Abstract
Epilepsy is the fourth most common neurological disorder, but current treatment options provide limited efficacy and carry the potential for problematic side effects. There is an immense need to develop new therapeutic interventions in epilepsy, and targeting areas outside the seizure focus for neuromodulation has shown therapeutic value. While not traditionally associated with epilepsy, anatomical, clinical, and electrophysiological studies suggest the cerebellum can play a role in seizure networks, and importantly, may be a potential therapeutic target for seizure control. However, previous interventions targeting the cerebellum in both preclinical and clinical studies have produced mixed effects on seizures. These inconsistent results may be due in part to the lack of specificity inherent with open-loop electrical stimulation interventions. More recent studies, using more targeted closed-loop optogenetic approaches, suggest the possibility of robust seizure inhibition via cerebellar modulation for a range of seizure types. Therefore, while the mechanisms of cerebellar inhibition of seizures have yet to be fully elucidated, the cerebellum should be thoroughly revisited as a potential target for therapeutic intervention in epilepsy.
Keywords: Channelrhodopsin, halorhodopsin, temporal lobe epilepsy, deep brain stimulation, fastigial nucleus, thalamus
1. Introduction
Roughly one in 27 people will develop epilepsy at some point in their lifetime, yet almost half of all epilepsy patients do not experience sufficient seizure relief with current treatment options [1]. There is a clear need for the development of new therapeutic interventions in epilepsy. Many current efforts focus on deep brain and on-demand stimulation for seizure control [2–6]. While some stimulation approaches target the seizure focus directly, stimulation of brain areas outside the seizure focus has also shown promise [3, 7–10]; one such area of interest is the cerebellum [11]. There is evidence to suggest the cerebellum is engaged during seizures [12–19], and cerebellar impairments have been observed in patients with epilepsy [20–23]. While early research outlines an unclear and potentially inconsistent relationship between the cerebellum and seizures, more recent work has renewed enthusiasm in the cerebellum as a promising target. In the present review, we discuss evidence for a role for the cerebellum in epilepsy and seizures, early animal work and clinical trials in which the cerebellar cortex was electrically stimulated, and recent studies using targeted optogenetic approaches in animal models of epilepsy. We argue that the increased specificity offered by on-demand optogenetic approaches in timing, direction of modulation, and cell populations engaged improves interpretability of results and may account for the more robust inhibition of seizures achieved with cerebellar modulation. While the precise mechanisms of cerebellar influence over seizures have yet to be fully elucidated, there is a clear rationale to revisit the cerebellum as a potential therapeutic target for intervention in epilepsy.
2. The cerebellum and seizure networks
2.1. Cerebellar changes associated with epilepsy
The cerebellum has not been traditionally associated with epilepsy or seizures, likely in part due to early studies conducted in the 1930s, in which electrical stimulation of the cerebellum did not readily produce seizures [24]. However, sufficient data has accumulated in the decades (nearly a century) since those original studies to firmly show that this view point needs to be updated. Recognizing a potential role for the cerebellum in epilepsy and seizures can provide a fuller understanding of seizure networks and the condition more broadly, and, importantly, ultimately provide new avenues to treatment.
Alterations in the cerebellum have been routinely noted with epilepsy, including disrupted functional and structural connectivity [25–28], changes in volume [29–37], and altered perfusion [38]. For example, atrophy of Purkinje cells (Box 1) is observed in postmortem tissue of patients with chronic epilepsy [39, 40]. Drug exposure alone cannot fully explain cerebellar alterations in epilepsy, as animal studies also reveal cerebellar alterations. A recent study examined structural alterations across the entire brain in two different rodent models of acoustically evoked seizures (the Genetically Epilepsy Prone Rat and the Wistar Audiogenic Rat models), to examine if there were any anatomical areas of overlap (i.e., any convergence in pathology) [33]. Interestingly, the cerebellum (specifically, the midline cerebellum) was the only area of structural changes unifying these two rodent models, highlighting the potential importance of the cerebellum to the observed epilepsy phenotype [33]. This data suggests that other observations of structural changes in the cerebellum in epilepsy are unlikely to be one-off or incidental findings. Rather, changes in the cerebellum may be a uniquely unifying feature across epilepsies.
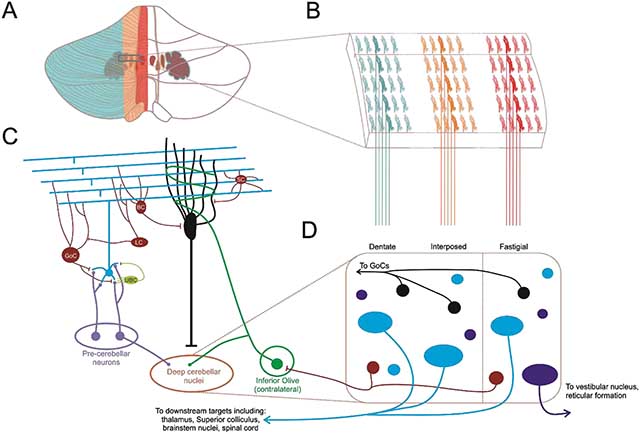
Box 1. Overview of cerebellar circuitry.
The cerebellum contains roughly 80% of the neurons in the central nervous system [165]. Classically considered a motor control structure, the cerebellum is essential for the production of smooth continuous movements [166] as well as the processing and correction of motor errors [167], but accumulating evidence suggests it also plays a role in higher order functions and cognitive processes [145, 146]. Located inferior to the occipital cortex and dorsal to the pons and medulla, the cerebellum integrates inputs in the cerebellar cortex (illustrated in panel A, modified from [166]), which generally exhibits a high degree of homogeneity in terms of cytoarchitecture [168], and sends outputs to downstream structures in the deep cerebellar nuclei (though Purkinje cells have been noted to project to other areas including the locus coeruleus [138, 156]). Note that integration also likely occurs in the nuclei, which receive collateral input from fibers projecting to the cerebellar cortex. The cerebellum (both the cerebellar cortex and the cerebellar nuclei) receives inputs via mossy fibers originating from a large number of sites including the spinal cord, brainstem nuclei, and cerebral cortex (including motor and sensory areas) via the pontine nuclei and reticular formation (light purple in panel C) [109, 127, 169]. These ascending mossy fibers synapse onto cerebellar granule cells (blue in panel C). Granule cells provide the first of two major excitatory synaptic inputs onto Purkinje cells (black in panel C). Axons from granule cells ascend into the molecular layer of the cerebellar cortex, where they then bifurcate and form parallel fibers. The parallel fibers then pass orthogonally through the expansive but almost two dimensional dendritic trees of Purkinje cells, which are oriented sagittally along the cerebellar cortex ([109, 169], illustrated in panel B). Purkinje cells also receive powerful synaptic inputs from climbing fibers originating in the contralateral inferior olive (green in panel C) [170]. There are several types of inhibitory interneurons within the cerebellar cortex (red in panel C). For example, Golgi cells receive excitatory inputs from parallel fibers and inhibit granule cells. Two other populations, Basket and stellate cells, provide inhibitory input to Purkinje cells. Other populations of both inhibitory and excitatory interneurons exist in the cerebellar cortex including lugaro cells and unipolar brush cells, respectively ([159] Panel C). Either directly or indirectly, these neurons shape the high frequency (50 to 150 spikes/s) simple spike output of Purkinje cells. Importantly, Purkinje cells sustain a high (~60Hz) baseline firing rate due to intrinsic channel conductances [171].
Purkinje cells project to and inhibit the deep cerebellar nuclei, which exhibit a far more heterogeneous cytoarchitecture and contain both local and projection excitatory and inhibitory neurons, many of which have yet to be fully characterized (illustrated in panel D). All four of the deep cerebellar nuclei: fastigial, globose and emboliform (often referred to together as the interposed nucleus), and dentate contain glutamatergic projection neurons (blue in panel D) which send excitatory output to numerous downstream structures including descending motor tracts, the cerebral cortex via the thalamus, the superior colliculus, and brainstem nuclei such as those of the reticular formation. The fastigial nucleus also contains a unique population of large, glycinergic projection neurons (purple in the panel D), which project to and inhibit ipsilateral brainstem and vestibular nuclei [155]. The deep cerebellar nuclei also contain a population of GABAergic projection neurons (red in panel D), which send inhibitory input to the inferior olive, creating a closed loop along with climbing fiber input from the inferior olive to cerebellar cortex [172–174]. GABA/Glycine co-releasing nucleocortical neurons (black) provide an inhibitory feedback loop back to the cerebellar cortex [175]. There is also a population of glutamatergic neurons that project to the cerebellar cortex via mossy fibers [176]. Finally, two populations of local interneurons have been identified: GABA/glycinergic inhibitory and small glutamatergic interneurons [154].
Functionally, the cerebellum exhibits a longitudinal organization, in which Purkinje cells integrate specific afferent inputs and project to a specific deep cerebellar nucleus (fastigial, interposed, or dentate) depending on where the Purkinje cells are positioned on the medial-lateral plane (panels A-B). Purkinje cells in and near the midline (vermis) project to and inhibit the fastigial deep cerebellar nucleus (outlined in red in the figure) as well as the vestibular nucleus; Purkinje cells in the cerebellar hemispheres predominantly project to the dentate nucleus (outlined in blue), and Purkinje cells in the intermediate regions between the vermis and lateral hemispheres predominantly project to the interposed nuclei (outlined in yellow) [109]. Classically, the midline cerebellum is considered to be part of the functional region known as the vestibulocerebellum, essential for maintenance of balance and equilibrium and coordinating eye movements [166]. Emerging evidence indicates a role for the vermis beyond these functions, however, and the midline cerebellum has also been associated with limbic structures [177]. The intermediate zones, along with portions of the midline are considered part of the spinocerebellum, contributing to fine control of limb movements [166]. The lateral cerebellar hemispheres are enlarged in humans and nonhuman primates and are considered part of the cerebrocerebellum due to the large number of feedback loops formed by inputs from cortex via pontine nuclei and outputs via the thalamus [166]. However, the full spectrum of downstream structures targeted by deep cerebellar nuclear outputs have not been characterized, and there is a large amount of evidence suggesting the cerebellum and its different subregions are highly engaged in processes beyond those outlined here.
It is important to emphasize that changes in the cerebellum are found in human patients, and that these alterations can have major implications for patient welfare. A recent study [29] found substantial gray matter loss in the cerebellum in SUDEP (sudden unexpected death in epilepsy) cases prior to the SUDEP event compared to healthy controls and low-risk patients. Additionally, these findings remained when controlling for phenytoin use, indicating that it is unlikely to simply reflect exposure to antiseizure drugs. Subjects at high risk of SUDEP also had reduced gray matter volume, specifically in the midline cerebellum (i.e., vermis). Therefore, there are structural changes in epilepsy in the cerebellum that cannot be fully explained by drug use and, importantly, can have major consequences for the patient.
There is also evidence to suggest a relationship between seizures themselves and cerebellar activity. An increase in cerebellar blood flow during seizures has been noted in patients with temporal lobe epilepsy [41], frontal lobe epilepsy [42, 43], and other partial epilepsies [38, 44]. Experimentally, increased metabolic activity in the cerebellum has also been reported for seizures induced by administration of PTZ [45, 46], focal penicillin [47], or electrical stimulation [48, 49]. Moreover, cerebellar neuronal activity can be correlated (including phase locked spiking) with seizure activity across a range of seizure types, including spontaneous hippocampal seizures occurring in the intrahippocampal kainate model [50], electrically induced hippocampal [15, 51] or cortical [52] seizure events, spike and wave events in rodent models of absence epilepsy [13, 14], and neocortical seizures occurring after penicillin injection [16–19, 53]. Altered firing of cerebellar neurons during seizure events has been observed prior to overt motor manifestations of seizures [50], as well as during absence seizures [14], arguing against the interpretation that cerebellar engagement simply reflects motor aspects of seizures. Cerebellar engagement during seizures is also not limited to animal models. Similar results have been observed during recordings from the deep cerebellar nuclei of human patients with epilepsy, including with cerebellar engagement occurring prior to or without generalization in two patients [24]. A third patient showed synchronous spike and wave activity in the cerebral and cerebellar nuclei leads, reinforcing the idea that cerebellar engagement can occur in a range of seizure types [24].
Not only can the cerebellum participate in seizure events, there are also noted clinical cases where the seizure focus itself appears to have been within the cerebellum, typically in patients with cerebellar lesions or tumors [54–63]. Additionally, injection of ouabain (an inhibitor of Na/K-ATPase) into the cerebellar cortex or injection of picrotoxin (a noncompetitive antagonist of GABAA receptors) into the fastigial nuclei of rats has been reported to induce seizures which progress to generalized tonic-clonic seizures [64, 65]. Similarly, mice genetically prone to spike and wave discharges showed an increase in absence seizure frequency when muscimol (a GABAA agonist) was injected into the cerebellar nuclei [66], and mice with a loss of P/Q channels in the cerebellum display absence epilepsy [67, 68]. Together these data point to the cerebellum not only as a passive participant in seizures, but also as a possible driver of seizure activity, across a range of seizure phenotypes.
Clearly, there is now ample evidence for the involvement of the cerebellum not only in epilepsy, but also in seizure networks. Therefore, the classical view that the cerebellum is one of the few brain structures that does not seize needs to be updated.
2.2. Conventional cerebellar modulation and seizure control
Not only does research suggest that the cerebellum can be engaged during seizures, other work suggests that cerebellar interventions may be able to inhibit ictal activity.
At the most crude level, the effects of cerebellectomy or cerebellar cortical lesions on seizures have been investigated. This work, perhaps unsurprisingly given the nature of the intervention, has produced mixed results. Some studies, examining the impact of cerebellectomy on penicillin-induced seizures, report an increase in the frequency of seizures [69, 70]. This finding can be interpreted as reflecting a native seizure-suppressive role of the cerebellum [24, 71]. However, other studies found no effect of cerebellectomy on epileptiform activity induced by penicillin [72] or electrical stimulation [73]. Additionally, lesions to either dorsal or ventral aspects of the cerebellar dentate nucleus have also been reported to decrease seizures in human [74]. These conflicting results are difficult to interpret. Experiments with more refined intervention methods are likely to provide greater insights.
Several early studies dating back to the 1950s electrically targeted the cerebellum in animal models of epilepsy to assess whether cerebellar stimulation could disrupt seizure events (Table 1). Here too, however, experimental data is rife with conflicting results. For example, in some studies, seizures induced by cobalt application [75–77], penicillin [70, 78, 79], PTZ [80], or electrical stimulation of the hippocampus, amygdala, or cortex [81–85] were suppressed by stimulation of either the midline cerebellar cortex [76, 77, 82–84] or deep cerebellar nuclei [75, 84, 86]. However, others found that cerebellar stimulation instead had no effect or actually evoked seizures or prolonged seizure duration for cobalt [75, 86, 87], alumina-gel [88, 89], penicillin [79, 90], PTZ [90], hyperbaric oxygen [91], or electrically evoked seizures [83, 92–94]. The widespread nature of these conflicting results suggest that differences in the model or type of seizure were unlikely to underlie differences in results. However, methodological differences may have been key -- a range of stimulation parameters, locations, and electrode configurations were used. The broad range and combinations of these variables, and the unfortunate situation that these variables were not consistently reported, make it difficult to glean insight by comparing results across studies. Additionally, all of these studies examining the impact of electrical stimulation of the cerebellum on seizures were done in an open-loop, rather than on-demand, manner, which creates another important experimental caveat.
Table 1:
Summary of cerebellar stimulation studies in animal models of epilepsy. Abbreviations: pw = pulse width.
| Study | Target | Number of patients | Stimulation protocols | Outcome |
|---|---|---|---|---|
| Bidzinski et. al, 1982 | Hemispheres | 14 | 1–7V; 10Hz; 1msec pw; 30min-1hr duration | 5 patients seizure free, 6 patients with reduced seizures, 2 patients with slight improvement, 1 patient with no change |
| Chkhenkeli et. al, 2004 | Dentate nucleus | 54 | 6–8mA; 50–100Hz; 2msec pw; 10sec epochs | >50% reduction in seizures |
| Cooper et. al, 1973 | Cerebellar cortex | 32 | 1msec pw; other parameters not reported | Over half of patients had >50% reduction in seizure frequency |
| Davis et. al, 1983 | Midline cerebellar cortex | 32 | 10–180Hz; other parameters not reported | 19 patients seizure free, 8 patients with reduced seizures, 4 patients with no change, 1 patient with increased seizures |
| Davis et. al, 1992 | Midline cerebellar cortex | 30 | 1–1.4mA; 150Hz; 0.5msec pw | >50% patients seizure free |
| Gilman et. al, 1977 | Anterior lobe, hemispheres | 6 | 10Hz; 1msec pw | 5 patients with reduced seizures |
| Klun et, al, 1987 | Hemispheres | 6 | 5–50Hz; 0.6msec pw | 3 patients seizure free, 3 patients with reduced seizures |
| Levy et. al, 1979 | Not given | 6 | 3–10V; 10Hz | 3 patients with reduced seizures, 2 patients with no change, 1 patient with increased seizures |
| Sramka et. al, 1976 | Dentate nucleus | 4 | 10V; 10 or 100Hz; 1msec pw; 3 min duration | Temporary improvement |
| Van Buren et. al, 1978 | Cerebellar cortex | 5 | Range | No change |
| Velasco et. al, 2005 | Midline cerebellar cortex | 5 | 3.8mA; 10Hz; 0.45msec pw | 41% mean decrease in seizure frequency |
| Wright et. al, 1984 | Cerebellar cortex | 12 | 7mA; 10Hz | No change |
Even when the work utilized the same models and was reported by the same authors in the same publication, mixed results could occur. In some cases, however, these divergent results begin to provide some potential insight into the mixed results in the literature. For example, Maiti and colleagues reported that electrically induced seizures could be inhibited by stimulation of the cerebellar cortex, but were exacerbated by stimulation targeting the downstream deep cerebellar nucleus [34]. Similarly, Godlevski and colleagues report that high frequency stimulation of the cerebellar cortex could inhibit ictal events while low frequency stimulation of the same area facilitated ictal discharges [95]. This suggests that successful interventions may be a complex interaction between stimulation protocol (e.g. high versus low frequency stimulation) and intervention location (e.g. cerebellar cortex versus nuclei).
Electrical stimulation of the cerebellum for epilepsy treatment has also been examined in human clinical trials (Table 2). As with the animal work, electrical stimulation of the cerebellum in humans has been done entirely with open-loop approaches, where stimulation occurred without regard for ictal or interictal state. Also as seen with animal work, electrical stimulation for seizure suppression in human patients has been done with a variety of methods, and produced very mixed results. Offering hope, in one study, a majority of patients (71%) were seizure free after >10 years of cerebellar cortical stimulation [96]. Another study showed cerebellar cortical stimulation produced a greater than 50% reduction in seizure frequency in over half of patients tested [97]. Similarly, a different study reported stimulation of the anterior lobules and cerebellar hemispheres resulted in a significant decrease in seizure frequency in 5 out of 6 patients tested [98], other studies reported that targeting the dentate nuclei was also effective at reducing seizure frequency [99, 100], and separate studies examining chronic cerebellar stimulation reported seizure freedom in at least half of patients [101, 102].
Table 2:
Summary of human cerebellar stimulation studies for the treatment of epilepsy.
| Study | Target | Epilepsy model | Stimulation protocols | Electrode information | Animal model | Results |
|---|---|---|---|---|---|---|
| Babb et. al, 1974 | Vermis, fastigial, dentate | Cobalt: Hippocampus | 1.0mA; 45Hz; 0.6msec pw | 1mm between tips | Cat | Fastigial vermal stimulation inhibited epileptic activity. Dentate could either inhibit or prolong seizures |
| Cooke et. al, 1955 | Cerebellar cortex and nuclei | Electrical stimulation: cortex | 40V; 20–300Hz; 5–10sec duration | Not stated | Cat | Stimulation could either inhibit or prolong epileptic activity |
| Dauth et. al, 1974 | Vermis | Cholarose, electrical stimulation: cortex | 3–5mA; 200Hz; 1msec pw; 0.2–10sec duration | Not stated | Cat | Inhibition of epileptic activity |
| Dow et. al, 1962 | Lobules V-VII | Cobalt: Cortex | 1–5V; 20–50 or 200–400Hz; 0.3–1 msec pw; 1–3sec duration | Not stated | Rat | Inhibition of epileptic activity |
| Fanardjian et. al, 1963 | Not stated | Electrical stimulaiton: Hippocampus | 50V; 300Hz; 0.1msec pw | Not stated | Cat | Stimulation could inhibit epileptic activity |
| Godlevsky et. al, 2004 | Nodulus, uvula | Penicillin: Systemic | 10–12Hz or 100– 300Hz; 0.5msec pw or 0.25msec pw | 0.12mm diameter 1.0mm between tips | Rat | Low frequency stimulation evoked seizures, higher frequency stimulation inhibited seizures |
| Grimm et. al. 1970 | Fastigial, dentate | Cobalt: Cortex | 0.6–0.9V; 250–300Hz; 1msec pw | Not stated | Monkey | Failed to inhibit epileptic activity |
| Hablitz, 1975 | Vermis, hemispheres | Alumina-Gel: Cortex | 1–10V; 5–15Hz or 100Hz; 1msec pw, 1–30sec duration | 5mm diameter | Monkey | Low frequency stimulation failed to inhibit seizures, higher frequency stimulation evoked seizures |
| Hablitz, 1976 | Vermis | Penicillin: Systemic | 0.25–2.0mA; 10 or 100Hz; 1msec pw; 10 sec duration | 3mm diameter | Cat | Inhibition of epileptic activity |
| Hemmy et. al, 1977 | Hemispheres, dentate nucleus | Electrical stimulation: cortex | 10mA; 4–100Hz; 1msec pw; | 1mm diameter disc electrodes (cortex); bipolar electrodes (denate) | Monkey | Failed to inhibit epileptic activity |
| Hutton et. al, 1972 | Vermis, paramedian lobules, dentate | Penicillin: Cortex | 0.3–5V; 200Hz | Not stated | Cat | Inhibition of epileptic activity |
| Iwata et. al, 1959 | Vermis | Electrical stimulation: Hippocampus | 5–15V; 30–100Hz; 1msec pw; 30sec duration | Not stated | Cat | Inhibition of epileptic activity |
| Kreindler, 1962 | Paleo and neocerebellum | Penicilin: Cortex | 0.25–3mA; 2.5Hz; 1msec pw | Not stated | Cat | Stimulation could either inhibit or prolong epileptic activity |
| Lockard et. al, 1979 | Vermis | Alumina-Gel: Cortex | 2.0mA; 10Hz; 1msec pw; 10min duration | 3 electrodes, 2mm apart | Monkey | Increased seizure frequency, decreased interictal bursting |
| Maiti et. al, 1975 | Vermis, fastigial | Electrical stimulation: Hippocampus and amygdala | 5–12V; 1–10, 30–200Hz; 0.1–1msec pw; 5–15sec duration | Not stated | Monkey, Cat | Vermal stimulation inhibited epileptic activity, fastigial stimulation prolonged epileptic activity |
| Mutani et. al, 1969 | Vermis lobules III-V | Cobalt: Amygdala and Hippocampus | 6–8V; 100Hz; 0.6msec pw; 1 second duration | 1–2mm between tips | Cat | Inhibition of epileptic activity |
| Myers et. al, 1975 | Paleo and neocerebellum | Penicillin, pTZ, enflurane, cholarose | 8V; 1–250Hz | 2mm between tips | Cat | Failed to inhibit epileptic activity |
| Reimer et. al, 1967 | Vermis, hemispheres | Cobalt: Cortex | 1–7.5V; 4–300Hz; 0.1msec pw; 1–10sec duration | 1mm between tips | Cat | Initiated or prolonged epileptic activity |
| Rucci et. al, 1968 | Anterior lobules | Hyperbaric oxygen | 4–5V; 30–300Hz; 1msec pw | Not stated | Rat | Stimulation could either inhibit or prolong epileptic activity |
| Snider et. al, 1974 | Anterior lobules, fastigial | Electrical stimulation: Cortex and Hippocampus | 0.5–3mA; 8–300Hz; 5sec duration | Not stated | Monkey | Inhibition of epileptic activity |
| Wada et. al, 1974 | Cerebellar cortex | Electrical stimulation: amygdala | Not stated | Monkey | Failed to inhibit epileptic activity |
While these successful studies suggest promise of targeting the cerebellum in epilepsy, other studies produced more mixed effects, with some patients showing no change or even increased seizure frequency with stimulation of the cerebellar cortex [101, 103–105] (Table 2). Double blind clinical trials in patients with both partial and generalized seizures showed no significant effect of electrical stimulation of the superior cerebellar cortex on the frequency of behavioral seizures [106, 107], seizure severity [107] or patient EEG [106]. Therefore, moving forward, it will be important to have a clearer picture of the source(s) of the disparate findings, to allow successful, robust inhibition of seizures in the future.
3. Potential contributions to heterogeneous effects of electrical stimulation
Why might these initial animal and clinical studies have produced such mixed effects? We focus on four sources of complexity in interpreting the results of studies using cerebellar stimulation to inhibit seizures: 1) lack of cell-type specificity and 2) lack of direction of modulation specificity, combined with 3) disparate experimental/intervention variables, and finally, 4) lack of temporal specificity.
3.1. Lack of cell type specificity
One major limitation of electrical stimulation is that it is often unclear which cell types are being modulated and how. Electrical stimulation can alter activity in local excitatory or inhibitory cells, efferent axons, afferent inputs, or combinations of these depending in part on stimulation parameters used. The effects of the electrical stimulation utilized in previous studies on cerebellar activity are thus highly difficult to interpret. In the case of electrical stimulation applied to the cerebellar cortex, the activity of Purkinje cells can be directly modulated, as can the activity of inhibitory stellate, basket, golgi, and lugaro cells (Box 1provides a brief overview of cerebellar anatomy). Additionally, parallel fibers, climbing fibers, and even mossy fibers can be impacted. Similarly, both excitatory and inhibitory input from cerebellar nuclei to the cortex can be altered, as could axons from neuromodulatory regions such as the locus coeruleus or raphe nuclei. Antidromic spiking can therefore also produce changes in nuclear neurons (directly), brain regions like the locus coeruleus or raphe which could produce neuromodulatory changes in other brain regions, the inferior olives (which provide climbing fiber inputs to the cerebellar cortex), and pontine neurons providing mossy fiber inputs to the cerebellum. To further complicate matters, climbing fiber inputs to Purkinje cells produce complex spikes, which can have longer term consequences due to synaptic plasticity and other potential mechanisms (for review, see [108]). Additionally, both inferior olive input and mossy fibers have collaterals to the cerebellar nuclei, providing an additional potential route for influence over cerebellar output that would need to be considered when electrically stimulating the cerebellar cortex.
Similar heterogeneous effects are possible when targeting the deep cerebellar nuclei, which contain several cell types, including excitatory projection neurons, inhibitory projection neurons, excitatory and inhibitory interneurons, as well as mossy fiber, climbing fiber, neuromodulatory, and Purkinje cell inputs, all of which might be affected by electrical stimulation (Box 1, Panel C&D). Increasing the specificity of intervention can improve the interpretability of results, and has the potential to provide needed insights into when or how cerebellar modulation can be an effective strategy for seizure control.
3.2. Direction of modulation
Electrical stimulation often produces mixed effects on excitability, in part due to the lack of cell-type specificity discussed above. Without a clear picture of the effects of stimulation, results are difficult to interpret, and the direction of modulation of cerebellar neurons may be critical for effective intervention. In the case of cerebellar cortical stimulation, differences in electrode position, orientation, or stimulation parameters can have differing effects on Purkinje cell firing [109]. For example, a study found that Purkinje cell responses to electrical stimulation at a variety of frequencies and train durations can be excitatory, inhibitory, or more complex alternating patterns of activation and suppression based on the distance from the stimulating electrode and Purkinje cell recorded [110, 111]. Interestingly, 0.5 Hz surface stimulation failed to produce pure activation in any Purkinje cells recorded [111]. Similar mixed effects are also likely when targeting the deep cerebellar nuclei, especially given the more heterogeneous cytoarchitecture as compared to the cerebellar cortex (See Box 1). The complexity of responses to electrical stimulations likely contributes to the observations that different stimulation parameters can inhibit or exacerbate seizures in the same animal models [95, 112]. For example, while 0.5 Hz stimulation of the cerebellar cortex can produce a mix of suppression and more complex changes in firing, 10 Hz stimulation produces suppression in almost all Purkinje cells recorded [111]. In this view, the mixed results of cerebellar stimulation in both animal models and human patients could be due to heterogeneous effects of electrical stimulation on activity. Increasing the specificity of intervention to evoke either pure excitation or inhibition (in the desired cell populations), for example with techniques such as optogenetics, may not only provide greater insight, it may also allow consistent seizure inhibition. Despite the challenges of electrical stimulation, however, it may be possible to find appropriate settings to also allow consistent seizure inhibition with electrical stimulation. This would provide a more immediate translational opportunity than optogenetic techniques.
3.3. Disparate experimental variables
As discussed above, electrical stimulation can cause varied responses depending on the location, stimulation parameters (including amplitude, pulse width, and frequency of stimulation), and other experimental variables. In considering the disparate findings as to the effectiveness of cerebellar stimulation for seizure suppression, it is therefore certainly worth noting the large range of methods used in previous literature (Tables 1 and 2). Theoretically, some insight might be gleaned by comparing across studies. However, this is hampered by two large factors. 1) There is inconsistent reporting of key variables. Sometimes relevant information is not reported at all (Tables 1 and 2), and sometimes ranges are given that are so large as to significantly hamper interpretability (e.g., 4–100 Hz [93]). 2) There are a large number of dimensions to explore. This may be the largest hurdle. Without a systematic study, disentangling the effects of any one parameter on outcomes becomes extremely difficult. For example, if study X differs from study Y in regard to location, pulse width, and frequency, how does one decide which variable is the key variable? This is especially difficult as variables may interact with one another.
Even without taking into account potential effects of epilepsy type or model on outcomes, the number of dimensions with regard to stimulation parameters to explore results in a huge parameter space, which is simply too sparsely sampled by the current literature. As noted above, studies in which a given parameter was varied within the study, to explore the impact of that parameter, provide some level of insight and hope for clarification. However, these studies are few and far between, and explore a very limited subset of the parameter space. What would be most beneficial in this regard would be a much larger undertaking – one that explored the impact of changing combinations of stimulation parameters in a systematic and thorough way, covering a large swath of the parameter space, in the same study. This would allow researchers to identify which parameters are critical and which combination of parameters allows for robust, consistent, seizure suppression. Effective settings may also be epilepsy type, or perhaps even individual, specific. There are strategies that provide a mechanism to do a thorough, rationale, and data driven search of the parameter space to identify which combinations of settings do, and do not, work [6, 113]. In some circumstances, such approaches can even be applied at an individual subject level. Experiments providing a large coverage of the parameter space, allowing direct comparisons of important experimental variables, will provide needed insight into this complex, but very relevant issue.
3.4. Closed versus open loop modulation
Previous work examining the effects of electrical stimulation of the cerebellum on seizures relied on open-loop stimulation protocols, in which electrical stimulation was applied on a regular basis irrespective of ongoing seizure activity. This can result in a misalignment of the intervention, limiting efficacy. It may also result in ‘over-stimulation’, with intervention occurring during interictal or other periods not needing intervention. Such continuous stimulation has the potential to carry negative side effects such as mal-adaptive plasticity, which may reduce the efficacy of electrical stimulation or even result in a kindling-like phenomenon. One alternative is to implement closed-loop on-demand interventions in which stimulation is applied only in an ‘as-needed’ basis, i.e., only during or immediately prior to seizure events (Figure 1). In addition to limiting potential mal-adaptive plasticity or other negative side-effects of stimulation, the improved temporal alignment of closed-loop approaches may simply be more effective [114, 115]. Therefore, on-demand interventions may not only allow more interpretable results, but may also have significant clinical benefits. Closed-loop on-demand interventions are now used both clinically [2, 116] and experimentally [50, 66, 114, 115, 117–119].
Figure 1. Closed-loop optogenetic interventions align intervention to the time of seizures.
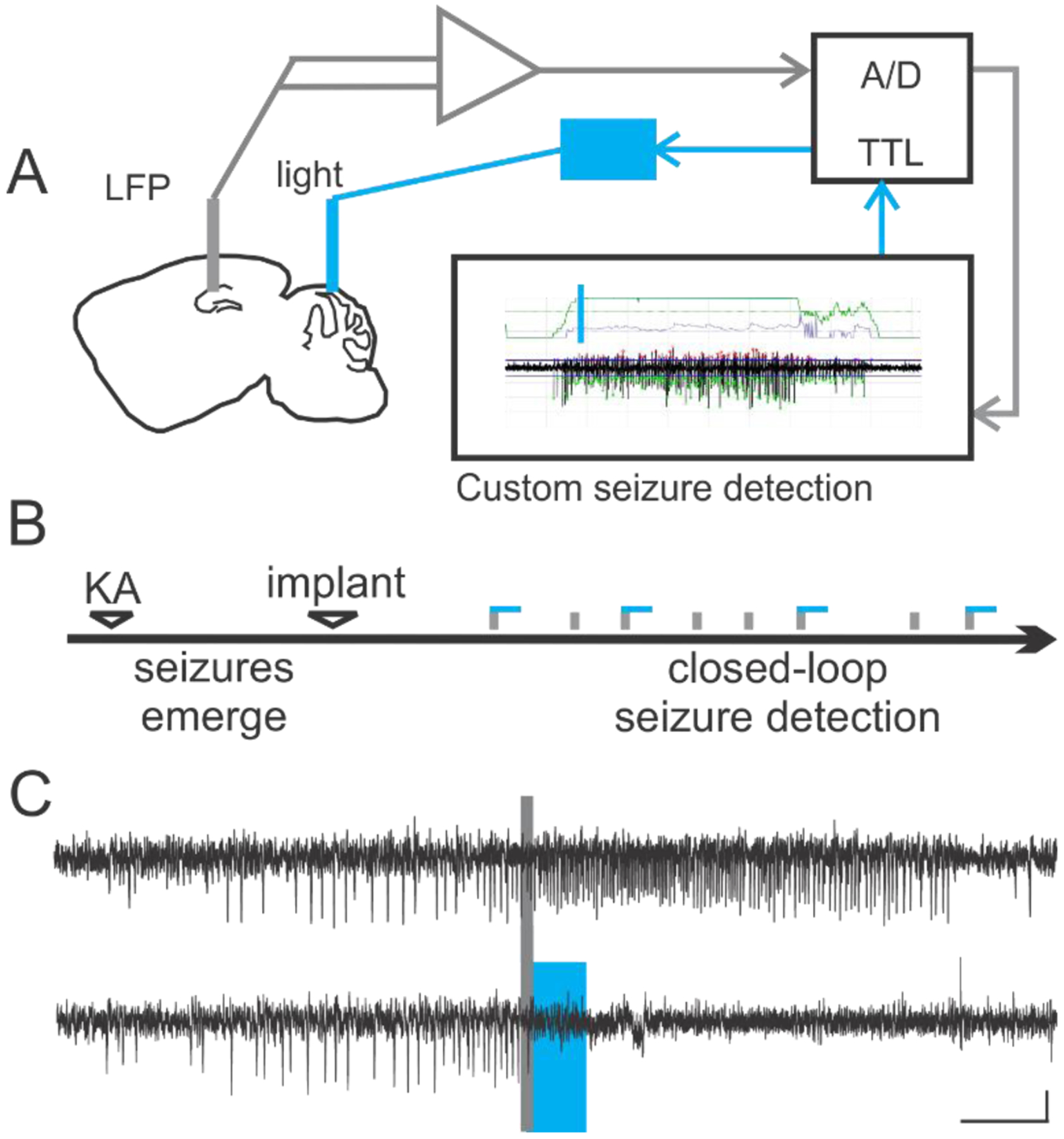
A) Schematic of an example on-demand optogenetic intervention strategy. Chronic hippocampal LFP recordings (near the presumed seizure focus) are digitized and fed into seizure detection software, allowing on-line detection through user-specified criteria, such as ictal spike frequency. Seizure detection in turn automatically triggers light delivery, for example to the cerebellum, for optogenetic interventions. B) On-demand optogenetic intervention can be implemented during the chronic phase of epilepsy in the intrahippocampal kainate model, such that spontaneous seizures which occur weeks after the initial insult are detected (gray vertical bars in the schematic denote detected seizures) and trigger light delivery. Blue horizontal lines in the schematic indicate light delivery. Note that only approximately half of detected events are followed by blue lines: detected events not receiving light intervention can serve as no-light internal controls. C) Example electrographic seizure events in the intrahippocampal kainate mouse model of temporal lobe epilepsy which were detected on-line (denoted by gray bar) and were either randomly selected to not receive light (top trace) or receive 3 seconds of pulsed light delivery to the cerebellar cortex (bottom trace, light delivery denoted by blue box). Scale bar: 5s, 0.05mV. Panels B-C reproduced from [50].
4. Renewed interest with more targeted approaches
As outlined above, the complex effects of electrical stimulation on both the populations of neurons recruited as well as the direction of modulation of cells may contribute to the inconsistency of cerebellar electrical stimulation in the attenuation of seizures. Improvement of the specificity of interventions in i) cell populations engaged, ii) direction of modulation, and iii) temporal alignment relative to seizure events may therefore improve efficacy of cerebellar modulation, and, importantly, interpretability of results. Optogenetics allows for the targeting of specific cell populations for either direct excitation or inhibition using transgenic and/or viral approaches [120]. When combined with on-demand light delivery, optogenetics also provides temporal specificity, and therefore overcomes many of the challenges in interpretability discussed above. The development and implementation of these techniques to increase the specificity and targeting of neuronal populations has led to renewed interest in the cerebellum as a target for therapeutic intervention in epilepsy. In the following subsections, we describe findings obtained using optogenetic techniques to modulate the cerebellum in mouse models of temporal lobe and absence epilepsy. A summary of findings from these studies is also provided in Table 3.
Table 3:
Summary of optogenetic interventions targeting the cerebellum in animal models of epilepsy.
| Study | Target | Epilepsy model | Stimulation protocols | Opsin, targeting | Animal model | Results |
|---|---|---|---|---|---|---|
| Krook-Magnuson et. al, 2014 | Vermis, simplex lobules IV/V | Intrahippocampal kainate (chronic phase) | 7Hz, 1Hz; 3sec duration | ChR in parvalbumin expressing neurons, Purkinje cells | Mouse | Seizure inhibition with all parameters |
| Krook-Magnuson et. al, 2014 | Vermis, simplex lobules IV/V | Intrahippocampal kainate (chronic phase) | 7Hz, 1Hz; 3sec duration | HR in parvalbumin expressing neurons, Purkinje cells | Mouse | Seizure inhibition with all parameters |
| Streng et. al, 2019 | Fastigial nucleus | Intrahippocampal kainate (chronic phase) | 1Hz, 7Hz, 10Hz; 3sec duration or single 50msec pulse | HR in VGluT2-expresisng neurons | Mouse | No effect |
| Streng et. al, 2019 | Fastigial nucleus | Intrahippocampal kainate (chronic phase) | 1Hz, 7Hz, 10Hz; 3sec duration or single 50msec pulse | ChR in VGluT2-expresisng neurons | Mouse | Seizure inhibition with all parameters |
| Kros et. al, 2015 | Medial and lateral deep cerebellar nuclei | tottering (tg) | Single 30–300msec pulse | ChR2 under hSyn promoter | Mouse | Seizure inhibition with all parameters |
| Kros et. al, 2015 | Medial and lateral deep cerebellar nuclei | C3h/HeOuJ | Single 30–300msec pulse | ChR2 under hSyn promoter | Mouse | Seizure inhibition with all parameters |
4.1. On-demand optogenetic modulation of cerebellar neurons in a mouse model of temporal lobe epilepsy
Using closed-loop optogenetic approaches, Krook-Magnuson et al selectively modulated cerebellar Purkinje cells in an on-demand fashion in the intrahippocampal kainate mouse model of chronic temporal lobe epilepsy [50] (Figure 2). On-demand optogenetic excitation of Purkinje cells in lobules IV/V of vermis (midline) or the simplex (either ipsilateral or contralateral to the presumed seizure focus) robustly inhibited spontaneous seizures recorded from the hippocampus (Fig. 2B) [50]. This showed that optogenetic excitation of Purkinje cells could be an effective strategy to inhibit seizures. These findings also suggested that, when optogenetically manipulating Purkinje neurons, multiple locations of intervention can be effective. Moreover, inhibition of seizures was achieved with short or long light pulses, indicating that a specific frequency of light delivery was not key to success with this optogenetic approach.
Figure 2. Excitation or inhibition of the cerebellar cortex can attenuate hippocampal seizures, while excitation of the deep cerebellar nuclei is required for successful seizure intervention.
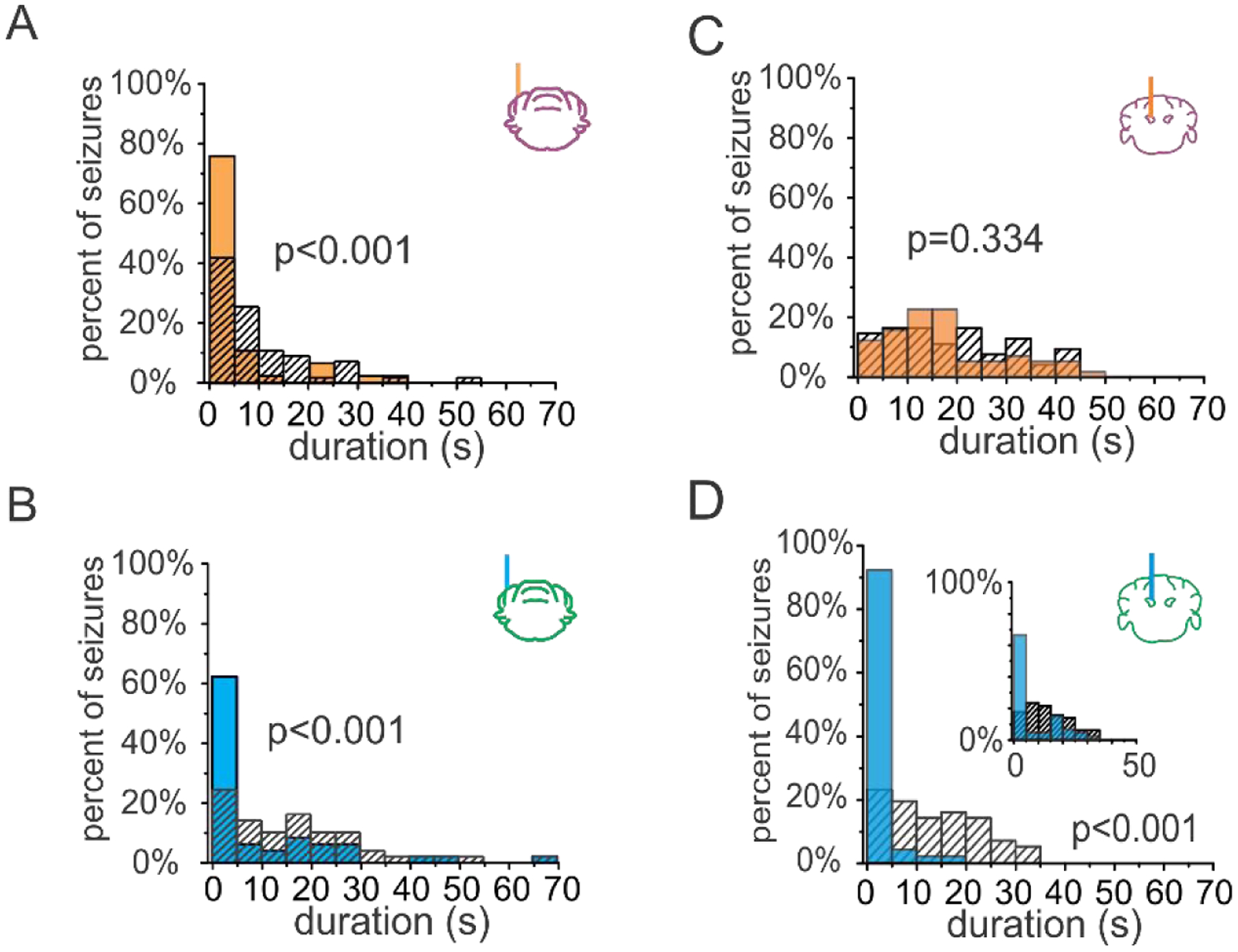
A-B) Example post-detection seizure durations for animals receiving three seconds of pulsed on-demand optogenetic intervention targeting the cerebellar cortex. Both on-demand inhibition (orange bars in A) and excitation (blue bars in B) of the cerebellar cortex robustly attenuate hippocampal seizures. Hashed bars: no light internal controls. C-D) Direction of modulation matters when instead targeting the cerebellar nuclei. C) Post-detection seizure durations for an example animal illustrating that on-demand inhibition of the fastigial nucleus fails to attenuate hippocampal seizures. D) Conversely, on-demand excitation of the fastigial nucleus robustly attenuates seizures. Even a single 50 msec pulse of blue light delivered to the fastigial nucleus is sufficient to reduce hippocampal seizure duration (inset, p < 0.01). P values from two sample Kolmogorov-Smirnov tests. Panels A-B reproduced and modified with permission from [50], panels C-D reproduced and modified with permission from [126]tr).
On-demand optogenetic inhibition of Purkinje cells, in the same cerebellar regions, was also tested [50]. Surprisingly, a reduction in seizure duration was also observed when Purkinje cells were inhibited rather than excited (Fig. 2A). This suggests that, when optogenetically manipulating Purkinje neurons, the direction of modulation is also not a critical factor for aborting hippocampal seizures. How could both excitation and inhibition of Purkinje cells be effective? Notably, it has been shown that optogenetic excitation of Purkinje cells can be followed by brief pauses in Purkinje cell firing, and, conversely, that optogenetic inhibition of Purkinje cells can be followed by brief periods of increased firing after light offset [50, 121]. The ability of both optogenetic excitation and inhibition of Purkinje cells to stop seizures may be a consequence of this phenomenon. However, optogenetic excitation and inhibition of Purkinje cells are not uniformly equivalent. This is evident when considering seizure frequency. In addition to truncating on-going hippocampal seizures, optogenetic excitation, but not inhibition, of the vermis produced a unique increase in time to next seizure, which, though brief, far outlasted the duration of the light intervention [50]. This finding highlights that the direction of modulation of Purkinje cells can, in certain circumstances, have functionally important consequences. It further indicates that that the midline cerebellar cortex can not only cause immediate changes (truncating ongoing seizures), but can also have longer lasting impacts which influence ictogenicity.
Recently, we used similar on-demand optogenetic methods to instead target the downstream fastigial nucleus. The fastigial nucleus gets inhibitory input from Purkinje cells, and contains a large variety of neuronal types (Box 1). Among these nuclear neuron types are glutamatergic projection neurons, which project to areas including the thalamus, superior colliculus, and brainstem nuclei including the reticular formation. In contrast to findings targeting Purkinje neurons described above, optogenetic inhibition of glutamatergic nuclear neurons had no effect on seizures (Fig. 2C). However, optogenetic excitation of glutamatergic nuclear neurons produced robust inhibition of seizures (Fig. 2D). This inhibition of seizures was sufficiently strong and immediate that a single 50 ms pulse of light was able to significantly shorten seizures (Fig. 2D, inset). These findings provide important additional insight into how cerebellar modulation may be inhibiting hippocampal seizures. Specifically, they indicate that excitation, but not inhibition, of nuclear neurons is able to inhibit seizures. Therefore, the direction of modulation of the nuclear neurons may be a critical factor in successful interventions when targeting the cerebellum. When targeting the cerebellar cortex, the brief pauses in Purkinje cell firing after optogenetic excitation may allow for disinhibition in the nuclei [121–123], and thereby successful seizure inhibition.
These experiments further illustrate how combinations of experimental factors can be especially relevant, in this case location and direction of modulation – when optogenetically targeting the cerebellar cortex, the direction of modulation was not a critical factor in reducing seizure duration; when targeting the nuclei, it was absolutely critical. It also provides a potential explanation for why previous electrical stimulation studies could have produced such mixed results: stimulation parameters that ultimately failed to excite nuclear neurons were unlikely to have been successful.
An additional important point is illustrated in our work using optogenetics to target the fastigial nucleus. Using viral approaches, we were able to either target the fastigial nucleus broadly, without cell type specificity, or specifically target glutamatergic neurons (Figure 3A–B). While both methods were able to successfully terminate hippocampal seizures (Figure 3C–D), we found that optogenetic excitation of glutamatergic nuclear neurons selectively provided greater seizure inhibition than optogenetic excitation of nuclear neurons broadly (Figure 3E–F, 66% versus 39% reduction, respectively). This highlights an important benefit optogenetic approaches can provide over electrical stimulation or many pharmacological approaches. Cell-type specificity of intervention improves interpretability of findings, and, as seen here, can improve outcomes.
Figure 3. Viral targeting of nuclear neurons in mouse models of temporal lobe and absence epilepsy.
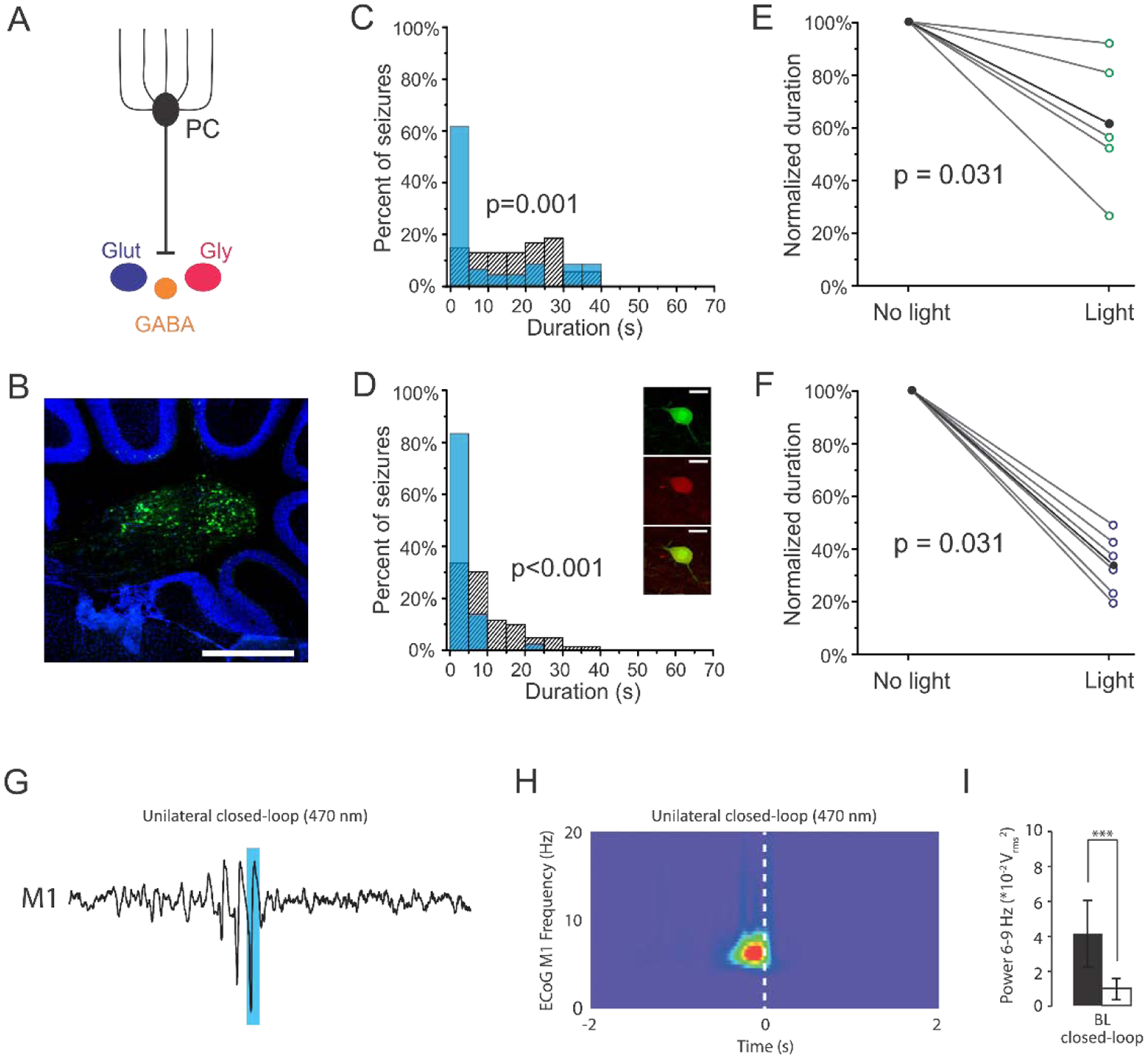
A) Viral approaches allowed for broadly targeting nuclear neurons or selective targeting of glutamatergic nuclear neurons (dark blue). B) GFP expression in nuclear neurons following injection of cre-dependent virus in a VGluT2-cre mouse. Scale bar: 500μm. C) Post-detection seizure durations for an example animal, illustrating that light delivery significantly reduces seizure duration when virally targeting the fastigial nucleus broadly (in this example, a 44% reduction, p = 0.001, two sample Kolmogorov-Smirnov test). Blue bars: events receiving light intervention; hashed bars: no-light internal controls. D) Seizure inhibition is also achieved by selectively targeting glutamatergic nuclear neurons (in this example, an 81% reduction, p < 0.001, two sample Kolmogorov-Smirnov test). Inset: Immunocytochemistry confirmed selective expression in glutamatergic neurons following injection of cre-dependent virus in VGluT2-cre animals (Top- green: GFP, middle- red: VGluT2 immunohistochemistry, Bottom- overlay. Scale bar: 70μm.) E-F) Selective targeting of glutamatergic neurons in the fastigial nucleus (F) produces significantly greater seizure attenuation than targeting fastigial neurons more broadly (E; broad targeting versus selective targeting: p = 0.026, Mann-Whitney). Each open circle represents one animal; black data points represent mean. G) Closed-loop optogenetic excitation of the deep cerebellar nuclei (targeting all nuclear neurons broadly using a viral approach) attenuates generalized spike and wave discharges in primary motor cortex in the C3H/HeOuJ mouse model. Blue bar indicates timing of light delivery. H) Wavelet spectrogram of the electrocorticograph during closed-loop stimulation, showing cessation of the GSWD event was time-locked to the onset (dashed bar) of intervention. I) Closed-loop intervention significantly reduces motor cortex band power associated with the GSWDs (p < 0.05, repeated measures ANCOVA). Panels A-F reproduced with modification and permission from [126]. Panels G-I reproduced with permission from [66].
4.2. Optogenetic and pharmacological cerebellar modulation in models of absence epilepsy
As discussed in previous sections, cerebellar modulation with electrical stimulation has been applied to a wide range of seizure types. Similarly, on-demand optogenetic approaches have not only been applied in the intrahippocampal mouse model of temporal lobe epilepsy, but also in mouse models of absence epilepsy. Kros and colleagues [66] found that on-demand optogenetic activation of cerebellar nuclear neurons was effective at attenuating generalized spike and wave discharges (GSWDs) in both tottering (tg) and inbred C3H/HeOuJ mouse lines, with spike and wave events terminating at the onset of light delivery (Figure 3G–I). The efficacy of on-demand cerebellar modulation in attenuating seizure events in two very different forms of epilepsy (temporal lobe versus thalamocortical absence epilepsy), with different underlying neural circuitry, suggests that the cerebellum may be a broadly applicable candidate for therapeutic intervention in epilepsy. This may make the cerebellum an especially attractive candidate in cases where the seizure focus is unknown, progressing, manifold, or otherwise inaccessible.
While this study did not look at optogenetic inhibition of nuclear neurons, pharmacological inhibition of nuclear neurons via application of the GABAA-agonist muscimol was examined [66]. Consistent with the idea discussed above that excitation of nuclear neurons is required for seizure reduction, pharmacological inhibition consistently increased, rather than decreased, the frequency of GSWDs. The pharmacological approach lacked the temporal precision, as well as the cell-type specificity, that an on-demand optogenetic approach can provide, and therefore is more difficult to confidently interpret. However, pharmacological excitation (/disinhibition), via application of the GABAA-antagonist gabazine, was able to significantly decrease GSWD occurrence (but, interestingly, had no effect on duration, in contrast to optogenetic excitation). It will be informing to see, in future studies, what the impact is of on-demand inhibition of (different populations of neurons in) the cerebellar nuclei on GSWDs.
6. Mechanisms of cerebellar inhibition of seizures
A major outstanding question is the mechanism(s) by which cerebellar modulation is able to attenuate seizures. One hypothesis regarding the mechanisms behind electrical stimulation of the cerebellum for epilepsy is that Purkinje cell activation serves to reduce excitatory output from the cerebellar nuclei to the thalamus and thereby reduce cortical excitability [124]. However, this hypothesis is in conflict with the observations described above that 1) pharmacological inhibition of deep cerebellar nuclei increases the frequency of GSWDs [66, 125], 2) pharmacological excitation of deep cerebellar nuclei decreases the frequency of GSWDs [66], 3) optogenetic excitation of deep cerebellar nuclei inhibits GSWDs [66], 4) optogenetic inhibition of deep cerebellar nuclei has no apparent effect on hippocampal seizures [126], while 5) optogenetic excitation of deep cerebellar nuclei inhibits hippocampal seizures [126]. Therefore, it appears that the reverse of the hypothesized mechanism is actually at play: effective cerebellar stimulation for inhibition of seizures may require increased output from excitatory projections for the deep cerebellar nuclei. Beyond that, little is currently known.
How would increased output from cerebellar nuclei neurons inhibit seizures? Neurons in the deep cerebellar nuclei project to numerous downstream structures (Figure 4) including various thalamic nuclei, the superior colliculus (which is of particular interest given its potential role as a regulator of ictal activity [9]), the pontine and medullary reticular formation, the locus coeruleus, and the amygdala [127–129]. This broad connectivity makes disentangling potential pathways influencing seizures difficult.
Figure 4. A few cerebellar output channels of potential relevance to seizure networks. The deep cerebellar nuclei contain numerous downstream projections to both ascending and descending structures, including but not limited to the thalamus, superior colliculus, amygdala, and reticular formation. The projections from deep cerebellar nuclei to these areas are visualized here from mice injected with viruses inducing GFP expression in nuclear neurons similar to the studies outlined in Figure.
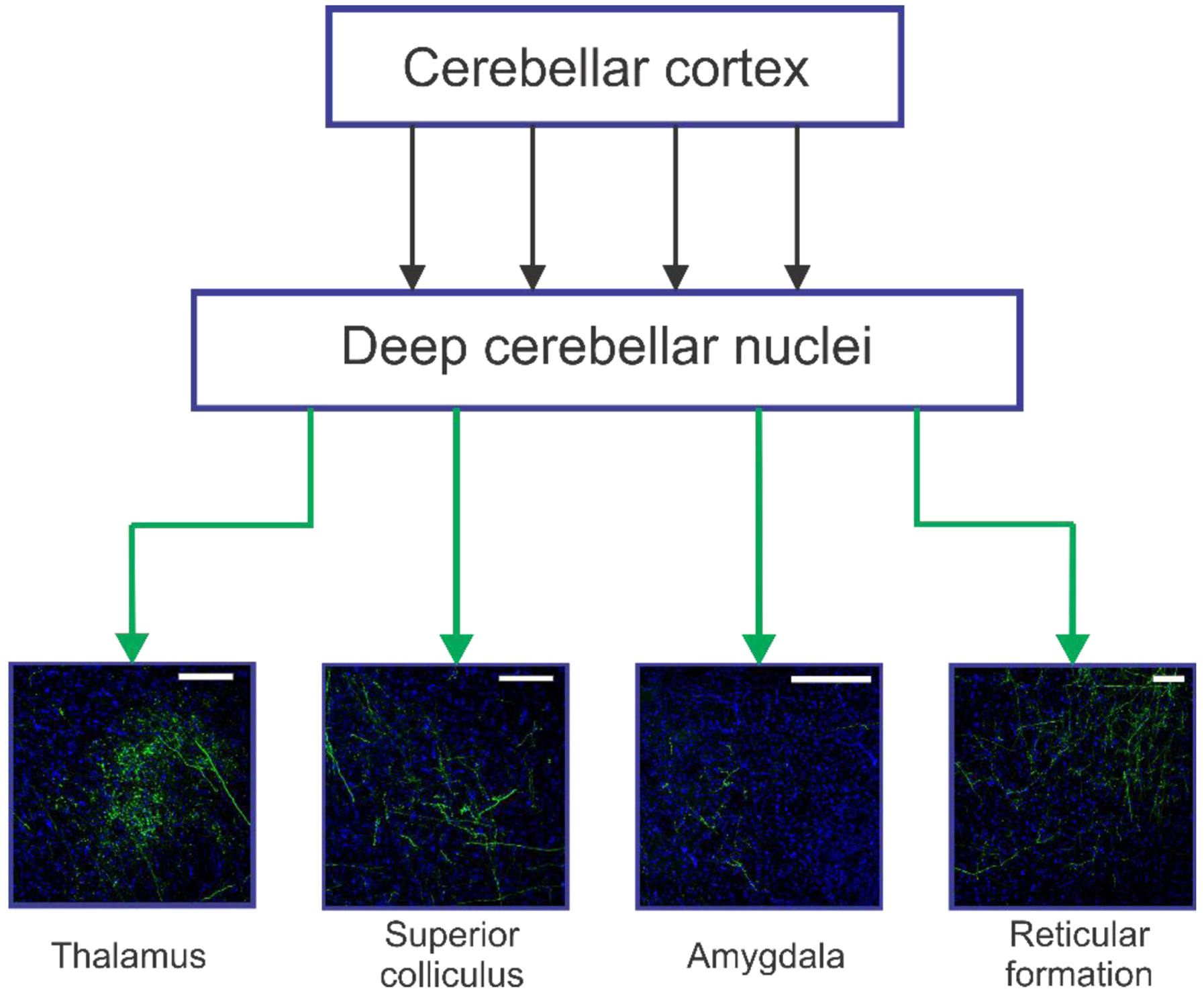
3. Scale bars: 100μm (Thalamus, Superior colliculus), 200μm (Amygdala, Reticular formation)
In the case of absence epilepsy, given the thalamic role in absence seizures [130, 131], a potential straight-forward explanation is the direct connection from the deep cerebellar nuclei to thalamic nuclei [132]. Altering excitatory input to the thalamus could, for example, shift thalamic neurons from phasic to tonic firing [133]. In the case of hippocampal seizures, however, things become more complicated.
While early research, using techniques such as examination of degenerating fibers [129] or time delays in responses recorded from the hippocampus after electrical stimulation in the cerebellum [75, 82], suggested there may be a direct connection from the cerebellum to the hippocampus, more recent studies have failed to find evidence for a direct, monosynaptic, connection [134–136]. Once a multi-synaptic pathway is considered, there are a great many potential routes for cerebellar modulation to influence hippocampal networks, including via the locus coeruleus, the septum, and potentially the thalamus [136–139].
A lack of a direct, mono-synaptic, connection does not imply that the cerebellum cannot have a strong impact on hippocampal networks. Indeed, a recent paper optogenetically manipulating the cerebellum in healthy animals found both increased bold signal in the hippocampus and altered neuronal firing in the hippocampus with multiunit recordings [140]. Suggesting a functional significance of these (indirect) connections, chronic cerebellar deficits can impact spatial encoding in the hippocampus [141, 142]. Similarly, cerebellar cortical activity has been shown to be synchronized with hippocampal oscillations during certain conditions [136, 143, 144]. While there are many scenarios that could produce coherent oscillations in these brain regions (including both the hippocampus and the cerebellum getting a common source of input), it minimally suggests that the two structures may be collaborating under certain conditions [136]. More generally, there has also been a great deal of accumulating evidence suggesting a role for the cerebellum in more cognitive functions, including hippocampal-dependent processes [145, 146]. Determining which, of the many possible pathways, underlie the functional connection between the cerebellum and the hippocampus in healthy animals, and the ability for cerebellar modulation to inhibit temporal lobe seizures, will require significant additional efforts. Notably, different pathways may underlie the seizure suppressive effects of cerebellar modulation and the functional connectivity that appears to be relevant to spatial navigation.
An additional possibility is that seizure disruption is due to a more global brain state change, rather than a specific effect on the thalamus or hippocampus. The cerebellum projects to numerous areas associated with the control of brain states, and for example, has been implicated in regulation of sleep-wake cycles [147, 148]. Brain states, including sleep-wake cycles, are known to impact seizure susceptibility for a range of seizures types [149–153]. In the context of seizure disruption, a sufficient change in brain state could be theoretically achieved in a variety of manners. Seizure suppression, even if ultimately associated with a fairly global brain state change, could result from activation of a specific cerebellar output pathway (e.g., the reticular formation). In this scenario, stimulating that pathway selectively could replicate the benefits seen with cerebellar modulation. Alternatively, there may be a requirement for simultaneous modification of multiple downstream targets through divergent output pathways of the cerebellum, such that no single output pathway is able to replicate the seizure inhibition benefits seen with direct cerebellar modulation. This question (single cerebellar output pathway versus requirement for simultaneous modulation of multiple pathways) is addressable with currently available techniques, and is an important question to examine. If the cerebellum is impacting seizures through a specific target structure, that structure may in turn be a potential therapeutic target.
Finally, it is worth noting that excitatory projections from the cerebellar nuclei are not the only way for the cerebellum to influence downstream structures. There are also inhibitory projection neurons from the cerebellar nuclei [154], including, but not limited to, a population of glycinergic neurons in the fastigial nucleus which project ipsilaterally to vestibular and reticular neurons [155]. Additionally, while Purkinje cells classically project to neurons in the deep cerebellar (and vestibular) nuclei, they also can have direct projections to other regions (bypassing the cerebellar nuclei) including the locus coeruleus [138] and medial parabrachial nucleus [156], although much less is known about these connections. Similarly, although somewhat controversial, a population of neurons in the cerebellar granule cell layer or white matter, which appear to have projections out of the cerebellar cortex, has been noted in several species [157–161]. The projection target of these cells is essentially unknown, and provide another potential route of influence. However, findings that selective optogenetic activation of glutamatergic nuclear neurons was able to inhibit seizures (Figure 3, Table 3[126]) suggests that the bulk of seizure inhibition may be mediated by excitatory projections from the cerebellar nuclei.
6. Translational strategies
While optogenetics represents a powerful tool to selectively manipulate neuronal populations, it is still far from being successfully implemented in human epilepsy patients [115, 162]. It may, however, be possible to use the insights gleaned from on-demand optogenetic work in animal models to improve electrical stimulation efforts targeting the cerebellum. For example, in the case of cerebellar cortical stimulation, if electrical stimulation can be tuned such that it results in inhibition of Purkinje cells and thereby activation of downstream nuclear neurons, it would perhaps be more consistently effective in attenuating seizures. Additional benefits, including surgical benefits, may be achieved by directly targeting the nuclei [163, 164]. Determining the specific parameters to allow robust inhibition of seizures with electrical stimulation, unfortunately, will not be trivial. However, there are strong potential benefits, and, as mentioned above, tools available to allow a strategic and fairly comprehensive search of the parameter space. For example, Bayesian parameter optimization may be one powerful approach to tackle the dauntingly large number of combinations of potential stimulation parameters [113]. Given that optogenetic work provides strong evidence that cerebellar modulation can produce robust seizure inhibition, the challenge becomes determining how to achieve similar results with methods more readily clinically available.
7. Conclusions
While the cerebellum is an area of the brain not traditionally associated with epilepsy, ample evidence suggests that it can play an important role in seizure networks. This includes changes in cerebellar activity during seizures and cerebellar abnormalities associated with epilepsy. Early stimulation efforts targeting the cerebellum for seizure control, while initially promising, produced mixed results, and the cerebellum has not been substantially revisited as a potential therapeutic target for epilepsy until recently. The use of more targeted approaches such as closed-loop optogenetics appear to have greatly increased the efficacy of cerebellar modulation in attenuating seizures. These results renew excitement in the cerebellum’s potential as a possible target for therapeutic intervention. The precise mechanisms by which cerebellar modulation attenuates seizures have yet to be fully elucidated, and future efforts will need to determine if electrical stimulation parameters can be optimized to provide consistent, robust, inhibition of seizures.
Highlights.
Cerebellar alterations are often observed with epilepsy, including structural changes and modulation of cerebellar activity during seizures.
The cerebellum itself has been identified as a seizure focus in a number of case reports.
The cerebellum was a target of early interest for therapeutic stimulation to disrupt seizures, but animal and human studies using electrical stimulation produced mixed effects.
Recent work utilizing more targeted, closed-loop interventions reveal that the cerebellum can powerfully inhibit seizures in multiple models of epilepsy.
Acknowledgments
This work was supported in part by an American Epilepsy Society Postdoctoral Fellowship (MLS), NIH R01-NS104071, a University of Minnesota McKnight Land-Grant Professorship award, and the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative.
Abbreviations
- GSWDs
Generalized spike and wave discharges
- SUDEP
sudden unexplained death in epilepsy
- GFP
Green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interests
Reference list
- [1].England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav 2012;25: 266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O’Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, Seale CG. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015;84: 810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, Group SS. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010;51: 899–908. [DOI] [PubMed] [Google Scholar]
- [4].Pakdaman H, Amini Harandi A, Abbasi M, Karimi M, Arami MA, Mosavi SA, Haddadian K, Rezaei O, Sadeghi S, Sharifi G, Gharagozli K, Bahrami P, Ashrafi F, Kasmae HD, Ghassemi A, Arabahmadi M, Behnam B. Vagus nerve stimulation in drug-resistant epilepsy: the efficacy and adverse effects in a 5-year follow-up study in Iran. Neurol Sci 2016;37: 1773–1778. [DOI] [PubMed] [Google Scholar]
- [5].Valentin A, Garcia Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, Torres N, Pastor J, Selway R, Sola RG, Alarcon G. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 2013;54: 1823–33. [DOI] [PubMed] [Google Scholar]
- [6].Nagaraj V, Lee ST, Krook-Magnuson E, Soltesz I, Benquet P, Irazoqui PP, Netoff TI. Future of seizure prediction and intervention: closing the loop. J Clin Neurophysiol 2015;32: 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elder C, Friedman D, Devinsky O, Doyle W, Dugan P. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open 2019;4: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, Labar D, Sperling MR, Sharan A, Sandok E, Handforth A, Stern JM, Chung S, Henderson JM, French J, Baltuch G, Rosenfeld WE, Garcia P, Barbaro NM, Fountain NB, Elias WJ, Goodman RR, Pollard JR, Troster AI, Irwin CP, Lambrecht K, Graves N, Fisher R, Group SS. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015;84: 1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Soper C, Wicker E, Kulick CV, N’Gouemo P, Forcelli PA. Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol Dis 2016;87: 102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeidler Z, Krook-Magnuson E. One Site to Rule Them All: Toward a Master Regulator of Ictal Activity. Epilepsy Curr 2016;16: 170–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miterko LN, Baker KB, Beckinghausen J, Bradnam LV, Cheng MY, Cooperrider J, DeLong MR, Gornati SV, Hallett M, Heck DH, Hoebeek FE, Kouzani AZ, Kuo SH, Louis ED, Machado A, Manto M, McCambridge AB, Nitsche MA, Taib NOB, Popa T, Tanaka M, Timmann D, Steinberg GK, Wang EH, Wichmann T, Xie T, Sillitoe RV. Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 2009;132: 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kandel A, Buzsaki G. Cerebellar neuronal activity correlates with spike and wave EEG patterns in the rat. Epilepsy Res 1993;16: 1–9. [DOI] [PubMed] [Google Scholar]
- [14].Kros L, Lindeman S, Eelkman Rooda OHJ, Murugesan P, Bina L, Bosman LWJ, De Zeeuw CI, Hoebeek FE. Synchronicity and Rhythmicity of Purkinje Cell Firing during Generalized Spike-and-Wave Discharges in a Natural Mouse Model of Absence Epilepsy. Front Cell Neurosci 2017;11: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mitra J, Snider RS. Effects of hippocampal afterdischarges on Purkinje cell activity. Epilepsia 1975;16: 235–43. [DOI] [PubMed] [Google Scholar]
- [16].Caveness WF, Kosaka K, Hosokawa S, O’Neill RR. Cerebral-cerebellar paroxysmal activity in experimental focal seizures. Ann Neurol 1977;1: 287–9. [DOI] [PubMed] [Google Scholar]
- [17].Gartside IB. The activity of cerebellar neurones during epileptiform activity induced by penicillin in the cerebral cortex of the rat. Electroencephalogr Clin Neurophysiol 1979;46: 189–96. [DOI] [PubMed] [Google Scholar]
- [18].Julien RM, Laxer KD. Cerebellar responses to penicillin-induced cerebral cortical epileptiform discharge. Electroencephalogr Clin Neurophysiol 1974;37: 123–32. [DOI] [PubMed] [Google Scholar]
- [19].Wray DV, Hablitz JJ. Neuronal firing patterns during generalized penicillin epilepsy in the awake cat. Brain Res Bull 1977;2: 317–21. [DOI] [PubMed] [Google Scholar]
- [20].Marcian V, Filip P, Bares M, Brazdil M. Cerebellar Dysfunction and Ataxia in Patients with Epilepsy: Coincidence, Consequence, or Cause? Tremor Other Hyperkinet Mov (N Y) 2016;6: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Leifer D, Cole DG, Kowall NW. Neuropathologic asymmetries in the brain of a patient with a unilateral status epilepticus. J Neurol Sci 1991;103: 127–35. [DOI] [PubMed] [Google Scholar]
- [22].Soffer D, Melamed E, Assaf Y, Cotev S. Hemispheric brain damage in unilateral status epilepticus. Ann Neurol 1986;20: 737–40. [DOI] [PubMed] [Google Scholar]
- [23].Tan N, Urich H. Postictal cerebral hemiatrophy: with a contribution to the problem of crossed cerebellar atrophy. Acta Neuropathol 1984;62: 332–9. [DOI] [PubMed] [Google Scholar]
- [24].Niedermeyer E, Uematsu S. Electroencephalographic recordings from deep cerebellar structures in patients with uncontrolled epileptic seizures. Electroencephalogr Clin Neurophysiol 1974;37: 355–65. [DOI] [PubMed] [Google Scholar]
- [25].Boscolo Galazzo I, Storti SF, Barnes A, De Blasi B, De Vita E, Koepp M, Duncan JS, Groves A, Pizzini FB, Menegaz G, Fraioli F. Arterial Spin Labeling Reveals Disrupted Brain Networks and Functional Connectivity in Drug-Resistant Temporal Epilepsy. Front Neuroinform 2018;12: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li R, Hu C, Wang L, Liu D, Liu D, Liao W, Xiao B, Chen H, Feng L. Disruption of functional connectivity among subcortical arousal system and cortical networks in temporal lobe epilepsy. Brain Imaging Behav 2019. [DOI] [PubMed] [Google Scholar]
- [27].Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J, Jr., Stern JM. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 2014;55: 137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buijink AW, Caan MW, Tijssen MA, Hoogduin JM, Maurits NM, van Rootselaar AF. Decreased cerebellar fiber density in cortical myoclonic tremor but not in essential tremor. Cerebellum 2013;12: 199–204. [DOI] [PubMed] [Google Scholar]
- [29].Allen LA, Vos SB, Kumar R, Ogren JA, Harper RK, Winston GP, Balestrini S, Wandschneider B, Scott CA, Ourselin S, Duncan JS, Lhatoo SD, Harper RM, Diehl B. Cerebellar, limbic, and midbrain volume alterations in sudden unexpected death in epilepsy. Epilepsia 2019;60: 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gessaga EC, Urich H. The cerebellum of epileptics. Clin Neuropathol 1985;4: 238–45. [PubMed] [Google Scholar]
- [31].Sandok EK, O’Brien TJ, Jack CR, So EL. Significance of cerebellar atrophy in intractable temporal lobe epilepsy: a quantitative MRI study. Epilepsia 2000;41: 1315–20. [DOI] [PubMed] [Google Scholar]
- [32].Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Bye AM. Cerebral and cerebellar volume reduction in children with intractable epilepsy. Epilepsia 2000;41: 1456–62. [DOI] [PubMed] [Google Scholar]
- [33].Lee Y, Rodriguez OC, Albanese C, Santos VR, Cortes de Oliveira JA, Donatti ALF, Fernandes A, Garcia-Cairasco N, N’Gouemo P, Forcelli PA. Divergent brain changes in two audiogenic rat strains: A voxel-based morphometry and diffusion tensor imaging comparison of the genetically epilepsy prone rat (GEPR-3) and the Wistar Audiogenic Rat (WAR). Neurobiol Dis 2018;111: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marcian V, Marecek R, Koritakova E, Pail M, Bares M, Brazdil M. Morphological changes of cerebellar substructures in temporal lobe epilepsy: A complex phenomenon, not mere atrophy. Seizure 2018;54: 51–57. [DOI] [PubMed] [Google Scholar]
- [35].Newham BJ, Curwood EK, Jackson GD, Archer JS. Pontine and cerebral atrophy in Lennox-Gastaut syndrome. Epilepsy Res 2016;120: 98–103. [DOI] [PubMed] [Google Scholar]
- [36].Alvim MK, Coan AC, Campos BM, Yasuda CL, Oliveira MC, Morita ME, Cendes F. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia 2016;57: 621–9. [DOI] [PubMed] [Google Scholar]
- [37].Wiest R, Estermann L, Scheidegger O, Rummel C, Jann K, Seeck M, Schindler K, Hauf M. Widespread grey matter changes and hemodynamic correlates to interictal epileptiform discharges in pharmacoresistant mesial temporal epilepsy. J Neurol 2013;260: 1601–10. [DOI] [PubMed] [Google Scholar]
- [38].Bohnen NI, O’Brien TJ, Mullan BP, So EL. Cerebellar changes in partial seizures: clinical correlations of quantitative SPECT and MRI analysis. Epilepsia 1998;39: 640–50. [DOI] [PubMed] [Google Scholar]
- [39].Crooks R, Mitchell T, Thom M. Patterns of cerebellar atrophy in patients with chronic epilepsy: a quantitative neuropathological study. Epilepsy Res 2000;41: 63–73. [DOI] [PubMed] [Google Scholar]
- [40].Spielmeyer W The anatomic substratum of the convulsive state. Archives of Neurology and Psychiatry 1930;23: 869–875. [Google Scholar]
- [41].Shin WC, Hong SB, Tae WS, Seo DW, Kim SE. Ictal hyperperfusion of cerebellum and basal ganglia in temporal lobe epilepsy: SPECT subtraction with MRI coregistration. J Nucl Med 2001;42: 853–8. [PubMed] [Google Scholar]
- [42].Seto H, Shimizu M, Watanabe N, Wu Y, Kageyama M, Kamisaki Y, Morijiri M, Tonoya Y, Kurachi M. Contralateral cerebellar activation in frontal lobe epilepsy detected by ictal Tc-99m HMPAO brain SPECT. Clin Nucl Med 1997;22: 194–5. [DOI] [PubMed] [Google Scholar]
- [43].Laich E, Kuzniecky R, Mountz J, Liu HG, Gilliam F, Bebin M, Faught E, Morawetz R. Supplementary sensorimotor area epilepsy. Seizure localization, cortical propagation and subcortical activation pathways using ictal SPECT. Brain 1997;120 ( Pt 5): 855–64. [DOI] [PubMed] [Google Scholar]
- [44].Won JH, Lee JD, Chung TS, Park CY, Lee BI. Increased contralateral cerebellar uptake of technetium-99m-HMPAO on ictal brain SPECT. J Nucl Med 1996;37: 426–9. [PubMed] [Google Scholar]
- [45].Rubin EH, Ferrendelli JA. Distribution and regulation of cyclic nucleotide levels in cerebellum, in vivo. J Neurochem 1977;29: 43–51. [DOI] [PubMed] [Google Scholar]
- [46].Ferrendelli JA, Blank AC, Gross RA. Relationships between seizure activity and cyclic nucleotide levels in brain. Brain Res 1980;200: 93–103. [DOI] [PubMed] [Google Scholar]
- [47].Collins RC, Kennedy C, Sokoloff L, Plum F. Metabolic anatomy of focal motor seizures. Arch Neurol 1976;33: 536–42. [DOI] [PubMed] [Google Scholar]
- [48].Ferrendelli JA, McDougal DB,. The effect of electroshock on regional CNS energy reserves in mice. J Neurochem 1971;18: 1197–205. [DOI] [PubMed] [Google Scholar]
- [49].McCandless DW, Feussner GK, Lust WD, Passonneau JV. Metabolite levels in brain following experimental seizures: the effects of maximal electroshock and phenytoin in cerebellar layers. J Neurochem 1979;32: 743–53. [DOI] [PubMed] [Google Scholar]
- [50].Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Heath RG, Dempesy CW, Fontana CJ, Fitzjarrell AT. Feedback loop between cerebellum and septal-hippocampal sites: its role in emotion and epilepsy. Biol Psychiatry 1980;15: 541–56. [PubMed] [Google Scholar]
- [52].Fernandez-Guardiola A, Manni E, Wilson JH, Dow RS. Microelectrode recording of cerebellar and cerebral unit activity during convulsive afterdischarge. Exp Neurol 1962;6: 48–69. [DOI] [PubMed] [Google Scholar]
- [53].Hablitz JJ, Wray DV. Cerebellar unit activity during generalized penicillin epilepsy in the awake cat. Exp Neurol 1977;56: 189–99. [DOI] [PubMed] [Google Scholar]
- [54].Boop S, Wheless J, Van Poppel K, McGregor A, Boop FA. Cerebellar seizures. J Neurosurg Pediatr 2013;12: 288–92. [DOI] [PubMed] [Google Scholar]
- [55].Harvey AS, Jayakar P, Duchowny M, Resnick T, Prats A, Altman N, Renfroe JB. Hemifacial seizures and cerebellar ganglioglioma: an epilepsy syndrome of infancy with seizures of cerebellar origin. Ann Neurol 1996;40: 91–8. [DOI] [PubMed] [Google Scholar]
- [56].Lascano AM, Lemkaddem A, Granziera C, Korff CM, Boex C, Jenny B, Schmitt-Mechelke T, Thiran JP, Garibotto V, Vargas MI, Schaller K, Seeck M, Vulliemoz S. Tracking the source of cerebellar epilepsy: hemifacial seizures associated with cerebellar cortical dysplasia. Epilepsy Res 2013;105: 245–9. [DOI] [PubMed] [Google Scholar]
- [57].Martins WA, Paglioli E, Hemb M, Palmini A. Dysplastic Cerebellar Epilepsy: Complete Seizure Control Following Resection of a Ganglioglioma. Cerebellum 2016;15: 535–41. [DOI] [PubMed] [Google Scholar]
- [58].Mesiwala AH, Kuratani JD, Avellino AM, Roberts TS, Sotero MA, Ellenbogen RG. Focal motor seizures with secondary generalization arising in the cerebellum. Case report and review of the literature. J Neurosurg 2002;97: 190–6. [DOI] [PubMed] [Google Scholar]
- [59].Chae JH, Kim SK, Wang KC, Kim KJ, Hwang YS, Cho BK. Hemifacial seizure of cerebellar ganglioglioma origin: seizure control by tumor resection. Epilepsia 2001;42: 1204–7. [DOI] [PubMed] [Google Scholar]
- [60].Jayakar PB, Seshia SS. Involuntary movements with cerebellar tumour. Can J Neurol Sci 1987;14: 306–8. [DOI] [PubMed] [Google Scholar]
- [61].al-Shahwan SA, Singh B, Riela AR, Roach ES. Hemisomatic spasms in children. Neurology 1994;44: 1332–3. [DOI] [PubMed] [Google Scholar]
- [62].Gupta S, Jayalakshmi S, Lingappa L, Konanki R, Vooturi S, Sudhakar P, Panigrahi M. Ictal FDGPET and SPECT in hemifacial seizures due to cerebellar epilepsy-Case report. Neurol India 2019;67: 169–172. [DOI] [PubMed] [Google Scholar]
- [63].Soysal DD, Caliskan M, Aydin K, Nayir A, Karabocuoglu M, Citak A, Uzel N. Isolated cerebellar involvement in a case of posterior reversible leukoencephalopathy. Clin Radiol 2006;61: 983–6. [DOI] [PubMed] [Google Scholar]
- [64].Davidson DL, Barbeau A. Locomotor activity and seizures induced by picrotoxin in the fastigeal nucleus of the rat. Exp Neurol 1975;49: 622–7. [DOI] [PubMed] [Google Scholar]
- [65].Davidson DL, Tsukada Y, Barbeau A. Ouabain induced seizures: site of production and response to anticonvulsants. Can J Neurol Sci 1978;5: 405–11. [DOI] [PubMed] [Google Scholar]
- [66].Kros L, Eelkman Rooda OH, Spanke JK, Alva P, van Dongen MN, Karapatis A, Tolner EA, Strydis C, Davey N, Winkelman BH, Negrello M, Serdijn WA, Steuber V, van den Maagdenberg AM, De Zeeuw CI, Hoebeek FE. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol 2015;77: 1027–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Maejima T, Wollenweber P, Teusner LU, Noebels JL, Herlitze S, Mark MD. Postnatal loss of P/Q-type channels confined to rhombic-lip-derived neurons alters synaptic transmission at the parallel fiber to purkinje cell synapse and replicates genomic Cacna1a mutation phenotype of ataxia and seizures in mice. J Neurosci 2013;33: 5162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mark MD, Maejima T, Kuckelsberg D, Yoo JW, Hyde RA, Shah V, Gutierrez D, Moreno RL, Kruse W, Noebels JL, Herlitze S. Delayed postnatal loss of P/Q-type calcium channels recapitulates the absence epilepsy, dyskinesia, and ataxia phenotypes of genomic Cacna1a mutations. J Neurosci 2011;31: 4311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gartside IB. The effects of cerebellectomy on a penicillin epileptogenic focus in the cerebral cortex of the rat. Electroencephalogr Clin Neurophysiol 1978;44: 373–9. [DOI] [PubMed] [Google Scholar]
- [70].Hutton JT, Frost JD, Jr., Foster J. the influence of the cerebellum in cat penicillin epilepsy. Epilepsia 1972;13: 401–8. [DOI] [PubMed] [Google Scholar]
- [71].Utterback RA. Parenchymatous Cerebellar Degeneration Complicating Diphenylhydantoin (Dilantin) Therapy. Archives of Neurology and Psychiatry 1958;80: 180–181. [Google Scholar]
- [72].Testa G, Pellegrini A, Giaretta D. Effects of electrical stimulation and removal of cerebellar structures in an experimental model of generalized epilepsy. Epilepsia 1979;20: 447–54. [DOI] [PubMed] [Google Scholar]
- [73].Barrionuevo G, Pechadre JC, Gautron M, Guiot F. Negative effects of chronic hemicerebellectomy of epileptiform after-discharges elicited by focal cortical stimulation in baboons (Papio papio). Electroencephalogr Clin Neurophysiol 1978;44: 232–5. [DOI] [PubMed] [Google Scholar]
- [74].Fraioli B, Guidetti. Effects of stereotactic lesions of the dentate nucleus of the cerebellum in man. Appl Neurophysiol 1975;38: 81–90. [DOI] [PubMed] [Google Scholar]
- [75].Babb TL, Mitchell AG Jr., Crandall PH. Fastigiobulbar and dentatothalamic influences on hippocampal cobalt epilepsy in the cat. Electroencephalogr Clin Neurophysiol 1974;36: 141–54. [DOI] [PubMed] [Google Scholar]
- [76].Dow RS, Fernandez-Guardiola A, Manni E. The influence of the cerebellum on experimental epilepsy. Electroencephalogr Clin Neurophysiol 1962;14: 383–98. [DOI] [PubMed] [Google Scholar]
- [77].Mutani R, Bergamini L, Doriguzzi T. Experimental evidence for the existence of an extrarhinencephalic control of the activity of the cobalt rhinencephalic epileptogenic focus. Part 2. Effects of the paleocerebellar stimulation. Epilepsia 1969;10: 351–62. [DOI] [PubMed] [Google Scholar]
- [78].Hablitz JJ. Intramuscular penicillin epilepsy in the cat: effects of chronic cerebellar stimulation. Exp Neurol 1976;50: 505–14. [DOI] [PubMed] [Google Scholar]
- [79].Kreindler A Active Arrest Mechanisms of Epileptic Seizures. Epilepsia 1962;3: 329–337. [Google Scholar]
- [80].Strain GM, Van Meter WG, Brockman WH. Elevation of seizure thresholds: a comparison of cerebellar stimulation, phenobarbital, and diphenylhydantoin. Epilepsia 1978;19: 493–504. [DOI] [PubMed] [Google Scholar]
- [81].Fanardjian VV, Donhoffer H. An Electrophysiological Study of Cerebellohippocampal Relationships in the Unrestrained Cat. Acta Physiol Acad Sci Hung 1964;24: 321–33. [PubMed] [Google Scholar]
- [82].Iwata K, Snider RS. Cerebello-hippocampal influences on the electroencephalogram. Electroencephalogr Clin Neurophysiol 1959;11: 439–46. [DOI] [PubMed] [Google Scholar]
- [83].Maiti A, Snider RS. Cerebellar control of basal forebrain seizures: amygdala and hippocampus. Epilepsia 1975;16: 521–33. [DOI] [PubMed] [Google Scholar]
- [84].Snider RS. Cerebellar modifications of abnormal discharges in cerebral sensory and motor areas. New York: Plenum Press; 1974. [Google Scholar]
- [85].Dauth GW, Carr D., Gilman S. Cerebellar cortical stimulation effects on EEG activity and seizure after-discharge in anesthetized cats. New York: Plenum Press; 1974. [Google Scholar]
- [86].Grimm RJ, Frazee JG, Bell CC, Kawasaki T, Dow RS. Quantitative studies in cobalt model epilepsy: the effect of cerebellar stimulation. Int J Neurol 1970;7: 126–40. [PubMed] [Google Scholar]
- [87].Reimer GR, Grimm RJ, Dow RS. Effects of cerebellar stimulation on cobalt-induced epilepsy in the cat. Electroencephalogr Clin Neurophysiol 1967;23: 456–62. [DOI] [PubMed] [Google Scholar]
- [88].Lockard JS, Ojemann GA, Congdon WC, DuCharme LL. Cerebellar stimulation in alumina-gel monkey model: inverse relationship between clinical seizures and EEG interictal bursts. Epilepsia 1979;20: 223–34. [DOI] [PubMed] [Google Scholar]
- [89].Hablitz JJ, McSherry JW, Kellaway P. Cortical seizures following cerebellar stimulation in primates. Electroencephalogr Clin Neurophysiol 1975;38: 423–6. [DOI] [PubMed] [Google Scholar]
- [90].Myers RR, Burchiel KJ, Stockard JJ, Bickford RG. Effects of acute and chronic paleocerebellar stimulation on experimental models of epilepsy in the cat: studies with enflurane, pentylenetetrazol, penicillin, and chloralose. Epilepsia 1975;16: 257–67. [DOI] [PubMed] [Google Scholar]
- [91].Rucci FS, Giretti ML, La Rocca M. Cerebellum and hyperbaric oxygen. Electroencephalogr Clin Neurophysiol 1968;25: 359–71. [DOI] [PubMed] [Google Scholar]
- [92].Cooke PM, Snider RS. Some cerebellar influences on electrically-induced cerebral seizures. Epilepsia 1955;4: 19–28. [DOI] [PubMed] [Google Scholar]
- [93].Hemmy DC, Larson SJ, Sances A, Jr., Millar EA. The effect of cerebellar stimulation on focal seizure activity and spasticity in monkeys. J Neurosurg 1977;46: 648–53. [DOI] [PubMed] [Google Scholar]
- [94].Wada JA. Progressive seizure recruitment in subhuman primates and effect of cerebellar stimulation upon developed vs. developing amygdaloid seizures. Bol Estud Med Biol 1974;28: 285–301. [PubMed] [Google Scholar]
- [95].Godlevskii LS, Stepanenko KI, Lobasyuk BA, Sarakhan EV, Bobkova LM. The effects of electrical stimulation of the paleocerebellar cortex on penicillin-induced convulsive activity in rats. Neurosci Behav Physiol 2004;34: 797–802. [DOI] [PubMed] [Google Scholar]
- [96].Davis R, Emmonds SE. Cerebellar stimulation for seizure control: 17-year study. Stereotact Funct Neurosurg 1992;58: 200–8. [DOI] [PubMed] [Google Scholar]
- [97].Cooper IS. Effect of chronic stimulation of anterior cerebellum on neurological disease. Lancet 1973;1: 206. [DOI] [PubMed] [Google Scholar]
- [98].Gilman S, Clinical, morphological, biochemical, and physiological effects of cerebellar stimulation. : Macel Dekker; 1977. [Google Scholar]
- [99].Sramka M, Fritz G, Galanda M, Nadvornik P. Some observations in treatment stimulation of epilepsy. Acta Neurochir (Wien) 1976: 257–62. [DOI] [PubMed] [Google Scholar]
- [100].Chkhenkeli SA, Sramka M, Lortkipanidze GS, Rakviashvili TN, Bregvadze E, Magalashvili GE, Gagoshidze T, Chkhenkeli IS. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg 2004;106: 318–29. [DOI] [PubMed] [Google Scholar]
- [101].Davis R, Gray E, Engle H, Dusnak A. Reduction of Intractable Seizures Using Cerebellar Stimulation. Applied Neurophysiology 1983;46: 57–61. [DOI] [PubMed] [Google Scholar]
- [102].Klun B, Stojanovic V, Strojnik P, Stanic U, Vodovnik L, Zirovnik S. Chronic Cerebellar Stimulation in the Treatment of Epilepsy. Berlin, Heidelberg: Springer; 1987. [Google Scholar]
- [103].Levy LF, Auchterlonie WC. Chronic cerebellar stimulation in the treatment of epilepsy. Epilepsia 1979;20: 235–45. [DOI] [PubMed] [Google Scholar]
- [104].Velasco F, Carrillo-Ruiz JD, Brito F, Velasco M, Velasco AL, Marquez I, Davis R. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia 2005;46: 1071–81. [DOI] [PubMed] [Google Scholar]
- [105].Bidzinski J, Bacia T, Ostrowski K, Czarkwiani L. [Effect of cerebellar cortical electrostimulation on the frequency of epileptic seizures in severe forms of epilepsy]. Neurol Neurochir Pol 1981;15: 605–9. [PubMed] [Google Scholar]
- [106].Van Buren JM, Wood JH, Oakley J, Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg 1978;48: 407–16. [DOI] [PubMed] [Google Scholar]
- [107].Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry 1984;47: 769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Streng ML, Popa LS, Ebner TJ. Complex Spike Wars: a New Hope. Cerebellum 2018;17: 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Eccles JC, Ito M, Szentágothai Jn. The cerebellum as a neuronal machine. Berlin, New York: etc.: Springer-Verlag; 1967. [Google Scholar]
- [110].Granit R, Phillips CG. Effects on Purkinje cells of surface stimulation of the cerebellum. J Physiol 1957;135: 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dauth GW, Dell S, Gilman S. Alteration of Purkinje cell activity from transfolial stimulation of the cerebellum in the cat. Neurology 1978;28: 654–60. [DOI] [PubMed] [Google Scholar]
- [112].Bantli H, Bloedel JR, Anderson G, McRoberts R, Sandberg E. Effects of stimulating the cerebellar surface on the activity in penicillin foci. J Neurosurg 1978;48: 69–84. [DOI] [PubMed] [Google Scholar]
- [113].Grado LL, Johnson MD, Netoff TI. Bayesian adaptive dual control of deep brain stimulation in a computational model of Parkinson’s disease. PLoS Comput Biol 2018;14: e1006606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Good LB, Sabesan S, Marsh ST, Tsakalis K, Treiman D, Iasemidis L. Control of synchronization of brain dynamics leads to control of epileptic seizures in rodents. Int J Neural Syst 2009;19: 173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nat Neurosci 2015;18: 331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Krook-Magnuson E, Gelinas JN, Soltesz I, Buzsaki G. Neuroelectronics and Biooptics: Closed-Loop Technologies in Neurological Disorders. JAMA Neurol 2015;72: 823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun 2013;4: 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Armstrong C, Krook-Magnuson E, Oijala M, Soltesz I. Closed-loop optogenetic intervention in mice. Nat Protoc 2013;8: 1475–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci 2013;16: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Christenson Wick Z, Krook-Magnuson E. Specificity, Versatility, and Continual Development: The Power of Optogenetics for Epilepsy Research. Front Cell Neurosci 2018;12: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lee KH, Mathews PJ, Reeves AM, Choe KY, Jami SA, Serrano RE, Otis TS. Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron 2015;86: 529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Brown ST, Raman IM. Sensorimotor Integration and Amplification of Reflexive Whisking by Well-Timed Spiking in the Cerebellar Corticonuclear Circuit. Neuron 2018;99: 564–575 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Gauck V, Jaeger D. The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. J Neurosci 2000;20: 3006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zangiabadi N, Ladino LD, Sina F, Orozco-Hernandez JP, Carter A, Tellez-Zenteno JF. Deep Brain Stimulation and Drug-Resistant Epilepsy: A Review of the Literature. Front Neurol 2019;10: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kros L, Eelkman Rooda OHJ, De Zeeuw CI, Hoebeek FE. Controlling Cerebellar Output to Treat Refractory Epilepsy. Trends Neurosci 2015;38: 787–799. [DOI] [PubMed] [Google Scholar]
- [126].Streng ML, Krook-Magnuson E. Excitation, but not inhibition, of the fastigial nucleus provides powerful control over temporal lobe seizures. J Physiol 2020;598: 171–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Faull RL, Carman JB. The cerebellofugal projections in the brachium conjunctivum of the rat I. The contralateral ascending pathway. J Comp Neurol 1978;178: 495–517. [PubMed] [Google Scholar]
- [128].Sakai K, Touret M, Salvert D, Leger L, Jouvet M. Afferent projections to the cat locus coeruleus as visualized by the horseradish peroxidase technique. Brain Res 1977;119: 21–41. [DOI] [PubMed] [Google Scholar]
- [129].Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol 1974;45: 268–87. [DOI] [PubMed] [Google Scholar]
- [130].Chen M, Guo D, Xia Y, Yao D. Control of Absence Seizures by the Thalamic Feed-Forward Inhibition. Front Comput Neurosci 2017;11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Huguenard JR. Neuronal circuitry of thalamocortical epilepsy and mechanisms of antiabsence drug action. Adv Neurol 1999;79: 991–9. [PubMed] [Google Scholar]
- [132].Gornati SV, Schafer CB, Eelkman Rooda OHJ, Nigg AL, De Zeeuw CI, Hoebeek FE. Differentiating Cerebellar Impact on Thalamic Nuclei. Cell Rep 2018;23: 2690–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sorokin JM, Davidson TJ, Frechette E, Abramian AM, Deisseroth K, Huguenard JR, Paz JT. Bidirectional Control of Generalized Epilepsy Networks via Rapid Real-Time Switching of Firing Mode. Neuron 2017;93: 194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rochefort C, Lefort JM, Rondi-Reig L. The cerebellum: a new key structure in the navigation system. Front Neural Circuits 2013;7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci 2009;32: 413–34. [DOI] [PubMed] [Google Scholar]
- [136].Yu W, Krook-Magnuson E. Cognitive Collaborations: Bidirectional Functional Connectivity Between the Cerebellum and the Hippocampus. Front Syst Neurosci 2015;9: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bohne P, Schwarz MK, Herlitze S, Mark MD. A New Projection From the Deep Cerebellar Nuclei to the Hippocampus via the Ventrolateral and Laterodorsal Thalamus in Mice. Front Neural Circuits 2019;13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 2015;524: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Watson TC, Obiang P, Torres-Herraez A, Watilliaux A, Coulon P, Rochefort C, Rondi-Reig L. Anatomical and physiological foundations of cerebello-hippocampal interaction. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Choe KY, Sanchez CF, Harris NG, Otis TS, Mathews PJ. Optogenetic fMRI and electrophysiological identification of region-specific connectivity between the cerebellar cortex and forebrain. Neuroimage 2018;173: 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lefort JM, Vincent J, Tallot L, Jarlier F, De Zeeuw CI, Rondi-Reig L, Rochefort C. Impaired cerebellar Purkinje cell potentiation generates unstable spatial map orientation and inaccurate navigation. Nat Commun 2019;10: 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Rochefort C, Arabo A, Andre M, Poucet B, Save E, Rondi-Reig L. Cerebellum shapes hippocampal spatial code. Science 2011;334: 385–9. [DOI] [PubMed] [Google Scholar]
- [143].McAfee SS, Liu Y, Sillitoe RV, Heck DH. Cerebellar Lobulus Simplex and Crus I Differentially Represent Phase and Phase Difference of Prefrontal Cortical and Hippocampal Oscillations. Cell Rep 2019;27: 2328–2334 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wikgren J, Nokia MS, Penttonen M. Hippocampo-cerebellar theta band phase synchrony in rabbits. Neuroscience 2010;165: 1538–45. [DOI] [PubMed] [Google Scholar]
- [145].Popa LS, Hewitt AL, Ebner TJ. The cerebellum for jocks and nerds alike. Front Syst Neurosci 2014;8: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sokolov AA, Miall RC, Ivry RB. The Cerebellum: Adaptive Prediction for Movement and Cognition. Trends Cogn Sci 2017;21: 313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Canto CB, Onuki Y, Bruinsma B, van der Werf YD, De Zeeuw CI. The Sleeping Cerebellum. Trends Neurosci 2017;40: 309–323. [DOI] [PubMed] [Google Scholar]
- [148].Cunchillos JD, De Andres I. Participation of the cerebellum in the regulation of the sleep-wakefulness cycle. Results in cerebellectomized cats. Electroencephalogr Clin Neurophysiol 1982;53: 549–58. [DOI] [PubMed] [Google Scholar]
- [149].Ewell LA, Liang L, Armstrong C, Soltesz I, Leutgeb S, Leutgeb JK. Brain State Is a Major Factor in Preseizure Hippocampal Network Activity and Influences Success of Seizure Intervention. J Neurosci 2015;35: 15635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Fujita S, Toyoda I, Thamattoor AK, Buckmaster PS. Preictal activity of subicular, CA1, and dentate gyrus principal neurons in the dorsal hippocampus before spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci 2014;34: 16671–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Khan S, Nobili L, Khatami R, Loddenkemper T, Cajochen C, Dijk DJ, Eriksson SH. Circadian rhythm and epilepsy. Lancet Neurol 2018;17: 1098–1108. [DOI] [PubMed] [Google Scholar]
- [152].Purnell BS, Thijs RD, Buchanan GF. Dead in the Night: Sleep-Wake and Time-Of-Day Influences on Sudden Unexpected Death in Epilepsy. Front Neurol 2018;9: 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Sedigh-Sarvestani M, Thuku GI, Sunderam S, Parkar A, Weinstein SL, Schiff SJ, Gluckman BJ. Rapid eye movement sleep and hippocampal theta oscillations precede seizure onset in the tetanus toxin model of temporal lobe epilepsy. J Neurosci 2014;34: 1105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Uusisaari MY, Knopfel T. Diversity of neuronal elements and circuitry in the cerebellar nuclei. Cerebellum 2012;11: 420–1. [DOI] [PubMed] [Google Scholar]
- [155].Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, du Lac S. Glycinergic projection neurons of the cerebellum. J Neurosci 2009;29: 10104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Hashimoto M, Yamanaka A, Kato S, Tanifuji M, Kobayashi K, Yaginuma H. Anatomical Evidence for a Direct Projection from Purkinje Cells in the Mouse Cerebellar Vermis to Medial Parabrachial Nucleus. Front Neural Circuits 2018;12: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Muller T Large nerve cells with long axons in the granular layer and white matter of the murine cerebellum. J Anat 1994;184 ( Pt 2): 419–23. [PMC free article] [PubMed] [Google Scholar]
- [158].Flace P, Lorusso L, Laiso G, Rizzi A, Cagiano R, Nico B, Ribatti D, Ambrosi G, Benagiano V. Calbindin-D28K immunoreactivity in the human cerebellar cortex. Anat Rec (Hoboken) 2014;297: 1306–15. [DOI] [PubMed] [Google Scholar]
- [159].Schilling K, Oberdick J, Rossi F, Baader SL. Besides Purkinje cells and granule neurons: an appraisal of the cell biology of the interneurons of the cerebellar cortex. Histochem Cell Biol 2008;130: 601–15. [DOI] [PubMed] [Google Scholar]
- [160].Laine J, Axelrad H. Morphology of the Golgi-impregnated Lugaro cell in the rat cerebellar cortex: a reappraisal with a description of its axon. J Comp Neurol 1996;375: 618–40. [DOI] [PubMed] [Google Scholar]
- [161].Crook JD, Hendrickson A, Erickson A, Possin D, Robinson FR. Purkinje cell axon collaterals terminate on Cat-301+ neurons in Macaca monkey cerebellum. Neuroscience 2007;149: 834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Walker MC, Kullmann DM. Optogenetic and chemogenetic therapies for epilepsy. Neuropharmacology 2019: 107751. [DOI] [PubMed] [Google Scholar]
- [163].Fountas KN, Kapsalaki E, Hadjigeorgiou G. Cerebellar stimulation in the management of medically intractable epilepsy: a systematic and critical review. Neurosurg Focus 2010;29: E8. [DOI] [PubMed] [Google Scholar]
- [164].Wathen CA, Frizon LA, Maiti TK, Baker KB, Machado AG. Deep brain stimulation of the cerebellum for poststroke motor rehabilitation: from laboratory to clinical trial. Neurosurg Focus 2018;45: E13. [DOI] [PubMed] [Google Scholar]
- [165].Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol 1992;326: 549–60. [DOI] [PubMed] [Google Scholar]
- [166].Kandel ER. Principles of neural science. 5th ed. New York: McGraw-Hill; 2013. [Google Scholar]
- [167].Popa LS, Streng ML, Hewitt AL, Ebner TJ. The Errors of Our Ways: Understanding Error Representations in Cerebellar-Dependent Motor Learning. Cerebellum 2016;15: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 2006;7: 511–22. [DOI] [PubMed] [Google Scholar]
- [169].Itō M. The cerebellum and neural control. New York: Raven Press; 1984. [Google Scholar]
- [170].Llinas RR. The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits 2013;7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 1997;17: 4517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Teune TM, Van der Burg J, De Zeeuw CI, Voogd J, Ruigrok TJH. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: A light microscopic and ultrastructural triple-tracer study in the rat. Journal of Comparative Neurology 1998;392: 164–178. [DOI] [PubMed] [Google Scholar]
- [173].Bengtsson F, Hesslow G. Cerebellar control of the inferior olive. Cerebellum 2006;5: 7–14. [DOI] [PubMed] [Google Scholar]
- [174].Kenyon GT, Medina JF, Mauk MD. A mathematical model of the cerebellar-olivary system I: self-regulating equilibrium of climbing fiber activity. J Comput Neurosci 1998;5: 17–33. [DOI] [PubMed] [Google Scholar]
- [175].Ankri L, Husson Z, Pietrajtis K, Proville R, Lena C, Yarom Y, Dieudonne S, Uusisaari MY. A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Gao Z, Proietti-Onori M, Lin Z, Ten Brinke MM, Boele HJ, Potters JW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI. Excitatory Cerebellar Nucleocortical Circuit Provides Internal Amplification during Associative Conditioning. Neuron 2016;89: 645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Schmahmann JD. The cerebellum and cognition. Neurosci Lett 2019;688: 62–75. [DOI] [PubMed] [Google Scholar]


