Abstract
Background:
Screening for prenatal drug use is recommended. The NIDA-modified ASSIST(NM-ASSIST) is a screener for drug use that has not yet been validated with pregnant women. This study aims to assess the substance-specific diagnostic validity of the NM-ASSIST (not including tobacco or alcohol) in pregnant women and determine optimal cut-points for substance-specific Substance Involvement(SI) scores.
Methods:
Five-hundred (500) pregnant women were recruited from two obstetric practices as part of a larger study of substance use screeners. Participants completed the NM-ASSIST and provided urine and hair samples for testing. Receiver operating characteristic (ROC) curves were derived to determine the optimal SI score cut-points for each drug.
Findings:
Prevalence estimates of prenatal drug use as determined by hair/urine drug testing were: cannabis(32.0%), cocaine (9.9%), benzodiazepines(1.0%), prescription opioids(4.3%) and street opioids (1.7%). The proportion of participants screening positive based on optimal SI score cut-points were as follows: cannabis (39.1%), cocaine(2.3%), benzodiazepines(0.8%), prescription opioids(2.7%), and street opioids (1.7%). There were no screen positives for amphetamines but six(1.2%) women had a positive amphetamine hair or urine test. Optimal cut-points to identify prenatal drug use were: cannabis, 2 (AUC=0.87;sensitivity=0.82; specificity=0.85;DOR=26.9); cocaine, 2(AUC=0.58; sensitivity=0.17; specificity=0.99;DOR=29.0); benzodiazepines, 15(AUC=0.59;sensitivity=0.20; specificity=0.99; DOR=38.8); prescription opioids, 3(AUC=0.61; sensitivity=0.25; specificity=0.98;DOR=18.3); and street opioids, 4(AUC=0.55;sensitivity=0.13;specificity=0.99;DOR=9.3).
Conclusions:
The NM-ASSIST reliably distinguished pregnant women who use cannabis from those who do not, but performed poorly for all other substances. More research is needed to identify screeners that reliably detect all prenatal drug use. Although more cost-prohibitive, a combination of self-report and toxicological screening may be preferable for detecting prenatal drug use.
Keywords: pregnancy, screening, NIDA-modified ASSIST, drug use, cannabis, opioids
1. Introduction
Prenatal substance use is a concern given the risks conferred to the woman and offspring.1,2 As such, the American College of Obstetricians and Gynecologists (ACOG) recommends universal screening for prenatal substance use in the United States (U.S.). Several screening tools exist, some validated in pregnant women and some not.3 For example, the 4P’s Plus© has been validated in pregnant women but is copyrighted and requires a licensing fee.4,5 Similarly, the Substance Use Risk Profile-Pregnancy (SURP-P) Scale has been validated for use in pregnancy to screen for high risk of overall illicit drug use but does not elicit substance-specific responses for opioids, stimulants, hallucinogens, or sedatives.6
The NIDA modified ASSIST (NM-ASSIST)7 is an enhancement of the World Health Organization (WHO) Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) Version (V) 3.0 for identifying substance use disorders.8 The NM-ASSIST asks about use of cannabis, cocaine, stimulants, opioids, inhalants, sedatives, hallucinogens and “other drug” in lifetime and past 3 months, and also probes for symptoms of dependence on these drugs. A Substance Involvement (SI) score is computed for each drug and used to classify respondents into low-risk (SI score 0–3), moderate-risk (SI score 4–26) and high-risk (SI score 27+) for drug use based on past 3 months history.7 In its current form, the NM-ASSIST is combined with the NIDA Quick Screen in clinical settings and are together referred to as the NIDA Quick Screen/ASSIST.
There are limitations to the use of self-reported drug use screening data in the general population and specifically in high-risk populations such as pregnant women because of stigma, fear of legal consequences and the resultant likelihood of underreporting drug use.9-12 Given this background, self-report screening tools needs to be validated against a gold standard to provide metrics of accuracy of drug use detection. While the WHO ASSIST is validated for screening for substance use in adults and adolescents,13 including a recently developed adaption to the Tobacco, Alcohol, Prescription medication, and other Substance use Tool (TAPS) for online use,14,15 validation of its use among pregnant women is only recently emerging.16-19
Ondersma et al (2019)17 compared diagnostic accuracy of five screening tools for prenatal substance use in a large sample of pregnant women, the tools included the Substance Use Risk Profile—Pregnancy (also known as the SURP‐P), CRAFFT, 5Ps (parents, peers, partner, pregnancy, past), Wayne Indirect Drug Use Screener (also known as WIDUS) and the National Institute on Drug Abuse (NIDA) Quick Screen and found that the NIDA Quick Screen showed high specificity but low sensitivity; the 5Ps - high sensitivity and low specificity; and for the CRAFFT, SURP‐P and 5Ps - the highest area AUC for alcohol (0.67, 0.66 and 0.62 respectively), and the WIDUS had the highest AUC for illicit drugs and opioids (0.70 and 0.69, respectively). Furthermore, Chang et al (2019)16 evaluated the same five questionnaires for accuracy in identification of substance use disorders – rather than mere substance use – and found accuracy as measured by AUC for all substance use was highest in CRAFFT then SURP-P, 5P’s, WIDUS, and NIDA Quick Screen in descending order.
Coleman-Cowger et al (2019)18 evaluated the accuracy of three screening tools – 4P’s Plus©, SURP-P and the NIDA Quick Screen/ASSIST - in identifying substance use among a diverse sample of pregnant women and found that the SURP-P and 4P’s Plus© had higher sensitivity and negative predictive values than the NIDA Quick Screen/ASSIST. Trocin et al (2019)19 conducted a qualitative assessment of the same three screening tools and evaluated perceptions in prenatal clinical staff related to characteristics such as questionnaire length, tone, ease of comprehension and other characteristics, and found some support for the qualitative features of the 4P’s Plus© in comparison to the SURP-P and NIDA Quick Screen/ASSIST.
Ideally, screening tools such as the NM-ASSIST should take into account a diversity of cultures and settings where substance use occurs. As such, the NM-ASSIST warrants further validation in various populations, including vulnerable populations such as pregnant women, who may be less likely than the general population to report drug use because of worries about stigma or legal consequences.12 As far as we know, prior to the current project, no prior validation of the NM-ASSIST has been conducted in pregnant women. It is therefore especially important to validate the NM-ASSIST for use in pregnancy given the associated risks of prenatal substance use to birth outcomes.28,29
To date, no substance-specific validation is available in the literature for the NM-ASSIST in pregnant women in the U.S using biologic testing such as hair and urine drug testing as reference standard. Our sister study (Coleman-Cowger et al, 2019)18 compares global diagnostic validity of the NM-ASSIST with the 4P’s Plus and the SURP-P (Substance Use Risk Profile – Pregnancy) for qualitative drug screening for any drug use, rather than substance-specific; and reveals that the overall specificity and test-retest reliability of the NM-ASSIST compares favorably with the 4P’s Plus© and the SURP-P, but had an inferior overall sensitivity.3,18 The logical next step, therefore, is to validate the substance specific diagnostic qualities of the NM-ASSIST in pregnant women and provide indices for each relevant substance. The aim of this report is to assess the substance-specific diagnostic validity (sensitivity, specificity, area under the curve (AUC) and diagnostic odds ratio (DOR)) of the NM-ASSIST in pregnant women for cannabis, cocaine, benzodiazepines, opioids (street and prescription) and amphetamines; and determine optimal cut-points for substance-specific SI scores in pregnant women in a high substance use setting.
2. Methods
2.1. Participants
This study was conducted at 2 prenatal practices in Baltimore, Maryland. The methodology of this study has been previously published.3 Five hundred (500) pregnant women presenting for prenatal care appointments were consecutively recruited into the study from January 2017 to January 2018, with equitable groups across the three trimesters of pregnancy. Inclusion criteria were 1) confirmed pregnancy, 2) hair length of at least 3 cm, 3) age 18 years or older, and 4) ability to communicate in English. The study was approved by the Institutional Review Boards (IRB) of Battelle Memorial Institute and the University of Maryland School of Medicine.
2.2. Measures
2.2.1. Index Test: NIDA-modified ASSIST
The NM-ASSIST is used in persons 18 years or older and may be delivered as an interview or completed by patients. The NM-ASSIST is used in conjunction with the NIDA Quick Screen which asks about past year drug use – if a negative response is given, the screening ends. However, a positive screen on the Quick Screen is followed up with the NM-ASSIST, after which risk scores are calculated.7 The NM-ASSIST asks about past lifetime and past 3-month drug use. The NM-ASSIST then asks questions relating to problematic use or use that would qualify as a substance use disorder (see Table 1). There are substance-specific SI scores associated with the responses and a global score of 0–3 is considered low-risk, 4–26 medium risk and 27 or higher considered high risk. Table 1 shows the NIDA Quick Screen/ASSIST questions and scoring of responses. Our analysis validates only the NM-ASSIST in pregnant women.
Table 1:
NIDA Quick Screen/ASSIST
|
Quick Screen Response options for each substance are: never, once or twice, monthly, weekly, and daily or almost daily. For purposes of validation, both the Quick Screen and ASSIST were given to all participants to complete. |
1. In the past year, how often have you used the following? a. Five or more alcohol drinks in a day for men or 4 or more alcohol drinks in a day for women, b. tobacco products, c. prescription drugs for non-medical reasons, and d. illegal drugs. |
|
NM-ASSIST Substances assessed are: tobacco products; alcohol; cannabis; cocaine; amphetamine-type stimulants (ATS); sedatives and sleeping pills (benzodiazepines); hallucinogens; inhalants; opioids; and “other” drugs. Responses to items (2) through (7) are summed to create a Substance Involvement (SI) score for each substance. (Response options of no, yes but not in the past 3 months, and yes in the past 3 months for items 6-8.) Each SI score is classified as: lower risk (scores 0-3), moderate risk (scores 4-26), or high risk (scores 27+). For validation purposes, moderate and high risk were considered positive screens |
1. In your lifetime, which of the following substances have you used? (response options of yes/no); |
| 2. In the past three months, how often have you used the substances you mentioned? (response options of never, once or twice, monthly, weekly, and daily or almost daily for items 2-5) | |
| 3. In the past three months, how often have you had a strong desire or urge to use (each substance)? | |
| 4. (During the past three months, how often has your use of (each substance) led to health, social, legal or financial problems? | |
| 5. During the past three months, how often have you failed to do what was normally expected of you because of your use of (each substance)? | |
| 6. Has a friend or relative or anyone else ever expressed concern about your use of (each substance)? | |
| 7. Have you ever tried to control, cut down or stop using (each substance)? | |
| 8. Have you ever used any drug by injection? |
2.2.2. Reference Test: Urine and Hair Drug Testing
Combined results from urine and hair drug testing were used as the reference standard for detecting past 90-day drug use i.e. urine to capture drug use in the past 1–2 weeks and hair to capture drug use in the past 90 days.30,31 The Alere iCup® 14-Panel urine multi-drug test was used to determine the presence of 14 substances including opioids, cocaine, barbiturates, benzodiazepines, amphetamines, cannabis and tricyclic antidepressants. Hair samples were processed in a commercial laboratory where confirmatory testing for drug metabolites was done. We also collected data from electronic health records (EHR) on all currently prescribed drugs which helped in distinguishing legitimate use of prescription medications such as opioids, sedatives and antidepressants from misuse.
2.3. Procedures
Women visiting the obstetric practices for prenatal care appointments were approached by research staff and given a brief description of the study to determine their interest in participating. If eligible, the staff obtained informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization (for urine collection, hair drug testing and prescription drug data abstraction from the EMR). Informed consent detailed that a certificate of confidentiality was obtained for this study and no study results would be shared with clinical practice staff. The study process lasted 20–30 minutes.
2.4. Statistical Analysis
We analyzed substance specific SI scores by demographic characteristics. We utilized receiver operator curve (ROC) analysis to evaluate the drug-specific diagnostic accuracy of the NM-ASSIST screen (index test). We computed the sensitivity, specificity, optimum cut-points for SI scores, area under the curve (AUC) and diagnostic odds ratio for detecting substance use in pregnancy. For each drug category in this population, we conducted analysis in the following manner for the NM-ASSIST: sensitivity was the probability that a woman who is using drugs while pregnant (as determined by biologic testing) is a screen positive on the NM-ASSIST; specificity was the probability that a woman who is not using drugs while pregnant is a screen negative on the NM-ASSIST; and diagnostic odds ratios were computed as the ratio of the odds of a positive NM-ASSIST screen in women who use drugs in pregnancy relative to the odds of a positive NM-ASSIST screen in women who do not use drugs in pregnancy (shown in Table 3).32 We used hair and urine drug testing as the reference test; a positive drug test in either urine or hair was considered positive.
Table 3.
Prevalence, AUC, Sensitivity and Specificity for Cannabis, Cocaine, Benzodiazepines, Opioids and Amphetamines Use, n=483
| Drug | AUC | Prenatal# Drug Use, n (%) |
NM-ASSIST Cut-Point |
Screen Positive on NM-ASSIST, n (%) |
Sensitivity (95% CI) |
Specificity (95% CI) |
Diagnostic Odds Ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| Cannabis | 0.9 | 151 (32%) | 2 | 189 (39.1%) | 82.1 (75.1, 87.9) | 85.4 (81.1, 89.1) | 26.9 (16.0, 45.0) |
| Cocaine | 0.6 | 47 (9.9%) | 2 | 11 (2.3%) | 17.0 (7.7, 30.8) | 99.3 (98.0, 99.9) | 29.0 (8.0, 105.0) |
| Prescription Opioids | 0.6 | 20 (4.3%) | 3 | 13 (2.7%) | 25.0 (8.7, 49.1) | 98.2 (96.5, 99.2) | 18.3 (5.6, 60.4) |
| Street Opioids | 0.6 | 8 (1.7%) | 4 | 8 (1.7%) | 12.5 (0.3, 52.7) | 98.5 (96.9, 99.4) | 9.3 (0.0, 68.1) |
| Benzodiazepines | 0.6 | 5 (1.0%) | 15 | 4 (0.8%) | 20.0 (0.5, 71.6) | 99.4 (98.1, 99.9) | 38.8 (0.0, 357.0) |
Amphetamines: 0 screen positives, but true prevalence of 1.2% (6 positive biologic drug tests)
Urine or hair sample tested positive for substances
Screen Positives on NM-ASSIST based on cut-point derived from ROC analysis
To establish the most optimal SI score cut-point of the NM-ASSIST, the ROC curve was used. Cut-points which maximized sensitivity were chosen for each substance in ROC analysis. We calculated the area under the curve (AUC), which is the probability estimate of the discriminative power of the NM-ASSIST and can take any value from 0 to 1, with 1 representing a perfect diagnostic test and 0.5 representing a non-discriminating test (no better than a coin toss).33
Regarding substances for which both index and reference tests were administered, we present results only for substances for which there was at least one positive screen on the index test (NM-ASSIST). For example, even though the index test (NM-ASSIST) asks about other substances such as hallucinogens or prescription stimulants, no participants screened positive for these substances in either the index or the reference tests. Regarding substances for which no reference testing was performed – alcohol and tobacco use (nicotine) – no analyses of diagnostic accuracy were performed. All participants received the index test (NM-ASSIST) irrespective of whether they were initially positive on the NIDA Quick Screen. All statistical analyses were conducted with STATA version 13 and SAS 9.4 software.
3. Results
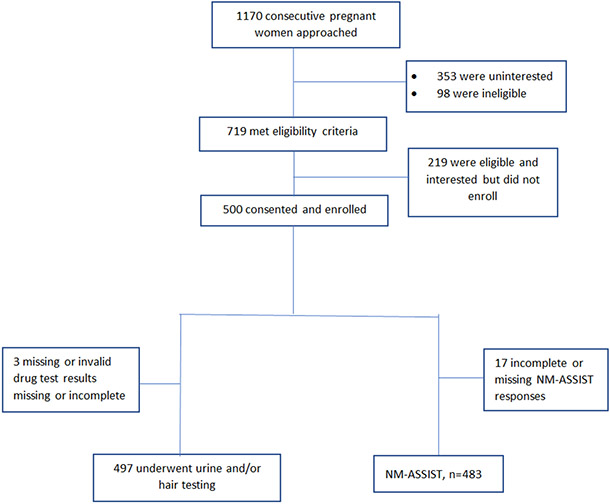
Of the 500 pregnant women enrolled, 483 (97%) were included in the final analyses as shown in Figure 1, with 17 excluded for missing data in key variables, either for the index test (NM-ASSIST) or for drug testing. Table 2 shows the median SI score for cannabis, cocaine, benzodiazepines and opioids, by demographic information of age, race, trimester, education, parity and gravidity.
Figure 1.
Flow Diagram
Table 2.
NIDA SI Scores by Substance and Demographic Information (N=483)
| Cannabis | Cocaine | Prescription Opioids |
Street Opioids |
Benzodiazepines | |||
|---|---|---|---|---|---|---|---|
| Variable | Categories | n (%) | Median (Range) |
Median (Range) |
Median (Range) |
Median (Range) |
Median (Range) |
| Age | 18-25 | 170 (35.2) | 0 (0, 37) | 0 (0, 6) | 0 (0, 26) | 0 (0, 39) | 0 (0, 22) |
| 26+ | 313 (64.8) | 0 (0, 33) | 0 (0, 37) | 0 (0, 39) | 0 (0, 39) | 0 (0, 37) | |
| Race | African American/Black | 346 (71.6) | 0 (0, 37) | 0 (0, 37) | 0 (0, 33) | 0 (0, 33) | 0 (0, 33) |
| Caucasian/White | 102 (21.1) | 0 (0, 23) | 0 (0, 37) | 0 (0, 39) | 0 (0, 39) | 0 (0, 37) | |
| Other/Multiracial | 35 (7.3) | 0 (0, 6) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| Trimester | 1st | 150 (31.1) | 0 (0, 33) | 0 (0, 35) | 0 (0, 33) | 0 (0, 28) | 0 (0, 15) |
| 2nd | 173 (35.8) | 0 (0, 37) | 0 (0, 26) | 0 (0, 6) | 0 (0, 6) | 0 (0, 12) | |
| 3rd | 160 (33.1) | 0 (0, 33) | 0 (0, 37) | 0 (0, 39) | 0 (0, 39) | 0 (0, 37) | |
| Education | Less than High School | 83 (17.2) | 3 (0, 33) | 0 (0, 35) | 0 (0, 33) | 0 (0, 28) | 0 (0, 0) |
| High School Graduate | 199 (41.2) | 0 (0, 37) | 0 (0, 37) | 0 (0, 39) | 0 (0, 39) | 0 (0, 37) | |
| Some College | 84 (17.4) | 0 (0, 27) | 0 (0, 26) | 0 (0, 12) | 0 (0, 6) | 0 (0, 0) | |
| College Graduate | 115 (23.8) | 0 (0, 6) | 0 (0, 6) | 0 (0, 0) | 0 (0, 0) | 0 (0, 12) | |
| Unavailable | 2 (0.4) | 5.5 (0, 11) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| Parity1 | 0 | 90 (18.6) | 0 (0, 37) | 0 (0, 26) | 0 (0, 6) | 0 (0, 0) | 0 (0, 15) |
| 1 or 2 | 227 (47.0) | 0 (0, 33) | 0 (0, 37) | 0 (0, 39) | 0 (0, 39) | 0 (0, 37) | |
| 3 or 4 | 48 (9.9) | 1 (0, 28) | 0 (0, 37) | 0 (0, 9) | 0 (0, 33) | 0 (0, 33) | |
| 5 or more | 13 (2.7) | 6 (0, 29) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| NA3 | 105 (21.7) | 0 (0, 23) | 0 (0, 35) | 0 (0, 33) | 0 (0, 2) | 0 (0 0) | |
| Gravidity2 | 0 | 108 (22.4) | 0 (0, 23) | 0 (0, 35) | 0 (0, 33) | 0 (0, 2) | 0 (0, 12) |
| 1 or 2 | 189 (39.1) | 0 (0, 37) | 0 (0, 30) | 0 (0, 33) | 0 (0, 28) | 0 (0, 15) | |
| 3 or 4 | 110 (22.8) | 0 (0, 33) | 0 (0, 37) | 0 (0, 39) | 0 (0, 39) | 0 (0, 37) | |
| 5 or more | 75 (15.6) | 0 (0, 29) | 0 (0, 26) | 0 (0, 26) | 0 (0, 39) | 0 (0, 22) | |
| NR4 | 1 (0.2) | 11 (11, 11) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
Parity is the response to the question "How many of these pregnancies resulted in a fullterm delivery?" in reference to the question below.
Gravidity is the response to the question "Not counting your current pregnancy, how many times have you been pregnant (including any pregnancies that resulted in abortions, miscarriages or stillbirths)?"
NA indicates that the question was not applicable based on responses to previous questions (i.e. having no previous pregnancies).
NR indicates that the participant gave no response.
Median SI scores were notable only for cannabis: pregnant women with less than high school education had a median (range) SI score of 3 (0, 33); and for multiparous women with 5 or more births, it was 6 (0, 29).
Table 3 shows prevalence of prenatal drug use as determined by either hair or urine drug testing (reference standard). Prevalence estimates were: cannabis =32.0%, cocaine =9.9%, benzodiazepines =1.0%, prescription opioids =4.3%, and street opioids =1.7%. Using SI score cut-points that maximized sensitivity, the NM-ASSIST classified pregnant women as screen positives as follows: cannabis =39.1%, cocaine =2.3%, benzodiazepines =0.8%, prescription opioids =2.7%, and street opioids =1.7%. There were no screen positives for amphetamines but 6 (1.2%) women had positive amphetamine hair or urine test. Sensitivity estimates (95% CI) were: cannabis = 82.1 (75.1, 87.9), cocaine = 17.0 (7.7, 30.8), benzodiazepines = 20.0 (0.5, 71.6), prescription opioids = 25.0 (8.7, 49.1), and street opioids = 12.5 (0.3, 52.7), and Specificity estimates were: cannabis = 85.4 (81.1, 89.1), cocaine = 99.3 (98.0, 99.9), benzodiazepines = 99.4 (98.1, 99.9), prescription opioids = 98.2 (96.5, 99.2) and street opioids = 98.5 (96.9, 99.4).
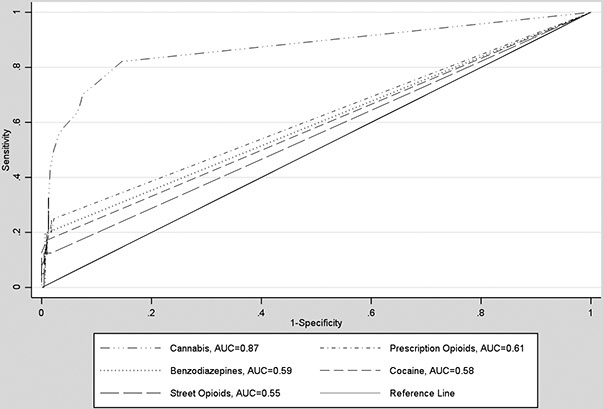
Figure 2 shows receiver operator characteristic curves (ROC) for substance-specific diagnostic accuracy of the NM-ASSIST. Area under the curve (see Table 3) was highest for cannabis, 0.87; followed by prescription opioids, 0.61; benzodiazepines, 0.59; cocaine, 0.58; and street opioids, 0.55. Diagnostic odds ratio (DOR) was highest for benzodiazepines, 38.8 (0.0, 357.0); followed by cocaine, 29.0 (8.0, 105.0); cannabis, 26.9 (16.0, 45.0); prescription opioids, 18.3 (5.6, 60.4); and street opioids, 9.3 (0.0, 68.1).
Figure 2.
Receiver operator characteristic curves (ROC) for substance-specific diagnostic accuracy of the NM-ASSIST
4. Discussion
In screening for prenatal drug use, diagnostic validity denotes how well a particular screener measures what it purports to measure (i.e. drug use in the current pregnancy). Prior validations of the WHO ASSIST did not report substance specific diagnostic accuracy of the screener for substance use in pregnancy, with biologic testing as a reference. As such, ours is the first study to examine the performance of the NM-ASSIST by drug in pregnant women. This study further evaluates the performance of the screener for commonly used drugs in a population of pregnant women in an urban population of the U.S.
Our findings in this population, based on ROC analysis, suggest that the NM-ASSIST had the highest discriminative value for cannabis use (AUC=0.87), but demonstrated lower than desirable AUC values for cocaine (AUC=0.58), benzodiazepines (AUC=0.59), prescription opioids (AUC=0.61) and street opioids (AUC=0.55). A prior validation of the ASSIST in primary care and drug use treatment populations of adults (not pregnant women) using the MINI-Plus (not biological testing) as reference standard showed that the ASSIST had an AUC of 0.9 for cannabis, 0.9 for amphetamines and 1.0 for opioids (all categorized together).21 However, the referenced study obtained a third of its sample from persons seeking treatment for substance use disorder, and thus had a higher prevalence of substance use.
For sensitivity, the ability to correctly identify users, the NM-ASSIST similarly performed best for cannabis, with a sensitivity of 82%. The sensitivity was lower than desirable for prescription opioids (25.0%), benzodiazepines (20.0%), cocaine (17.0%) and street opioids (12.5%). A prior validation by Newcombe et al (2005) in primary care adults shows a similar sensitivity for cannabis (85%), but a much higher sensitivity for opioids (100%).21 Regarding specificity, that is, the ability of the screener to correctly identify nonusers, the NM-ASSIST performed best for opioids, benzodiazepines and cocaine (specificity=98–99%), followed by cannabis (85%). The validation by Newcombe et al (2005) finds specificity for cannabis to be 83% and for all opioids to be 91%.21 The reference standard in the above referenced study was the MINI-plus; our study utilizes the gold standard - biologic drug screening with urine and hair samples - as reference.
Given that the use of the NM-ASSIST in this population for substance-specific validation has not produced both superior sensitivity and specificity for any particular substance (highest sensitivity for cannabis, highest specificity for benzodiazepines), we utilized the DOR to evaluate the effectiveness of the NM-ASSIST as a binary (positive/negative) classifier of prenatal drug use. We found that the NM-ASSIST is an effective binary classifier for prenatal use of cannabis, cocaine and benzodiazepines, given the values of DOR of 26.9, 29.0, and 38.8 respectively, but not for opioids (prescription opioids, 18.3; and street opioids, 9.3). The DOR is a robust measure that is independent of prevalence and is the ratio of the odds of a positive NM-ASSIST screen in prenatal drug users relative to the odds of a positive NM-ASSIST screen in non-users. DOR values higher than 20 are considered good for screening tools.34
Our study has significant strengths. The sample size of 500 pregnant women attains a sufficiently large sample for this kind of analysis in a high-prevalence urban setting. Additionally, we utilized biologic testing of urine and hair samples which represent the gold standard for detecting substance use. In addition, our population represents two prenatal clinics and therefore is more likely to be representative of a population that would be using these screeners. Most previous studies of prenatal drug use have over-selected substance users and included many women already accessing treatment for substance use, which is not the target population for a screener.
Despite the study’s strengths, there are limitations worth noting. The NM-ASSIST is only used clinically as a follow up to a positive screen on the NIDA Quick Screen; however, for validation purposes, we included all respondents in our analysis, irrespective of whether they were considered positive on the NIDA Quick Screen or not. While this does not detract from the drug-specific validation, it limits its direct clinical application within the context of the combined NIDA-modified Quick Screen/ASSIST. Our study is drawn from a prenatal clinic population with a higher than average prevalence of prenatal drug use when compared with nationwide results from the National Survey on Drug Use and Health (NSDUH) thus limiting its external validity;35 for example, Ahrnsbrak et al (2017)36 reported prevalence of prenatal substance of 20%, compared to our study population for which total substance use prevalence approached 40%. However, primary care prenatal settings such as these are ideal for testing the NM-ASSIST in pregnancy, given that it mimics the intended real-world use.
Furthermore, our study only validated the NM-ASSIST for cannabis, cocaine, benzodiazepines, opioids, and to a certain extent, amphetamines, given that there were very few or no screen positives for the other substances such as hallucinogens and inhalants. Nevertheless, we retained an analysis of benzodiazepines and street opioids despite the low prevalence in this population because we would expect similar findings in pregnant women in non-treatment settings, which is the setting in which the NM-ASSIST will be most frequently deployed for screening for prenatal drug use. To mitigate this concern about unique prevalence rates, we included substance-specific DOR estimates, which is a robust measure that is independent of prevalence. Additionally, we did not validate alcohol and tobacco use with biologic testing, mostly because the NM-ASSIST does not include items on alcohol and smoking (unlike the WHO ASSIST V3); nor did we validate the NIDA Quick Screen in general. Finally, drug testing with hair and urine samples, although considered the gold standard, have some limitations. Both urine and hair testing often produce false positives and do not inform about timing or dosage of substance use.37 Hair sample testing does not include benzodiazepines, barbiturates or tricyclic antidepressants; additionally, hair samples allow for detecting substance use for up to 90 days after last use compared to only 5–14 days for urine.30 By combining both urine and hair sample testing as gold standard, we recognized the imperfections of each method and hoped to maximize the relative strengths of each, providing a stronger reference test for validating the NM-ASSIST than either alone.
From a broader clinical perspective, it is pertinent to re-evaluate the p use of self-reports for prenatal substance use screening versus simply implementing universal toxicological testing. Despite the well-documented limitations of utilizing self-report for identifying prenatal drug use such as under-reporting, we believe that self-report screeners such as the NM-ASSIST maintain a unique role.38-41 Even though urine testing can be universally deployed, albeit at significantly higher cost, it only captures recent drug use. Hair sample testing, which can detect drug use for up to 90 days after use, may be difficult to deploy universally in a clinical setting given that it requires gas chromatography–mass spectrometry (GCMS) which is expensive and not timely, precluding the possibility of combining hair and urine sampling in clinical settings as replacement for self-report. Yonkers et al (2011)39 found that self-report may be a better indicator of past-month prenatal substance use than urine testing which capture only about 1–2 weeks, noting that women accurately self-reported recent substance use in general, but also tended to report that they used at a time that would be outside the testing window of urine toxicology e.g. using a month ago would be detected by self-report but not urine testing. Self-report may also allow for more of a collaborative clinical conversation around prenatal substance use than toxicology screening, as the former incorporates the patient’s voice and requires mutual trust. Christmas et al (1992)42 suggested that using a screening combination of self-report and universal urine toxicology could identify more current users than either technique alone, with none being clearly more accurate than the other. These two reports suggest that, at the very least, self-report retains a crucial role, and though a combination of the two would be better, it is likely to require more resources than either alone, with universal toxicology being more cost-prohibitive to deploy than self-report.
5. Conclusion
The NM-ASSIST demonstrated substantial validity as a screener for prenatal cannabis use, which is the most common substance used in pregnancy. However, the NM-ASSIST demonstrated a variable performance for cocaine, benzodiazepines, opioids and amphetamines. Despite these findings, we recommend that further analyses of diagnostic validity of the NM-ASSIST should be conducted in other contexts within pregnancy, such as in rural, suburban and perhaps native populations, to ensure that it serves as a robust tool for detecting prenatal drug use. Because of the NM-ASSIST’s variability in performance between drugs, its utility in any practice setting would likely depend on the practice-specific prevalence of substance use. Given the increasing prevalence and high risk associated with opioid use in pregnancy, further studies to validate screening modalities for opiate use in pregnancy are warranted. Finally, while SI score cut-points are important, clinical judgment is still recommended for deciding which patients are considered for an intervention; for example, a patient who reports polysubstance use but does not obtain a high enough SI score for any particular drug may be a candidate for further intervention.
6. Acknowledgement
EAO and VCC conceived the study. EAO, VCC, ENP and KM participated in the drafting of the manuscript and each approved the final draft. We acknowledge Kelsy Kinderknecht for assisting with statistical analysis.
7. Funding
The Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01DA041328.
Footnotes
Conflicts of Interest
None declared.
8. References
- 1.SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of national findings. In: NSDUH Series H-46, HHS Publication No.(SMA) 13–4795. Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, MD; 2013. [Google Scholar]
- 2.Forray A Substance use during pregnancy. F1000Research. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman-Cowger VH, Oga EA, Peters EN, Trocin K, Koszowski B, Mark K. Comparison and validation of screening tools for substance use in pregnancy: a cross-sectional study conducted in Maryland prenatal clinics. BMJ Open. 2018;8(2):e020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasnoff I, Wells A, McGourty R, Bailey L. Validation of the 4P's Plus© screen for substance use in pregnancy validation of the 4P's Plus. Journal of Perinatology. 2007;27(12):744. [DOI] [PubMed] [Google Scholar]
- 5.Oga EA, Peters EN, Mark K, Trocin K, Coleman-Cowger VH. Prenatal Substance Use and Perceptions of Parent and Partner Use Using the 4P’s Plus Screener. Maternal and child health journal. 2019;23(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yonkers KA, Gotman N, Kershaw T, Forray A, Howell HB, Rounsaville BJ. Screening for prenatal substance use: development of the Substance Use Risk Profile-Pregnancy scale. Obstetrics and gynecology. 2010;116(4):827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIDA. Smoking, and Substance Involvement Screening Test: NM-ASSIST. In.
- 8.WHO. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–1194. [DOI] [PubMed] [Google Scholar]
- 9.Midanik LT. Validity of self‐reported alcohol use: a literature review and assessment. British journal of addiction. 1988;83(9):1019–1029. [DOI] [PubMed] [Google Scholar]
- 10.Zuckerman B, Amaro H, Cabral H. Validity of self-reporting of marijuana and cocaine use among pregnant adolescents. The Journal of pediatrics. 1989;115(5):812–815. [DOI] [PubMed] [Google Scholar]
- 11.Garg M, Garrison L, Leeman L, et al. Validity of self-reported drug use information among pregnant women. Maternal and child health journal. 2016;20(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RH, Zatzick DF, Anders TF. The detection and treatment of psychiatric disorders and substance use among pregnant women cared for in obstetrics. American journal of psychiatry. 2001;158(2):213–219. [DOI] [PubMed] [Google Scholar]
- 13.McNeely J, Strauss SM, Wright S, et al. Test–retest reliability of a self-administered Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in primary care patients. Journal of substance abuse treatment. 2014;47(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeely J, Wu L-T, Subramaniam G, et al. Performance of the tobacco, alcohol, prescription medication, and other substance use (TAPS) tool for substance use screening in primary care patients. 2016;165(10):690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NIDA. Tobacco, Alcohol, Prescription medication, and other Substance use Tool (TAPS). https://www.drugabuse.gov/taps/#/. Published 2018. Accessed August 6, 2018.
- 16.Chang G, Ondersma SJ, Blake-Lamb T, Gilstad-Hayden K, Orav EJ, Yonkers KA. Identification of substance use disorders among pregnant women: A comparison of screeners. Drug and Alcohol Dependence. 2019:107651. [DOI] [PubMed] [Google Scholar]
- 17.Ondersma SJ, Chang G, Blake‐Lamb T, et al. Accuracy of five self‐report screening instruments for substance use in pregnancy. Addiction. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman-Cowger VH, Oga EA, Peters EN, Trocin KE, Koszowski B, Mark K. Accuracy of three screening tools for prenatal substance use. Obstetrics and gynecology. 2019;133(5):952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trocin KE, Weinstein NI, Oga EA, Mark KS, Coleman-Cowger VH. Prenatal Practice Staff Perceptions of Three Substance Use Screening Tools for Pregnant Women. Journal of addiction medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotham E, Ali R, White J, Sullivan T, Robinson J. Investigation of the alcohol, smoking, and substance involvement screening test (the ASSIST) version 3.0 in pregnancy. Addictive Disorders & Their Treatment. 2013;12(3):123–135. [Google Scholar]
- 21.Newcombe DA, Humeniuk RE, Ali R. Validation of the world health organization alcohol, smoking and substance involvement screening test (ASSIST): report of results from the Australian site. Drug and alcohol review. 2005;24(3):217–226. [DOI] [PubMed] [Google Scholar]
- 22.Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction. 2008;103(6):1039–1047. [DOI] [PubMed] [Google Scholar]
- 23.Humeniuk R, Ali R, Organization WH, Group APIS. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and pilot brief intervention [electronic resource]: a technical report of phase II findings of the WHO ASSIST Project. 2006. [Google Scholar]
- 24.Gryczynski J, Kelly SM, Mitchell SG, Kirk A, O'grady KE, Schwartz RP. Validation and performance of the A lcohol, S moking and S ubstance I nvolvement S creening T est (ASSIST) among adolescent primary care patients. Addiction. 2015;110(2):240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'grady KE, Gryczynski J, Mitchell SG, Ondersma SJ, Schwartz RP. Confirmatory factor analysis of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in community health center patients. The American journal on addictions. 2016;25(4):259–263. [DOI] [PubMed] [Google Scholar]
- 26.Tiburcio Sainz M, ROSETE-MOHEDANO MG, Natera Rey G, Martinez Velez NA, Carreno Garcia S, Perez Cisneros D. Validity and Reliability of the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) in University Students. Adicciones. 2016;28(1). [DOI] [PubMed] [Google Scholar]
- 27.Soto-Brandt G, Huidobro RP, Artigas DH, et al. Evidencia de validez en Chile del alcohol, smoking and substance involvement screening test (ASSIST). Adicciones. 2014;26(4):291–302. [DOI] [PubMed] [Google Scholar]
- 28.Oga EA, Mark K, Coleman-Cowger VH. Cigarette Smoking Status and Substance Use in Pregnancy. Maternal and child health journal. 2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman-Cowger VH, Oga E, Peters EN, Mark K. Prevalence and associated birth outcomes of co-use of Cannabis and tobacco cigarettes during pregnancy. Neurotoxicology and teratology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuPont RL, Baumgartner WA. Drug testing by urine and hair analysis: complementary features and scientific issues. Forensic Science International. 1995;70(1–3):63–76. [DOI] [PubMed] [Google Scholar]
- 31.Ledgerwood DM, Goldberger BA, Risk NK, Lewis CE, Price RK. Comparison between self-report and hair analysis of illicit drug use in a community sample of middle-aged men. Addictive behaviors. 2008;33(9):1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. Journal of clinical epidemiology. 2003;56(11):1129–1135. [DOI] [PubMed] [Google Scholar]
- 33.Šimundić A-M. Measures of diagnostic accuracy: basic definitions. Ejifcc. 2009;19(4):203. [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive care medicine. 2003;29(7):1043–1051. [DOI] [PubMed] [Google Scholar]
- 35.SAMHSA. SAMHSA’s Annual Mental Health, Substance Use Data Provide Roadmap for Future Action. In:2018.
- 36.Ahrnsbrak R, Bose J, Hedden S, Lipari R, Park-Lee E. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration (SAMHSA) NSDUH Data Review https://wwwsamhsagov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016htm Published September 2017. 2017. [Google Scholar]
- 37.Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. Journal of analytical toxicology. 2014;38(7):387–396. [DOI] [PubMed] [Google Scholar]
- 38.Roberts SC, Nuru-Jeter A. Women’s perspectives on screening for alcohol and drug use in prenatal care. Women’s Health Issues. 2010;20(3):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yonkers KA, Howell HB, Gotman N, Rounsaville BJ. Self-report of illicit substance use versus urine toxicology results from at-risk pregnant women. Journal of substance use. 2011;16(5):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weatherby NL, Needle R, Cesari H, et al. Validity of self-reported drug use among injection drug users and crack cocaine users recruited through street outreach. Evaluation and Program planning. 1994;17(4):347–355. [Google Scholar]
- 41.Harrison L, Hughes A. The validity of self-reported drug use: Improving the accuracy of survey estimates. NIDA research monograph. 1997;167:1–16. [PubMed] [Google Scholar]
- 42.Christmas JT, Knisely JS, Dawson KS, Dinsmoor MJ, Weber SE, Schnoll SH. Comparison of questionnaire screening and urine toxicology for detection of pregnancy complicated by substance use. Obstetrics and gynecology. 1992;80(5):750–754. [PubMed] [Google Scholar]