Abstract
In a number of mouse models of hereditary deafness, therapeutic transgene delivery to the cochlea and vestibular organs using adeno-associated viral vectors (AAVs) has shown striking rescue of hearing and balance. However, only a subset of AAV capsids have shown efficacy in transducing both inner hair cells and outer hair cells, and it is also not clear which of these can be translated to treatment of human inner ear. We recently reported efficient transgene expression of a GFP reporter in a non-human primate cochlea, in both inner and outer hair cells, following injection of the AAV9 capsid variant PHP.B via the round window membrane (RWM). However efficiency was poor at a lower dose. To further define the transduction potential of AAV9-PHP.B, we have performed a dosing study in the cynomolgus monkey and assessed vector-encoded GFP expression. Three animals were injected in both ears and four doses were tested. We describe a transmastoid surgical approach needed to access the RWM of this common primate model. We found that AAV9-PHP.B transduced nearly 100% of both IHCs and OHCs, from base to apex, at the higher doses (3.5×1011 and 7×1011 vector genomes). However, at lower doses there was a steep reduction in viral transduction. Thus, AAV9-PHP.B efficiently transduces the IHCs and OHCs of nonhuman primates, and should be considered as an AAV capsid for inner ear gene therapy in humans.
1. Introduction
Hair cells in the cochlea are responsible for amplifying and detecting sound. They are of two types: outer hair cells (OHCs) amplify basilar membrane vibration for quiet sounds, and inner hair cells (IHCs) sense the vibration and convey the neural signal to spiral ganglion neurons. Hair cells are compromised in the majority of inherited forms of deafness, and so gene therapy vectors to treat these disorders must affect transgene expression in both OHCs and IHCs. Gene therapy using adeno-associated virus (AAV) delivery has been shown to be effective in treating some forms of inherited deafness in mouse models, and offers great potential for patient treatment. However, two issues remain: First, many AAV serotypes tested in vivo in mouse after round window membrane (RWM) injection (such as AAV1, AAV8, and AAV9) target the IHCs, but do not target the OHCs efficiently (Akil, Seal et al. 2012, Askew, Rochat et al. 2015, Chien, Isgrig et al. 2016, Gyorgy, Sage et al. 2017). In recent years, our group and others have demonstrated that novel vectors such as AAV9-PHP.B and Anc80L65 are capable of eliciting more efficient transduction in both IHCs and OHCs in mice (Landegger, Pan et al. 2017, Gyorgy, Meijer et al. 2019, Tan, Chu et al. 2019). Second, while efficient transduction of hair cells has been shown in rodents with AAV, their potential has not been adequately explored in non-human primates (NHPs), a more relevant therapeutic model for human treatment. Thus significant challenges remain before AAV-based vectors can be translated to the clinic. Recently our group reported robust transgene expression in cochlea of a cynomolgus monkey (Macaca fascicularis) using an AAV9 capsid variant, PHP.B, injected via the RWM (Gyorgy, Meijer et al. 2019). PHP.B was originally selected for its ability to transduce the brains of mice after intravenous injection (Deverman, Pravdo et al. 2016). However, while the enhancement was confirmed in the mouse strain in which the library was selected, this enhancement was not observed in NHPs (Hordeaux, Wang et al. 2018, Matsuzaki, Konno et al. 2018). Surprisingly, PHP.B mediated efficient transgene expression of a GFP reporter in many cochlear cells including both inner and outer hair cells of an NHP. However, a second monkey injected with a lower dose showed limited transduction with no obvious transduction of hair cells (Gyorgy, Meijer et al. 2019). The difference might be attributed to steep dose dependency, to a pre-existing immunity (i.e. neutralizing antibodies) to the AAV vector, or to the technical reproducibility of RWM injections in larger mammals.
To further define the transduction potential of AAV9-PHP.B in primates, we performed a dosing study in the cynomolgus monkey inner ear using RWM injection of AAV9-PHP.B, and assessed GFP expression. We found that AAV9-PHP.B efficiently transduces the IHCs and OHCs of nonhuman primates, but shows a striking dose dependency.
Because the narrow ear canal of cynomolgus monkeys does not allow trans-canal access to the RWM for vector injection, we used a transmastoid approach to expose the RWM. Here we also describe the surgical approach in detail.
2. Methods
2.1. AAV vector production and purification
For vector production, we used an AAV plasmid driving GFP as previously described (Gyorgy, Meijer et al. 2019). Briefly, the single-stranded (ss) transgene cassette consisted of AAV2 inverted terminal repeats (ITRs) flanking a hybrid cytomegalovirus immediate-early/chicken beta actin promoter (CBA), GFP cDNA, a woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and SV40 and bovine growth hormone polyA signal sequences.
Production of the AAV9.PHP.B vector was done in HEK293T cells. Eighteen tissue culture dishes (15 cm diameter) were used (1.5×107 cells per plate), with cells cultured in DMEM containing 10% fetal bovine serum (FBS) and 100 U/ml of penicillin, 100 μg/ml streptomycin, and 292 μg/ml L-glutamine (Invitrogen). Twenty-four hours after plating, cells were triple transfected using the calcium phosphate method, adding to each plate GFP transgene plasmid (10 μg), pGG-PHP.B rep/cap plasmid (12 μg) (a kind gift of Dr. Miguel Sena-Esteves, University of Massachusetts Medical School), and pAdΔF6 (helper plasmid, 26 μg). Cell media was replaced the day after transfection with DMEM containing 2% FBS. Seventy-two hours post transfection, AAV was purified from clarified cell lysate by iodixanol density-gradient ultracentrifugation.
2.2. Mouse housing and breeding
All mouse experiments were performed in compliance with ethical regulations approved by the Animal Care Committee of Harvard Medical School. Wild-type pregnant C57BL/6 females were obtained from Jackson Laboratory, and their P1 pups were used for AAV9-PHP.B-GFP injections.
2.3. Mouse RWM injection in neonatal mice
Pups were anesthetized by hypothermia and then kept on an ice pack during the procedure. As previously described (Gyorgy, Sage et al. 2017), a small incision was made underneath the external ear. The incision was enlarged, and soft tissues were retracted using an eyelid retractor to expose the bulla. The round window niche was located visually. For GFP expression experiments 1.2 μL of AAV9-PHP.B-CBA-GFP vector solution was injected with a micropipette needle at a rate of 60 nL/min. The surgical incision was closed with 2–3 sutures using a 7–0 Vycril surgical suture. At P6, mice were euthanized to assess vector transduction.
2.4. Mouse cochlear immunostaining and imaging
For immunostaining, cochleas were fixed with 4% formaldehyde in PBS for 1 hr, washed three times with PBS to remove fixative, and then blocked and permeabilized with 10% donkey serum with 0.3% Triton X-100 for 1 hr at 22 C. To label hair cells, rabbit polyclonal anti-MYO7A antibodies (Proteus Biosciences, Cat. No. 25–6790) were diluted 1:500 in 10% donkey serum (NGS)/0.1% Triton X-100/PBS and incubated overnight at 4 C followed by several rinses in PBS. Next, samples were incubated in blocking solution for 30 min at room temperature, and incubated 4–6 hrs at room temperature with a donkey anti-rabbit immunoglobulin G (IgG) secondary antibody conjugated to Alexa Fluor 593 in a 1:500 dilution in blocking solution. To stain hair-bundle actin, we used phalloidin conjugated to Alexa Fluor 405 (Life Technologies) (1:40). GFP was detected via its intrinsic fluorescence. Tissues were mounted on a Colorfrost glass slide (Thermo Fisher Scientific) using Prolong Gold Antifade mounting medium (Thermo Fisher Scientific). Imaging for GFP fluorescence was performed with a Nikon Ti2 inverted spinning disk confocal using a Plan Apo λ 20x/0.8 objective and Plan Apo λ 60x/1.4 oil objective.
2.5. NHP surgical procedures
2.5.1. Overview.
NHP studies were performed at Charles River Laboratories (Montreal, ON, Canada) according to animal use guidelines and approved procedures. Four female (1.5–5 y.o., weighing 3–5 kg) cynomolgus monkeys (Macaca fascicularis) were studied. Animals were considered acclimated to the environment at the time of transfer to the study. Each animal was healthy and without history of ear infection, ear surgery, signs of imbalance, or other risk factors for loss of inner ear or middle ear function.
Three female monkeys received transmastoid/trans-RWM injection in both cochleas of AAV9-PHP.B-GFP with doses of 1×1011 VG (n=1), 2×1011 VG (n=1), 3.5×1011 VG (n=2), and 7×1011 VG (n=2); the fourth animal served as a vehicle (PBS) control (see Table 1).
Table 1.
Volume and dose of AAV9-PHP.B-CBA-GFP administered to each NHP.
| Cynomolgus monkey | Right ear dose | Left ear dose |
|---|---|---|
| #1 (ID#2501) | 3.5 × 1011 VG (10ul) | 7.0 × 1011 VG (20ul) |
| #2 (ID#3501) | 1.0 × 1011 VG (10ul) | 3.5 × 1011 VG (10ul) |
| #3(ID#4501) | 2.0 × 1011 VG (10ul) | 7.0 × 1011 VG (20ul) |
| #4(ID#1501) | PBS (10ul) | PBS (20ul) |
2.5.2. Pre- and post-operative anesthesia, analgesia, and antibiotic administration.
The animals were anesthetized by intramuscular injection of a cocktail (ketamine, 10 mg/kg; xylazine, 0.6 mg/kg; and glycopyrrolate, 0.01 mg/kg) following overnight food deprivation. Animals were intubated, and maintained with oxygen and isoflurane during surgery. Eye lubricant was administered prior to surgery, during surgery as deemed necessary and following surgery. During the surgery, animals received warmed lactated Ringer’s solution intravenously (10mL/kg/h; volume and rate adjusted as necessary) to improve recovery.
Dexamethasone (1 mg/kg intramuscular) was given once on the day of surgery, at least 30 min before incision, and again beginning ~24 hours later to reduce fibrosis and inflammatory responses associated with surgery and to reduce nausea (0.5 mg/kg intramuscular every 8–12 hours for two additional days, a total of 5 injections). Local anesthesia for skin incision was achieved using subcutaneous injection of 1.0 mg/kg of bupivacaine 0.25% prior to incision and during wound closure. Cefazolin (20 mg/kg intravenously) was given prior to surgery and every 90–120 min until wound closure. Hydromorphone (0.1 mg/kg intramuscular) was given twice (on the day of surgery at least 30 minutes prior incision and again 2–4 hours later). Buprenorphine SR (0.2 mg/kg subcutaneous) was provided 2–4 h after the last dose of hydromorphone and again three days later until the animal appeared to be pain-free without medication. After the last dexamethasone administration, animals were treated with meloxicam (0.2 mg/kg,, followed by 0.1 mg/kg for 2 days).
Daily wound checks were performed throughout the study. Animals were monitored for evidence of pain and recovery and treated accordingly following completion of the regimen outlined above.
2.5.3. Surgical approach.
All viral inner ear administrations were performed via the facial recess approach, similar to that used by Dai et al.(Dai, Lehar et al. 2017) with round window injection under general inhalational endotracheal anesthesia (Fig. 1).
Fig. 1.
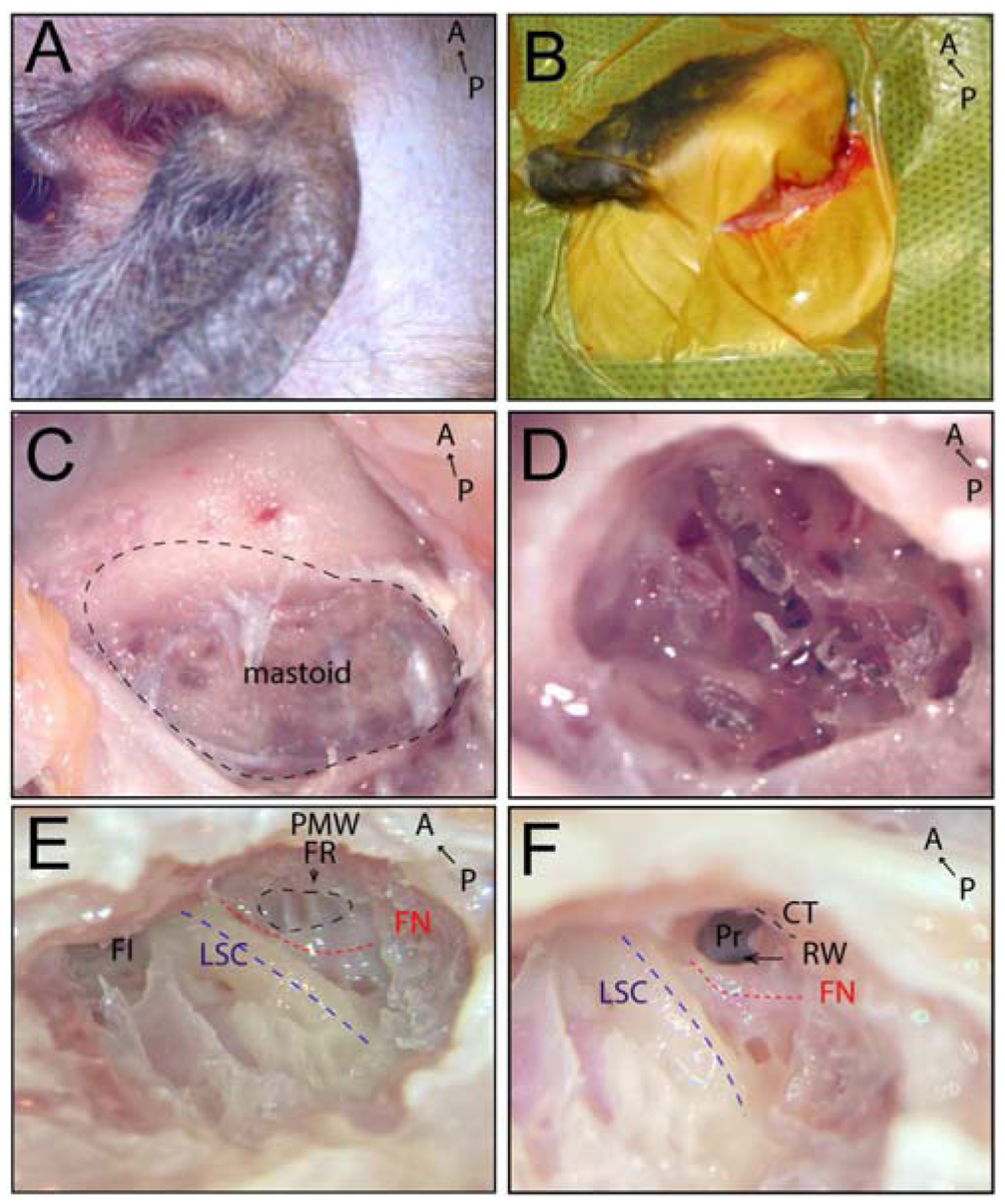
Transmastoid surgical approach to expose the round window (RW) in the right ear. A) Right mastoid region of cynomolgus monkey. B) Retroauricular incision (2 cm) made behind the pinna. C) Exposed mastoid bone (black dashed line) after removal of postauricular muscles. D) Cortical mastoidectomy. E) Approach to facial recess in the mastoid; facial nerve shown as a red dashed line, facial recess outlined with a black dashed line, lateral semicircular canal shown as a blue dashed line. F) Opening the facial recess to approach the RW; facial nerve shown as a red dashed line, lateral semicircular canal shown as a blue dashed line, arrow indicates RW. A, anterior; CT, chorda tympani (black dashed line); FI, fossa incudis; FN, facial nerve (red dashed line); PMW, posterior meatal wall; FR, facial recess (black dashed line); LSC, lateral semicircular canal (blue dashed line); P, posterior; Pr, promontory; RW, round window.
Following a retroauricular incision (Fig. 1A, B), approximately 2 cm in length, the mastoid bone was exposed (Fig. 1C). A cortical mastoidectomy was carried out with microsurgical cutting, diamond burrs and continuous suction-irrigation (Fig. 1D). Bone removal was started along an imaginary line between the digastric ridge and the horizontal semicircular canal. The mastoid antrum was opened, and the lateral semicircular canal was identified as the most important landmark in the temporal bone (Fig. 1E). It allows the dissection to proceed toward delineating the fallopian canal and the osseous labyrinth. The fossa incudis is a second reliable landmark for identifying the level of the facial recess.
When the fossa incudis was reached, a 1- to 2-mm facial recess was then identified (Fig. 1F). Understanding that the facial nerve is most vulnerable at this stage, a low drill speed and continuous fluid irrigation were used at this area to minimize thermal injury to the facial nerve. A thin layer of bone was preserved over the facial nerve. The widest portion of the facial recess rarely exceeds 3 mm. If the facial recess width was <1 mm at the plane of the round window, the chorda tympani nerve was identified and separated from the main trunk of the facial nerve with a 1-mm diameter diamond burr and then retracted and/or resected. The width of the facial recess was enlarged at the level of the round window. Then the facial recess was opened to approach the round window niche (Fig. 1F). Through the opening, the round window membrane was observed.
Under direct visualization through the operating microscope, viral vector injection was performed manually at 0.5 μL/min for 20 min (for 10 μL volume), or for 40 min (for 20 μL volume) using a Hamilton syringe connected via a thin, relatively noncompliant silicone tube to a 29-gauge, ~2-cm-long, sharp-end stainless steel injection needle.
Dose volumes of 10 or 20 μL were administered. The syringe and catheter were filled with sterile saline, and the needle backfilled with 30 μL of vector separated from saline by an air bubble in the catheter. A micromanipulator was used to position and hold the needle in place during the infusion. Once the injection completed, the surgical site was gently flushed with warm saline. The temporal muscles were approximated with interrupted tension sutures (far-near near-far sutures of 3–0 PDS) followed by continuous subcutaneous and subcuticular sutures of absorbable suture material (4–0 PDS). The skin surrounding the surgical site was cleaned with saline in order and a topical antibiotic ointment administered to the surgical site immediately after surgery. The surgical wounds were monitored daily for proper healing for two weeks.
Four weeks post-surgery, the animals were anesthetized by isoflurane anesthesia and were perfused with PBS followed by a fixation in neutral buffered 10% formalin. Temporal bones were extracted and then were retained in neutral buffered 10% formalin for at least 48 h.
2.6. Temporal bone dissection and decalcification.
We used a protocol that minimizes decalcification time and enhances the quality of the histological and immunohistochemical evaluation. Temporal bone dissections and visualization of anatomical structures were performed under a microscope. Following skin and subcutaneous tissue removal, postauricular muscles were removed from the temporal bone. Important landmarks related to the medial and lateral anatomical surfaces of temporal bone were identified (Fig. 2A, B). After determining whether the bone was left or right, it was secured in a temporal bone holder in a surgical position. The zygomatic root was anterior and mastoid tip was inferior. Cortical mastoidectomy was performed and air cells were identified (Fig. 2C, D). The mastoid antrum was opened, and the lateral semicircular canal, facial nerve, and facial recess were located (Fig. 2E, F). Next, posterior tympanotomy was performed for approaching structures within tympanic cavity. Intact tympanic membrane, annulus, facial nerve, and promontory were identified after tegmental wall was removed (Fig. 2G). Next, the lateral wall, squama, and petrous bone were removed. Middle ear structures were clearly visible (Fig. 2H, I). Cochlea and vestibular parts of inner ear were dissected from surrounding bone (Fig. 2J, K).
Fig. 2.
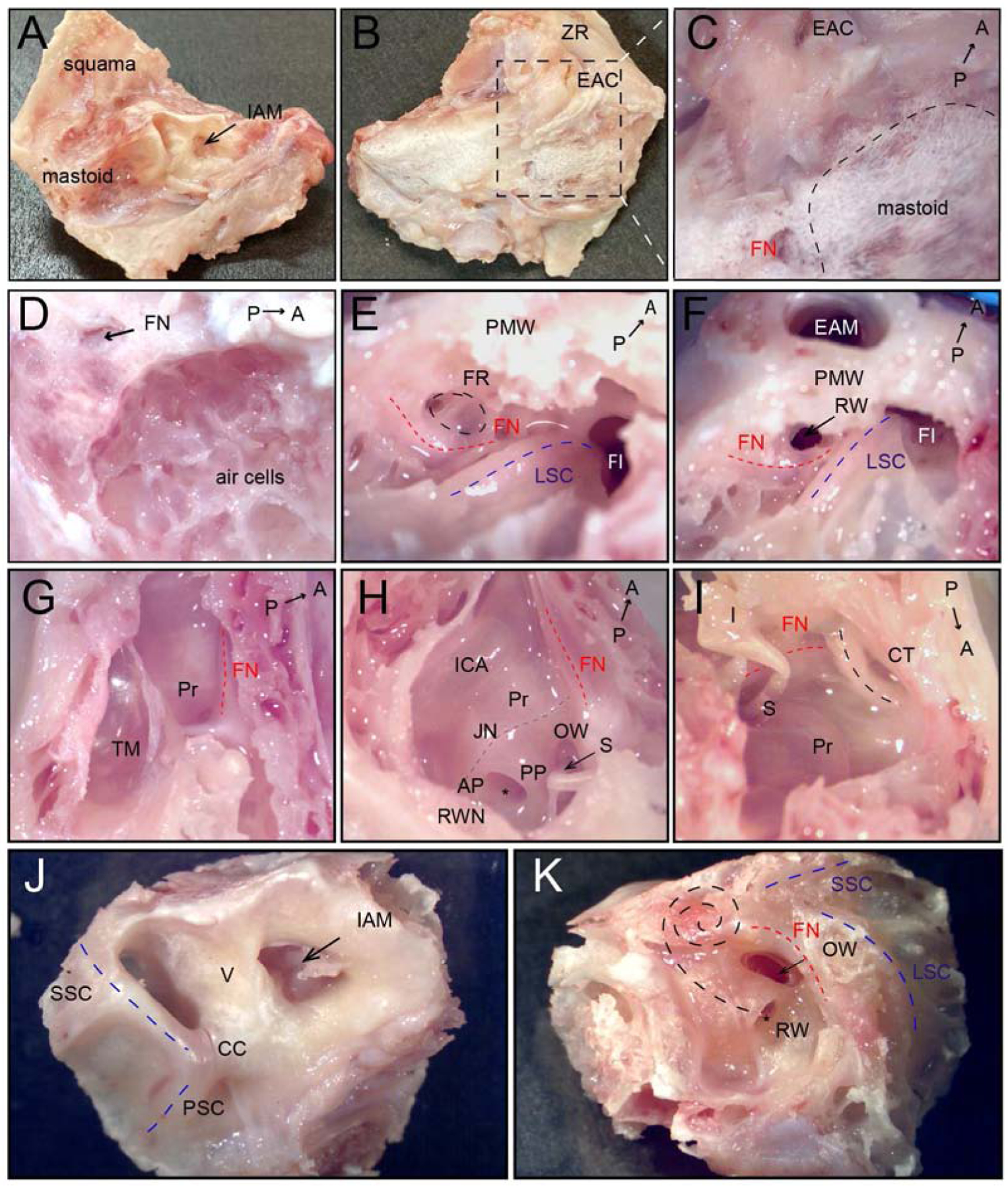
Dissection and visualization of anatomical structures of the middle and inner ear in a cynomolgus monkey left temporal bone. A) Medial surface of the temporal bone, arrow indicates internal auditory meatus. B) Lateral surface of the temporal bone after removal of postauricular muscles. C) High magnification of mastoid region (black dashed line). D) After cortical mastoidectomy; air cells are identified. E) Approach to facial recess in the mastoid; facial nerve—red dashed line, facial recess—black dashed line, lateral semicircular canal—blue dashed line. F) Facial recess opened to access the RW; arrow points to RW. G) Tegmental wall removed, view from the top. H) Lateral and tegmental walls removed, view from posterior to anterior; Jacobson nerve with inferior tympanic artery—grey dashed line. I) Lateral and tegmental walls removed, view from anterior to posterior; black dashed line—chorda tympani nerve. J) Medial surface of the cochlea and the vestibular of inner ear; blue dashed lines—posterior and superior semicircular canal, arrow indicates internal auditory meatus. K) The lateral surface of the cochlea and the vestibular of inner ear; black dashed lines indicate the projection of cochlear turns. A, anterior; AP, anterior pillar; CC, common crus, CT, Chorda tympany; EAC, external auditory canal; EAM, external auditory meatus; FI, fossa incudis; FN, facial nerve (red dashed line); FR, facial recess (black dashed line); I, incus; IAM, internal auditory meatus; ICA, internal carotid artery; JN, Jacobson nerve with inferior tympanic artery; LSC, lateral semicircular canal (blue dashed line); OW, oval window, P, posterior; PMW, posterior meatal wall, PSC, posterior semicircular canal (black dashed line); PP, posterior pillar; Pr, promontory; RW, round window; RWN, round window niche; S, stapes; SSC, superior semicircular canal (black dashed line); TM, tympanic membrane, V, vestibule; ZR, zygomatic root.
Tissues were then transferred to fresh 10% EDTA for 10–15 days until fully decalcified, embedded in paraffin blocks, serial-sectioned at 6 μm, and mounted on glass slides.
2.7. Cochlear histology and imaging
Cochlear sections were deparaffinized in xylene, 100% ethanol and 95% ethanol washes before being rehydrated in deionized water (diH2O), then were permeabilized in 0.5% Triton X-100 in PBS for 30 minutes, washed in diH2O, and incubated in 3% hydrogen peroxide for 10 minutes. Sections were blocked with 5% goat serum diluted in Tris-buffered saline-Tween 20 (TBST) for 1 hour at room temperature. The blocking solution was removed via suction and anti-GFP primary antibody (rabbit anti-GFP D5.1, Cell Signaling Technologies #2956) at a 1:1000 dilution in Signal Stain antibody diluent was applied and incubated overnight at 4 °C. Sections were washed and then incubated in SignalStain Boost Detection Reagent (anti-rabbit HRP, #8114, Cell Signaling Technologies) at room temperature for 30 minutes.
After three washes, SignalStain diaminobenzidine (DAB) substrate was added to slides for 40–45 seconds. The slides were immersed in diH2O to stop the DAB reaction and counterstained with hematoxylin for 3 minutes. The sections were flooded with diH2O and then dehydrated in 95% ethanol, 100% ethanol and xylene washes. SignalStain mounting medium was used to mount the slides.
For H&E-stained slides, hemotoxylin was applied above, followed by destaining in acid ethanol (0.5% HCl in 70% ethanol). Eosin was applied for 40 seconds, followed by rinsing in water and dehydration with ethanol and xylene. Mounting was performed as above.
The stained cochlear sections were imaged on either an Olympus VS120 Virtual Slide Microscope or a KEYENCE BZ-X800 microscope. A 2× overview of each slide was taken before 10× images of the organ of Corti regions were taken. Quantification of the efficiency of IHC and OHC transduction was carried out blinded by two investigators. Where possible, alternate sections were taken in order to ensure even analysis of hair cell transduction numbers and to avoid double counting. For each ear, 20–22 sections centered on the modiolus were counted. Higher magnification images were taken at 20× on the KEYENCE BZ-X800, and at 40× on the Olympus VS120.
2.8. Anti-AAV9 neutralizing antibody serum analysis
Blood was collected one day before surgery and immediately upon the terminal endpoint three weeks post injection. Serum was obtained and stored at −80°C until AAV neutralizing antibody analysis was performed. Heat-inactivated serum samples from all monkeys were evaluated for the presence of neutralizing antibodies to AAV9 using an in vitro NAB50 transduction assay in HEK293 cells performed by the Human Immunology Core of the University of Pennsylvania. The limit of detection of the assay was 1/5 serum dilution.
2.9. Statistics
To compare sample groups, we used one-way ANOVA with multiple-comparison correction. For normality testing, we used the Shapiro-Wilk test. For statistical testing, we used GraphPad Prism software. p values < 0.05 were considered statistically significant.
3. Results
Before testing any individual preparation of drug/biologic, including AAV, in NHPs it is important to first test in mice to (1) confirm the potency of the preparation and (2) ensure the absence of contaminants. P1 C57BL/6 mice were injected via the RWM with 5×1010 VG of ssAAV9-PHP.B-CBA-GFP. They were euthanized 5 days later at P6 and their cochleas were dissected, mounted and stained for GFP expression analysis. We observed robust GFP expression in both IHCs and OHCs, lateral wall and spiral limbus in the apical, middle and basal turns of the cochlea (Fig. 3). These results were similar to previous work by our group (Gyorgy, Meijer et al. 2019), and therefore, we used this preparation of AAV9-PHP.B-CBA-GFP for the NHP study.
Fig. 3.
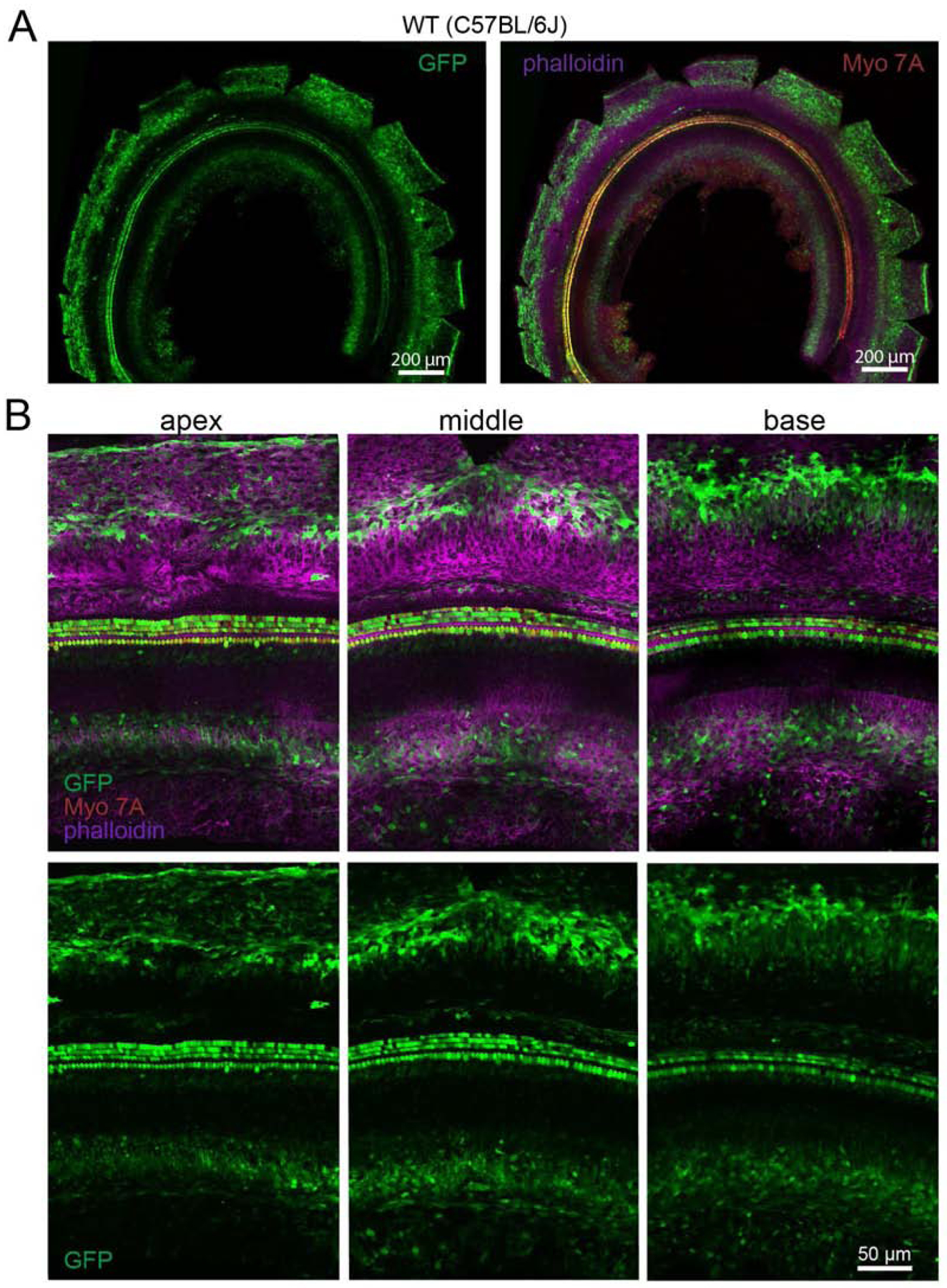
Transduction efficiency in C57BL/6J mice of ssAAV9-PHP.B-CBA-GFP after neonatal RWM injection. A) Low-magnification images of the cochlea showing transduction level. Animals were injected at P1 with 5×1010 VG and the cochlea was dissected and mounted at P6. The left panel shows GFP expression only (green); the right panel shows GFP expression (green), an anti-MYO7A antibody (red) and phalloidin-stained actin (magenta). B) High-magnification images of apical, middle and basal regions of the cochlea. Upper panel shows GFP expression (green), as well as MYO7A (red) and phalloidin (magenta); lower panel shows GFP expression only (green).
To further characterize the efficacy of AAV9-PHP.B in primates, we injected ssAAV9-PHP.BCBA-GFP through the RWM of cynomolgus monkeys using a transmastoid surgical approach (see Methods), and assessed GFP expression. Three adult/juvenile female animals were injected with vector and one animal received vehicle (PBS). Four doses were tested (Table 1).Three female macaques received RWM injection of AAV9-PHP.B-GFP with doses of 1×1011 VG (n=1 ear), 2×1011 VG (n=1), 3.5×1011 VG (n=2), and 7×1011 VG (n=2). Three weeks later, the animals were euthanized, and the cochleas from temporal bones were extracted, decalcified over 10–15 days, paraffin-embedded, sectioned for histological analysis and stained with an anti-GFP antibody (Fig. 5,6) or H&E (Fig. 4).
Fig. 5.
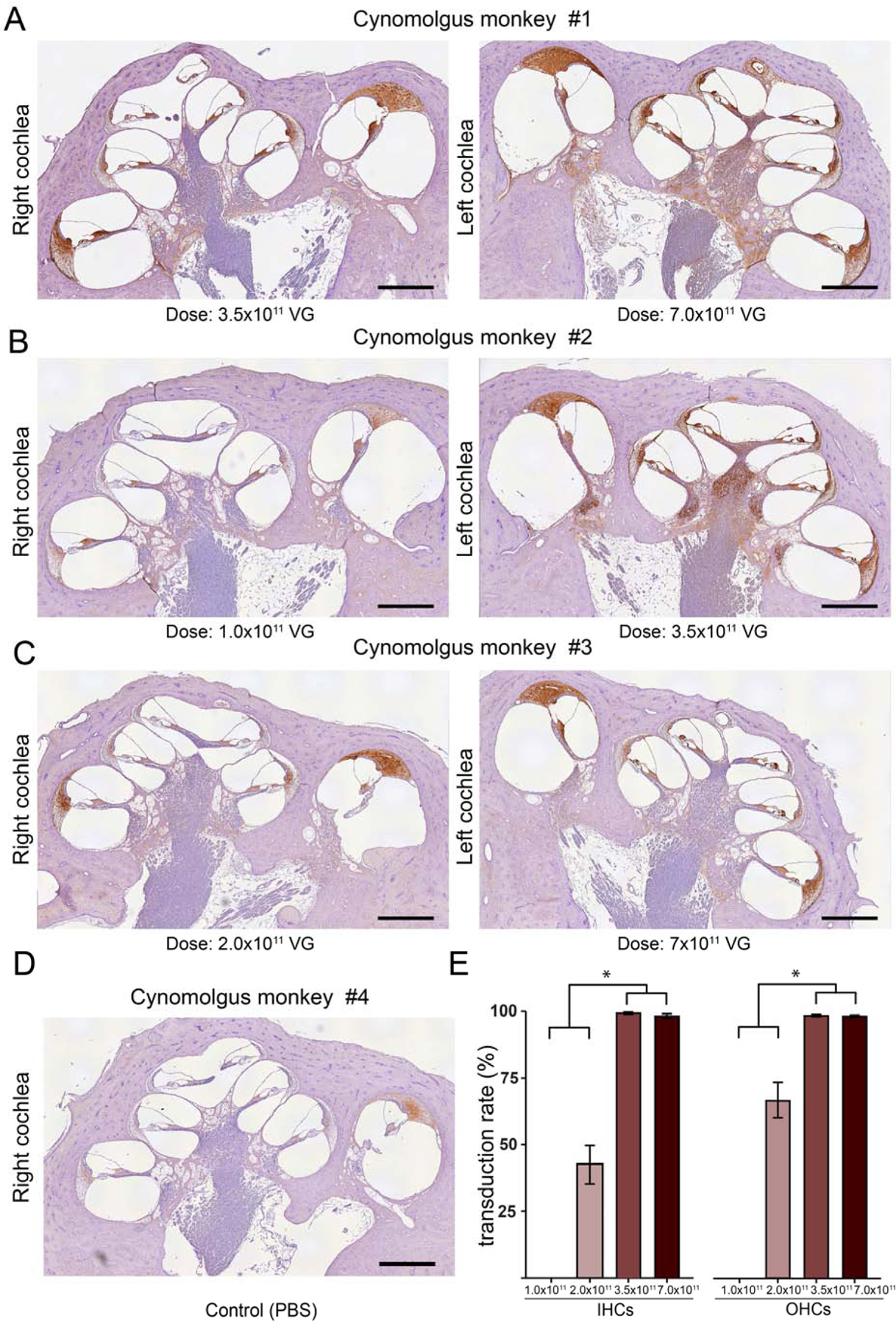
Transduction profile with different doses of AAV9.PHP.B-CBA-GFP in cynomolgus monkeys. A-D) Low-magnification images of the injected cochleas. Anti-GFP staining was detected with a horseradish-peroxidase-conjugated secondary antibody and developed using diaminobenzidine (brown areas). Slides were counterstained with hematoxylin. A) Cynomolgus monkey #1. Right ear was injected with 3.5 × 1011 VG (10 μl); left ear was injected with 7.0 × 1011 VG (20 μl); B) Cynomolgus monkey #2. Right ear was injected with 1.0 × 1011 VG (10 μl); left ear was injected with 3.5 × 1011 VG (10 μl); C) Cynomolgus monkey #3. Right ear was injected with 2.0 × 1011 VG (10 μl); left ear was injected with 7.0 × 1011 VG (20 μl); D) Cynomolgus monkey #4. Right ear was injected with PBS (10 μl). E) Quantification of transduction efficiency in IHCs and OHCs. Data shown as mean ± s.e.m., * p<0.05 by one-way ANOVA with multiple comparisons correction. Scale bar 1.0 mm.
Fig. 6.
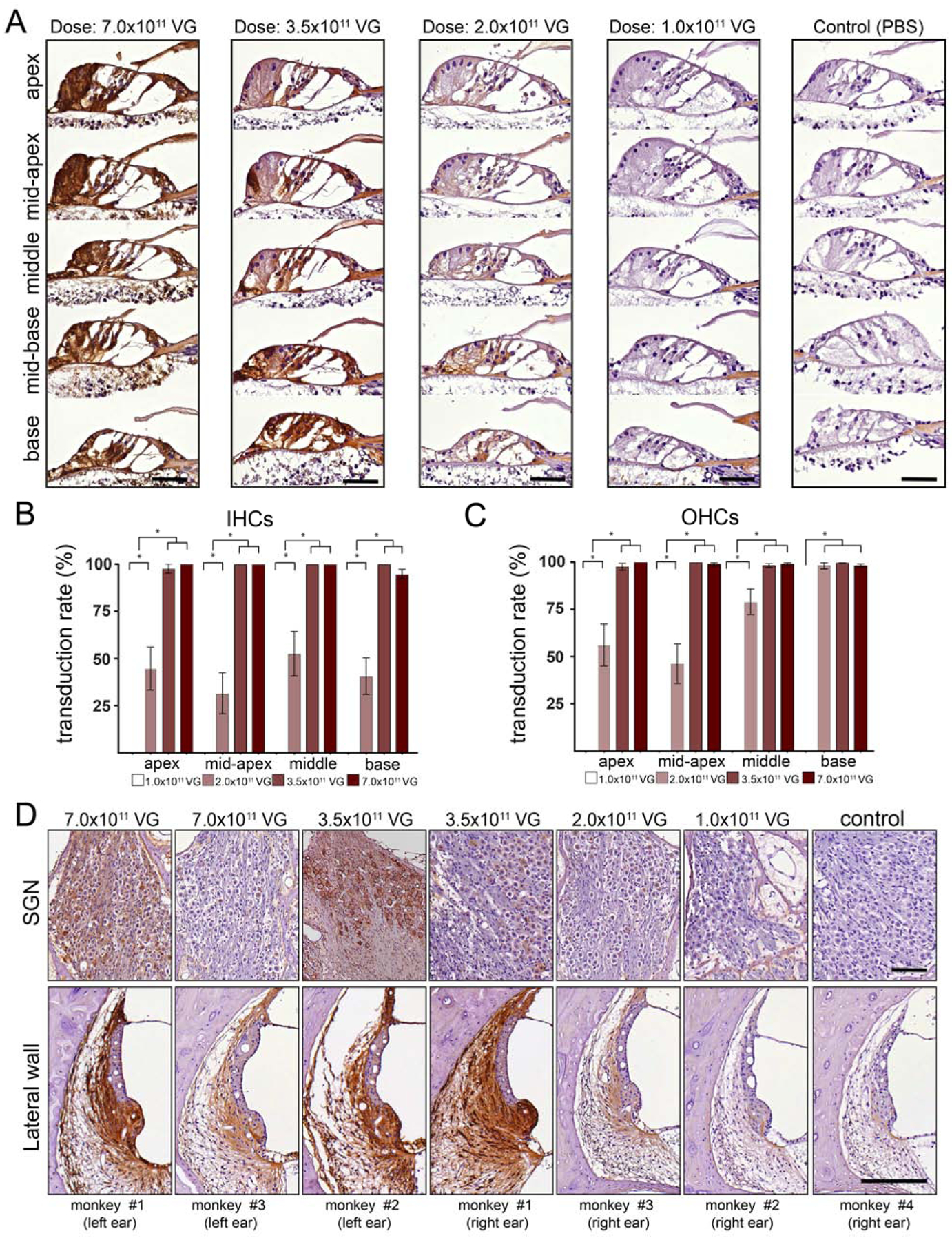
Transduction profile with different doses of AAV9.PHP.B-CBA-GFP in organ of Corti, spiral ganglion region and lateral wall. A) Higher magnification images of the organ of Corti from base to apex. Robust anti-GFP immunoreactivity was observed at the higher doses (3.5×1011 and 7.0×1011 VG) (first and second panel). Moderate GFP immunoreactivity was observed at 2.0 ×1011 VG (third panel). Lack of anti-GFP immunoreactivity was observed at 1.0 ×1011 VG (fourth panel). Ear injected with PBS (control) lacks GFP-specific immunoreactivity (fifth panel). Scale bar 50 μm. B,C) Regional quantitation of transduction efficiency in IHCs (B) and OHCs (C). D) anti-GFP immunoreactivity of the SGNS and lateral wall at high magnification. Data shown as mean ± s.e.m., * p<0.05 by one-way ANOVA with multiple comparisons correction. Scale bar 150 μm.
Fig 4.
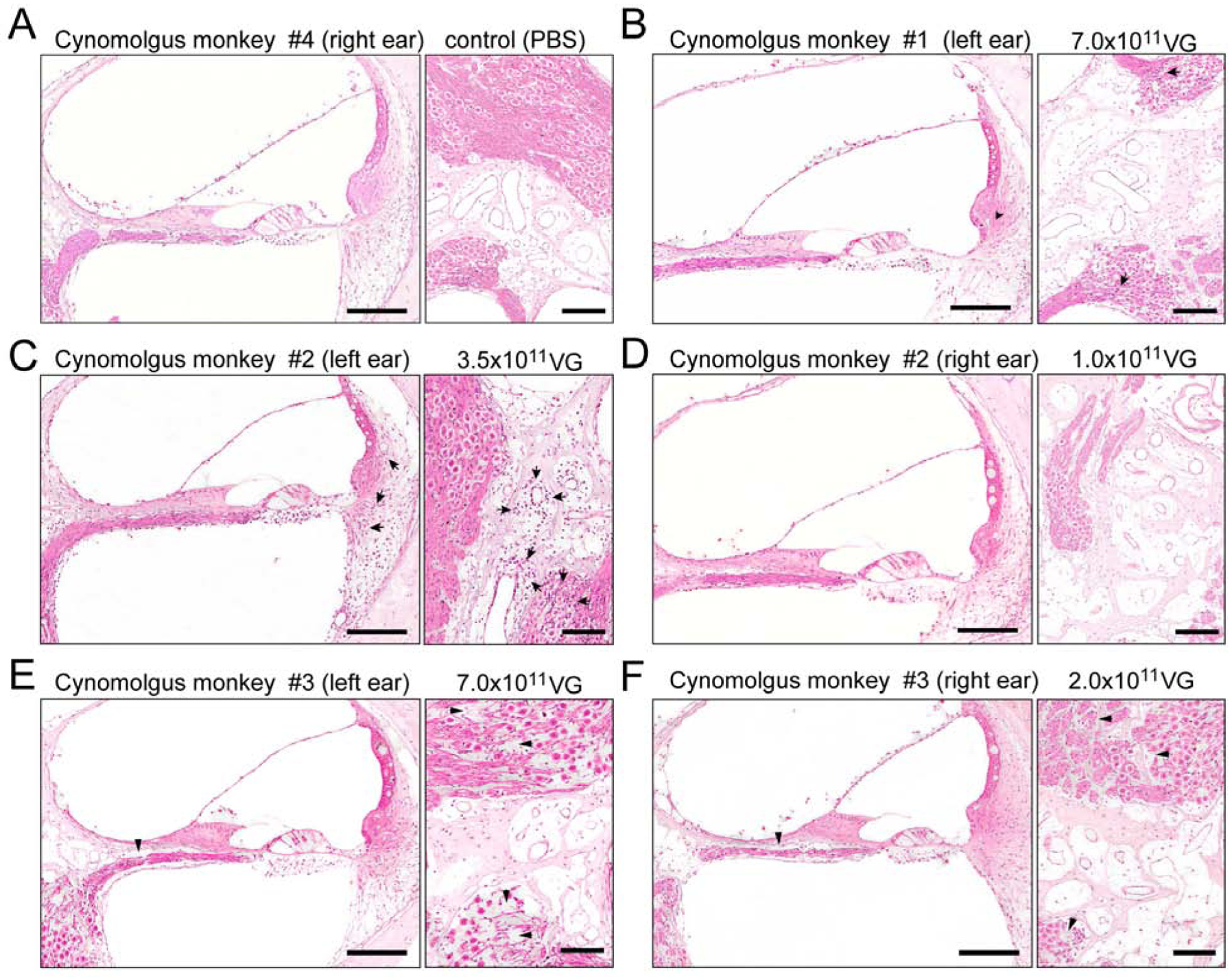
Hematoxylin and eosin staining of the injected ears of cynomolgus monkeys administered with 1×1011 VG, 2×1011 VG, 3.5×1011 VG, and 7.0×1011 VG of AAV9-PHP.B-CBA-GFP, respectively. A) Cynomolgus monkey #4. The right ear, injected with PBS (10 μl), shows no indication of immune infiltration in the organ of Corti, lateral wall (left panel) or spiral ganglion region (right panel). B) Cynomolgus monkey #1. The left ear (7.0 × 1011 VG; 20 μl) shows minimal focal perivascular mononuclear cell infiltration (arrows) in the spiral ligament (left panel) and in the spiral ganglion region (right panel). C) Cynomolgus monkey #2. The left ear (3.5 × 1011 VG; 10 μl) shows perivascular mild multifocal mononuclear cell infiltrates (mostly lymphocytes) in spiral ligament (left panel) and in the spiral ganglion region, mostly perivascular (arrows). D) Cynomolgus monkey #2. The right ear (1.0 × 1011 VG; 10 μl) shows no indication of immune infiltration in the organ of Corti, lateral wall (left panel) or spiral ganglion region (right panel). E) Cynomolgus monkey #3. The left ear (7.0 × 1011 VG; 20 μl) shows loss of nerve fibers and spiral ganglion neurons in the basal turn of the cochlea (right panel, arrowheads) and no indication of immune response in the organ of Corti or lateral wall (left panel). F) Cynomolgus monkey #3. The right ear (2.0 × 1011 VG; 10 μl), shows loss of nerve fibers and spiral ganglion neurons in the basal turn of the cochlea (right panel, arrowheads) and no indication of immune response in the organ of Corti or lateral wall (left panel). Scale bar 150 μm.
From each ear, 2–3 hematoxylin and eosin (H&E)-stained sections were analyzed by a veterinary pathologist (Fig. 4). Small changes associated with surgery and vector injection were observed in all cochleas. There was no indication of immune infiltration in the control ears that received PBS (Fig. 4A) and or in ears treated with 1×1011 (10 μL) and 2×1011 VG (10 μL) of AAV9-PHP.B-CBA-GFP (Fig. 4D, F). The left ear of cynomolgus monkey #2, which received 3.5×1011 VG (10 μL) of AAV9-PHP.B-CBA-GFP, showed mild multifocal mononuclear (mostly lymphocytic) cell infiltration, concentrated perivascular and mainly seen in the spiral ligament, in the spiral ganglia region (Fig. 4C) and at the periphery of the cochlear nerve. The left ear of cynomolgus monkey #1, which received 7.0 × 1011 VG (20 μL), showed minimal focal perivascular mononuclear cell infiltration in the spiral ligament and in the spiral ganglion region (Fig. 4B). The HCs, stria vascularis, and spiral ganglion neurons had normal morphology. The left ear of cynomolgus monkey #3, which received 7.0 × 1011 VG (10 μL), showed no indication of immune infiltration (Fig. 4E). The same was found for the right ear, which received 2×1011 VG (10 μl) of AAV9-PHP.B-CBA-GFP (Fig. 4F). Both ears of cynomolgus monkey #3 showed very focal atrophy of the organ of Corti with hair cell loss, loss of nerve fibers and spiral ganglion neurons at the basal turn of the cochlea. In some animals we observed moderate distension of Reissner’s membrane into scala vestibule, a possible sign of endolymphatic hydrops (Fig. 4 B,C,E). This was apparent in monkey#1 (cochleas injected with 3.5×1011 and 7.0×1011 VG), monkey #2 (left cochlea; 3.5×1011 VG) and monkey #3 (left cochlea; 7.0×1011 VG).
We used an anti-GFP antibody to assess GFP expression. We observed robust GFP immunoreactivity throughout the cochleas administrated with high doses of 7.0×1011 VG (Fig. 5A, monkey #1 left cochlea; Fig. 5C, monkey #3 left cochlea) and 3.5×1011 VG (Fig. 5A, monkey #1 right cochlea; Fig. 5B, monkey #2 left cochlea). GFP staining was also present in the supporting cells of the organ of Corti, the spiral ligament, the spiral limbus, spiral ganglion neurons, and Reissner’s membrane.
However, at lower doses, there was a steep reduction in vector transduction. We observed moderate anti-GFP staining in the cochlea injected with 2.0×1011 VG (Fig. 5C, monkey #3 right cochlea) and very limited transduction in a cochlea that received 1×1011 VG (Fig. 5B, monkey#2 right cochlea). Only low levels of nonspecific staining were observed in the lateral wall in the ear administrated with PBS (Fig. 5D). Importantly, there was no background staining in the HCs in the vehicle-administered animal, making interpretation of transgene expression in these cells unambiguous.
Quantification of vector transduction efficiency in IHCs and OHCs (Fig. 5E) showed that at the higher doses (3.5×1011 and 7×1011 VG), AAV9-PHP.B transduced nearly 100% of both IHCs and OHCs. In a cochlea administrated with 2×1011 VG we observed ~50% of IHCs and ~65% of OHCs transduced, and no transduction of IHCs and OHCs in a cochlea injected with 1×1011 VG. The steep dose dependency probably explains the limited transduction we previously reported with a lower dose of ssAAV9-PHP.B (1×1011 VG; (Gyorgy, Meijer et al. 2019)).
Next, we analyzed higher magnification images of the organ of Corti from base to apex (Fig. 6A), which confirmed robust GFP immunoreactivity in IHCs and OHCs, border cells, inner and outer phalangeal cells, pillar cells, Hensen’s cells and Claudius’ cells, with no gradient from base to apex at the higher doses (3.5×1011 and 7.0×1011 VG) (Fig. 6A, first and second panel). Anti-GFP staining decreased in the cochlea administered with 2.0 ×1011 VG (Fig. 6A, third panel) and was not detected in the cochlea administered with 1.0 ×1011 VG (Fig. 6A, fourth panel) or with PBS (Fig. 6A, fifth panel).
Quantification of transduction efficiency in IHCs (Fig. 6B) and OHCs (Fig. 6C) in apical, mid-apical, middle and basal turns of cochlea revealed no gradient in the transduction efficacy in hair cells in all analyzed regions in cochleas injected with 7.0×1011 or 3.5×1011, and showed a base (high transduction) to apex (low transduction) gradient in OHCs in cochlea injected with 2.0 ×1011 VG of ssAAV9-PHP.B-CBA-GFP.
To assess GFP expression in spiral ganglion neurons (SGNs) and the lateral wall, we analyzed high magnification images from cochleas that received different doses of AAV9-PHP.B-CBA-GFP. Interestingly, monkey #1 (7.0×1011 VG in the right ear) showed robust anti-GFP immunoreactivity in SGNs, while monkey #3 (same dose) showed much lower anti-GFP staining in SGNs. We observed the same variability of GFP expression in monkey #2 and monkey #1 injected with 3.5×1011 VG.
In the lateral wall, widespread and consistent transduction was observed in cochleas that received higher doses of ssAAV9-PHP.B-CBA-GFP. As mentioned above, there was low background staining in the lateral wall in the monkey injected with PBS and the staining intensity was similar to this in the animal injected with 1×1011 VG.
The role of pre-existing neutralizing antibodies (NAbs) in affecting transduction efficiency of AAV in the inner ear is unclear in NHPs. We observed that all animals had detectable, but relatively low levels of plasma NAbs ranging from 1:20 to 1:40 (Table 2). Since we observed high transduction efficiency at all doses above 1×1011 VG, it did not appear that these levels of antibodies blocked transduction, although it is unclear what levels are present in the perilymph, which are likely much lower than those in plasma. We also evaluated the level of antibodies induced by intra-cochlear injection of AAV9-PHP.B, by measuring anti-AAV9 antibodies from blood drawn before euthanasia (3 weeks post injection). Anti-AAV9 antibody titers increased in all NHPs following AAV9-PHP.B-CBAGFP injection regardless of virus dosage.
Table 2.
Pre- and post-injection levels of neutralizing antibodies (NAb50) in treated NHPs. Number sindicate serum dilution at which relative luminescence units (RLUs) from an AAV9 vector encoding luciferase were reduced 50% compared to control wells (AAV9 incubated without serum) in HEK293 cells.
| Cynomolgus monkey | Pre-injection NAb50 | Three weeks post-injection NAb50 |
|---|---|---|
| #1 | 1:40 | 1:320 |
| #2 | 1:20 | 1:640 |
| #3 | 1:20 | 1:80 |
The strongest immune response, around 32-fold increase of anti-AAV9 antibody titer post-injection, was observed in cynomolgus monkey #2 which received 3.5×1011 VG (10 μl) of AAV9-PHP.B-CBA-GFP to the right ear and 1.0×1011 VG (10 μl) to the left ear. Cynomolgus monkey #1, administrated with 3.5×1011 VG (10 μl) of AAV9-PHP.B-CBA-GFP to the right ear and 7.0×1011 VG (20 μl) to the left ear, showed an 8-fold increase post-injection; cynomolgus monkey #3, injected with 2.0×1011 VG (10 μl) of AAV9-PHP.B-CBA-GFP to the right ear and 7.0×1011 VG (20 μl) to the left ear, showed only a 4-fold increase.
4. Discussion
We found that AAV9-PHP.B is an effective vector for targeting hair cells of the cochlea in NHPs at a range of doses from 2–7×1011 VG. PHP.B can effectively mediate near total transduction of hair cells at doses at and above 3.5×1011 VG. This confirms the result from our previous work (Gyorgy, Meijer et al. 2019), in which PHP.B showed a lack of hair cell transduction at 1×1011 VG and very high efficiency of transduction at 3×1011 VG, and expands upon that study with a wider variety of doses. This reveals a steep gradient in efficiency with increasing doses.
Development of gene therapies for inherited hearing loss has been slowed by a lack of highly effective vectors for targeting hair cells, in particular OHCs. While several new vectors have now been investigated to overcome this barrier, such as exo-AAV (Gyorgy, Sage et al. 2017), Anc80 (Landegger, Pan et al. 2017), PHP.B (Gyorgy, Meijer et al. 2019, Kim, Ryu et al. 2019, Lee, Nist-Lund et al. 2020), and more recently AAV-ie (which contains the PHP.B 7-mer peptide; (Tan, Chu et al. 2019), comprehensive examination of these vectors’ effectiveness in NHPs had not yet been demonstrated. Although this study represents only a limited sample (six cochleas in three monkeys), it is a vital step in defining the transduction capacity of PHP.B and its usefulness in gene therapy for hearing loss.
The presence of preexisting circulating NAbs against the AAV serotype is one of the exclusion criterion in clincal trials of systemically delivered vector, because even low titers (<1:10) of NAbs can abrogate gene transfer by intravenously administered AAV (Scallan, Jiang et al. 2006, Wang, Calcedo et al. 2011, Calcedo, Franco et al. 2015). In other organs and fluids, such as cerebral spinal fluid (CSF), the concentration of antibodies is much lower than in the plasma. Antibody levels in brain have been shown to be 0.6% of that in plasma (Treleaven, Tamsett et al. 2012). In fact Gray et al. demonstrated efficient transduction of spinal cord motor neurons after intrathecal delivery of AAV9 encoding GFP even at a plasma anti-AAV NAb titer of 1:128 (Gray, Nagabhushan Kalburgi et al. 2013). Thus compartmentalized organs and tissues, such as the inner ear, may be less prone to neutralization by low-to-moderate serum antibody titers. The NHPs in our study had low levels of circulating anti-AAV9 NAbs (1:20–1:40) and this did not appear to abrogate gene transfer, as we observed efficient transduction at all doses above 1×1011 VG. It will be important to continue to correlate antibody titers with gene transfer with AAV9-PHP.B in future studies, and it would also be useful to measure and compare antibody titers in plasma with those in the perilymph, the inner ear fluid receiving vector injected through the RWM. We also measured NAb levels three weeks after vector injection. Titers increased by 8 to 32-fold following injection with AAV9-PHP.B vector. The inter-animal differences may be due to several factors, but likely depend on how much vector leaked into the systemic circulation for B cell activation.
We also examined cochlear tissue from vector- and PBS-injected animals for pathology associated with the surgery and vector. In general the inner ear architecture and cellular anatomy were maintained in vector- and PBS-treated animals, although in cochleas injected with higher doses of AAV.9 PHP.B we observed minimal or mild endolymphatic hydrops (Salt and Plontke 2010) which might result from transgene expression toxicity, post-operative trauma following RWM injection, or inflammation in the inner ear (Ferster, Cureoglu et al. 2017). In one vector-injected monkey there was some loss of nerve fibers and spiral ganglion neurons and focal atrophy of the organ of Corti with hair cell loss in the cochlear base in both cochleas, indicating chronic degeneration events in the inner ear, perhaps not related to the surgery.
In two of six vector-injected ears (one at 3.5×1011, one at 7×1011 VG) we observed mild-to-minimimal multifocal mononuclear cell infiltration in the spiral ganglia region and at the periphery of the cochlear nerve. This did not prevent transgene expression in the time frame examined, although it could be an immune reaction to the robust GFP expression in the spiral ganglia region in those cochleas.
We intentionally kept the post-injection period relatively short (3 weeks) to minimize the chance of immunological responses to GFP, which is known to be immunogenic especially in NHPs (Ciesielska, Hadaczek et al. 2013, Ramsingh, Gray et al. 2018). Future comparison of AAV9-PHP.B capsid encoding GFP with one devoid of transgene expression will determine whether the infiltrate is coming from capsid or transgene product (or both). Additionally, we envisage that future studies will measure the toxicity of clinically-relevant transgenes injected into NHP ears, ideally using techniques such as auditory brainstem response (ABR) before and after surgery. As the anaesthetic required for performing ABR carries an additional risk of mortality, we did not perform it for this study. Most important will be long term expression studies using PHP.B with clinically-relevant transgenes.
5. Conclusions
AAV9-PHP.B efficiently transduces the IHCs and OHCs of nonhuman primates, but shows a striking dose dependency. Together, these data support a feasible path towards clinical development of gene therapy for hereditary hearing loss with AAV9-PHP.B capsid.
Highlights.
Research Article: Preclinical testing of AAV9-PHP.B for transgene delivery to the non-human primate cochlea
AAV9-PHP.B capsid mediates efficient transgene expression in the non-human primate inner ear
Transgene expression in inner and outer hair cells is nearly 100% at doses above 2e11 vector genomes/ear.
AAV9-PHP.B capsid should be considered as a clinical candidate delivery vehicle for hereditary deafnesses
Acknowledgments
We appreciate advice from Vladimir K. Berezovskii (Harvard Medical School; supported by NIH grant P30-EY12196) and Ronna Hertzano (University of Maryland School of Medicine). We appreciate the use of microscopes in the Harvard Medical School Neurobiology Imaging Facility (supported by NIH P30-NS072030) and of the Nikon Ti2 inverted spinning disk confocal at the Harvard Medical School MicRoN Microscopy Core. We thank Cole W. Peters (Harvard Medical School) for providing materials. We thank Daniel J. Lee, David H. Jung and Elliot D. Kozin (Massachusetts Eye and Ear) for the opportunity to participate in a temporal bone dissection course. We thank Dr. Jessica Chichester of the University of Pennsylvania Human Immunology Core for performing the AAV9 neutralization assays. This work was supported by NIH grants DC002281 and DC016932 (to D.P.C.), and DC017117 (to C.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
C.A.M. has a financial interest in Chameleon Biosciences, Inc., a company developing an enveloped AAV vector platform technology for repeated dosing of systemic gene therapy. C.A.M. interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. C.A.M. and D.P.C. have filed a patent application related to the use of AAV9-PHP.B for inner ear gene delivery.
References
- Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH and Lustig LR (2012). “Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy.” Neuron 75(2): 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew C, Rochat C, Pan B, Asai Y, Ahmed H, Child E, Schneider BL, Aebischer P and Holt JR (2015). “Tmc gene therapy restores auditory function in deaf mice.” Sci Transl Med 7(295): 295ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Franco J, Qin Q, Richardson DW, Mason JB, Boyd S and Wilson JM (2015). “Preexisting Neutralizing Antibodies to Adeno-Associated Virus Capsids in Large Animals Other Than Monkeys May Confound In Vivo Gene Therapy Studies.” Hum Gene Ther Methods 26(3): 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WW, Isgrig K, Roy S, Belyantseva IA, Drummond MC, May LA, Fitzgerald TS, Friedman TB and Cunningham LL (2016). “Gene Therapy Restores Hair Cell Stereocilia Morphology in Inner Ears of Deaf Whirler Mice.” Mol Ther 24(1): 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS and Forsayeth J (2013). “Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses.” Mol Ther 21(1): 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Lehar M, Sun DQ, Rvt LS, Carey JP, MacLachlan T, Brough D, Staecker H, Della Santina AM, Hullar TE and Della Santina CC (2017). “Rhesus Cochlear and Vestibular Functions Are Preserved After Inner Ear Injection of Saline Volume Sufficient for Gene Therapy Delivery.” J Assoc Res Otolaryngol 18(4): 601–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP and Gradinaru V (2016). “Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain.” Nat Biotechnol 34(2): 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster APO, Cureoglu S, Keskin N, Paparella MM and Isildak H (2017). “Secondary Endolymphatic Hydrops.” Otol Neurotol 38(5): 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ and Jude Samulski R (2013). “Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates.” Gene Ther 20(4): 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B, Meijer EJ, Ivanchenko MV, Tenneson K, Emond F, Hanlon KS, Indzhykulian AA, Volak A, Karavitaki KD, Tamvakologos PI, Vezina M, Berezovskii VK, Born RT, O’Brien M, Lafond JF, Arsenijevic Y, Kenna MA, Maguire CA and Corey DP (2019). “Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate.” Mol Ther Methods Clin Dev 13: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B, Sage C, Indzhykulian AA, Scheffer DI, Brisson AR, Tan S, Wu X, Volak A, Mu D, Tamvakologos PI, Li Y, Fitzpatrick Z, Ericsson M, Breakefield XO, Corey DP and Maguire CA (2017). “Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV.” Mol Ther 25(2): 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux J, Wang Q, Katz N, Buza EL, Bell P and Wilson JM (2018). “The Neurotropic Properties of AAVPHP.B Are Limited to C57BL/6J Mice.” Mol Ther 26(3): 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Ryu N, Kim HM, Kim YR, Lee B, Kwon TJ, Bok J and Kim UK (2019). “Targeted Gene Delivery into the Mammalian Inner Ear Using Synthetic Serotypes of Adeno-Associated Virus Vectors.” Mol Ther Methods Clin Dev 13: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegger LD, Pan B, Askew C, Wassmer SJ, Gluck SD, Galvin A, Taylor R, Forge A, Stankovic KM, Holt JR and Vandenberghe LH (2017). “A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear.” Nat Biotechnol 35(3): 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Nist-Lund C, Solanes P, Goldberg H, Wu J, Pan B, Schneider BL and Holt JR (2020). “Efficient viral transduction in mouse inner ear hair cells with utricle injection and AAV9-PHP.B.” Hear Res: 107882. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y, Konno A, Mochizuki R, Shinohara Y, Nitta K, Okada Y and Hirai H (2018). “Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain.” Neurosci Lett 665: 182–188. [DOI] [PubMed] [Google Scholar]
- Ramsingh AI, Gray SJ, Reilly A, Koday M, Bratt D, Koday MT, Munson PM, Murnane R, Smedley J, Hu Y, Messer A and Fuller DH (2018). “Sustained AAV9-mediated expression of a non-self protein in the CNS of non-human primates after immunomodulation.” PLoS One 13(6): e0198154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN and Plontke SK (2010). “Endolymphatic hydrops: pathophysiology and experimental models.” Otolaryngol Clin North Am 43(5): 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, Couto LB and Pierce GF (2006). “Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice.” Blood 107(5): 1810–1817. [DOI] [PubMed] [Google Scholar]
- Tan F, Chu C, Qi J, Li W, You D, Li K, Chen X, Zhao W, Cheng C, Liu X, Qiao Y, Su B, He S, Zhong C, Li H, Chai R and Zhong G (2019). “AAV-ie enables safe and efficient gene transfer to inner ear cells.” Nat Commun 10(1): 3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treleaven CM, Tamsett TJ, Bu J, Fidler JA, Sardi SP, Hurlbut GD, Woodworth LA, Cheng SH, Passini MA, Shihabuddin LS and Dodge JC (2012). “Gene transfer to the CNS is efficacious in immune-primed mice harboring physiologically relevant titers of anti-AAV antibodies.” Mol Ther 20(9): 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Bell P, Lin J, Grant RL, Siegel DL and Wilson JM (2011). “Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors.” Hum Gene Ther 22(11): 1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]


