethnicity, gender, weight status, and colorectal cancer screening. Journal of Obesity. 2011;314619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jandorf L, Braschi C, Ernstoff E, Wong CR, Thelemaque L, Winkel G, Thompson HS, Redd WH, Itzkowitz SH. Culturally targeted patient navigation for increasing African Americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong MC, Ching JY, Lm TY, Luk AK, Hirai HW, Griffiths SM, Chan FK, Sung JJ. Prospective cohort study of compliance with faecal immunochemical tests for colorectal cancer screening in Hong Kong. Preventive Medicine. 2013;57(3):227–31. [DOI] [PubMed] [Google Scholar]
- 14.Palmer RC, Chhabra D, McKinney S . Colorectal cancer screening adherence in African-American men and women 50 years of age and older living in Maryland. Journal of Community Health. 2011;36(4):517–24. [DOI] [PubMed] [Google Scholar]
- 15.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Munoz R, Lau C, Somsouk M, El-Nachef N, Hawyard RA. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Archives of Internal Medicine. 2012; 172(7):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers RE, Bittner-Fagan H, Daskalakis C, et al. A randomized controlled trial of a tailored navigation and a standardized intervention in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers RE, Sifri R, Daskalakis C, DiCarlo M, et al. Increasing colon cancer screening in primary care among African Americans. J Natl Cancer Inst. 2014;106(12):dju344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–32. [PubMed] [Google Scholar]
- 19.Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening among African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2005;14:2855–61. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7(4):355–86. [DOI] [PubMed] [Google Scholar]
- 21.Myers RE, Stello B, Daskalakis C, Sifri R, Gonzalez ET, DiCarlo M, Johnson MB, Hegarty SE, Shaak K, Rivera A, Gorkils-Molina L, Petrich A, Careyva B, de-Ortiz R, Diaz L. Decision support and navigation to increase colorectal cancer screening among Hispanic patients. Cancer Epidemiol Biomarkers Prev. 2019;28(2):384–91. [DOI] [PubMed] [Google Scholar]
- 22.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes & Control. 2007;19(4):339–59. [DOI] [PubMed] [Google Scholar]
- 23.Ioannou G. Predictors of colorectal cancer screening participation in the United States. The American Journal of Gastroenterology. 2003;98(9):2082–91 [DOI] [PubMed] [Google Scholar]
- 24.Power E, Miles A, Wagner CV, Robb K, Wardle J. Uptake of colorectal cancer screening: system, provider and individual factors and strategies to improve participation. Future Oncology. 2009;5(9):1371–88. [DOI] [PubMed] [Google Scholar]
- 25.Frederiksen BL, Jørgensen T, Brasso K, Holten I, Osler M. Socioeconomic position and participation in colorectal cancer screening. British Journal of Cancer. 2010;103(10):1496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, et al. Predictors of nonadherence to screening colonoscopy. Journal of General Internal Medicine. 2005;20(11):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nursing. 1995; 18(5):385–92. [PubMed] [Google Scholar]
- 28.Wang H-L, Christy SM, Skinner CS, Champion VL, Springston JK, Perkins SM, Tong Y, Krier C, Gebregziabher N, Rawl SM. Predictors of stage of adoption for colorectal cancer screening among African American primary care patients. Cancer Nursing. 2014;37(4):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shokar NK, Carlson CA, Wller SC. Factors associated with racial/ethnic differences in colorectal cancer screening. J Am Board Fam Med 2008;21(5)414–26. [DOI] [PubMed] [Google Scholar]
- 30.Jerant AF, Franks P, Jackson JE, Doescher MP. Age-related disparities in cancer screening: analysis of 2001 Behavioral Risk Factor Surveillance System data. Ann Fam Med 2004;2(5):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelto D, Sly JR, Winkel G, Redd WH, Thompson HS, Itzkowitz SH, Jandorf L. Predicting colonoscopy completion among African American and Latino/a participants in a patient navigation program. J Racial Ethn Health Disparities. 2015;2(1):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waghray A, Jain A, Waghray N. Colorectal cancer screening in African Americans: practice patterns in the United States. Are we doing enough? Gastroenterology. 2016;4(2):136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.