Abstract
Lacosamide, an anti-epileptic drug prescribed for children with refractory focal epilepsy, is generally well tolerated, with dose-dependent adverse effects. We describe 4 children who developed a movement disorder in conjunction with the initiation and/or uptitration of lacosamide. Three patients developed dyskinesias involving the face or upper extremity while the fourth had substantial worsening of facial tics. The patients all had histories suggestive of opercular dysfunction: three had seizure semiologies including hypersalivation, facial and upper extremity clonus while the fourth underwent resection of polymicrogyria involving the opercula. Onset, severity, and resolution of dyskinesias correlated with lacosamide dosing. These cases suggest that pediatric patients with dysfunction of the opercular cortex are at increased risk for developing drug-induced dyskinesias on high-dose lacosamide therapy. Practitioners should be aware of this potential side effect and consider weaning lacosamide or video EEG for differential diagnosis, particularly in pediatric patients with underlying opercular dysfunction.
Keywords: Lacosamide, dyskinesia, intractable epilepsy, focal epilepsy, operculum
1.1. Introduction
Lacosamide, an anti-epileptic drug (AED) prescribed for refractory focal epilepsy, selectively enhances slow inactivation of voltage gated sodium channels, thus decreasing the proportion of sodium channels available for depolarization.1 Lacosamide is generally well tolerated, with dose-dependent adverse effects including dizziness, headaches and nausea.2 New oral motor tics in a pediatric patient on lacosamide have been reported,3 but lacosamide-related movement disorders have not been well-described. We report four children with focal epilepsy who developed facial or upper limb dyskinesias while taking lacosamide and discuss possible underlying pathophysiological mechanisms.
Methods
The four children were active patients at Lucile Packard Children’s Hospital between March 2016 and March 2018 when this series was written. The families were approached by their treating physicians and each provided verbal consent to be interviewed by the authors regarding the movement disorder. Case reports are IRB exempt. Each family provided the authors with home videos of the movements of concern. These histories and videos were reviewed by all authors, including a pediatric movement disorder specialist (JAO).
2.1. Patient 1
A 10-year-old right-handed boy with attention deficit hyperactivity disorder (ADHD), autism, and idiopathic hyperinsulinemia presented for epilepsy management. His seizures began at 3 months of age with facial clonus, head turn to either side, whole body myoclonus, and altered consciousness, frequently followed by right-sided weakness. Neurologic exam showed dystonic posturing of the right arm and right upgoing plantar response. Initially all seizures occurred in the setting of hypoglycemia but after 2 years, glucose control stabilized with gastric dextrose infusions, yet seizures continued. Magnetic resonance imaging (MRI) was unremarkable and electroencephalogram (EEG) showed bilateral independent centrotemporal spikes waves. Genetic testing showed a heterozygous, maternally-inherited, likely-benign variant of unknown significance in GRIN2A (c.3228 C>A; p.Asn1076Lys).
Seizures were refractory to levetiracetam, carbamazepine and oxcarbazepine and valproic acid was discontinued due to hyperammonemia. At 8 years old, he became seizure free with lacosamide (11.2mg/kg/day), but then developed weekly episodes of right-sided facial contractions associated with intermittent, non-rhythmic, highly variable amplitude tongue protrusion and mild jaw opening with some lip pursing in addition to “gargling” sounds and slow, non-rhythmic, upper trunk extension of varying intensity, lasting 5–20 minutes. Movements occurred exclusively in wakefulness and waxed and waned in severity. Though unable to speak during episodes, he could follow commands and immediately returned to neurologic baseline. Given concern for seizures, lacosamide was uptitrated (14.3mg/kg/day), but episodes increased to daily. Video EEG demonstrated no epileptiform correlate, and upon review by a pediatric movement disorder specialist, episodes were recognized as most consistent with complex drug-induced dyskinesias. After lacosamide wean (to 8.7mg/kg/day), dyskinesias decreased in frequency and resolved by 9 months.
2.2. Patient 2
A 7-year-old, left-handed girl with perinatal brain injury and subsequent microcephaly, mild intellectual disability, spastic diplegia, and cortical visual impairment, as well as sensorineural hearing loss secondary to Connexin gene 26 [GJB2] mutation, presented with refractory focal seizures. She had one prolonged febrile seizure at 10 months and was subsequently seizure free until age 7, when she developed hour-long, complex focal seizures characterized by leftward eye and head deviation, clonic jerking of the left arm, hypersalivation, and choking. EEG showed bifrontal slowing with intermixed, irregular bifrontal sharp waves as well occipital spike-waves evoked by eye closure. Prior imaging found periventricular and bilateral parietooccipital T2 hyperintensities.
The patient failed levetiracetam monotherapy (inefficacy) and oxcarbazepine (rash), so started lacosamide (4.8mg/kg/day) at age 13 years. After one week, she developed daily, 5-minute episodes of spontaneous, involuntary, non-suppressible, non-rhythmic movements of variable amplitude and frequency, involving the left hand and wrist, best categorized as dyskinesias. Episodes involve intermittent non-rhythmic finger and wrist flexion movements with finger flexion of variable frequency and intensity occurring primarily at MCP joints of second through 5th digits, occasionally involving PIP joint flexion, also accompanied by intermittent slow alternating wrist flexion and extension, intermittent gentle ulnar deviation that has some dystonic features, and intermittent thumb/palm adduction. With lacosamide uptitratration (6.1mg/kg/day), episodes increased in frequency and duration, lasting 30 minutes and occurring consistently 90 minutes after the dose. With lacosamide wean (2.5mg/kg/day), the dyskinesias resolved.
2.3. Patient 3
A 4-year-old right-handed girl with intellectual disability and Potocki-Lupski (17p11.2 duplication) Syndrome presented with nocturnal seizures of left arm clonus, hypersalivation, and facial twitching followed by prolonged weakness. EEG showed independent multifocal epileptiform discharges with a right-sided predominance and background slowing. Seizures were refractory to levetiracetam (80mg/kg/day) but responded to lacosamide (11mg/kg/day). With lacosamide initiation, she developed episodes of intermittent, distractible right eye blinking, exacerbated by stress, at the time thought most likely to be simple motor tic.
At 8 years old, the patient had a language regression and EEG demonstrated electrical status of epilepticus of slow wave sleep (ESES), consistent with Landau-Kleffner Syndrome. Levetiracetam and lacosamide were increased (LEV: 100mg/kg/day; LCM: 17mg/kg/day). Within one month, right eye blinking evolved to 30-minute episodes of unprovoked, non-distractible, non-painful, slow semi-rhythmic low amplitude right face hemicontraction with intermittent chewing alternating with briefly sustained jaw opening during which rotary and extensor dyskinetic tongue movements are seen; patient remains conscious and responsive throughout episode. There was no epileptiform correlate on video EEG during these episodes of dyskinesia. Lacosamide was decreased (10mg/kg/day) and steroids were initiated. ESES resolved and language returned to baseline within 3 months. Dyskinetic movements markedly improved with lacosamide wean and resolved when lacosamide was replaced with valproic acid.
2.4. Patient 4
A 3-month-old boy presented with flexor spasms and subtle left hemiparesis. MRI demonstrated diffuse right frontal, perisylvian, and opercular polymicrogyria. EEG showed independent bilateral spike-waves with seizures arising exclusively from the right hemisphere. After failing levetiracetam, phenobarbital, oxcarbazepine, clobazam, and the ketogenic diet, he became seizure free after focal perisylvian resection at 14 months and stopped all medications. Eighteen months post-operatively, he developed non-distractible eyelid fluttering occurring every several months, lasting days at a time, and increasing during periods of stress; after negative EEG, he was diagnosed with motor tics.
Two years post-operatively, he developed new seizures (left arm extension, left leg flexion, and stereotyped vocalization) and oromotor apraxia. Seizures were controlled with clobazam (10mg/kg/day) and chronic blinking tics persisted. At four years old, he developed another seizure semiology (unresponsiveness and inappropriate ictal laughter) but became seizure free when clobazam was replaced with lacosamide (7.5mg/kg/day). However, his chronic blinking tics increased to daily episodes.
At age 6 years old, in the setting of new inattentiveness and frequent, sleep-potentiated spike waves on EEG, lacosamide was increased (11mg/kg/day). His oral apraxia worsened, and his blinking tics became disruptive and continuous. With lacosamide wean (8.8mg/kg/day), the blinking tics decreased to brief, daily episodes.
3.1. Discussion
These cases suggest that pediatric epilepsy patients with dysfunction of the opercular cortex may be at increased risk for developing lacosamide-induced dyskinesias. The operculum includes the motor cortex responsible for control of the face and mouth. Pathology in this region can lead to the well-established opercular syndrome, characterized by loss of voluntary facial musculature control but preservation of automatic-emotional movements.4,5 Three of our patients had seizure semiology suggesting opercular onset – including hypersalivation, facial twitching, and choking – and the fourth patient had perisylvian pathology (Figure 1) and opercular resection. The opercular region contributes to movement control through the cingulo-opercular circuit, which is postulated to play a role in motor task maintenance. Together with fronto-parietal circuits, these complex motor networks are uniquely linked in children compared to adults,6 and dysregulation and/or immaturity of these circuits has been implicated in complex movement disorders. For example, it is postulated that abnormal connectivity with and within the cingulo-opercular network in children with Tourette Syndrome may lead to impaired suppression of unwanted behaviors such as tics.7 Impaired inhibition of a number of different motor circuits has been implicated in various movements disorders of childhood.8 Thus, immaturity coupled with epilepsy-related dysfunction of cingulo-opercular circuitry in affected children may decrease ability to suppress unwanted or unnecessary movements.
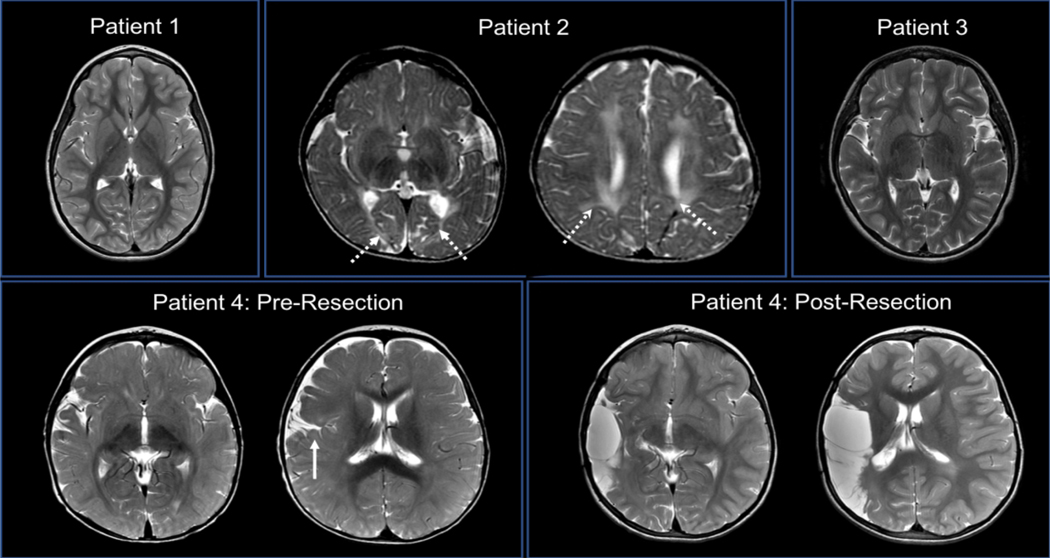
Figure 1:
Neuroimaging of the Opercular Region. MRI T2 sequences. Patients 1 (age 8 years) and 3 (age 10 years) had no obvious intracranial pathology. Patient 2 (age 10 months) had periventricular (left image, arrows) and parietooccipital (right image, arrows) white matter injury, but no opercular pathology. Patient 4’s pre-resection scan (age 3 months) found diffuse right hemispheric polymicrogyria (arrow), while the second scan (age 2 years and 8 months) demonstrated the extent of the resection, including the right opercular region.
Though this retrospective case series cannot establish that lacosamide causes movement disorders, the correlation between onset and progression of abnormal movements with medication initiation and uptitration implicates lacosamide as a potential etiology. Furthermore, three patients’ movements resolved with lacosamide cessation, and for one, dyskinesias temporally correlated with lacosamide dosing. Patient 4 was unique in that he had facial motor tics before starting lacosamide, so was at high risk for re-emergence of tics regardless of lacosamide initiation, but his tics worsened and improved in a dose-dependent fashion.
Interestingly, our patients experienced onset of abnormal movements at typical doses of lacosamide (6–11mg/kg/day), though two developed bothersome movements only at high doses (>15mg/kg/day).
There is one other report of lacosamide related oral motor tics3 in a child with refractory focal epilepsy, which remitted following lacosamide cessation. Other AEDs, especially sodium channel inhibitors,9 are known to cause movement disorders. Dyskinesia is a rare but well documented complication of phenytoin.10 Certain populations are at higher risk for AED-related movement disorders. Children with basal ganglia injury or intellectual disability have an increased risk of phenytoin-induced chorea,11,12 and patients with severe myoclonic epilepsy of infancy are vulnerable to phenytoin-induced choreoathetosis.13 Similar to patient 3 in our series, children with severe language dysfunction, pre-existing hyperkinetic movement disorders, and Landau-Kleffner syndrome are susceptible to orofacial tics as a side effect of lamotrigine therapy.14,15
Our patients’ movement disorders are best described as dyskinesias affecting the face and/or upper limbs, with some features suggestive of complex motor tics and/or hemifacial spasm. The complex appearance of these movements may reflect a combination of underlying pathophysiologic mechanisms. We postulate that alteration of sodium channel signalling by lacosamide in the already abnormal motor cortex of a child with epilepsy could alter both cortical output to target tissues such as the basal ganglia and cortical receptivity to input from movement regulatory centers such as the thalamus. This may contribute to disruption in tonic motor inhibition, lowering the threshold for emergence of tics and other dyskinesias. Sodium channel dysfunction can contribute to emergence of multiple abnormal movements as can been seen in genetic channelopathies16 and with other sodium channel modulating medications.17 The role of sodium channel modulation in movement disorders is certainly complex and incompletely understood. In one case report, a patient’s paroxysmal kinesigenic dyskinesias (PKD) were successfully treated with lacosamide. The underlying etiology of PKD is thought to be related to dysregulation of sodium channels, which in theory is corrected by sodium channel blockers. There is evidence of reduced transcallosal inhibition in PKD, corrected with agents such as carbamazepine.18 Impaired intracortical inhibition may play a role in epilepsy19,20; and impaired inhibitory circuitry likely plays a role in a number of pediatric movement disorders, as previously discussed. The specific effects of lacosamide on motor circuitry inhibition in children with epilepsy is unknown, though lacosamide decreases motor cortex excitability in adults.21 While aberrant inhibition may be associated with dyskinesias regardless of etiology, key differences in the specific circuity involved for a particular individual may explain how the same drug may either relieve or provoke similar appearing movements in different disease states22. While the exact pathophysiologic mechanisms of lacosamide-related abnormal movements are unknown, our patients provide additional examples of drug-induced dyskinesias and provide further evidence for a role of sodium channel signalling in movement control in children.
4.1. Limitations
Our conclusions are limited by small patient numbers and lack of a systematic ascertainment. Furthermore, certain movement disorders (i.e. transient tics) are common in children and these are often exacerbated by concurrent acute and/or chronic illnesses such as epilepsy. In this setting, we cannot conclude that lacosamide directly causes onset/exacerbation of abnormal movements, but rather note the temporal and dose-dependent association.
5.1. Conclusions
In summary, we describe 4 children with refractory focal epilepsy related to underlying opercular dysfunction who developed a dyskinetic movement disorder in conjunction with the initiation and/or uptitration of lacosamide. For each of these patients, there were initial concerns that abnormal movements were seizures, especially given that prior seizures and the new dyskinesias involved the face. Video EEG was crucial in distinguishing this condition from recurrent seizures. Practitioners should be aware of this side effect of lacosamide, particularly in epilepsy patients with underlying opercular dysfunction. In similar cases, physicians may consider reducing the lacosamide dose and performing video EEG if symptoms do not resolve.
Table 1:
Seizure vs. Dyskinesia Semiology
| Patient (age LCM initiated, gender) | Seizure Semiology | Dyskinesia Semiology | Dyskinesia Frequency | Dyskinesia Correlation with LCM |
|---|---|---|---|---|
| 1 (8y, M) | Facial twitching (either side), head deviation, whole body myoclonus & AMS. | Orofacial dyskinesias: Right facial contractions, intermittent gargling, & brief truncal extension | Weekly for 5–20min | Began w discontin |
| 2 (13y, F) | Deviation of eye & head to left, left arm clonus, choking & hypersalivation. | Left hand dyskinesias: Left hand finger flexion and wrist flexion/extension | Daily for 5min | Began w frequenc resolved |
| 3 (8y, F) | Facial twitching (either side), left arm clonus, hypersalivation. | Orofacial dyskinesias: Right eye twitching, evolving to right face hemicontraction with extensor & rotatory tongue movements | Up to 30 min | Began w uptitratio resolved |
| 4 (6y, M) | Pre-resection: Flexor spasms. Post-resection: (a) Left arm & leg extension, vocalization. (b) AMS & inappropriate laughter. |
Prior to LCM: Facial motor tic: Bilateral eye fluttering With LCM: Hemifacial dyskinesia: Eye-fluttering & forceful blinking. |
Clusters for several days in a row, occurring every other month Daily for 20 min |
Pre-existing tics increased in frequency with initiation, became constant & disabling with uptitration, & decreased with wean |
AMS = altered mental status; BL=bilateral; D/c=discontinuation, LCM= lacosamide
8.1 Acknowledgements and Funding
This work was conducted with support from a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083) and (UL1 TR 001085) (FB).
Footnotes
Disclosure of conflicts of interest
None of the authors has any conflict of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Ortiz de la Rosa JS, Ladino LD, Rodríguez PJ, Rueda MC, Polanía JP, Castañeda AC. Efficacy of lacosamide in children and adolescents with drug-resistant epilepsy and refractory status epilepticus: A systematic review. Seizure. 2018;56:34–40. doi: 10.1016/j.seizure.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 2.Buck ML, Goodkin HP. Use of lacosamide in children with refractory epilepsy. J Pediatr Pharmacol Ther. 2012;17(3):211–219. doi: 10.5863/1551-6776-17.3.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilhoto LMFF, Loddenkemper T, Gooty VD, et al. Experience with lacosamide in a series of children with drug-resistant focal epilepsy. Pediatr Neurol. 2012;16(1):15–19. doi: 10.1016/j.pediatrneurol.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 4.Bakar M, Kirshner HS, Niaz F. The opercular-subopercular syndrome: Four cases with review of the literature. Behav Neurol. 1998;11(2):97–103. doi: 10.1155/1998/423645 [DOI] [PubMed] [Google Scholar]
- 5.Szabó N, Hegyi Á, Boda M, et al. Bilateral Operculum Syndrome in Childhood.J Child Neurol. 2009;24(5):544–550. doi: 10.1177/0883073808327841 [DOI] [PubMed] [Google Scholar]
- 6.Fair DA, Dosenbach NUF, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci. 2007. doi: 10.1073/pnas.0705843104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church JA, Fair DA, Dosenbach NUF, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009. doi: 10.1093/brain/awn223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert RW. Tic modulation using sensory tricks. Tremor Other Hyperkinet Mov (N Y). 2013. doi: 10.7916/d81g0kzr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaccara G, Cincotta M, Borgheresi a, Balestrieri F. Adverse motor effects induced by antiepileptic drugs. Epileptic Disord. 2004;6(3):153–168. http://www.ncbi.nlm.nih.gov/pubmed/15504714. [PubMed] [Google Scholar]
- 10.Lee CH, Li JY. Phenytoin intoxication and upper facial dyskinesia: An unusual presentation. Mov Disord. 2008;23(8):1188–1189. doi: 10.1002/mds.22004 [DOI] [PubMed] [Google Scholar]
- 11.Koukkari MW, Vanefsky MA, Steinberg GK, Hahn JS. Phenytoin-Related Chorea in Children With Deep Hemispheric Vascular Malformations. J Child Neurol. 1996;11(6):490–491. doi: 10.1177/088307389601100617 [DOI] [PubMed] [Google Scholar]
- 12.Harrison MB, Lyons GR, Landow ER. Phenytoin and dyskinesias: A report of two cases and review of the literature. Mov Disord. 1993;8(1):19–27. doi: 10.1002/mds.870080104 [DOI] [PubMed] [Google Scholar]
- 13.Saito Y, Oguni H, Awaya Y, Hayashi K, Osawa M. Phenytoin-Induced Choreoathetosis in Patients with Severe Myoclonic Epilepsy in Infancy. Neuropediatrics. 2001;32(5):231–235. doi: 10.1055/s-2001-19116 [DOI] [PubMed] [Google Scholar]
- 14.Sotero de Menezes MA, Rho JM, Murphy P, Cheyette S. Lamotrigine-induced tic disorder: report of five pediatric cases. Epilepsia. 2000;41(7):862–867. doi: 10.1111/j.1528-1157.2000.tb00254.x [DOI] [PubMed] [Google Scholar]
- 15.Burd L, Kerbeshian J, Fisher W, Gascon G. Anticonvulsant medications: an iatrogenic cause of tic disorders. Can J Psychiatry. 1986;31(5):419–423. http://www.ncbi.nlm.nih.gov/pubmed/3731009. [DOI] [PubMed] [Google Scholar]
- 16.Meisler MH, Kearney JA, Sprunger LK, MacDonald BT, Buchner DA, Escayg A. Mutations of voltage-gated sodium channels in movement disorders and epilepsy. Novartis Found Symp. 2002;241:72–81; discussion 82–6, 226–232. doi:11771652 [PubMed] [Google Scholar]
- 17.Chalhub EG, Devivo DC, Volpe JJ. Phenytoin-induced dystonia and choreoathetosis in two retarded epileptic children. Neurology. 1976;26(5):494–498. http://www.ncbi.nlm.nih.gov/pubmed/944401. [DOI] [PubMed] [Google Scholar]
- 18.Mir P, Huang YZ, Gilio F, et al. Abnormal cortical and spinal inhibition in paroxysmal kinesigenic dyskinesia. Brain. 2005. doi: 10.1093/brain/awh342 [DOI] [PubMed] [Google Scholar]
- 19.Badawy RAB, Strigaro G, Cantello R. TMS, cortical excitability and epilepsy: The clinical impact. Epilepsy Res. 2014. doi: 10.1016/j.eplepsyres.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 20.Bauer PR, De Goede AA, Stern WM, et al. Long-interval intracortical inhibition as biomarker for epilepsy: A transcranial magnetic stimulation study. Brain. 2018. doi: 10.1093/brain/awx343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang N, Rothkegel H, Peckolt H, Deuschl G. Effects of lacosamide and carbamazepine on human motor cortex excitability: A double-blind, placebo-controlled transcranial magnetic stimulation study. Seizure. 2013. doi: 10.1016/j.seizure.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 22.Gittis AH, Kreitzer AC. Striatal microcircuitry and movement disorders. Trends Neurosci. 2012. doi: 10.1016/j.tins.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]