Abstract
Background:
We recently reported that a novel CXCR5+IFN-γ+CD8+ T cell subset significantly inhibits posttransplant alloantibody production in a murine transplant model. These findings prompted the current study to investigate the association of human CD8+ T cells with the same phenotype with the development of de novo donor-specific antibody (DSA) after kidney transplantation.
Methods:
In the current studies, we prospectively and serially analyzed peripheral blood CD8+ and CD4+ T cell subsets and monitored for the development of de novo DSA in kidney transplant recipients during the first year posttransplant. We report results on 95 first-time human kidney transplant recipients with 1-year follow-up.
Results:
Twenty-three recipients (24.2%) developed de novo DSA within 1-year posttransplant. Recipients who developed DSA had significantly lower quantities of peripheral CXCR5+IFN-γ+CD8+ T cells (p=0.01) and significantly lower ratios of CXCR5+IFN-γ+CD8+ T cell to combined CD4+ Th1/Th2 cell subsets (IFN-γ+CD4+ and IL-4+CD4+ cells; p=0.0001) compared to recipients who remained DSA-negative over the first year posttransplant.
Conclusion:
Our data raise the possibility that human CXCR5+IFN-γ+CD8+ T cells are a homologue to murine CXCR5+IFN-γ+CD8+ T cells (termed antibody-suppressor CD8+ T cells) and that the quantity of CXCR5+IFN-γ+CD8+ T cells (or the ratio of CXCR5+IFN-γ+CD8+ T cells to Th1/Th2 CD4+ T cells) may identify recipients at risk for development of DSA.
Introduction
Kidney transplantation is the optimal treatment for patients with end stage renal disease (ESRD) and increases patient survival and quality of life. While advances in immunosuppression have reduced the incidence of acute cellular rejection and short-term graft survival has improved over the last 20 years,1 long-term survival of kidney allografts remains unchanged.2,3 A pathogenic role for MHC-directed alloantibody after kidney transplant is well established.4,5 A previous retrospective study at our center reported that 22% of first time kidney transplant recipients developed de novo donor-specific antibody (DSA) and that graft survival was significantly worse in recipients with de novo DSA compared to recipients that did not develop de novo DSA.6 These results are consistent with other published studies [13–27% incidence of de novo DSA7-10] and the development of DSA posttransplant correlates with worse long-term graft outcomes after kidney transplant7,11-19 as well as after other cell20-22 and solid organ transplants.23-27
While the immune mechanisms which regulate DSA production in transplant recipients are not well understood, new immunoregulatory pathways have been identified in mouse models. Our group first reported that a novel subset of IFN-γ+CD8+ T cells negatively regulates the magnitude of IL-4-dependent (Th2 driven) IgG1 alloantibody produced following hepatocyte transplant.28 This subset also downregulates combined Th1- and Th2-driven alloantibody (IgG1, IgG2b, IgG2c, and IgG3) produced after islet or skin transplant in mice (unpublished findings). These antibody-suppressing CD8+ T cells negatively regulate humoral responses by killing alloprimed IgG+ B cells29 and downregulating alloprimed CD4+ T cells.28 Recent studies reveal that antibody-suppressor CD8+ T cells not only require IFN-γ but also require CXCR5.30
Based on the foundation of our experimental data, we hypothesized that antibody-suppressor CD8+ T cells could exist in humans and impact the development of DSA in kidney transplant recipients. To begin to investigate this hypothesis, we prospectively and serially monitored peripheral blood T cell subsets in first-time kidney transplant recipients and correlated results with the development of de novo DSA during the first year posttransplant. To our knowledge, this is the first prospective study to report an association between the quantity of human peripheral CXCR5+IFN-γ+CD8+ T cells (as well as the ratio of this subset to combined IFNγ+CD4+ and IL-4+CD4+ T cell quantity) with de novo DSA production in the first year after kidney transplant.
Materials and Methods
Study Participants.
Participation in this study was offered (by informed consent) to prospective first-time kidney transplant recipients, 18 years of age or older, transplanted at The Ohio State University Comprehensive Transplant Center (OSU CTC) beginning in August of 2015 through March of 2017. Ninety-five patients enrolled in the study have a minimum of 1-year follow-up posttransplant and comprise the study group for this report. All recipients in this study were DSA-negative before transplant. The study population characteristics are shown in Table 1.
Table 1.
Demographics and Characteristics of the First-Time Kidney Transplant Study Population.
| Number of Recipients | |||||
|---|---|---|---|---|---|
| Demographics and Characteristics | All Recipients (n=95) | DSA negative (n=72) | DSA positive (n=23) | P values | |
| Demographics of transplant patients | |||||
| Median Age (average ± standard deviation) | 52.7±12.1 | 53.4±11.8 | 50.8±12.9 | p=0.37 | |
| Female | 39 (41.1%) | 32 (44.4%) | 7 (30.4%) | p=0.23 | |
| African American | 20 (21.1%) | 15 (20.8%) | 5 (21.7%) | p=0.92 | |
| Characteristics of transplant patients | |||||
| Primary cause of renal disease | Diabetes Mellitus | 36 (37.9%) | 29 (40.3%) | 7 (30.4%) | p=0.87 |
| Hypertensive Nephrosclerosis | 12 (12.6%) | 11 (15.3%) | 1 (4.3%) | ||
| Focal Glomerular Sclerosis | 12 (12.6%) | 7 (9.7%) | 5 (21.7%) | ||
| Polycystic Kidneys | 6 (6.3%) | 5 (6.9%) | 1 (4.3%) | ||
| Autoimmune Disease (IgA Nephropathy, Lupus, anti-GBM, Wegener’s) | 12 (12.6%) | 8 (11.1%) | 4 (17.4%) | ||
| Others | 17 (17.9%) | 12 (16.7%) | 5 (21.7%) | ||
| Donor Type | Deceased | 31 (32.6%) | 24 (33.3%) | 7 (30.4%) | p=0.92 |
| Living Related | 18 (18.9%) | 13 (18.1%) | 5 (21.7%) | ||
| Living Unrelated | 46 (48.4%) | 35 (48.6%) | 11 (47.8%) | ||
| Pretransplant Dialysis | 78 (82.1%) | 59 (81.9%) | 19 (82.6%) | p=0.99 | |
| HLA Donor/Recipient Mismatch (avg ± stdev)a | 6.2±2.1 | 6.0±2.1 | 6.8±2.1 | p=0.06 | |
| Pretransplant calculated Panel Reactive Antibody (cPRA)b | |||||
| Number of Recipients with Pretransplant cPRA >0% | 25 (26.3%) | 20 (27.8%) | 5 (21.7%) | p=0.77 | |
Donor-recipient HLA mismatches were based on A, B, C, DR, and DQ antigens
Pretransplant cPRA for DSA-negative recipients (median cPRA= 47.5%, ranging from 2% to 100%, p=ns) and DSA-positive recipients (median cPRA= 27.8%, ranging from 4% to 94%)
Clinical Protocols.
All recipients were transplanted following a negative flow cytometric T and B cell crossmatch. All 95 recipients included in this study received standard immunosuppression as per OSU CTC protocol. The routine immunosuppression regimen during the study period included posttransplant treatment with antithymocyte globulin induction (ATG, 1.25 mg/kg per day, up to a maximum of 125 mg for each dose on days 1–5 posttransplant) and a rapid steroid taper (500 mg, 200 mg, 125 mg, 50 mg and 25 mg on days 0 through 4 posttransplant, respectively). Peripheral T cells were undetectable early posttransplant (day 5) but repopulated in the peripheral blood by 1-month posttransplant. Steroid-free maintenance therapy included calcineurin inhibition (CNi) with cyclosporine (Neoral) and mTOR inhibition (mTORi) with sirolimus or everolimus (Rapamune or Zortress). Immunosuppressive therapy was adjusted based on established target immunosuppression levels, clinical conditions, laboratory results and individual side effects.
Serial Monitoring.
Serial monitoring of immunosuppression levels included twice weekly C2 levels and mTORi trough levels for the first 3 months posttransplant, weekly levels between 3–6 months, twice monthly levels between 6 to 12 months posttransplant. Overall, sample capture rate was 81% for recipient C2 levels and mTORi levels.
DSA Determination.
Recipients were monitored for the development of DSA by serial testing pretransplant and at 1, 3, 6, 9 and 12 months after kidney transplant and for clinical indication. Blood samples were analyzed by OSU Tissue Typing Laboratory to determine the presence of DSA. The detection of anti-HLA antibodies was performed with LABScreen single-antigen class I and II beads (One Lambda), using a Labscan 200 (Luminex) instrument. The assay was performed according to the manufacturer’s protocol. The OSU Tissue Typing Laboratory utilizes a normalized MFI threshold of 2000 for reporting HLA class I and II DSA but also includes MFI values less than 2000 for clinical interpretation. DSA results reported in this study include MFI greater than 2000 in the majority of cases (n=22) though 1 recipient did have initial detection of DSA with MFI less than 2000 (Figure 1). Blood samples used for DSA determination were obtained concurrently with clinical samples drawn at the time of routine clinic visits.
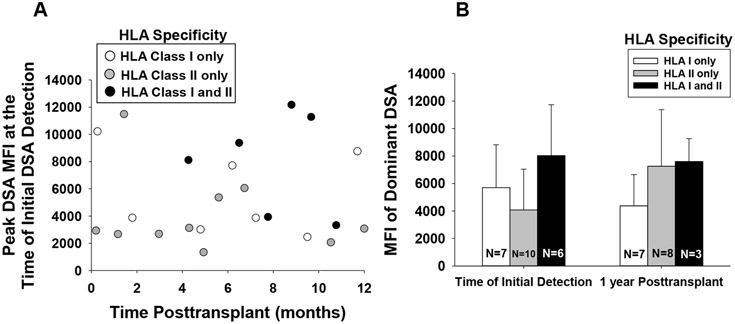
Figure 1. Time of Initial Detection, MFI, and HLA specificity of de novo DSA in First Time Kidney Transplant Recipients.
All study participants (n=95) were tested for DSA in serum samples collected during regularly scheduled posttransplant clinic visits with their CTC clinician provider. Sample analysis to detect DSA was performed by The Ohio State University Tissue Typing Laboratory with LABScreen single-antigen class I and II beads. De novo DSA was detected in 24 percent (23/95) of recipients over the first year posttransplant. A) Initial detection of de novo DSA occurred on average 6.0±3.7 months posttransplant. The mean fluorescence intensity (MFI) ± standard deviation of the dominant DSA specificity at the time of initial detection was 5605±3518. 10 recipients had specificity to HLA class II, 7 had specificity to HLA class I, and 6 recipients had specificity to both HLA class I and II. B) MFI of the dominant (highest MFI) DSA specificity at 1 year posttransplant is shown for recipients who had DSA with specificity for HLA class I, HLA class II or both HLA class I and HLA class II (dominant specificity shown for this latter group represents HLA Class I (n=2) or HLA Class II (n=4). Data was censored for recipients that no longer had DSA (n=3) or had graft loss (n=2) by 1-year posttransplant. Graphed data represents geometric mean ± standard error.
Flow Cytometric Analysis of PBMCs.
Peripheral blood samples were collected pretransplant (within 24 hours before transplant) and at 1, 3, 6, 9 and 12 months posttransplant for immunophenotyping of peripheral blood monocytes (PBMCs). Procurement of PBMCs was performed similarly to the NIH Clinical Trials in Organ Transplantation (CTOT) validated methods.31,32 PBMC samples were frozen down in cell freezing media. As in previous studies,33 we find no significant difference between the flow cytometric analyses performed on fresh or frozen PBMCs (data not shown). PBMCs were thawed, washed and incubated with Brefeldin, Ionomycin, and 2 ng/mL PMA (low dose) for 4 hours. PBMCs were treated with anti-FcγR mAb (Fc-block) and subsequently stained for extracellular markers (CD4- clone RPA-T4, CD8- clone RPA-T8, CD44- clone G44–26, and CXCR5- clone RF8B2 all from Becton Dickinson). Intracellular staining was performed following manufacture’s recommendations for IFN-γ (clone 45.B3) and IL-4 (clone MP4–25D2, both from Becton Dickinson). Cells were permeabilized using FIX & PERM Cell Fixation and Cell Permeabilization kit by manufacture’s recommendations (Thermofisher). No cytokine expression was observed in flow cytometric analysis of unstimulated PBMCs. CD8+ PBMCs were predominately CD8+CD3+ T cells (~95%; data not shown). Lymphocyte populations as well as CD4+ T cells or CD8+ T cells were utilized for gating. IFN-γ+CD8+ T cells were predominately terminally differentiated effector CD8+ T cells (CD62L−CD45RO−CD44+) (Figure S1). CXCR5+IFN-γ+CD8+ T cells similarly expressed predominately a terminally differentiated effector phenotype (not shown). The collection rate of peripheral blood samples for immunophenotyping was similar between DSA-positive and DSA-negative recipients (Figure S2).
Statistics.
Baseline demographic and clinical characteristics were summarized and compared between DSA-positive and DSA-negative groups using t-tests or Wilcoxon rank-sum tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. Outcomes were transformed to the natural log scale prior to modeling due to violations in the normality of residuals assumption where appropriate. Linear mixed models that account for repeated measures over time within recipients were fit for each of the longitudinally measured outcomes and clinical characteristics (mTORi, WBC, etc.) respectively to test for group differences. Model based back transformed geometric means ± 1 standard error of the mean were plotted at each time point. The models each included main effects for group and time and a group by time interaction effect. Missing data were considered to be missing at random. Kaplan-Meier plots were presented for time to event data and compared between study groups using log-rank tests. All hypothesis testing was conducted at a 5% type I error rate (alpha = 0.05). Statistical analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Study Approval:
This study was reviewed and approved by the OSU Wexner Medical Center Institutional Review Board for human subject research (IRB protocol 2011H0256). All study participants provided written informed consent prior to their participation in the study.
Results
Incidence of DSA in the first year posttransplant in first-time kidney transplant recipients.
Ninety-five first-time kidney transplant recipients who enrolled in the study reached 1-year follow-up. Demographics and characteristics of the study population are shown in Table 1. Within the first year posttransplant, 24.2% (23/95) of the study recipients developed de novo DSA. There were no significant differences in age, gender, or race in DSA-positive and DSA-negative recipients. Similarly, there was no significant difference in the primary cause of renal disease, donor type (living or deceased donor), recipients requiring dialysis prior to transplant, or the number of donor-recipient HLA mismatches (A, B, C, DR, and DQ). In addition, there was no significant difference in the level of sensitization as reflected by pretransplant calculated panel reactive antibody (cPRA). No recipients had DSA prior to transplant.
Characteristics of de novo DSA: Time of initial DSA detection in the first year posttransplant, quantity, and HLA specificity.
Initial DSA detection occurred throughout the observation period and on average 6.0±3.7 months posttransplant (Figure 1). At the time of initial DSA detection, the quantity of DSA as reflected by the mean fluorescence intensity (MFI) of the dominant DSA specificity was 5605±3518 [mean±sd, range of MFI from 1337 to 12 172; Figure 1A]. Of the 23 DSA-positive recipients, 10 initially tested positive for DSA specific for HLA class II alloantigens, 7 tested positive for DSA specific to HLA class I alloantigens, and the remaining 6 recipients had DSA with specificity to both HLA class I and II alloantigens. HLA class I and HLA class II specificities are shown in Figure S3. At 1-year posttransplant, DSA-positive recipients with functioning grafts had DSA specific for HLA class II (n=8), HLA class I (n=7), both HLA class I and II (n=3) and 3 no longer had DSA and 2 experienced graft loss (Figure 1B). The mean MFI for the dominant DSA specificity at 1 year was 5309±3822.
Development of de novo DSA is inversely associated with the quantity of peripheral blood CXCR5+IFN-γ+CD8+ T cells.
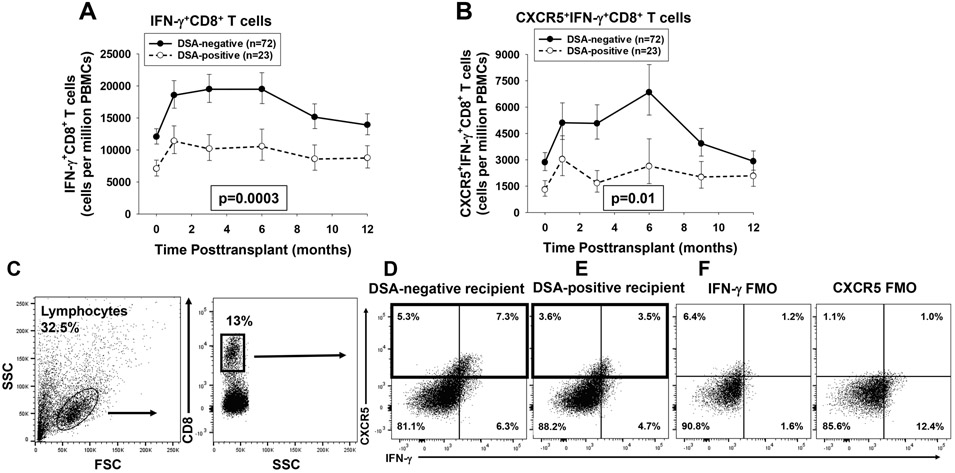
In order to investigate the association between circulating T cells subsets and development of de novo DSA in humans, we serially monitored the quantity of CD4+ and CD8+ T cell subsets in first-time kidney transplant recipients. Altogether we analyzed the quantity of IFN-γ+CD8+ T cells (n=95 recipients, 402 samples) and CXCR5+IFN-γ+CD8+ T cells (n=95 recipients, 320 samples). Recipients who developed de novo DSA (DSA-positive recipients) had significantly lower quantities of both IFN-γ+CD8+ T cells (1.6 to 1.9 fold lower at all time points) and CXCR5+IFN-γ+CD8+ T cells (1.4 to 3.0 fold lower at all time points) compared to recipients who did not develop de novo DSA (DSA-negative recipients) over the 12-month time-period posttransplant (p=0.0003 and p=0.01, respectively; Figure 2A,B). The difference in quantity of these CD8+ T cell subsets was not only significant over the 1-year posttransplant time course but also at baseline since DSA-positive compared to DSA-negative recipients had lower quantity of IFN-γ+CD8+ T cells (1.7 fold lower, p=0.008) as well as lower quantity of CXCR5+IFN-γ+CD8+ T cells (2.1 fold lower, p=0.04) in the pretransplant (time 0) blood sample. The quantity of peripheral CD8+ T cell subsets in individual profiles did not fluctuate significantly prior to DSA detection however sample collections occurred 1–3 months prior to initial DSA detection in most recipients and may not reflect immune cell subset changes immediately before or during DSA development (data not shown). The quantity of CXCR5+IFN-γ+CD8+ T cells were not significantly different in DSA-positive recipients who developed both class I and class II antibodies (n=6) or in those who later lost DSA (n=3) compared to the overall DSA-positive group. Approximately 40–70% of peripheral blood CXCR5+CD8+ T cells expressed IFN-γ+ and approximately 35–60% of circulating IFN-γ+CD8+ T cells expressed CXCR5 (Figure 2C-E).
Figure 2. DSA-positive recipients have significantly lower quantity of peripheral blood IFN-γ+CD8+ T cells and CXCR5+IFN-γ+CD8+ T cells than DSA-negative recipients.
Peripheral blood from DSA-positive recipients (n=23) and DSA-negative recipients (n=72) was analyzed using flow cytometry to determine the quantity of IFN-γ+CD8+T cells and CXCR5+IFN-γ+CD8+ T cells. A) IFN-γ+CD8+ T cells were significantly lower in quantity (1.6 to 1.9 fold) in DSA-positive recipients over the first year posttransplant (p=0.0003). B) CXCR5+IFN-γ+CD8+ T cells were also significantly lower in quantity (1.4- to 3.0-fold) in the DSA-positive recipients (p=0.01). Graphed data represents geometric mean ± standard error. C) Representative flow plots were initially gated on lymphocytes and CD8+ T cells. D) A flow plot for a representative DSA-negative recipient (3 months posttransplant) shows that CXCR5+CD8+ T cells comprise 12.6% of the total peripheral CD8+ T cells (boxed area) and 58% of these were IFN-γ+. E) A flow plot for a representative DSA-positive recipient (3 months posttransplant) shows that CXCR5+CD8+ T cells comprise 7.1% of total peripheral blood CD8+ T cells (boxed area) and 50% of these were IFN-γ+. F) Flow minus 1 (FMO) controls were used for setting the positive gates and indicating background staining for IFN-γ and CXCR5.
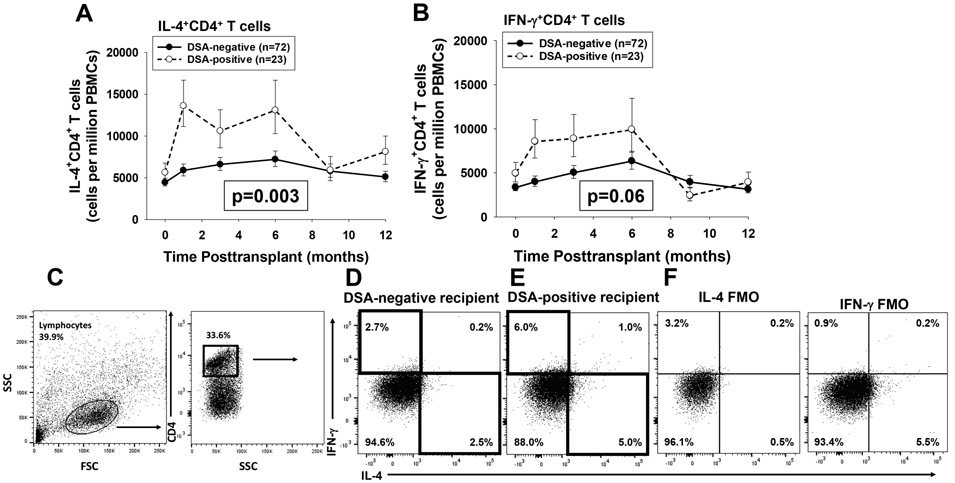
Published studies report that posttransplant DSA in human transplant recipients includes multiple isotypes known to require both Th1 and Th2 driven CD4+ T cell “help.”34,35 Therefore, we analyzed serial quantities of Th1 and Th2 CD4+ T cell subsets in this population of first-time kidney transplant recipients. In contrast to the inverse association detected for CD8+ T cell subsets, we found a direct association between the quantity of peripheral Th1 and Th2 cytokine expressing CD4+ T cells and the development of de novo DSA. DSA-positive recipients, compared to DSA-negative recipients, had higher quantities of peripheral IL-4+CD4+ T cells (p=0.003) and trending to higher quantities of IFN-γ+CD4+ T cells (p=0.06) over the 12-month time-period (Figure 3A-C). The associations observed for CD8+ and CD4+ T cell subsets with posttransplant DSA could not be attributed to differences in the overall quantity of CD8+ T cells or CD4+ T cells in DSA-positive and DSA-negative recipients (Figure S4A,B). White blood cell counts were higher in the DSA-positive compared to DSA-negative groups but overall were in the normal range (4–10 × 103) in both groups throughout the study period (Figure S4C). No difference was observed in the quantities of IL-17+CD4+ T cells, IL-10+FoxP3+CD4+ T cells, or IL-10+CD4+ T cell subsets between DSA-positive and DSA-negative recipients (Figure S5).
Figure 3. DSA-positive recipients have higher quantity of peripheral blood Th1 and Th2 CD4+ T cells than DSA-negative recipients.
Peripheral blood from DSA-positive recipients (n=23) and DSA-negative recipients (n=72) was analyzed using flow cytometry to determine the quantity of IFN-γ+CD4+ T cells (Th1) and IL-4+CD4+ T cells (Th2). A) A significantly higher quantity of IL-4+CD4+ T cells (Th2) was detected in DSA-positive compared to DSA-negative recipients over the first year posttransplant (p=0.003). B) A higher quantity of IFN-γ+CD4+ T Cells (Th1) was detected in DSA-positive compared to DSA-negative recipients (p=0.06). Graphed data represents geometric mean ± standard error. C-E) Representative flow plots for IFN-γ+CD4+ and IL-4+CD4+ T Cells in a DSA-negative and a DSA-positive recipient 3 months posttransplant are shown. Cells were gated on lymphocytes and CD4+ T cells. The DSA positive recipient had ~2 fold more (5.0% vs. 2.5%) IL-4+CD4+ T cells and ~2 fold more IFN-γ+CD4+T cells (6.0% vs. 2.7%). F) Flow minus 1 (FMO) controls were used for setting the positive gates and indicating background staining for IL-4 and IFN-γ.
In order to analyze the potential for bias based on the calculation method of immune cell subset quantity per million PBMCs, we performed additional quantification of immune cell subsets as a percentage of total CD8+ T cells or as a percentage of total CD4+ T cells between DSA-negative and DSA-positive recipients. We found similar patterns in serial T cell subset quantities regardless of the method of calculation (data not shown).
Development of de novo DSA is inversely associated with the ratio of peripheral CD8+ to CD4+ Th1/Th2 cell subsets.
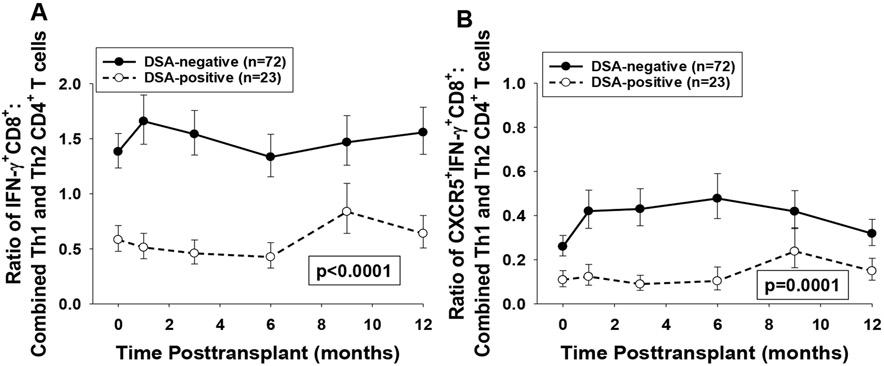
We next analyzed the ratio of CD8+ T cell subsets (IFN-γ+CD8+ or CXCR5+IFN-γ+CD8+) to combined Th1 and Th2 CD4+ T cell subsets [IFN-γ+CD4+ (Th1) and IL-4+CD4+ (Th2)] in peripheral blood mononuclear cells (PBMCs) of DSA-positive and DSA-negative recipients. We found that DSA-positive recipients exhibited a significantly lower ratio of IFN-γ+CD8+ T cells to combined CD4+ Th1 and Th2 cells (1.8 to 3.4 fold lower at all time points) as well as a lower ratio of CXCR5+IFN-γ+CD8+ T cells to combined CD4+ Th1 and Th2 cells (1.8 to 4.8 fold lower for all time points) compared to DSA-negative recipients over the 12-month time-period posttransplant (p<0.0001 and p=0.0001, respectively; Figure 4A,B). In addition, this association between lower ratio of both IFN-γ+CD8+ (2.4 fold lower, p=0.0002) and CXCR5+IFN-γ+CD8+ T cell subsets (2.4-fold lower, p=0.02) to combined Th1 and Th2 CD4+ T cells subsets in DSA-positive compare to DSA-negative recipients was also observed at baseline (before transplant).
Figure 4. DSA-positive recipients have lower ratio of peripheral blood IFN-γ+CD8+ T cells or CXCR5+IFN-γ+CD8+ T cells to combined Th1/Th2 CD4+ T cells than DSA-negative recipients.
Peripheral blood from DSA-positive recipients (n=23) and DSA-negative recipients (n=72) was analyzed using flow cytometry to determine the quantity of IFN-γ+CD4+ T cells (Th1), IL-4+CD4+ T cells (Th2), IFN-γ+CD8+T cells, and CXCR5+IFN-γ+CD8+ T cells. Ratios of both CD8+ T cell subsets to the combined Th1 and Th2 CD4+ T cells were then calculated. A) Ratio of the quantity of IFN-γ+CD8+T cells to the combined quantity of Th1 and Th2 CD4+ T cell subsets and B) Ratio of the quantity of CXCR5+IFN-γ+CD8+ T cells to the combined quantity of Th1 and Th2 CD4+ T cell subsets were significantly lower in DSA-positive recipients compared to DSA-negative recipients over the first year posttransplant (1.8- to 3.4-fold and 1.8- to 4.8-fold, respectively; p<0.0001 and p=0.0001, respectively). Graphed data represents geometric mean ± standard error.
Association of Baseline Peripheral Immune Cell Subset Quantity and Ratios with de novo DSA posttransplant.
Prompted by the observed associations between baseline pretransplant CD8+ T cell subset quantity and ratios of CD8+ to CD4+ T cell subsets, we next investigated the predictive value and discriminatory ability of pretransplant peripheral blood immune cell subset quantity with the development of de novo DSA after kidney transplantation. We analyzed receiver operating characteristic (ROC) curves and found that a pretransplant 1) quantity of CXCR5+IFN-γ+CD8+ T cells less than 3300 per million PBMCs (AUC statistic=0.81), 2) a CXCR5+IFN-γ+CD8+ T cells : combined Th1/Th2 CD4+ T cell ratio of less than 0.3 (AUC statistic=0.76), and 3) a IFN-γ+CD8+ T cells : combined Th1/Th2 CD4+ T cell ratio of less than 1.5 (AUC statistic=0.72 were associated with the development of de novo DSA (Table 2). In addition, the sensitivity for each of these 3 immune cell subsets was over 90%. Other immune cell subsets analyzed had less favorable AUC statistic (<0.70)36-38 (Table 2). This data suggests that baseline quantity (CXCR5+IFN-γ+CD8+ T cells) and/or ratios (CXCR5+IFN-γ+CD8+ T cells or IFN-γ+CD8+ T cells to combined Th1/Th2 CD4+ T cells) of pretransplant peripheral blood immune cell subsets may be predictive of de novo DSA production posttransplant.
Table 2.
Predictive Values and Discriminatory Ability of Pre-Transplant Peripheral Blood Immune Cell Subset Quantity and Ratios with Development of de novo DSA in the First Year Posttransplant.
| Immune cell subsets (pretransplant) | AUC statistic (95% confidence interval) |
Optimal threshold (based on Youden index) |
Sensitivity | Specificity |
|---|---|---|---|---|
| CXCR5+IFN-γ+CD8+ T cells | 0.81 (0.69 - 0.92) | < 3300 cells per million PBMCs | 0.93 | 0.62 |
| Ratio of CXCR5+IFN-γ+CD8+ T cells to combined Th1/Th2 cells | 0.76 (0.63 - 0.88) | < 0.3 | 0.93 | 0.62 |
| Ratio of IFN-γ+CD8+ T cells to combined Th1/Th2 cells | 0.72 (0.60 - 0.83) | < 1.5 | 0.91 | 0.43 |
| CXCR5+CD8+ T cells | 0.71 (0.57 - 0.85) | < 20,481 cells per million PBMCs | 0.81 | 0.59 |
| IFN-γ+CD8+ T cells | 0.70 (0.58 - 0.82) | < 10,365 cells per million PBMCs | 0.74 | 0.63 |
| CD8+ T cells | 0.64 (0.51 - 0.78) | < 164,694 cells per million PBMCs | 0.83 | 0.44 |
| CD4+ T cells | 0.63 (0.50 - 0.75) | > 523,165 cells per million PBMCs | 0.74 | 0.56 |
| IFN-γ+CD4+ T cells (Th1) | 0.59 (0.46 - 0.72) | > 2,296 cells per million PBMCs | 0.87 | 0.35 |
| IL-10+CD4+ T cells | 0.58 (0.44 - 0.72) | > 5,909 cells per million PBMCs | 0.48 | 0.74 |
| IL-4+CD4+ T cells (Th2) | 0.58 (0.44 - 0.72) | > 6,553 cells per million PBMCs | 0.61 | 0.60 |
| FoxP3+IL-10+CD4+ T cells (Treg) | 0.53 (0.39 - 0.66) | > 342 cells per million PBMCs | 1.00 | 0.12 |
| IL-17+CD4+ T cells (Th17) | 0.48 (0.34 - 0.63) | > 1,773 cells per million PBMCs | 0.22 | 0.90 |
Immunosuppression Drug Levels in DSA-positive and DSA-negative Recipients.
All recipients received induction therapy with lymphocyte depleting agent, a short-term (5 day) steroid taper followed by combination calcineurin (CNi) and mTOR inhibition (mTORi) maintenance immunosuppression without steroids. No differences in serum immunosuppression drug levels for CNi (cyclosporine 2 hour peak) or mTORi (trough) were detected between DSA-positive and DSA-negative recipients in the first year posttransplant (Figure S6A,B). Thus the differences in T cell subset quantity and development of DSA posttransplant cannot be attributed to differences in immunosuppression regimen or exposure.
Posttransplant Infections in DSA-positive and DSA-negative Recipients.
We analyzed the development of infections in transplant recipients over the first year posttransplant. Altogether 45 percent (43/95) of transplant recipients developed 1 or more bacterial (25/43) and/or viral (22/43) infections during the 12-month follow-up (Table 3). Bacterial and viral infections occurred most commonly in the first 4 months posttransplant (Figure S7). Infections occurred more frequently in DSA-positive (14/23, 61%) compared to DSA-negative (29/72, 40%) recipients (p=0.03). Recipients in the DSA-positive group developed infections both prior to (8/14) and after (6/14) initial detection of DSA. In the 8 DSA-positive recipients who developed infections prior to DSA detection, infection preceded DSA detection by 3.5±1.8 months. Only 2 of the 8 recipients had an infection closer to the time of initial DSA detection (1 month prior to DSA detection). Thus, development of DSA in the majority of recipients did not occur in the context of proximate infections in this study. Most recipients with infection developed only 1 bacterial infection (17/25) and/or 1 viral infection (21/22) during the study period. Only 5 recipients developed CMV viremia (4 DSA-negative and 1 DSA-positive recipients); and only 1 of the 5 who developed CMV viremia had D+/R- serology. The rate of CMV infection in DSA-negative (4/72 or 5.5%) and in DSA-positive recipients (1/23 or 4.3%) were similar. Fungal infections were infrequent (3/43) and occurred in recipients with multiple infections.
Table 3.
Infections in DSA-positive and DSA-negative Primary Kidney Transplant Recipients in the First Year Posttransplant.
| Number of Recipients with Infections (in the first year post transplant) | |||||
|---|---|---|---|---|---|
| All Recipients (n=95) | DSA negative (n=72) | DSA positive (n=23) | P values | ||
| Recipients with Bacterial and/or Viral Infection | 43 (45.3%)a | 29 (40.3%) | 14 (60.9%) | p=0.03 | |
| Recipients with Bacterial Infectione | 25 (26.3%) | 16 (22.2%)b | 9 (39.1%)c | p=0.07 | |
| Infection site | Urinary Tract Infection | 21 (22.1%) 5 with urosepsis | 14 (19.4%) 3 with urosepsis | 7 (30.4%) 2 with urosepsis | p=0.01 |
| Surgical Wound Infection | 6 (6.3%) | 2 (2.8%) | 4 (17.4%) | p=0.03 | |
| C. Diff. Colitis | 3 (3.2%) | 2 (2.8%) | 1 (4.3%) | p=0.57 | |
| Bacteremia (including urosepsis) | 8 (8.4%) | 4 (5.6%) | 4 (17.4%) | p=0.14 | |
| Recipients with Viral Infectionf | 22 (23.2%)d | 15 (20.8%)d | 7 (30.4%) | p=0.54 | |
| Viremia | Symptomatic Viremia | 10 (10.5%) | 7 (9.7%) | 3 (13%) | p=0.70 |
| Asymptomatic Viremia | 13 (13.7%) | 9 (12.5%) | 4 (17.4%) | p=0.51 | |
4 recipients had both viral and bacterial infections (2 DSA-negative and 2 DSA-positive recipients)
3 DSA-negative recipients with multiple bacterial infections
5 DSA-positive recipients with multiple bacterial infections
1 DSA-negative recipient with 1 symptomatic viral infection and 1 asymptomatic viremia
Bacterial pathogens included Enterococcus faecium (17), Escherichia coli (7), Klebsiella pneumonia (4), Staphylococcus aureus (4), Pseudomonas aeruginosa (4), Clostridium difficile (3), Staphylococcus epidermidis (1), Dermabacter hominis (1), and Citrobacter freundii (1).
Viral pathogens included BKV (12), CMV (5), EBV (2), influenza (2), HSV (1), and Varicella zoster (1)
The quantity of peripheral CD8+ T cell subsets as well as Th1 and Th2 CD4+ T cell subsets in individual profiles generally did not fluctuate in DSA-positive and DSA-negative recipients with infection compared to those without infection. A subset of DSA-positive recipients with infection (7/14) compared to DSA-positive recipients without infection had a slight increase in quantity of CXCR5+IFN-γ+CD8+ T cells noted at 9 and 12 months posttransplant. However, as shown in Figure S7, most infections occurred early post transplant.
De novo DSA and Clinical Outcomes.
One-Year Patient and Graft Survival.
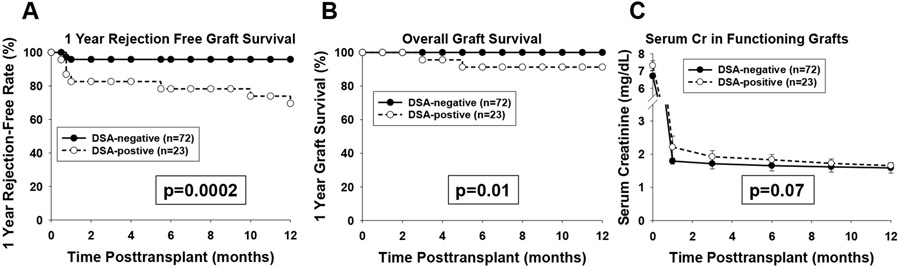
One-year patient survival is 100% in both groups. One-year rejection-free graft survival was lower in DSA-positive (69.6%) compared to DSA-negative (95.8%) recipients (p=0.0002; Figure 5A). One-year overall graft survival was lower in DSA-positive (91%) compared to DSA-negative (100%) recipients (p=0.01; Figure 5B). No significant difference in serum creatinine was detected between DSA-positive and DSA-negative recipients with functioning grafts (p= ns, Figure 5C; serum creatinine values censored for delayed graft function or graft loss).
Figure 5. Rejection-Free Graft Survival, Overall Graft Survival, and Serial Serum Creatinine of Functioning Allografts in DSA-positive and DSA-negative Recipients over the First Year Posttransplant.
95 kidney transplant recipients were monitored for rejection, graft function and survival over the first year posttransplant. A) One year rejection-free graft survival was significantly lower in DSA-positive recipients (69.6%, n=23) compared to DSA-negative recipients (95.8%, n=72; p=0.0002). B) Overall graft survival was significantly lower in DSA-positive recipients (91%, n=23) compared to DSA-negative recipients (100%, n=72; p=0.01). C) Serum creatinine (mg/dL) before transplant and after transplant in recipients with functioning kidney allografts at 1, 3, 6, 9 and 12 months posttransplant (censored for recipients with dialysis dependent delayed graft function or graft loss) was similar between DSA-positive and DSA-negative recipients over the first year posttransplant. Delayed graft function occurred in both DSA-positive (5/23) and DSA-negative (5/72) recipients. Graphed serum creatinine represents geometric mean ± standard error.
First Year Hospital Readmissions.
Overall 67 percent (64/95) of recipients were readmitted to the hospital in the first year posttransplant. First year hospital readmissions were more frequent in DSA-positive (19/23, 82.6%) compared to DSA-negative (45/72, 62.5%) recipients (p=0.004). Among recipients readmitted to the hospital in the first year posttransplant, DSA-positive recipients had more hospital readmissions (4.1±2.9) compared to DSA-negative recipients (2.3±1.5; p=0.002, Table 4).
Table 4.
Readmissions, Biopsies, and Outcomes in DSA-positive and DSA-negative Primary Kidney Transplant Recipients in the First Year Posttransplant.
| Number of Recipients | |||||
|---|---|---|---|---|---|
| Patient Outcomes | All Recipients (N=95) | DSA negative (n=72) | DSA positive (n=23) | P value | |
| Hospital Readmissions | 64 (67.4%) | 45 (62.5%) | 19 (82.6%) | p=0.004 | |
| Readmissions per patienta | 2.7±2.2 | 2.3±1.5 | 4.1±2.9 | p=0.002 | |
| Kidney Biopsy | 31 (32.6%) | 14 (19.4%) | 17 (74.0%) | p<0.0001 | |
| Acute Rejection | 10 (10.5%) | 3 (4.2%) | 7 (30.4%) | p=0.002 | |
| Rejection | CMR | 5 (5.3%) | 3 (4.2%) | 2 (8.7%) | - |
| AMR | 1 (1.1%) | 0 (0%) | 1 (4.3%) | - | |
| Mixed CMR/AMR | 4 (4.2%) | 0 (0%) | 4 (17.4%) | - | |
| Graft loss | 2 (2.1%) | 0 (0%) | 2 (8.7%) | p=0.057 | |
Mean ± standard deviation of readmissions is shown for recipients with readmission
Acute Rejection (AR) in the First Year Posttransplant.
In the first year posttransplant 33 percent (31/95) of transplant recipients were biopsied for diagnostic purposes. DSA-positive recipients had a significantly higher frequency of renal biopsies (17/23, 74%) compared to DSA-negative recipients (14/72, 19%; p<0.0001; Table 4). The overall incidence of acute rejection (AR) was 11% (10/95) (5 cell-mediated rejection, 1 antibody mediated rejection, and 4 combined cellular and antibody mediated rejection). The incidence of acute rejection was significantly higher in DSA-positive recipients (7/23, 30.4%) compared to DSA-negative recipients (3/72, 4.2%; p=0.002). Of the 7 DSA-positive recipients who developed biopsy-proven AR, 2 had cell-mediated rejection (CMR), 1 had antibody-mediated rejection (AMR), and 4 had mixed rejection (CMR and AMR). All of the DSA-positive (n=2) and DSA-negative (n=3) recipients with only CMR responded to antirejection therapy; all 5 received a steroid taper (2 mg/kg) and 1/2 DSA-positive and 2/3 DSA-negative recipients with CMR also received ATG and methylprednisolone bolus. Antirejection treatment in all recipients was accompanied by an improvement in serum creatinine (4.1±1.3 to 1.7±0.3 mg/dL; p=0.001).
Five DSA-positive recipients had biopsy-proven AMR (1 AMR alone; 4 with mixed AMR and CMR). The biopsies in these DSA-positive recipients demonstrated histologic features of AMR including peritubular margination, and peritubular capillary C4d staining. Recipients who were treated for mixed AMR/CMR all received a steroid taper (2 mg/kg) and some also received a variety of other agents including ATG (n=3/4), IVIG (n=3/4), methylprednisolone bolus (n=1/4), velcade (n=2/4), plasmapheresis (n=2/4), eculizumab (n=1/4), and/or belatacept (n=1/4). The recipient who had AMR alone was treated with steroid taper, plasmapheresis, IVIG, velcade, and belatacept. Three of the 5 recipients (1 AMR alone and 2 with mixed AMR and CMR) responded to therapy with an improvement of serum creatinine; 1 without significant change in DSA and 2 with a ~5-fold reduction in MFI of the dominant DSA specificity. The remaining 2 recipients with mixed AMR and CMR were nonresponsive to therapeutic interventions and remained dialysis-dependent despite reduction in DSA (DSA MFI decreased from 10 220 to 3224 in one and from 3907 to undetectable in the other), and both lost their allografts by 3 and 5 months posttransplant, respectively. No significant differences in peripheral Th1 or Th2 CD4+ T cell subsets or IFN-γ+ or CXCR5+IFN-γ+ CD8+ T cell subsets were observed among DSA-positive recipients with rejection and/or graft loss compared to DSA-positive recipients without rejection and/or graft loss (data not shown).
Peripheral blood analysis of immune cell subsets in recipients with rejection (n=10) did not coincide with treatment of rejection in any recipients. Treatment for rejection is unlikely to have impacted quantification of peripheral immune cell subsets since PBMC samples were obtained either before initiation of treatment for rejection or at least 1-month following treatment of rejection. In the samples obtained after treatment of rejection (on average 2.4±0.7 months after initiation of antirejection therapy), we noted no significant difference between total CD4+ or CD8+ T cells or T cell subset quantities before or after the treatment.
Discussion
Despite the use of powerful immunosuppressive medications, a substantial number (13–27%) of renal transplant recipients develop DSA.7-10 The high frequency of DSA specific to HLA class II alloantigens (16/23 recipients) observed in this study is consistent with prior publications showing a high frequency of HLA class II directed DSA occurring within the first year after kidney transplant.6,12,14,39 While we surmised that all recipients in this study developed de novo DSA based on the absence of DSA detected pretransplant, it is recognized that early DSA production (less than 3 months posttransplant) could be an amnestic response in some cases. However, the few recipients (n=5) who developed early DSA were young and/or had significant donor/recipient HLA disparity (not shown) which Tambur et al. noted are factors more likely associated with de novo DSA production.40 It is well known that long-term kidney allograft survival is reduced with recipients who develop de novo DSA.7,11-19 Several reports conclude that no current immunosuppressive regimen effectively prevents the development of DSA,4,41 while others infer relative efficacy of calcineurin inhibitors (CNi) based on the observation that low CNi trough levels42 or conversion of kidney transplant patients from CNi to mTOR inhibitor (mTORi) based therapy43-46 is associated with the development of DSA production and decreased graft function. However, these clinical data are difficult to interpret in aggregate and comparisons between studies are flawed due to the retrospective nature of the studies, the variability in immunosuppression treatment regimens (doses, timing, combination therapies, use of induction agents), and variability in reporting isotype, specificity and timing of DSA measurement after transplant.
In the current study, we serially monitored immune profiles of first-time kidney transplant recipients treated with a uniform immunosuppressive regimen and who manifested equivalent immunosuppression levels of CNi and mTORi throughout the first year posttransplant. In this prospective longitudinal study, we found a significant and novel inverse association between the quantity of peripheral CXCR5+IFN-γ+CD8+ T cells as well as IFN-γ+CD8+ T cells with the development of de novo DSA. Since the risk of developing DSA is likely a balance between mechanisms which promote and those which downregulate humoral alloimmunity, we determined the ratio of CD8+ to CD4+ T cell subsets. We found that the ratio of CXCR5+IFN-γ+CD8+ T cells as well as IFN-γ+CD8+ T cells to combined Th1 and Th2 CD4+ T cell subsets was significantly lower in recipients who developed DSA. Overall these results suggest that immune monitoring of CD8+ T cell subset quantities and/or CD8+:CD4+ T cell subset ratios in kidney transplant recipients may be useful to predict immunologic risk for development of DSA. In future studies, it will be interesting to determine if the quantity of these CD8+ T cell subsets are also significantly reduced in sensitized versus nonsensitized dialysis patients awaiting transplant.
These studies also highlight intriguing similarities between humoral immune responses in mice and humans. The inverse relationship observed in this study between the quantity of circulating human peripheral blood CXCR5+IFNγ+CD8+ T cells (expressing the phenotype associated with antibody-suppressor CD8+ T cells in mice) with the development of DSA after kidney transplant is consistent with an antibody-suppressor function. In murine experimental studies, antibody-suppressor CD8+ T cells downregulate alloantibody production by inhibiting alloprimed CD4+ T cells subsets and by perforin- and FasL-dependent killing of alloprimed B cells.28 We recently reported that CXCR5, a chemokine receptor important for germinal center homing, is essential for antibody-suppressor CD8+ T cell function in mice.30 Indeed, we found that alloantibody suppression by adoptively transferred CXCR5+IFN-γ+CD8+ T cells into transplant recipients is accompanied by a reduction in the number of B cells and cytokine-expressing CD4+ TFH cells in the germinal center (GC).30 In our study, approximately 65% of human peripheral blood CXCR5+IFNγ+CD8+ T cells express LAMP-1 (CD107a) (not shown), a marker of perforin/granzyme degranulation, consistent with cytotoxic effector mechanism.47 Altogether, these data lend support to the hypothesis that there is a human homolog of murine antibody-suppressor CD8+ T cells. In support of this hypothesis is the observation that human CXCR5+CD8+ T cells are detected in lymphoid tissue (tonsillar GC) and that these human CXCR5+CD8+ T cells decrease CD4+ TFH and plasma cell differentiation in in vitro coculture.48 However, in other studies, human CXCR5+CD8+ T cells detected in human tonsillar tissue were determined to be noncytolytic effector memory cells which could be stimulated to produce cytokines and increase survival and function of B cells in vitro to a modest extent.49 More in depth studies are necessary to determine if these observed differences reflect the activity of phenotypically and functionally distinct subsets of human CXCR5+CD8+ T cells.
Precedence exists for detection of lymphoid GC T cell populations in the periphery. For example, circulating CD4+ T follicular helper (cTFH) cells with capacity to provide B cell help are detected in both mice and humans.50-53 These cells share some but not all phenotypic markers and cytokine expression profile associated with GC CD4+ TFH cells.50 Circulating TFH cells are reported to circulate to the lymph node and have been correlated with B cell maturation to plasma cells and with antibody production.51-53 Of note, we did not detect any differences in cTFH cells or B cell subsets including IgG+ B cells and plasma cells between DSA-positive and DSA-negative recipients (not shown).
Overall our data demonstrate that development of de novo DSA is associated with higher hospital readmissions, higher rate of acute rejection and inferior kidney transplant survival. Diagnosis of AMR precipitated by the detection of DSA requires performance of a renal biopsy which carries some procedural risk and may identify antibody mediated pathology at an advanced stage with less likelihood for successful treatment. Strategies to predict risk of developing posttransplant humoral alloimmunity may be useful to identify recipients who could benefit from higher target levels of immunosuppression to avoid the development of DSA or more intense monitoring of DSA in order to diagnose AMR at an earlier stage. Success in reducing the incidence of DSA posttransplant would be expected to translate to improved clinical outcomes.
To our knowledge this is the first prospective study to report an inverse association between the quantity of human peripheral CXCR5+IFNγ+CD8+ T cell subsets, expressing the phenotype identified for antibody-suppressor CD8+ T cells in mice, with de novo DSA production in the first year after kidney transplant. These results support the rationale for future larger scale studies in transplant recipients to corroborate these findings and to investigate the function of these human CXCR5+IFN-γ+CD8+ T cells, their susceptibility to immunosuppressive agents and their biomarker potential in diverse populations.
Supplementary Material
Acknowledgments
The authors thank the OSU Clinical Trials Management Office (CTMO) for their assistance with study administrative support and informed consent. The authors thank the OSU CTC nursing staff for their assistance with coordination of study sample collection.
Financial Disclosure: This work was supported by the Ohio State University Wexner Medical Center Department of Surgery Lockwood Career Development Award (JMZ), the OSU Division of Transplant Surgery, the OSU College of Medicine, the OSU College of Medicine Roessler research scholarship (MWB) and the National Institutes of Health grants AI139913 and CA016058.
Abbreviations Page
- AMR
antibody-mediated rejection
- BKV
BK virus
- CMV
cytomegalovirus
- CNi
calcineurin inhibition
- cTFH cells
circulating follicular helper T cells
- DSA
donor specific antibody
- EBV
Epstein-Barr virus
- ESRD
end staged renal disease
- HSV
herpes simplex virus
- IFN
interferon
- IL
interleukin
- PRA
panel reactive antibody
- MFI
mean fluorescence intensity
- MST
median survival time
- mTORi
mechanistic target of rapamycin inhibition
Footnotes
Disclaimer: All authors declare no conflicts of interest.
References
- 1.Sá H, Leal R, Rosa MS. Renal transplant immunology in the last 20 years: a revolution towards graft and patient survival improvement. Int Rev Immunol. 2017;36(3):182–203. [DOI] [PubMed] [Google Scholar]
- 2.Ponticelli C Progression of renal damage in chronic rejection. Kidney Int Suppl. 2000;75:S62–S70. [PubMed] [Google Scholar]
- 3.Baluja P, Haragsim L, Laszik Z. Chronic allograft nephropathy. Adv Chronic Kidney Dis. 2006;13(1):56–61. [DOI] [PubMed] [Google Scholar]
- 4.Puttarajappa C, Shapiro R, Tan HP. Antibody-mediated rejection in kidney transplantation: a review. J Transplant. 2012;2012:193724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenzuela NM, Reed EF. Antibodies in transplantation: the effects of HLA and non-HLA antibody binding and mechanisms of injury. Methods Mol Bio. 2013;1034:41–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelletier RP, Hennessy PK, Adams PW, et al. Clinical significance of MHC-reactive alloantibodies that develop after kidney or kidney-pancreas transplantation. Am J Transplant. 2002;2(2):134–141. [DOI] [PubMed] [Google Scholar]
- 7.Lefaucheur C, Viglietti D, Bentlejewski C, et al. IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J Am Soc Nephrol. 2016;27(1):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devos JM, Gaber AO, Teeter LD, et al. Intermediate-term graft loss after renal transplantation is associated with both donor-specific antibody and acute rejection. Transplantation. 2014;97(5):534–540. [DOI] [PubMed] [Google Scholar]
- 9.Heilman RL, Nijim A, Desmarteau YM, et al. De novo donor-specific human leukocyte antigen antibodies early after kidney transplantation. Transplantation. 2014;98(12):1310–1315. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JE, Gralla J, Cagle L, et al. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011;91(10):1103–1109. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Liang LW, Gjertson DW, et al. Development of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunction. Transplantation. 2005;79(5):591–598. [DOI] [PubMed] [Google Scholar]
- 12.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12(5):1157–1167. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo LG, Campbell PM, Sis B, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9(11):2532–2541. [DOI] [PubMed] [Google Scholar]
- 14.Guidicelli G, Guerville F, Lepreux S, et al. Non-complement-binding de novo donor-specific anti-HLA antibodies and kidney allograft survival. J Am Soc Nephrol. 2016;27(2):615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubert O, Loupy A, Hidalgo L, et al. Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol. 2017;28(6):1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths EJ, Nelson RE, Dupont PJ, et al. Skewing of pretransplant anti-HLA class I antibodies of immunoglobulin G isotype solely toward immunoglobulin G1 subclass is associated with poorer renal allograft survival. Transplantation. 2004;77(11):1771–1773. [DOI] [PubMed] [Google Scholar]
- 17.Khovanova N, Daga S, Shaikhina T, et al. Subclass analysis of donor HLA-specific IgG in antibody-incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure. Transpl Int. 2015;28(12):1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamdani G, Goebel JW, Brailey P, et al. IGG3 anti-HLA donor-specific antibodies and graft function in pediatric kidney transplant recipients. Pediatr Transplant. 2018;22(5):e13219. [DOI] [PubMed] [Google Scholar]
- 19.Worthington JE, Martin S, Al-Husseini DM, et al. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation. 2003;75(7):1034–1040. [DOI] [PubMed] [Google Scholar]
- 20.Piemonti L, Everly MJ, Maffi P, et al. Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes. 2013;62(5):1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drachenberg CB, Odorico J, Demetris AJ, et al. Banff schema for grading pancreas allograft rejection: working proposal by a multi-disciplinary international consensus panel. Am J Transplant. 2008;8(6):1237–1249. [DOI] [PubMed] [Google Scholar]
- 22.Jorns C, Nowak G, Nemeth A, et al. De novo donor-specific HLA antibody formation in two patients with Crigler-Najjar syndrome type I following human hepatocyte transplantation with partial hepatectomy preconditioning. Am J Transplant. 2016;16(3):1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank R, Molina MR, Wald JW, et al. Correlation of circulating donor-specific anti-HLA antibodies and presence of C4d in endomyocardial biopsy with heart allograft outcomes: a single-center, retrospective study. J Heart Lung Transplant. 2013;32(4):410–417. [DOI] [PubMed] [Google Scholar]
- 24.Kozlowski T, Rubinas T, Nickeleit V, et al. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011;17(4):357–368. [DOI] [PubMed] [Google Scholar]
- 25.Tsai HL, Island ER, Chang JW, et al. Association between donor-specific antibodies and acute rejection and resolution in small bowel and multivisceral transplantation. Transplantation. 2011;92(6):709–715. [DOI] [PubMed] [Google Scholar]
- 26.Girnita AL, McCurry KR, Iacono AT, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23(10):1135–1141. [DOI] [PubMed] [Google Scholar]
- 27.Mittal S, Page SL, Friend PJ, et al. De novo donor-specific HLA antibodies: biomarkers of pancreas transplant failure. Am J Transplant. 2014;14(7):1664–1671. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerer JM, Pham TA, Sanders VM, et al. CD8+ T cells negatively regulate IL-4-dependent, IgG1-dominant posttransplant alloantibody production. J Immunol. 2010;185(12):7285–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerer JM, Pham TA, Wright CL, et al. Alloprimed CD8(+) T cells regulate alloantibody and eliminate alloprimed B cells through perforin- and FasL-dependent mechanisms. Am J Transplant. 2014;14(2):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerer JM, Ringwald BA, Elzein SM, et al. Antibody-suppressor CD8+ T cells require CXCR5. Transplantation. 2019;103(9):1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashoor I, Najafian N, Korin Y, et al. Standardization and cross validation of alloreactive IFNγ ELISPOT assays within the clinical trials in organ transplantation consortium. Am J Transplant. 2013;13(7):1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hricik DE, Augustine J, Nickerson P, et al. Interferon gamma ELISPOT testing as a risk-stratifying biomarker for kidney transplant injury: results from the CTOT-01 multicenter study. Am J Transplant. 2015;15(12):3166–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg A, Song LY, Wilkening C, et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol. 2009;16(8):1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa AO, Henry S, Marója FM, et al. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin Exp Immunol. 1998;111(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Male D, Brostoff J, Roth DB, et al. Immunology. 7th ed. Amsterdam, the Netherlands: Elsevier Ltd; 2006. [Google Scholar]
- 36.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology. 2003;229(1):3–8. [DOI] [PubMed] [Google Scholar]
- 37.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283–298. [DOI] [PubMed] [Google Scholar]
- 38.Lüdemann L, Grieger W, Wurm R, et al. Glioma assessment using quantitative blood volume maps generated by T1-weighted dynamic contrast-enhanced magnetic resonance imaging: a receiver operating characteristic study. Acta Radiol. 2006;47(3):303–310. [DOI] [PubMed] [Google Scholar]
- 39.Ntokou IS, Iniotaki AG, Kontou EN, et al. Long-term follow up for anti-HLA donor specific antibodies postrenal transplantation: high immunogenicity of HLA class II graft molecules. Transpl Int. 2011;24(11):1084–1093. [DOI] [PubMed] [Google Scholar]
- 40.Tambur AR, Campbell P, Claas FH, et al. Sensitization in Transplantation: Assessment of Risk (STAR) 2017 working group meeting report. Am J Transplant. 2018;18(7):1604–1614. [DOI] [PubMed] [Google Scholar]
- 41.Bartel G, Schwaiger E, Böhmig GA. Prevention and treatment of alloantibody-mediated kidney transplant rejection. Transpl Int. 2011;24(12):1142–1155. [DOI] [PubMed] [Google Scholar]
- 42.Wiebe C, Rush DN, Nevins TE, et al. Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol. 2017;28(11):3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz San Millán JC, López-Hoyos M, Segundo DS, et al. Predictive factors of allosensitization in renal transplant patients switched from calcineurin to mTOR inhibitors. Transpl Int. 2014;27(8):847–856. [DOI] [PubMed] [Google Scholar]
- 44.Kamar N, Del Bello A, Congy-Jolivet N, et al. Incidence of donor-specific antibodies in kidney transplant patients following conversion to an everolimus-based calcineurin inhibitor-free regimen. Clin Transplant. 2013;27(3):455–462. [DOI] [PubMed] [Google Scholar]
- 45.Croze LE, Tetaz R, Roustit M, et al. Conversion to mammalian target of rapamycin inhibitors increases risk of de novo donor-specific antibodies. Transpl Int. 2014;27(8):775–783. [DOI] [PubMed] [Google Scholar]
- 46.Liefeldt L, Brakemeier S, Glander P, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant. 2012;12(5):1192–1198. [DOI] [PubMed] [Google Scholar]
- 47.Donia M, Kjeldsen JW, Andersen R, et al. PD-1+ polyfunctional T cells dominate the periphery after tumor-infiltrating lymphocyte therapy for cancer. Clin Cancer Res. 2017;23(19):5779–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu F, Neelapu SS. CXCR5+CD8+ T cells are localized in B cell follicles and germinal centers and exhibit regulatory and anti-tumor function. J Immunother Cancer. 2015;3(suppl 2):P321. [Google Scholar]
- 49.Quigley MF, Gonzalez VD, Granath A, et al. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol. 2007;37(12):3352–3362. [DOI] [PubMed] [Google Scholar]
- 50.Thornhill JP, Fidler S, Klenerman P, et al. The role of CD4+ T follicular helper cells in HIV infection: from the germinal center to the periphery. Front Immunol. 2017;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sage PT, Alvarez D, Godec J, et al. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124(12):5191–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forcade E, Kim HT, Cutler C, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127(20):2489–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.