Abstract
This pilot study examined whether a combined aerobic resistance exercise program reduced fatigue and the potential inflammatory and epigenetic mechanisms in patients with head and neck cancer (HNC) receiving intensity-modulated radiotherapy. The exercise group (N=12) received a 3-month supervised aerobic resistance exercise intervention that was initiated before a 6-week radiotherapy regimen; the control group (N=14) received standard care. Fatigue was measured using Multidimensional Fatigue Inventory-20; physical function measures included a 6-minute walk distance (6MWD), chair stands, bicep curls, and hand grip strength. Inflammatory markers and DNA methylation data were acquired using standardized protocol. Patients were mostly white (93%) and male (81%) with a mean age of 57 years. At the end of the intervention, the exercise group had a marginal decrease in fatigue compared with the control (−5.0 vs. 4.9; P = 0.10). The exercise group had a significantly greater improvement in 6MWD (29.8 vs. −55.5 meters; P = 0.04), and a marginally smaller decline in hand grip (−0.3 vs. −5.8 lbs; P = 0.05) at the end of the intervention than the control. No significant difference in inflammatory markers was observed between groups. Lower plasma interleukin (IL) 6, IL1 receptor antagonist, tumor necrosis factor α (TNFα), soluble TNF receptor II and C-reactive protein were significantly associated with increased 6MWD, chair stand, and bicep curl at the end of the intervention (p<0.05). Among the 1,152 differentially methylated sites (DMS) after intervention (p<0.001), 163 DMS were located in gene promoter regions. Enrichment analysis suggested that the top 10 upstream regulators were associated with tumor (HNF4A, RPP38, HOXA9, SAHM1, CDK7, NDN, RPS15) and inflammation (IRF7, CRKL, ONECUT1). The top 5 diseases or functions annotations of the 62 hypermethylated DMS indicated anti-tumor and anti-inflammatory effects that might be linked to exercise. These findings suggest that exercise may improve physical performance and reduce fatigue, which could be further linked to decreased inflammation, during active radiotherapy for HNC patients. Larger studies are warranted.
Keywords: fatigue, exercise, inflammation, DNA methylation
Introduction
Head and neck cancers (HNC) are the 6th most common cancers worldwide.1 A continuous rise in the incidence of HNCs, due to the increased incidence of human papillomavirus (HPV) infection, has been noted in the past decade, and there were more than 65,410 new estimated HNC cases in 2019 in the US alone.2 Fatigue, the most common and distressing cancer symptom,3–5 is a prognostic indicator for poor tumor response6 and lower survival7–9 rates among patients with HNC.7–9 HNC patients typically receiving intensity-modulated radiotherapy (IMRT) experience greater fatigue than conventional-radiotherapy (RT).10,11 Combined chemotherapy with IMRT produces even more severe fatigue, likely from the synergistic effects of multimodality treatment.12,13 Given its high prevalence and adverse effects, reducing fatigue will improve quality of life for patients with HNC.
Since no reliable or evidenced-based pharmacological interventions currently exist.14,15 the National Comprehensive Cancer Network (NCCN) has recommended exercise for managing cancer-related fatigue, Although there is robust evidence of the beneficial effect of exercise on fatigue across many cancer sites,16,17 data on patients with HNC are limited.18,19 Two pilot intervention studies that have evaluated either aerobic or resistance exercise during active treatment have demonstrated their safety and potential for improving physical function and lowering fatigue within 4–12 weeks.18,19 More recently, a randomized aerobic resistance exercise study for 148 patients with HNC reported a significant decrease in fatigue for the exercise group.20 However, the underlying mechanisms responsible for the beneficial effects have yet to be identified. Preliminary but inconclusive evidence suggests that inflammation may play a key role linking exercise with fatigue and cancer survival.21–26 Additionally, given the reversible process of epigenetic regulation (e.g., DNA methylation), these non-DNA sequence changes may be the underlying mechanisms of the positive exercise effects observed in this population.27–30 Thus, we proposed this pilot study to evaluate the effects of a combined exercise approach on fatigue in patients with HNC undergoing active treatment and, more importantly, the potential inflammatory and epigenetic changes related to exercise and its effect on fatigue.
Method
Study design
This was a pilot study with patients assigned to a 12-week combined aerobic resistance exercise or a control program. All patients were enrolled before a 6-week IMRT and the study continued for an additional 6 to 7 weeks after cancer treatment completion. Surgery usually occurred one month before the IMRT. Twenty-six patients were in the study using sequential sampling: initial patients were enrolled into the control group (n=14), and the next 12 patients were in the exercise group.
Participants
Patients were recruited at the Winship Cancer Institute Radiation Oncology Clinics of Emory Healthcare. The major inclusion criteria were: histological proof of squamous cell carcinoma of the head and neck regions; any clinical stage with no distant metastasis; currently not exercising more than 150-minutes per week; Body Mass Index (BMI) <40, ≥ 21 years of age; no major organ disease; and being scheduled to receive IMRT. The primary exclusion criteria were simultaneous primaries; previous invasive malignancies; pregnant women; patients with major psychiatric disorders according to medical records or those who did not understand English; and those with physical limitations that would prohibit exercise participation. Other exclusion criteria that might confound the relationship between fatigue and inflammation were chronic medical conditions involving the immune system (e.g., HIV, hepatitis B or C) and regular use of immunosuppressive medications. The study was approved by the Emory University Institutional Review Board and all participants signed a written informed consent prior to enrollment.
Measurement
Symptom and Social Behavioral Measures
Demographic and clinical variables
were collected at baseline and/or follow up as appropriate through administration of standardized patient reported questionnaires or standardized abstraction forms for medical chart review. Variables included age, sex, race, marital status, smoking status, alcohol status, body mass index (BMI), HPV status, primary cancer site, cancer stage (TNM), radiation dose, and chemotherapy regimen. These variables were chosen for their potential influence on fatigue.31–36 HPV status was defined as either HPV or p16 positive per chart review.
Fatigue
was measured using the Multidimensional Fatigue Inventory (MFI)-20. The MFI is a 20-item self-report instrument that covers five dimensions: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity.37 The total score, ranging from 20 to 100 (higher score indicates more fatigue) is calculated as the sum of the five dimensions, and each dimension is the sum of the four items. MFI-20 has well-established validity and reliability (α=0.84) in use with patients with cancer receiving RT.37,38 Additionally, fatigue was measured in a weekly basis using the Visual Analog Fatigue Scale ranging from 0 to 10 recommended by the NCCN cancer-related fatigue guideline for exercise monitoring, with 0 indicating no fatigue, 1–3 representing mild fatigue, 4–6 meaning moderate fatigue, and 7–10 being severe fatigue.39
Muscle and Physical Function
Six Minute Walk Test (6MWT)
is a simple, well-validated test that has been used extensively as a measure of submaximal functional capacity for participants including HNC.40 Participants were instructed to walk along a flat 100 foot hallway for 6 minutes, and the distance walked (meters) was recorded.41 An improvement of 35–42 meters has been previously reported as a significant change in studies examining a walking intervention in HNC undergoing concurrent chemoradiotherapy.42,43
30 second Chair Stand Test
is a simple, well-validated, and reliable method to quantify lower extremity muscle strength and endurance for participants including cancer patients.44,45 Participants were asked to sit in a standardized chair and asked to rise from a sitting to standing position as many times as they could during 30 seconds.
Hand Grip Strength
test evaluates overall strength using a dynamometer (Jamar, Bolingbrook, Illinois) and a standardized protocol for both hands. Three measures were taken for each hand and the average pounds used for grip strength.46,47
30 second maximal arm curl test
was measured by lifting a 3 kg and 4 kg dumbbell for women and men, respectively, to assess arm strength. Participants sat in a straight back chair and were asked to perform as many arm curls as possible in 30 seconds. In addition, maximal muscle strength has been associated with the 30 second arm curl performance and 30 second chair rise in persons with HNC.48
Laboratory Measures
Whole blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes for the isolation of plasma and buffy coat. Whole blood was separated into plasma and buffy coat by centrifugation at 1000 × g for 10 minutes at 4°C, and then plasma and buffy coat were aliquoted into siliconized polypropylene tubes and stored at −80°C until batched assays for inflammatory biomarkers and DNA extraction, respectively.
Inflammatory Biomarkers:
Plasma concentrations of C-reactive protein (CRP), interleukin 1 receptor antagonist (IL1ra), IL6, IL10, tumor necrosis factor α (TNFα), soluble TNF receptor 2 (sTNFR2), monocyte chemoattractant protein 1 (MCP1) were determined in duplicate using multiplex assays (Meso Scale Discovery, Rockville, MD). Supplemental Table 1 displays the coefficient of variation and detection limits of inflammatory markers. All cytokines and their receptors as well as CRP have been reliably found to be elevated in association with cancer-related fatigue by our studies and others.32,49–52
Genome-wide DNA Methylation:
DNA was extracted from buffy coat using standardized protocol (QIAamp DNA Blood Mini Kit: Qiagen). DNA quantification was determined using the Quant-iT dsDNA broad range assay kit (ThermoFisher). DNA of greater concentration than 20μg/ml were used for methylation analysis using MethylationEPIC BeadChip (Illumina) at the Emory Integrated Genomics Core.
Intervention
Aerobic exercise
A home-based program was used with participants instructed to walk 5 times per-week for a minimum of 30 minutes per-day at moderate intensity for 12 weeks. This protocol was based on current exercise recommendations for cancer.53,54 Participants were instructed on Borg’s Rate of Perceived Exertion between 12–13, a reliable and validated measure for determining exercise intensity.55 A multi-sensory wristband (Fitbit Charge 2™) was provided to participants to monitor their steps with a goal of increasing steps each week by 5% if fatigue level was stable. If fatigue levels were increasing, participants were asked to walk the same number of steps as the previous week if possible. The Fitbit data were synchronized using the Fitabase analytics system (Small Steps Labs, San Diego, CA, USA) to enable our team to remotely monitor steps and give feedback on their walking progress during weekly clinic visits or by telephone.
Progressive Resistance Training
Participants were instructed to perform the resistance exercises using color bands 2 times per week, but not on consecutive days to avoid muscle fatigue and soreness.54,56,57 Resistance training targeted major upper and lower body muscle groups: wall push-ups, standing row, chest press, horizontal fly biceps curl, hip abduction, hip diagonal, leg press, heel raises and wall squats. If there were prior injuries or limitations in any muscle group, alternative resistance exercises were performed to ensure safety. The resistance levels were increased from light (5–10 lbs) to low moderate (15–20 lbs) when the participant could perform 2 sets at current band level with Borg’s Rate of Perceived Exertion between 12–13, indicating moderate exertion. Participants spent 20–30 minutes initially that could be increased to 45 to 60 minutes. Each week a member of the research team met with each participant to review exercises and to determine progress recorded on their resistance exercise logs.
Control
Participants in the control group received standard care and were not provided any specific exercise goals and instruction. To test feasibility of an attention control, the last 5 participants enrolled into the control group received instruction on stretching and flexibility and advised to return to pre-treatment exercise level after the completion of cancer treatment. These five participants in the control group were also provided a Fitbit Charge 2.
Adherence monitoring
Participants with a Fitbit were asked to wear the Fitbit on all days during the study period and to synchronize their device at least twice per week for the research team to remotely monitor steps. An exercise log was provided for participants in the intervention group to document the resistance exercises performed, number of sets and repetitions, band color used for that session during the exercise.
Data analysis
Descriptive statistics were performed for all measures prior to analysis. Baseline group differences were tested using non-parametric Wilcoxon Mann Whitney U test, Fisher’s exact test, or Fisher’s Freeman-Halton test. Nonparametric Wilcoxon Mann Whitney U tests were used for the outcome differences between groups and between time points. Additionally, we performed a mixed model repeated-measures analysis with maximum-likelihood estimation to examine intervention effects by assessing differences in mean changes in fatigue and physical function at the 3-month follow-up visit between the exercise and control groups. Spearman correlation analysis was used to examine the association among fatigue, physical function, and inflammatory markers. Inflammatory markers were log transformed for data analysis.
The DNA methylation data were preprocessed and normalized using minfi R package.58 Quality control was conducted through getQC function with default parameters to identify samples with abnormal signals.
Normalization was performed using functional normalization incorporating sex information. Probe loci with SNPs were removed. In order to examine the differentially methylated sites (DMS) between exercise and control groups over time, we used limma R package,59 which uses linear models with the Empirical Bayes Moderated test for group difference.60 In the linear mixed effects model, the methylation level of each probe at each time was the response with the intervention group (exercise vs. control), time and their interaction as fixed effects and subject as a random effect term. Given the sample size and pilot exploratory nature, p value < 0.001 was used to select top DMS between the two groups over time. Ingenuity Pathway Analysis (IPA) package was used for enrichment analysis of related genes.
Results
Baseline characteristics
The two groups were well-balanced for baseline demographic and clinical characteristics (see Table 1). The mean age of patients was 57±11 years (median: 55; range: 44), and the majority were male (81%), Caucasian (92%) and married (73%). Fifty-eight percent had a history of drinking, and 61% were smokers or had a history of smoking. Clinically, most patients were in an advanced stage of cancer (84% stage III or IV); 50% were HPV or p16 positive; approximately half were oropharyngeal cancer (54%); and half received chemotherapy (54%). Among patients receiving chemotherapy, 9 had cisplatin, and 4 had carboplatin/paclitaxel or others. The only statistically significant difference between the exercise group and the control group was the radiation dose: patients in the exercise group received relative higher dose than the control, 68±3 vs. 64±5, respectively (p=0.02). There was no difference in fatigue levels between the two groups at baseline, 45±15 vs. 50±15, for the exercise and the control respectively (p=0.33). Similarly, no significant differences were noticed between the two groups by physical function tests at baseline. For patients in the control group, no significant differences in demographic/clinical variables, fatigue, and inflammation were observed at baseline and the end of the intervention between those enrolled earlier into standard care and those enrolled later into the attention control.
Table 1.
Baseline demographic and clinical characteristics between the intervention and control groups
| Intervention N=12 Mean (SD) or N (%) | Control N=14 | P value | ||
|---|---|---|---|---|
| Age | 57 (10) | 56 (12) | 0.67 | |
| Sex | Male | 9 (75) | 12 (86) | |
| Female | 3 (25) | 2 (14) | 0.64 | |
| Race | White | 11 (92) | 13 (93) | |
| Non-White | 1 (8) | 1 (7) | 1.00 | |
| Marital status* | Married | 8 (67) | 11 (79) | |
| Not Married | 4 (33) | 3 (21) | 0.67 | |
| Smoking status | Never smoker | 3 (25) | 7 (50) | |
| Ever/current smoker | 9 (75) | 7 (50) | 0.25 | |
| Alcohol status | Never drinker | 5 (42) | 6 (43) | |
| Ever drinker | 7 (58) | 8 (57) | 1.00 | |
| Body mass index | 26.89 (4.20) | 26.80 (3.15) | 0.94 | |
| HPV status | Unrelated | 6 (50) | 7 (50) | |
| Related | 6 (50) | 7 (50) | 1.00 | |
| Cancer site | Oropharynx | 6 (50) | 8 (57) | |
| non-Oropharynx | 6 (50) | 6 (43) | 1.00 | |
| Stage | II | 3 (25) | 1 (7) | |
| III | 3 (25) | 4 (29) | ||
| IV | 6 (50) | 9 (64) | 0.48 | |
| Treatment | Radiotherapy only | 2 (17) | 0 (0) | |
| Radiotherapy & Surgery | 3 (24) | 7 (50) | ||
| Radiotherapy & Chemotherapy | 5 (42) | 4 (29) | ||
| Radiotherapy, Chemotherapy & Surgery | 2 (17) | 3 (21) | 0.35 | |
| Radiation dose (Gy) | 68 (3) | 64 (5) | 0.02 | |
Married includes patients married or living as married; Unmarried includes patients single, separated, divorced, or widowed.
Note. HPV related tumors were defined as either HPV or p16 positive; HPV unrelated tumors were defined as both HPV and p16 negative or unknown.
Fatigue and physical function changes between the two groups
Table 2 provides the comparison of the mean changes in fatigue (MFI total) at the end of the intervention by the two groups. By the end of the 3-month intervention, the exercise group had a marginal decrease in fatigue score compared with the control group (−5.0 vs. 4.9; P = 0.10). We also used generalized estimating equations (GEE) model to explore whether the MFI fatigue total score and subscale scores would be different by the two groups with time, group, and the interaction term between time and group as predictors. Using the GEE model, fatigue total score (p=0.041), and subscales of physical fatigue (p=0.030) and reduced motivation (p=0.036) were significantly lower in the exercise group than the control group over time. Further Mann-Whitney U test showed that physical fatigue of MFI was significantly lower in the exercise group compared to the control group (9.25 ± 4.09 vs. 12.43 ± 3.63; p=0.036) at the end of intervention, while the fatigue total score and the reduced motivation were marginally significantly lower in the exercise group then the control group (p=0.053, p=0.060, respectively).
Table 2.
Effect of exercise versus control on fatigue and physical function at baseline and changes at 3 months
| Intervention | Control | Exercise Effect | P value | |
|---|---|---|---|---|
| Fatigue* | N=12 | |||
| Baseline | 44.50 (14.74) | 49.84 (15.13) | 0.33 | |
| 3 months change | −5.02 (−13.64, 3.61) | 4.87 (−3.10, 12.85) | −9.89 (−21.76, 1.97) | 0.10 |
| 6MWD (meters) | N=9 | N=4 | ||
| Baseline | 448.00 (76.27) | 398.50 (94.28) | 0.40 | |
| 3 months change | 29.79 (−14.19, 73.76) | −55.52 (−122.62, 11.58) | 85.31 (3.59, 167.02) | 0.04 |
| Chair Stand (times) | N=9 | N=4 | ||
| Baseline | 13.56 (3.13) | 11.25 (1.89) | 0.20 | |
| 3 months change | 2.06 (0.66, 3.46) | 0.87 (−1.30, 3.03) | 1.19 (−1.47, 3.85) | 0.34 |
| Arm Curl (times) | N=9 | N=4 | ||
| Baseline | 24.11 (4.37) | 19.25 (8.54) | 0.19 | |
| 3 months change | −1.82 (−6.16, 2.52) | −1.15 (−7.86, 5.56) | −0.67 (−8.93, 7.59) | 0.86 |
| Hand Grip (lbs) | N=9 | N=4 | ||
| Baseline | 32.44 (11.14) | 36.25 (17.23) | 0.64 | |
| 3 months change | −0.32 (−3.38, 2.74) | −5.78 (−10.39, −1.17) | 5.46 (−0.09, 11.02) | 0.05 |
Note. Baseline scores are presented as means (standard deviation). 3-month changes are presented as least square means and 95% confidence interval from mixed model analysis adjusting for baseline score. These bold values are statistically significant (p<0.05). 6MWD= 6-minute walk distance. SD = stand deviation. Physical function tests were added later into the study, only a subset of participants had the physical function tests, plus 3 missing for intervention group.
Fatigue was measured by using Multidimensional Fatigue Inventory-20.
Table 2 also provides the comparison of the mean changes in physical function scores at the end of the intervention by the two groups. Since physical function tests were added later into the study, only a subset of the control group had the physical function tests. At the end of the 3-month intervention, the exercise group had a significantly greater improvement in 6-minute-walk-distance (29.8 vs. −55.5 meters; p = 0.04) compared to the control group.
Furthermore, the exercise group had a marginally smaller decline in hand grip strength (−0.3 vs. −5.8 lbs; p = 0.05) at the end of intervention compared to the control group. Other physical function tests, including chair stand and maximal arm curl, did not show statistical significances between the two groups.
Inflammation and its association with fatigue and physical function
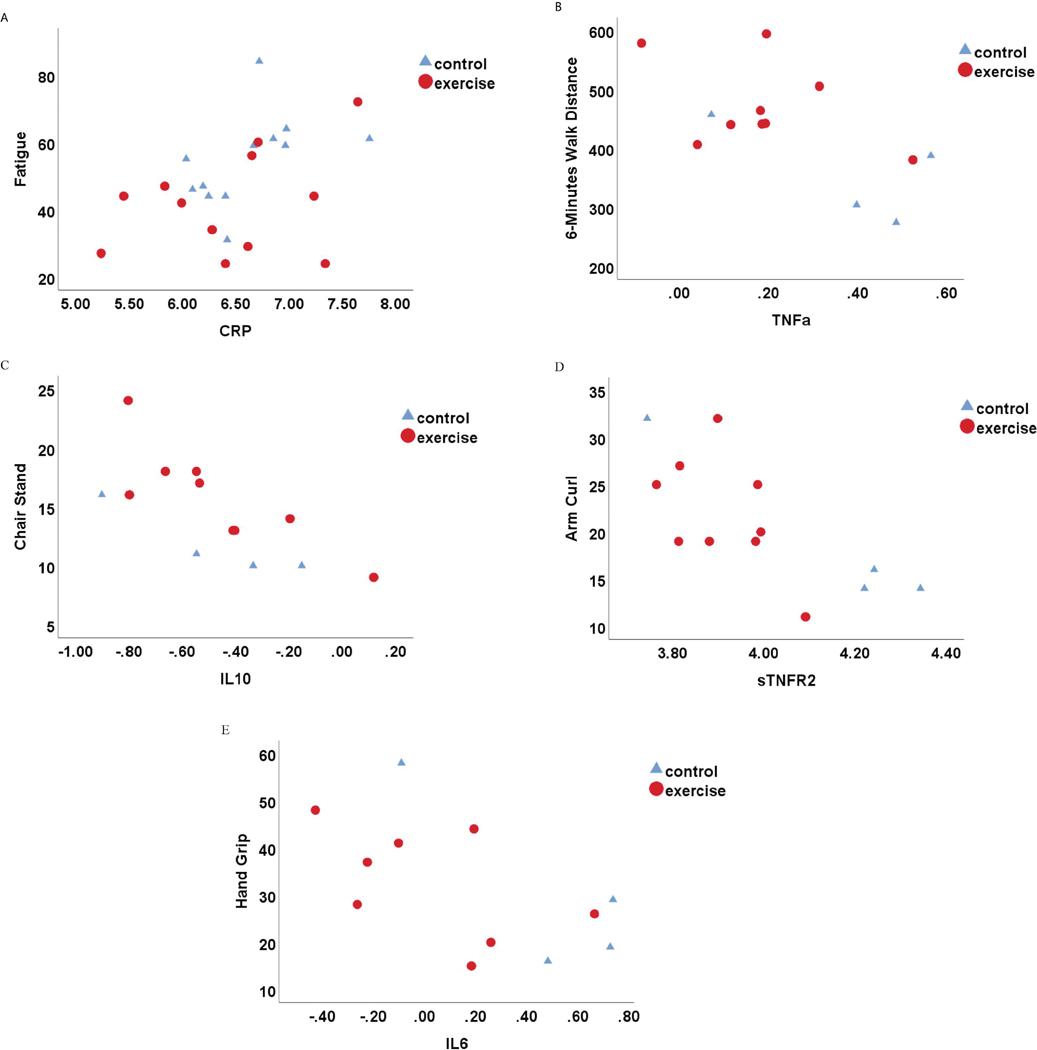
Despite that we did not observe significant differences in inflammatory markers between the two groups, we did find significant associations between inflammatory markers and fatigue as well as most of the physical function tests. CRP was significantly higher in fatigued patients (r=0.468, p=0.021; see Table 3 and Figure 1A). Among the subscales of MFI, higher physical fatigue was significantly associated with higher CRP (r=0.521, p=0.009) and TNFα (r=0.472, p=0.020); and higher reduced activity was significantly associated with higher CRP (r=0.417, p=0.043). For the physical function tests, lower CRP, IL6, IL1ra, TNFα, and sTNFRII were significantly associated with increased 6MWD, chair stand, and bicep curl tests at the end of the intervention (p<0.05; see Table 3 and Figure 1B-E). Given the small sample size and no difference in inflammatory markers between the two groups, the correlation analysis combined patients from both groups.
Table 3.
Correlation coefficients among fatigue, physical function and inflammation at 3 months
| Fatigue* | 6MWD (meters) | Chair Stand (times) | Arm Curl (times) | Hand Grip (lbs) | |
|---|---|---|---|---|---|
| CRP (pg/ml) | 0.468 (0.021) | −0.467 (0.108) | −0.522 (0.067) |
−0.621
(0.023) |
−0.286 (0.344) |
| IL1ra (pg/ml) | 0.109 (0.611) | −0.396 (0.181) | −0.688 (0.009) |
−0.682
(0.010) |
−0.231 (0.448) |
| IL6 (pg/ml) | 0.298 (0.167) | −0.741 (0.006) | −0.662 (0.019) |
−0.676
(0.016) |
−0.497 (0.101) |
| IL10 (pg/ml) | 0.173 (0.420) | −0.374 (0.209) | −0.779 (0.002) |
−0.899
(<0.001) |
−0.379 (0.201) |
| TNFα (pg/ml) | 0.394 (0.057) | −0.516 (0.071) | −0.588 (0.034) |
−0.666
(0.013) |
−0.099 (0.748) |
| sTNFR2 (pg/ml) | 0.172 (0.421) | −0.505 (0.078) | −0.522 (0.067) |
−0.724
(0.005) |
−0.445 (0.128) |
| MCP1 (pg/ml) | 0.321 (0.126) | −0.165 (0.590) | −0.307 (0.308) | −0.455 (0.118) |
−0.033 (0.915) |
| Fatigue | − | −0.335 (0.264) | −0.488 (0.091) | −0.420 (0.153) |
−0.440 (0.133) |
Note: N=24 for fatigue (missing inflammatory cytokines in two patients); N=13 for physical functions (Physical function tests were added later into the study, only a subset of participants had the physical function tests, plus 4 missing for intervention group). Parenthesis is the p value. These bold values are statistically significant (p<0.05). 6MWD= 6-minute walk distance, CRP = C-reactive protein, IL1ra = interleukin 1 receptor antagonist, IL6 = interleukin 6, MCP1= monocyte chemoattractant protein 1, sTNFR2 = soluble tumor necrosis factor receptor 2, TNFα = tumor necrosis factor α.
Fatigue was measured by using Multidimensional Fatigue Inventory-20
Figure 1.
Scatter plots for the associations between inflammatory markers and fatigue as well as physical function measures at end of the intervention. A, fatigue (Multidimensional Fatigue Inventory 20); B, 6-minute walk distance; C, chair stand; D, arm curl; E, hand grip. Note. CRP = C-reactive protein, IL10 = interleukin 10, IL6 = interleukin 6, sTNFR2 = soluble tumor necrosis factor receptor 2, TNFα = tumor necrosis factor α.
DNA methylation change during the interventional period
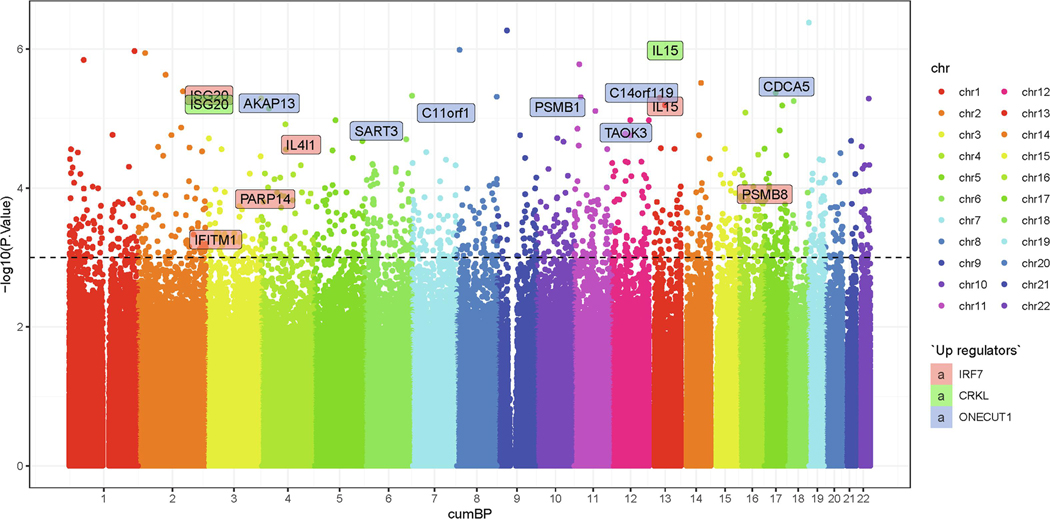
DNA methylation changes were explored in our study. After quality control, there are 835,424 methylation sites; among them, we found 1,152 DMS after intervention (p<0.001; see Figure 2). Since different methylation patterns in gene promoters are important for the regulation of gene expression,61 we examined methylation patterns at the promoter regions. Among the 1,152 DMS, 163 DMS were located at the promoter regions; enrichment analysis suggested that the top 10 upstream regulators were linked to tumor: Hepatocyte Nuclear Factor 4 Alpha (HNF4A), Ribonuclease P/MRP Subunit P38 (RPP38), Homeobox A9 (HOXA9), stapled αhelical peptide derived from Maml1 (SAHM1), Cyclin-dependent kinase 7 (CDK7), Necdin (NDN), and Ribosomal Protein S15 (RPS15), and inflammation: Interferon regulatory factor 7 (IRF7), CRK Like (CRKL), and One Cut Homeobox 1 (ONECUT1; see supplemental Table 2). Further analysis on the 62 hypermethylated DMS showed that apoptosis of CD4+ T-lymphocytes (p= 5.13E-05), activation of vascular endothelial tissue (p= 2.95E-04), loss of telomeres (p= 3.68E-04), and cell proliferation of lymphoma cell lines (p=6.28E-04) were among the top 5 diseases and functions annotations.
Figure 2.
Manhattan Plot of the DNA methylation sites and genes in the top 3 inflammation-related upstream regulators of the promoter regions.
Aerobic and resistance exercise adherence
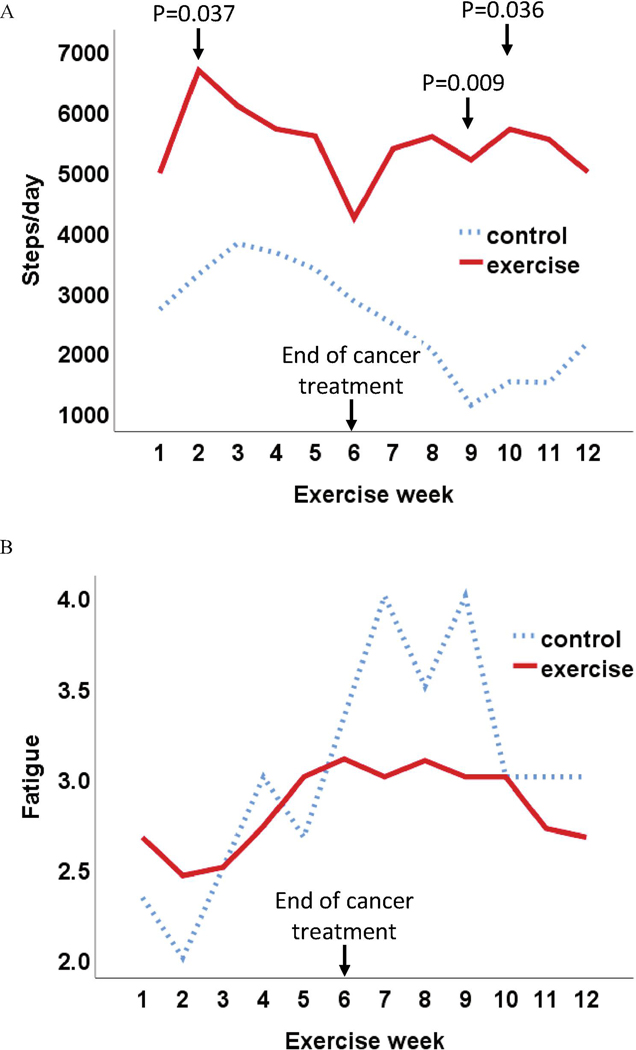
The average number of steps at week 1 was 2689 ± 1122 in the controls and 4952 ± 2490 among the intervention group (p=0.104; Figure 3A). The steps in the exercise group increased in week 2 and was significantly higher than the control group in week 2, week 9, week 10 (p=0.037, 0.009, 0.036, respectively). At the end of cancer treatment (week 6), the average steps in the exercise group decreased to 4206 ± 2605 from-week 5 of 5567 ± 3132, while the control had a continuous drop during the 6 weeks of cancer treatment. The changes in weekly step data (Figure 3A) were relatively consistent with the changes in weekly fatigue data (measured by using Visual Analog Fatigue Scale; Figure 3B) for both groups: more steps were associated with lower fatigue (GEE model on weekly fatigue with week, group, and steps/week as predictors: beta for steps/week=−7.772E-5, p<0.001). For the resistance exercise, approximately 70% of patients (n=8) completed at least once/week of progressive resistance training, and no statistically significant differences in fatigue and physical function tests were observed between patients completed at least once/week than the others. Among them, patients did twice/week in 40% weeks and did once/week in 22% weeks of the 12-week intervention.
Figure 3.
Average Steps and Fatigue Over 12 Weeks in Exercise and Control Groups. A: average step per day; B: average fatigue (Visual Analog Fatigue Scale). Note: fatigue 1: no fatigue, 2: mild fatigue; 3: moderate fatigue; 4: severe fatigue.
Discussion
This study is among the first to evaluate the biological mechanisms of a combined aerobic resistance exercise program on fatigue for patients with HNC. Although this pilot study was not powered to detect group differences, we did find a trend of reduced fatigue levels and a significant improvement in physical function such as 6MWD in the exercise group, compared to the control group. Additionally, a consistent association between decreased physical function and increased inflammation was observed. The DNA methylation results further suggested that exercise may be linked to the methylation changes involved in tumor growth and inflammation.
Evidence of the effect of exercise on cancer-related fatigue is sparse for patients with HNC, particularly those undergoing active treatment. We found a trend of decreased fatigue measured by using the MFI, particularly in the physical fatigue subscale, and a significant increase in 6MWD and no significant side effects occurred. Additionally, more steps/week was also associated with lower fatigue measured using Visual Analog Fatigue Scale. These findings are consistent with current evidence. For instance, two pilot studies using either aerobic or resistance exercise have shown positive results on cancer-related fatigue in HNC.18,19 Consistent with our findings, a recent randomized trial using a combined approach of aerobic resistance exercise in HNC patients also showed a significant beneficial effect on fatigue and physical function,20 suggesting that exercise programs in patients with HNCs are feasible and potentially beneficial.
Our findings demonstrated a consistent and relatively strong association between increased physical function measures and decreased inflammatory markers. The biological mechanisms linking exercise to cancer outcomes such as fatigue are not well-understood and the evidence of the inflammatory mechanisms of exercise to date is inconsistent and preliminary.22,23,25,62 Our published work on HNC has shown a strong association between increased fatigue and increased peripheral inflammation.63,64 This association indicates that interventions targeting inflammatory signaling pathways might be able to reduce fatigue. Although this pilot study had an insufficient sample size to demonstrate a significant difference in inflammatory markers between the exercise and control groups, our combined group data show a statistically significant correlation between physical function, such as 6MWD, and inflammation. Additionally, our data show that the exercise group had a significantly lower physical fatigue measured using a subscale of MFI compared to the control. This lower physical fatigue was also associated with lower inflammation such as CRP and TNFα in our data. Taken together, our findings suggest that inflammation may indeed play an important role in the association between exercise and cancer-related fatigue.
Future research is recommended to establish this association in larger samples of HNC patients. DNA methylation is a well-studied epigenetic modification and has been characterized in various disease processes including cancer, psychiatric symptoms, and inflammation.65–68 Methylation occurs at different locations of genes and promoter methylation is highly dynamic for transcription or gene expression.61,69 In our enrichment analysis, three of the top ten upstream regulators in the promoter regions of our DMS-located genes were associated with inflammation. Specifically, the diseases and functions annotation of the hypermethylated DMS indicated that exercise may have anti-inflammatory effects as suggested by potential suppression on the loss of telomere length and the activation of vascular endothelial tissue, both of which have been linked to increased inflammation.70,71 Although evidence is sparse, a few studies have also suggested that exercise may introduce anti-inflammatory effect through DNA methylation changes in inflammatory-associated genes. For instance, DNA methylation of apoptosis-associated speck-like protein containing a CARD (ASC), a vital component of the inflammasome, was higher in an exercise group compared with the control group. This increased methylation in ASC suggested an anti-inflammatory effect as IL-1β was also lower in the exercise group than the control.72,73 Significant hypermethylation of the TNF gene (a pro-inflammatory cytokine) and hypomethylation of IL-10 gene (an anti-inflammatory cytokine) were also reported in those who maintained increased physical activity energy expenditure.74
Our data also indicated that tumor growth was linked to the top 10 upstream regulators in the promoter regions of the DMS-located genes. Exercise may deliver anti-tumor effects through the down regulation of the apoptosis of CD4+ T-lymphocytes and cell proliferation of lymphoma cell lines. T-cell death caused by tumors is a key mechanism for tumors to evade effective immunosurveillance.75 Our data appear to support that exercise may suppress t-cell death, along with reducing lymphoma cell proliferation, to generate the anti-tumor effects. Additionally, HNF4A was the most significant upstream regulator in our data. As a well-established key regulator of liver development and function, HNF4A is linked to oncogenesis through the inflammatory pathways.76 HNF4A is also essential for lipid and glucose metabolism,77,78 which are important for tumor progression and inflammation.79–81 Thus, our data suggest that exercise may contribute to anti-tumor and anti-inflammatory effects through regulating lipid and glucose metabolism. Despite the beneficial effects of exercise on fatigue and cancer survival,82,83 the mechanisms of this beneficial effect are not well understood. The findings of our study may shed light on the mechanism although large studies are warranted.
The study had several limitations. Because of the pilot nature of the study, the sample size was small and limited our ability to test for group differences using more comprehensive analyses, which may have biased our findings. In addition, the study was not randomized, and data collection was not blinded. However, the two groups were well-balanced for major demographic and clinical variables, which justified our analysis plan to use non-parametric tests for group differences. Sensitivity analysis using repeated measures of ANOVA also revealed similar findings. Blood samples were not collected under fasting condition in the morning due to patients’ schedules with oncologists; however, our previous studies have shown that the time of blood collection does not have a significant impact on the results.84 With our low cutoff, the top ten upstream regulators in the promoter regions of the CPG site located-genes are still interpretable. The interpretation of the methylation results should be cautious as the blood cell type was not control; supplemental Table 3 compared cell proportions between the two groups at baseline and the end of intervention, which did not show significant differences between the groups. Given the fact of sparse evidence regarding the effect of exercise on fatigue, our pilot study may still provide preliminary information about the potential mechanisms of exercise on reducing fatigue.
Conclusion
Effective management of cancer-related fatigue, the most common and distressing side effect from cancer and its treatment, benefits cancer patients by improving quality of life and survival rates. Revealing the potential mechanisms of the symptom management for fatigue may provide a more targeted therapeutic strategy than is currently available. Findings from this pilot study are consistent with other studies suggesting a potential role of exercise for improving physical function performance and reducing fatigue that could be further linked to decreased inflammation during active radiotherapy for HNC patients-- a highly fatigued but understudied population. Our exploratory DNA methylation results further suggest potential involvement of tumor growth and inflammation as the mechanisms of exercise in cancer patients. Large intervention studies are warranted to examine the mechanisms associated with exercise and fatigue in cancer populations.
Supplementary Material
Highlights.
Exercise improved physical performance during radiotherapy for head and neck cancer
Exercise may improve fatigue for patients with head and neck cancer
Physical performance was negatively associated with inflammation
DNA methylation related to tumor growth and inflammation associated with exercise
Acknowledgements:
The authors are grateful for the support from participants and research staff.
Funding
This research was supported in part by the University Research Committee at Emory University and NIH/NCI P30CA138292.
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzmaurice C, Dicker D, Pain A, et al. The Global Burden of Cancer 2013. JAMA oncology. 2015;1(4):505–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Janaki MG, Kadam AR, Mukesh S, et al. Magnitude of fatigue in cancer patients receiving radiotherapy and its short term effect on quality of life. J Cancer Res Ther. 2010;6(1):22–26. [DOI] [PubMed] [Google Scholar]
- 4.Solberg Nes L, Ehlers SL, Patten CA, Gastineau DA. Self-regulatory Fatigue in Hematologic Malignancies: Impact on Quality of Life, Coping, and Adherence to Medical Recommendations. Int J Behav Med. 2013;20(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein D, Bennett B, Friedlander M, Davenport T, Hickie I, Lloyd A. Fatigue states after cancer treatment occur both in association with, and independent of, mood disorder: a longitudinal study. BMC Cancer. 2006;6:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HC, Janjan NA, Mendoza TR, et al. Temporal patterns of fatigue predict pathologic response in patients treated with preoperative chemoradiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75(3):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackerstaff AH, Rasch CR, Balm AJ, et al. Five-year quality of life results of the randomized clinical phase III (RADPLAT) trial, comparing concomitant intra-arterial versus intravenous chemoradiotherapy in locally advanced head and neck cancer. Head Neck. 2012;34(7):974–980. [DOI] [PubMed] [Google Scholar]
- 8.Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer. 2004;100(2):425–432. [DOI] [PubMed] [Google Scholar]
- 9.Montazeri A Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulliford SL, Miah AB, Brennan S, et al. Dosimetric explanations of fatigue in head and neck radiotherapy: An analysis from the PARSPORT Phase III trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;104(2):205–212. [DOI] [PubMed] [Google Scholar]
- 11.Powell C, Schick U, Morden JP, et al. Fatigue during chemoradiotherapy for nasopharyngeal cancer and its relationship to radiation dose distribution in the brain. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013. [DOI] [PubMed] [Google Scholar]
- 12.Jereczek-Fossa BA, Santoro L, Alterio D, et al. Fatigue during head-and-neck radiotherapy: prospective study on 117 consecutive patients. Int J Radiat Oncol Biol Phys. 2007;68(2):403–415. [DOI] [PubMed] [Google Scholar]
- 13.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. [DOI] [PubMed] [Google Scholar]
- 14.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane database of systematic reviews (Online). 2010(7):CD006704. [DOI] [PubMed] [Google Scholar]
- 15.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. Journal of the National Cancer Institute. 2008;100(16):1155–1166. [DOI] [PubMed] [Google Scholar]
- 16.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134(5):700–741. [DOI] [PubMed] [Google Scholar]
- 17.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clinical oncology (Royal College of Radiologists (Great Britain)). 2010;22(3):208–221. [DOI] [PubMed] [Google Scholar]
- 18.Aghili M, Farhan F, Rade M. A pilot study of the effects of programmed aerobic exercise on the severity of fatigue in cancer patients during external radiotherapy. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2007;11(2):179–182. [DOI] [PubMed] [Google Scholar]
- 19.Rogers LQ, Anton PM, Fogleman A, et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck. 2013;35(8):1178–1188. [DOI] [PubMed] [Google Scholar]
- 20.Samuel SR, Maiya AG, Fernandes DJ, et al. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support Care Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley GA, Kelley KS. Exercise and cancer-related fatigue in adults: a systematic review of previous systematic reviews with meta-analyses. BMC Cancer. 2017;17(1):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. Journal of the National Cancer Institute. 2012;104(11):815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen JF, Tolver A, Andersen JL, Rorth M, Daugaard G, Hojman P. Resistance training does not protect against increases in plasma cytokine levels among germ cell cancer patients during and after chemotherapy. J Clin Endocrinol Metab. 2014;99(8):2967–2976. [DOI] [PubMed] [Google Scholar]
- 24.Jones SB, Thomas GA, Hesselsweet SD, Alvarez-Reeves M, Yu H, Irwin ML. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer prevention research (Philadelphia, Pa.). 2013;6(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lof M, Bergstrom K, Weiderpass E. Physical activity and biomarkers in breast cancer survivors: a systematic review. Maturitas. 2012;73(2):134–142. [DOI] [PubMed] [Google Scholar]
- 26.Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21(7):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima K, Takeoka M, Mori M, et al. Exercise effects on methylation of ASC gene. International journal of sports medicine. 2010;31(9):671–675. [DOI] [PubMed] [Google Scholar]
- 28.Nitert MD, Dayeh T, Volkov P, et al. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61(12):3322–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronn T, Volkov P, Davegardh C, et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS genetics. 2013;9(6):e1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng H, Irwin ML, Lu L, et al. Physical activity and breast cancer survival: an epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res Treat. 2012;133(1):127–135. [DOI] [PubMed] [Google Scholar]
- 31.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24(6):968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell SA. Cancer-related fatigue: state of the science. PM & R : the journal of injury, function, and rehabilitation. 2010;2(5):364–383. [DOI] [PubMed] [Google Scholar]
- 34.Fang FM, Tsai WL, Chien CY, et al. Changing quality of life in patients with advanced head and neck cancer after primary radiotherapy or chemoradiation. Oncology. 2005;68(4–6):405–413. [DOI] [PubMed] [Google Scholar]
- 35.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral oncology. 2013;49(4):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao C, Hanlon A, Zhang Q, et al. Risk factors for clinician-reported symptom clusters in patients with advanced head and neck cancer in a phase 3 randomized clinical trial: RTOG 0129. Cancer. 2014;120(6):848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. [DOI] [PubMed] [Google Scholar]
- 38.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4):413–427. [DOI] [PubMed] [Google Scholar]
- 39.NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Cancer-Related Fatigue. 2019; Version 1.2019 — March 12, 2019:https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf, 2019.
- 40.ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt ME, Wiskemann J, Krakowski-Roosen H, et al. Progressive resistance versus relaxation training for breast cancer patients during adjuvant chemotherapy: design and rationale of a randomized controlled trial (BEATE study). Contemporary clinical trials. 2013;34(1):117–125. [DOI] [PubMed] [Google Scholar]
- 42.Javaheri PA, Nekolaichuk C, Haennel R, Parliament MB, McNeely ML. Feasibility of a pedometer-based walking program for survivors of breast and head and neck cancer undergoing radiation therapy. Physiotherapy Canada. Physiotherapie Canada. 2015;67(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuel SR, Maiya GA, Babu AS, Vidyasagar MS. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. The Indian journal of medical research. 2013;137(3):515–520. [PMC free article] [PubMed] [Google Scholar]
- 44.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. The American journal of medicine. 1985;78(1):77–81. [DOI] [PubMed] [Google Scholar]
- 45.Kampshoff CS, Chinapaw MJ, Brug J, et al. Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC medicine. 2015;13:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. The journal of supportive oncology. 2009;7(5):158–167. [PMC free article] [PubMed] [Google Scholar]
- 47.ACSM’s Guidelines for Exercise Testing and Prescription / American College of Sports Medicine. 9th Edition ed. Baltimore: Lippincott, Williams, & Wilkins; 2014. [Google Scholar]
- 48.Lonbro S, Dalgas U, Primdahl H, et al. Lean body mass and muscle function in head and neck cancer patients and healthy individuals--results from the DAHANCA 25 study. Acta oncologica (Stockholm, Sweden). 2013;52(7):1543–1551. [DOI] [PubMed] [Google Scholar]
- 49.Lyon DE, McCain NL, Pickler RH, Munro C, Elswick RK Jr., Advancing the biobehavioral research of fatigue with genetics and genomics. J Nurs Scholarsh. 2011;43(3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyes-Gibby CC, Spitz MR, Yennurajalingam S, et al. Role of inflammation gene polymorphisms on pain severity in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2636–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orre IJ, Reinertsen KV, Aukrust P, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71(3):136–141. [DOI] [PubMed] [Google Scholar]
- 53.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. [DOI] [PubMed] [Google Scholar]
- 54.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and science in sports and exercise. 2010;42(7):1409–1426. [DOI] [PubMed] [Google Scholar]
- 55.Borg GA. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 56.Lonkvist CK, Lonbro S, Vinther A, et al. Progressive resistance training in head and neck cancer patients during concomitant chemoradiotherapy -- design of the DAHANCA 31 randomized trial. BMC Cancer. 2017;17(1):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lonkvist CK, Vinther A, Zerahn B, et al. Progressive resistance training in head and neck cancer patients undergoing concomitant chemoradiotherapy. Laryngoscope investigative otolaryngology. 2017;2(5):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aryee MJ, Jaffe AE, Corrada-Bravo H, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics (Oxford, England). 2014;30(10):1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. [DOI] [PubMed] [Google Scholar]
- 61.Varley KE, Gertz J, Bowling KM, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome research. 2013;23(3):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogers LQ, Fogleman A, Trammell R, et al. Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: pilot randomized controlled trial. Psychooncology. 2015;24(3):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao C, Beitler JJ, Higgins K, et al. Fatigue is Associated with Inflammation in Patients with Head and Neck Cancer Before and After Intensity-Modulated Radiation Therapy. Brain, Behavior, and Immunology. 2015;(in Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao C, Beitler JJ, Higgins KA, et al. Associations among human papillomavirus, inflammation, and fatigue in patients with head and neck cancer. Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ptak C, Petronis A. Epigenetic approaches to psychiatric disorders. Dialogues in clinical neuroscience. 2010;12(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Booij L, Wang D, Levesque ML, Tremblay RE, Szyf M. Looking beyond the DNA sequence: the relevance of DNA methylation processes for the stress-diathesis model of depression. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368(1615):20120251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143):433–440. [DOI] [PubMed] [Google Scholar]
- 68.Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun. 2014;38:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nature reviews. Genetics. 2008;9(6):465–476. [DOI] [PubMed] [Google Scholar]
- 70.Wong JY, De Vivo I, Lin X, Fang SC, Christiani DC. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS One. 2014;9(1):e87348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. Journal of leukocyte biology. 2005;77(4):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butts B, Butler J, Dunbar SB, Corwin E, Gary RA. Effects of Exercise on ASC Methylation and IL-1 Cytokines in Heart Failure. Medicine and science in sports and exercise. 2018;50(9):1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butts B, Gary RA, Dunbar SB, Butler J. Methylation of Apoptosis-Associated Speck-Like Protein With a Caspase Recruitment Domain and Outcomes in Heart Failure. Journal of cardiac failure. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw B, Leung WC, Tapp HS, Fitzpatrick AL, Saxton JM, Belshaw NJ. A change in physical activity level affectsleukocyte DNA methylation of genes implicated in cardiovas-cular disease in the elderly. Paper presented at: Proc Physiol Soc 2014. [Google Scholar]
- 75.Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death & Differentiation. 2008;15(1):70–79. [DOI] [PubMed] [Google Scholar]
- 76.Hatziapostolou M, Polytarchou C, Aggelidou E, et al. An HNF4α-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147(6):1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Molecular and cellular biology. 2001;21(4):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu H Crosstalk of HNF4alpha with extracellular and intracellular signaling pathways in the regulation of hepatic metabolism of drugs and lipids. Acta Pharm Sin B. 2016;6(5):393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hay N Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nature Reviews Cancer. 2016;16(10):635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Disease Models & Mechanisms . 2013;6(6):1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflammation Research. 2000;49(10):497–505. [DOI] [PubMed] [Google Scholar]
- 82.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. [DOI] [PubMed] [Google Scholar]
- 83.Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. Journal of physiotherapy. 2016;62(2):68–82. [DOI] [PubMed] [Google Scholar]
- 84.Xiao C, Beitler JJ, Higgins KA, et al. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav Immun. 2016;52:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.