Abstract
To better understand the potential function of carotenoids in the chemoprevention of cancers, mechanistic understanding of carotenoid action on genetic and epigenetic signaling pathways is critically needed for human studies. The use of appropriate animal models are the most justifiable approaches to resolve mechanistic issues regarding protective effects of carotenoids at specific organs and tissue sites. While the initial impetus for studying the benefits of carotenoids in cancer prevention was their antioxidant capacity and pro-vitamin A activity, significant advances have been made in the understanding of the action of carotenoids with regards to other mechanisms. This review will focus on two common carotenoids, provitamin A carotenoid β-cryptoxanthin and non-provitamin A carotenoid lycopene, as promising chemopreventive agents or chemotherapeutic compounds against cancer development and progression. We reviewed animal studies demonstrating that β-cryptoxanthin and lycopene effectively prevent the development or progression of various cancers and the potential mechanisms involved. We highlight recent research that the biological functions of β-cryptoxanthin and lycopene are mediated, partially via their oxidative metabolites, through their effects on key molecular targeting events, such as NF-κB signaling pathway, RAR/PPARs signaling, SIRT1 signaling pathway, and p53 tumor suppressor pathways. The molecular targets by β-cryptoxanthin and lycopene, offer new opportunities to further our understanding of common and distinct mechanisms that involve carotenoids in cancer prevention.
Keywords: Carotenoids, β-cryptoxanthin, lycopene, cancer prevention, molecular mechanisms
Introduction
Dietary intervention is one of the main strategies for preventing cancer development and increasing cancer survival rates. Despite the earlier unexpected findings of the human intervention trials conducted to determine the chemoprotective effect of high doses of β-carotene on the incidences of lung cancer in smokers, which found no protective effects or even harmful effects, supporting evidence indicates that the protective roles of fruits and vegetables rich in carotenoids in cancer prevention continues to be reported in human epidemiological studies and in mechanistic studies using cell culture and animal models. There are three provitamin A carotenoids (β-carotene, α-carotene, and β-cryptoxanthin) and three non-provitamin A carotenoid (lycopene, lutein and zeaxanthin) that can be found routinely in human plasma and tissues. These carotenoids have been studied for their potential beneficial roles against cancer development (e.g., lung, liver, prostate, breast, colorectal and stomach). The reader is referred to accompanying review article for both preclinical and epidemiological data on carotenoids in cancer prevention in this special issue [1]). Carotenoids are lipophilic plant pigments with polyisoprenoid structure, typically containing a series of conjugated double bonds in the central chain of the molecule, which makes them susceptible to oxidative and enzymatic cleavage and formation of potentially bioactive metabolites. Within the past years, we have gained greater knowledge of the biological effects of carotenoids, particularly the impact of oxidation on these carotenoids and the potential for beneficial effects of small quantities or harmful effects of large quantities of the resulting metabolic products. In particular, it is important to understand the protective roles of carotenoids and their derivatives in the process of chronic disease development, with special attention to their metabolism and biological actions, molecular targets, dose effects, and organ-specific effects. In this review, we focus on recent animal studies for potential underlying mechanisms for chemopreventive effects of β-cryptoxanthin and lycopene in tumorigenesis, dependent and independent of the carotenoid cleavage enzymes, in particularly, in the lung and liver. The reader is referred also to recent review articles regarding other common carotenoids, such as β-carotene [2], lutein [2–4], and zeaxanthin [2–4]. These studies help us understand the common and distinct molecular mechanisms by which carotenoids and their metabolites protect against the development of cancers in different tissues.
1. Chemistry and metabolism of β-cryptoxanthin and lycopene and the role of BCO1 and BCO2 beyond carotenoid cleavage activity
1.1. Chemistry and metabolism of β-cryptoxanthin and lycopene
β-Cryptoxanthin is a pro-vitamin A carotenoid primarily found in citrus fruits such as tangerines, oranges, mandarins, and papaya, and vegetables, such as sweet red peppers and butternut squash [5, 6]. Lycopene is the pigment principally responsible for the characteristic deep-red color of ripe tomato and tomato products, and watermelon. β-Cryptoxanthin and lycopene are two of the six most abundant carotenoids in human plasma and tissues [7]. Indeed, epidemiological studies provided evidence that β-cryptoxanthin and lycopene may act as anti-carcinogenic agents against certain types of cancers, including those of the lung, colon and prostate [1].
In mammalians, two carotenoid cleavage enzymes are involved in apocarotenoid biosynthesis and include beta-carotene-15,15’-oxygenase (BCO1) for retinoid [8, 9], and beta-carotene-9’,10’-oxygenase (BCO2) for apo-10’-carotenoids [10, 11]. The reader is referred to accompanying review article in this special issue that discuss carotenoid metabolism for details [12]. BCO1 cleaves β-cryptoxanthin at the 15,15′ double bond, which is critical for vitamin A production [13], while BCO2 cleaves β-cryptoxanthin at the 9’,10’ double bond to generate apo-10’-carotenoids [9, 14] (Fig. 1). Lycopene [15], lutein and zeaxanthin [14] can be preferentially cleaved by BCO2 to form their metabolite, apo-10’-carotenoids, also referred to as apo-10’-lycopenoid from lycopene cleavage (Fig. 2). When BCO2 was ablated in mice, the levels of lycopene were significantly elevated in hepatic and adipose tissues, as compared to wild-type mice, providing further evidence that lycopene is preferentially cleaved by BCO2 [16]. Both BCO1 and BCO2 are highly expressed in the liver and other peripheral tissues. β-Cryptoxanthin feeding resulted in a significant yellow coloration of the liver and adipose tissue due to accumulation of β-cryptoxanthin in the absence of either BCO1 [12] or both BCO1 and BCO2 [17].
Figure 1.
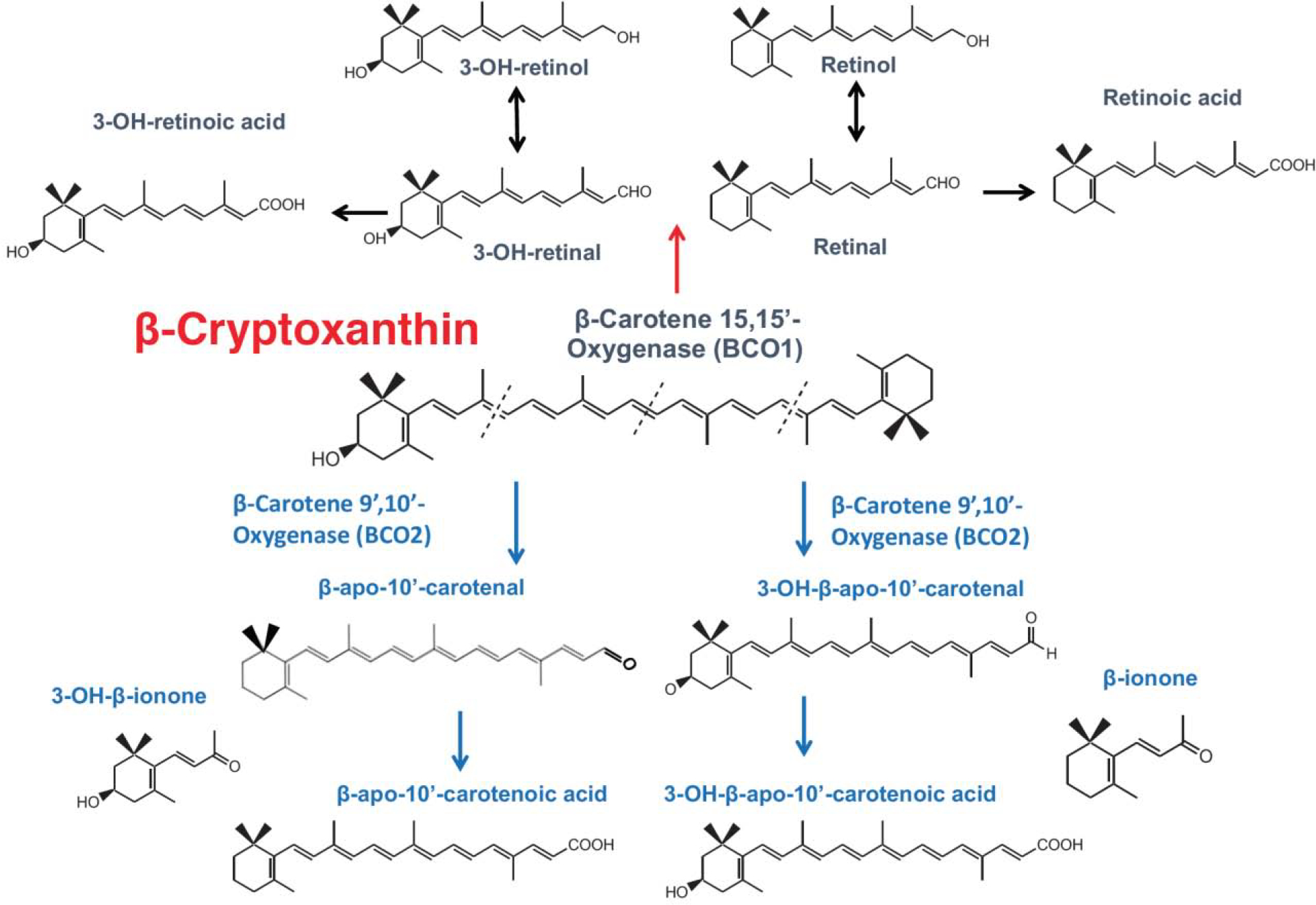
Metabolism of β-cryptoxanthin.
Figure 2.
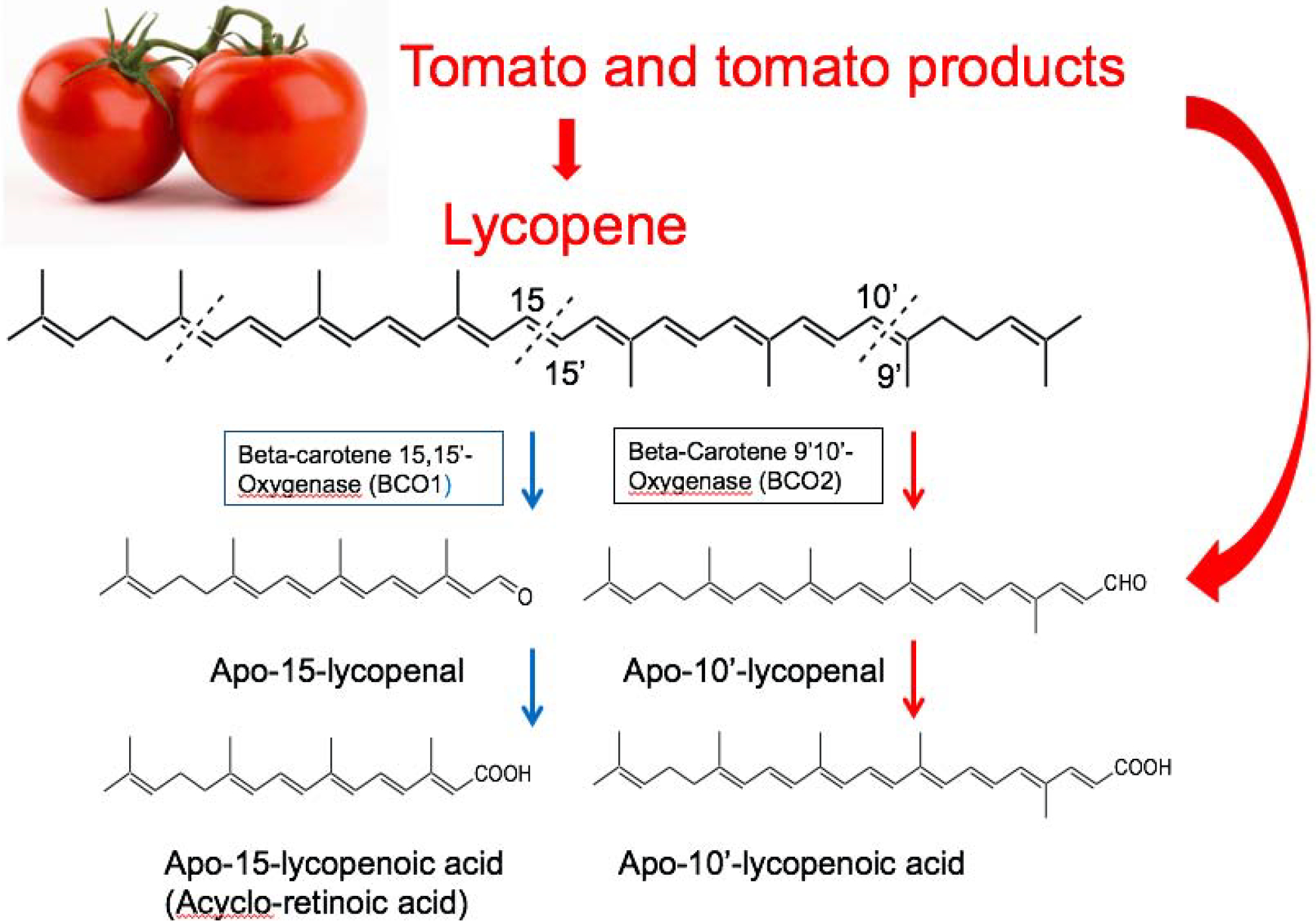
Metabolism of lycopene (modified from [204])
1.2. Genetic variants of BCO1/BCO2 in animals and humans
Single nucleotide polymorphisms (SNPs) of the BCO1/BCO2 genes related to a variety of conditions have been reported in humans. Previous studies have reported that BCO1 gene SNPs are common in humans and related to impaired catalytic activity in the bioconversion of β-carotene to vitamin A [18], although no significant association has been observed between BCO1 SNPs and the risk of various cancers such as breast [19], prostate [19], and lung cancer [20]. A recent study has shown that BCO1 SNPs are associated with plasma lycopene in response to the consumption of tomato juice in prostate cancer patients [21]. The Age-Related Eye Disease Study demonstrated that the BCO2 rs2250417 SNP is strongly related to age-related eye disease [22]. BCO2 SNPs have also been associated with alterations in the status of human and animal carotenoid levels, pro-inflammatory cytokine IL-18 expression [23], and susceptibility to ischemic stroke [24]. Higher serum levels of carotenoids have been reported to be associated with decreased risk of several cancers [25, 26]. However, whether the conflicting clinical trial results regarding the chemo-preventive effects of carotenoids might be due to the existence BCO1/BCO2 polymorphisms and the potential function of BCO1/BCO2 in cancers in humans need further investigation.
1.3. The role of BCO1 and BCO2 other than carotenoid cleavage activity
Recent studies using BCO1 and BCO2 knockout (KO) mice indicate that BCO1/BCO2 play diverse roles in many physiological processes [27, 28] beyond their carotenoid cleavage activities; BCO1 is involved in regulating lipid accumulation [29], insulin [16], steroid metabolism [30], and heart function [31], while BCO2 is involved in modulating mitochondria [8, 27], and anemia [28]. BCO2 also regulates nutrient metabolism, hypothalamic mitochondrial function, and local oxidative stress and inflammation [32]. We have showed that BCO1/BCO2 double knockout (BCO1/BCO2 DKO) mice exhibited mild liver steatosis and had significantly higher levels of hepatic cholesterol and triglycerides compared to wild-type (WT) animals [33]. Interestingly, microRNAs (miR-34a, miR-33, and miR-122) related to lipid and cholesterol metabolism were altered in BCO1/BCO2 DKO mice [33]. Moreover, hepatic oxidative stress markers, including HO-1, SOD1, SOD2, GPX, and catalase, were significantly altered in BCO1/BCO2 DKO mice [33]. The mRNA levels of sirtuin 1 (SIRT1) and farnesoid X receptor protein were impaired in BCO1/BCO2 DKO mice [33], indicating that BCO1/BCO2 could play an important role in maintaining normal hepatic lipid and cholesterol homeostasis, potentially through the activation of the miR-34a/SIRT1 pathway.
In terms of tumorigenesis, emerging cell culture studies and a xenograft study have indicated that the BCO1 and BCO2 enzymes might serve as tumor suppressor genes [34–36]. The authors observed that the inhibition of BCO1 in colon cancer cells increased certain characteristics of colon cancer including cell migration and invasion by up-regulating metalloproteinase 7 and 28 [34]. BCO1 overexpression in human neuroblastoma cells led to the suppression of cancer stemness and metastasis in human neuroblastoma cells [36]. BCO2 overexpression in prostate cancer cells also inhibited colony formation and proliferation [35]. However, there were no significant associations between BCO1 SNPs and various cancers risk such as breast [19], prostate cancer [19], and lung cancer have been observed in clinical data [20]. The ablation of BCO2 significantly attenuated the anti-prostate cancer effect in lycopene or tomato-fed groups, but the absence of BCO2 did not contribute to prostate tumorigenesis in control diet-fed group [37]. We have recently demonstrated that there were no significant differences in diethylnitrosamine (DEN)-initiated and a high-refined carbohydrate-diet-promoted hepatocellular carcinoma (HCC) development between WT and BCO1/BCO2 double KO mice [38]. Although this finding may not be universally applicable to other different cancer models, we provide the in vivo evidence that BCO1 and BCO2 do not play a major role as tumor promoters or tumor suppressors [38]. More relevant evidence involving use of a murine knockout model is necessary for better understanding the roles of BCO1 and BCO2 in cancer development at different organs.
2. The effects of β-cryptoxanthin and lycopene in cancer prevention
2.1. β-Cryptoxanthin and lycopene in cancer prevention
Recent data from the National Health and Nutrition Examination Survey III [39] and other epidemiological studies [40, 41], including seven large well-implemented cohorts [42], have shown that a high serum level of β-cryptoxanthin is associated with a lower risk of lung cancer death in current smokers. Importantly, the protective effect of β-cryptoxanthin was independent of the consumption of other nutrients found in fruits and vegetables, such as vitamin C, folate, and other carotenoids including α-carotene, lycopene, lutein, and zeaxanthin [39, 42]. These data support the concept that the beneficial effects anti-lung cancer effects of β-cryptoxanthin are unique and that β-cryptoxanthin, rather than β-carotene, is an effective preventive agent. The protective role of β-cryptoxanthin-rich fruits and vegetables in the prevention of cancer development has been demonstrated in human epidemiological studies, indicating that higher levels of serum β-cryptoxanthin are associated with a lowered risk of several cancers including lung cancer [42], squamous cell carcinoma of the esophagus [43], and bladder cancer [44]. A recent clinical intervention study has shown that consumption of β-cryptoxanthin-enriched juice with a carotenoid mixture capsule for 2.5 years led to a lowered risk of liver cancer among hepatitis patients with cirrhosis [45]. Animal studies have shown that β-cryptoxanthin exerts protective effects against the development of several cancers including lung cancer [46–48], colon cancer [49], gastric cancer [50], urinary bladder carcinogenesis [51], and liver cancer [38] by targeting multiple molecular mechanisms. These studies raise an important question whether the protective effect of β-cryptoxanthin against cancer development is due to intact β-cryptoxanthin or its metabolites such as vitamin A or apo-carotenoids. In the molecular mechanism section below, we highlight the major targets of β-cryptoxanthin that can lead to the suppression of the development or progression of various cancers. There are multiple avenues of evidence from both in vitro and in vivo experiments, indicating that the preventive effects of β-cryptoxanthin were likely due to biological activities of their intact molecules and metabolites produced by BCO1 and BCO2 against cancer development.
Although studies are inconsistent, epidemiologic studies suggested that higher intake of tomato products and lycopene are associated with lowered risk of prostate cancer [52, 53]. Animal studies using ferrets and mice have suggested that lycopene or the metabolites of lycopene supplementation may be therapeutic agents that can prevent the development of cancers such as lung cancer [54–56], liver cancer [57, 58], skin tumorigenesis [59], and prostate cancer [60] via multiple molecular pathways. There were several animal studies using whole foods intervention approach, such as using tomato powder or tomato paste which enriched in lycopene, to investigate the role of tomato consumption in the development of liver [61] and prostate cancer [62, 63]. Numerous oxidative metabolites of lycopene have been identified in both in vitro and in vivo systems, raising the possibility that the chemopreventive effect of lycopene could be, at least in part, due to its metabolites [64]. Indeed, apolycopenoids exist in tomatoes and tomato products, and a series of apolycopenoids including apo-10’-lycopenal have been identified in human plasmas of individuals who consumed tomato juice [65]. In mammalian tissues, lycopene can be cleaved by BCO2, producing apo-10’-lycopenal that can be oxidized into apo-10’-lycopenoic acid [11]. Recent study demonstrated that lycopene feeding inhibited hepatic tumorigenesis via differential mechanisms depending on BCO2 in mice [58]. This suggests that lycopene and the metabolites of lycopene may activate different molecular pathways, which may lead to the same preferable results in tumor outcomes. Here, we reviewed most recent studies and summarized the molecular mechanisms by which tomato consumption, lycopene, and metabolites of lycopene prevents the development of cancers.
2.2. Molecular mechanisms underlying β-cryptoxanthin and lycopene suppression of tumorigenesis
2.2.1. The NF-κB signaling pathway
Nuclear factor-κB (NF-κB) is a ubiquitous nuclear transcription factor that plays a pivotal role in various pathological processes such as inflammation, cell growth and survival, immune response, and development [66, 67]. NF-κB has been shown to activate the expression of genes involved in cell proliferation and cell survival [68]. It has been shown that tumor samples from lung cancer patients had significantly high levels of NF-κB in both non-small lung cancer and small cell lung cancer [69], which was correlated with cancer progression and poor prognosis [70]. Emerging pre-clinical evidence has demonstrated the tumor promoting role of NF-κB activation in lung inflammation, and tumorigenesis [71, 72]. Several factors can activate the NF-κB signaling pathway including tobacco-specific N-nitrosamine, cigarette smoke condensate, tumor necrosis factor (TNF)-α, and oxidative stress [73, 74]. Additionally, certain therapies for lung cancer, including systemic chemotherapy or radiation, which have been shown to induce NF-κB activation, decreased the efficacy of these treatments. [75]. Thus, it is important to identify dietary components targeting the NF-κB signaling pathway.
It has been showed that male ferrets exposed to tobacco smoke induced significantly higher levels of inflammation, oxidative stress DNA damage, and squamous metaplasia in the lung tissues, which were significantly improved by β-cryptoxanthin supplementation ((10 mg/kg diet and 20 mg/kg diet) in a dose-dependent manner [47]. Using immunohistochemistry analysis, the protein expression levels of inflammation markers including TNF-α, NF-κB, and activator protein-1 (AP-1) were significantly reduced by β-cryptoxanthin supplementation compared with the cigarette smoke alone ferrets [47]. Indeed, β-cryptoxanthin supplementation increased lung and plasma β-cryptoxanthin levels in a dose-dependent manner, suggesting that β-cryptoxanthin itself may have protective effects against lung lesions potentially by regulating the NF-κB pathway [47]. β-Cryptoxanthin supplementation also inhibited tobacco carcinogen (NNK)- and nicotine-induced IL-6 expression, emphysema and lung cancer development in AJ mice [46]. These results suggest that β-cryptoxanthin can serve as an effective dietary component against smoke-induced lung cancer by suppressing the NF-κB and inflammation pathway (Fig. 3).
Figure 3. Potential molecular mechanisms underlying β-cryptoxanthin prevention of cancer development/progression.
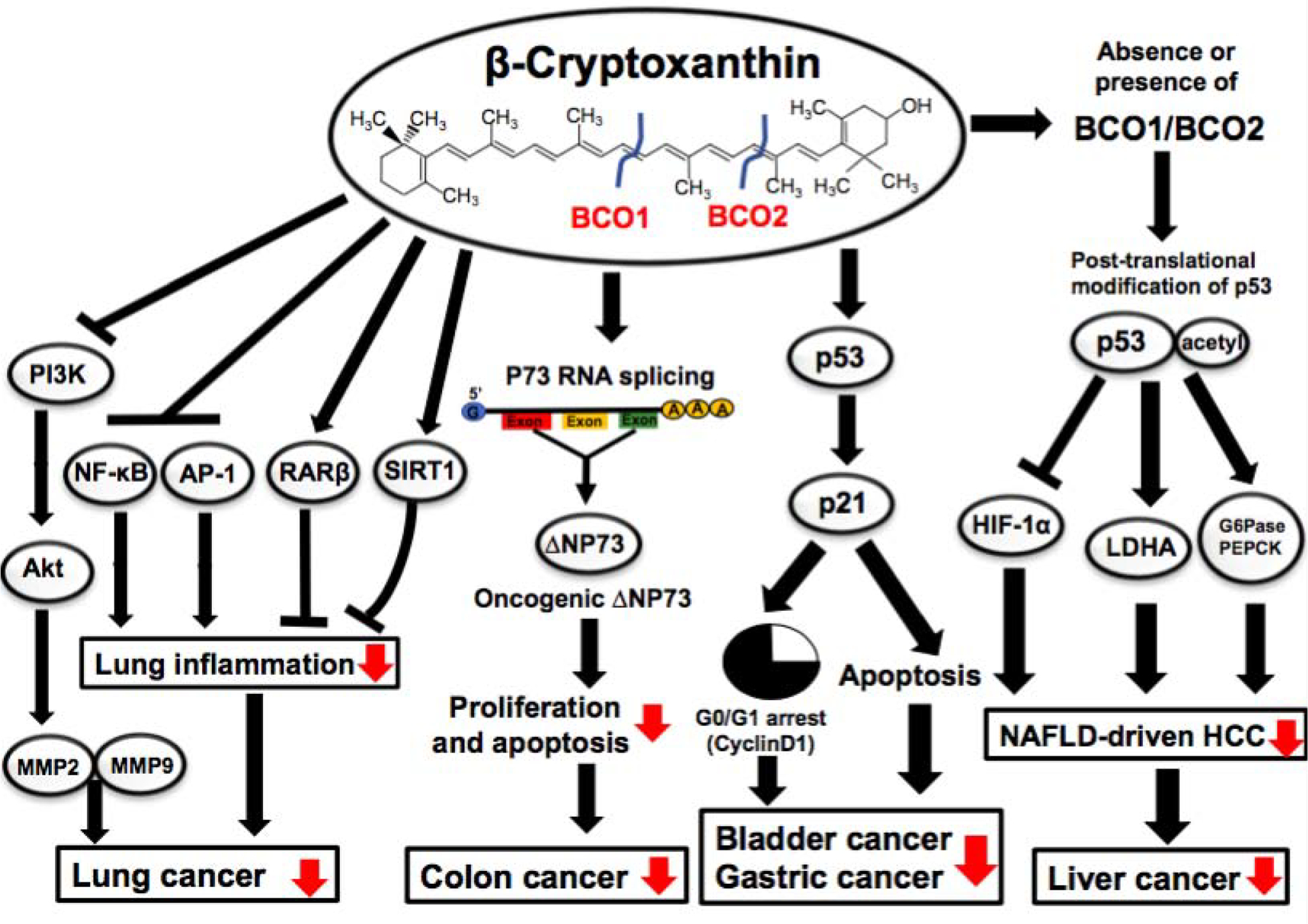
β-Cryptoxanthin supplementation inhibits lung inflammation by reducing nuclear NF-κB and AP-1 and also suppresses lung cancer development by restoring RARβ, SIRT1, and NAChRs/PI3K/Akt pathway in lung cancer murine models; β-cryptoxanthin treatment regulates p73 RNA splicing, producing less oncogenic truncated ΔNP73, thereby suppressing proliferation and contributing to the inhibition of colon cancer in mice; β-cryptoxanthin increases acetylated-p53, an active form of p53, thereby inhibiting HIF-1α, LDHA, and increasing G6Pase and PEPCK in hepatic tumors and leading to the suppression of HCC in WT and BCO1/BCO2 DKO mice; β-cryptoxanthin upregulates p53 and induces G0/G1 cell cycle arrest and apoptosis in gastric and bladder cancer in mice. AP-1, activator protein 1; G6Pase, glucose-6 phosphatase; HIF-1α, hypoxia-inducible factor 1α; LDHA, lactate dehydrogenase; MMP, matrix metallopeptidase; PEPCK, phosphoenolpyruvate carboxykinase; RAR, retinoic acid receptor; SIRT1, sirtuin1.
Lycopene treatment inhibits NF-κB activation in cells and animal models by down-regulating the binding ability of NF-κB [76–78]. The study by Kolberg et al. used the nude mice bearing human prostate cancer cells to investigate the effects of a 10% tomato paste diet on the development of prostate cancer and its underlying mechanisms. Although there were no significant differences in tumor size between vehicle control-fed mice and tomato diet-fed mice, tomato paste feeding effectively suppressed TNF-α-induced NF-κB activity [62]. Interestingly, lycopene supplementation (100 mg/kg diet) for 24 weeks activated different protective mechanisms in liver tumorigenesis depending on the presence or absence of BCO2 [58]. In WT mice, but not in BCO2 KO mice, the protective effects of lycopene supplementation against hepatic tumorigenesis were associated with lycopene-mediated inhibition of NF-κB phosphorylation in WT mice [58]. Furthermore, lycopene metabolite, apo-10’-lycopenoic acid supplementation (10 mg/kg diet) significantly inhibited hepatic inflammation and liver cancer based on the protein expression levels of pro-inflammatory markers, including NF-κB, compared with high-fat diet-fed mice without supplementation [57]. The inhibitory effect of apo-10’-lycopenoic acid in NF-κB protein expression was correlated with a reduction in IL-6 protein levels and liver tumor volume [57]. In this in vivo study, the levels of apo-10’-lycopenoic acid in the liver tissues were in the picomolar range, which is significantly lower than the levels of lycopene in the liver in rats (7.5–17.6 nmol/g liver) [79] or in humans (0.1–20.7 nmol/g liver) [80]. These data indicate that metabolites of lycopene may play potential roles in regulating NF-κB mediated hepatic inflammation and liver tumorigenesis. However, the regulation of NF-κB by lycopene and its metabolites remains unclear and should be further investigated in future studies.
2.2.2. Retinoic acid signaling pathway
Retinoic acid (RA) is a critical bioactive metabolite of vitamin A [81]. Importantly, RA and its derivatives has been regarded as potential chemopreventive agents owing to their ability to inhibit proliferation and induce apoptosis, differentiation, and anti-oxidant effects in cancers [82]. RA activity is mostly mediated via RA receptors (RARs) and retinoid X receptors (RXRs), which are members of the nuclear receptor superfamily but differ in their amino-and carboxyl-terminal domain sequence and retinoid-binding specificity [83, 84]. Downregulation of RA signaling has been observed in several cancer cell types including head and neck [85, 86], breast [87, 88], esophagus [89], prostate [90], lung [91–93], and cirrhosis and liver cancer [94]. It has been reported that cigarette smoke reduces RAR-β2 expression with morphological changes in the lung tissues of animals [95] and that the combination of cigarette smoke and (4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) treatment leads to aberrant methylation of RARs in murine lung tumors [96]. Activation of the RAR signaling pathway is considered a potential therapeutic strategy for cancer prevention [97]. As the bioavailability of various natural RAR ligands (such as all-trans RA) are affected by various factors, such as retinoid binding proteins involved in transport, uptake, storage, and sequestration of retinoid metabolic enzymes [98], it is important to identify natural RAR ligands other than RA to treat various chronic diseases [99]. β-Cryptoxanthin has been reported to be a novel natural RAR ligand [46, 100]. It has been demonstrated that β-cryptoxanthin treatment (1–20 μmol/L) suppressed the growth of lung cancer cells by up-regulating RARβ expression in a dose-dependent manner [101]. The doses of β-cryptoxanthin treatment used in this study were above the β-cryptoxanthin levels in serum collected from fasting subjects (0.05–0.52 μmol/L) [102]. However, the estimated intracellular β-cryptoxanthin levels (0.04–7.4 μmol/L) in cells treated with 1–20 μmol/L of β-cryptoxanthin were much lower than the β-cryptoxanthin levels in the cell culture medium [101]. This effect was similar to the physiological effects achieved with dietary sources in animal studies. For example, serum β-cryptoxanthin levels (~17.38 nmol/g tissue) were much higher than lung β-cryptoxanthin levels (~0.16 nmol/g tissue) in lung cancer murine model [48]. β-Cryptoxanthin treatment (1–20 μmol/L) also activated retinoic acid response elements (RARE)-driven transcription activity, suggesting that the activation of the retinoid signaling pathway by β-cryptoxanthin could be due to β-cryptoxanthin or its metabolites [101] (Fig. 3). Matsumoto et al. demonstrated that β-cryptoxanthin had high binding activity against RAR in an in vitro CoA-BAP system, indicating that β-cryptoxanthin itself might be a natural RAR ligand [99]. Interestingly, β-cryptoxanthin supplementation (10 and 20 mg/kg diet), which are equivalent to daily consumption of 3–5 raw tangerines daily in human, which are equivalent to daily consumption of 3–5 raw tangerines daily in human, restored the down-regulated RARβ by tobacco carcinogen (NNK) and nicotine exposure to normal, without significant changes of retinoid levels in A/J lung cancer mouse model [46]. Similarly, Quesada-Gómez et al. demonstrated that β-cryptoxanthin treatment (0.5 mM) reduced vessel formation by potentially up-regulating RAR levels in mice [103].
One of the important aspects is that the beneficial and adverse effects of carotenoids may be due to their metabolites or decomposition products [104]. In addition to RA, carotenoid metabolites can activate RARE-driven transcription activity. Previous studies demonstrated that acycloretinoic acid (Fig. 2) activates the transcription of RARβ and inhibit cancer cell proliferation in vitro [105, 106]. Due to the similarity in chemical structures between apo-10’-lycopenoic acid and acycloretinoic acid, we have demonstrated that apo-10’-lycopenoic acid can transactivate RARβ using a reporter vector containing the RARβ promoter fragment in the promoter region of luciferase gene [55]. When the RARE in RARβ promoter was mutated, the ability of apo-10’-lycopenoic acid to transactivate RARβ promoter was abolished [55]. These results suggest that activation of RARs may account for the growth inhibitory effect of apo-10’-lycopenoic acid. Indeed, apo-10’-lycopenoic acid (Fig. 2) supplementation (1 to 120 mg/kg diet) inhibited the number of NNK-induced lung tumors in mice in a dose-dependent manner, suppressed the growth of lung cancer cell lines, and decreased cell cycle progression in the G1 phase by decreasing cyclin E [55]. Importantly, apo-10’-lycopenoic acid increased RARβ mRNA expression in a dose-dependent manner, and plasma apo-10’-lycopenoic acid levels were correlated with apo-10’-lycopenoic acid dose in the diet [55]. It is possible that anti-proliferation effects of lycopene or metabolites of lycopene in cancer may be achieved via nuclear receptors other than RAR/RXR. For example, apo-10’-lycopenoic acid (2.5 to 40 μM) inhibited cancer cell motility and angiogenesis by upregulating peroxisome proliferator-activated receptor γ (PPARγ) [107] (Fig. 4), which is involved in controlling angiogenesis, tumor progression, and metastasis [108]. In terms of apo-carotenoids, the combination treatment of β-carotene (30 μmol/L) and its metabolite, apo-14’-carotenoic acid (1 to 10 μmol/L), increased RARβ expression levels in human bronchial epithelial cells with or without benzopyrene, a primary lung carcinogen from cigarette smoke [109]. A recent study showed that apo-14′-carotenoic acid is present endogenously in the human plasma and increased after carrot juice rich in β-carotene supplementation in human subjects [110]. Apo-14′-carotenoic acid is a moderate activator of RAR-transactivation in reporter cell lines and apo-14′-carotenoic acid can potently activate retinoid signaling in DR5/RARE-reporter mice [110]. The authors concluded that apo-14′-carotenoic acid alone or in combination with its metabolite, all-trans-13,14-dihydroretinoic acid, could be an alternative pathway for potent RAR-mediated signaling [110]. Taken together, these results indicate that activation of RARs by carotenoids themselves or their metabolites can contribute to the suppression the cancer development, especially lung cancer.
Figure 4.
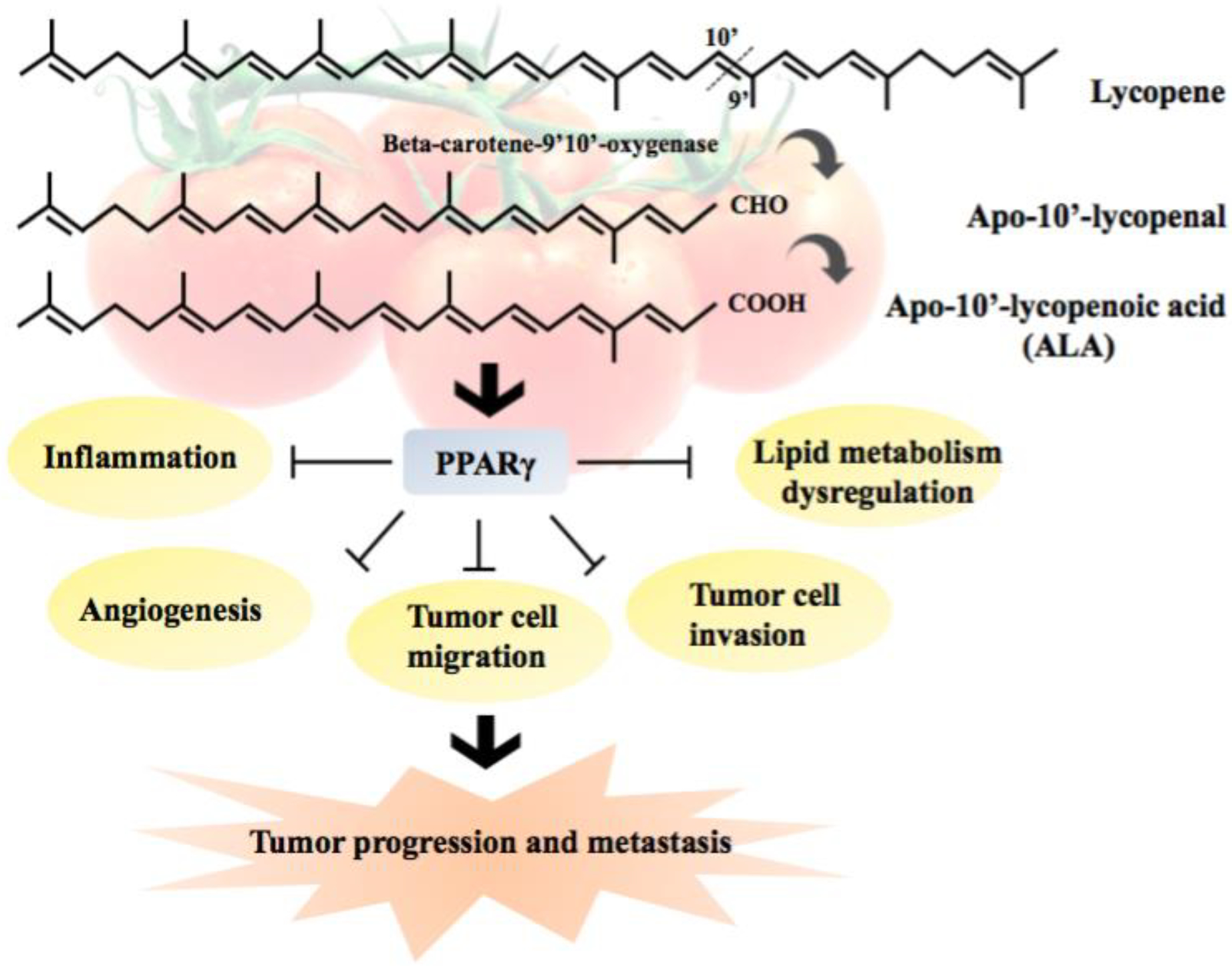
Molecular mechanisms by which apo-10’-lycopenoic acid up-regulates PPARγ signaling pathway and inhibits tumor progression and metastasis (modified from [107])
2.2.3. SIRT1 signaling pathway
SIRT1, a nicotine adenosine dinucleotide (NAD+)-dependent protein-deacetylase, plays important biological roles in several processes including circadian rhythms, inflammation, lipid/glucose metabolism, and immune function [111–113]. SIRT1 can be activated in response to various conditions including calorie restriction, fasting, changes in NAD+ levels, and resveratrol [114]. Nicotinamide phosphoribosyltransferase (NAMPT), the rate limiting NAD biosynthetic enzyme, and NAD+ display circadian oscillation, as they are regulated by the circadian machinery in peripheral tissues such as lung, liver and adipose tissues [115]. NAMPT-mediated NAD+ synthesis has been shown to be significantly decreased in peripheral metabolic organs by high fat diet [116]. Smoke exposure can decrease SIRT1 levels in the nucleus, thereby contributing to the increased acetylation of nuclear RelA/p65 and IL-18 secretion [117]. Recent study reported that tobacco carcinogen (NNK)- and nicotine-induced lung cancer and emphysema were ameliorated with β-cryptoxanthin supplementation (10 mg/kg diet and 20 mg/kg diet) in AJ mice [46]. Moreover, NNK and nicotine treatment significantly reduced the protein levels of SIRT1 in lung tissues, which recovered to normal levels following β-cryptoxanthin supplementation in AJ mice [46]. It has been reported that SIRT1 directly controls lung inflammation [118]; SIRT1−/− mice showed higher levels of inflammation after ambient particulate matter exposure, which is intimately related to increased NF-κB acetylation [118]. The reduction in SIRT1 protein levels by NNK and nicotine treatment was accompanied by elevated IL-6, which was decreased by β-cryptoxanthin supplementation [46]. β-Cryptoxanthin supplementation suppressed the development of NAFLD by potentially up-regulating SIRT1 protein levels in the liver and inhibiting pro-inflammatory cytokine IL-6 mRNA expression along with up-regulation of SIRT1 mRNA expression in the mesenteric adipose tissues of BCO1/BCO2 DKO mice [119]. Collectively, these results suggest that β-cryptoxanthin is a potential dietary factor that plays an important role in restoring SIRT1 protein levels, thereby contributing to the amelioration of inflammation and carcinogenesis (Fig. 5).
Figure 5. Molecular mechanisms underlying β-cryptoxanthin and lycopene regulates SIRT1, a nicotine adenosine dinucleotide (NAD+)-dependent protein-deacetylase, thereby inhibiting western diet (high fat/refined carbohydrate/excessive sugar)-promoted inflammation and carcinogenesis.
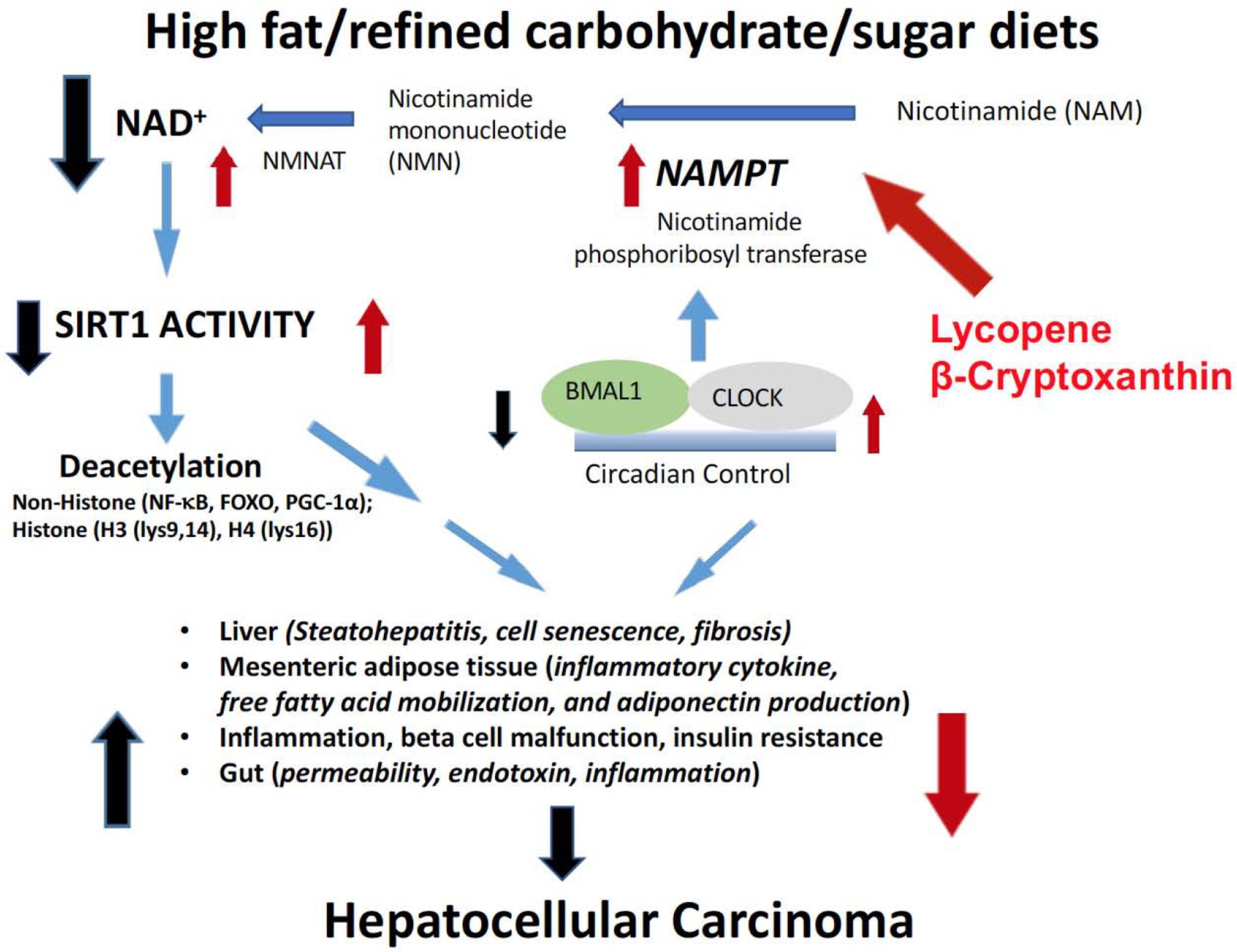
High fat/refined carbohydrate/sugar diets impaired SIRT1 activity, thereby increasing susceptibility of many diseases, including non-alcoholic steatohepatitis and HCC, by targeting multiple organs. Carotenoids may restore western diet-reduced SIRT1 deacetylase activity and NAD+ levels by up-regulating nicotinamide phosphoribosyltransferase (NAMPT). NAD+, nicotine adenosine dinucleotide; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide mononucleotide adenylyltransferase; NAMPT, nicotinamide phosphoribosyltransferase; HCC, hepatocellular carcinoma; SIRT1, sirtuin1.
Emerging studies have demonstrated the importance of circadian rhythms and oncogenic processes [120]. Oncogenic processes can inhibit the balance maintained by the circadian clock machinery, thus facilitating uncontrolled proliferation and promoting an inflammatory tumor microenvironment [121]. SIRT1 is intimately linked to circadian rhythms, as it regulates the transcription of clock genes including Bmal1, Per2, and Cry [122] (Fig. 5). Thus, it is important to identify dietary factors that can induce NAMPT and SIRT1 activity and restore circadian rhythmicity, thereby preventing inflammation and tumorigenesis (Fig. 5). Recent study showed that tomato powder containing substantial lycopene inhibits high-fat diet-induced hepatic steatosis, inflammation, and hepatic tumorigenesis [61]. This is associated with increased SIRT1 activity, NAMPT expression, and AMPK phosphorylation, as well as restored high-fat diet-reduced circadian clock genes (CLOCK, PER2, and CRY2) to the normal levels of the control group [61]. Moreover, the protective effects of tomato powder feeding were associated with decreased hepatic inflammatory foci and pro-inflammatory biomarkers (IL-6, IL-12a, MCP-1, and inducible NO synthase (iNOS)). Since metabolic modulations by SIRT1 have been shown to protect against inflammation by reducing IL-6 and TNF-α expression, potentially via NF-kB signaling [123], the study indicates that tomato powder feeding can inhibit hepatic inflammatory responses by the SIRT1 signaling pathway. It is also possible that the beneficial effects of tomato powder on liver tumorigenesis in the BCO1/BCO2 DKO mice are due to apo-lycopenoids that are produced by other unidentified carotenoid cleavage enzymes in tomatoes or gut bacteria. In plants, the production of apocarotenoids from carotenoids is catalyzed by a family of carotenoid cleavage dioxygenases which cleave the 9’,10’ double bonds of carotenoids including lycopene (57). A series of apolycopenoids including apo-10’-lycopenal have been identified in human plasmas of humans who consumed tomato juice for 8 weeks (4 to 8 ounces delivering 21.9 mg of lycopene/day) (58). This notion was supported by the previous report that the anti-carcinogenesis effects of apo-10’-lycopenoic acid in liver cancer are intimately related to up-regulation of hepatic SIRT1 and modulation of SIRT1 target genes [57]. Apo-10’-lycopenoic acid supplementation significantly decreased hepatic inflammation markers (inflammatory foci, IL-6, TNF-α, and NF-κB) and the proliferation marker, cyclin D1, while cleaved-PARP protein levels increased following apo-10’-lycopenoic acid supplementation [57]. These data suggest that anti-proliferation and pro-apoptotic effects of apo-10’-lycopenoic acid can be mediated by SIRT1 pathway.
However, SIRT1 has been reported to have dual effects on carcinogenesis depending on cancer cell lines and animal models [124]; For example, SIRT1 has been suggested as a potential tumor suppressor in human skin tumors [125], whereas it has been suggested as a potential tumor promoter in the ovary, breast, stomach, and pancreas cancer [126–129]. The role of SIRT1 in liver cancer alsoremains controversial [130–133]. Therefore, whether SIRT1 acts as a tumor suppressor or tumor promoter in tumorigenesis and furthermore, how carotenoids and their metabolites differently regulates SIRT1 levels in tumors vs. non-tumor tissues as well as potential mechanisms involved need further investigation.
2.2.4. Nicotinic Acetylcholine Receptor (nAChR) α7 signaling pathway
Nicotine is the major component in cigarette smoke that can interact with nAChR [134–136]. Ionotropic nicotinic acetylcholine receptors (AChR) are expressed in both neuronal and non-neuronal cells. α7-nAChR is a major subtype of AChR, which is associated with chronic obstructive pulmonary disease and lung cancer development [134, 136, 137]; it can react with NNK and nicotine and is a proposed molecular target [135, 138–141]. Indeed, recent studies utilizing the ferrets, an animal model that closely mimic human lung cancer, have demonstrated that ferrets develop pulmonary preneoplastic and plastic lesions, and upregulate α7-nAChR expression in the bronchial epithelial cells and pulmonary carcinogenesis following NNK exposure [142]. Antagonizing α7-nAChR has been reported to inhibit lung tumor growth and tumor cell migration [139, 143].
Recent study demonstrated that carotenoids can inhibit α7-nAChR signaling pathway as a promising strategy to prevent development of smoke-related carcinogenesis [48, 144]. β-Cryptoxanthin inhibited cell migration and invasion of α7-nAChR positive cancer cells by inhibiting the α7-nAChR/PI3K/AKT signaling pathway, but not in α7-nAChR nagative cancer cells in vitro [48] (Fig. 6). Moreover, β-cryptoxanthin supplementation effectively reduced nicotine-promoted emphysema and NNK-induced adenocarcinoma multiplicity without significant changes in vitamin A (retinol and retinyl palmitate) levels in both mouse lungs and serums [48, 145]. In this study, β-cryptoxanthin supplementation in mice 2 weeks prior to NNK injection effectively reduced lung tumor multiplicity by 52 – 63%, suggesting that β-cryptoxanthin was effective in suppressing tumor promotion [48]. Collectively, β-cryptoxanthin suppress cancer cell motility and lung tumorigenesis via down-regulation of α7-nAChR/PI3K/AKT signaling (Fig. 6).
Figure 6. Molecular mechanisms underlying β-cryptoxanthin inhibits α7-nAChR, thereby leading to the inhibition of lung cancer progression and metastasis.
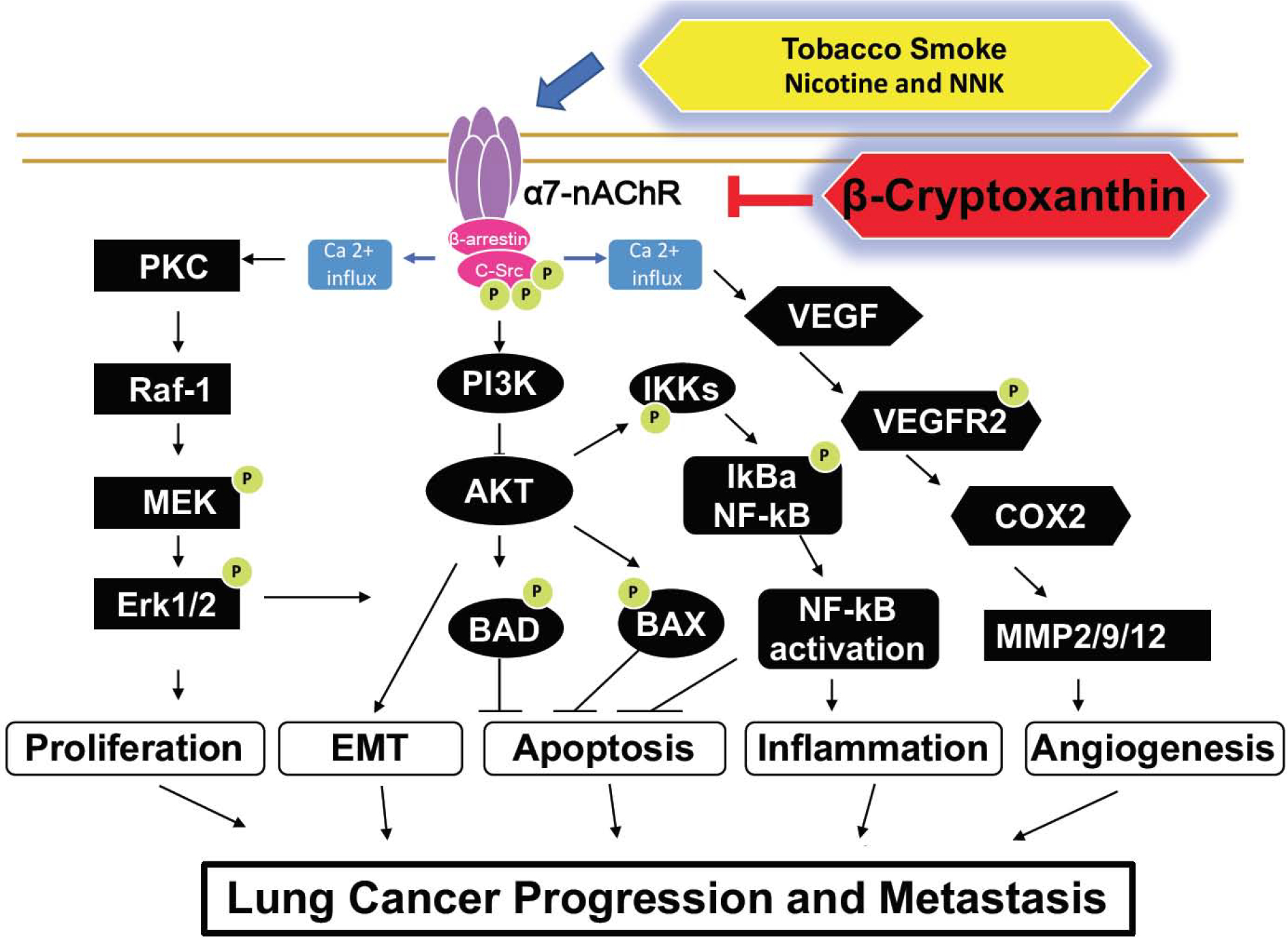
The binding of nicotine and NNK, a tobacco carcinogen derived from nictine to α7-nAChR might induce the formation of an oligomeric complex, including Src, β-arrestin, and α7-nAChR. This can lead to the elevation of cytosolic calcium influx and activation of the MEK/ERK signaling pathway, thereby promoting lung cancer proliferation. Nicotine also phosphorylates VEGFR2, which in turn increases COX and induces MMPs, which can cause cancer cell invasion. Nicotine can activate NF-κB by activation of the PI3K/Akt pathway, which may increase inflammation, survival, and proliferation. β-Cryptoxanthin inhibits lung cancer progression and metastasis by inhibiting α7-nAChR in mice. α7-nAChR, α7 nicotinic acetylcholine receptor; BCO1, β-carotene 15,15’-dioxygenase; BCO2, β-carotene 9’,10’-oxygenase; COX2, cyclooxygenase2; MMP, matrix metallopeptidase; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Lycopene also has been shown to suppress the α7-nAChR signaling pathway. Lycopene supplementation significantly decreased NNK-induced α7-nAChR and its downstream target proteins, including metalloproteinase (MMP)-2, cyclin D1, and NF-κB, in ferrets and decreased the NNK-induced mortality and pathological changes in lung tissues [144], indicating that lycopene could prevent the angiogenesis, proliferation, and inflammation by regulating pulmonary α7-nAChR. These results suggest that β-cryptoxanthin and lycopene can provide beneficial effects against smoke carcinogen-induced lung injury by inhibiting the α7-nAChR signaling pathway. The exactly mechanism(s) how carotenoids regulate α7-nAChR signaling pathway is unclear.
2.2.5. Tumor suppressor proteins p53 and p73
p53 is a tumor suppressor gene that plays important roles in senescence, cell-cycle arrest, and apoptosis under various cellular stress conditions [146]. Many cancers exhibit loss of p53, which contributes to chemotherapy resistance [147]; thus, re-activation of p53 is a potent strategy for treating cancer. p73, a member of the p53 transcription factor family, regulates similar target genes [148]. Several small molecules that activate p73 have been reported to have anti-tumorigenic effects [149, 150]. p73 undergoes mRNA splicing to produce ΔNp73, an oncogenic truncated p73 that negatively regulates p53 and p73 and is responsible for chemo-resistance [151].
Anti-carcinogenic effects of β-cryptoxanthin were proved to regulate the levels of ΔNp73 [49]. San et al. showed that the lymphocytes of healthy volunteers who were administered a juice containing 0.75 mg of β-cryptoxanthin for one month exhibited downregulation of ΔNp73 in their lymphocytes [49]. β-cryptoxanthin treatment enhanced the anti-carcinogenic effects of oxaliplatin in colon cancer cell lines (HCT116, SW480) by decreasing the expression of ΔNp73 [49]. Moreover, β-cryptoxanthin alone or the combination treatment of β-cryptoxanthin and oxaliplatin significantly suppressed tumor development along with significant inhibition of ΔNp73 levels in the kidneys, normal colonic mucosa, and xenograft tumors compared to the control group [49]. These results suggest that β-cryptoxanthin affords protective effects against colon cancer growth by regulating the oncogenic p73 truncated isoform and might have broader effects on different tissues.
Post-translational modification of p53 has been gaining extensive interest; acetylation and phosphorylation of p53 have been reported to stabilize and activate p53 [152]. Recently, we found that β-cryptoxanthin supplementation significantly increased the protein levels of acetylated-p53, an active form of p53, in liver tumor tissues, thereby inhibiting the development of liver cancer in both WT and BCO1/BCO2 DKO mice [38]. It has been well established that p53 regulates apoptosis and cell proliferation; we found that the protein levels of cyclinD1 and anti-apoptosis marker, Bcl-xl, were significantly reduced in hepatic tumors by β-cryptoxanthin supplementation in both WT and BCO1/BCO2 DKO mice [38]. Gao et al. have shown that β-cryptoxanthin supplementation inhibited gastric carcinogenesis by inducing G0/G1 arrest and suppressing cyclinD, E, cyclin-dependent kinases, potentially by increasing p53 protein levels [50]. Miyazawa et al. investigated that β-cryptoxanthin feeding decreased the multiplicity and incidence of preneoplastic and neoplastic region of bladder cancer was reduced, in conjunction with inhibition of cyclin-D-positive cells [51]. Coral et al. also found that β-cryptoxanthin treatment inhibited the number of colon cancer cells in the S-phase largely due to the anti-proliferative activity of β-cryptoxanthin rather than apoptosis [49]. Anti-proliferative effects of β-cryptoxanthin treatment have been reported in both normal and tumor cells [101, 153]. Collectively, these results suggest that β-cryptoxanthin might be a potential natural bioactive component that can be used in combination with conventional chemotherapeutic agents; it negatively regulates ΔNp73, but activates p53 potentially by regulating post-translational modification in cancer. However, further studies are required to unravel the mechanisms underlying β-cryptoxanthin regulation of p53 post-translational modification.
It is well accepted that the loss of function of p53 by mutation results in enhanced proliferative activity and tumor progression. Mutation of p53 can lead to increased mutant p53 proteins, which have a longer half-life than wild-type p53 protein, thereby accumulating in cancer cells [154]. Furthermore, increased p53 protein may serve as a useful indicator for poor prognosis of several cancers, including lung cancer [155–157]. Moreover, p53 plays critical roles in cellular responses to various cellular stresses such as genotoxic and oncogenic stress [158]. In response to different genotoxic stresses, various protein kinases, such as p38 MAPK and JNKs, phosphorylate p53, especially at serine 15, leading to its activation [159]. Previous study has shown that p53 gene expression in the lung was significantly increased in smoke-exposed ferrets, while low dose of β-carotene restored p53 expression to normal levels compared to the control group [56]. Increased total p53 and phosphorylated-p53 protein levels were significantly induced by cigarette smoke in the gastric mucosa of ferrets, which were significantly decreased by lycopene supplementation in a dose-dependent manner, compared with the cigarette smoke alone group [160]. These data further indicate that either low- or high-dose lycopene supplementation may play a critical role in the prevention of cigarette smoke exposure-related alteration in p53 and its target genes involved in apoptosis and proliferation.
2.2.6. Carotenoid metabolites and the insulin-like growth factor-1 (IGF-1) pathway
The insulin-like growth factors (IGFs) are mitogens involved in regulating cell proliferation, differentiation, and apoptosis [161]. The downstream pathway of the IGF-1R signaling involves the activation of both phosphatidylinositol 3’-kinase (PI3K)/Akt/protein kinase B and Ras/Raf/MAPK pathways. Disruptions of normal IGF-1 system components lead to hyperproliferation and survival signals and have been implicated in the development of various tumor types. Several lines of evidence implicate IGF-1 and its receptor, IGF-1R in lung cancer and other malignancies [161, 162]. Epidemiological evidence indicates that increased levels of IGF-1, reduced levels of IGF binding protein-3, or an increased ratio of IGF-1 to IGF binding protein-3 in circulation are associated with an increased risk for the development of several common cancers, including those of the breast, prostate, colon, and lung [162].
It has been reported that the IGF-1 stimulated cell growth was reduced by physiological concentrations of lycopene in endometrial, mammary (MCF-7) and lung (NCI-H226) cancer cells [163, 164]. Lycopene treatment was also associated with an increase in membrane-associated IGF binding proteins [164]. Two studies in humans have also shown that higher intake of cooked tomatoes or lycopene was significantly associated with lower circulating levels of IGF-1 and higher levels of IGF binding protein-3 [165]. In an animal study, lycopene supplementation reduced local prostatic IGF-1 expression in the Dunning prostate cancer model [166]. Lycopene supplementation in smoke-exposed ferrets inhibits lung squamous metaplasia by up-regulating IGF-binding protein 3 [167], which is a potent inhibitor of both PI3K/Akt/PKB and MAPK signaling pathways [168] and regulates the bioactivity of IGF-I by sequestering it away from its receptor in the extracellular milieu, thereby inhibiting the mitogenic and anti-apoptotic action of IGF-I (Figure 3). Furthermore, the changes of IGF-I binding protein-3 by lycopene supplementation in the plasma of the ferrets were associated the increased apoptosis and diseased cell proliferation in the lungs of smoke-exposed ferrets. Although the mechanism by which lycopene increases the level of IGF binding protein-3 remains to be elucidated, these results demonstrate the importance of IGF binding protein-3 in the regulation of smoke-induced lung lesion, proliferation and apoptosis, suggesting that IGF binding protein-3 is a molecular target of lycopene for the prevention of lung cancer. These studies support the notion that the lycopene may exert biological functions in cancer prevention potentially by regulating IGF signaling pathway. However, the molecular properties of lycopene and its metabolites need more investigation and knowledge of their metabolic pathway, dose effects, and tissue specificity.
2.2.7. Regulation of energy metabolism in tumors
Cancer cells tend to have altered energy metabolism, known as the Warburg effect, which is characterized by higher glucose uptake and glycolysis rather than oxidative phosphorylation [169]. The Warburg effect has been widely examined in human cancers, including hepatocellular carcinoma (HCC) [170], and is closely associated with the aggressiveness and high proliferation of the cancer [171, 172]. Gupta et al. demonstrated that the N-Nitrosodiethylamine alone treatment led to poorly differentiated HCC, which was inhibited following lycopene treatment in mice [173]. Additionally, the lycopene-mediated improvement of histopathology and ultrastructure was associated with inhibition of the activities of glycolytic enzymes, including hexokinase and phosphoglucose isomerase, compared with the N-Nitrosodiethylamine-induced HCC group [173]. These findings suggest that lycopene has the potential to inhibit HCC development by targeting glucose metabolism in the liver. The protein levels of lactate dehydrogenase is an important enzyme regulating glycolysis and HCC proliferation [174]. The enhancing gluconeogenesis in malignant HCC has been reported to suppress glycolysis and HCC progression [175]. Very recently, we found that β-cryptoxanthin concentrate feeding (10 mg/kg diet) significantly reduced HCC numbers, average tumor size, and total tumor volume in both WT and BCO1/BCO2 DKO mice [38]. The dose of β-cryptoxanthin used in this study was within a physiological range based on the levels of β-cryptoxanthin in WT-HRCD+β-cryptoxanthin mice. The mean β-cryptoxanthin levels (0.14 nmol/g tissue) in the liver tissues of WT-HRCD+β-cryptoxanthin mice were comparable to the β-cryptoxanthin levels in human livers (0.1–3.5 nmol/g tissue) [80]. The chemopreventive effects of β-cryptoxanthin were associated with increased gluconeogenesis markers (phosphoenolpyruvate carboxykinase, glucose 6-phosphatase) and decreased protein levels of a glycolysis marker (lactate dehydrogenase) and of the hypoxia-inducible factor-1α and its downstream targets, matrix metalloproteinase 2/9 in tumors [38]. Additionally, the β-cryptoxanthin-mediated alteration in glucose metabolism was associated with up-regulation of p53 acetylation [38]. It has been shown that p53 suppresses lactate dehydrogenase expression [176] and up-regulates phosphoenolpyruvate carboxykinase 1 and glucose 6-phosphatase [177]. Thus, it is plausible that β-cryptoxanthin feeding increases p53 acetylation and leads to modulation of glucose metabolism, which may contribute to the inhibition of HCC progression inhibition in both WT and BCO1/BCO2 DKO mice. There is a great need for experimental studies to further clarify the underlying mechanistic actions of β-cryptoxanthin.
2.2.8. Modulation of the Reverse Cholesterol Transport mechanism
The role of cholesterol in cancer has received increasing attention recently [178]. A growing number of studies have demonstrated that cancer cells exhibit abnormal cholesterol accumulation, affecting various oncogenic cellular signaling pathways [179, 180]. In vitro studies have demonstrated that lycopene treatment inhibits cholesterol synthesis in macrophage cell lines [181], as well as in prostate PC-3, lung BEN, and colon HCT-116 and HT-29 cancer cell lines by inhibiting the mevalonate pathway [180].
Lycopene treatment significantly reduced cancer growth in these cancer cell lines by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase expression [180]. A recent study using a ferret model showed that lycopene-mediated beneficial effects against smoke-induced chronic bronchitis, emphysema, and preneoplastic lesions, are attributed to the improvement of cholesterol homeostasis through reverse cholesterol transport [54]. Additionally, the changes in reverse cholesterol transport markers following lycopene supplementation were associated with the regulation of nuclear hormone receptors, such as PPARα and LXR [54]. Similarly, Yang et al. showed that lycopene treatment significantly inhibited human prostate cancer cells and reduced cellular cholesterol levels by activating PPAR-LXR-ABCA1 [182]. Taken together, these results suggest that lycopene plays a potential role in cholesterol metabolism in cancer, which may contribute to the inhibition of cancer development.
2.2.9. Regulation of microbiome and cancer
Emerging studies indicate that the human gut microbiota plays a role in regulating inflammation, immune response, various metabolism, and cancer [183]. The liver is directly exposed to metabolites produced by the gut microbiota through portal circulation [184]. In the HCC mouse model, the intestinal microbiota has been shown to induce Toll-like receptors and inflammation, which may contribute to the HCC progression [185]. Moreover, alteration of gut microbiota composition induced by genetic or dietary obesity significantly increased deoxycholic acid, a metabolite of the gut microbiota, which may cause DNA damage [186]. The enterohepatic circulation of deoxycholic acid affects the senescence-associated secretory phenotype, thereby inducing inflammation and the carcinogenesis process in the liver [186]. Interestingly, inhibition of deoxycholic acid or gut microbiota prevented the development of HCC in obese mice [186], indicating that components and metabolites produced by the gut microbiota play an important role in the development of obesity-induced HCC. A recent clinical study showed that parcitipants who were administered different dose of lycopene (7 mg/day and 30 mg/day) for one month exhibited a dose-dependent increase in gut microbiota composition [187], suggesting that lycopene has prebiotic potential. A recent study using BCO1/BCO2 DKO mice that were fed either high-fat diet alone or high-fat diet with dietary tomato powder for 24 weeks revealed that tomato powder supplementation significantly decreased hepatic inflammatory foci, hepatic steatosis, and hepatic tumorigenesis, as compared with the high-fat diet alone-fed mice [61] (Fig. 7). In addition, tomato powder supplementation increased gut microbiota richness and diversity, but decreased Clostridium species, which are known to increase deoxycholic acid and decrease natural killer T cells [188, 189]. These studies suggest that carotenoids prevent inflammation with potential modulating gut microbiota and inhibits HCC development. The reader is referred to a recent review article regarding the effects of other common carotenoids, astaxanthin and β-carotene on gut microbiota dysbiosis [190]. Future studies are needed to investigate the role of lycopene in the gut microbiome, immune function, and cancer prevention.
Figure 7. The proposed mechanisms of how tomato powder containing lycopene regulates microbiome and inhibits the development of hepatic inflammation and hepatocellular carcinoma (modified from [205]).
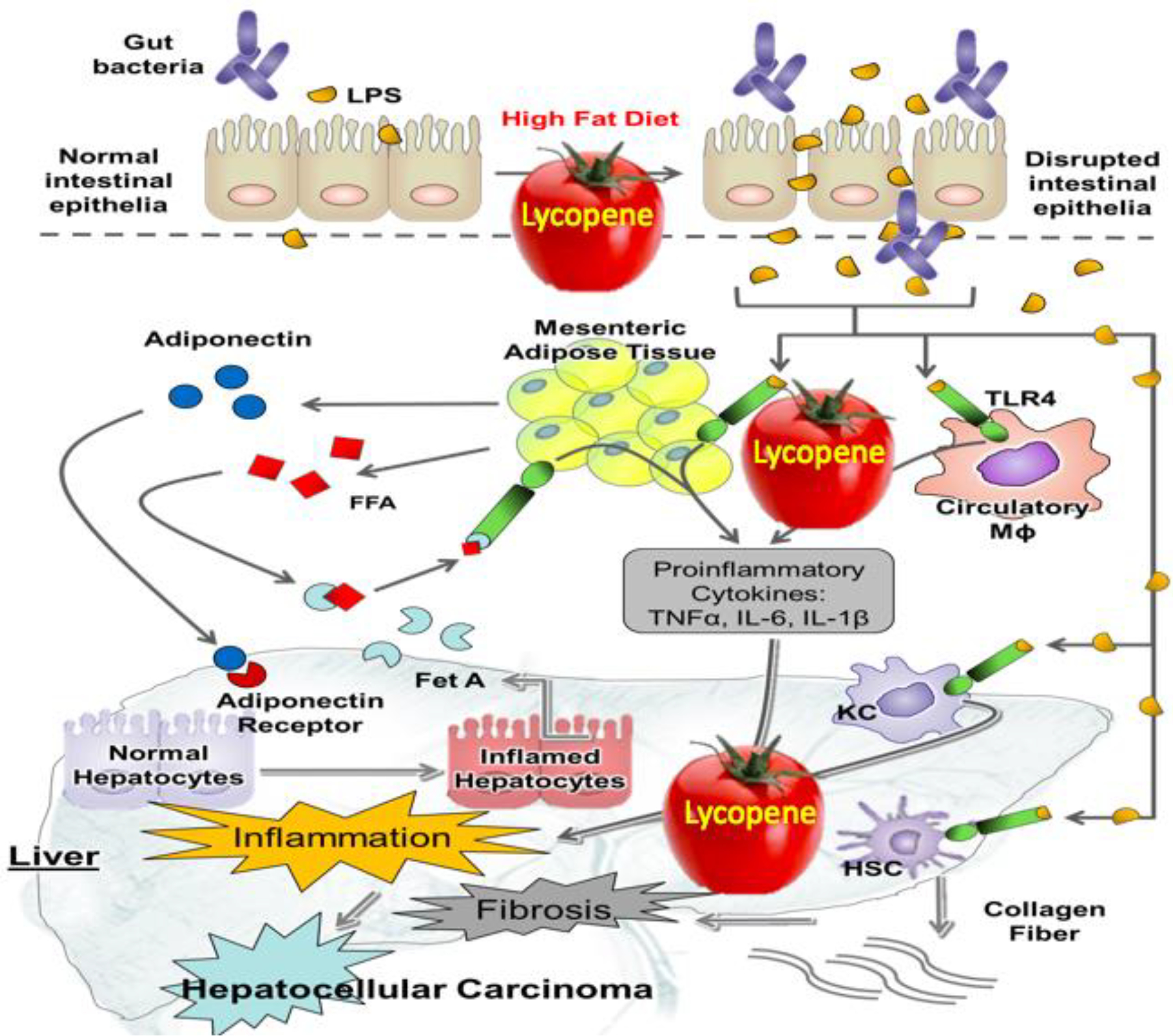
A high-fat diet can impair the intestinal epithelium, which may lead to hepatic inflammation and HCC by increasing lipopolysaccharides (LPS). Obesity can induce adipocyte hypertrophy, which may facilitate releasing free fatty acids (FFA) into the circulation. Circulatory FFA can activate Toll-like receptor 4 (TLR4)-mediated inflammatory signaling pathways in macrophages and adipocyte. Tomato powder supplementation reverses these reactions, thereby attenuating hepatic inflammation, hepatic steatosis, and HCC. FFA, free fatty acids; HCC, hepatocellular carcinoma; LPS, lipopolysaccharides; TLR4, toll-like receptor 4.
2.2.10. Cancer stem cell and cancer cell niche in tumorigenesis
Cancer stem cells are a subset of cells that have stem-like characteristics in tumors that are responsible for self-renewal, tumor relapse, and drug resistance [191]. β-Carotene, a pro-vitamin A carotenoid, has been shown to inhibit neuroblastoma tumorigenesis by modulating cancer stem cell markers including Oct3/4 and delta-like 1 homologue and hypoxic tumor microenvironment marker, hypoxia-inducible factor-1α, in neuroblastoma tumors [192]. Our preliminary data showed that β-cryptoxanthin can suppress cancer stem cell marker CD133 in the liver tumors of WT and BCO1/BCO2 DKO mice (unpublished), suggesting that β-cryptoxanthin itself plays a role in inhibiting cancer stemness in HCC. Animal studies investigating the effects of β-cryptoxanthin or lycopene on cancer stem cell characteristics are still lacking and thus pre-clinical studies are highly encouraged to explore this.
Cancer cell niche, including nutrient supply and hypoxic tumor microenvironment, play important roles in obesity-driven carcinogenesis. Accumulating evidence in recent years has drawn attention in the role of lipid droplets in cancer development [193–195]; lipid-rich cancer cells exhibit more aggressive behavior and chemotherapy resistance in animal and human studies [193–195]. Lipid droplets are intimately related to inflammation and hypoxic tumor cells, which can lead to the development and progression of neoplastic regions [196, 197]. Hypoxic tumor microenvironment triggers cancer cells to uptake fatty acids from the outside [198]. Moreover, cancer cells can uptake lipids secreted by extra-vesicles secreted from nearby hypoxic cells [199]. Recently, we found that the average liver tumor size was significantly correlated with hepatic steatosis assessed in the non-tumor region, and importantly, hepatic steatosis scores were significantly decreased in the livers of WT and BCO1/BCO2 DKO mice treated with β-cryptoxanthin supplementation compared with the high-refined carbohydrate diet littermates [119]. The protein levels of hypoxia-inducible factor-1α and its downstream targets, metalloproteinase (MMP)-2 and 9, were significantly suppressed by β-cryptoxanthin feeding in the hepatic tumors of both WT and BCO1/BCO2 DKO mice [38]. It is unclear whether the inhibition of lipid accumulation by β-cryptoxanthin in the surrounding tissue of hepatic tumor affects the development of hepatic tumor growth.
Inflammatory tumor microenvironment is also important in tumor growth. Natural killer cells are the primary innate immune cells with a major role in directing tumor killing. Emerging evidence have been focusing on natural killer cell-based cancer immunotherapy [200]. Further, natural killer cells are a major target in immunotherapy owing to their ability to detect and remove cancer stem cells [201, 202]. Previously, it has been demonstrated that β-cryptoxanthin oral administration (1.5 μg/kg for one week) significantly reversed a stress-induced decrease in the cytotoxic activity of natural killer cells in mice, demonstrating a potential effect of β-cryptoxanthin on natural killer cells [203]. More recently, lycopene feeding significantly increased inflammatory cells including natural killer cells in tumor tissues and reduced prostate cancer tumor burden [60], indicating that lycopene may promote the immune system in cancer. These data suggest that β-cryptoxanthin and lycopene may have potential roles in immune system in tumor microenvironment in mice. Taken together, β-cryptoxanthin and lycopene might constitute good chemopreventive agent candidates that can regulate tumor microenvironment, thereby affecting cancer development.
3. Conclusions and future perspectives
Epidemiologic studies have shown that dietary intake patterns with increased consumption of fruits and vegetables rich in carotenoids and, thus, increased serum levels of carotenoids have been associated with a decreased development of cancers. We reviewed the animal experimental evidence which has shown that dietary β-cryptoxanthin and lycopene exert comprehensive chemopreventive and therapeutic effects. This review opens several new research questions around the effects of β-cryptoxanthin and lycopene in carcinogenesis research. Future research should include mechanistic and translational pre-clinical experimental studies with both male and female animal models. The anticancer effects of β-cryptoxanthin and lycopene are associated with multiple signaling pathways, including p53, NF-κB, SIRT1, α7-nAChR, IGF-1, and retinoid signaling pathway. Recent studies indicated that the anti-cancer effects of lycopene are related to regulating gut microbiome, cholesterol metabolism, and energy metabolism in tumors in animal models. Emerging studies revealed that β-cryptoxanthin and lycopene are good candidates to regulate tumor microenvironment. Cross-talk between cancer cells and surrounding tumor microenvironment is very important in cancer initiation and progression; thus, animal studies investigating whether β-cryptoxanthin or lycopene feeding differentially regulate these aforementioned pathways in cancer cells and non-cancerous cells are necessary in the future. Further investigation, using animal cancer models, into the effect of β-cryptoxanthin and lycopene on immune function should be conducted. Unraveling these questions will be helpful when considering β-cryptoxanthin and lycopene as potential dietary bioactive treatment and immunotherapy aid for cancers. Further investigations elucidating how β-cryptoxanthin and lycopene or the combination prevent tumorigenesis in animal models are needed to provide insight into the future usage of β-cryptoxanthin and lycopene as a potential bioactive component either by itself or together with cancer treatment medications.
Acknowledgements
This work was supported by the NIH CA104932 grant, and the U.S. Department of Agriculture grant 1950-51000-064S and NIFA/AFRI [2017-67017-26363]. Any opinions, findings, conclusions, and recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the sponsors.
Abbreviations:
- BCO1
β-Carotene-15,15′-oxygenase
- BCO2
β-carotene-9′,10′-oxygenase
- SNP
single nucleotide polymorphism
- BCO1/BCO2 DKO
BCO1−/−/BCO2−/− double knockout
- WT
wild-type
- SIRT1
sirtuin 1
- DEN
diethylnitrosamine
- IGF-1
insulin-like growth factor-1
- RA
retinoic acid
- RARs
RA receptors
- RXRs
RA X receptors
- NNK
nicotine-derived nitrosamine ketone
- RARE
retinoic acid response element
- NF-κB
nuclear factor-κB
- TNF-α
tumor necrosis factor α
- AP-1
activator protein 1
- NAD
nicotine adenosine dinucleotide
- NAFLD
non-alcoholic fatty liver disease
- HCC
hepatocellular carcinoma
- nAChR
nicotinic acetylcholine receptor
- PPAR
peroxisome proliferator-activated receptor
- NAMPT
nicotinamide phosphoribosyltransferase
- PI3K
phosphatidylinositol 3’-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest
References
- [1].Rowles JL, Erdman JW, Carotenoids and their role in cancer prevention (in this special issue), Biochim Biophys Acta Mol Cell Biol Lipids., (2020). [DOI] [PubMed] [Google Scholar]
- [2].Moran NE, Mohn ES, Hason N, Erdman JW Jr., Johnson EJ, Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids, Adv Nutr, 9 (2018) 465–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Giordano E, Quadro L, Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health, Arch Biochem Biophys, 647 (2018) 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Harrison EH, Quadro L, Apocarotenoids: Emerging Roles in Mammals, Annu Rev Nutr, 38 (2018) 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boon CS, McClements DJ, Weiss J, Decker EA, Factors influencing the chemical stability of carotenoids in foods, Crit Rev Food Sci Nutr, 50 (2010) 515–532. [DOI] [PubMed] [Google Scholar]
- [6].Maiani G, Caston MJ, Catasta G, Toti E, Cambrodon IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, Bohm V, Mayer-Miebach E, Behsnilian D, Schlemmer U, Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans, Mol Nutr Food Res, 53 Suppl 2 (2009) S194–218. [DOI] [PubMed] [Google Scholar]
- [7].Burri BJ, Beta-cryptoxanthin as a source of vitamin A, J Sci Food Agric, 95 (2015) 1786–1794. [DOI] [PubMed] [Google Scholar]
- [8].von Lintig J, Vogt K, Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal, J Biol Chem, 275 (2000) 11915–11920. [DOI] [PubMed] [Google Scholar]
- [9].Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J, A mitochondrial enzyme degrades carotenoids and protects against oxidative stress, FASEB J, 25 (2011) 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J, Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A, J Biol Chem, 276 (2001) 14110–14116. [DOI] [PubMed] [Google Scholar]
- [11].Anstee QM, Goldin RD, Mouse models in non-alcoholic fatty liver disease and steatohepatitis research, Int J Exp Pathol, 87 (2006) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].von Lintig J, Moon J, Lee J, Ramkumar S, Carotenoid metabolism at the intestinal barrier, Biochim Biophys Acta Mol Cell Biol Lipids, (2019) 158580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J, Two carotenoid oxygenases contribute to mammalian provitamin A metabolism, J Biol Chem, 288 (2013) 34081–34096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang XD, Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9′,10′-monooxygenase, Arch Biochem Biophys, 506 (2011) 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD, The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo, J Biol Chem, 281 (2006) 19327–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ford NA, Elsen AC, Erdman JW Jr., Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice, Nutr Res, 33 (2013) 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lim JY, Liu C, Hu KQ, Smith DE, Wu D, Lamon-Fava S, Ausman LM, Wang XD, Xanthophyll beta-Cryptoxanthin Inhibits Highly Refined Carbohydrate Diet-Promoted Hepatocellular Carcinoma Progression in Mice, Mol Nutr Food Res, (2019) e1900949. [DOI] [PubMed] [Google Scholar]
- [18].Lietz G, Oxley A, Leung W, Hesketh J, Single nucleotide polymorphisms upstream from the beta-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers, J Nutr, 142 (2012) 161S–165S. [DOI] [PubMed] [Google Scholar]
- [19].Hendrickson SJ, Lindstrom S, Eliassen AH, Rosner BA, Chen C, Barrdahl M, Brinton L, Buring J, Canzian F, Chanock S, Clavel-Chapelon F, Figueroa JD, Gapstur SM, Garcia-Closas M, Gaudet MM, Haiman CA, Hazra A, Henderson B, Hoover R, Husing A, Johansson M, Kaaks R, Khaw KT, Kolonel LN, Le Marchand L, Lissowska J, Lund E, McCullough ML, Peplonska B, Riboli E, Sacerdote C, Sanchez MJ, Tjonneland A, Trichopoulos D, van Gils CH, Yeager M, Kraft P, Hunter DJ, Ziegler RG, Willett WC, Plasma carotenoid- and retinol-weighted multi-SNP scores and risk of breast cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium, Cancer Epidemiol Biomarkers Prev, 22 (2013) 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].He F, Xiao RD, Lin T, Xiong WM, Xu QP, Li X, Liu ZQ, He BC, Hu ZJ, Cai L, Dietary patterns, BCMO1 polymorphisms, and primary lung cancer risk in a Han Chinese population: a case-control study in Southeast China, BMC Cancer, 18 (2018) 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moran NE, Thomas-Ahner JM, Fleming JL, McElroy JP, Mehl R, Grainger EM, Riedl KM, Toland AE, Schwartz SJ, Clinton SK, Single Nucleotide Polymorphisms in beta-Carotene Oxygenase 1 are Associated with Plasma Lycopene Responses to a Tomato-Soy Juice Intervention in Men with Prostate Cancer, J Nutr, 149 (2019) 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meyers KJ, Mares JA, Igo RP Jr., Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK, Blodi B, Gehrs K, Tinker L, Wallace R, Robinson J, LeBlanc ES, Sarto G, Bernstein PS, SanGiovanni JP, Iyengar SK, Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS), Invest Ophthalmol Vis Sci, 55 (2014) 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].He M, Cornelis MC, Kraft P, van Dam RM, Sun Q, Laurie CC, Mirel DB, Chasman DI, Ridker PM, Hunter DJ, Hu FB, Qi L, Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels, Arterioscler Thromb Vasc Biol, 30 (2010) 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhao TY, Li Z, Lei S, Huang L, Yang L, Associations for BCO2, PCSK9, and TR1B1 Polymorphism and Lifestyle Factors with Ischemic Stroke: A Nested Case-Control Study, Yonsei Med J, 60 (2019) 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abar L, Vieira AR, Aune D, Stevens C, Vingeliene S, Navarro Rosenblatt DA, Chan D, Greenwood DC, Norat T, Blood concentrations of carotenoids and retinol and lung cancer risk: an update of the WCRF-AICR systematic review of published prospective studies, Cancer Med, 5 (2016) 2069–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM, Travier N, Olsen A, Tjonneland A, Overvad K, Rinaldi S, Romieu I, Brennan P, Boutron-Ruault MC, Perquier F, Cadeau C, Boeing H, Aleksandrova K, Kaaks R, Kuhn T, Trichopoulou A, Lagiou P, Trichopoulos D, Vineis P, Krogh V, Panico S, Masala G, Tumino R, Weiderpass E, Skeie G, Lund E, Quiros JR, Ardanaz E, Navarro C, Amiano P, Sanchez MJ, Buckland G, Ericson U, Sonestedt E, Johansson M, Sund M, Travis RC, Key TJ, Khaw KT, Wareham N, Riboli E, van Gils CH, Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort, Am J Clin Nutr, 103 (2016) 454–464. [DOI] [PubMed] [Google Scholar]
- [27].Wu L, Guo X, Hartson SD, Davis MA, He H, Medeiros DM, Wang W, Clarke SL, Lucas EA, Smith BJ, von Lintig J, Lin D, Lack of beta, beta-carotene-9′, 10′-oxygenase 2 leads to hepatic mitochondrial dysfunction and cellular oxidative stress in mice, Mol Nutr Food Res, 61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lobo GP, Isken A, Hoff S, Babino D, von Lintig J, BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway, Development, 139 (2012) 2966–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A, CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice, J Biol Chem, 282 (2007) 33553–33561. [DOI] [PubMed] [Google Scholar]
- [30].van Helden YG, Godschalk RW, Swarts HJ, Hollman PC, van Schooten FJ, Keijer J, Beta-carotene affects gene expression in lungs of male and female Bcmo1 (−/−) mice in opposite directions, Cell Mol Life Sci, 68 (2011) 489–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee SA, Jiang H, Trent CM, Yuen JJ, Narayanasamy S, Curley RW Jr., Harrison EH, Goldberg IJ, Maurer MS, Blaner WS, Cardiac dysfunction in beta-carotene-15,15′-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism, Am J Physiol Heart Circ Physiol, 307 (2014) H1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo X, Wu L, Lyu Y, Chowanadisai W, Clarke SL, Lucas EA, Smith BJ, He H, Wang W, Medeiros DM, Lin D, Ablation of beta,beta-carotene-9′,10′-oxygenase 2 remodels the hypothalamic metabolome leading to metabolic disorders in mice, J Nutr Biochem, 46 (2017) 74–82. [DOI] [PubMed] [Google Scholar]
- [33].Lim JY, Liu C, Hu KQ, Smith DE, Wang XD, Ablation of carotenoid cleavage enzymes (BCO1 and BCO2) induced hepatic steatosis by altering the farnesoid X receptor/miR-34a/sirtuin 1 pathway, Arch Biochem Biophys, 654 (2018) 1–9. [DOI] [PubMed] [Google Scholar]
- [34].Pham DN, Leclerc D, Levesque N, Deng L, Rozen R, beta,beta-carotene 15,15′-monooxygenase and its substrate beta-carotene modulate migration and invasion in colorectal carcinoma cells, Am J Clin Nutr, 98 (2013) 413–422. [DOI] [PubMed] [Google Scholar]
- [35].Gong X, Marisiddaiah R, Zaripheh S, Wiener D, Rubin LP, Mitochondrial beta-Carotene 9′,10′ Oxygenase Modulates Prostate Cancer Growth via NF-kappaB Inhibition: A Lycopene-Independent Function, Mol Cancer Res, 14 (2016) 966–975. [DOI] [PubMed] [Google Scholar]
- [36].Kim YS, Gong X, Rubin LP, Choi SW, Kim Y, beta-Carotene 15,15′-oxygenase inhibits cancer cell stemness and metastasis by regulating differentiation-related miRNAs in human neuroblastoma, J Nutr Biochem, 69 (2019) 31–43. [DOI] [PubMed] [Google Scholar]
- [37].Tan HL, Thomas-Ahner JM, Moran NE, Cooperstone JL, Erdman JW Jr., Young GS, Clinton SK, beta-Carotene 9’,10′ Oxygenase Modulates the Anticancer Activity of Dietary Tomato or Lycopene on Prostate Carcinogenesis in the TRAMP Model, Cancer Prev Res (Phila), 10 (2017) 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lim JY, Liu C, Hu KQ, Smith DE, Wu D, Lamon-Fava S, Ausman LM, Wang XD, Xanthophyll beta-Cryptoxanthin Inhibits High-Refined Carbohydrate Diet-Promoted Hepatocellular Carcinoma Progression in Mice, Mol Nutr Food Res, (2019) e1900949. [DOI] [PubMed] [Google Scholar]
- [39].Min KB, Min JY, Serum carotenoid levels and risk of lung cancer death in US adults, Cancer Sci, 105 (2014) 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yuan JM, Ross RK, Chu XD, Gao YT, Yu MC, Prediagnostic levels of serum beta-cryptoxanthin and retinol predict smoking-related lung cancer risk in Shanghai, China, Cancer Epidemiol Biomarkers Prev, 10 (2001) 767–773. [PubMed] [Google Scholar]
- [41].Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC, Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study, Cancer Epidemiol Biomarkers Prev, 12 (2003) 890–898. [PubMed] [Google Scholar]
- [42].Mannisto S, Smith-Warner SA, Spiegelman D, Albanes D, Anderson K, van den Brandt PA, Cerhan JR, Colditz G, Feskanich D, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Miller AB, Rohan TE, Virtamo J, Willett WC, Hunter DJ, Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies, Cancer Epidemiol Biomarkers Prev, 13 (2004) 40–48. [DOI] [PubMed] [Google Scholar]
- [43].De Stefani E, Brennan P, Boffetta P, Ronco AL, Mendilaharsu M, Deneo-Pellegrini H, Vegetables, fruits, related dietary antioxidants, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay, Nutr Cancer, 38 (2000) 23–29. [DOI] [PubMed] [Google Scholar]
- [44].Zeegers MP, Goldbohm RA, van den Brandt PA, Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study, Br J Cancer, 85 (2001) 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nishino H, Murakoshi M, Tokuda H, Satomi Y, Cancer prevention by carotenoids, Arch Biochem Biophys, 483 (2009) 165–168. [DOI] [PubMed] [Google Scholar]
- [46].Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM, Wang XD, beta-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice, Cancer Prev Res (Phila), 6 (2013) 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu C, Bronson RT, Russell RM, Wang XD, beta-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets, Cancer Prev Res (Phila), 4 (2011) 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Iskandar AR, Miao B, Li X, Hu KQ, Liu C, Wang XD, beta-Cryptoxanthin Reduced Lung Tumor Multiplicity and Inhibited Lung Cancer Cell Motility by Downregulating Nicotinic Acetylcholine Receptor alpha7 Signaling, Cancer Prev Res (Phila), 9 (2016) 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].San Millan C, Soldevilla B, Martin P, Gil-Calderon B, Compte M, Perez-Sacristan B, Donoso E, Pena C, Romero J, Granado-Lorencio F, Bonilla F, Dominguez G, beta-Cryptoxanthin Synergistically Enhances the Antitumoral Activity of Oxaliplatin through DeltaNP73 Negative Regulation in Colon Cancer, Clin Cancer Res, 21 (2015) 4398–4409. [DOI] [PubMed] [Google Scholar]
- [50].Gao M, Dang F, Deng C, beta-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer, Eur J Pharmacol, 859 (2019) 172528. [DOI] [PubMed] [Google Scholar]
- [51].Miyazawa K, Miyamoto S, Suzuki R, Yasui Y, Ikeda R, Kohno H, Yano M, Tanaka T, Hata K, Suzuki K, Dietary beta-cryptoxanthin inhibits N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in male ICR mice, Oncol Rep, 17 (2007) 297–304. [PubMed] [Google Scholar]
- [52].Chen J, Song Y, Zhang L, Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta-analysis of prospective studies, J Nutr Sci Vitaminol (Tokyo), 59 (2013) 213–223. [DOI] [PubMed] [Google Scholar]
- [53].Rowles JL 3rd, Ranard KM, Applegate CC, Jeon S, An R, Erdman JW Jr., Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis, Prostate Cancer Prostatic Dis, 21 (2018) 319–336. [DOI] [PubMed] [Google Scholar]
- [54].Mustra Rakic J, Liu C, Veeramachaneni S, Wu D, Paul L, Chen CO, Ausman LM, Wang XD, Lycopene Inhibits Smoke-Induced Chronic Obstructive Pulmonary Disease and Lung Carcinogenesis by Modulating Reverse Cholesterol Transport in Ferrets, Cancer Prev Res (Phila), 12 (2019) 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lian F, Smith DE, Ernst H, Russell RM, Wang XD, Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo, Carcinogenesis, 28 (2007) 1567–1574. [DOI] [PubMed] [Google Scholar]
- [56].Liu C, Russell RM, Wang XD, Low dose beta-carotene supplementation of ferrets attenuates smoke-induced lung phosphorylation of JNK, p38 MAPK, and p53 proteins, J Nutr, 134 (2004) 2705–2710. [DOI] [PubMed] [Google Scholar]
- [57].Ip BC, Hu KQ, Liu C, Smith DE, Obin MS, Ausman LM, Wang XD, Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice, Cancer Prev Res (Phila), 6 (2013) 1304–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ip BC, Liu C, Ausman LM, von Lintig J, Wang XD, Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice, Cancer Prev Res (Phila), 7 (2014) 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Koul A, Bansal MP, Chaudhary H, Chugh NA, Lycopene enriched tomato extract suppresses chemically induced skin tumorigenesis in mice, Int J Vitam Nutr Res, (2019) 1–21. [DOI] [PubMed] [Google Scholar]
- [60].Jiang LN, Liu YB, Li BH, Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression, Asian J Androl, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xia H, Liu C, Li CC, Fu M, Takahashi S, Hu KQ, Aizawa K, Hiroyuki S, Wu G, Zhao L, Wang XD, Dietary Tomato Powder Inhibits High-Fat Diet-Promoted Hepatocellular Carcinoma with Alteration of Gut Microbiota in Mice Lacking Carotenoid Cleavage Enzymes, Cancer Prev Res (Phila), 11 (2018) 797–810. [DOI] [PubMed] [Google Scholar]
- [62].Kolberg M, Pedersen S, Bastani NE, Carlsen H, Blomhoff R, Paur I, Tomato paste alters NF-kappaB and cancer-related mRNA expression in prostate cancer cells, xenografts, and xenograft microenvironment, Nutr Cancer, 67 (2015) 305–315. [DOI] [PubMed] [Google Scholar]
- [63].Applegate CC, Rowles JL 3rd, Erdman JW Jr., Can Lycopene Impact the Androgen Axis in Prostate Cancer?: A Systematic Review of Cell Culture and Animal Studies, Nutrients, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, Mao H, Wei M, Clark KR, Mendell JR, Caligiuri MA, Jacob ST, Mendell JT, Ghoshal K, Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver, J Clin Invest, 122 (2012) 2871–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kopec RE, Riedl KM, Harrison EH, Curley RW Jr., Hruszkewycz DP, Clinton SK, Schwartz SJ, Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma, J Agric Food Chem, 58 (2010) 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Karin M, Cao Y, Greten FR, Li ZW, NF-kappaB in cancer: from innocent bystander to major culprit, Nat Rev Cancer, 2 (2002) 301–310. [DOI] [PubMed] [Google Scholar]
- [67].Nakajima S, Kitamura M, Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response, Free Radic Biol Med, 65 (2013) 162–174. [DOI] [PubMed] [Google Scholar]
- [68].Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, McDowell HP, Unitt JF, Sullivan E, Grimley R, Benson N, Broomhead D, Kell DB, White MR, Oscillations in NF-kappaB signaling control the dynamics of gene expression, Science, 306 (2004) 704–708. [DOI] [PubMed] [Google Scholar]
- [69].Karin M, Greten FR, NF-kappaB: linking inflammation and immunity to cancer development and progression, Nat Rev Immunol, 5 (2005) 749–759. [DOI] [PubMed] [Google Scholar]
- [70].Jin X, Wang Z, Qiu L, Zhang D, Guo Z, Gao Z, Deng C, Wang F, Wang S, Guo C, Potential biomarkers involving IKK/RelA signal in early stage non-small cell lung cancer, Cancer Sci, 99 (2008) 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Deng J, Fujimoto J, Ye XF, Men TY, Van Pelt CS, Chen YL, Lin XF, Kadara H, Tao Q, Lotan D, Lotan R, Knockout of the tumor suppressor gene Gprc5a in mice leads to NF-kappaB activation in airway epithelium and promotes lung inflammation and tumorigenesis, Cancer Prev Res (Phila), 3 (2010) 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T, Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma, Nature, 462 (2009) 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Anto RJ, Mukhopadhyay A, Shishodia S, Gairola CG, Aggarwal BB, Cigarette smoke condensate activates nuclear transcription factor-kappaB through phosphorylation and degradation of IkappaB(alpha): correlation with induction of cyclooxygenase-2, Carcinogenesis, 23 (2002) 1511–1518. [DOI] [PubMed] [Google Scholar]
- [74].Lingappan K, NF-kappaB in Oxidative Stress, Curr Opin Toxicol, 7 (2018) 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nakanishi C, Toi M, Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs, Nat Rev Cancer, 5 (2005) 297–309. [DOI] [PubMed] [Google Scholar]
- [76].Huang CS, Fan YE, Lin CY, Hu ML, Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1, J Nutr Biochem, 18 (2007) 449–456. [DOI] [PubMed] [Google Scholar]
- [77].Hadad N, Levy R, The synergistic anti-inflammatory effects of lycopene, lutein, beta-carotene, and carnosic acid combinations via redox-based inhibition of NF-kappaB signaling, Free Radic Biol Med, 53 (2012) 1381–1391. [DOI] [PubMed] [Google Scholar]
- [78].Gouranton E, Thabuis C, Riollet C, Malezet-Desmoulins C, El Yazidi C, Amiot MJ, Borel P, Landrier JF, Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue, J Nutr Biochem, 22 (2011) 642–648. [DOI] [PubMed] [Google Scholar]
- [79].Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD, Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats, Int J Cancer, 126 (2010) 1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Schmitz HH, Poor CL, Wellman RB, Erdman JW Jr., Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue, J Nutr, 121 (1991) 1613–1621. [DOI] [PubMed] [Google Scholar]
- [81].Wang XD, Caudill MA, Vitamin A In: Biochemical, physiological, and molecular aspects of human nutrition, Elsevier Inc, 2018. [Google Scholar]
- [82].di Masi A, Leboffe L, De Marinis E, Pagano F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P, Nervi C, Retinoic acid receptors: from molecular mechanisms to cancer therapy, Mol Aspects Med, 41 (2015) 1–115. [DOI] [PubMed] [Google Scholar]
- [83].Chambon P, The retinoid signaling pathway: molecular and genetic analyses, Semin Cell Biol, 5 (1994) 115–125. [DOI] [PubMed] [Google Scholar]
- [84].Pfahl M, Vertebrate receptors: molecular biology, dimerization and response elements, Semin Cell Biol, 5 (1994) 95–103. [DOI] [PubMed] [Google Scholar]
- [85].Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R, Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues, Cancer Res, 54 (1994) 3580–3587. [PubMed] [Google Scholar]
- [86].Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, Hong WK, Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin, N Engl J Med, 332 (1995) 1405–1410. [DOI] [PubMed] [Google Scholar]
- [87].Widschwendter M, Berger J, Daxenbichler G, Muller-Holzner E, Widschwendter A, Mayr A, Marth C, Zeimet AG, Loss of retinoic acid receptor beta expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer, Cancer Res, 57 (1997) 4158–4161. [PubMed] [Google Scholar]
- [88].Xu XC, Sneige N, Liu X, Nandagiri R, Lee JJ, Lukmanji F, Hortobagyi G, Lippman SM, Dhingra K, Lotan R, Progressive decrease in nuclear retinoic acid receptor beta messenger RNA level during breast carcinogenesis, Cancer Res, 57 (1997) 4992–4996. [PubMed] [Google Scholar]
- [89].Xu XC, Liu X, Tahara E, Lippman SM, Lotan R, Expression and up-regulation of retinoic acid receptor-beta is associated with retinoid sensitivity and colony formation in esophageal cancer cell lines, Cancer Res, 59 (1999) 2477–2483. [PubMed] [Google Scholar]
- [90].Lotan Y, Xu XC, Shalev M, Lotan R, Williams R, Wheeler TM, Thompson TC, Kadmon D, Differential expression of nuclear retinoid receptors in normal and malignant prostates, J Clin Oncol, 18 (2000) 116–121. [DOI] [PubMed] [Google Scholar]
- [91].Dahl C, Guldberg P, Abildgaard C, Evaluating the Role of RARbeta Signaling on Cellular Metabolism in Melanoma Using the Seahorse XF Analyzer, Methods Mol Biol, 2019 (2019) 171–180. [DOI] [PubMed] [Google Scholar]
- [92].Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, Kurie JM, Hong WK, Lotan R, Suppression of retinoic acid receptor beta in non-small-cell lung cancer in vivo: implications for lung cancer development, J Natl Cancer Inst, 89 (1997) 624–629. [DOI] [PubMed] [Google Scholar]
- [93].Xu XC, Lee JS, Lee JJ, Morice RC, Liu X, Lippman SM, Hong WK, Lotan R, Nuclear retinoid acid receptor beta in bronchial epithelium of smokers before and during chemoprevention, J Natl Cancer Inst, 91 (1999) 1317–1321. [DOI] [PubMed] [Google Scholar]
- [94].Cortes E, Lachowski D, Rice A, Chronopoulos A, Robinson B, Thorpe S, Lee DA, Possamai LA, Wang H, Pinato DJ, Del Rio Hernandez AE, Retinoic Acid Receptor-beta Is Downregulated in Hepatocellular Carcinoma and Cirrhosis and Its Expression Inhibits Myosin-Driven Activation and Durotaxis in Hepatic Stellate Cells, Hepatology, 69 (2019) 785–802. [DOI] [PubMed] [Google Scholar]
- [95].Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M, Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke, J Natl Cancer Inst, 91 (1999) 60–66. [DOI] [PubMed] [Google Scholar]
- [96].Vuillemenot BR, Pulling LC, Palmisano WA, Hutt JA, Belinsky SA, Carcinogen exposure differentially modulates RAR-beta promoter hypermethylation, an early and frequent event in mouse lung carcinogenesis, Carcinogenesis, 25 (2004) 623–629. [DOI] [PubMed] [Google Scholar]
- [97].Hayashi K, Yokozaki H, Naka K, Yasui W, Lotan R, Tahara E, Overexpression of retinoic acid receptor beta induces growth arrest and apoptosis in oral cancer cell lines, Jpn J Cancer Res, 92 (2001) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Napoli JL, Biosynthesis and metabolism of retinoic acid: roles of CRBP and CRABP in retinoic acid: roles of CRBP and CRABP in retinoic acid homeostasis, J Nutr, 123 (1993) 362–366. [DOI] [PubMed] [Google Scholar]
- [99].Matsumoto A, Mizukami H, Mizuno S, Umegaki K, Nishikawa J, Shudo K, Kagechika H, Inoue M, beta-Cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages, Biochem Pharmacol, 74 (2007) 256–264. [DOI] [PubMed] [Google Scholar]
- [100].Hara H, Takahashi H, Mohri S, Murakami H, Kawarasaki S, Iwase M, Takahashi N, Sugiura M, Goto T, Kawada T, beta-Cryptoxanthin Induces UCP-1 Expression via a RAR Pathway in Adipose Tissue, J Agric Food Chem, (2019). [DOI] [PubMed] [Google Scholar]
- [101].Lian F, Hu KQ, Russell RM, Wang XD, Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression, Int J Cancer, 119 (2006) 2084–2089. [DOI] [PubMed] [Google Scholar]
- [102].Sowell AL, Huff DL, Yeager PR, Caudill SP, Gunter EW, Retinol, alpha-tocopherol, lutein/zeaxanthin, beta-cryptoxanthin, lycopene, alpha-carotene, trans-beta-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection, Clin Chem, 40 (1994) 411–416. [PubMed] [Google Scholar]
- [103].Quesada-Gomez JM, Santiago-Mora R, Duran-Prado M, Dorado G, Pereira-Caro G, Moreno-Rojas JM, Casado-Diaz A, beta-Cryptoxanthin Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells Through Retinoic Acid Receptor, Mol Nutr Food Res, 62 (2018). [DOI] [PubMed] [Google Scholar]
- [104].Liu C, Russell RM, Wang XD, Alpha-tocopherol and ascorbic acid decrease the production of beta-apo-carotenals and increase the formation of retinoids from beta-carotene in the lung tissues of cigarette smoke-exposed ferrets in vitro, J Nutr, 134 (2004) 426–430. [DOI] [PubMed] [Google Scholar]
- [105].Kotake-Nara E, Kim SJ, Kobori M, Miyashita K, Nagao A, Acyclo-retinoic acid induces apoptosis in human prostate cancer cells, Anticancer Res, 22 (2002) 689–695. [PubMed] [Google Scholar]
- [106].Ben-Dor A, Nahum A, Danilenko M, Giat Y, Stahl W, Martin HD, Emmerich T, Noy N, Levy J, Sharoni Y, Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells, Arch Biochem Biophys, 391 (2001) 295–302. [DOI] [PubMed] [Google Scholar]
- [107].Cheng J, Miao B, Hu KQ, Fu X, Wang XD, Apo-10′-lycopenoic acid inhibits cancer cell migration and angiogenesis and induces peroxisome proliferator-activated receptor gamma, J Nutr Biochem, 56 (2018) 26–34. [DOI] [PubMed] [Google Scholar]
- [108].Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN, Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells, J Biol Chem, 276 (2001) 29681–29687. [DOI] [PubMed] [Google Scholar]
- [109].Prakash P, Liu C, Hu KQ, Krinsky NI, Russell RM, Wang XD, Beta-carotene and beta-apo-14’-carotenoic acid prevent the reduction of retinoic acid receptor beta in benzo[a]pyrene-treated normal human bronchial epithelial cells, J Nutr, 134 (2004) 667–673. [DOI] [PubMed] [Google Scholar]
- [110].Aydemir G, Dominguez M, de Lera AR, Mihaly J, Torocsik D, Ruhl R, Apo-14 - Carotenoic Acid Is a Novel Endogenous and Bioactive Apo-Carotenoid, Nutrients, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bellet MM, Masri S, Astarita G, Sassone-Corsi P, Della Fazia MA, Servillo G, Histone Deacetylase SIRT1 Controls Proliferation, Circadian Rhythm, and Lipid Metabolism during Liver Regeneration in Mice, J Biol Chem, 291 (2016) 23318–23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A, Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders, Cell Signal, 25 (2013) 1939–1948. [DOI] [PubMed] [Google Scholar]
- [113].He M, Tan B, Vasan K, Yuan H, Cheng F, Ramos da Silva S, Lu C, Gao SJ, SIRT1 and AMPK pathways are essential for the proliferation and survival of primary effusion lymphoma cells, J Pathol, 242 (2017) 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Allard JS, Perez E, Zou S, de Cabo R, Dietary activators of Sirt1, Mol Cell Endocrinol, 299 (2009) 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Imai S, “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging, Biochim Biophys Acta, 1804 (2010) 1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yoshino J, Mills KF, Yoon MJ, Imai S, Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice, Cell Metab, 14 (2011) 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I, SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease, Am J Respir Crit Care Med, 177 (2008) 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wu Z, Liu MC, Liang M, Fu J, Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure, Blood, 119 (2012) 2422–2429. [DOI] [PubMed] [Google Scholar]
- [119].Lim JY, Liu C, Hu KQ, Smith DE, Wu D, Lamon-Fava S, Ausman LM, Wang XD, Dietary beta-Cryptoxanthin Inhibits High-Refined Carbohydrate Diet-Induced Fatty Liver via Differential Protective Mechanisms Depending on Carotenoid Cleavage Enzymes in Male Mice, J Nutr, 149 (2019) 1553–1564. [DOI] [PubMed] [Google Scholar]
- [120].Sulli G, Lam MTY, Panda S , Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment, Trends Cancer, 5 (2019) 475–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell, 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [122].Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U, SIRT1 regulates circadian clock gene expression through PER2 deacetylation, Cell, 134 (2008) 317–328. [DOI] [PubMed] [Google Scholar]
- [123].Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D, SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells, Biochem Biophys Res Commun, 427 (2012) 191–196. [DOI] [PubMed] [Google Scholar]
- [124].Song NY, Surh YJ, Janus-faced role of SIRT1 in tumorigenesis, Ann N Y Acad Sci, 1271 (2012) 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ming M, Shea CR, Guo X, Li X, Soltani K, Han W, He YY, Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C, Proc Natl Acad Sci U S A, 107 (2010) 22623–22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, Nicosia SV, Zhang X, Deacetylation of cortactin by SIRT1 promotes cell migration, Oncogene, 28 (2009) 445–460. [DOI] [PubMed] [Google Scholar]
- [127].Sung JY, Kim R, Kim JE, Lee J, Balance between SIRT1 and DBC1 expression is lost in breast cancer, Cancer Sci, 101 (2010) 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Feng AN, Zhang LH, Fan XS, Huang Q, Ye Q, Wu HY, Yang J, Expression of SIRT1 in gastric cardiac cancer and its clinicopathologic significance, Int J Surg Pathol, 19 (2011) 743–750. [DOI] [PubMed] [Google Scholar]
- [129].Zhao G, Cui J, Zhang JG, Qin Q, Chen Q, Yin T, Deng SC, Liu Y, Liu L, Wang B, Tian K, Wang GB, Wang CY, SIRT1 RNAi knockdown induces apoptosis and senescence, inhibits invasion and enhances chemosensitivity in pancreatic cancer cells, Gene Ther, 18 (2011) 920–928. [DOI] [PubMed] [Google Scholar]
- [130].Wu Q, Wang Y, Qian M, Qiao Y, Zou S, Chen C, Zhang X, Chen Y, Zhao Y, Zhu G, Chen Y, Sun F, Wang J, Pan Q, Sirt1 suppresses Wnt/betaCatenin signaling in liver cancer cells by targeting betaCatenin in a PKAalpha-dependent manner, Cell Signal, 37 (2017) 62–73. [DOI] [PubMed] [Google Scholar]
- [131].Liu J, Wu W, Jin J, A novel mutation in SIRT1-AS leading to a decreased risk of HCC, Oncol Rep, 34 (2015) 2343–2350. [DOI] [PubMed] [Google Scholar]
- [132].Liu L, Liu C, Zhang Q, Shen J, Zhang H, Shan J, Duan G, Guo D, Chen X, Cheng J, Xu Y, Yang Z, Yao C, Lai M, Qian C, SIRT1-mediated transcriptional regulation of SOX2 is important for self-renewal of liver cancer stem cells, Hepatology, 64 (2016) 814–827. [DOI] [PubMed] [Google Scholar]
- [133].Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL, SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis, Ann Surg Oncol, 19 (2012) 2011–2019. [DOI] [PubMed] [Google Scholar]
- [134].Thunnissen FB, Acetylcholine receptor pathway and lung cancer, J Thorac Oncol, 4 (2009) 943–946. [DOI] [PubMed] [Google Scholar]
- [135].Grando SA, Connections of nicotine to cancer, Nat Rev Cancer, 14 (2014) 419–429. [DOI] [PubMed] [Google Scholar]
- [136].Niu XM, Lu S, Acetylcholine receptor pathway in lung cancer: New twists to an old story, World J Clin Oncol, 5 (2014) 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Brown KC, Perry HE, Lau JK, Jones DV, Pulliam JF, Thornhill BA, Crabtree CM, Luo H, Chen YC, Dasgupta P, Nicotine induces the up-regulation of the alpha7-nicotinic receptor (alpha7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway, The Journal of biological chemistry, 288 (2013) 33049–33059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zhao Y, The Oncogenic Functions of Nicotinic Acetylcholine Receptors, J Oncol, 2016 (2016) 9650481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhang C, Yu P, Zhu L, Zhao Q, Lu X, Bo S, Blockade of alpha7 nicotinic acetylcholine receptors inhibit nicotine-induced tumor growth and vimentin expression in non-small cell lung cancer through MEK/ERK signaling way, Oncol Rep, 38 (2017) 3309–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Grozio A, Paleari L, Catassi A, Servent D, Cilli M, Piccardi F, Paganuzzi M, Cesario A, Granone P, Mourier G, Russo P, Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: a promising prospective in anti-cancer drug development, Int J Cancer, 122 (2008) 1911–1915. [DOI] [PubMed] [Google Scholar]
- [141].Egleton RD, Brown KC, Dasgupta P, Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis, Trends Pharmacol Sci, 29 (2008) 151–158. [DOI] [PubMed] [Google Scholar]
- [142].Aizawa K, Liu C, Veeramachaneni S, Hu KQ, Smith DE, Wang XD, Development of ferret as a human lung cancer model by injecting 4-(Nmethyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK), Lung Cancer, 82 (2013) 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Pepper C, Tu H, Morrill P, Garcia-Rates S, Fegan C, Greenfield S, Tumor cell migration is inhibited by a novel therapeutic strategy antagonizing the alpha-7 receptor, Oncotarget, 8 (2017) 11414–11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Aizawa K, Liu C, Tang S, Veeramachaneni S, Hu KQ, Smith DE, Wang XD, Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation, Int J Cancer, 139 (2016) 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM, Wang XD, Beta-Cryptoxanthin Restores Nicotine-Reduced Lung SIRT1 to Normal Levels and Inhibits Nicotine-Promoted Lung Tumorigenesis and Emphysema in A/J Mice, Cancer Prev Res (Phila), 6 (2013) 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hong B, van den Heuvel AP, Prabhu VV, Zhang S, El-Deiry WS, Targeting tumor suppressor p53 for cancer therapy: strategies, challenges and opportunities, Curr Drug Targets, 15 (2014) 80–89. [DOI] [PubMed] [Google Scholar]
- [147].Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B, Disruption of p53 in human cancer cells alters the responses to therapeutic agents, J Clin Invest, 104 (1999) 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Fontemaggi G, Kela I, Amariglio N, Rechavi G, Krishnamurthy J, Strano S, Sacchi A, Givol D, Blandino G, Identification of direct p73 target genes combining DNA microarray and chromatin immunoprecipitation analyses, J Biol Chem, 277 (2002) 43359–43368. [DOI] [PubMed] [Google Scholar]
- [149].Wang W, Kim SH, El-Deiry WS, Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts, Proc Natl Acad Sci U S A, 103 (2006) 11003–11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lu C, Wang W, El-Deiry WS, Non-genotoxic anti-neoplastic effects of ellipticine derivative NSC176327 in p53-deficient human colon carcinoma cells involve stimulation of p73, Cancer Biol Ther, 7 (2008) 2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wilhelm MT, Rufini A, Wetzel MK, Tsuchihara K, Inoue S, Tomasini R, Itie-Youten A, Wakeham A, Arsenian-Henriksson M, Melino G, Kaplan DR, Miller FD, Mak TW, Isoform-specific p73 knockout mice reveal a novel role for delta Np73 in the DNA damage response pathway, Genes Dev, 24 (2010) 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bode AM, Dong Z, Post-translational modification of p53 in tumorigenesis, Nat Rev Cancer, 4 (2004) 793–805. [DOI] [PubMed] [Google Scholar]
- [153].Wu C, Han L, Riaz H, Wang S, Cai K, Yang L, The chemopreventive effect of beta-cryptoxanthin from mandarin on human stomach cells (BGC-823), Food Chem, 136 (2013) 1122–1129. [DOI] [PubMed] [Google Scholar]
- [154].Mattioni M, Soddu S, Prodosmo A, Visca P, Conti S, Alessandrini G, Facciolo F, Strigari L, Prognostic role of serum p53 antibodies in lung cancer, BMC Cancer, 15 (2015) 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, Sudo S, Ju J, Sakuragi N, Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis, Oncogene, 32 (2013) 3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Melling N, Norrenbrock S, Kluth M, Simon R, Hube-Magg C, Steurer S, Hinsch A, Burandt E, Jacobsen F, Wilczak W, Quaas A, Bockhorn M, Grupp K, Tachezy M, Izbicki J, Sauter G, Gebauer F, p53 overexpression is a prognosticator of poor outcome in esophageal cancer, Oncol Lett, 17 (2019) 3826–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zhang J, Xu Z, Yu L, Chen M, Li K, Assessment of the potential diagnostic value of serum p53 antibody for cancer: a meta-analysis, PLoS One, 9 (2014) e99255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Fu X, Wu S, Li B, Xu Y, Liu J, Functions of p53 in pluripotent stem cells, Protein Cell, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].She QB, Chen N, Dong Z, ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation, J Biol Chem, 275 (2000) 20444–20449. [DOI] [PubMed] [Google Scholar]
- [160].Liu C, Russell RM, Wang XD, Lycopene supplementation prevents smoke-induced changes in p53, p53 phosphorylation, cell proliferation, and apoptosis in the gastric mucosa of ferrets, J Nutr, 136 (2006) 106–111. [DOI] [PubMed] [Google Scholar]
- [161].Yu H, Rohan T, Role of the insulin-like growth factor family in cancer development and progression, J Natl Cancer Inst, 92 (2000) 1472–1489. [DOI] [PubMed] [Google Scholar]
- [162].Giovannucci E, Insulin-like growth factor-I and binding protein-3 and risk of cancer, Horm Res, 51 (suppl 3) (1999) 34–41. [DOI] [PubMed] [Google Scholar]
- [163].Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y, Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene, Nutr Cancer, 24 (1995) 257–266. [DOI] [PubMed] [Google Scholar]
- [164].Karas M, Amir H, Fishman D, Danilenko M, Segal S, Nahum A, Koifmann A, Giat Y, Levy J, Sharoni Y, Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells, Nutr Cancer, 36 (2000) 101–111. [DOI] [PubMed] [Google Scholar]
- [165].Holmes MD, Pollak MN, Willett WC, Hankinson SE, Dietary Correlates of Plasma Insulin-like Growth Factor I and Insulin-like Growth Factor Binding Protein 3 Concentrations, Cancer Epidemiol Biomarkers Prev, 11 (2002) 852–861. [PubMed] [Google Scholar]
- [166].Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, Wertz K, Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model, Faseb J, 18 (2004) 1019–1021. [DOI] [PubMed] [Google Scholar]
- [167].Liu C, Lian F, Smith DE, Russell RM, Wang XD, Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets, Cancer Res, 63 (2003) 3138–3144. [PubMed] [Google Scholar]
- [168].Lee HY, Chun KH, Liu B, Wiehle SA, Cristiano RJ, Hong WK, Cohen P, Kurie JM, Insulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancer, Cancer Res, 62 (2002) 3530–3537. [PubMed] [Google Scholar]
- [169].Liberti MV, Locasale JW, The Warburg effect: how does it benefit cancer cells?, Trends in biochemical sciences, 41 (2016) 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Mello T, Simeone I, Galli A, Mito-Nuclear Communication in Hepatocellular Carcinoma Metabolic Rewiring, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Liberti MV, Locasale JW, The Warburg Effect: How Does it Benefit Cancer Cells?, Trends Biochem Sci, 41 (2016) 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kitamura K, Hatano E, Higashi T, Narita M, Seo S, Nakamoto Y, Yamanaka K, Nagata H, Taura K, Yasuchika K, Nitta T, Uemoto S, Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis, J Hepatol, 55 (2011) 846–857. [DOI] [PubMed] [Google Scholar]
- [173].Gupta P, Bhatia N, Bansal MP, Koul A, Lycopene modulates cellular proliferation, glycolysis and hepatic ultrastructure during hepatocellular carcinoma, World J Hepatol, 8 (2016) 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhang HF, Wang YC, Han YD, MicroRNA34a inhibits liver cancer cell growth by reprogramming glucose metabolism, Mol Med Rep, 17 (2018) 4483–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ma R, Zhang W, Tang K, Zhang H, Zhang Y, Li D, Li Y, Xu P, Luo S, Cai W, Ji T, Katirai F, Ye D, Huang B, Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma, Nat Commun, 4 (2013) 2508. [DOI] [PubMed] [Google Scholar]
- [176].Zhou Y, Niu W, Luo Y, Li H, Xie Y, Wang H, Liu Y, Fan S, Li Z, Xiong W, Li X, Ren C, Tan M, Li G, Zhou M, p53/Lactate dehydrogenase A axis negatively regulates aerobic glycolysis and tumor progression in breast cancer expressing wild-type p53, Cancer Sci, 110 (2019) 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I, A crucial role for adipose tissue p53 in the regulation of insulin resistance, Nat Med, 15 (2009) 1082–1087. [DOI] [PubMed] [Google Scholar]
- [178].Silvente-Poirot S, Poirot M, Cholesterol metabolism and cancer: the good, the bad and the ugly, Curr Opin Pharmacol, 12 (2012) 673–676. [DOI] [PubMed] [Google Scholar]
- [179].Bang B, Gniadecki R, Gajkowska B, Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes, Exp Dermatol, 14 (2005) 266–272. [DOI] [PubMed] [Google Scholar]
- [180].Palozza P, Colangelo M, Simone R, Catalano A, Boninsegna A, Lanza P, Monego G, Ranelletti FO, Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines, Carcinogenesis, 31 (2010) 1813–1821. [DOI] [PubMed] [Google Scholar]
- [181].Fuhrman B, Elis A, Aviram M, Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages, Biochem Biophys Res Commun, 233 (1997) 658–662. [DOI] [PubMed] [Google Scholar]
- [182].Yang CM, Lu IH, Chen HY, Hu ML, Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARgamma-LXRalpha-ABCA1 pathway, J Nutr Biochem, 23 (2012) 8–17. [DOI] [PubMed] [Google Scholar]
- [183].Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF, The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies, Science, 359 (2018) 1366–1370. [DOI] [PubMed] [Google Scholar]
- [184].Szabo G, Gut-liver axis in alcoholic liver disease, Gastroenterology, 148 (2015) 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF, Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4, Cancer Cell, 21 (2012) 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N, Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome, Nature, 499 (2013) 97–101. [DOI] [PubMed] [Google Scholar]
- [187].Wiese M, Bashmakov Y, Chalyk N, Nielsen DS, Krych L, Kot W, Klochkov V, Pristensky D, Bandaletova T, Chernyshova M, Kyle N, Petyaev I, Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons, Biomed Res Int, 2019 (2019) 4625279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Jia B, Commentary: Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells, Front Immunol, 10 (2019) 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF, Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells, Science, 360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lyu Y, Wu L, Wang F, Shen X, Lin D, Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis, Exp Biol Med (Maywood), 243 (2018) 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Valent P, Bonnet D, De Maria R, Lapidot T, Copland M, Melo JV, Chomienne C, Ishikawa F, Schuringa JJ, Stassi G, Huntly B, Herrmann H, Soulier J, Roesch A, Schuurhuis GJ, Wohrer S, Arock M, Zuber J, Cerny-Reiterer S, Johnsen HE, Andreeff M, Eaves C, Cancer stem cell definitions and terminology: the devil is in the details, Nat Rev Cancer, 12 (2012) 767–775. [DOI] [PubMed] [Google Scholar]
- [192].Lim JY, Kim YS, Kim KM, Min SJ, Kim Y, Beta-carotene inhibits neuroblastoma tumorigenesis by regulating cell differentiation and cancer cell stemness, Biochem Biophys Res Commun, 450 (2014) 1475–1480. [DOI] [PubMed] [Google Scholar]
- [193].Espinosa de los Monteros A, Hellmen E, Ramirez GA, Herraez P, Rodriguez F, Ordas J, Millan Y, Lara A, Martin de las Mulas J, Lipid-rich carcinomas of the mammary gland in seven dogs: clinicopathologic and immunohistochemical features, Vet Pathol, 40 (2003) 718–723. [DOI] [PubMed] [Google Scholar]
- [194].Ramos CV, Taylor HB, Lipid-rich carcinoma of the breast. A clinicopathologic analysis of 13 examples, Cancer, 33 (1974) 812–819. [DOI] [PubMed] [Google Scholar]
- [195].Rak S, De Zan T, Stefulj J, Kosovic M, Gamulin O, Osmak M, FTIR spectroscopy reveals lipid droplets in drug resistant laryngeal carcinoma cells through detection of increased ester vibrational bands intensity, Analyst, 139 (2014) 3407–3415. [DOI] [PubMed] [Google Scholar]
- [196].Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA, Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms, Nat Rev Cancer, 13 (2013) 759–771. [DOI] [PubMed] [Google Scholar]
- [197].Freitas I, Pontiggia P, Barni S, Bertone V, Parente M, Novarina A, Roveta G, Gerzeli G, Stoward P, Histochemical probes for the detection of hypoxic tumour cells, Anticancer Res, 10 (1990) 613–622. [PubMed] [Google Scholar]
- [198].Ackerman D, Simon MC, Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment, Trends Cell Biol, 24 (2014) 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Schlaepfer IR, Nambiar DK, Ramteke A, Kumar R, Dhar D, Agarwal C, Bergman B, Graner M, Maroni P, Singh RP, Agarwal R, Deep G, Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation, Oncotarget, 6 (2015) 22836–22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Suen WC, Lee WY, Leung KT, Pan XH, Li G, Natural Killer Cell-Based Cancer Immunotherapy: A Review on 10 Years Completed Clinical Trials, Cancer Invest, 36 (2018) 431–457. [DOI] [PubMed] [Google Scholar]
- [201].Hu W, Wang G, Huang D, Sui M, Xu Y, Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities, Front Immunol, 10 (2019) 1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Barao I, Murphy WJ, The immunobiology of natural killer cells and bone marrow allograft rejection, Biol Blood Marrow Transplant, 9 (2003) 727–741. [DOI] [PubMed] [Google Scholar]
- [203].Terao R, Murata A, Sugamoto K, Watanabe T, Nagahama K, Nakahara K, Kondo T, Murakami N, Fukui K, Hattori H, Eto N, Immunostimulatory effect of kumquat (Fortunella crassifolia) and its constituents, beta-cryptoxanthin and R-limonene, Food Funct, 10 (2019) 38–48. [DOI] [PubMed] [Google Scholar]
- [204].Stice CP, Xia H, Wang XD, Tomato lycopene prevention of alcoholic fatty liver disease and hepatocellular carcinoma development, Chronic Dis Transl Med, 4 (2018) 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Ip BC, Wang XD, Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention, Nutrients, 6 (2013) 124–162. [DOI] [PMC free article] [PubMed] [Google Scholar]


