Abstract
Rationale:
The elderly experience profound systemic responses after stroke, which contribute to higher mortality and more severe long-term disability. Recent studies have revealed that stroke outcomes can be influenced by the composition of gut microbiome. However, the potential benefits of manipulating the gut microbiome after injury is unknown.
Objective:
To determine if restoring youthful gut microbiota after stroke aids in recovery in aged subjects, we altered the gut microbiome through young fecal transplant gavage (young FTG) in aged mice after experimental stroke. Further, the effect of direct enrichment of selective bacteria producing short-chain fatty acids (SCFAs) was tested as a more targeted and refined microbiome therapy.
Methods And Results:
Aged male mice (18–20 months) were subjected to ischemic stroke by middle cerebral artery occlusion (MCAO). We performed FTG three days after MCAO using young donor biome (2–3 months) or aged biome (18–20 months). At day 14 after stroke, aged stroke mice receiving young FTG had less behavioral impairment, and reduced brain and gut inflammation. Based on data from microbial sequencing and metabolomics analysis demonstrating that young fecal transplants contained much higher SCFA levels and related bacterial strains, we selected four SCFA-producers (Bifidobacterium longum, Clostridium symbiosum, Faecalibacterium prausnitzii and Lactobacillus fermentum) for transplantation. These SCFA-producers alleviated post-stroke neurological deficits and inflammation, and elevated gut, brain and plasma SCFA concentrations in aged stroke mice.
Conclusions:
This is the first study suggesting that the poor stroke recovery in aged mice can be reversed via “post-stroke bacteriotherapy” following the replenishment of youthful gut microbiome via modulation of immunologic, microbial and metabolomic profiles in the host.
Subject Terms: Aging, Basic Science Research, Inflammation, Ischemic Stroke, Translational Studies
Keywords: Ischemic stroke, aging, brain-gut axis, gut microbiome, fecal microbiota transplantation, inflammation, metabolomics
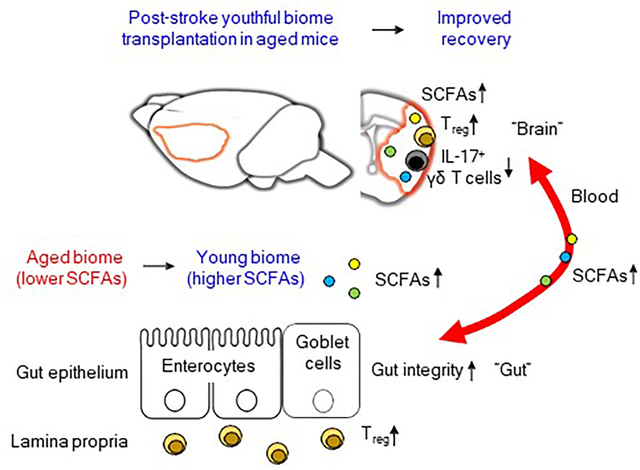
Graphical Abstract
Ischemic stroke is a leading cause of morbidity and mortality in the elderly. Recent studies have emphasized the importance of understanding the cross-talk between the microbiota, the gut and the brain in stroke. Here, we manipulated the gut microbiota after stroke to test our hypothesis that gut microbiota rejuvenation even at a delayed time-point after stroke would improve functional recovery in aged animals. Post-stroke restoration of youthful microbiota in aged mice using fecal transplant gavage from young donor mice (young FTG) improved behavioral recovery. Young FTG enhanced gut integrity and conferred a protective phenotype in both gut and brain T cells, as reflected by the increase in regulatory T (Treg) cells. Interestingly, young FTG reduced pro-inflammatory IL-17 in brain γδ T cells. To test if the beneficial effects of young FTG are mediated by short-chain fatty acids (SCFAs), we transplanted a cocktail of four bacterial strains of SCFA-producers. Transplantation of SCFA-producers in combination with inulin significantly reduced functional impairment and neuroinflammation in recipient aged stroke mice. Enrichment of the gut with SCFA-producers increased SCFA levels in the brain and plasma. This is the first demonstration that “bacteriotherapy” is a viable post-stroke treatment option in the aged.
INTRODUCTION
Recent demonstration of bidirectional communications between the brain and the gut have opened new areas of investigation for stroke and other neurological diseases. This bidirectional communication, termed the microbiota-gut-brain (MGB) axis, provides novel avenues for both the prevention and treatment of stroke.1–6 Following stroke, communications from the brain to the gut (top-down signaling) via the MGB axis likely occur through sympathetic and parasympathetic efferent fibers that innervate the gut directly or indirectly through the enteric nervous system.3, 5, 6 Although the exact mechanism is not well understood, it is increasingly evident that stroke alters gut motility, increases gut permeability, activates resident immune cells in the gut, and shifts the gut microbiome to one that is more toxic, a state that we designate as dysbiotic.1–4, 6, 7
A dysbiotic gut microbiome in turn appears to communicate to the brain through the MGB axis (bottom-up signaling) to exacerbate the detrimental effects of the stroke.2, 3 This bottom-up signaling likely involves migration of pro-inflammatory cells from the gut to the brain via the blood. Other potential mechanisms to signal the brain include afferent parasympathetic and sensory networks originating in the gut, or by bacteria, bacterial toxins, or bacterial metabolites that gain access to the blood through a disrupted gut barrier.2, 3 Regardless of the signaling mechanisms for the bidirectional MGB communication, a feedforward mechanism is thought to occur where the stroke brain is responsible for gut dysbiosis and gut inflammation, and subsequently the gut dysbiosis feeds back to promote neuroinflammation following stroke.2, 3 This cycle hinders recovery once a stroke has occurred.
Interfering with this cycle by altering the gut microbiome through fecal transplant gavage (FTG) or antibiotics prior to or at the time of experimental stroke can significantly affect recovery.3, 4 However, it is not known if an anti-inflammatory FTG is efficacious in improving outcome when conducted hours to days after stroke, a time when the critical window for other treatments has passed. If post-stroke alteration in the gut microbiome were efficacious, then individuals lacking a healthy or anti-inflammatory gut microbiome at the time of stroke could benefit from therapy. The study of stroke and the gut microbiome is especially significant in the aged, a population that is at risk for stroke.8, 9 Outcomes after stroke are also worse in aged mice compared to young mice.1 Complicating matters even more, aging alone is accompanied by gut dysbiosis.4 Thus, it is important to conduct studies in aged animals to target those conditions most relevant to the human stroke population. To this end, we conducted all studies in aged (18–20 months) mice. Given the above, we tested the hypothesis that less toxic microbiota from young mice could improve outcomes in aged mice even when FTG is delayed for three days after experimental stroke.
A second component of this study investigates short-chain fatty acid (SCFA)-producing bacteria and the SCFAs they produce. SCFAs, which are generated by bacteria metabolizing non-digestible fiber in the gut,10 serve to stabilize the gut through activation of G protein-coupled receptors and inhibition of histone deacetylases.11 We have previously shown that fecal SCFAs decrease ~70% in aged mice and in the feces of young mice after FTG with a fecal suspension from aged mice.4 Therefore, we tested the hypothesis that we could restore SCFAs concentrations through probiotics and prebiotics and improve outcomes in aged mice following experimental stroke.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed methods are available in the Online Data Supplement.
RESULTS
Gut bacterial profiles in recipient aged stroke mice replicate the donor profile.
Aged male mice (18–20 months) were subjected to a 60-minute transient middle cerebral artery occlusion (MCAO) and gavaged with streptomycin (Abx) for two consecutive days. On days 3 and 4 after stroke (days 1 and 2 after Abx treatment), fresh fecal microbiota from non-stroke young mice (2–3 months; young FTG) or non-stroke aged mice (18–20 months; aged FTG) were transplanted by oral gavage. Antibiotics were used to reduce the bacterial load allowing for more efficient colonization of newly introduced bacteria (P=0.0002; Online Figure I). Behavioral testing was conducted on days 3, 7 and 14 after stroke.
First, we profiled the gut bacterial composition after both FTGs using 16S rRNA sequencing. Linear discriminant effect size (LEfSe) analysis revealed that the composition of the gut microbiota between young and aged donor mice had significant differences as shown in the cladogram (Figure 1A). Additionally, we found that members of Bifidobacteriaceae, a family in the Actinobacteria class (e.g., Bifidobacterium and Bifidobacteriaceae), and members of Clostridiaceae, a family in the Clostridia class (e.g., Clostridium and Clostridiaceae), were enriched in young microbiota. Importantly, data including principal coordinates analysis (PCoA) plots on unweighted and weighted UniFrac revealed that the microbiome of transplanted mouse recipients reflected the microbiome of the donors 10–11 days after FTG [Figure 1B (P=0.042 for unweighted and P=0.007 for weighted UniFrac) and 1C]; whereas FTG without antibiotics did not affect gut microbiota profiles in recipient mice (Online Figure I). Aged stroke mice colonized with young microbiome contained lower abundances of Bacteroides and Prevotella, potential pathobionts,12–14 when compared to those gavaged with aged FTG (Online Figure I).
Figure 1. Composition of the fecal microbiota of young and aged donor mice and clustering in recipient aged stroke mice.
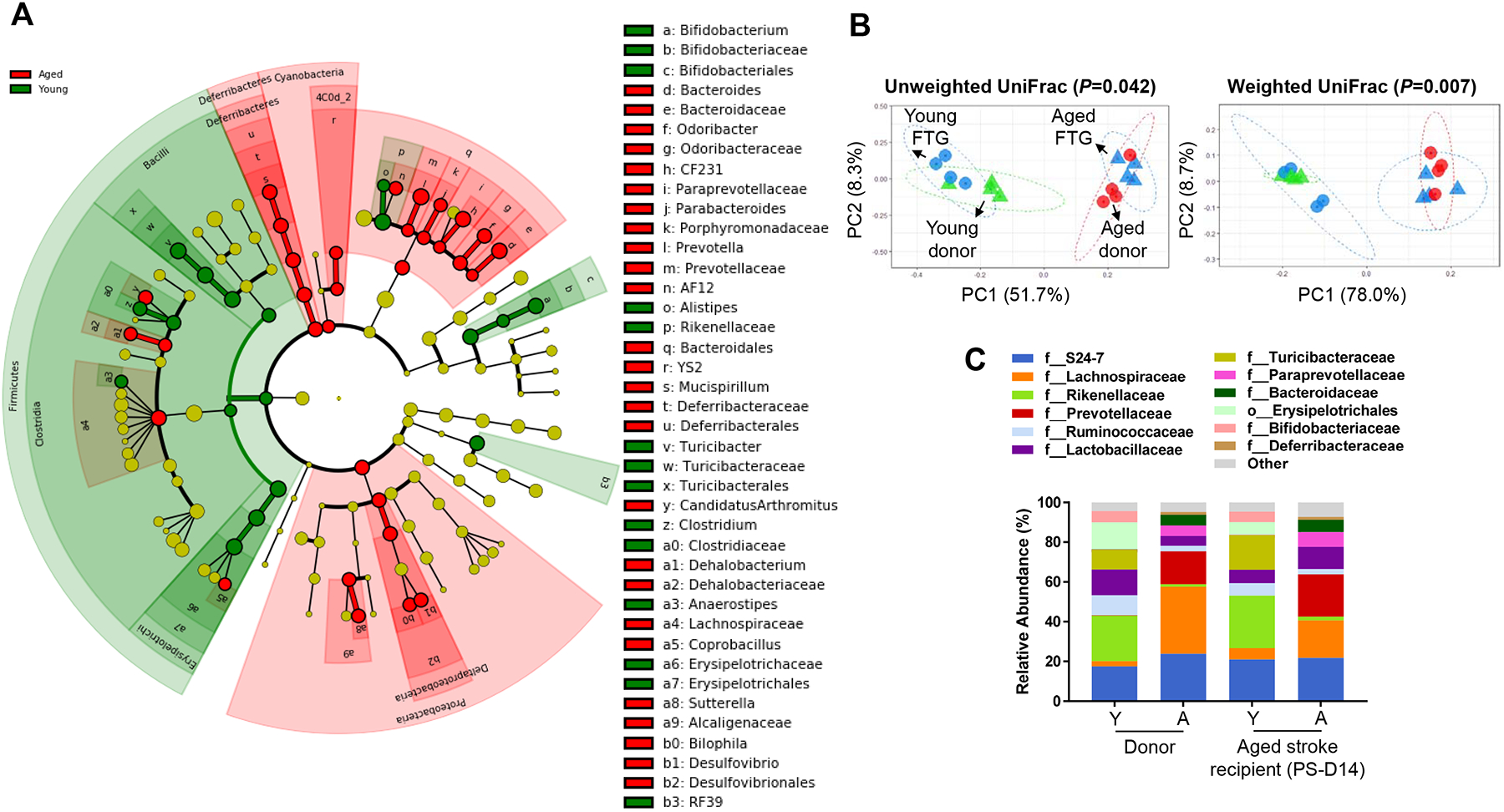
A, Overall representation of bacterial profiles in young (2–3 months) and aged (18–20 months) donor mice at the baseline, by linear discriminant effect size (LEfSe) analysis (n=4 per group). B, Principal coordinates analysis (PCoA) of fecal microbiota from the donor (young: 2–3 months, aged: 18–20 months) and recipient stroke mice (18–20 months) with fecal transplant gavage (FTG) using unweighted (R2=0.2567) and weighted (R2=0.4562) UniFrac distances (n=4 per group). C, Bacterial composition at the family level in transplanted recipients cluster according to the donor profiles at day 14 post-MCAO (n=4 per group).
Post-stroke restoration of youthful microbiota improves behavioral recovery in aged mice independent of infarct size.
To assess the impact of a youthful gut microbiota, we first evaluated the physiological changes in aged recipient mice engrafted with young or aged FTG by day 14 after stroke (Figure 2A). Stroke patients and mice after MCAO experience physiological changes such as weight loss.1, 15 Mice in our study lost weight (~11%) by day 3 after stroke. By day 14 after MCAO, aged mice with young biome exhibited recovery to their pre-stroke body weight (~3% weight gain), while aged FTG mice did not return to their original body weight after 14 days (Figure 2B). However, no statistical difference between the groups was observed.
Figure 2. Post-stroke transplanting of young fecal microbiome improves behavior in aged mice.
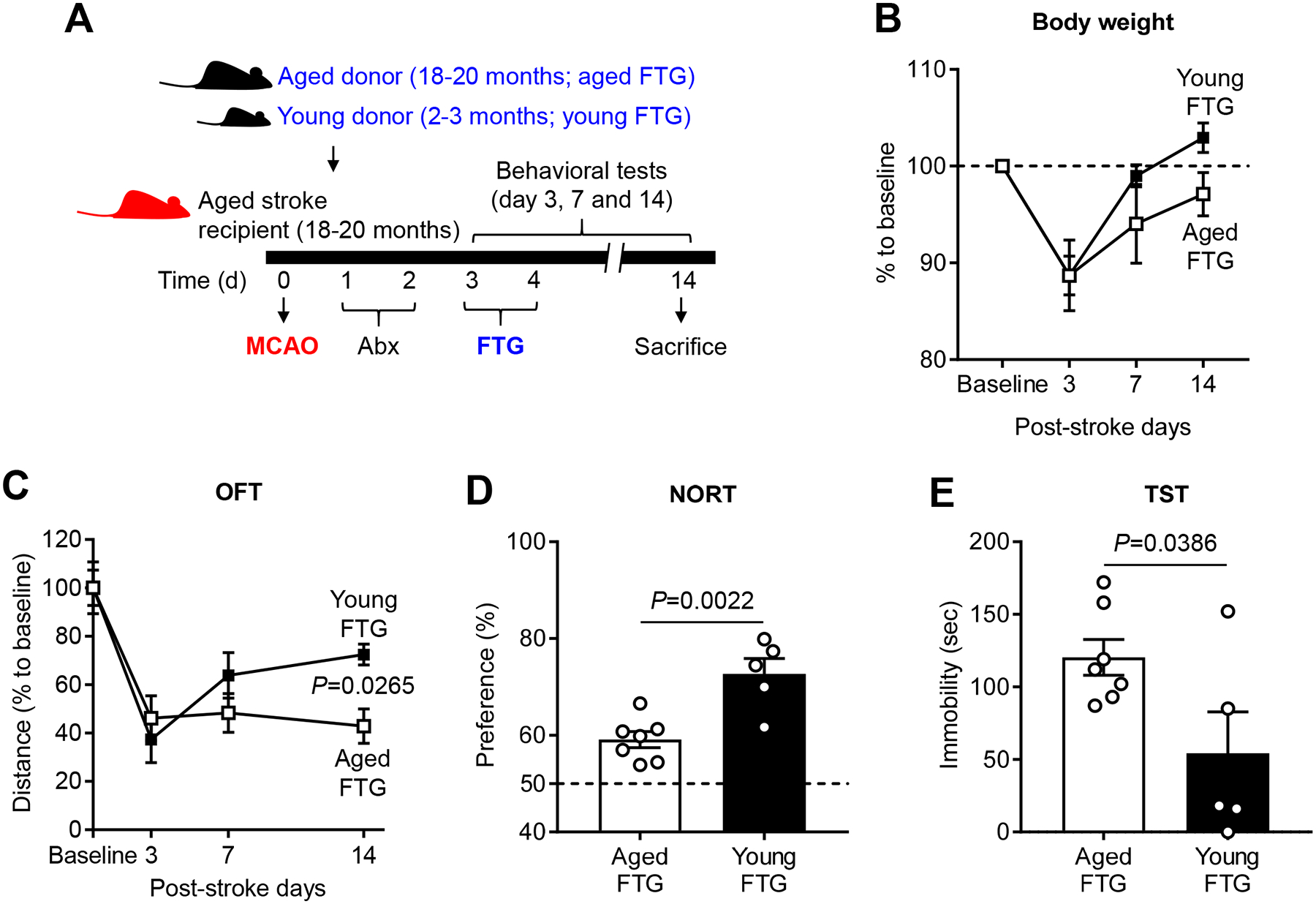
A, The experimental protocol for FTG from young (2–3 months) and aged (18–20 months) donor mice into recipient aged stroke mice (18–20 months). Changes of body weight (B), total locomotor activity using open field test (OFT, C), cognitive function using novel objective recognition test (NORT, D) and depression-like behaviors using tail suspension test (TST, E) after young (n=5) and aged FTG (n=7) on day 14 following MCAO. Throughout, error bars represent mean±SEM. For the repeated measurement study, a linear mixed model was used to account for within-subject correlation (B and C). Group comparisons were further performed at each time adjusted for multiple testing. For two-group comparisons, Student’s t test was used after the normality of data was confirmed by the Shapiro-Wilk normality test (D and E).
To test whether a young microbiota improved outcomes after stroke in aged mice, we performed a battery of behavioral tests including: (1) open field test (OFT) for spontaneous locomotor activity; (2) novel objective recognition test (NORT) for cognitive function; (3) tail suspension test (TST) for post-stroke depressive-like phenotypes; (4) hang wire test for grip strength; and (5) neurological deficit score (NDS) as a measure of overall post-stroke deficits. At day 14 after stroke, we did not observe a significant difference in the NDS or in the hangwire test between the groups (Online Figure II). However, aged stroke mice with young FTG demonstrated improved locomotor activity in OFT (P=0.0265; Figure 2C) and better post-stroke cognition in the NORT (P=0.0022; Figure 2D) as compared to aged mice transplanted with aged FTG. We further determined the effect of FTG on post-stroke depressive phenotypes, which is a common psychiatric comorbidity after stroke.15, 16 Interestingly, young microbiota significantly reduced immobility in the TST, which is suggestive of anti-depressive-like effect (P=0.0386; Figure 2E). Importantly, there was no significant difference in infarct damage, as measured by brain atrophy at 14 days post-stroke, between the young microbiome transplanted group (19.7±3.1%) compared to aged FTG mice (20.3±2.5%) (Online Figure II). These findings demonstrate that the improved stroke outcomes seen in aged mice with young FTG in terms of activity (OFT), memory (NORT) and depression-like behavior (TST) are independent of infarct size.
Post-stroke young FTG confers a protective phenotype in intestinal T cells and enhances gut integrity.
In the gut, microbes shape the local immune system by communicating with several types of T lymphocytes especially the regulatory T (Treg) cells found in the intestinal lamina propria (LP), which are anti-inflammatory and suppress immune responses.17–20 To determine if externally transplanted microbiome can modulate intestinal Treg cells, we first analyzed LP of small (SI) and large intestines (LI) after FTG in stroke mice. Flow cytometry analysis revealed that aged stroke mice with young FTG did not have a significant statistical difference in the frequency of CD4+ T cells in either the SI or LI LP. However, we found that young FTG significantly increased CD4+Foxp3+ Treg cells in the SI LP (P=0.0362), but not the LI LP, as compared to aged stroke mice with aged FTG (Figure 3A and 3B). Levels of CD3+ T cells (T cell lineage), CD8+ cytotoxic T cells, CD19+ B cells, NK1.1+ natural killer cells, Th17 cells and γδ T cells did not differ between young and aged FTG groups (Online Figure III). These data indicate that intestinal T cells in aged stroke mice acquired a regulatory phenotype after the introduction of youthful microbiota via FTG.
Figure 3. Young FTG increases intestinal regulatory T (Treg) cells in the lamina propria (LP) and enhances mucin productions in the epithelium of aged stroke mice.
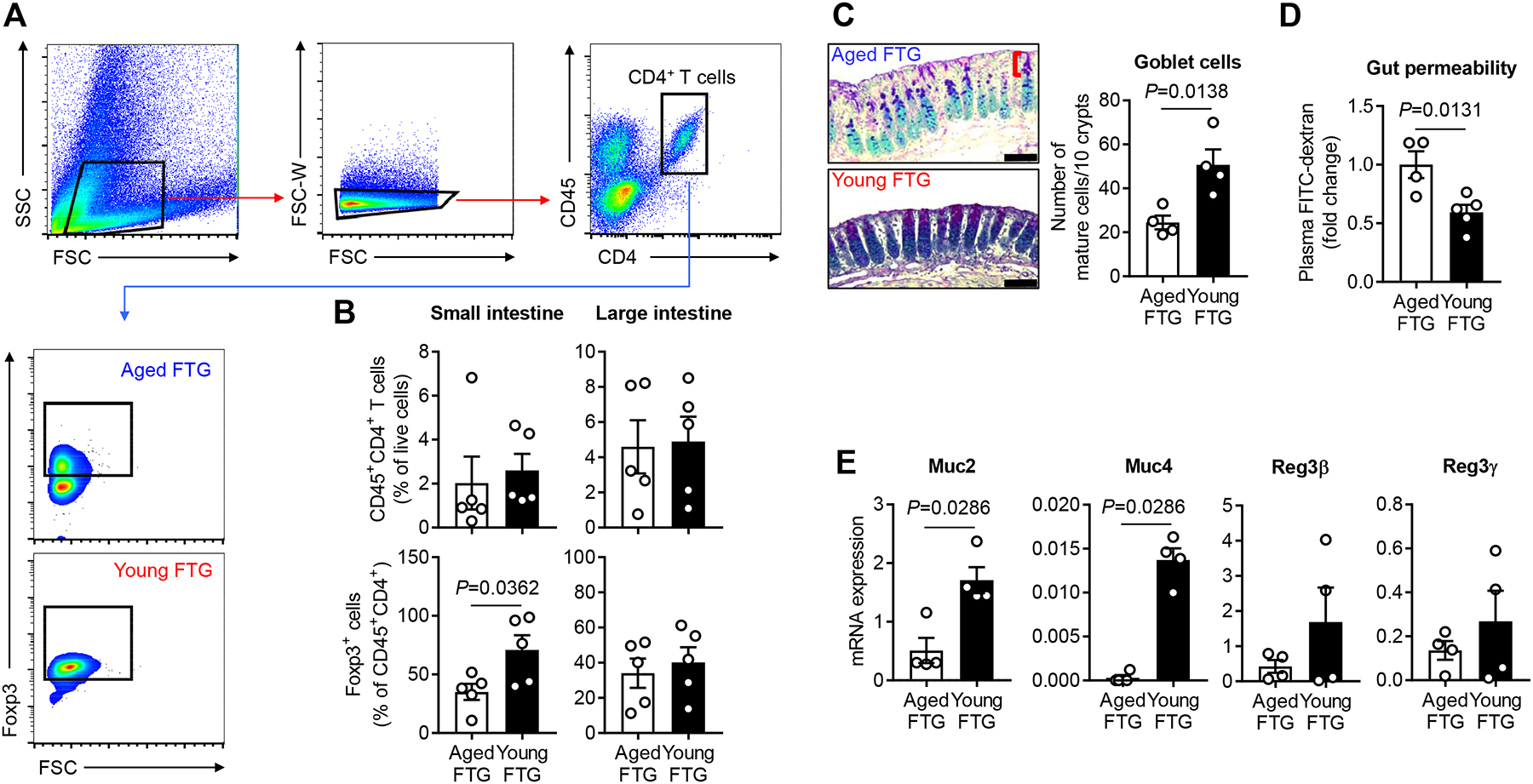
A, Representative flow cytometry plots to identify Treg cells in the intestinal LP of aged stroke mice. CD45+CD4+Foxp3+ cells in the LP of both small intestine (SI) and large intestine (LI) were gated and analyzed as Treg cells. An amine reactive Live/Dead Aqua viability stain was used to identify live and dead cells. Only live cells were gated for the analysis. Graphs represent percentages of Foxp3+ cells of CD4+ T cells in the SI LP. B, Flow cytometric analysis of CD4+ T cells and Treg cells in the intestinal LP of aged stroke mice at day 14 after MCAO (n=5 per group). C, Alcian blue and periodic acid-Schiff (AB-PAS) staining of the LI of aged stroke mice at post-MCAO day 14 (n=4 per group) and the number of mature goblet cells per 10 upper crypts/mouse are quantified. The bracket indicates the upper crypt possessing mature cells. Scale bars, 50 μm. D, FITC-dextran intestinal permeability assay at day 14 after MCAO in aged mice with aged (n=4) and young FTG (n=5). Data were normalized to aged FTG controls. E, The relative mRNA abundance for epithelial mucin genes and Reg3 genes of the LI in aged stroke mice at post-MCAO day 14 (n=4 per group). Throughout, error bars represent mean±SEM. Student’s t test (B, C and D) and Mann-Whitney U test (E) were used based on the normality of data assessed by the Shapiro-Wilk normality test.
Next, we sought to elucidate interactions between transplanted microbiome and the intestinal epithelial cell layer in aged stroke mice as this layer is in contact with luminal microbiota. In the intestine, these epithelial layers maintain homeostasis and play a central role in inflammation by allowing the host’s immune cells to interact with the microbiota in the lumen.21, 22 The mucus layer, a protective net-like polymer formed by goblet cell-producing mucins (Muc), covers the gut epithelium to protect the host.23 Histochemical staining using alcian blue and periodic acid-Schiff (AB-PAS) showed that young FTG enhanced the number of mature goblet cells in upper crypts which produce mucins in the LI (P=0.0138; Figure 3C). Further, we found that the intestinal barrier integrity at post-stroke day 14 was increased in mice with young FTG compared to aged FTG, as reflected by decreased efflux of circulating fluorescein isothiocyanate (FITC)-dextran (P=0.0131; Figure 3D). Next, we isolated epithelial cells (ECs) from the LI from mice that received FTG from young and aged donors. We examined mucin gene expression by investigating Muc2 and Muc4, which are responsible for inner and outer layers of mucus. Compared to aged stroke mice that received aged FTG, aged stroke mice with young FTG showed a significantly higher level of Muc2 (3.4-fold increase, P=0.0286) and Muc4 expression (44.8-fold increase, P=0.0286) (Figure 3E). Next, we examined changes in epithelial-antimicrobial peptides (AMPs), since transplanted fecal material including potential pathogens can provoke unwanted immune responses in the host epithelium. We isolated LI ECs and tested the gene expression of regenerating islet-derived protein (Reg) family-specific AMP proteins Reg3β and Reg3γ. We observed no significant difference in Reg3 gene expression between young and aged FTG groups (Figure 3E). Taken together, these data suggest that young FTG transplantation was protective in both the SI and LI of aged animals after stroke.
Post-stroke young FTG increases Treg cells and decreases IL-17+ γδ T cells in the aged brain.
Next, we addressed whether young FTG can alter populations of Treg cells in the aged stroke brain, which we observed in the SI. Interestingly, flow cytometry analysis showed that young FTG significantly enhances Foxp3 expression in CD4+ T cells in the ipsilateral stroke hemisphere of aged stroke mice compared to aged FTG groups (P=0.0190; Figure 4A and 4B). This data indicates a potential role of youthful microbiome in regulating neuroinflammation after stroke in aged mice.
Figure 4. Young FTG increases Treg cells and reduces IL-17 production of γδ T cells in the brain of aged stroke mice.
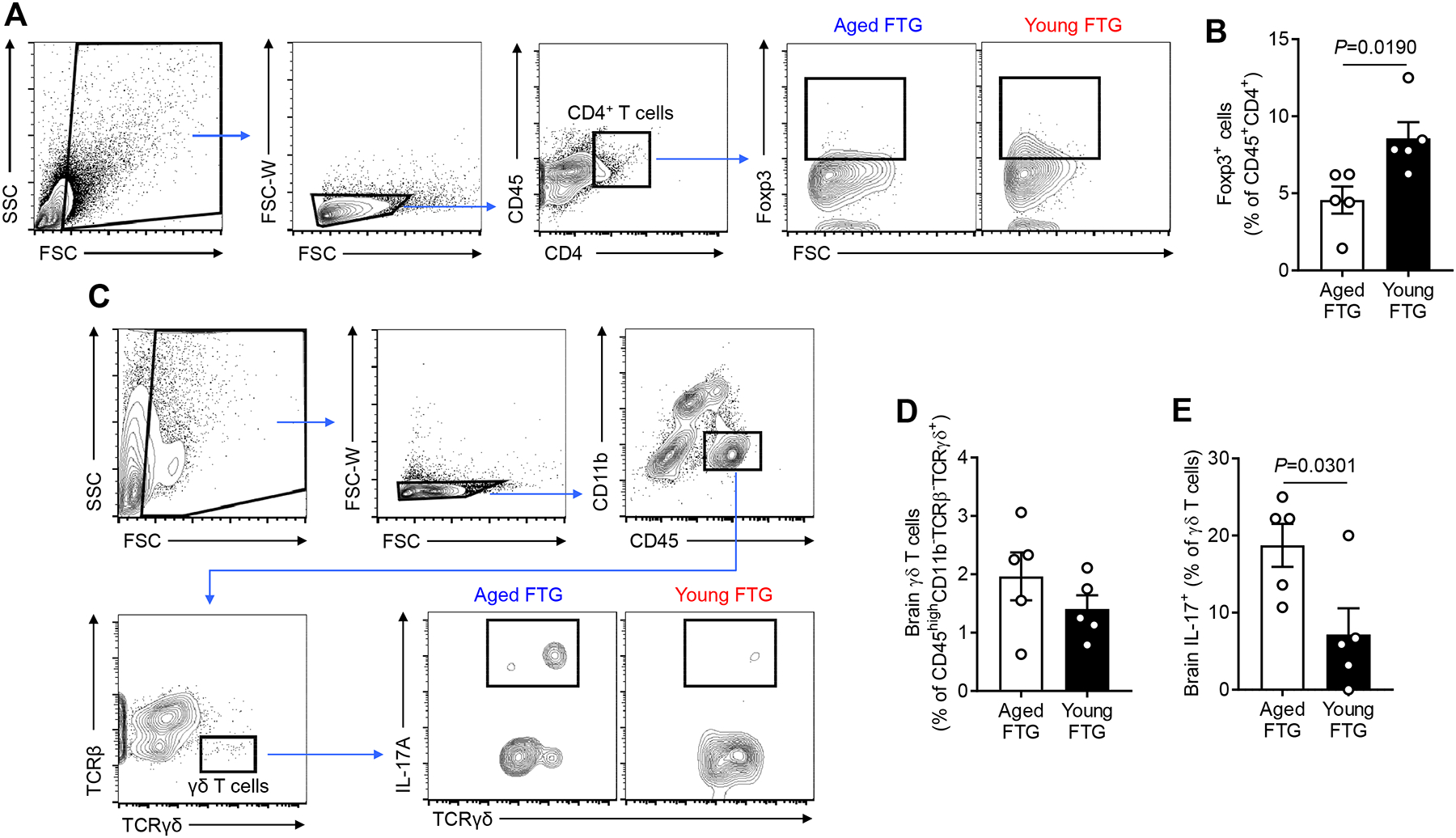
A, Representative flow cytometry plots to identify Treg cells (CD45+CD4+Foxp3+) in the brain of aged stroke mice. B, Flow cytometric analysis of Treg cells in the brain of aged stroke mice at day 14 after MCAO (n=5 per group). C, Representative flow cytometry plots to identify γδ T cells (CD45highCD11b−TCRβ−TCRγδ+) in the brain of aged stroke mice. IL-17+ γδ T cells (CD45highCD11b−TCRβ−TCRγδ+IL-17+) were analyzed at post-MCAO day 14. An amine reactive Live/Dead Aqua viability stain was used to identify live and dead cells. The ipsilateral stroke hemisphere was used for the cell isolation and only live cells were gated for the analysis for both (A) and (C). Flow cytometric analysis of brain γδ T cells (D) and IL-17+ γδ T cells (E) of aged stroke mice at day 14 after MCAO (n=5 per group). Throughout, error bars represent mean±SEM. Student’s t test (B, D and E) was used after the normality of data was confirmed by the Shapiro-Wilk normality test.
A recent study has shown that the meninges contain a repertoire of immune cells, especially γδ T cells, which are involved in stroke-induced brain inflammation.2 γδ T cells play a pivotal role in aggravating injury after brain ischemia by secreting IL-17.24, 25 Thus, we asked whether FTG altered γδ T cells in the brain after stroke. At day 14 after stroke (i.e., post-FTG day 10), we did not observe any difference in the frequency of γδ T cells (CD45highCD11b−TCRβ−TCRγδ+) in the ipsilateral stroke hemisphere of aged stroke mice that received young FTG as compared to aged stroke mice that received aged FTG (Figure 4C and 4D). However, the IL-17 protein expression of γδ T cells (CD45highCD11b−TCRβ−TCRγδ+IL-17+) was lower in aged stroke mice with young FTG compared to aged mice treated with aged FTG (2.6-fold, P=0.0301; Figure 4E). No significant differences in the frequency of other brain cells, including microglia (CD45intCD11b+ cells) and myeloid-lineage cells (CD45+CD11bhigh cells) (Online Figure IV) were observed. However, the immunohistochemistry analysis revealed the reduction of microglial activation, i.e., decreased Iba1 and increased P2RY12 expression, in the young FTG group compared to aged FTG-treated mice brains (Online Figure IV). The observed sensitivity of P2RY12 expression in our young and aged FTG groups is consistent with prior studies reporting a downregulation of P2RY12 by activated microglia in response to various central nervous system injury models in mice and humans.26–29 Future investigations will be required to elucidate molecular mechanisms that mediate the effects of aged microbiome on P2RY12 expression by microglia. Taken together, our data supports that the beneficial effects of young FTG in aged stroke mice is mediated through peripheral (i.e., gut-related) mechanisms and through modulation of central immunity by regulating the inflammatory response in the brain.
Post-stroke oral gavage with SCFA-producers improves recovery in aged mice.
Based on our previous findings showing that pre-treatment of young biome containing higher SCFAs contributes to better stroke outcomes in recipient mice compared to an aged biome with less SCFAs,4 we hypothesized that increasing SCFAs post-stroke could improve recovery. To this end, we first determined if SCFAs differed in fecal samples used for FTG in this study. We measured fecal SCFA concentrations in naïve young mice (2–3 months) and aged mice (18–20 months) and assessed the concentrations of primary metabolites including acetate, propionate and butyrate. Mass spectrometry analysis revealed an inverse relation between aging and SCFA concentrations: the amount of some fecal SCFAs including acetate (P=0.0010), propionate (P=0.0002) and butyrate (P=0.0133) significantly decreased with age (Figure 5A). These data prompted us to test if augmentation of selective SCFA-producing bacteria (SCFA-producers) could be a viable post-stroke treatment option to reduce functional impairments in aged mice.
Figure 5. Fecal SCFA concentrations decrease with age and the combined treatment of SCFA-producing bacteria and prebiotic inulin after stroke ameliorates behavioral disorders in aged mice.
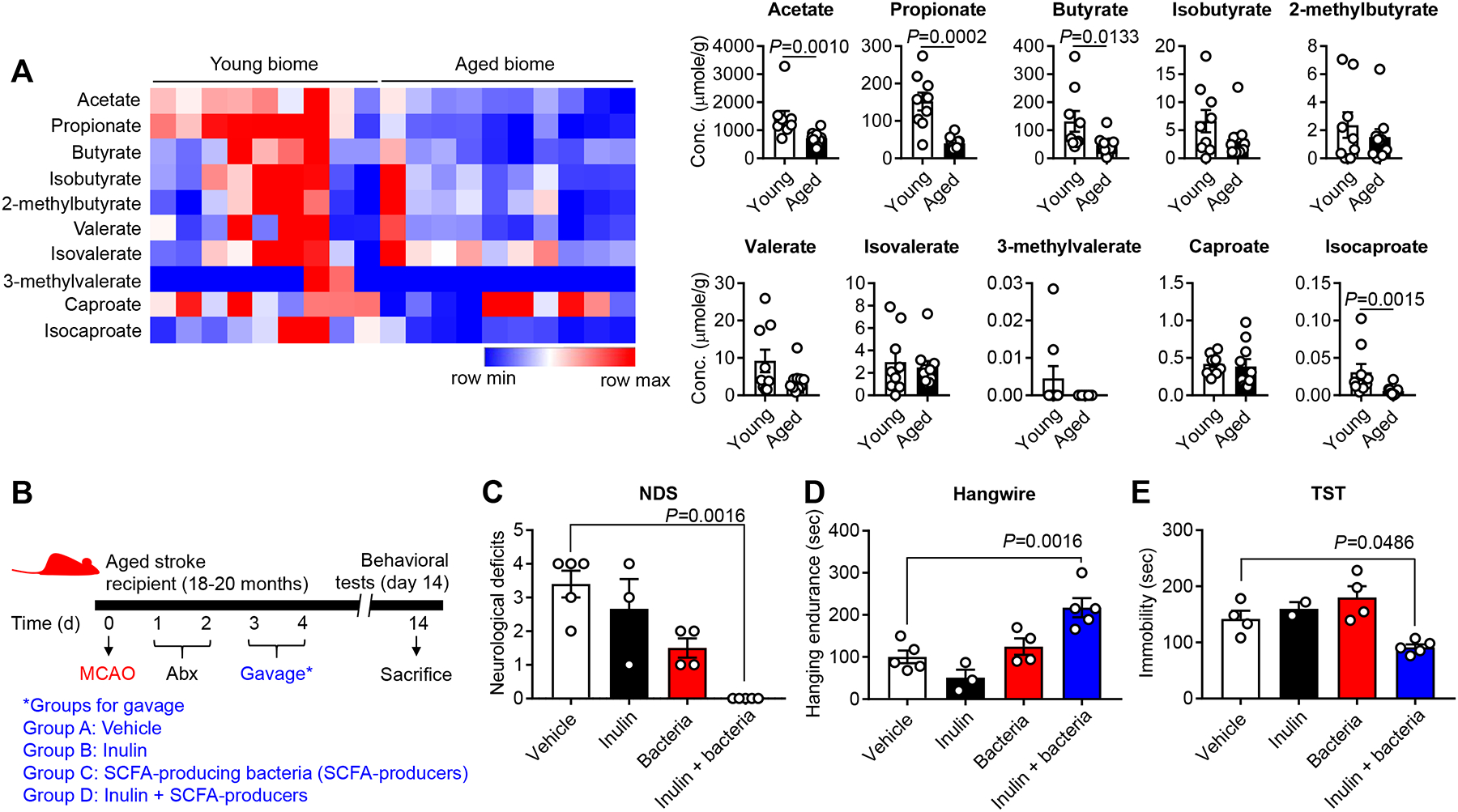
A, Aging decreases the concentration of primary SCFAs in fecal samples of young (2–3 months) and aged (18–20 months) mice. Fecal samples were collected and analyzed using mass spectrometry. Graphs represent changes of individual SCFAs (young biome, n=9; aged biome, n=10). B, To examine the effect of inulin and SCFA-producers on stroke outcome, four separate groups of mice were orally gavaged with 1) vehicle, 2) inulin, 3) SCFA-producers and 4) inulin+SCFA-producers. For SCFA-producers, the cocktail of Bifidobacterium longum (1×107), Clostridium symbiosum (5×106), Faecalibacterium prausnitzii (1×106) and Lactobacillus fermentum (1×109) were freshly cultured and gavaged to mice. Neurological deficit score (NDS) (C), hangwire test (D) and TST (E) were performed at day 14 after MCAO (vehicle, n=5; inulin alone, n=3; SCFA-producers alone, n=4; inulin+SCFA-producers, n=5). For the TST, two mice from vehicle- and inulin-treated group (one for each group) which showed spinning due to severe neurological deficits were excluded. Overall P value for NDS, hangwire test and TST was 0.0001, 0.0004 and 0.0036, respectively. Throughout, error bars represent mean±SEM. For group comparisons, Mann-Whitney U test (A) and ordinary one-way ANOVA or Kruskal-Wallis test (C, D and E) were used based on the normality of data assessed by the Shapiro-Wilk normality test, followed by Dunnett’s multiple comparison test.
To increase SCFA concentrations, we treated mice post-stroke with a prebiotic (inulin) and a cocktail of four primary SCFA-producing bacterial strains (Bifidobacterium longum, Clostridium symbiosum, Faecalibacterium prausnitzii and Lactobacillus fermentum). Notably, two genera, Bifidobacterium and Clostridium, were identified as a microbial signature for young microbiome, compared to aged microbiome (Figure 1A). Inulin, a soluble fiber that can reach the colon in an intact state due to its resistance to enzymatic degradation in the upper gut and SI, is a substrate that supports enhanced SCFA production by the above bacteria.30 We employed a similar protocol (Figure 5B) to that used for the FTG studies (Figure 2A).
To test our hypothesis, we first examined the effects of inulin and SCFA-producers on behavioral outcomes after stroke, using four separate groups of mice treated with: 1) vehicle (sterile culture media), 2) inulin alone, 3) SCFA-producers alone and 4) the combination of inulin+SCFA-producers. Aged stroke mice with inulin+SCFA-producers had significantly improved neurological deficit scores by day 14 post-stroke compared to the vehicle-treated control animals (P=0.0016; Figure 5C). In addition, this group of mice had significant improvements in their motor coordination and strength in the hangwire test, when compared to vehicle controls (P=0.0016; Figure 5D). Similar to the effect seen after young FTG, SCFA-producers with inulin had a significant anti-depressive effect in aged stroke mice (P=0.0486; Figure 5E). Interestingly, we did not find significant behavioral benefits in aged stroke mice given either inulin alone or bacteria alone suggesting a synergistic effect of inulin with bacteria.
Brain IL-17+ γδ T cells is reduced in aged stroke mice with SCFA-producers and inulin.
Next, we assessed whether the oral gavage with SCFA-producers and inulin had a similar anti-inflammatory effect in the brain of aged stroke mice to that seen in young FTG-treated mice. We focused on the change in IL-17+ γδ T cells and analyzed brain immune cells using flow cytometry with the same gating strategy used in the FTG study (Figure 4C and 6A). Interestingly, we found that bacteria and inulin treatment significantly reduced the IL-17+ γδ T cells, not γδ T cells, at post-stroke day 14 (Figure 6B to 6D). Brain atrophy at 14 days post stroke was not different between the groups (19.2% vs. 16.1%, data not shown).
Figure 6. Transplanting SCFA-producers reduces brain IL-17 production of γδ T cells in the aged stroke mice.
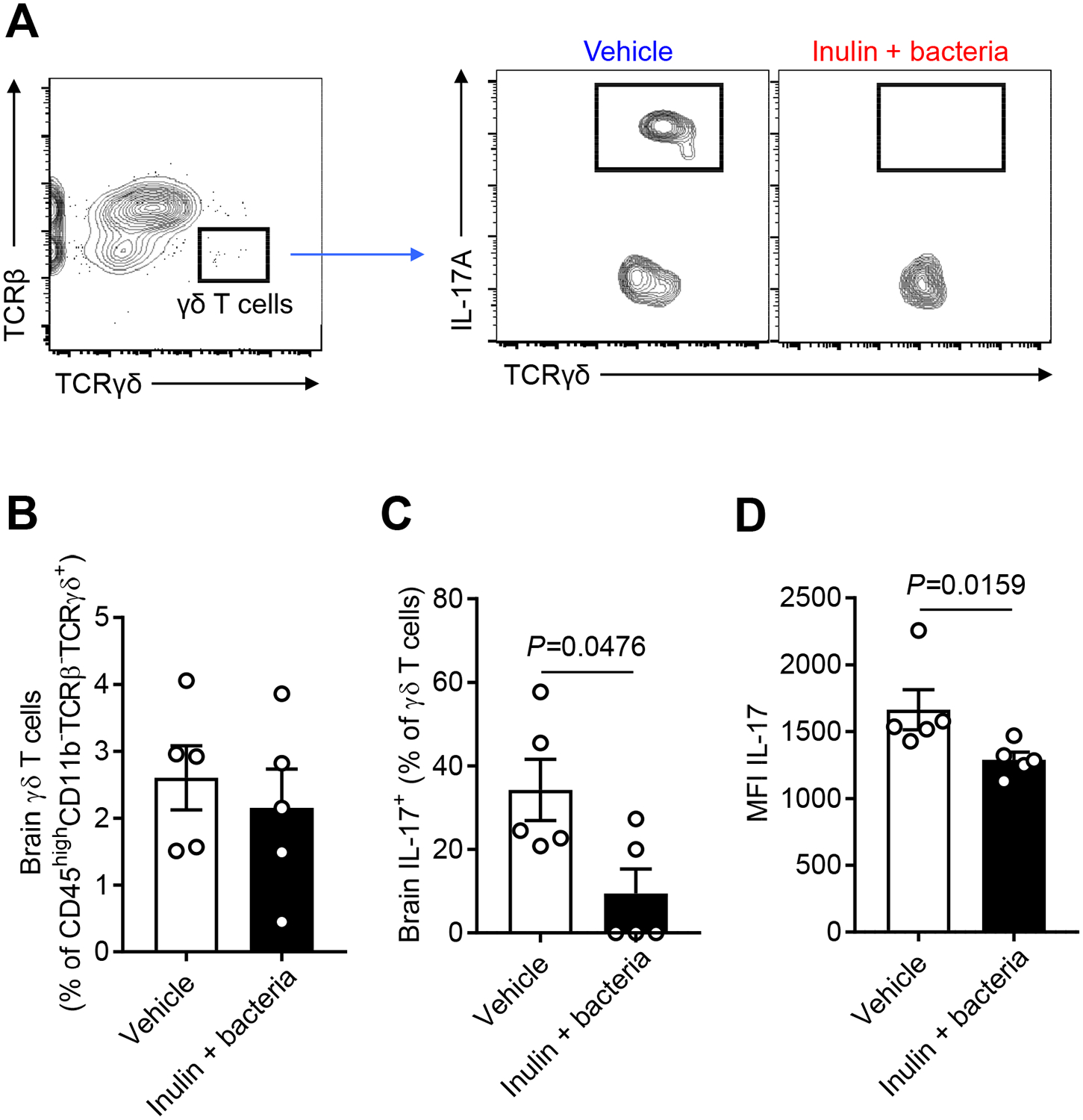
A, Representative flow cytometry plots to identify γδ T cells in the brain (ipsilateral stroke hemisphere) of aged stroke mice. The same gating strategy used in FTG study was employed. Brain γδ T cells (B) and IL-17+ γδ T cells (C) of aged stroke mice were analyzed at day 14 after MCAO (n=5 per group). The IL-17 median fluorescence intensity (MFI) was calculated (D). Throughout, error bars represent mean±SEM. Mann-Whitney U test (B, C and D) was used because the normality of data was rejected by the Shapiro-Wilk normality test.
Enhanced gut SCFAs alters systemic and brain SCFA profiles in aged stroke mice.
We hypothesized that the beneficial bacterial strains directly contributed to the maintenance of intestinal integrity. Mice treated with SCFA-producing bacteria and inulin had a healthier anatomic profile compared to stroke-induced gut injury occurring in the absence of SCFA-producing bacteria (Online Figure V). We then tested if SCFAs could stimulate ECs including goblet cells to synthesize protective mucins.31, 32 Immunohistochemistry for Muc2 proteins showed that aged stroke mice receiving SCFA-producers and inulin had significantly enhanced Muc2 proteins expressed in goblet cells of the LI, along the epithelial lining in the crypts, compared to the vehicle-treated group (P=0.0339; Online Figure V).
Finally, we profiled changes in SCFAs in the gut of aged stroke mice that received SCFA-producers and inulin by metabolomic analysis from fecal samples. The primary metabolites that were increased in this group were acetate (P=1.2E-08), propionate (P=0.0003), butyrate (P=0.0140), isobutyrate (P=0.0033) and 2-methylbutyrate (P=8.9E-06), when compared to the vehicle group (Figure 7). In addition, as shown in Online Figure VI, treatment with SCFA-producing bacteria and inulin increased the fecal concentrations of valerate (P=0.0205), isovalerate (P=0.0071), 3-methylvalerate (P=0.0003), caproate (P=2.0E-09) and isocaproate (P=0.0003). Thus, we questioned whether this increase in gut SCFAs can result in a concomitant increase of brain and circulating blood SCFA levels. Surprisingly, the mice that showed increased fecal SCFAs had significantly higher levels of acetate (P=0.0212), butyrate (P=5.3E-05), isobutyrate (P=0.0025), 2-methylbutyrate (P=0.0025), valerate (P=0.0004), isovarelate (P=0.0025), 3-methylvalerate (P=0.0177), caproate (P=0.0019) and isocaproate (P=0.0248) in the brain (Figure 7 and Online Figure VI). In plasma from the same mice, the concentrations of acetate (P=0.0203), propionate (P=0.0246), butyrate (P=0.0003), 2-methylbutyrate (P=0.0059), isovalerate (P=0.0037), 3-methylvalerate (P=0.0093) and caproate (P=0.0140) were higher in the bacteria-treated group compared to the vehicle group (Figure 7 and Online Figure VI). Taken together, this suggests that SCFAs might be the key component from the young microbiome that benefits post-stroke recovery in aged mice.
Figure 7. SCFA profiles in SCFA-producers-treated aged stroke mice.
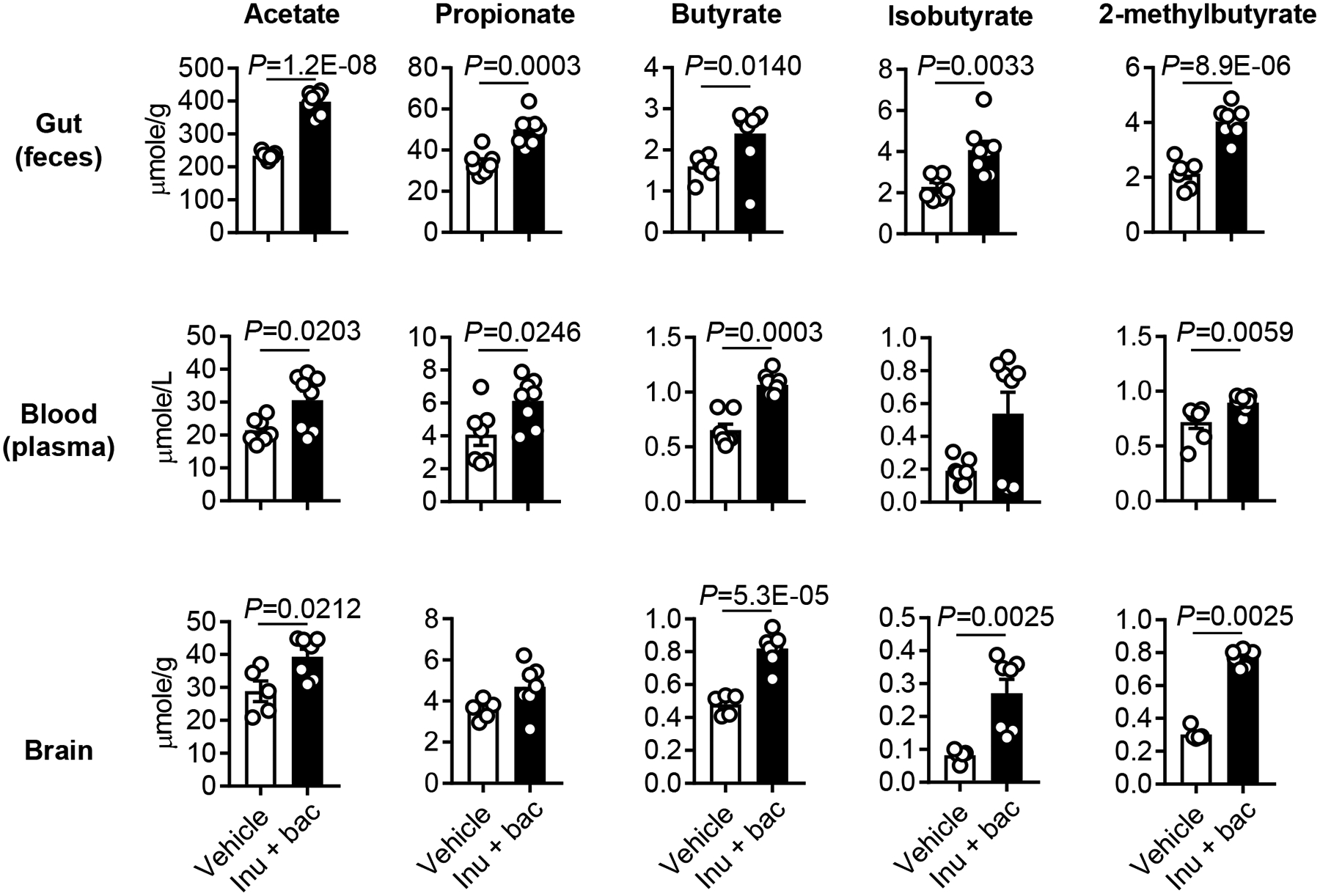
At day 14 after MCAO and Abx (days 1 and 2) and inulin+bacteria (days 3 and 4) treatment, the samples were collected from aged stroke mice. Changes of fecal (vehicle, n=7; inulin+bacteria, n=8), plasma (vehicle, n=7; inulin+bacteria, n=8) and brain (vehicle, n=5; inulin+bacteria, n=7) metabolites were assessed using mass spectrometry. Throughout, error bars represent mean±SEM. Mann-Whitney U test (fecal butyrate, plasma butyrate, isobutyrate and 2-methylbutyrate and brain isobutyrate and 2-methylbutyrate) and Student’s t test (other metabolites) were used based on the normality of data assessed by the Shapiro-Wilk normality test.
DISCUSSION
In this study, we determined that FTG of young microbiota significantly improved outcomes in aged mice, even when the FTG was performed several days following experimental stroke. This protection is related to the enhancement of the integrity of the gut barrier and attenuation of the inflammatory response in both the gut and brain. A decrease in bacterially-derived SCFAs in the aged microbiome is in part responsible for the enhanced immune state and poorer outcomes after stroke seen in aged mice. Supporting this idea is the finding that stroke outcomes significantly improved after SCFAs were restored to the levels seen in the young microbiome using SCFA-producing bacteria (probiotics) and a food source (prebiotic) for these bacteria. Of significance, these effects on outcome were not related to the chronological age of the mice but were related to the “age” of the microbiome.
We found that post-stroke transplantation of youthful microbiota into aged mice had a number of beneficial effects on behavioral recovery. Importantly, to control for infarct volumes between groups, we delayed altering the microbiota composition until 72 hours after stroke, at which point the histological infarct is mature in this model. This strategy specifically addressed the effects on recovery. Our data showed that these beneficial effects of young FTG were independent of infarct volume since no differences in infarct size was observed between the FTG groups at post-stroke day 14. Second, the toxicity of an aged microbiome is related to an enhanced immune response by the host. Gut microbes are known to program CD4+ T cells to differentiate into anti-inflammatory regulatory T (Treg) cells or pro-inflammatory effector T cells.33–35 In our study, a young biome augmented the frequency of intestinal Treg cells compared to an aged microbiome 14 days after stroke.
An important finding of this study is the reduction of brain IL-17+ γδ T cells seen after young FTG in aged mice after stroke. Meningeal IL-17-producing γδ T cells of intestinal origin have been implicated in the severity of stroke outcome.2 They are normally present in small numbers in the circulating blood and account for 3–5% of all circulating T lymphocytes.36 γδ T cells also occupy highly unique niches both in innate and adaptive immunity; they respond to perturbations earlier than other types of T cells such as αβ T cells37 as they do not require specific T cell receptor (TCR) activation. Strikingly, aged mice given young FTG had significantly decreased IL-17+ γδ T cells in the brain after stroke, indicating an attenuation of brain inflammation. We did not observe a reduction in proinflammatory γδ T cells in the gut after stroke, which has been reported previously in young mice.2 This discrepancy is possibly due to the different age of mice used in this study. Of note, we have shown that aging significantly alters the immune response to stroke.1, 38 In conclusion, this data provides a proof-of-concept that targeting the aged gut by youthful microbiota transplantation can improve both gut and brain health.
The gut epithelium consists of a single layer of ECs that are responsible for mucosal immunity and homeostasis and play an integral role in the dialogue with luminal bacteria and antigens.21 Goblet cells are a unique cell population in the epithelium and exclusively produce mucins on the apical side of the gut to form a physical barrier between the lumen and host tissues.23 Importantly, aging is associated with the altered production of mucus.39 Our previous finding has also shown the negative effects of age on gut permeability and stroke outcome.1 An intriguing finding in this study was that the replenishment of youthful biome restored the capacity of aged mice to enhance expression of the mucin genes, Muc2 and Muc4, in ECs of the LI. Thus, restoring mucins with probiotics would be a promising therapeutic option in other age-related diseases to protect against bacterial challenge.
A decrease in bacterially-derived SCFAs in the aged microbiome appears to be at least partially responsible for the enhanced immune state and poor outcomes after stroke. The conversion of luminal environments by antibiotics and dietary components (e.g., non-digestible fiber) into metabolites is an important factor in mucosal immunity and overall homeostasis of the gut.10, 32, 40 One class of these critical metabolites is the SCFAs, protective molecules which are decreased in aged mice or young mice with an aged microbiome. Thus, we chose to investigate microbiota-derived SCFAs as a potential mechanism by which youthful biome enhances recovery after stroke in aged mice. SCFAs and several other metabolites are known to be difficult to detect by currently available unbiased metabolic screening assays,41 but targeted LC-MS metabolomics analysis can accurately measure changes in SCFA concentrations. We employed targeted LC-MS metabolomics to analyze the major SCFAs, butyrate, propionate and acetate and found that the concentrations of these SCFAs were significantly reduced with age. This is consistent with clinical studies showing a decrease in acetate levels in fecal samples of stroke patients.42 We then determined if exogenous enhancement of SCFAs would replicate the benefits seen in aged mice given young biome transplants. To evaluate the effects of SCFAs on recovery, we transplanted a cocktail of SCFA-producing probiotics, B. longum, C. symbiosum, F. prausnitzii and L. fermentum and inulin into aged mice three days after stroke and compared these to controls given culture media and inulin or SCFA producers alone. SCFAs act locally at the mucosal layer to maintain gut function and barrier integrity, increase the protective mucus layer, alter T lymphocyte populations, modulate antibody secretion, and can potentially gain access to the systemic circulation to modulate cytokine secretion.10 SCFAs are also known to regulate leukocyte trafficking from the gut to external-intestinal tissues, e.g., the uvea.43 They have been reported to control brain microglia function and maturation, and are instrumental in development and maintenance of the blood-brain barrier.44 Furthermore, a recent study showed that SCFAs, e.g., propionic acid, play a potent immunomodulatory role in multiple sclerosis, by regulating the balance of T cells.45 We found that SCFAs were increased in the gut with inulin+bacteria, and ameliorated stroke-induced intestinal damage by stimulating ECs to produce mucin, which supports gut barrier function.40, 46 Importantly, transplanting bacteria with their substrate, inulin, into aged stroke mice was sufficient to improve neurological deficits, motor function, grip strength and depressive phenotypes when compared to mice treated with inulin alone, SCFA-producing alone, or vehicle. This suggests that providing bacteria with an appropriate substrate is needed to enhance behavioral recovery. Of note, it further indicates that newly introduced bacteria are viable in the recipient mice and are able to metabolize inulin. We then tested if bacteria from the gut can also alter brain levels of SCFAs. Strikingly, we found an increase in several SCFAs in both the brain and plasma of mice after gavage of SCFA-producers with inulin. Interestingly, it was recently found that intraperitoneal delivery of acetate-encapsulated liposomal nanoparticles protected rats from ischemic stroke. This strategy allows for a longer half-life, as acetate, as with other SCFAs, has a short half-life in the blood.47 Our approach, which replaced the bacteria with SCFA-producers and provided them with the substrate needed to produce SCFAs, is likely a more translational approach to enhance the durability of the production of these beneficial metabolites. One limitation of laboratory based microbiome studies is that all mice are raised in a clean and tightly controlled environment, their microbiome may not accurately represent the diversity of gut microbes seen in wild animals. Another limitation is that although we have performed multiple testing adjustment for post-hoc group comparisons, we did not adjust across the entire body of work, as this was an exploratory study.
In conclusion, our findings demonstrate that restoration of a youthful gut microbiome, even days after stroke, can reduce inflammation and enhance stroke recovery in aged animals. This is mediated in large part by bacterially produced metabolites, specifically SCFAs. Our study strongly supports the idea that targeting “bottom-up signaling” (gut to brain) can enhance post-stroke recovery in aged subjects. Our results indicate that post-stroke rejuvenation of gut microbiome in aged mice dramatically improves gut integrity and enhances host immunity. Finally, the beneficial effects of SCFA/probiotics may have direct benefit in other acute neuronal injury models. This study thus provides the first direct experimental evidence that microbiota composition can be therapeutically exploited to improve recovery after stroke.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Aging is a major risk factor for stroke.
Both stroke and aging induce gut dysbiosis.
Gut microbiota and microbial-derived metabolites are implied in various neurological diseases including stroke.
What New Information Does This Article Contribute?
Post-stroke microbiota reconstitution in aged mice using fecal transplant gavage (FTG) from young mice, which contains higher levels of short-chain fatty acid (SCFA)-producing bacteria, improves functional and behavioral outcomes.
Young FTG enhances gut integrity, expands regulatory T (Treg) cells in the gut and brain, and diminishes IL-17 production by brain γδ T cells after stroke.
Transplantation of selective SCFA-producing bacterial with the prebiotic inulin reproduces the beneficial effects of young FTG on stroke recovery in aged stroke mice.
ACKNOWLEDGMENTS
We thank all of the members in the BRAINS laboratory at UTHealth for technical assistance and discussion.
SOURCES OF FUNDING
This study was supported by grants from the NIH/NINDS RF1AG058463 (to L.D.M. and R.M.B.), R01NS103592 (to L.D.M. and R.M.B.), R01NS094543 (to L.D.M.), the American Heart Association 15SDG23250025 (to V.R.V.), NIH grant DK56338, which supports the Texas Medical Center Digestive Diseases Center (to V.R.V., D.J.D. and B.P.G.), and an award from the American Heart Association and the American Brain Foundation 19POST34410076 (to J.L.). The metabolomics core was supported by CPRIT Proteomics and Metabolomics Core Facility (to N.P.) (RP170005), NIH (P30-CA125123), and Dan L. Duncan Cancer Center.
Nonstandard Abbreviations and Acronyms:
- AMP
antimicrobial peptide
- EC
epithelial cell
- FTG
fecal transplant gavage
- LEfSe
linear discriminant effect size
- LI
large intestine
- LP
lamina propria
- MCAO
middle cerebral artery occlusion
- MGB
microbiota-gut-brain
- Muc
mucin
- NDS
neurological deficit score
- NORT
novel objective recognition test
- OFT
open field test
- PCoA
principal coordinates analysis
- Reg
regenerating islet-derived protein family
- SCFA
short-chain fatty acid
- SI
small intestine
- Treg
regulatory T cell
- TST
tail suspension test
Footnotes
DISCLOSURES
None.
SUPPLEMENTAL MATERIALS
REFERENCES
- 1.Crapser J, Ritzel R, Verma R, Venna VR, Liu F, Chauhan A, Koellhoffer E, Patel A, Ricker A, Maas K, Graf J, McCullough LD. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany NY). 2016;8:1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδt cells. Nature medicine. 2016;22:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. The Journal of Neuroscience. 2016;36:7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Annals of neurology. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, Wong CHY. Translocation and dissemination of commensal bacteria in post-stroke infection. Nature Medicine. 2016;22:1277. [DOI] [PubMed] [Google Scholar]
- 6.Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Scientific Reports. 2018;8:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durgan DJ, Lee J, McCullough LD, Bryan RM. Examining the role of the microbiota-gut-brain axis in stroke. Stroke. 2019;50:2270–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentsen L, Christensen L, Christensen A, Christensen H. Outcome and risk factors presented in old patients above 80 years of age versus younger patients after ischemic stroke. Journal of Stroke and Cerebrovascular Diseases. 2014;23:1944–1948 [DOI] [PubMed] [Google Scholar]
- 9.Saposnik G, Cote R, Phillips S, Gubitz G, Bayer N, Minuk J, Black S. Stroke outcome in those over 80. Stroke. 2008;39:2310–2317 [DOI] [PubMed] [Google Scholar]
- 10.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345 [DOI] [PubMed] [Google Scholar]
- 11.Pluznick JL. Microbial short-chain fatty acids and blood pressure regulation. Current Hypertension Reports. 2017;19:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruseler-van Embden JG, Both-Patoir HC. Anaerobic gram-negative faecal flora in patients with crohn’s disease and healthy subjects. Antonie van Leeuwenhoek. 1983;49:125–132 [DOI] [PubMed] [Google Scholar]
- 13.HARTLEY MG, HUDSON MJ, SWARBRICK ET, HILL MJ, GENT AE, HELLIER MD, GRACE RH. The rectal mucosa-associated microflora in patients with ulcerative colitis. Journal of Medical Microbiology. 1992;36:96–103 [DOI] [PubMed] [Google Scholar]
- 14.Gorvitovskaia A, Holmes SP, Huse SM. Interpreting prevotella and bacteroides as biomarkers of diet and lifestyle. Microbiome. 2016;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson RG, Jorge RE. Post-stroke depression: A review. American Journal of Psychiatry. 2015;173:221–231 [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe LM, Doran SJ, Mwilambwe-Tshilobo L, Conti LH, Venna VR, McCullough LD. Social isolation after stroke leads to depressive-like behavior and decreased bdnf levels in mice. Behavioural Brain Research. 2014;260:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung H, Pamp Sünje J, Hill Jonathan A, Surana Neeraj K, Edelman Sanna M, Troy Erin B, Reading Nicola C, Villablanca Eduardo J, Wang S, Mora Jorge R, Umesaki Y, Mathis D, Benoist C, Relman David A, Kasper Dennis L. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory t cells by indigenous clostridium species. Science. 2011;331:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geuking Markus B, Cahenzli J, Lawson Melissa AE, Ng Derek CK, Slack E, Hapfelmeier S, McCoy Kathy D, Macpherson Andrew J. Intestinal bacterial colonization induces mutualistic regulatory t cell responses. Immunity. 2011;34:794–806 [DOI] [PubMed] [Google Scholar]
- 20.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature. 2013;504:446. [DOI] [PubMed] [Google Scholar]
- 21.Artis D Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Reviews Immunology. 2008;8:411. [DOI] [PubMed] [Google Scholar]
- 22.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nature Reviews Immunology. 2014;14:667. [DOI] [PubMed] [Google Scholar]
- 23.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nature Reviews Microbiology. 2011;9:265. [DOI] [PubMed] [Google Scholar]
- 24.Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Hölscher C, Glatzel M, Korn T, Gerloff C, Tolosa E, Magnus T. Neutralization of the il-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120:3793. [DOI] [PubMed] [Google Scholar]
- 25.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17–producing γδt cells in the delayed phase of ischemic brain injury. Nature Medicine. 2009;15:946. [DOI] [PubMed] [Google Scholar]
- 26.Klein B, Mrowetz H, Barker CM, Lange S, Rivera FJ, Aigner L. Age influences microglial activation after cuprizone-induced demyelination. Frontiers in Aging Neuroscience. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of alzheimer’s disease. Cell. 2017;169:1276–1290.e1217 [DOI] [PubMed] [Google Scholar]
- 28.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nature Medicine. 2017;23:1018–1027 [DOI] [PubMed] [Google Scholar]
- 29.Mildner A, Huang H, Radke J, Stenzel W, Priller J. P2y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia. 2017;65:375–387 [DOI] [PubMed] [Google Scholar]
- 30.Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. European Journal Of Clinical Nutrition. 2009;63:1277. [DOI] [PubMed] [Google Scholar]
- 31.Finnie IA, Dwarakanath AD, Taylor BA, Rhodes JM. Colonic mucin synthesis is increased by sodium butyrate. Gut. 1995;36:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frankel W, Lew J, Su B, Bain A, Klurfeld D, Einhorn E, MacDermott RP, Rombeau J. Butyrate increases colonocyte protein synthesis in ulcerative colitis. Journal of Surgical Research. 1994;57:210–214 [DOI] [PubMed] [Google Scholar]
- 33.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraj P, Ignatowicz L. The mechanisms shaping the repertoire of cd4+ foxp3+ regulatory t cells. Immunology. 2017;153:290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russler-Germain EV, Rengarajan S, Hsieh CS. Antigen-specific regulatory t-cell responses to intestinal microbiota. Mucosal Immunology. 2017;10:1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poggi A, Zocchi MR. Γδ t lymphocytes as a first line of immune defense: Old and new ways of antigen recognition and implications for cancer immunotherapy. Frontiers in Immunology. 2014;5:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chien Y-h, Meyer C, Bonneville M. Γδ t cells: First line of defense and beyond. Annual Review of Immunology. 2014;32:121–155 [DOI] [PubMed] [Google Scholar]
- 38.Ritzel RM, Lai Y-J, Crapser JD, Patel AR, Schrecengost A, Grenier JM, Mancini NS, Patrizz A, Jellison ER, Morales-Scheihing D, Venna VR, Kofler JK, Liu F, Verma R, McCullough LD. Aging alters the immunological response to ischemic stroke. Acta Neuropathologica. 2018;136:89–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elderman M, Sovran B, Hugenholtz F, Graversen K, Huijskes M, Houtsma E, Belzer C, Boekschoten M, de Vos P, Dekker J, Wells J, Faas M. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLOS ONE. 2017;12:e0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilderink R, Verseijden C, Seppen J, Muncan V, van den Brink GR, Lambers TT, van Tol EA, de Jonge WJ. The scfa butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial hdac. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2016;310:G1138–G1146 [DOI] [PubMed] [Google Scholar]
- 41.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19:848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura YK, Janowitz C, Metea C, Asquith M, Karstens L, Rosenbaum JT, Lin P. Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Scientific Reports. 2017;7:11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the cns. Nature Neuroscience. 2015;18:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, Bader V, Haase S, Kaisler J, David C, Schneider R, Troisi R, Zent D, Hegelmaier T, Dokalis N, Gerstein S, Del Mare-Roumani S, Amidror S, Staszewski O, Poschmann G, Stühler K, Hirche F, Balogh A, Kempa S, Träger P, Zaiss MM, Holm JB, Massa MG, Nielsen HB, Faissner A, Lukas C, Gatermann SG, Scholz M, Przuntek H, Prinz M, Forslund SK, Winklhofer KF, Müller DN, Linker RA, Gold R, Haghikia A. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180:1067–1080.e1016 [DOI] [PubMed] [Google Scholar]
- 46.van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall H, Johansson MEV, Hansson GC. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019:gutjnl-2018–317571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.So PW, Ekonomou A, Galley K, Brody L, Sahuri-Arisoylu M, Rattray I, Cash D, Bell JD. Intraperitoneal delivery of acetate-encapsulated liposomal nanoparticles for neuroprotection of the penumbra in a rat model of ischemic stroke. International journal of nanomedicine. 2019;14:1979–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. Rigor guidelines: Escalating stair and steps for effective translational research. Translational stroke research. 2013;4:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (adp-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. Journal of Cerebral Blood Flow & Metabolism. 2005;25:502–512 [DOI] [PubMed] [Google Scholar]
- 50.Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, Eberl G, Baldassano RN, Laufer TM, Elson CO, Sonnenberg GF. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria–specific cd4+ t cells. Science. 2015;348:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J, Park EJ, Yuki Y, Ahmad S, Mizuguchi K, Ishii KJ, Shimaoka M, Kiyono H. Profiles of microrna networks in intestinal epithelial cells in a mouse model of colitis. Scientific Reports. 2015;5:18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganesh BP, Hall A, Ayyaswamy S, Nelson JW, Fultz R, Major A, Haag A, Esparza M, Lugo M, Venable S, Whary M, Fox JG, Versalovic J. Diacylglycerol kinase synthesized by commensal lactobacillus reuteri diminishes protein kinase c phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. Mucosal Immunology. 2018;11:380–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganesh BP, Klopfleisch R, Loh G, Blaut M. Commensal akkermansia muciniphila exacerbates gut inflammation in salmonella typhimurium-infected gnotobiotic mice. PLOS ONE. 2013;8:e74963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J, Lin K, Sequeira C, Borchers CH. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Analytica Chimica Acta. 2015;854:86–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


