Abstract
Background:
Sepsis-induced multiple organ failure (MOF) has plagued surgical intensive care units (ICU) for decades. Early nutrition (principally enteral) improves hospital outcomes of high risk, ICU patients. The purpose of this study is to document how the growing epidemic of Chronic Critical Illness (CCI) patients respond to adequate evidence-based ICU nutrition.
Methods:
This retrospective post hoc subgroup analysis of an ongoing sepsis database identified 56 CCI patients who received early, adequate nutritional per an established surgical ICU protocol compared with 112 matched rapid recovery (RAP) patients.
Results:
The matched CCI and RAP groups had similar baseline characteristics. Serial biomarkers showed that CCI patients remained persistently inflamed with ongoing stress metabolism and that despite receiving evidence-based protocol (EBP) nutrition, had persistent catabolism and immunosuppression with more secondary infections. More CCI patients were discharged to poor non-home destinations (i.e. skilled nursing facilities, long-term acute care, hospice) (81% vs 29%, p<0.05). At 12 month follow up, CCI patients had worse functional status by Zubrod score (3.17 vs 1.62, p<0.001) and Short Physical Battery Testing (4.78 vs 8.59, p <0.02), worse health-related quality of life by EQ-5D-3L descriptive measures (9.07 vs 7.45, p <0.003) and lower survival (67% vs 92%, p<0.05).
Conclusions:
Despite early, adequate, evidence-based ICU nutrition, septic surgical ICU patients who develop CCI exhibit: persistent inflammation, immunosuppression, and catabolism with unacceptable long-term morbidity and mortality. While current evidence based ICU nutrition may improve short-term ICU outcomes, novel adjuncts are needed to improve long-term outcomes for CCI patients.
Keywords: chronic critical illness, Persistent Inflammation Immunosuppression Catabolism Syndrome, nutrition, sepsis biomarkers
INTRODUCTION:
Sepsis-induced multiple organ failure (MOF) has plagued intensive care units (ICUs) for decades. Seminal reports in the 1980s described MOF as “septic auto-cannibalism” with unremitting hypermetabolism causing acute protein malnutrition and progressive organ failure with in-hospital mortality > 80%. This provided the rationale for the early use of parenteral nutrition (PN) in high risk ICU patients to achieve early positive caloric and nitrogen balance. Unfortunately, multiple clinical trials extending into the 2000s failed to show this strategy improved outcomes and some have shown early PN to be harmful. However, clinical trials have consistently shown that early enteral nutrition (EEN) improves outcomes and as a result EEN became the standard of care in late 1990s. Additionally, other fundamental advances in ICU care (i.e. resuscitation, ventilation, dialysis, etc.) progressively reduced in-hospital MOF mortality. Most recently in the 2000s, successful implementation of the Surviving Sepsis Campaign’s evidence-based guidelines (EBGs) for early treatment of sepsis substantially reduced early mortality and late MOF deaths virtually disappeared. As a result, MOF now most often leads to lingering chronic critical illness (CCI) with induced frailty, long-term disabilities, and indolent death.
In 2012, University of Florida (UF) Sepsis Critical Illness Research Center (SCIRC) investigators coined the term Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS) to provide a mechanistic framework in which to study CCI in septic surgical ICU patients. In other words, PICS represents the pathobiology driving patients to a new phenotype of perpetual multiorgan dysfunction and a subgroup of CCI. In 2014, the SCIRC investigators initiated a NIH funded five year prospective longitudinal cohort study to define the epidemiology, dysregulated immunity, and long-term outcomes of newly diagnosed sepsis in surgical ICU patients. Patients were categorized into three clinical trajectories of 1) early death, 2) rapid recovery (RAP) and 3) CCI (see methods for definitions). Early deaths were surprisingly low at 4 %, and 62% of septic patients experienced rapid recovery (RAP = discharged alive prior to day 14 of ICU stay), but a notably high 34% developed CCI and experienced poor long-term outcomes. Biomarker existence of PICS has been demonstrated in this CCI cohort, and others have confirmed this (1–8). It seems prudent to provide early, adequate, evidence-based ICU nutrition to these newly described CCI and PICS populations, but the effects of this approach have not been previously described.
The purpose of this study was to determine how CCI patients respond to receiving early, adequate, ICU nutrition by an established evidence-based protocol (EBP) (Supplement 1). Previous studies demonstrate that CCI patients have persistent low grade inflammation and catabolism (4–6). Thus, we hypothesized that despite best practice using our nutritional EBP in delivering adequate calories that CCI patients compared to the RAP patients would: 1) remain inflamed, immunosuppressed, and catabolic with ongoing stress metabolism; 2) have higher discharge rates to poor dispositions; 3) have worse functional and quality of life outcomes; and 4) lower long-term survival.
Methods:
This study is a retrospective post hoc subgroup analysis of the UF SCIRC sepsis database approved by the UF Institutional Review Board (IRB:201702261) and is currently registered with clinicaltrials.gov ( NCT02276417). Details of the study design as well as the clinical and laboratory standard operating procedure (SOPs) utilized have been published (2). In brief, overall cohort inclusion criteria included: 1) age ≥18 years; 2) clinical diagnosis of sepsis as defined by 2001 consensus guidelines; and 3) entrance into an electronic medical record (EMR) evidence based sepsis SOPs. Exclusion criteria eliminated patients whose baseline immunosuppression, end-stage comorbidities, or severe injuries would be a primary determinant of their long-term outcomes and thus confound outcome assessment. Clinical data was collected into an established MOF database. Blood and urine samples were collected for biomarkers (described below) at 12 hours, one, four, seven, and 14 days, and weekly thereafter while hospitalized. Study patients are grouped by three predefined clinical trajectories of 1) early death, 2) RAP, and 3) CCI. Early death is defined as death within 14 days of sepsis onset. CCI was defined as an ICU stay greater than or equal to 14 days with evidence of persistent organ dysfunction based upon components of the Sequential Organ Failure Assessment (SOFA) score. The rapid recovery or RAP patients were those discharged from the ICU within 14 days with resolution of organ dysfunction. Discharge disposition was classified based on known associations with long-term outcomes as either “good” (home with or without health care services, or rehabilitation facility) or “poor” (long-term acute care centers [LTAC], skilled nursing facilities [SNFs], another acute care hospital, hospice, or inpatient death). Among survivors, follow-up assessments were performed at 3, 6, and 12 months for mortality (with cross-check validation via the United States Social Security Death Index), health related quality of life (HRQOL, measured by EuroQol-5D-3L descriptive and Utility Index), physical function (measured by Short Physical Performance Battery [SPPB]), and performance status was measured by WHO/Zubrod score. EQ-5D-3L is a descriptive system comprised of five dimensions to determine HRQOL: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Utility scores or index are on a scale of 0 (health state close to death) to 1 (full health) (9). The EQ-5D-3L is an established valid tool used in critical care survivors or as a surrogate-completed proxy to measure HRQOL (10). Patients were asked to retrospectively report their baseline (i.e., pre-admission, within the past 4 weeks) level of functioning, as well as, their current state at 3, 6, and 12 months. When the patient was unavailable, the measure was completed by an available proxy. SPPB an objective assessment tool for evaluating lower extremity function and is based on a timed short-distance (4 meter) walk, repeated chair stands, and balance test. Zubrod scores range is from zero to five, with increasing score reflecting worse performance status: 0) asymptomatic and fully active; 1) symptomatic but completely ambulatory; 2) symptomatic with <50% in bed during the day; 3) symptomatic with >50% in bed, but not bedbound; 4) bedbound, completely disabled, and incapable of any self-care; and 5) death. Baseline (i.e., pre-hospitalization) performance status was based upon patient/proxy reported 4-week recall assessment as soon as possible after sepsis onset.
Patients were managed using standardized sepsis SOPs based on the Surviving Sepsis Campaign’s EBGs, supplemented by other UF developed evidenced-based ICU clinical care SOPs including an ICU nutrition protocol based on Society of Critical Care Medicine/American Association of Parenteral and Enteral Nutrition (SCCM/ASPEN) ICU Nutrition EBG (see Supplement 1). In brief, per our ICU nutrition protocol, patients are started on early enteral nutrition (within 24 hours) and advanced to a goal rate to achieve 25kcal/kg/day unless on vasopressors or in bowel discontinuity. Most of these patients qualify to receive Impact 1.5 for the first week and are then switched to Osmolite 1.5 for the remainder of the hospital stay until they can be transitioned to oral diets. In addition to the enteral tube feeds, protein is supplemented to achieve 1.5–2g/kg/d ideal body weight (IBW). Parenteral nutrition (PN) is started at day 7 if enteral nutrition in not possible, supplemental PN started if not able to achieve at least 60% of goal calories secondary to enteral feeding intolerance, or on day 4 in those patients deemed high risk for malnutrition by NUTRIC score (11–13). PN amino acids were provided at a similar rate of 1.5–2g/kg/d protein (IBW) in a 2-in-1 mixture with carbohydrates, and 1.0g/kg/d soy based lipid twice a week.
Design of This Study:
This is a post hoc analysis of the UF SCIRC sepsis database that identified 56 CCI patients that survived the 4 weeks in the ICU during which time they received nutritional support as per the ICU nutrition protocol described above. To improve statistical power, the 56 CCI patients were matched (2:1) by age, gender, APACHE II score, and Charlson Comorbidity Index to identify 112 RAP patients. The SCIRC database was than queried to obtain data specific to these cohorts including a) baseline characteristics (demographics, body mass index [BMI], comorbidities, admission diagnosis, APACHE II scores, sepsis severity, and site of infection), b) outcomes (ICU days, hospital days, incidence acute kidney injury [AKI] by KDIGO score, incidence of MOF by Sequential Organ Failure Assessment [SOFA] score, incidence of secondary infections), c) post discharge disposition classified as “good” or “poor”, and d) biomarker reflecting underlying pathobiology of MOF and PICS including persistent inflammation (interleukin [IL]-6, IL-8), stress metabolism (glucagon-like peptide 1 [GLP-1] and albumin), immunosuppression (absolute lymphocyte count [ALC] and soluble programmed death ligand one [sPDL-1]), and catabolism (urinary 3-methyhistidine [3-MH]), e) functional outcomes including HRQOL (measured by EQ-5D-3L- descriptive and utility Index), physical function (measured by SPPB) and performance status (measured by WHO/Zubrod score) and f) 12 month mortality (6, 14).
Approval was also obtained from the UF IRB to perform a focused retrospective review of the electronic medical records (EMR) to obtain nutritional support related data for the 56 CCI patients to determine total calories received for each day of macronutrients (protein or amino acid, carbohydrate, lipids). Weekly totals were then represented as a percentage over the 4 week ICU stay divided by goal calories based on SCCM/ASPEN ICU Nutrition EBG of 25kcal/kg/day (12). For calories to be classified as received the by patient, calories had to be documented in three areas: nursing documentation, daily Ins and Out section, and daily ICU flow sheets. The enteral supplements (tube feeds and protein additives) were identified and calories were calculated from milliliters provided and caloric content of tube feeds per milliliter. In the event that PN was provided the dietician and pharmacy documentation of calories was used to calculate total calories provided daily. Calorie counts were provided for patients tolerating oral diets. Adequate nutrition (primary outcome) was defined as receiving >75% of goal calories over 4 weeks in the surgical ICU.
Statistical Analysis:
Data for baseline characteristics are presented as frequency and percentage for categorical data, mean and standard deviations for normally distributed data, as well as, median and 25th/75th percentiles for non-normally distributed data. Student’s t-test, ANOVA and Kruskal–Wallis tests were used for comparison of continuous variables as appropriate. Measured biomarkers were compared using non-parametric rank tests to determine significant differences between groups at each time point. Mixed model analysis was also performed to determine differences between groups over time. As this is a post hoc analysis of a prospective observational study, there is no power calculation.
Results:
Baseline characteristics of all patients and the CCI versus RAP cohorts are depicted in Table 1. Overall the study patients were predominantly Caucasian males of advance age (mean 60 years) with high BMI (mean 29), significant comorbidities, and severe illness (mean APACHE II 18.5). By design the CCI and RAP cohorts were matched for age, gender, APACHE II, and Charlson Comorbidity Index, otherwise there were no significant differences except for a higher incidence septic shock in CCI patients (39% vs 22%). There was no difference in primary source of sepsis between cohorts.
Table 1.
Baseline Characteristics
| Overall (n=168) | CCI (n=56) | RAP (n=112) | P-value | |
|---|---|---|---|---|
| Male, n (%) | 96 (57.1) | 32 (57.1) | 64 (57.1) | 1 |
| Age in years, mean (SD) | 60.1 (15.4) | 60.4 (14.4) | 59.9 (16) | 0.8902 |
| Age ≥ 65 years, n (%) | 76 (45.2) | 26 (46.4) | 50 (44.6) | 0.8702 |
| Race, n (%) | 0.7847 | |||
| Caucasian (White) | 149 (88.7) | 50 (89.3) | 99 (88.4) | |
| African American | 16 (9.5) | 5 (8.9) | 11 (9.8) | |
| Asian | 1 (0.6) | 0 (0) | 1 (0.9) | |
| Other | 1 (0.6) | 1 (1.8) | 0 (0) | |
| American Indian | 1 (0.6) | 0 (0) | 1 (0.9) | |
| BMI, median (25th, 75th) | 29 (24.5, 36) | 29.6 (24.7, 37.5) | 28.4 (24.5, 34.8) | 0.4184 |
| Number of comorbidities, n (%) | 0.4441 | |||
| 0 | 39 (23.2) | 10 (17.9) | 29 (25.9) | |
| 1 | 50 (29.8) | 18 (32.1) | 32 (28.6) | |
| 2 | 38 (22.6) | 11 (19.6) | 27 (24.1) | |
| ≥3 | 41 (24.4) | 17 (30.4) | 24 (21.4) | |
| Charlson comorbidity index median (25th, 75th) | 3 (2, 5) | 3 (2, 5) | 3 (2, 5) | 0.606 |
| APACHE II, median (25th, 75th) | 18.5 (14, 23.5) | 20 (15, 26) | 18 (14, 23) | 0.1484 |
| Admission Diagnosis, n (%) | 0.3909 | |||
| Infection-Related | 53 (31.5) | 20 (35.7) | 33 (29.5) | |
| Planned Surgery | 34 (20.2) | 8 (14.3) | 26 (23.2) | |
| Trauma | 13 (7.7) | 6 (10.7) | 7 (6.2) | |
| Other Acute Medical Conditions | 68 (40.5) | 22 (39.3) | 46 (41.1) | |
| Sepsis Severity | 0.0284 | |||
| Sepsis | 44 (26.2) | 9 (16.1) | 35 (31.2) | |
| Severe Sepsis | 77 (45.8) | 25 (44.6) | 52 (46.4) | |
| Septic Shock | 47 (28) | 22 (39.3) | 25 (22.3) | |
| Primary Sepsis Diagnosis, n (%) | 0.682 | |||
| CLABSI | 4 (2.4) | 1 (1.8) | 3 (2.7) | |
| Intra-abdominal | 50 (29.8) | 20 (35.7) | 30 (26.8) | |
| NSTI | 27 (16.1) | 8 (14.3) | 19 (17.0) | |
| Urosepsis | 21 (12.5) | 4 (7.1) | 17 (15.2) | |
| Pneumonia | 27 (16.1) | 11 (19.6) | 16 (14.3) | |
| SSI | 33 (19.6) | 10 (17.9) | 23 (20.5) | |
| Other | 6 (3.6) | 2 (3.6) | 4 (3.6) |
Clinical outcomes of all patients and the CCI versus RAP cohorts are listed in Supplement Table 1. Overall the CCI cohort had a higher percentage of patients with MOF (77% vs 45%), as well as, higher median SOFA score (9 vs 7). T number of secondary, nosocomial infections per patient was higher in the CCI cohort (1.3 vs 0.3), as well as, number of secondary infections per 100 person hospital days (3.7 vs 1.6). The CCI patients that suffered a secondary infection were more likely to get a pneumonia or urinary tract infection compared with the RAP cohort. CCI patients had a significantly longer ICU and hospital stay, as well as, higher incidence of patients designated as “poor” discharge disposition to a non-home destination (81% vs 29%, p<0.05). One third of all CCI patients expired by the end of one year, versus only 8% for the RAP cohort (12-month survival CCI 67% vs RAP 92%, p<0.0001).
During the four week study period, the CCI cohort reached 76% of goal calories being recommended by the ICU EBP nutritional protocol and executed by the clinical care teams (surgeons, intensivists, dieticians, pharmacists, and bedside nurses). Excluding week 1 calories, when nutritional intake in some patients was limited because repeat operations were being performed (for surgical source control or to reestablish gastrointestinal continuity), patients were on vasoactive agents, and/or enteral nutrition was otherwise contraindicated, the CCI patients received 88% of goal calories for the remaining three weeks. Of the 56 CCI patients, 35 (63%) received four weeks of total enteral nutrition. As per SCCM/ASPEN EBG, the remaining 21 (37%) received either PN until enteral feeds were initiated, or as supplemental PN. Only three (5%) patients remained on PN by the end of the study period.
Differences in biomarker concentrations in CCI versus RAP sepsis survivors are shown in Figures 1–4.
Fig. 1. Inflammatory Biomarkers.
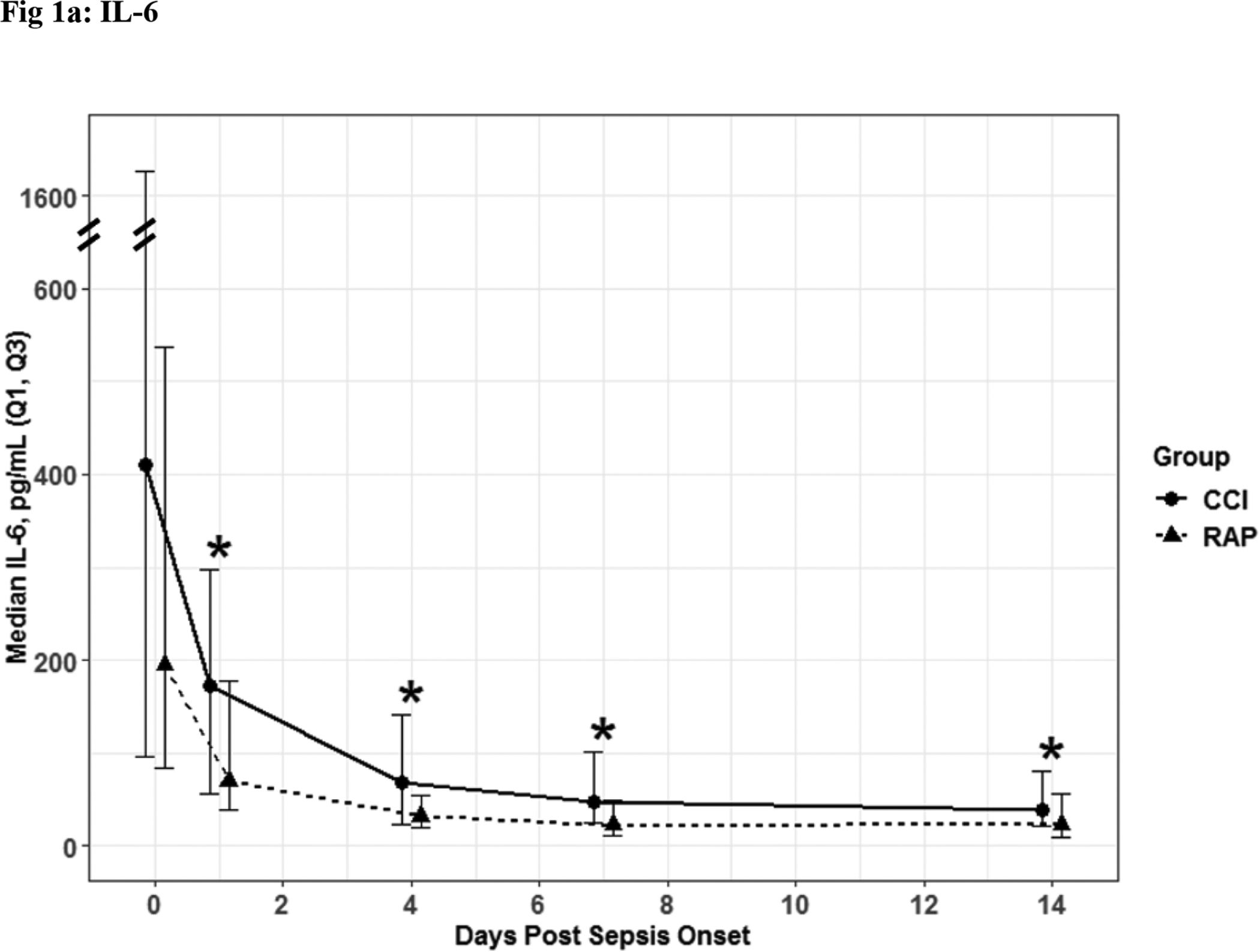
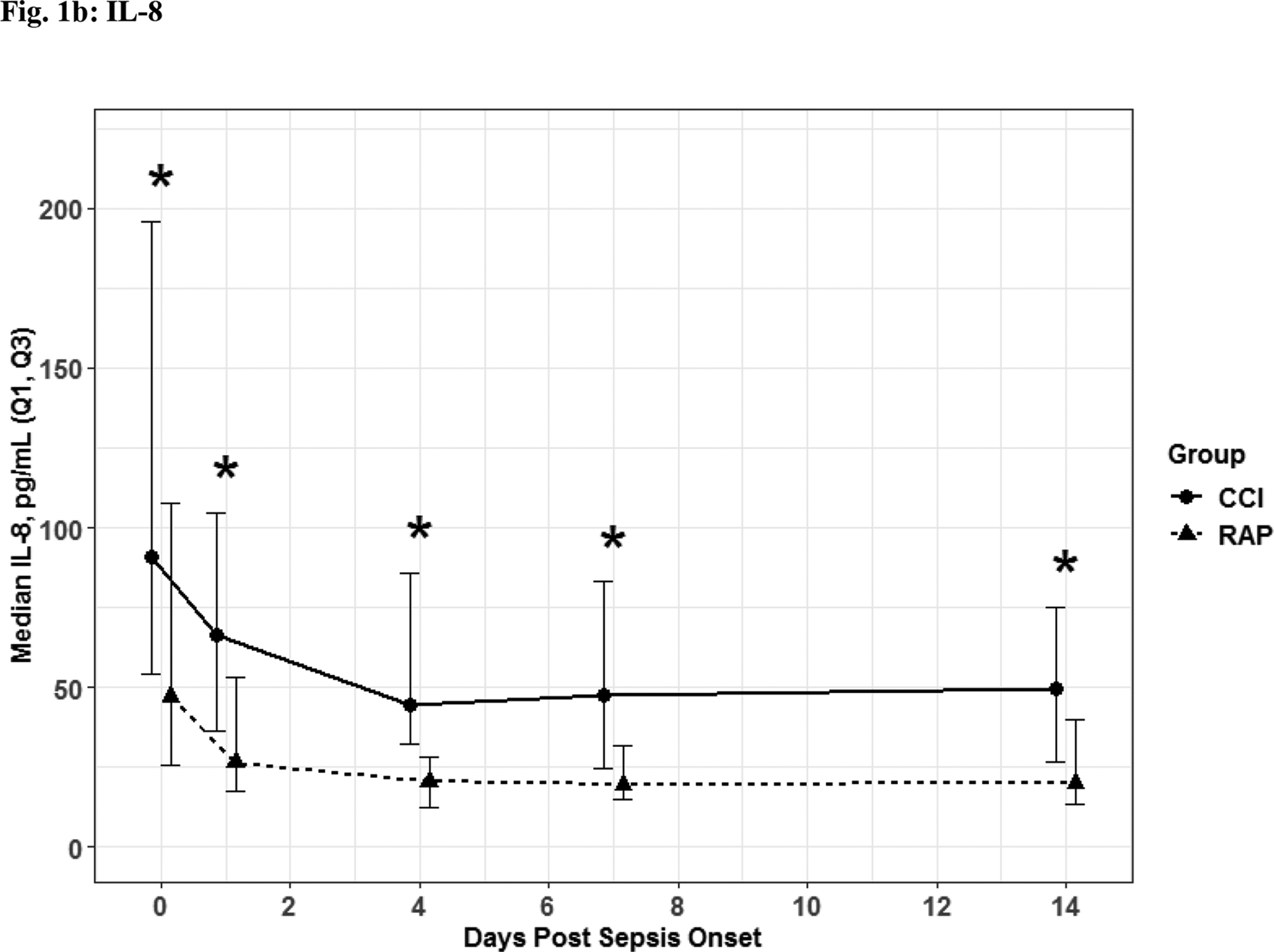
IL- 6 (Fig. 1a) and IL-8 (Fig. 1b) depicted as the median for each time point and for the representative days post sepsis onset, as well as, (*) denoting statistical significance. The solid line is CCI and dashed is RAP.
Fig. 4. Catabolism.
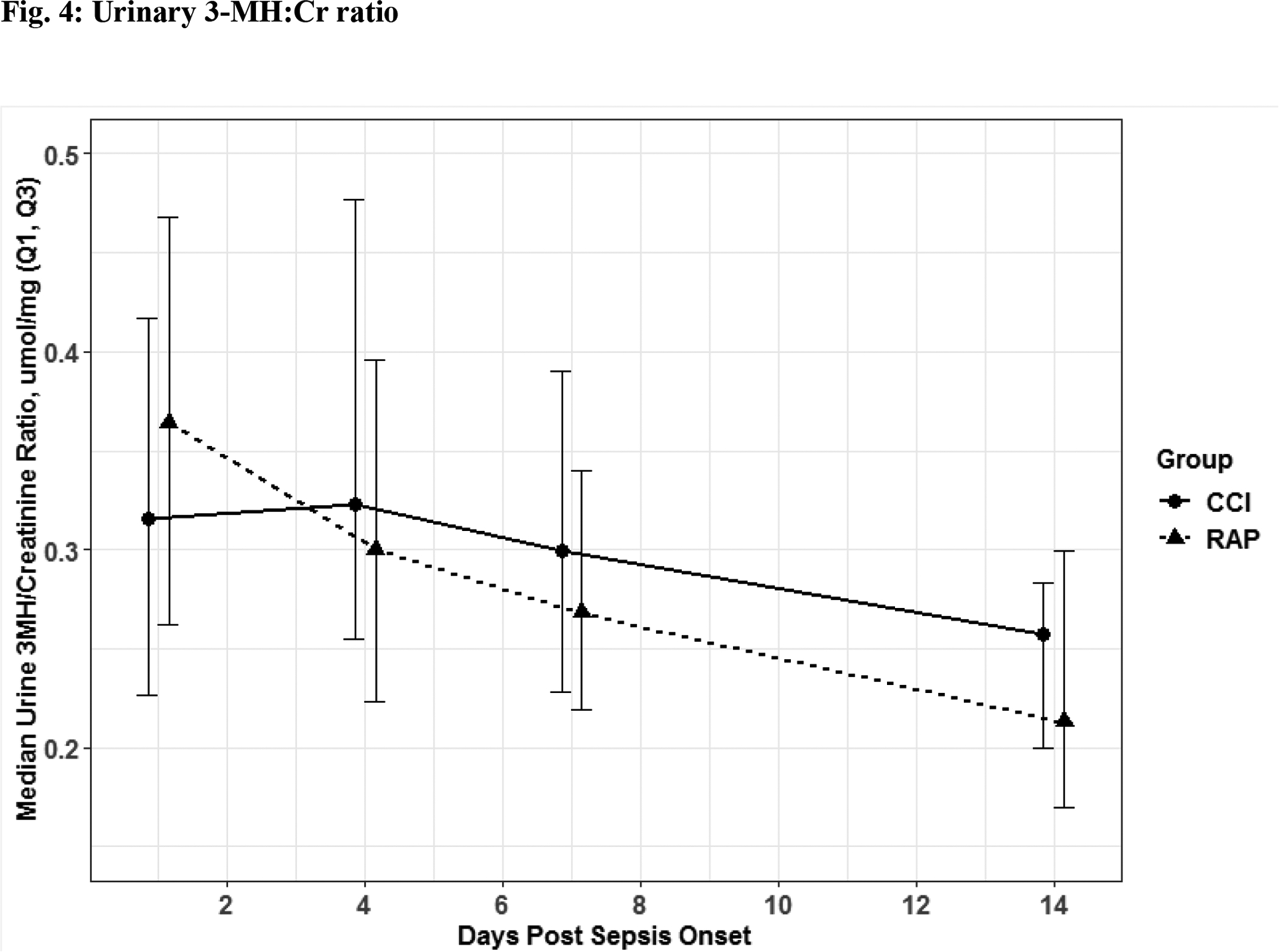
Urinary three methylhistidine to creatinine ratio. Though these values were not significant there was an obvious trend towards CCI patient having higher physiologic signs of catabolism when 3-MH excretion was expressed as a ratio to creatinine excretion to correct for any type of renal pathology like acute kidney injury. The solid line is CCI and dashed is RAP.
The 56 CCI (compared to the 112 RAP) sepsis survivors had evidence of persistent inflammation (significantly higher levels of IL-6, IL-8; Figure 1a–1b), with ongoing stress metabolism (higher levels of GLP-1 and lower serum albumin; Figure 2a–2b), immunosuppression (trend toward lower levels of ALC and higher sPDL-1; Figure 3a–3b), and a trend towards more catabolism (urinary 3-MH to creatinine ratio; Figure 4).
Fig. 2. Stress Metabolism.
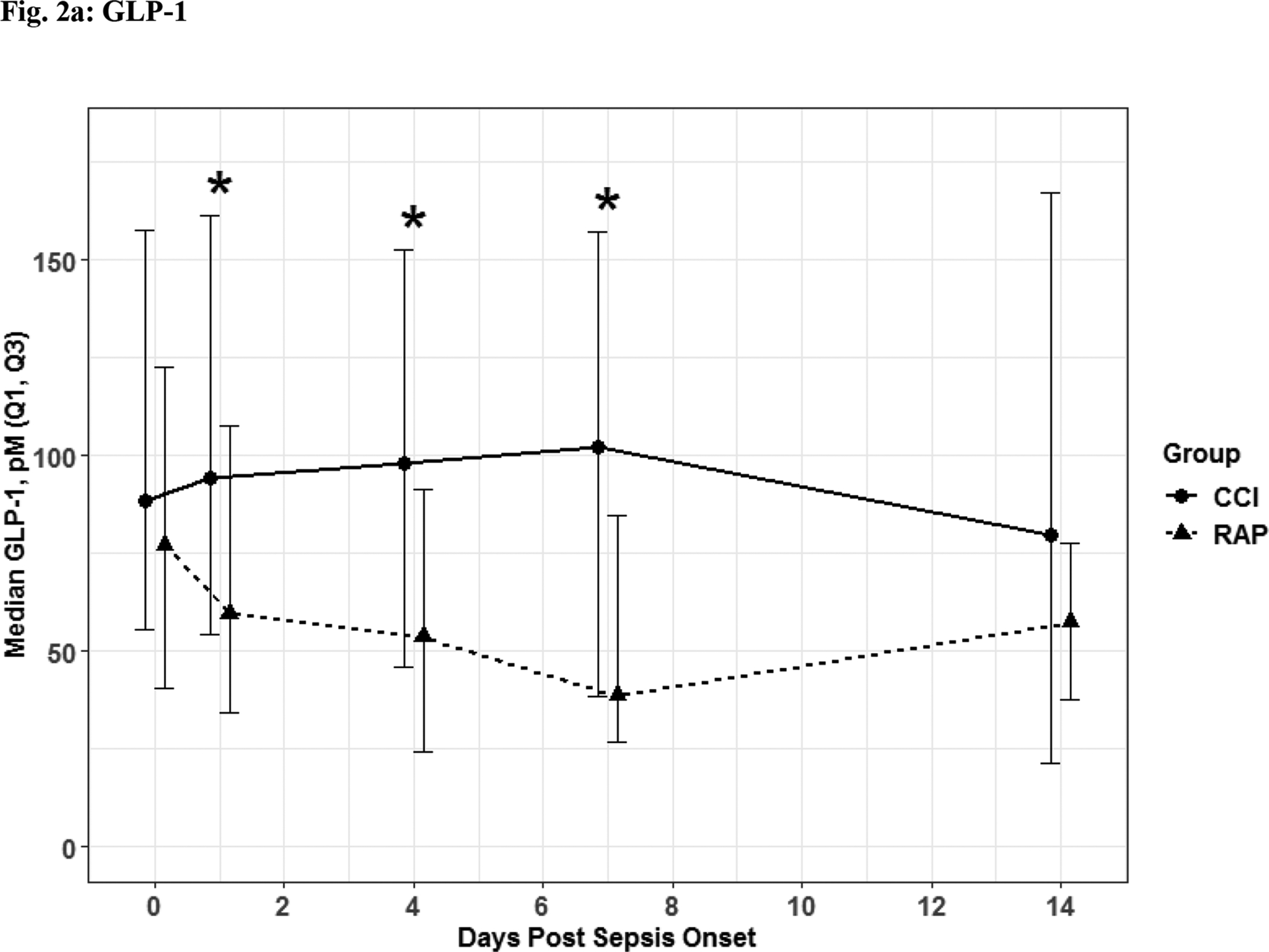
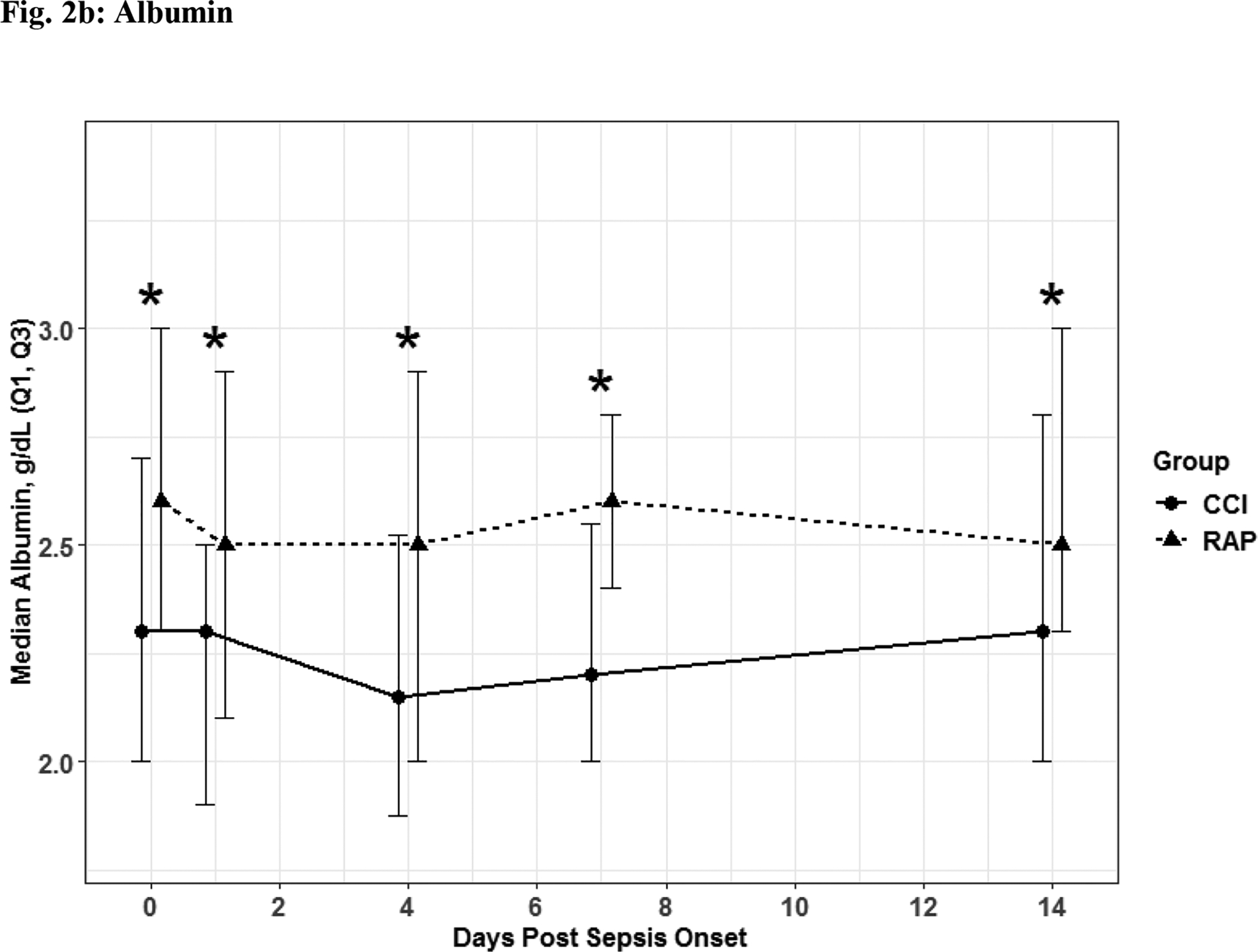
Glucagon-like Peptide 1 (Fig 2a) and albumin (Fig 2b) depicted as the median for each time point and for the representative days post sepsis onset, as well as, (*) denoting statistical significance. The solid line is CCI and dashed is RAP.
Fig. 3. Immunosuppression.
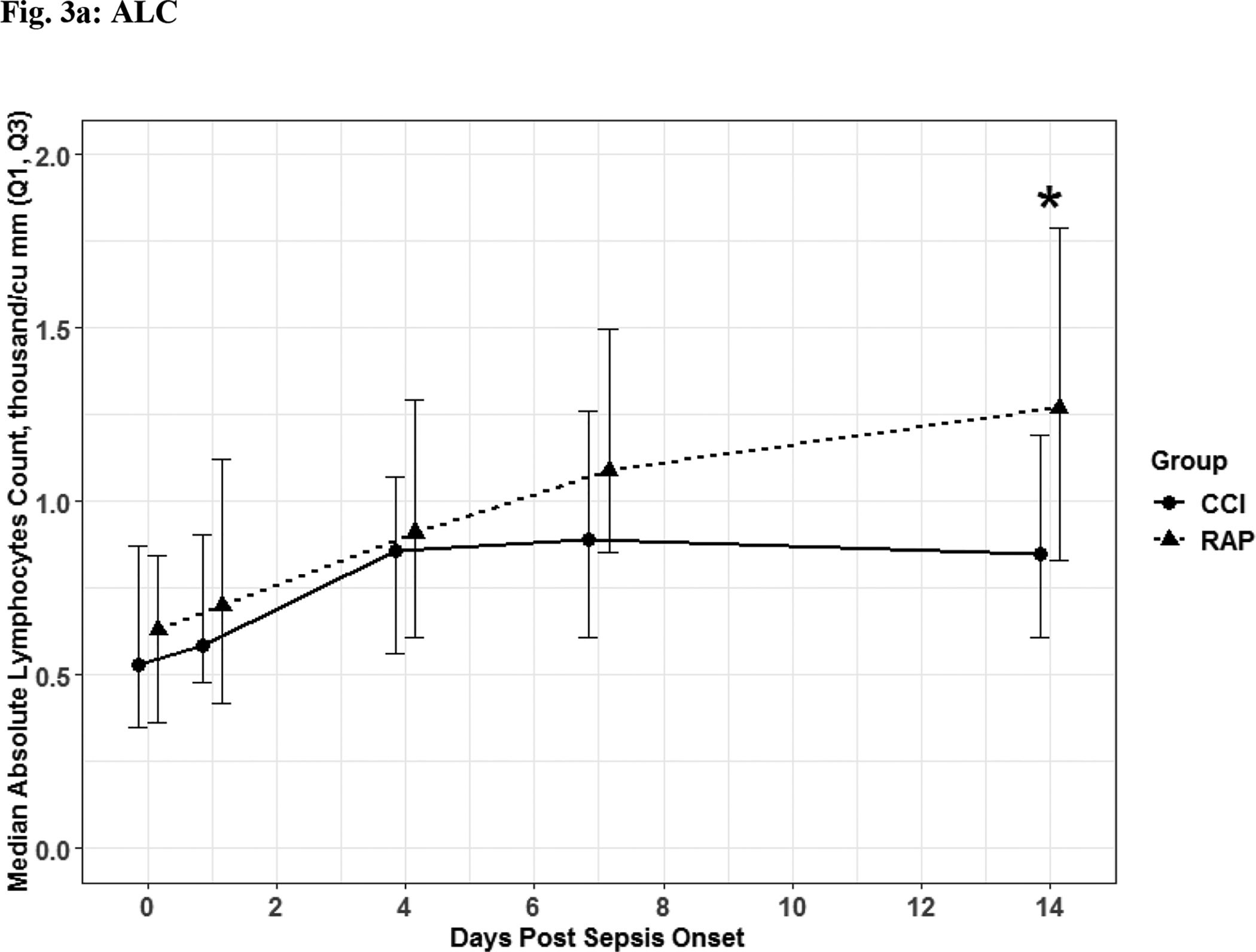
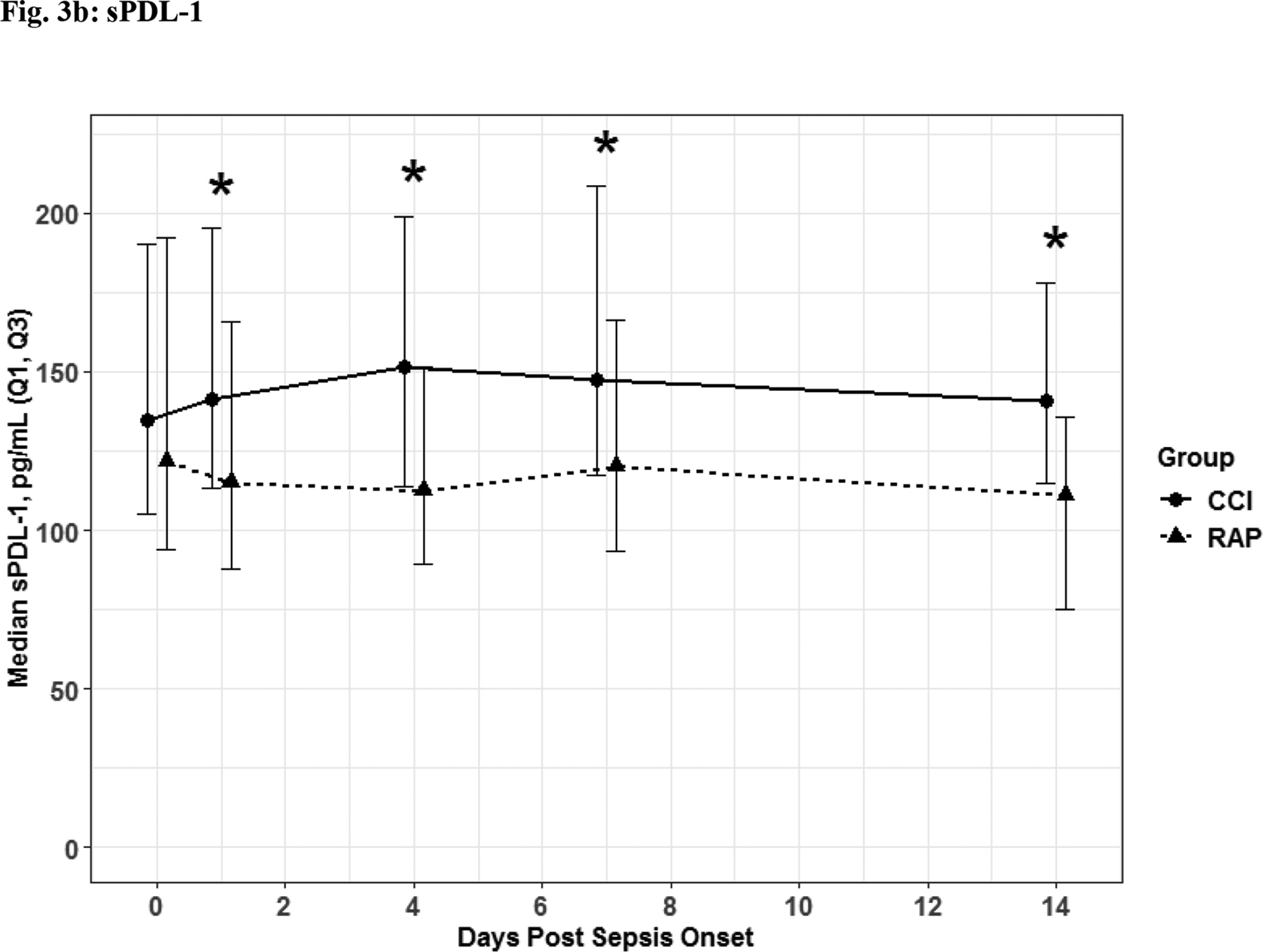
Absolute Lymphocyte Count (Fig 3a) Soluable Programmed Death Ligand 1 (Fig 3b) depicted as the median for each time point for the representative days post sepsis onset, as well as, (*) denoting statistical significance. The solid line is CCI and dashed is RAP.
Functional and QOL outcomes are listed in Figures 5–6.
Fig. 5. Functional Assessment.
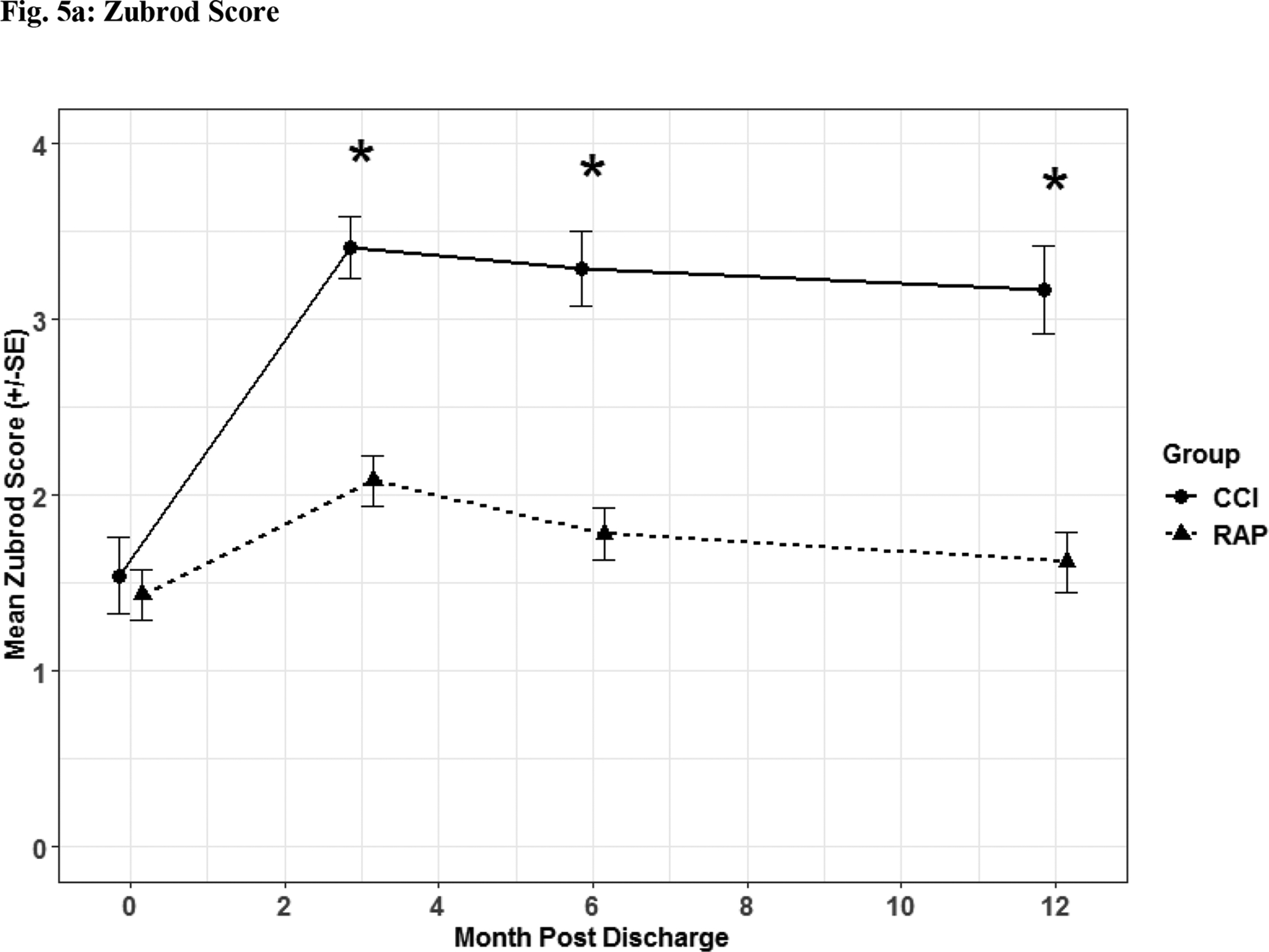
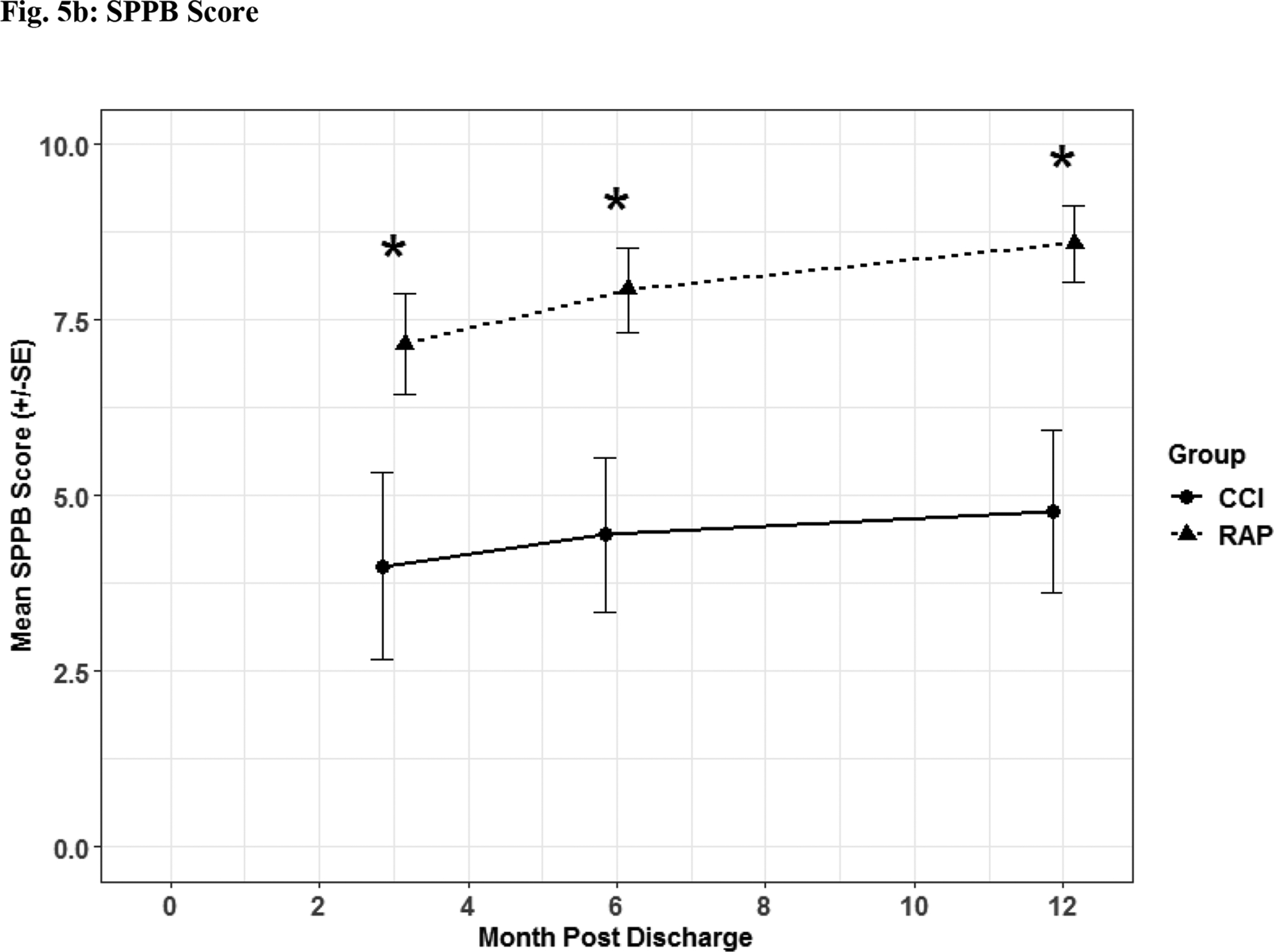
Zubrod (Fig 5a) and Short Physical Performance Battery (Fig 5b) scores depicted as the mean for each time point for the representative days post sepsis onset, as well as, (*) denoting statistical significance. The solid line is CCI and dashed is RAP.
Fig. 6. Quality of Life Assessment.
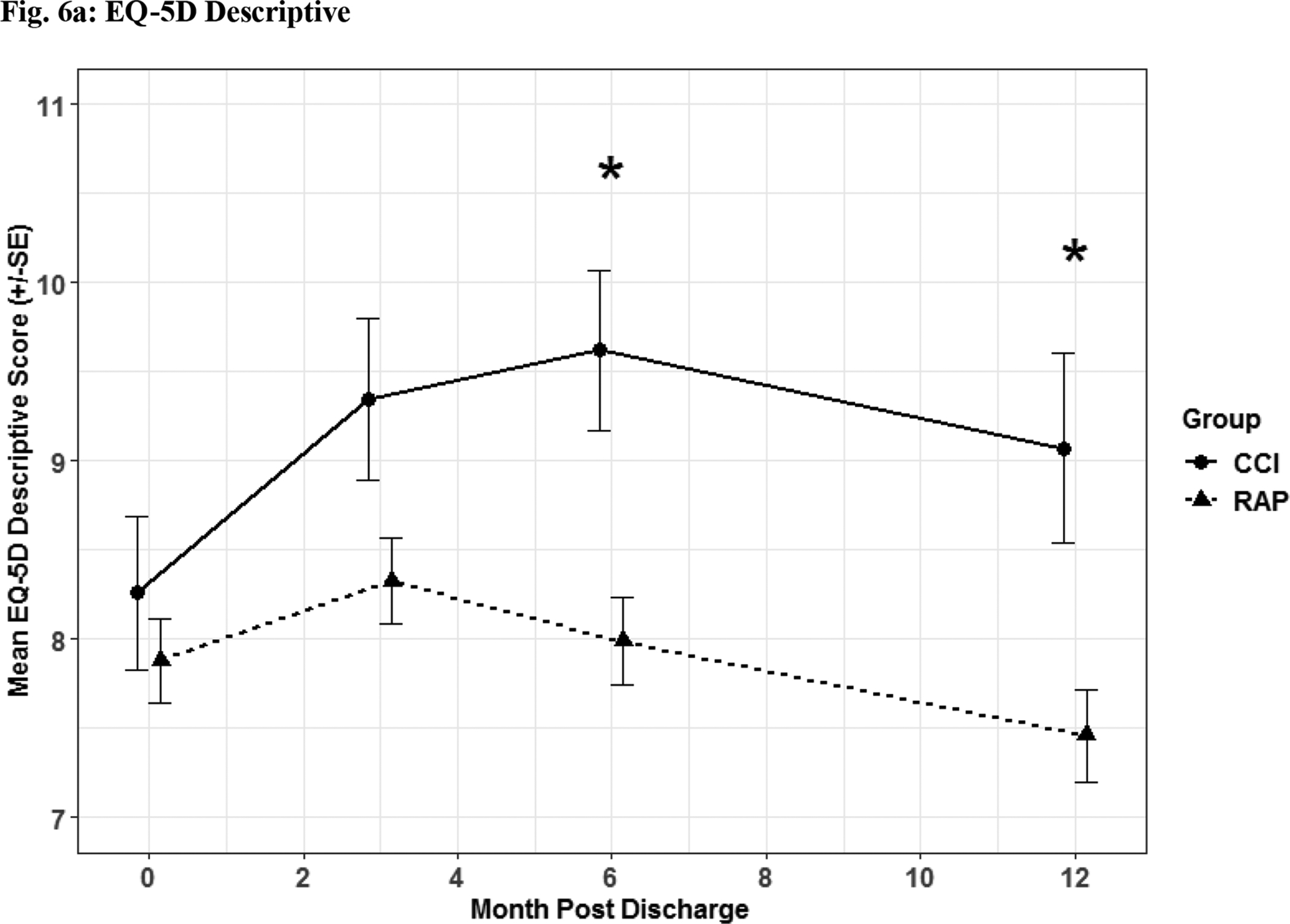
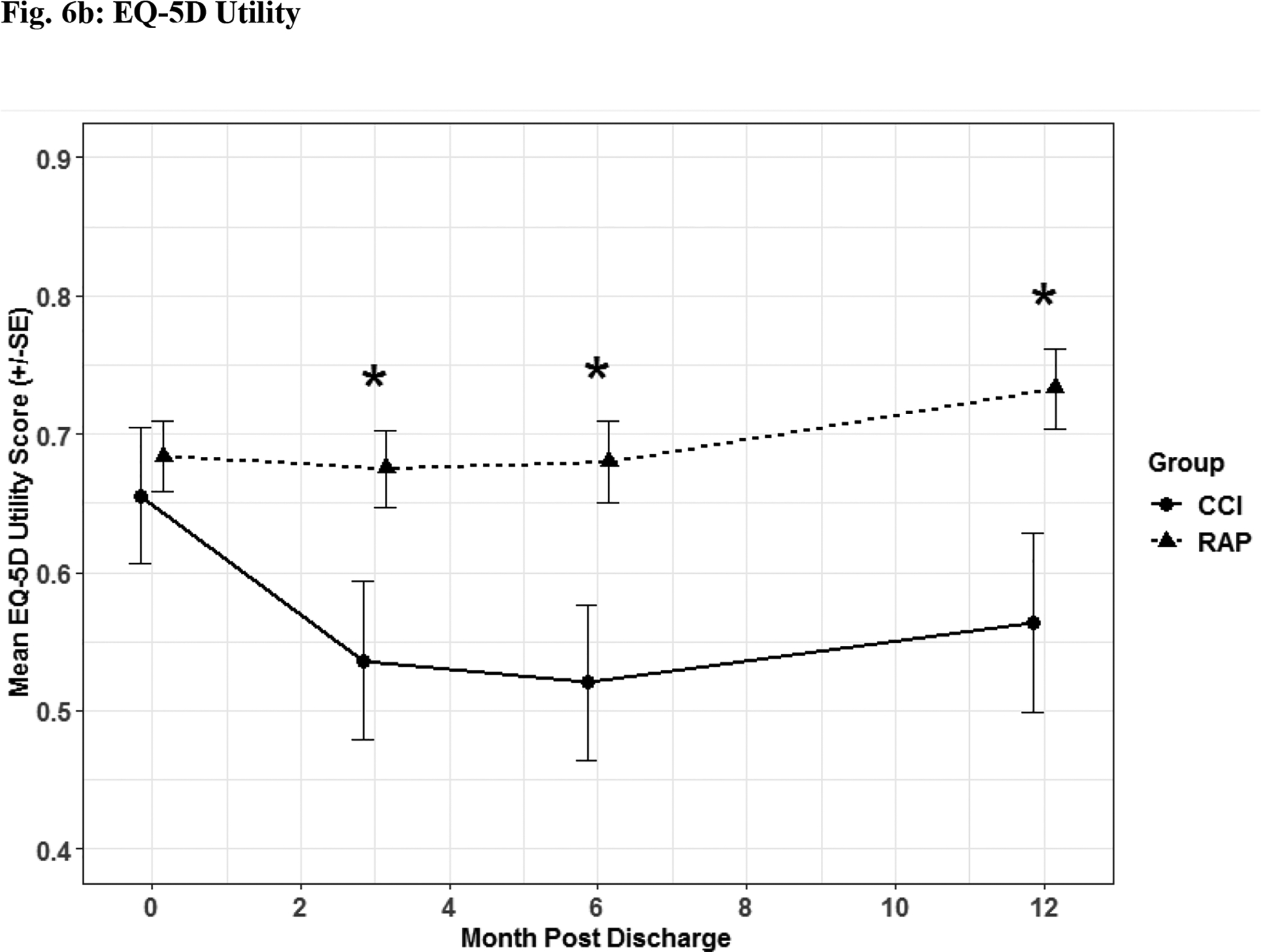
Quality of life assessment by EQ-5D descriptive (Fig 6a) and utility (Fig 6b) depicted as the mean for each time point for the representative days post sepsis onset, as well as, (*) denoting statistical significance. The solid line is CCI and dashed is RAP.
Patients suffering from CCI exhibited lower scores on functional assessment questionnaires identified via Zubrod (Figure 5a) and Short Physical Performance Battery scores (Figure 5b) when compared to RAP patients. When HRQOL post sepsis was assessed with EQ-5D-L questionnaire, CCI patients had significantly lower levels in descriptive (Figure 6a) and utility (Figure 6b) measures.
Discussion:
The major finding of this study is that despite receiving nutritional support consistent with the ASPEN/SCCM ICU Nutrition EBG, CCI sepsis survivors fail to achieve immunometabolic homeostasis and progress into a chronic malnourished state with dismal long-term outcomes compared with RAP patients. Based on existing evidence, these CCI patients most likely did benefit in the short-term from receiving early enteral nutrition, but improved adjuncts are needed in these CCI survivors to reduce low grade inflammation, promote anabolism and improve long-term outcomes. The CCI cohort (compared with RAP patients) have biomarker evidence of PICS. These CCI-PICS patients remain persistently inflamed (with higher IL-6 and IL-8 levels) and immunosuppressed (with decreased TLC and elevated sPDL-1 levels). They fail to restore metabolic homeostasis (reflected by elevated GLP-1) with an ongoing acute phase response (lower albumin levels) and persistent catabolism (trend towards higher the urinary 3-MH:Creatinine ratios). To date our UF SCIRC studies support the PICS paradigm as the pathobiologic explanation for the development of CCI after sepsis (4, 5, 7, 15–17). From a nutritional perspective, despite aggressive efforts to provide ICU EBG nutritional support, these patients have continued loss of lean muscle mass with severe functional disabilities, endure sepsis recidivism, and have poor dispositions to non-home destinations, high rates of hospital re-admissions, and poor one-year survival.
While there is virtually no nutritional support evidence specific to this growing epidemic of CCI-PICS patients, there is literature from other patient populations who experience similar persistent inflammation, muscle loss, and anabolic resistance including major burns, cancer cachexia, and sarcopenia. It is well documented that the hypermetabolic response to major burn injury with persistent inflammation and catabolism continues for months after injury. This lead Herndon et al. to recommend 2 grams/kg/day protein supplementation to compensate for this tremendous catabolic insult (18). In this population, Herndon et al. has also shown that additional anabolic interventions can reverse muscle breakdown and preserve lean body mass including: a) oxandralone (19), b) propranolol (20), and c) exercise programs (21).
Cancer cachexia is another difficult to treat, complex, catabolic state characterized by progressive weight loss, muscle atrophy, muscle loss, and frailty. Similar to our sepsis CCI cohort, this is mediated in part by the persistent expansion myeloid derived suppressor cells (MDSCs) and the elaboration of cytokines such as TNF-α, IL-1, IL-6, IL-8, and IFN-γ (22). Unfortunately, once a cancer patient reaches a cachectic state, few interventions are effective. However, early high protein intake has been shown to promote anabolism in cancer patients (23). Additionally, the use of various appetite stimulants have been investigated. The most notable are the progesterone analogues: megestrol acetate and medroxyprogesterone. Both are associated with improved quality of life, potentially minor weight gain, but no survival benefit (24, 25). Omega 3 polyunsaturated fatty acids (PUFAs) have also shown benefit in cancer patients to improve nutritional status and restore adaptive immunologic function, as well as, decrease inflammatory biomarkers (26). Similar to appetite stimulants, omega 3 PUFAs have been associated with improved quality of life among cancer patients, and positive treatment responses to anti-tumor medications (26). These findings make omega 3 PUFAs a potentially interesting nutritional component to augment standard of therapy when treating cancer patients with advanced disease.
Leucine is another anabolic agent that could serve as a nutritional adjunct to achieve optimal nitrogen balance for both cancer and CCI-PICS, as early high protein has proven to be of benefit (23). Leucine is an amino acid that stimulates anabolism through a special signaling pathway: mammalian Target of Rapamycin (mTOR) (27). Unfortunately, in the acute septic event mTOR is down regulated and relatively inactive to leucine (28). Teleologically, it makes sense not to be anabolic at a time of acute metabolic stress, but CCI-PICS patients already survived the acute septic insult. During the CCI phase (i.e. beyond 14 days), leucine and potential beta-hydroxy-beta-methylbutyrate (HMB: a metabolite of leucine) might help dampen, and even reverse, the catabolic state (29). Prolonged ICU stays only add chronicity to persistent catabolism, giving rise to ICU acquired weakness and the CCI-PICS phenotype (30). Leucine, alongside a regimen of Vitamin D and high protein, helps to preserve lean muscle mass in elderly patients (31). Leucine or HMB supplementation would hopefully increase muscle mass and strength to improve patients’ rehab potential, and restore some semblance of baseline function and independence once discharged from the ICU, as well as, possibly improve overall survival (32).
Lastly, similar to sepsis CCI-PICS survivors, sarcopenia is associated with low grade inflammation, catabolism, and anabolic resistance leading to frailty and poor long-term outcomes. It is this “inflammaging” described by Franceschi that contributes to age-related decline in functional status and increased morbidity and mortality (33). Anton et al. performed a meta-analysis to identify nutritional and pharmacologic interventions targeting chronic low-grade inflammation in older adults and determined that probiotics had the largest impact on decreasing IL-6 and CRP as biomarkers for inflammation. Angiotensin II receptor blockers and omega 3 fatty acids also significantly reduced these biomarkers, but to a lesser extent (34). In studies of non-critically ill and critically ill elderly patients, combined resistance exercise and higher protein diets (1.0 to 1.5 g/kg/d) are the most effective interventions (35). The Society of Sarcopenia, Cachexia, and Wasting Disease suggested that a combined approach of exercise and protein intake, possibly increasing supplemental leucine and creatine, and appropriate anabolic nutrition could at least slow the progression of sarcopenia in the aging population (36). A recent Protein Summit consensus statement recommended that protein supplementation as high as 1.2–2.5g/kg/d may be needed in the ICU setting to optimize nutrition, preserve muscle mass, and decrease mortality (37). Rondanelli et al. reported that in addition to exercise and protein supplementation, vitamin D and an improved omega 6:omega 3 PUFA ratio (either omega 3 supplementation directly or consumption of fish at least 4 times per week) had a positive impact on maintaining lean muscle mass in the elderly (38).
Omega 3 FA and Specialized Pro-Resolving Mediators (SPMs) offer potential benefits as direct nutritional adjuncts for CCI-PICS populations. These lipid mediators are thought to be the bioactive agent in the enzymatic conversion of Omega 3 PUFAs (39). They are further divided into three classes: resolvins, protectins, and maresins (macrophage-derived resolution mediators of inflammation). Resolvins are pluripotent inflammation resolving molecules that serve as an endogenous brake to inflammation through various mechanisms: decreasing neutrophil infiltration and diapedesis from post capillary venules, recruiting mononuclear cells, reducing platelet activation, decreasing adhesion molecules, and stimulating macrophage phagocytosis and efferocytosis (39, 40). However, this has not been described among surgical sepsis survivors. There is only one study of Medical ICU patients, which found that non-survivors after sepsis had higher levels of pro-inflammatory mediators compared with resolvins (41). It is now known that SPMs also promote tissue regeneration, reduce pain, increase clearance of cellular debris, and potentially limit ongoing organ injury by reducing fibrosis, which could help prevent CCI-PICS progression (42, 43). Serhan et al. stated, “New evidence is now available indicating that pathologic conditions associated with reduced SPMs can contribute to chronicity and magnitude of persistent inflammation” (44). It is within this context that we believe SPMs (specifically resolvins) could play a crucial role in thwarting the development of CCI-PICS by decreasing catabolism driven by persistent inflammation.
In addition to the recommendations above, standard ICU nutritional supplementation favors increasing protein doses to help offset the catabolic nature of critical illness. Studies have emerged showing benefit to early delivery of protein calories over non-protein supplements (45–48). The EBP for nutritional supplementation in our ICUs delivered high protein (1.5–2g/kg/d ideal body weight). Despite these efforts, to see maximal benefit, this needs to continue after enteral and parenteral nutrition ends and patients leave the ICU. This is exquisitely difficult to achieve solely based on a normal oral diet. Based on recent evidence, supplemental protein should be given throughout the day as four boluses. This stipulates at least 0.4g/kg/meal, but it could be helpful to increase to 0.55g/kg/meal, which correlates with 2.2g/kg/day (45, 47, 48). A recent protein summit consensus coincides with our prescription of protein, recommending 2g/kg/d for critically ill and persistently inflamed patients (37). In 2013, Wolfe and Deutz et al. described an “anabolic response” in which higher protein supplementation suppressed endogenous protein breakdown in a dose dependent manner (49). This has inordinate implications for CCI-PICS patients to combat catabolism by feeding them with high doses of protein.
Limitations:
This post hoc analysis has two primary limitations: 1) there is no power analysis as we included all CCI patients that had four weeks of nutritional data and survived during that study period, and 2) the four-week nutritional support calculation for RAP patients was not possible because these individuals were discharged from the ICU within 14 days. Nevertheless, the RAP cohort was treated with the same ICU nutrition protocols.
Conclusion:
Despite early, adequate, evidence-based ICU nutrition, septic patients who develop CCI exhibit 1) biomarker evidence of persistent inflammation, immunosuppression, catabolism (PICS), and stress metabolism, 2) poor long-term functional status with 3) worse discharge disposition and long-term survival. Although current evidence-based ICU nutrition may improve short-term ICU outcomes, novel adjuncts are needed to improve long-term outcomes for this epidemic of vulnerable CCI-PICS patients.
Supplementary Material
Clinical Relevancy Statement:
This manuscript describes the importance of developing new strategies to provide optimal nutrition for a growing population of CCI and PICS patients. Most level one evidence for ICU nutrition corresponds to the acute critical illness phase, i.e. patients remain in the ICU for several days or weeks, but are discharged to a good disposition. Unfortunately, providing nutritional support to patients that develop CCI is limited and there is no evidence for adjunctive therapy in patients that develop PICS. This manuscript provides evidence that current evidenced-based protocols need to be re-modeled to optimize nutritional therapies and adjuncts for patients that develop CCI and PICS.
Acknowledgements:
The investigators acknowledge the contribution of the Center for Sepsis and Critical Illness Award # P50 GM-111152 from the National Institute of General Medical Sciences (NIGMS), as well as, the hard work by each contributing author.
Abbreviations:
- AKI
Acute Kidney Injury
- CCI
Chronic Critical Illness
- EEN
Early Enteral Nutrition
- EMR
Electronic Medical Record
- EBG
Evidence-Based Guidelines
- EBPs
Evidence-Based Protocols
- ICU
Intensive Care Unit
- LTAC
Long-Term Acute Care
- MOF
Multiple Organ Failure
- PN
Parenteral Nutrition
- PICS
Persistent Inflammation Immunosuppression Catabolic Syndrome
- RAP
Rapid Recovery
- SCIRC
Sepsis Critical Illness Research Center
- SOFA
Sequential Organ Failure Assessment
- SPPB
Short Physical Performance Battery
- SNFs
Skilled Nursing Facilities
- SPMs
Specialized Pro-Resolving Mediators
- SOPs
Standard Operating Procedures
- UF
University of Florida
Footnotes
Conflict of Interest and Financial Disclosure Statement: No conflict of or competing interests have been declared and there were no funds provided for this manuscript.
References
- 1.Horiguchi H, Loftus TJ, Hawkins RB, Raymond SL, Stortz JA, Hollen MK, Weiss BP, Miller ES, Bihorac A, Larson SD, et al. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front Immunol. 2018;9:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Stortz JA, Brumback BA, Bihorac A, Segal MS, Anton SD, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loftus TJ, Mira JC, Stortz JA, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Brumback BA, Ungaro RF, Bihorac A, Leeuwenburgh C, et al. Persistent inflammation and anemia among critically ill septic patients. The journal of trauma and acute care surgery. 2019;86(2):260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mira JC, Cuschieri J, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Loftus TJ, Stortz JA, Raymond SL, Lanz JD, Hennessy LV, et al. The Epidemiology of Chronic Critical Illness After Severe Traumatic Injury at Two Level-One Trauma Centers. Critical care medicine. 2017;45(12):1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Leeuwenburgh C, Segal MS, Bihorac A, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. The journal of trauma and acute care surgery. 2018;84(2):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, Nacionales DC, Loftus TJ, Wang Z, Ozrazgat-Baslanti T, et al. Evidence for Persistent Immune Suppression in Patients Who Develop Chronic Critical Illness After Sepsis. Shock. 2018;49(3):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner AK, Ghita GL, Wang Z, Ozrazgat-Baslanti T, Raymond SL, Mankowski RT, Brumback BA, Efron PA, Bihorac A, Moore FA, et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival Among Early Survivors of Sepsis in Surgical ICUs. Critical care medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal M, Gabrielli A, Moore F. The evolution of nutritional support in long term ICU patients: from multisystem organ failure to persistent inflammation immunosuppression catabolism syndrome. Minerva anestesiologica. 2016;82(1):84–96. [PubMed] [Google Scholar]
- 9.McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. 2016;14(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinglas VD, Gifford JM, Husain N, Colantuoni E, Needham DM. Quality of life before intensive care using EQ-5D: patient versus proxy responses. Critical care medicine. 2013;41(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, Thibault R, Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–93. [DOI] [PubMed] [Google Scholar]
- 12.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN Journal of parenteral and enteral nutrition. 2016;40(2):159–211. [DOI] [PubMed] [Google Scholar]
- 13.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Critical care. 2011;15(6):R268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner AK, Ghita GL, Wang Z, Ozrazgat-Baslanti T, Raymond SL, Mankowski RT, Brumback BA, Efron PA, Bihorac A, Moore FA, et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival Among Early Survivors of Sepsis in Surgical ICUs. Critical care medicine. 2019;47(4):566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guirgis FW, Brakenridge S, Sutchu S, Khadpe JD, Robinson T, Westenbarger R, Topp ST, Kalynych CJ, Reynolds J, Dodani S, et al. The long-term burden of severe sepsis and septic shock: Sepsis recidivism and organ dysfunction. The journal of trauma and acute care surgery. 2016;81(3):525–32. [DOI] [PubMed] [Google Scholar]
- 16.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Critical care medicine. 2017;45(2):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal MD, Kamel AY, Rosenthal CM, Brakenridge S, Croft CA, Moore FA. Chronic Critical Illness: Application of What We Know. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2018;33(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–902. [DOI] [PubMed] [Google Scholar]
- 19.Porro LJ, Herndon DN, Rodriguez NA, Jennings K, Klein GL, Mlcak RP, Meyer WJ, Lee JO, Suman OE, Finnerty CC. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. Journal of the American College of Surgeons. 2012;214(4):489–502; discussion −4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. The New England journal of medicine. 2001;345(17):1223–9. [DOI] [PubMed] [Google Scholar]
- 21.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. Journal of applied physiology. 2001;91(3):1168–75. [DOI] [PubMed] [Google Scholar]
- 22.Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, et al. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Annals of surgery. 2017;265(4):827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, Erickson N, Laviano A, Lisanti MP, Lobo DN, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clinical nutrition. 2017;36(5):1187–96. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, Panzone F, Contu P. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15(2):200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz Garcia V, Lopez-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. The Cochrane database of systematic reviews. 2013(3):CD004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Liu L, Zhang Y, Wei J, Yang F. Effects of omega-3 fatty acids on patients undergoing surgery for gastrointestinal malignancy: a systematic review and meta-analysis. BMC Cancer. 2017;17(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beugnet A, Tee AR, Taylor PM, Proud CG. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem J. 2003;372(Pt 2):555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laufenberg LJ, Pruznak AM, Navaratnarajah M, Lang CH. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids. 2014;46(12):2787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holecek M Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. 2017;8(4):529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal MD, Rosenthal CM, Moore FA, Martindale RG. Persistent, Immunosuppression, Inflammation, Catabolism Syndrome and Diaphragmatic Dysfunction. Current Pulmonology Reports. 2017;6(1):54–7. [Google Scholar]
- 31.Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. The American journal of clinical nutrition. 2015;101(2):279–86. [DOI] [PubMed] [Google Scholar]
- 32.Delphan M, Lin T, Liesenfeld DB, Nattenmuller J, Bohm JT, Gigic B, Habermann N, Zielske L, Schrotz-King P, Schneider M, et al. Associations of branched-chain amino acids with parameters of energy balance and survival in colorectal cancer patients: Results from the ColoCare Study. Metabolomics. 2018;2018(14):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S4–9. [DOI] [PubMed] [Google Scholar]
- 34.Custodero C, Mankowski RT, Lee SA, Chen Z, Wu S, Manini TM, Hincapie Echeverri J, Sabba C, Beavers DP, Cauley JA, et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res Rev. 2018;46:42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anton SD, Hida A, Mankowski R, Layne A, Solberg LM, Mainous AG, Buford T. Nutrition and Exercise in Sarcopenia. Curr Protein Pept Sci. 2018;19(7):649–67. [DOI] [PubMed] [Google Scholar]
- 36.Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, Doehner W, Fearon KC, Ferrucci L, Hellerstein MK, et al. Nutritional recommendations for the management of sarcopenia. Journal of the American Medical Directors Association. 2010; 11(6):391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurt RT, McClave SA, Martindale RG, Ochoa Gautier JB, Coss-Bu JA, Dickerson RN, Heyland DK, Hoffer LJ, Moore FA, Morris CR, et al. Summary Points and Consensus Recommendations From the International Protein Summit. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2017;32(1_suppl):142S–51S. [DOI] [PubMed] [Google Scholar]
- 38.Rondanelli M, Faliva M, Monteferrario F, Peroni G, Repaci E, Allieri F, Perna S. Novel insights on nutrient management of sarcopenia in elderly. Biomed Res Int. 2015;2015:524948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenthal MD, Patel J, Staton K, Martindale RG, Moore FA, Upchurch GR Jr. Can Specialized Pro-resolving Mediators Deliver Benefit Originally Expected from Fish Oil? Current gastroenterology reports. 2018;20(9):40. [DOI] [PubMed] [Google Scholar]
- 40.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3:1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalli J, Colas RA, Quintana C, Barragan-Bradford D, Hurwitz S, Levy BD, Choi AM, Serhan CN, Baron RM. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations With Survival and Clinical Outcomes. Critical care medicine. 2017;45(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26(4):1755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. Journal of immunology. 2006;177(9):5902–11. [DOI] [PubMed] [Google Scholar]
- 44.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weijs PJ, Cynober L, DeLegge M, Kreymann G, Wernerman J, Wolfe RR. Proteins and amino acids are fundamental to optimal nutrition support in critically ill patients. Critical care. 2014;18(6):591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weijs PJ, Looijaard WG, Beishuizen A, Girbes AR, Oudemans-van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Critical care. 2014;18(6):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Compher C, Chittams J, Sammarco T, Nicolo M, Heyland DK. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit Care Med. 2017;45(2):156–63. [DOI] [PubMed] [Google Scholar]
- 48.Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C. Clinical Outcomes Related to Protein Delivery in a Critically Ill Population: A Multicenter, Multinational Observation Study. JPEN Journal of parenteral and enteral nutrition. 2016;40(1):45–51. [DOI] [PubMed] [Google Scholar]
- 49.Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clinical nutrition. 2013;32(2):309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


