Abstract
Alcohol and other substance use disorders (AUD and SUD) are complex diseases that are postulated to have a polygenic inheritance and are often comorbid with other disorders. The comorbidities may arise partially through genetic pleiotropy. Identification of specific gene variants accounting for large parts of the variance in these disorders has yet to be accomplished. We describe a flexible strategy that takes a variant-trait association database and determines if a subset of disease/straits are potentially pleiotropic with the disorder under study. We demonstrate its usage in a study of use disorders in two independent cohorts: alcohol, stimulants, cannabis (CUD), and multi-substance use disorders (MSUD) in American Indians (AI), and AUD and CUD in Mexican Americans (MA). Using a machine learning method with variants in GWAS catalog, we identified 229-246 pleiotropic variants for AI and 153-160 for MA for each SUD. Inflammation was the most enriched for MSUD and AUD in AI. Neurological disorder was the most significantly enriched for CUD in both cohorts, and for AUD and stimulants in AI. Of the select pleiotropic genes shared among substances-cohorts, multiple biological pathways implicated in SUD and other psychiatric disorders were enriched, including: neurotrophic factors, immune responses, extracellular matrix, and circadian regulation. Shared pleiotropic genes were significantly up-regulated in brain regions playing important roles in SUD, down-regulated in esophagus mucosa, and differentially regulated in adrenal gland. This study fills a gap for pleiotropy detection in under-studied admixed populations, and identifies pleiotropic variants that may be potential targets of interest for SUD.
Keywords: pleiotropy, substance use disorders, alcohol use disorders, comorbidity, genetics, admixed population
INTRODUCTION
Alcohol use disorders (AUD) and other substance use disorders (SUD) are complex diseases, and like many other complex diseases, are postulated to have polygenic mode of inheritance and are often comorbid with other disorders1-4. Rates of AUD and SUD also vary across ethnic groups and populations5. For instance, select American Indian tribes have been shown to have significantly higher rates of AUD than the general population in the U.S.6-8 These differences are likely due to both environmental and genetic factors.
Previous studies have shown that AUD may co-occur with other SUD such as cannabis, stimulants or nicotine use disorders5. There are a number of general theories that have been put forth to explain the psychobiological development of substance use disorders. Some are specific to particular substances such as gene variants that code for variation in drug metabolism. For instance, variants in the genes coding for the major alcohol metabolizing enzymes, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), have been consistently demonstrated to significantly affect drinking behavior and risk for AUD9. Whereas gene variants in CYP2A6, that induce a slower metabolism of nicotine, are associated with the tendency for less severe nicotine withdrawal symptoms and a higher likelihood of quitting smoking10.
Other sets of theories posit that common pathways and neurocircuitries underlie a whole host of addictive behaviors. It has been suggested that taking addictive drugs as well as behaviors such as compulsive eating or sexual behavior may at the cellular and circuit level be associated with “motivational drives” or “reward mechanisms” that are dependent on concentrations of dopamine in the striatum11,12. Although such reductionist theories are conceptually appealing, and some studies have provided evidence to suggest that stimulants and, to a certain extent, alcohol increase striatal dopamine levels, there is less evidence for dopamine involvement in opiate, nicotine or cannabis dependence13.
Alternatively, incentive sensitization theory of addiction proposes that brain systems mediating incentive salience (“wanting”) to drugs and associated cues are sensitized through long-lasting neuroadaptations14. Neuroadaptations in key neurochemical elements in the brain stress systems has also been suggested to underlie drug action15. The development of a substance use disorder has also been conceptualized, in recent theories, as a spiraling cycle of hedonic dysregulation not only involving brain systems that mediate positive reinforcements associated with the rewarding effects of the drug but also involving systems that affect negative reinforcements driven by aversive, negative affective states16.
AUD and SUD can also be comorbid with other psychiatric disorders, for instance schizophrenia, mood disorders (major depressive disorders (MDD), bipolar disorder), anxiety disorders (panic disorder, post-traumatic stress disorder, and generalized anxiety disorder), and externalizing disorders (antisocial personality disorder, conduct disorder, attention-deficit/hyperactivity disorder (ADHD))5,17,18. Additionally, AUD may co-occur with metabolic disorders19 or liver diseases20. There are a number of reasons why two or more diseases may co-occur. Since many mental disorders are common in the population they can co-occur by chance. Alternatively, one disease may induce or cause the other directly or indirectly, or they may share the same genetic, biological or environmental causes. While it is likely that a combination of factors may contribute to the comorbidity between complex diseases, one of the fundamental questions that this study attempts to address is whether there are shared genetic factors underlying AUD, various SUD, and other diseases. In particular, given the high rates of comorbidity between alcohol and other substance use disorders, and in light of the addiction theories, we ask whether there are common genetic substrates that underlie substance use disorder symptoms. Further, we suggest that there may be other diseases or traits that are genetically linked to SUD but are not readily apparent (such as risk for liver disease or pancreatitis).
When one genetic locus affects two or more traits, the affected traits are said to be pleiotropic21. Some genetic pleiotropy between diseases is expected, while others may be unsuspected. For instance, a gene product such as an extracellular matrix protein might influence cells in multiple organ systems throughout the organism. As a result, pleiotropy of complex disorders could theoretically manifest in a wide range of unexpected traits. For complex diseases, moreover, there is likely polygenic pleiotropy. There are a number of pleiotropy detection methods for complex human traits (see review22). They can be classified into within-cohort methods or cross-sample methods. The former includes genetic correlation methods such as genome-wide complex trait analysis (GCTA)23, multivariate genome-wide association studies (GWAS), and more recently phenome-wide association studies (PheWAS) where associations between each variant and all the traits in the dataset can be tested24. The latter includes an application of polygenetic risk scores (PRS)25, cross-trait linkage disequilibrium score regression (LDSR)26, and meta-analysis. LDSR analysis of large-scale GWAS studies of European and African Americans have indeed shown that AUD is genetically correlated with other substance use traits including: nicotine dependence, cannabis initiation, and multiple psychiatric traits and disorders such as depressive symptoms, MDD, ADHD, bipolar disorder, and schizophrenia27,28. While each method has its applicability, in general, the within-cohort methods require individuals in the same sample collection to have phenotypic data for all of the traits being investigated. The cross-sample methods can accommodate multiple samples and exclude within-sample correlations; however, they often demand GWAS summary statistics from populations of similar ancestral origins. These constraints pose challenges for the investigation of under-studied admixed populations, such as American Indians, where limited genetic studies exist for genetic correlation analyses using existing methods.
We propose a flexible framework that takes a database of known variant-trait/disease associations and determines if a subset of the traits and diseases are potentially pleiotropic with the disorder under study in a population of interest. We use these analyses to ask whether there are common genetic substrates that underlie substance use disorder severity, and whether other diseases or traits that are genetically linked to these disorders can be identified. To accomplish this we applied our methodology to the study of AUD and SUD in two independent populations at high risk for use disorders: American Indians and Mexican Americans. We used the entire set of variants in the NHGRI-EBI catalog of published GWAS that were associated with any common diseases or traits, and applied a machine learning technique to select a subset of the variants that are associated with SUD. The selected pleiotropic variants were then subjected to disease enrichment and functional network analyses.
MATERIALS AND METHODS
Participants
Two independent populations were studied: 742 Native Americans of extended pedigrees from an American Indian cohort (AI), and 547 Mexican Americans from a primarily second-generation young adult Mexican American cohort (MA). The population characteristics and the recruitment procedures of the two cohorts have been respectively described8,29-31. Their demographics are characterized in Table S1. The Scripps Research Institute Institutional Review Board (TSRI-IRB) approved the protocol of the study of both cohorts.
Phenotypes and genotypes
All participants were assessed using Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA)32,33, from which the phenotypes were derived. We used measures of the clinical course of alcoholism originally described by Schuckit and colleagues34. These measures were based on the relative order of the appearance of major “alcohol-related life events”. These life events have been shown to be highly similar and consistent across many different subgroups and populations, although age of onset and endorsement rates of individual events can differ29,35-42. We further extended these measures to quantitate life events during the clinical course of other substance use disorders. We also derived a metric that indexes the severity level of SUD progression based on the sequential occurrence of the events. The metric gives larger weights to the more severe events that occur later in the clinical course and are more associated with severe use disorder (see SI methods)37,43. For the MA cohort, we quantitated the severity levels of alcohol use disorders (AUD), and cannabis use disorders (CUD). For the AI cohort, we quantitated the severity levels of AUD, CUD, cocaine and stimulant use disorders, along with multi-substance use disorders (MSUD)44. Tables S2A, B, C list the substance-related life events in the clinical course of AUD (36 events), CUD (20 events), and cocaine & stimulant disorders (32 events) respectively. The MSUD was defined as having AUD and at least one other substance use disorder including cannabis, opioid, cocaine and other stimulants. For the MSUD phenotype, each substance was assigned a weight (alcohol=1, cannabis=2, sedative=4, non-heroin opioid=4, cocaine=5, stimulant=5, heroin opioid=10). An individual’s number of dependence symptoms for a particular drug was multiplied by the drug’s weight. The severity level of MSUD was determined by the total sum of weighted symptom counts of all drugs that an individual used.. The distributions of the phenotypes are shown in Figures S1 and S2. Phenotype correlations are shown in Table S3. The AI participants had low-coverage whole genome sequencing (LCWGS) on blood-derived DNAs45. The MA participants had exome genotyping with imputation46. Details are given in SI methods.
Variant-trait association database
We downloaded the NHGRI-EBI catalog of published GWAS release on 2018-09-15 v1.0.2 that contained 56301 single nucleotide polymorphisms (SNPs) and 71673 unique SNP-trait associations at p-values of less than 10−5 resulting from 3567 publications. Of the 53526 unique autosomal SNPs from the GWAS catalog, 46673 of these variants were found in the AI cohort with LCWGS; 31034 were found in the MA cohort with exome data. We refer to these variants as candidate variants (or candidate variant set). 29927 candidate variants were common to both cohorts; 16747 and 1107 were unique to AI and MA respectively. MA had fewer SNPs since exome data were available. 5746 autosomal SNPs from the GWAS catalog were not found in either cohort. These SNPs may represent alleles present in other populations but absent from the two population cohorts under study, or could possibly be unique intergenic variants in MA, or small insertions or deletions that our genotyping excluded. GWAS catalog mapped the reported traits to experimental factor ontology (EFO) terms that were classified into seventeen parent categories47, which we refer to as disease category. Table S4 shows the distributions of the candidate variants by their mapped disease category.
While GWAS catalog contained the variants of interest, to test the robustness of the method, we added 25% randomly chosen variants from each cohort to each candidate variant set forming a new variant set, and referred to the new set as candidate-random variant set. The randomly chosen variants were assigned disease category unknown with the following exception: if a random variant happened to be a variant in the GWAS catalog or in high LD ((∣D’∣ ≥ 0.8 & R2 ≥ 0.2) with a variant in the GWAS catalog, the random variant would be assigned to the same trait and disease category as those of the catalog variant. The candidate-random variant set has 58284 and 38731 variants for the AI and MA respectively.
Variant selection and pleiotropic disease enrichment analysis
To determine which variants (from the candidate variant set) were associated with a SUD trait, we used regularized multiple linear regression. Multiple-regression allows us to model the relations between the SUD trait and all variants of interest simultaneously. However, we have many more variants (explanatory variables) than samples (outcomes) resulting in insufficient degrees of freedom to estimate the full model. To resolve this problem and to avoid over-fitting, we used a technique called regularization that constrains the coefficient estimates. This is consistent with the idea that most variants have tiny or insignificant effects on the trait thus their effect sizes could be quickly reduced or even eliminated during the estimation. Specifically, we used L1 regularized regression method lasso48 that introduced a penalty parameter to allow some of the coefficients to be shrunken all the way to zero, resulting in a solution with a small number of the explanatory variables with non-zero coefficients. Those variants with non-zero coefficients are the “selected variants” from the candidate set that have the highest association with the phenotype. We performed this variant selection process for each SUD trait for the two cohorts, from each cohort’s respective candidate variant set and the candidate-random variant set. To control for the population structure and possible relatedness, we incorporated a genetic relationship matrix into the model. We further included age, age-squared, and sex as covariates. The penalty parameter of lasso controls how many variants might be selected in the end; this parameter was determined by a permutation-based selection procedure with type-I-error (false positive) rate set at 0.00549,50. We refer to the resulting set of variants as select pleiotropic variants (or pleiotropic variants) for each SUD phenotype and cohort.
For a set of select pleiotropic variants, the disease category enrichment is determined as the ratio of the percentage of the pleiotropic variants that are mapped to the disease category over the percentage of the candidate variants (or candidate-random variants) that are mapped to the category. The significance of the enrichments was obtained through permutation.
Details are given in SI methods.
Shared select pleiotropic loci
Once we obtained select pleiotropic variants (as defined above) for each SUD phenotype and cohort, we investigated whether any of these variants were shared between traits within the same cohort or between the two cohorts. To obtain select pleiotropic variants shared between traits and/or between cohorts, we took intersections between sets of the select pleiotropic variants while taking linkage disequilibrium (LD) structures into consideration. Essentially within the same cohort two variants were considered as the same locus if they were in LD with each other. Across the two cohorts, if two loci overlapped in their genomic positions, there were treated as one locus. Select pleiotropic variants within a certain LD threshold were consecutively merged, resulting into pleiotropic loci. Intersections were taken between the pleiotropic loci to finally determine the shared select pleiotropic loci (or shared pleiotropic loci) between SUD traits within the same cohort, or of the same trait between the cohorts. Details are given in SI methods.
Functional analyses
In the GWAS catalog, reported variants were mapped to genes. We used this information in the functional analysis. We refer to a gene that was mapped to a select pleiotropic variant as pleiotropic gene. Genes corresponding to the shared pleiotropic loci (as described above) are referred to as shared select (pleiotropic) genes. We subjected all shared pleiotropic genes to functional analyses.
Gene expression, gene ontology (GO) and pathway enrichment analyses were performed using GENE2FUNC in FUMA, in which enriched biological functions were extracted by testing against gene sets from MsigDB51 and WikiPathways52 using hypergeometric tests53. The tissue-specific differential gene expression test was conducted for shared pleiotropic genes against the entire list of genes that had significantly increased or decreased expressions in a certain tissue sample compared to all other samples. The analysis utilized tissue-specific transcriptome data across 53 tissue types from GTEx v754. Integrated network analysis was done with GeneMANIA55. We allowed the GeneMANIA to include 20 additional related genes. The following networks were included in this analysis: co-expressions, co-localizations, and consolidated pathways. Additionally, certain pleiotropic variants were tested against brain-specific cis-eQTLs database BRAINEAC56.
RESULTS
Select pleiotropic variants and disease enrichments
The select pleiotropic variant sets had 229-246 variants for the AI cohort, and 153-160 variants for the MA (see Table S5 for the complete list). Their mappings to the disease categories and enrichments are detailed in Tables S7 & S8. Figure 1 illustrates the enrichments for pleiotropic disease categories. Inflammatory measurement was the most enriched category for MSUD severity (p=0.016) and AUD severity in AI although it was less significant for AUD (p=0.065). Neurological disorder was the most enriched for the severity level of cannabis use disorder (CUD) in both AI (p=0.003) and MA (p=0.021), and was also significantly enriched for alcohol (p=0.029) and stimulants (p=0.024) use disorders but not for MSUD in AI. Figures 2 and S3 detail the traits being selected under each disease category.
Fig. 1.
Disease enrichments for each substance use disorder in select pleiotropic variants from the NHGRI-EBI GWAS catalog candidate variant set. The number in each bar indicates the number of SNPs selected for the substance use disorder. AL: alcohol use disorders; CS: cocaine & stimulant use disorders; MJ: cannabis (marijuana) use disorders; MS: multi-substance use disorders; AI: American Indian; MA: Mexican American. See Table S6 for exact values of enrichments and p-values (** p<0.05, * p<0.1).
Fig. 2.
Mapped GWAS traits and diseases in the categories of (A) Inflammatory measurement and (B) Neurological disorder of select pleiotropic variants for each substance in the AI and MA cohorts. Numbers in each cell indicate the number of select pleiotropic variants for the substance use disorder. Column labels: AL: alcohol use disorders; CS: cocaine & stimulant use disorders; MJ: cannabis (marijuana) use disorders; MS: multi-substance use disorders; Black font: American Indian; Magenta font: Mexican American.
Of note, variants associated with inflammatory measurement basophil count (Figure 2A) were selected across all substances in both cohorts. Three variants associated with c-reactive protein (CRP) measurement were selected for MSUD in AI. Candidate variants associated with alcohol dependence (Figure 2B), alcohol dependence measurement and consumption measurement (Figure S3M) were selected for AUD in both cohorts, including rs1229984 (ADH1B*2) for MA and rs1229978 downstream of ADH1C for AI (Table S5). Variant rs10392 on gene PPP1R16B, also associated with alcohol dependence, was selected for severities of alcohol, stimulants and cannabis use disorders in AI (Table S5). Among the neurological disorders (Figure 2B), schizophrenia and unipolar depression variants were selected for all substances in both cohorts. Several variants that were associated with multiple sclerosis or ADHD were also selected across substances in both AI and MA.
Other traits that were selected for all or all but one of the substances across the cohorts included: bipolar disorder, mental or behavioral disorder, migraine disorder (Figure 2B); alcohol drinking, smoking behavior, intelligence (Figure S3A); immune system disorders, psoriasis, ankylosing spondylitis (Figure S3H), and immune related digestive system disorders (Figure S3F); body mass index (Figure S3B); breast and prostate cancers (Figure S3C); coronary artery disease (Figure S3D), artery calcification (Figure S3N), mean corpuscular volume (MCV) and hemoglobin (MCH) (Figure S3G), as well as other cardiovascular risk measurements (Figure S3E); LDL (Figure S4I); type II diabetes (Figure S4K); and chronic obstructive pulmonary disease (COPD) (Figure S3L).
Shared select pleiotropic loci
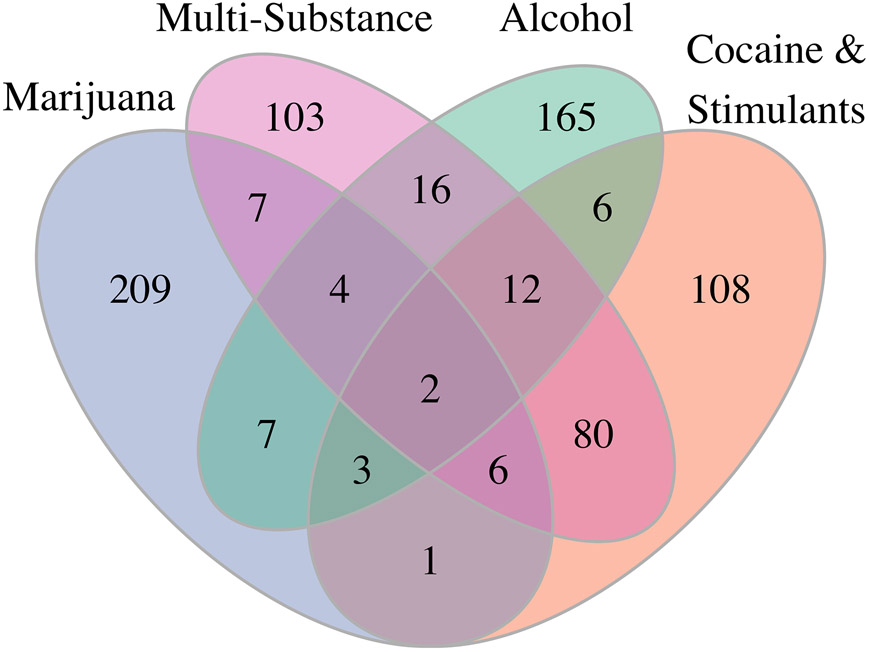
Figure 3 illustrates the number of select pleiotropic loci (pleiotropic variants taking LD into account) unique to one substance use disorder, or common to two or more substance use disorders in the AI cohort. Two variants were found in all four traits in AI: rs12156277 on gene CSMD1, and rs1524058 located between LOC105375236 and STARD3NL. 12 additional loci composed of 19 unique pleiotropic variants that were common to alcohol, stimulants and multi-substance use disorders but not cannabis use disorder (Table 1, Figure S4) in AI. Among the genes that these loci mapped to were: a vascular cell adhesion molecule gene VCAM1 whose expression was shown to be affected by alcohol and nicotine consumption57,58, and the EXT family gene, EXTL2, that may mediate axon regeneration59. Cannabis use disorder was found to have the most unique pleiotropic loci, whereas stimulants and multi-substance use disorders shared the most number (86) of pleiotropic loci (Figure S4). These are consistent with the finding that cannabis use severity level was least correlated with other traits while stimulants and multi-substance use severity levels were most correlated in AI (Table S3).
Fig. 3.
Numbers of unique or shared select pleiotropic loci (pleiotropic variants taking linkage disequilibrium into consideration) of the severity levels of alcohol, cannabis, cocaine & stimulants, and multi-substance use disorders in the American Indian cohort. The two variants that were selected by all four traits are rs12156277 on gene CSMD1, and intergenic variant rs1524058.
Table 1.
Shared select pleiotropic loci across alcohol, stimulants and multi-substance use disorders in the American Indian cohort.
| dbSNP ID | SNP | MAPPED GENEa | MAPPED TRAITSa |
|---|---|---|---|
| rs12048904 | 1:101331536 | VCAM1 - EXTL2 | multiple sclerosis |
| rs2698530 | 2:64503895 | LOC100507006 - LOC105374768 | iron biomarker measurement, total iron binding capacity, transferrin saturation measurement |
| rs1463104 | 4:88799710 | MEPE - HSP90AB3P | bone density |
| rs10005067 | 4:88852643 | HSP90AB3P - SPP1 | bone density |
| rs1524058b | 7:38136277 | LOC105375236 - STARD3NL | bone density |
| rs7668874 | 4:116841324 | PGAM4P2 - KRT18P21 | response to carboplatin |
| rs151283 | 5:122446619 | PRDM6 | heart rate response to recovery post exercise |
| rs186749 | 5:122454305 | PRDM6 | dense area measurement, mammographic density measurement, mammographic density percentage |
| rs7808252 | 7:150825839 | AGAP3 | loneliness measurement |
| rs12156277b | 8:3172400 | CSMD1 | loneliness measurement |
| rs6993244 | 8:8863059 | ERI1 | mean corpuscular hemoglobin |
| rs189798 | 8:8990577 | ERI1 - PPP1R3B | pathological myopia |
| rs378974 | 8:9026639 | LOC102724880 | reticulocyte count |
| rs440932 | 8:9026929 | LOC102724880 | reticulocyte count |
| rs7303433 | 12:23640479 | LOC101928441 - SOX5 | schizophrenia |
| rs4280164 | 14:24771285 | NOP9 | language impairment |
| rs13344313 | 19:18517767 | LRRC25 - SSBP4 | systemic lupus erythematosus |
| rs7258465 | 19:18533642 | SSBP4 | breast carcinoma |
| rs4808801 | 19:18571141 | ELL | breast carcinoma, estrogen-receptor negative breast cancer |
| rs9623320 | 22:41478482 | LOC391334 - MIR1281 | unipolar depression |
| rs727563 | 22:41867377 | ACO2 | crohn's disease, inflammatory bowel disease |
Mapped gene and traits are what were reported in the NHGRI-EBI GWAS catalog.
The two SNPs in bold font are also selected for the cannabis use disorder in AI.
AUD and CUD shared 17 pleiotropic loci consisting of 31 unique variants in MA (Table 2). Four loci (9 variants) were shared between AI and MA for AUD, and four loci (11 variants) for CUD (Table 3). The AUD loci included: variants on and near the ethanol metabolic genes ADH1B and ADH1C, two genes NIKIRAS1, LRRC25 involved in NF-κB signaling that are critical for innate immune responses and inflammation60, leucine-rich repeat and immunoglobulin-like gene LINGO1 that is implicated in numerous neurological and psychiatric disorders61, circadian nuclear receptor gene NR1D262, and a variant on SSBP4 that is also associated with Alzheimer’s and immune-mediated diseases63.
Table 2.
Shared select pleiotropic loci between alcohol and cannabis use disorders in the Mexican American cohort.
| dbSNP ID | SNP | MAPPED GENEa | MAPPED TRAITSa |
|---|---|---|---|
| rs2401137 | 1:8693324 | RERE | tonsillectomy risk measurement |
| rs12068123 | 1:8894346 | RPL23AP19 - ENO1 | tonsillectomy risk measurement |
| rs2479409 | 1:55504650 | BSND - PCSK9 | total cholesterol measurement, low density lipoprotein cholesterol measurement |
| rs9306895 | 2:20878153 | GDF7 - C2orf43 | prostate carcinoma |
| rs2289081 | 2:20881840 | GDF7 - C2orf43 | pulse pressure measurement |
| rs10170310 | 2:139278922 | SPOPL | response to olanzapine |
| rs58938945 | 2:139399354 | SPOPL - NXPH2 | mhpg measurement |
| rs13015447 | 2:167377978 | SCN7A - LOC107985958 | amyotrophic lateral sclerosis |
| rs163563 | 3:3086195 | CNTN4-AS1, CNTN4 | basophil percentage of leukocytes |
| rs163574 | 3:3096927 | CNTN4, CNTN4-AS1 | basophil count, basophil percentage of granulocytes |
| rs13082711 | 3:27537909 | UBA52P4 - LOC105377005 | smoking status measurement, systolic blood pressure, mean arterial pressure, diastolic blood pressure |
| rs1795648 | 3:55571760 | ERC2 | mental or behavioral disorder |
| rs652889 | 3:61794054 | PTPRG | qt interval |
| rs2001144 | 6:108407662 | OSTM1-AS1 | unipolar depression |
| rs9486815 | 6:108448046 | OSTM1-AS1 | unipolar depression |
| rs3105169 | 8:100495639 | VPS13B | smoking initiation |
| rs1487022 | 8:100529826 | VPS13B | cleft palate, cleft lip |
| rs2675609 | 10:63636531 | LOC107984237 - ARID5B | total blood protein measurement |
| rs35413307 | 13:112191778 | LOC107984618 - LOC105370369 | age at menarche |
| rs717344 | 16:5039248 | SEC14L5 | cancer |
| rs1558562 | 16:5061883 | SEC14L5 | cancer |
| rs2908668 | 16:5073773 | SEC14L5 - NAGPA | cancer |
| rs12599777 | 16:5079466 | NAGPA | blood protein measurement |
| rs346750 | 19:45737218 | EXOC3L2 | uric acid measurement |
| rs12982781 | 19:45786555 | MARK4 | platelet component distribution width |
| rs2248359 | 20:52791518 | CYP24A1 - LOC105372675 | multiple sclerosis |
| rs964293 | 20:52816717 | LOC105372675 - PFDN4 | colorectal cancer, hormone replacement therapy |
| rs131654 | 22:21917190 | UBE2L3 | systemic lupus erythematosus |
| rs5754217 | 22:21939675 | UBE2L3 | systemic lupus erythematosus, mean corpuscular volume, mean corpuscular volume |
| rs140496 | 22:21926456 | UBE2L3 | serum non-albumin protein measurement |
| rs4820091 | 22:21940189 | UBE2L3 | mean corpuscular volume |
Mapped gene and traits are what were reported in the NHGRI-EBI GWAS catalog.
Table 3.
Shared select pleotropic loci for AUD and CUD between the AI and MA cohorts.
| dbSNP ID | SNP | MAPPED GENEa | MAPPED TRAITSa |
|---|---|---|---|
| Alcohol Use Disorders | |||
| rs11129127 | 3:23943406 | NKIRAS1 | intelligence |
| rs2001209 | 3:23962646 | RPL15 | intelligence |
| rs7646501 | 3:24079291 | NR1D2 - NPM1P23 | intelligence |
| rs1229984 | 4:100239319 | ADH1B | alcohol dependence, alcohol drinking, alcohol consumption measurement, alcohol dependence measurement, body mass index, esophageal carcinoma, oropharynx cancer, pharynx cancer, oral cavity cancer, pulse pressure measurement, upper aerodigestive tract neoplasm |
| rs1229978 | 4:100256199 | ADH1B - ADH1C | alcohol dependence measurement |
| rs3935685 | 15:78034268 | LINGO1 | intelligence |
| rs1985157 | 19:18513594 | LRRC25 - SSBP4 | granulocyte percentage of myeloid white cells, mosquito bite reaction itch intensity measurement |
| rs13344313 | 19:18517767 | LRRC25 - SSBP4 | systemic lupus erythematosus |
| rs7258465 | 19:18533642 | SSBP4 | breast carcinoma |
| Cannabis Use Disorders | |||
| rs7595950 | 2:60143192 | RNA5SP94 - MIR4432HG | alcohol dependence, unipolar depression |
| rs6712720 | 2:60281608 | RNA5SP94 - MIR4432HG | monocyte early outgrowth colony forming unit |
| rs2561288 | 3:159674928 | IL12A-AS1 | celiac disease |
| rs1014486 | 3:159691112 | IL12A-AS1 | multiple sclerosis |
| rs4680534 | 3:159698945 | IL12A-AS1 | multiple sclerosis |
| rs1551277 | 7:47829317 | PKD1L1 | anxiety disorder |
| rs6967514 | 7:47896158 | PKD1L1 | colorectal health |
| rs346750 | 19:45737218 | EXOC3L2 | uric acid measurement |
| rs1130742 | 19:45452812 | APOC2, APOC4-APOC2 | blood protein measurement |
| rs8112909 | 19:46413408 | MYPOP - NANOS2 | c-x-c motif chemokine 10 measurement |
| rs12982781 | 19:45786555 | MARK4 | platelet component distribution width |
Mapped gene and traits are what were reported in the NHGRI-EBI GWAS catalog.
Candidate versus candidate-random variant sets
To test the robustness of the method, 25% randomly chosen variants from each cohort were added to the respective candidate variant set. Using the same selection process and parameter settings, 288—306 and 188—194 variants were selected for AI and MA. Table S8 details the numbers of shared or distinct pleiotropic loci of the candidate variant set and the candidate-random variant set. The two select pleiotropic variant sets largely overlap. Figure S5 illustrates the disease enrichments in the select pleiotropic variants for each trait. As expected, the unknown category (assigned to random variants not in high LD with candidate variants) was not enriched for any substance use disorders in either cohort. In fact, it was significantly depleted across substances for AI (p-value of depletion = 0.004—0.01). While variants in AI were from whole genome sequencing, variants in MA were obtained from imputed exome genotyping, which likely explains why the unknown category was slightly more enriched in MA (p=0.26, 0.56) than in AI (p=0.96-1.0).
The disease enrichment patterns are otherwise comparable between the select pleiotropic variants from the candidate variant set (Figure 1) and those from the candidate-random variant set (Figure S5), with one exception that more digestive system variants were selected from the candidate-random set for AUD in MA thus making it significantly enriched (p=0.011).
Functional analyses of shared pleiotropic genes
Across severity levels of four substance use disorders for AI and two substance use disorders for MA--making up six substance-cohort combinations--238 genes were selected for at least two substance-cohorts, of which 136 gene symbols were recognized by GeneMANIA, and 155 by GENE2FUNC. Neurotrophin transmembrane tyrosine kinase (TRK) receptor signaling pathway (FDR=5.2E-10), and negative regulation of axonogenesis (FDR=8.8E-9) were the top functional networks identified by GeneMANIA (Figure S6 and Table S9). The most enriched pathways identified by GENE2FUNC for the shared pleiotropic genes included: Reactome P75 neurotrophin receptor (NTR) mediated signaling pathway (p=0.019), matrisome (extracellular matrix and associated genes) (p=0.021), KEGG pathways cytokine-cytokine receptor interaction (p=0.039), and calcium signaling (p=0.039), melatonin metabolism and effects (p=2.8E-3), brain-derived neurotrophic factor (BDNF) signaling (p=3.1E-3), and TGF-β signaling (p=7.0E-3) in the WikiPathways database (Table S10). In the gene ontology (GO) enrichment tests, the most significant GO biological process was regulation of multicellular organismal development (p=1.6E-5), while extracellular space was the top GO cellular component (p=4.4E-4), and receptor binding the top GO molecular function (p=5.4E-4) (Table S11). Of note, out of the 155 shared select genes, 130 (84%) were involved in 940 enriched immunologic signatures (Table S12); 217 transcription factor targets were enriched involving 95 (61%) such genes (Table S13); and 82 microRNA targets were enriched involving 70 (45%) genes (Table S14).
In the tissue-specific gene expression analysis, the shared pleiotropic genes were most significantly differentially expressed in substantia nigra part (p=2.7E-5) of the brain tissue (significantly up-regulated), followed by esophagus mucosa (p=7.5E05) (significantly down-regulated), coronary artery (p=2.1E-4) (up-regulated), and adrenal gland (p=7.4E-4) (Figure S7). Other brain tissues that had significantly up-regulated shared pleiotropic genes were cortex (p=0.001), anterior cingulate cortex (p=0.003), amygdala (p=0.028), and nucleus accumbens (p=0.032) (Table S15), which all play significant roles in addiction as well as related psychiatric disorders.
DISCUSSION
We described a flexible approach to explore potential pleiotropy for complex disorders, and applied it to the study of alcohol, cocaine and stimulants, cannabis, and multi-substance use disorders in an American Indian and a Mexican American population. While the method is applicable to any population, it is particularly powerful for use in admixed populations where limited genetic studies are available for pleiotropy analyses using existing methods. We identified a set of pleiotropic variants that may be potential targets of interest for substance use disorders. The study of pleiotropy may help identify the unique polygenetic etiology of individual substance use disorders, as well as potentially uncover common substrates that may underlie addictive disorders in general.
Neurological disorders and inflammatory measurements were most enriched for pleiotropic variants
Neurological disorders were the most significantly enriched disease category in the select pleiotropic variants for the severity levels of alcohol, cocaine & stimulants, and cannabis use disorders in AI, as well as cannabis use disorders in MA. In particular, multiple pleiotropic variants were selected for schizophrenia, depression, ADHD, and multiple sclerosis across all substance-cohort combinations. Inflammatory measurement was the most enriched for the severity levels of MSUD and AUD in AI. Immune system disorders such as systemic lupus and psoriasis, inflammatory diseases such as IBD, celiac disease were selected for all substance-cohorts. Variants implicated in alcohol drinking and smoking behaviors were selected for all or nearly all substance-cohorts.
Shared pleiotropic loci captured genes common to severity levels of alcohol, cannabis, stimulants, and multi-substance use disorders in American Indians
Two variants were selected across all substances in AI. Variant rs12156277 is on CUB and sushi multiple domains 1 gene CSMD1. CSMD1 encodes a cell adhesion molecule highly expressed in the central nervous system (CNS). Another variant on the same gene has been associated with cannabis dependence in European American and African American meta-analysis64. Csmd1 was also associated with locomotor activity and shown to be causal as demonstrated in Csmd1 mutant mice65.
The intergenic variant rs1524058 found brain eQTL for genes SFRP4 (putamen p=1.3e-4), VPS41 (cerebellum p=7e-4), and ELMO1 (frontal cortex p=1e-3). SFRP4 was found differentially expressed in the nucleus accumbens of rhesus macaques with long-term cocaine self-administration66. SFRP4 expression was also elevated in astrocytes obtained from alcohol-treated animals (the only gene in the Differentiation, Cell Growth & Survival category)67. Multiple variants of ELMO1 were nominal-significant for multiple substance dependences (poly-substance in NIDA sample, alcohol dependence in COGA, methamphetamine dependence from JGIDA and a Taiwan cohort)68. ELMO1 mediates the regulation of dendrite morphogenesis by interacting with the adhesion-G protein-coupled receptor (GPCR) BAI3, which is involved in a signaling pathway controlling dendrite morphogenesis, and thus linked to psychiatric disorders69.
Shared select pleiotropic genes recapitulated functional networks underlying substance use disorders
Neurotrophic factors.
The most enriched network and pathway in the shared pleiotropic genes were neurotrophin signaling, such as TRK receptor, p75 NTR, and BDNF signaling pathways. BDNF has a well-known role in neurodevelopment and memory formation. It binds to TrkB and p75 receptors. Regulations of neurotrophic factors including BDNF are known to be involved in neuroadaptive process induced by drugs of abuse such as alcohol, opioid and methamphetamine, that may lead to addictive behaviors through motivational aspects of addiction70-72. There is some evidence that implicates neurotropic factors in drug sensitization14. Several of these enriched pathways contain gene LINGO1. Same variant on LINGO1 was selected for AUD in both AI and MA. LINGO1 is almost exclusively expressed in CNS and was implicated in numerous neurological and psychiatric disorders. Several variants on AKAP13 were selected for CUD and MSUD in AI and AUD in MA. The gene has been associated with a variety of substances and related disorders73.
Immune responses.
Cytokine-cytokine receptor interaction, TGF-β and SMAD signaling, and intestinal immune network were also among the most enriched pathways in shared pleiotropic genes. While substances often affect immune functions, evidence has shown that neuroimmune response also mediates usage of substances such as stimulants74 and alcohol75. Within the enriched pathways, several genes including CSF3R, TNFSF12/13, NGFR, NCOR2, ICOSLG, and SPP1 were selected for cocaine & stimulants and multi-substance use disorders in AI. IL12A, SKI, ZNF423, and SPP1 were selected for AUD in AI. IL12A, SKI, and ZNF324 were also selected for CUD in either or both cohorts. There are also overlaps between pathways: for instance, NGFR is part of the p75 NTR and BDNF signaling.
Extracellular matrix.
Matrisome was one of the highly enriched canonical pathways in shared pleiotropic genes, and the extracellular space was the most enriched GO cellular components. Increasing evidence suggests that aberrant ECM remodeling play crucial roles in development of addiction to drugs of abuse such as stimulants, opiates, and alcohol76. This is consistent with the characterization of addiction as a disorder of maladaptive neuroplasticity. Of the enriched matrisome genes, loci spanning MEPE and SPP1 were selected for the severity levels of alcohol, stimulants and multi-substance use disorders in AI. Variants near GDF7 were selected in both AUD and CUD in MA.
Cell adhesion molecules.
One component of the ECM is cell adhesion molecules (CAMs). Of the two variants selected in all substances in AI, one resides on CSMD1, a CAM gene. Other two variants on CSMD1 were selected for CUD in AI and MA. A number of other CAM genes were also selected. For instance, VCAM1 of immunoglobulin superfamily of CAMs, and CDH18 of cadherin superfamily of CAMs were both selected in three out of the four substances in AI. CDH13 was selected for cannabis in AI, and CDH6 by alcohol in MA. CDH13 has been associated with drug dependence and other addiction phenotypes by several studies77-79. Neurexin is another group of CAMs involved in the central organization of glutamatergic and GABAergic synapses and has been implicated previously in addiction as well as other neurodevelopmental disorders. Neurexin gene such as NRXN1 was selected for AUD in AI, and NRXN3 for AUD in MA. NRXN3 has been previously implicated in opioid, alcohol, drug and nicotine dependence80-82. Protein tyrosine phosphatase receptor type (PTPR) of CAMs such as PTPRD, PTPRM were selected for AUD in AI, PTPRT for CUD in AI, and PTPRG by both AUD and CUD in MA; PTP non-receptor gene PTPN5 was selected for MSUD in AI. PTPRD has been repeatedly implicated in various drug dependence and addiction-related phenotypes although with small effects. Although it’s not fully known what specific roles that CAMs play in addiction, it’s known that various CAMs play important roles in synaptic plasticity, axon growth, and regeneration. The variations in CAM genes might influence their expression during neuronal development or neural plasticity in response to addictive drugs83.
Circadian regulations.
Melatonin metabolism was the most enriched pathway in the WikiPathways. Exercise-induced circadian regulation was also significantly enriched. Melatonin is a neurohormone playing an important role in modulations of circadian rhythms as well as behavior and physiological functions84. Growing evidence has linked the disruption of circadian rhythms and variations in circadian genes to the development and progression of drug addiction85. Within the enriched pathways, cryptochrome circadian regulator CRY2 was selected for stimulants in AI and for cannabis in MA. Adrenergic receptor gene ADRB1 was selected for AUD and MSUD in AI. ERC2 was selected for both AUD and CUD in MA. SULT1AI was selected for stimulants and multi-substance in AI. SULT1AI has been associated with nicotine addiction86, and its expression was shown to be up-regulated by ethanol in rat liver and intestine87.
Methodological considerations and future directions
It should be noted that the pleiotropic variants that our method identified were based on cross-trait associations. As a result, they may not all represent true biological pleiotropic risk loci where causal variants of different traits are on the same gene or regulatory region. When applying the method, we used variants from the GWAS catalog. Some of the findings therefore could result from one variant tagging different causal variants through LD for different traits. In addition, the method does not distinguish between biological pleiotropy from mediated pleiotropy where a variant affects one trait directly that in turn affects another22. Some of our findings might in fact be caused by elevated levels of substance use. For instance, variants associated with mean corpuscular volume were selected across substance-cohorts, and variants associated with coronary artery calcification were associated with five out of six substance-cohorts. Elevated MCV is seen in over half of people with chronic excessive alcohol consumption88 as a result from the effect of alcohol on erythroblast development89. Aortic calcification has been positively correlated with heavy alcohol consumption as well90. Since smoking is a major risk factor for chronic obstructive pulmonary disease, that multiple COPD variants were selected for different substances might reflect the comorbidity between tobacco, alcohol and other drug use. To further identify the individual pleiotropic scenarios, statistical fine mapping and Mendelian randomization are needed computationally. Experimental studies will be required ultimately for confirmation.
Another limitation was inherent in the L1 regularization. When two variants are highly correlated, the L1 regularization selects one of them more or less randomly, which might eliminate the true causal variant. There are different variable (model) selection processes, for instance elastic-net, stepwise regression; each has its own limitations. It should also be noted that most of the select pleiotropic variants would not be associated with the substance use phenotypes at a genome-wide significant level if tested individually in GWAS. Our approach implicitly assumed polygenic inheritance in the context of pleiotropy, which is likely the case for complex disorders. Finally we acknowledge that we were limited by the sample sizes. However, we did identify 4 pleiotropic loci (9 variants) shared between AI and MA for the severity level of AUD, and 4 loci (11 variants) for CUD. We also validated our method by mixing candidate variants with randomly chosen variants; and our findings largely aligned with their biological implications.
In conclusion, we used a novel pleiotropic approach to study the genetic underpinnings of substance use disorders. We identified pleiotropic variants, genes, networks and pathways shared by various SUD in two independent populations. Our findings suggested common genetic substrates underlying primarily the neuroadaptation and immune response aspects of human substance use disorders.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge and thank the following people for their roles in 1) the genotyping effort: Scott Chasse, Piotr Mieczkowski, Ewa Patrycja Malc, Joshua Sailsbery, and Phil Owens; 2) quality control and imputation of MA genotypes: Whitney E. Melroy-Greif; and 3) recruiting participants and collection and preparation of the clinical data: David Gilder, Corinne Kim, Evie Phillips, Gina Stouffer, Susan Lopez, Linda Corey, Greta Berg, and Derek Wills.
This work was supported by the National Institutes of Health (NIH): National Institute on Alcohol Abuse and Alcoholism (NIAAA) under Grant K25 AA025095 to QP; NIAAA and National Center on Minority Health and Health Disparities (NCMHD) under Grant 5R37 AA010201 to CLE; National Institute on Drug Abuse (NIDA) under Grant R01 DA030976 to CLE, KCW; and NIAAA Grants R01 AA027316 and AA026248 to CLE. NIAAA, NCMHD and NIDA had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
DISCLOSURES
The authors report no conflicts of interest.
REFERENCES
- 1.Yu C, McClellan J. Genetics of Substance Use Disorders. Child and Adolescent Psychiatric Clinics of North America. 2016;25(3):377–385. [DOI] [PubMed] [Google Scholar]
- 2.Edenberg HJ, Foroud T. Chapter 32 - Genetics of alcoholism In: Sullivan EV, Pfefferbaum A, eds. Handbook of Clinical Neurology. Vol 125: Elsevier; 2014:561–571. [DOI] [PubMed] [Google Scholar]
- 3.Salvatore JE, Han S, Farris SP, Mignogna KM, Miles MF, Agrawal A. Beyond genome-wide significance: integrative approaches to the interpretation and extension of GWAS findings for alcohol use disorder. Addiction Biology. 2018:n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edenberg HJ, Gelernter J, Agrawal A. Genetics of Alcoholism. Current Psychiatry Reports. 2019;21(4):26. [DOI] [PubMed] [Google Scholar]
- 5.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of dsm-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions iii. JAMA Psychiatry. 2015;72(8):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72(8):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64(5):566–576. [DOI] [PubMed] [Google Scholar]
- 8.Ehlers CL, Gizer IR. Evidence for a genetic component for substance dependence in Native Americans. American Journal of Psychiatry. 2013;170(2):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Benowitz NL. Nicotine Addiction. New England Journal of Medicine. 2010;362(24):2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkow ND, Wise RA, Baler R. The dopamine motive system: implications for drug and food addiction. Nature Reviews Neuroscience. 2017;18:741. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Nong Z, Li Y, Huang J, Chen C, Huang L. Role of Dopamine Signaling in Drug Addiction. Current Topics in Medicinal Chemistry. 2017;17(21):2440–2455. [DOI] [PubMed] [Google Scholar]
- 13.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PRA. The dopamine theory of addiction: 40 years of highs and lows. Nature Reviews Neuroscience. 2015;16:305. [DOI] [PubMed] [Google Scholar]
- 14.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 Supplement 2(0965-2140 (Print)):S91–S117. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Buck CL, Cohen A, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob GF, Moal ML. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997;278(5335):52. [DOI] [PubMed] [Google Scholar]
- 17.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 18.Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. American Journal of Orthopsychiatry. 1996;66(1):17–31. [DOI] [PubMed] [Google Scholar]
- 19.Vancampfort D, Hallgren M, Mugisha J, et al. The Prevalence of Metabolic Syndrome in Alcohol Use Disorders: A Systematic Review and Meta-analysis. Alcohol and Alcoholism. 2016;51(5):515–521. [DOI] [PubMed] [Google Scholar]
- 20.Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Seminars in Liver Disease. 1988;8(1):12–25. [DOI] [PubMed] [Google Scholar]
- 21.Stearns FW. One Hundred Years of Pleiotropy: A Retrospective. Genetics. 2010;186(3):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackinger S, Zeggini E. Statistical methods to detect pleiotropy in human complex traits. Open biology. 2017;7(11):170125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. The American Journal of Human Genetics. 2011;88(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bush WS, Oetjens MT, Crawford DC. Unravelling the human genome–phenome relationship using phenome-wide association studies. Nature Reviews Genetics. 2016;17:129. [DOI] [PubMed] [Google Scholar]
- 25.Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk to disease from genome-wide association studies. Genome Research. 2007;17(10):1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nature Genetics. 2015;47:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walters RK, Adams MJ, Adkins AE, et al. Trans-ancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. bioRxiv. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kranzler HR, Zhou H, Kember RL, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nature Communications. 2019;10(1):1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehlers CL, Wall TL, Betancourt M, Gilder DA. The clinical course of alcoholism in 243 Mission Indians. American Journal of Psychiatry. 2004;161(7):1204–1210. [DOI] [PubMed] [Google Scholar]
- 30.Ehlers CL, Gilder DA, Wall TL, Phillips E, Feiler H, Wilhelmsen KC. Genomic screen for loci associated with alcohol dependence in Mission Indians. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;129B(1):110–115. [DOI] [PubMed] [Google Scholar]
- 31.Ehlers CL, Gilder DA, Criado JR, Caetano R. Sleep quality and alcohol-use disorders in a select population of young-adult Mexican Americans. Journal of studies on alcohol and drugs. 2010;71(6):879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55(2):149–158. [DOI] [PubMed] [Google Scholar]
- 33.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94(9):1361–1370. [DOI] [PubMed] [Google Scholar]
- 34.Schuckit MA, Smith TL, Anthenelli R, Irwin M. Clinical course of alcoholism in 636 male inpatients. American Journal of Psychiatry. 1993;150(5):786–792. [DOI] [PubMed] [Google Scholar]
- 35.Schuckit MA, Smith TL, Danko GP, Reich T, Bucholz KK, Bierut LJ. Similarities in the Clinical Characteristics Related to Alcohol Dependence in Two Populations. The American Journal on Addictions. 2002;11(1):1–9. [DOI] [PubMed] [Google Scholar]
- 36.Ehlers CL, Gizer IR, Vieten C, et al. Age at Regular Drinking, Clinical Course, and Heritability of Alcohol Dependence in the San Francisco Family Study: A Gender Analysis. The American Journal on Addictions. 2010;19(2):101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehlers CL, Stouffer GM, Corey L, Gilder DA. The clinical course of DSM-5 alcohol use disorders in young adult native and Mexican Americans. The American Journal on Addictions. 2015;24(8):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuckit MA, Anthenelli RM, Bucholz KK, Hesselbrock VM, Tipp J. The time course of development of alcohol-related problems in men and women. Journal of Studies on Alcohol. 1995;56(2):218–225. [DOI] [PubMed] [Google Scholar]
- 39.Venner KL, Miller WR. Progression of alcohol problems in a Navajo sample. Journal of Studies on Alcohol. 2001;62(2):158–165. [DOI] [PubMed] [Google Scholar]
- 40.Malcolm BP, Hesselbrock MN, Segal B. Multiple Substance Dependence and Course of Alcoholism among Alaska Native Men and Women. Substance Use & Misuse. 2006;41(5):729–741. [DOI] [PubMed] [Google Scholar]
- 41.Scott DM, Williams CD, Cain GE, et al. Clinical Course of Alcohol Dependence in African Americans. Journal of Addictive Diseases. 2008;27(4):43–50. [DOI] [PubMed] [Google Scholar]
- 42.Montane-Jaime LK, Shafe S, Joseph R, et al. The Clinical Course of Alcoholism in Trinidad and Tobago. Journal of Studies on Alcohol and Drugs. 2008;69(6):834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Q, Bizon C, Gizer IR, Wilhelmsen KC, Ehlers CL. Genetic loci for alcohol-related life events and substance-induced affective symptoms: indexing the “dark side” of addiction. Translational Psychiatry. 2019;9(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilder DA, Stouffer GM, Lau P, Ehlers CL. Clinical characteristics of alcohol combined with other substance use disorders in an American Indian community sample. Drug and Alcohol Dependence. 2016;161:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bizon C, Spiegel M, Chasse SA, et al. Variant calling in low-coverage whole genome sequencing of a Native American population sample. BMC Genomics. 2014;15(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norden-Krichmar TM, Gizer IR, Wilhelmsen KC, Schork NJ, Ehlers CL. Protective variant associated with alcohol dependence in a Mexican American cohort. BMC Medical Genetics. 2014;15(136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malone J, Holloway E, Adamusiak T, et al. Modeling sample variables with an Experimental Factor Ontology. Bioinformatics. 2010;26(8):1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tibshirani R Regression Shrinkage and Selection Via the Lasso. Journal of the Royal Statistical Society, Series B. 1994;58:267–288. [Google Scholar]
- 49.Ayers KL, Cordell HJ. Snp selection in genome-wide and candidate gene studies via penalized logistic regression. Genet Epidemiol. 2010;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arbet J, McGue M, Chatterjee S, Basu S. Resampling-based tests for Lasso in genome-wide association studies. BMC Genetics. 2017;18(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kutmon M, Riutta A, Nunes N, et al. WikiPathways: capturing the full diversity of pathway knowledge. Nucleic Acids Research. 2016;44(D1):D488–D494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nature Communications. 2017;8(1):1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.GTEx Consortium T The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348(6235):648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Research. 2010;38(suppl 2):W214–W220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramasamy A, Trabzuni D, Guelfi S, et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17(10):1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacanella E, Estruch R. The effect of alcohol consumption on endothelial adhesion molecule expression. Addict Biol. 2003;8(4):371–378. [DOI] [PubMed] [Google Scholar]
- 58.Ueno H, Pradhan S, Schlessel D, Hirasawa H, Sumpio BE. Nicotine enhances human vascular endothelial cell expression of ICAM-1 and VCAM-1 via protein kinase C, p38 mitogen-activated protein kinase, NF-kappaB, and AP-1. Cardiovasc Toxicol. 2006;6(1):39–50. [DOI] [PubMed] [Google Scholar]
- 59.Miyata S, Kitagawa H. Mechanisms for modulation of neural plasticity and axon regeneration by chondroitin sulphate. The Journal of Biochemistry. 2014;157(1):13–22. [DOI] [PubMed] [Google Scholar]
- 60.Feng Y, Duan T, Du Y, et al. LRRC25 Functions as an Inhibitor of NF-κB Signaling Pathway by Promoting p65/RelA for Autophagic Degradation. Scientific Reports. 2017;7(1):13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews JL, Fernandez-Enright F. A decade from discovery to therapy: Lingo-1, the dark horse in neurological and psychiatric disorders. Neuroscience & Biobehavioral Reviews. 2015;56:97–114. [DOI] [PubMed] [Google Scholar]
- 62.Cha HK, Chung S, Lim HY, Jung J-W, Son GH. Small Molecule Modulators of the Circadian Molecular Clock With Implications for Neuropsychiatric Diseases. Frontiers in Molecular Neuroscience. 2019;11:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yokoyama JS, Wang Y, Schork AJ, et al. Association Between Genetic Traits for Immune-Mediated Diseases and Alzheimer DiseaseGenetic Association Between Immune-Mediated Diseases and Alzheimer DiseaseGenetic Association Between Immune-Mediated Diseases and Alzheimer Disease. JAMA Neurology. 2016;73(6):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherva R, Wang Q, Kranzler H, et al. Genome-wide association study of cannabis dependence severity, novel risk variants, and shared genetic risks. JAMA Psychiatry. 2016;73(5):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzales NM, Seo J, Hernandez-Cordero AI, et al. Genome wide association study of behavioral, physiological and gene expression traits in a multigenerational mouse intercross. bioRxiv. 2017. [Google Scholar]
- 66.Vallender EJ, Goswami DB, Shinday NM, Westmoreland SV, Yao W-D, Rowlett JK. Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug and alcohol dependence. 2017;175:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trindade P, Hampton B, Manhães AC, Medina AE. Developmental alcohol exposure leads to a persistent change on astrocyte secretome. Journal of neurochemistry. 2016;137(5):730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uhl GR, Drgon T, Johnson C, et al. “Higher order” addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochemical Pharmacology. 2008;75(1):98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanoue V, Usardi A, Sigoillot SM, et al. The adhesion-GPCR BAI3, a gene linked to psychiatric disorders, regulates dendrite morphogenesis in neurons. Molecular Psychiatry. 2013;18:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koskela M, Bäck S, Võikar V, et al. Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiology of disease. 2017;97(Pt B):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction Motivation Reformulated: An Affective Processing Model of Negative Reinforcement. Psychological Review. 2004;111(1):33–51. [DOI] [PubMed] [Google Scholar]
- 72.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. NeuroMolecular Medicine. 2004;5(1):69–83. [DOI] [PubMed] [Google Scholar]
- 73.Uhl GR, Drgon T, Johnson C, et al. Molecular Genetics of Addiction and Related Heritable Phenotypes. Annals of the New York Academy of Sciences. 2008;1141(1):318–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo Iacono L, Catale C, Martini A, et al. From Traumatic Childhood to Cocaine Abuse: The Critical Function of the Immune System. Biological Psychiatry. 2018;84(12):905–916. [DOI] [PubMed] [Google Scholar]
- 75.Jacobsen JHW, Buisman-Pijlman FTA, Mustafa S, Rice KC, Hutchinson MR. The efficacy of (+)-Naltrexone on alcohol preference and seeking behaviour is dependent on light-cycle. Brain, Behavior, and Immunity. 2018;67:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beloate LN, Kalivas PW. Chapter 17 - Role of the Extracellular Matrix in Addiction In: Torregrossa M, ed. Neural Mechanisms of Addiction: Academic Press; 2019:247–258. [Google Scholar]
- 77.Liu Q-R, Drgon T, Johnson C, Walther D, Hess J, Uhl GR. Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141B(8):918–925. [DOI] [PubMed] [Google Scholar]
- 78.Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nature genetics. 2010;42(5):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drgonova J, Walther D, Hartstein GL, et al. Cadherin 13: human cis-regulation and selectively-altered addiction phenotypes and cerebral cortical dopamine in knockout mice. Molecular medicine (Cambridge, Mass). 2016;22:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riddick A, Gaffney J, Santana M, et al. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Human Molecular Genetics. 2007;16(11):1327–1334. [DOI] [PubMed] [Google Scholar]
- 81.Docampo E, Ribasés M, Gratacòs M, et al. Association of Neurexin 3 polymorphisms with smoking behavior. Genes, Brain and Behavior. 2012;11(6):704–711. [DOI] [PubMed] [Google Scholar]
- 82.Hishimoto A, Walther D, Liu Q-R, et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Human Molecular Genetics. 2007;16(23):2880–2891. [DOI] [PubMed] [Google Scholar]
- 83.Muskiewicz DE, Uhl GR, Hall FS. The Role of Cell Adhesion Molecule Genes Regulating Neuroplasticity in Addiction. Neural Plasticity. 2018;2018:9803764.29675039 [Google Scholar]
- 84.Onaolapo OJ, Onaolapo AY. Melatonin in drug addiction and addiction management: Exploring an evolving multidimensional relationship. World journal of psychiatry. 2018;8(2):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Logan RW, Williams WP 3rd, McClung CA. Circadian rhythms and addiction: mechanistic insights and future directions. Behavioral neuroscience. 2014;128(3):387–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Liu M, Li X, Zhang L, Fan R, Wang J. Prioritizing Genes Related to Nicotine Addiction Via a Multi-source-Based Approach. Molecular Neurobiology. 2015;52(1):442–455. [DOI] [PubMed] [Google Scholar]
- 87.Maiti S, Chen G. Ethanol up-regulates phenol sulfotransferase (SULT1A1) and hydroxysteroid sulfotransferase (SULT2A1) in rat liver and intestine. Archives of Physiology and Biochemistry. 2015;121(2):68–74. [DOI] [PubMed] [Google Scholar]
- 88.Ballard HS. Hematological Complications of Alcoholism. Alcoholism: Clinical and Experimental Research. 1989;13(5):706–720. [DOI] [PubMed] [Google Scholar]
- 89.Whitehead TP, Clarke CA, Bayliss RI, Whitfield AG. Mean red cell volume as a marker of alcohol intake. Journal of the Royal Society of Medicine. 1985;78(10):880–881. [PMC free article] [PubMed] [Google Scholar]
- 90.Mahajan H, Choo J, Masaki K, et al. Association of alcohol consumption and aortic calcification in healthy men aged 40–49 years for the ERA JUMP Study. Atherosclerosis. 2018;268:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.