Abstract
Purpose
Treatment of multiple myeloma (MM) has evolved tremendously and optimal utilization of available therapies will ensure maximal patient benefits.
Experimental Design
We report the SWOG randomized phase 2 trial (S1304) comparing twice-weekly low-dose (27 mg/m2; Arm 1) to high-dose carfilzomib (56 mg/m2; Arm 2), both with dexamethasone, administered for 12 cycles (11 months) for relapsed and/or refractory MM with up to six prior lines of therapy (NCT01903811). The primary endpoint was progression-free survival (PFS), and patients on Arm 1 could crossover to Arm 2 after progression on treatment.
Results
Among 143 enrolled patients, of whom 121 were eligible and analyzable, the overall response rate was 42.8%, with no significant difference between the arms (p=0.113). Also, neither the median PFS (5 months and 8 months, respectively; HR: 1.061, 80% Wald CI 0.821, 1.370; p=0.384) nor the median overall survival were significantly different (26 and 22 months, respectively; HR: 1.149, 80% Wald CI 0.841, 1.571; p=0.284). Sixteen patients crossed over to Arm 2 with a median PFS benefit of 3 months. Certain adverse events (AE) were more frequent in Arm 2, including fatigue, thrombocytopenia and peripheral neuropathy, but there was no significant difference in cardiopulmonary AEs.
Conclusions
This randomized trial did not support a benefit of fixed-duration, twice-weekly 56 mg/m2 dosing of carfilzomib over the 27 mg/m2 dose for the treatment of relapsed and/or refractory MM. However, treatment to progression in earlier patient populations with high-dose carfilzomib using different schedules should still be considered as part of the standard of care.
Introduction
Multiple myeloma (MM) is the second most common hematological malignancy, with more than 30,000 patients diagnosed in the United States (U.S.) every year.1 There have been tremendous improvements in outcomes of MM patients, with an estimated 5-year overall survival (OS) of 50.7%, as compared to only 34.6% less than two decades ago,2,3 mainly due to a better understanding of disease biology and the development of novel therapeutic agents.
Proteasome inhibitors represent one such category of anti-MM therapeutic agents.4 The ubiquitin proteasome pathway is a central component of the cellular protein-degradation machinery with essential functions in homeostasis, which include preventing the accumulation of misfolded or deleterious proteins.5 Inhibition of this pathway causes disruption of this homeostasis and intracellular accumulation of protein-degradation byproducts, leading to cell death. The first proteasome inhibitor, bortezomib, was approved by the FDA for treatment of patients with MM in 2003.6 Since then carfilzomib, and most recently ixazomib, have gained FDA approval.7 The utilization of these agents has evolved from single-agent to combination regimens, from later lines of therapy to earlier in the treatment paradigm of MM patients, and with changes in the dosage and mode of administration to deliver them in the safest and most efficacious manner.4,8 Amongst these changes, the utilization of carfilzomib has evolved substantially over time. Carfilzomib was initially approved as a single-agent for the treatment of relapsed and/or refractory MM (RRMM) in patients who had received at least two prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent (IMiD).9 The initially approved dose of carfilzomib was 20 mg/m2 intravenously (IV) administered as a single-agent on days 1, 2, 8, 9, 15 and 16 every 28 days for the first cycle, followed by 27 mg/m2 on the same schedule starting cycle 2 onwards for a total of 12 cycles. Since then, several clinical trials have led to significant changes in its usage, including escalating to 27 mg/m2 starting on day 8 of cycle 1, increasing the subsequent doses to 36 mg/m2 or 56 mg/m2 twice-weekly, using it in combination with other agents, and once-weekly at 70mg/m2.10–14 All these data resulted in changes to the FDA label for carfilzomib.15 The current FDA-approved clinical indications for carfilzomib are summarized in Table 1.
Table 1.
Current FDA-approved Variations of Carfilzomib in Relapsed and/or Refractory Multiple Myeloma
| Regimen | Dose | Schedule |
|---|---|---|
| Monotherapy | 20/27 mg/m2 | Twice-weekly |
| Carfilzomib, Lenalidomide, Dexamethasone | 20/27 mg/m2 | Twice-weekly |
| Monotherapy | 20/56 mg/m2 | Twice-weekly |
| Carfilzomib, Dexamethasone | 20/56 mg/m2 | Twice-weekly |
| Carfilzomib, Dexamethasone | 20/70 mg/m2 | Once-weekly |
Despite several clinical trials evaluating various carfilzomib-containing regimens in differing doses, schedules and clinical settings, no study has previously compared different doses of this agent on the same schedule in a randomized trial to understand their mutual safety and efficacy. The recently published randomized A.R.R.O.W. trial did compare two doses of carfilzomib, but they were administered in differing schedules, once-weekly (70 mg/m2) versus twice-weekly (27 mg/m2).13 SWOG undertook an intergroup randomized phase 2 clinical trial, S1304, to compare the safety and efficacy of low-dose (27 mg/m2) versus high-dose (56 mg/m2) carfilzomib with dexamethasone administered twice-weekly for RRMM (NCT01903811). We present here the primary results from this clinical trial.
Patients and Methods
This national, multicenter, open-label phase 2 randomized clinical trial was approved by the Cancer Therapy Evaluation Program (CTEP), relevant institutional review boards, and was conducted according to the Declaration of Helsinki. All patients provided written informed consent. Statistical analyses were conducted at the SWOG Statistics and Data Management Center in Seattle, WA.
Study Design
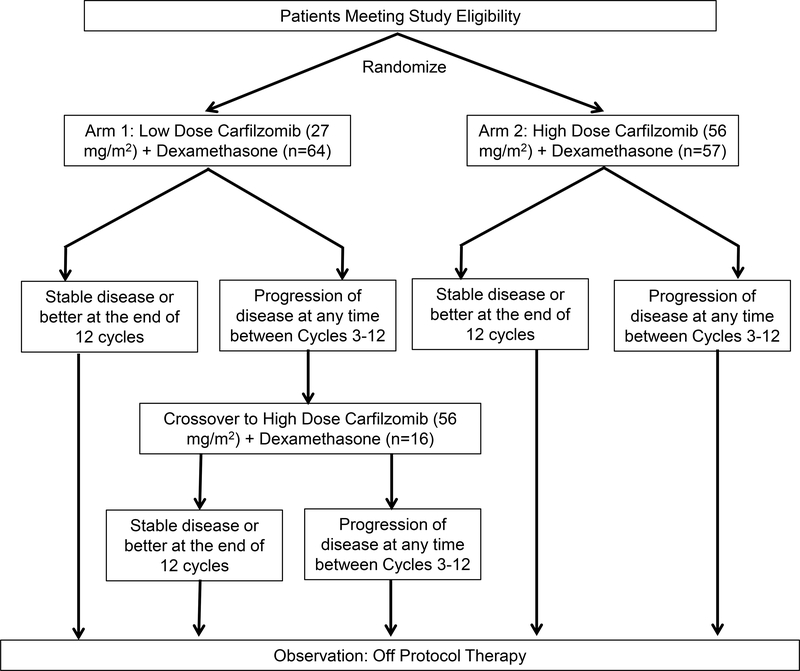
This randomized phase 2 trial compared low-dose carfilzomib plus dexamethasone (Arm 1) with high-dose carfilzomib plus dexamethasone (Arm 2) in RRMM. Patients who had disease progression any time between cycle 3 and 12 on arm 1 could crossover to high-dose carfilzomib. Each study cycle was 28 days long. The study schema is outlined in Figure 1. Treatment dose and schedule on the study arms included Arm 1 with carfilzomib 27 mg/m2 administered as a slow intravenous (IV) push (over 2–10 minutes) on days 1, 2, 8, 9, 15 and 16 of every cycle along with dexamethasone 20 mg IV, 30 minutes prior to every carfilzomib dose administration. Of note, on both, Arm 1 and Arm 2 carfilzomib was administered at a dose of 20 mg/m2 IV (over 2–10 minutes) for the first complete cycle, consistent with the then FDA label for carfilzomib. Arm 2 and the crossover arm had the same treatment schedule and regimen except that carfilzomib was administered at 56 mg/m2 IV over 30 minutes. The crossover arm was started directly at the 56 mg/m2 dose. Dose calculations were based on the patient’s actual body weight at baseline. Patients with a body surface area (BSA) >2.2 m2 received a dose based on BSA of 2.2 m2. Treating physicians were advised to administer IV fluid pre- and post-carfilzomib in accordance with the FDA label, with a caveat to monitor fluids along with the patient’s cardiopulmonary status and symptomatology. If a patient experienced grade ≥3 non-hematologic or grade 4 hematologic toxicity considered related to the study treatment during cycle 1 on Arm 1 or Arm 2, the second cycle of treatment was also administered at 20 mg/m2 of carfilzomib prior to escalation to the respective treatment dose for subsequent cycles. If a patient could not undergo dose escalation to the assigned treatment dose despite being at 20 mg/m2 for 2 cycles, they were to be removed from the protocol. All patients received antiviral prophylaxis.
Figure 1.
Study Schema Including Number of Eligible Plus Evaluable Patients in Every Arm
In addition to myeloma-related testing, all patients underwent cardiac monitoring including electrocardiography (EKG), echocardiography (ECHO) and cardiac biomarker laboratory testing (CK-MB, troponin, pro-BNP) every 12 weeks while on study. Additionally, all patients noted to have extramedullary disease on fluorodeoxyglucose positron emission tomography (PET) scan at baseline were to have a repeat PET scan at the end of treatment or at the time of achieving very good partial response (VGPR) or better, whichever occurred earlier.
Patients
Eligible patients were age ≥18 years with a confirmed diagnosis of RRMM. Those with POEMS syndrome, systemic amyloidosis or non-secretory MM were ineligible. Patients were required to have at least 1 but no more than 6 prior lines of anti-MM therapy.16 No prior carfilzomib treatment was permitted. A baseline PET scan was required within 28 days prior to registration on the trial. Other study requirements included adequate performance status (Zubrod performance status 0–2), lack of any other significant concomitant illnesses (with specific focus on cardiac comorbidities including, but not limited to class III or IV heart failure,17 unstable angina pectoris, myocardial infarction within the prior 6 months, or grade ≥3 cardiac arrhythmias), lack of grade >2 peripheral neuropathy and adequate organ function [total bilirubin ≤1.5 X upper limit of normal (UNL), SGOT/SGPT ≤3 X UNL, measured creatinine clearance ≥30 ml/min, absolute neutrophil count (ANC) ≥1,000 cells/mm3 without growth factor support, platelets ≥50,000 cells/mm3]. A left ventricular ejection fraction (LVEF) of ≥45% by ECHO was required within 28 days prior to registration on the trial. Prior anti-MM therapy including radiation therapy (XRT) had to be completed at least 21 days prior, although pulse steroids for a myeloma-related complication were allowed up to 7 days prior to registration.
Eligibility criteria for registration to the crossover portion of the trial required that the patient was initially randomized to the low-dose carfilzomib treatment (Arm 1) and had disease progression noted anywhere between the completion of 2–12 cycles of treatment. At least 14 but no more than 28 days were to have elapsed between the last day of treatment on Arm 1 and registration on the crossover arm. Patients must have recovered from all non-hematologic toxicities to grade ≤2 and from all hematologic toxicities to grade ≤3 prior to registration on the crossover arm. Patients must not have required any dose reductions on Arm 1 due to drug toxicity and must not have experienced a decrease in LVEF of >10% from baseline, or any LVEF decrease accompanied by clinical signs/symptoms of NYHA class III or IV heart failure, measured within 28 days prior to registration on the crossover arm.
Patients were stratified on the two treatment arms as per whether they had received 1–3 versus 4–6 prior therapies and whether they were refractory or not, as per International Myeloma Working Group (IMWG) criteria, to prior treatment with bortezomib.18
Objectives
The primary objective was to compare progression free survival (PFS) of two different doses of carfilzomib with dexamethasone (Arm 1 and 2) in the selected RRMM patients. A median PFS of 9 months was anticipated in Arm 1 based on information from previous trials. The study was designed with 90% power to detect a hazard ratio (HR) of 1.67, which corresponded to an increase in the median PFS from 9 to 15 months in Arm 2. These calculations were based on a one-sided stratified log rank test at level (alpha) of 0.1 and on the assumption that progressions would be exponentially distributed. Secondary objectives included evaluating the response rates, safety and median OS of patients on Arms 1 and 2 and also the response rates for patients who relapsed on low-dose carfilzomib treatment and subsequently crossed over to high-dose carfilzomib. Of note, the power calculations were to satisfy the primary objective of any clinically meaningful PFS benefit of Arm 2 over Arm 1.
Results
The clinical trial was activated on October 18, 2013, the first patient was randomized on February 12, 2014, and the last patient was randomized on November 6, 2015. These primary results of the clinical trial represent a median overall follow-up of 32 months (median 31 months on Arm 1 and 34 months on Arm 2). A total of 143 patients were enrolled on these 2 arms, of which 121 patients (64 on Arm 1 and 57 on Arm 2) were eligible and analyzable per the protocol. Twenty-one patients were deemed ineligible due to missing or untimely baseline eligibility laboratory evaluations (6 on Arm 1 and 11 on Arm 2), not having measurable disease at baseline (1 on each arm) and not completing or discontinuing prior therapy at least 28 days prior to randomization (1 on each arm). Furthermore, three patients (two of whom were ineligible due to missing baseline eligibility laboratory evaluations) were deemed inevaluable for having withdrawn from the study prior to receiving any treatment.
Patient and Treatment Characteristics
Baseline patient characteristics on the two study arms are summarized in Table 2. Of note, cytogenetic analysis was not uniformly available in all patients on the study. There were no significant differences noted in any of the baseline demographics, disease or previous treatment characteristics between Arms 1 and 2. The median number of prior lines of therapy was 3 on both arms. Among the eligible and analyzable patients, seven patients on Arm 1 and three patients on Arm 2 did not initiate cycle 2 and came off treatment before the end of cycle 1. Average carfilzomib dose per cycle of treatment on Arm 1 was 25 mg/m2 for the whole treatment and 27 mg/m2 after excluding cycle 1 (20 mg/m2 dose), while that on Arm 2 was 44.4 mg/m2 overall and 53.8 mg/m2 after excluding cycle 1. The median cumulative dose (including cycle 1 dosing) was 114.5 mg in Arm 1 and 244 mg in Arm 2 of the trial.
Table 2.
Patient Characteristics
| Factor | All Patients (n=121) | Arm 1 (n=64) | Arm 2 (n=57) | P-value* |
|---|---|---|---|---|
| Age ≥65 years (n=121) | 52% | 48% | 56% | 0.467 |
| Gender (n=121) | 0.584 | |||
| Female | 45% | 48% | 42% | |
| Male | 55% | 52% | 58% | |
| Performance Status >1 (n=121) | 4% | 3% | 5% | 0.666 |
| Myeloma Isotype (n=115) | ||||
| IgG | 52% | 59% | 44% | 0.137 |
| IgA | 15% | 8% | 22% | 0.039 |
| Light Chain Only | 15% | 15% | 15% | 1 |
| Creatinine Clearance <60 ml/min (n=121) | 34% | 36% | 32% | 0.701 |
| LDH ≥190 U/L (n=119) | 48% | 49% | 46% | 0.855 |
| Hemoglobin <10 g/dL (n=121) | 17% | 23% | 11% | 0.091 |
| Platelet Count <150 X 10^9/L (n=121) | 2% | 2% | 2% | 1 |
| ISS Disease Stage (n=121) | ||||
| Stage I | 35% | 31% | 39% | 0.447 |
| Stage II | 40% | 47% | 33% | 0.142 |
| Stage III | 25% | 22% | 28% | 0.528 |
| 4–6 Prior Lines of Therapy** (n=121) | 24% | 22% | 26% | 0.671 |
| Prior Therapy*** (n=120) | ||||
| Steroids | 99% | 100% | 99% | |
| IMiDs | 88% | 88% | 88% | |
| Lenalidomide | 84% | 86% | 82% | |
| Pomalidomide | 9% | 9% | 9% | |
| Thalidomide | 14% | 9% | 19% | |
| Proteasome Inhibitors | 84% | 88% | 80% | |
| Alkylators (Non-transplant) | 36% | 39% | 33% | |
| Stem Cell Transplant | 41% | 40% | 41% | |
| Other | 18% | 19% | 16% | |
| Refractory to Bortezomib** (n=121) | 50% | 50% | 49% | 1 |
| Serum M Spike >3 g/dL (n=120) | 16% | 20% | 11% | 0.211 |
| BM Plasma Cells >60% (n=119) | 21% | 22% | 20% | 0.823 |
P-values computed using Fisher’s exact test and representing a comparison between groups, not against the overall population
Stratification Factor
Prior Therapy (patients could have had more than one of these agents previously): IMiDs included thalidomide, lenalidomide and pomalidomide; Proteasome inhibitors included bortezomib and ixazomib; Alkylators included cyclophosphamide and melphalan in the non-transplant setting, Others included agents that were used in <5% patients on either arm and included monoclonal antibodies (monoclonal antibodies used as prior therapy in 7 cases).
Number of patients on whom data was available for each characteristic are shown in parenthesis in the respective rows.
LDH=Lactate dehydrogenase, M spike=Monoclonal spike, BM=Bone marrow
Efficacy Analysis
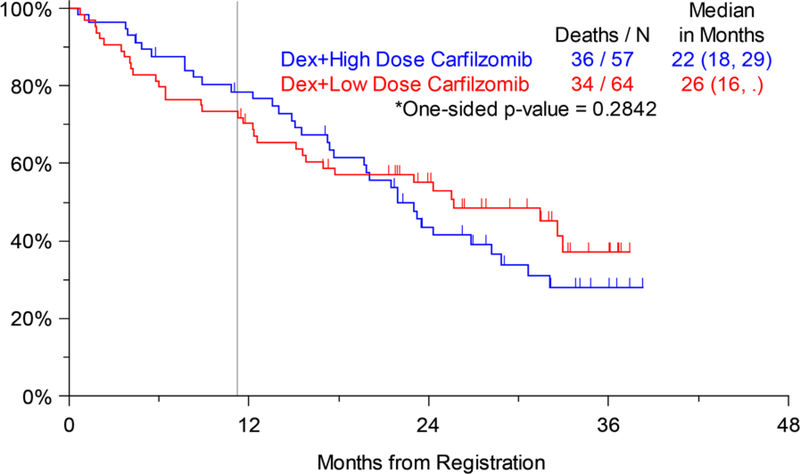
The primary objective of any significant PFS difference between Arms 1 and 2 was not statistically significant. Among the eligible and analyzable treatment population, 53 of the 64 patients on Arm 1 and 52 of the 57 patients on Arm 2 were noted to have disease progression, resulting in a median PFS of 5 months and 8 months, respectively (HR: 1.061, 80% Wald CI 0.821, 1.370; p=0.384) (Figure 2a). Similarly, OS was not significantly different between Arms 1 and 2. At the time of analysis, 34 of the 64 patients on Arm 1 and 36 of the 57 patients on Arm 2 had died, resulting in a median OS of 26 months and 22 months, respectively (HR: 1.149, 80% Wald CI 0.841, 1.571; p=0.284) (Figure 2b). Response assessment as per IMWG criteria was available in 112 patients (59 on Arm 1 and 53 on Arm 2). An overall response rate (ORR=confirmed partial response; PR or better) of 42.8% was seen in all patients (39% in Arm 1, 47% in Arm 2). Response rate categories by treatment arm are shown in Table 3. This data did not support the hypothesis that patients receiving high-dose carfilzomib with dexamethasone will have a significantly improved ORR as compared to those treated with low-dose carfilzomib and dexamethasone, based on a Cochran Mantel Haenszel test (two-sided p-value = 0.113). The median time to best response among patients who responded on either arm was 2.1 months on Arm 1 and 2.2 months for Arm 2.
Figure 2a.
Progression-free Survival (PFS) by Assigned Treatment Arm (Arm 1: Dexamethasone + Low-dose Carfilzomib, Arm 2: Dexamethasone + High-dose Carfilzomib); vertical line marks 11 months of active treatment on either arm of the trial.
Figure 2b.
Overall Survival (OS) by Assigned Treatment Arm (Arm 1: Dexamethasone + Low-dose Carfilzomib, Arm 2: Dexamethasone + High-dose Carfilzomib); vertical line marks 11 months of active treatment on either arm of the trial.
Table 3.
Response Rates as per International Myeloma Working Group (IMWG) Response Categories by Treatment Arm
| Response Category | Arm 1 (n=59) | Arm 2 (n=53) | Total (n=112) |
|---|---|---|---|
| Very Good Partial Response (VGPR) | 5 (8%) | 14 (26%) | 19 (17%) |
| Partial Response (PR) | 18 (31%) | 11 (21%) | 29 (26%) |
| Unconfirmed Partial Response (uPR) | 0 (0.0%) | 2 (4%) | 2 (2%) |
| Stable Disease (SD) | 24 (41%) | 19 (36%) | 43 (38%) |
| Progressive Disease | 12 (20%) | 7 (13%) | 19 (17%) |
A sensitivity analysis was performed for patients who initiated cycle 2 (the 1st cycle of treatment on the assigned study dose of carfilzomib (27 mg/m2 on Arm 1 and 56 mg/m2 on Arm 2), since carfilzomib was administered at a dose of 20 mg/m2 during cycle 1 on each of the arms. Of note, current standard practice is to escalate carfilzomib to the desired dose of 27 mg/m2 or 56 mg m2 starting at day 8 of cycle 1. This sensitivity analysis did not show a significant difference in PFS (median 10 months on Arm 1 and 7 months on Arm 2, one-sided stratified log-rank p=0.175, HR: 1.24, 80% Wald CI 0.924, 1.652) or OS (median 32 months on Arm 1 and 22 months on Arm 2, one-sided stratified log-rank p=0.197, HR: 1.30, 80% Wald CI 0.93, 1.83).
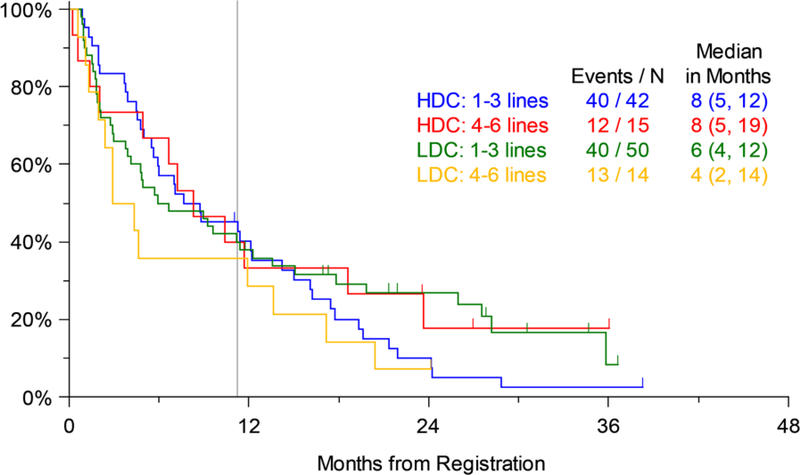
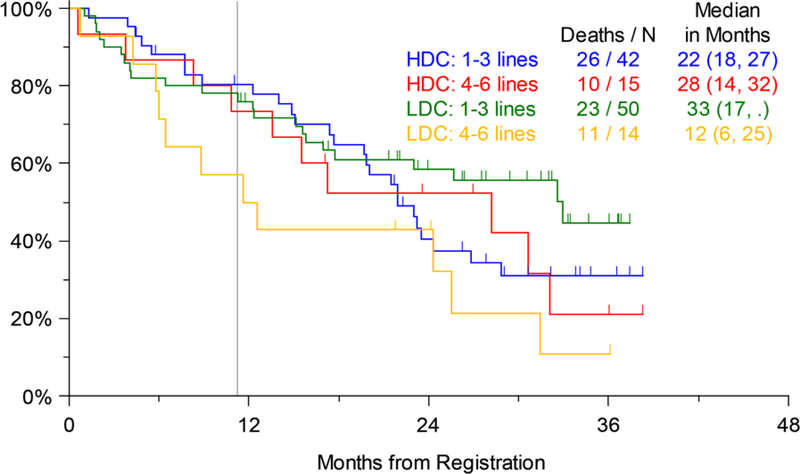
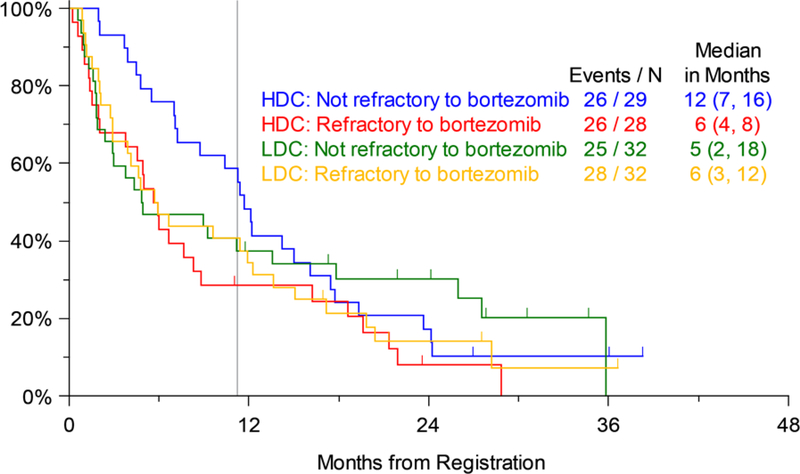
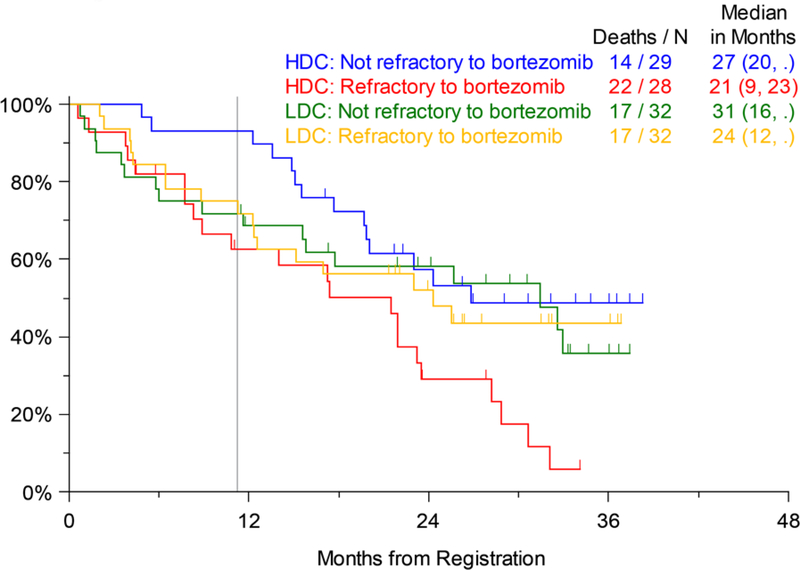
Efficacy analysis was done for the pre-specified study strata as well. There was no significant difference in PFS by number of prior lines of therapy (1–3 vs. 4–6) on Arms 1 or 2. Both these strata had a median PFS of 8 months on Arm 2, while the less heavily pretreated patients (1–3 prior lines of treatment) on Arm 1 had a median PFS of 6 months compared to only 4 months for those with 4–6 prior lines of therapy (not significant) (Figure 3a). Similarly, OS for patients on the two arms by prior lines of therapy was not significantly different, although similar to the trend for PFS, on Arm 1 the median OS for patients with 1–3 prior lines of therapy was 33 months compared to only 12 months for those with 4–6 prior lines of therapy. The median OS on Arm 2 was 22 months for patients with 1–3 prior lines and 28 months for those with 4–6 prior lines of therapy (Figure 3b). The largest numerical difference in OS between Arms 1 and 2 was noted for those with 4–6 prior lines of therapy (12 months and 28 months, respectively), but this was also not statistically significant (p=0.135). Similarly, looking at the pre-specified strata of disease refractory or not to prior bortezomib therapy, there were no significant differences in PFS (Figure 4a) or OS (Figure 4b) between Arms 1 and 2.
Figure 3a.
Progression-free Survival (PFS) by Number of Prior Lines of Therapy (LDC=Low-dose Carfilzomib; Arm 1, HDC=High-dose Carfilzomib; Arm 2); vertical line marks 11 months of active treatment on either arm of the trial.
Figure 3b.
Overall Survival (OS) by Number of Prior Lines of Therapy (LDC=Low-dose Carfilzomib; Arm 1, HDC=High-dose Carfilzomib; Arm 2); vertical line marks 11 months of active treatment on either arm of the trial.
Figure 4a.
Progression-free Survival (PFS) by Disease Refractory or Not to Previous Bortezomib Therapy (LDC=Low-dose Carfilzomib; Arm 1, HDC=High-dose Carfilzomib; Arm 2); vertical line marks 11 months of active treatment on either arm of the trial.
Figure 4b.
Overall Survival (OS) by Disease Refractory or Not to Previous Bortezomib Therapy (LDC=Low-dose Carfilzomib; Arm 1, HDC=High-dose Carfilzomib; Arm 2); vertical line marks 11 months of active treatment on either arm of the trial.
In order to see if there was any significant efficacy difference when patients were actively receiving treatment (11 months; 12 cycles), additional landmark sensitivity analyses were performed with patient data censored at 11 months from randomization. There was no significant difference for PFS (p=0.453) or OS (p=0.469) for this analysis as well. An analysis of OS was also done with censoring data for patients who crossed over to Arm 2 at the time of disease progression on Arm 1, and OS was again not significantly different (p=0.14).
A total of 16 patients on Arm 1 crossed over to high-dose carfilzomib with dexamethasone at the time of disease progression. Response assessment was available in 12 of these, with stable disease noted in seven and disease progression in five patients. Median PFS from the time of crossover was 3 months (range 2–6 months) in these patients, with 15 patients having undergone disease progression at the time of analysis. Median OS was 15 months (range 5–27 months) for these patients. Reasons for discontinuation of treatment on the crossover arm were increasing disease in seven, toxicity in eight and withdrawal of consent in one patient. We performed a sensitivity analysis by censoring patients on Arm 1 at the time of crossover to see if there was any OS benefit of high-dose carfilzomib with dexamethasone; the results were consistent with the primary endpoint analysis, showing no evidence of a difference between treatment arms (p=0.14).
Safety Analysis
Adverse events (AEs) on both study arms were compared and the ones that were at least possibly attributable to the study treatment and noted in >10% of patients on either arm are listed in Table 4. Hematological AEs were seen more frequently and with a higher grade than non-hematological AEs. Grade 3 or 4 hematological AEs that were seen in either arm included anemia, thrombocytopenia and lymphopenia, with anemia being the most common overall hematologic AE noted in the study. The most common non-hematological AE overall as well as grade ≥3 was fatigue. AE categories with notable differences in the frequency of grade ≥3 AEs between Arms 1 and 2 that were at least possibly attributed to the study treatment were compared with Fisher Exact test. There was a significantly higher number of patients with any Grade ≥3 AE in Arm 2 vs. Arm 1 (Arm 2: 37 vs, Arm 1: 25; p<0.001). Of these, fatigue and thrombocytopenia were by themselves significantly more frequently seen in Arm 2 as compared to Arm 1. Additionally, cardiopulmonary AEs were of special interest in the trial and so they were compared between the two study arms. These analyses included cardiovascular [including hypertension, acute coronary syndrome, arrhythmias, cardiac chest pain, congestive heart failure (CHF), cardiomyopathy] and pulmonary (including cough, dyspnea, hypoxia, pulmonary edema, respiratory failure) AEs reviewed as separate categories as well as analyzed together as one combined category of cardiopulmonary AEs. There was no significant difference noted for these between Arms 1 and 2. It was noted that three patients on Arm 1 and two patients on Arm 2 discontinued treatment secondary to new onset or significant worsening (defined as significant clinical deterioration in cardiac symptoms or >10% decrease in LVEF from baseline) in pre-existing CHF. Any grade hypertension was seen in 16% of patients, with no significant differences between the arms.
Table 4.
Treatment-Related Adverse Events At least Possibly Attributable to the Study Treatment and Occurring in >10% Patients on at least One of the Two Arms
| Adverse Event | Arm 1 (N=64) | Arm 2 (N=57) | ||
|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| Anemia | 39% | 13% | 53% | 16% |
| Fatigue | 45% | 2% | 52% | 14%* |
| Thrombocytopenia | 35% | 5% | 50% | 25%* |
| Leucopenia | 23% | - | 42% | 5% |
| Dyspnea | 21% | 4% | 36% | 9% |
| Nausea | 30% | 2% | 32% | 2% |
| Lymphopenia | 22% | 5% | 29% | 11% |
| Diarrhea | 23% | - | 25% | 2% |
| Neutropenia | 11% | - | 24% | 6% |
| Hyperglycemia | 7% | - | 24% | 2% |
| Peripheral Sensory Neuropathy | 9% | - | 23% | - |
| Insomnia | 12% | - | 20% | 2% |
| Constipation | 13% | - | 19% | - |
| Back Pain | 5% | - | 18% | 2% |
| Hypertension | 16% | 3% | 17% | 7% |
| Creatinine Increased | 13% | 4% | 17% | 4% |
| Vomiting | 17% | 2% | 11% | 2% |
| Transaminitis | 5% | - | 17% | 4% |
| Headache | 16% | - | 16% | - |
| Blurred Vision | 5% | 2% | 16% | - |
| Anorexia | 9% | - | 14% | 2% |
| Muscle Weakness | 8% | - | 14% | 2% |
| Dizziness | 11% | - | 13% | 2% |
| Pedal Edema | 13% | 2% | 9% | - |
| Hypokalemia | 13% | 2% | 6% | 2% |
| Hypoalbuminemia | 8% | - | 11% | - |
| Fever | 7% | - | 11% | - |
| Pneumonia | 7% | 5% | 11% | 11% |
| Cough | 6% | - | 11% | - |
| Flushing | 11% | - | 5% | - |
Statistically significant for grade ≥3 between Arms 1 and 2 by Fischer’s Exact Test.
At the time of data analysis 34 patients on Arm 1 and 36 patients on Arm 2 had died. Cause of death was MM-related in 73% (n=24) patients on Arm 1, 56% (n=20) on Arm 2, and 64% (n=44) for the overall study population. The cause of death for one patient on Arm 1 had not yet been classified.
Discussion
Clinical development of carfilzomib has involved several reported and ongoing clinical trials utilizing varying doses and schedules of the drug in many combination regimens.19,20 As a single agent or in combination with dexamethasone without a third anti-myeloma agent, carfilzomib is FDA-approved at the doses of 20/27 mg/m2 and 20/56 mg/m2, administered twice-weekly, and 20/70 mg/m2, administered once-weekly.14,21 Due to differences in the efficacy (median PFS) and clinical context (prior lines of therapy) in these trials, there had been questions regarding the optimal dose of carfilzomib, especially in combination with dexamethasone. This is highlighted by the fact that a recent randomized phase 3 clinical trial (ARROW) comparing twice-weekly and once-weekly dosing of carfilzomib, which lead to FDA approval of once-weekly (70 mg/m2) carfilzomib with dexamethasone, utilized 27 mg/m2 dose of this agent with dexamethasone in the twice-weekly comparator arm even though it is not a currently FDA-approved regimen at this dose and schedule.13 Furthermore, the ENDEAVOR study showed that, in a 923-patient randomized clinical trial, the 56 mg/m2 twice-weekly dose of carfilzomib with dexamethasone demonstrated superior OS to the comparator, bortezomib and dexamethasone, suggesting that 56 mg/m2 might be the optimal twice-weekly dose for carfilzomib.14 S1304 is the first clinical trial to report a direct comparison of the two doses of carfilzomib on the same treatment schedule of twice-weekly administration, in combination with dexamethasone.
Despite an assumption prior to initiating the trial that the higher dose of carfilzomib with dexamethasone would have higher efficacy than the lower dose, this randomized study failed to show such an effect. The higher carfilzomib dose did have slightly better VGPR rates but not significantly so, and this difference did not translate to a significantly higher PFS, the primary endpoint of the study, or OS. Of note, no confirmed complete responses (CRs) were reported on either arm due to lack of confirmatory bone marrow biopsies for response assessment. It should also be noted that both treatment arms utilized 20 mg/m2 carfilzomib for the whole first cycle, as was consistent with the FDA-approved label of the drug at the time of the clinical trial design (Reference ID: 3161927). Subsequently, the carfilzomib label has been updated to increase its dose to the target dose of 27 mg/m2 or 56 mg/m2 on day 8 of the first cycle, as long as the patient is tolerating therapy well. This may also have resulted in a slightly longer median time to best response noted in the current trial on either arm as compared to other carfilzomib-based trials, where the dose was escalated beyond 20 mg/m2 starting on day 8 of cycle 1. To eliminate any lack of efficacy on either arm due to this, we recommended that patients not be removed from the trial due to disease progression prior to the end of cycle 2, as long as it was considered safe for them to stay on treatment. Furthermore, we conducted additional analyses on patients who had received at least two cycles of therapy, including at least one cycle on the assigned treatment arm carfilzomib dose of 27 mg/m2 or 56 mg/m2, but this also did not isolate any efficacy difference.
There was a trend that efficacy of high-dose carfilzomib was maintained irrespective of the number of prior lines of therapy while low-dose carfilzomib seemed to have more benefit in patients with fewer (1–3) prior lines of therapy, but this was not statistically significant. Curiously, the median OS for the more heavily pre-treated patients (4–6 prior lines of therapy) was 28 months as compared to only 22 months for the group that had received less pre-treatment (1–3 prior lines of therapy). It could be possible that a higher level of proteasome inhibition is necessary in heavily pretreated patients, a hypothesis worth further investigation. Importantly, this was a subgroup analysis and not something that the study was powered to address. The trend was not that clear looking at patients refractory to prior bortezomib treatment or not, although there seemed to be better PFS and OS among patients not refractory to prior bortezomib, especially with high-dose carfilzomib (Arm 2). Again, it is important to note that the comparisons between these study strata were exploratory, and the study was adequately powered only to detect a pre-specified PFS benefit of Arm 2 over Arm 1. Furthermore, looking at the PFS and OS of all patients on both arms, it seemed that there was some separation of the survival curves while patients were on active treatment during the first 12 months after randomization (Figures 2a and 2b), but a post-hoc sensitivity analysis looking at only this study period did not show any significant survival difference between the arms. Of note, the current norm is not to discontinue carfilzomib-based treatment after a fixed number of treatment cycles, but to continue the regimen at least with maintenance dosing as long as the patient is deriving adequate clinical benefit and is tolerating the regimen well.
While the study plan included a crossover to the higher dose for those patients who had disease progression on the lower dose of carfilzomib, only 16 patients entered the crossover treatment. There were more patients on Arm 1 who had disease progression while on treatment, but they did not crossover based on patient or physician preference. At least in this small number of patients, the higher dose of carfilzomib was not able to provide any objective incremental responses. Analysis of the genomic, imaging and cardiac correlative study objectives is ongoing.
The safety profile from this trial was noted to be very similar to that from other studies, with no significant concern of cardiopulmonary AEs, even with the higher carfilzomib dose on this twice-weekly schedule. While a majority of the AEs noted on both arms did not have grade 3 or higher events, there were some AEs including thrombocytopenia, peripheral neuropathy and fatigue, which were significantly more evident in the high-dose carfilzomib arm. The incidence of grade ≥3 of any AE was noted to be significantly higher with 56 mg/m2 carfilzomib (65%) as compared to the 27 mg/m2 dose (39%, p=0.006). This suggests that the twice-weekly 56 mg/m2 dose of carfilzomib with dexamethasone results in increased toxicity without necessarily additional clinical benefit as compared to the 27 mg/m2 dose on the same schedule.
It is conceivable that a comparison of the outcomes from S1304 will be made with existing data on carfilzomib, especially the ENDEAVOR trial, where the 56 mg/m2 dose was used as the study arm, although it was compared with bortezomib treatment. The S1304 subset which is closest to the ENDEAVOR trial includes patients who were treated on Arm 2 and had 1–3 prior lines of therapy. However, the efficacy measures for this subset do not mimic the results from ENDEAVOR, and this is likely due to several factors, some of which were mentioned earlier. First, S1304 included patients with 1–6 prior lines of therapy (median of 3), while ENDEAVOR included patients with 1–3 previous treatments (median of 2). Most agents used against myeloma are more efficacious in earlier lines of therapy when the disease is less chemo-resistant. Second, ENDEAVOR excluded patients who were previously bortezomib-refractory while S1304 allowed such patients and they may have had disease with some cross-resistance to carfilzomib that reduced its efficacy. Third, S1304 continued carfilzomib dosing for up to a maximum of twelve cycles because that was the carfilzomib regimen which was approved at that time, while ENDEAVOR continued dosing until disease progression. Fourth, the subgroup of patients with 1–3 prior lines of therapy on the high-dose carfilzomib arm constitutes only 42 patients, and the trial was not powered to compare patients with 1–3 versus those with 4–6 prior therapies. Similarly, it was not designed or powered for cross-study comparisons, and it is possible that the small sample size of this cohort therefore contributes to the differences between S1304 and ENDEAVOR. Finally, the ENDEAVOR trial dosed carfilzomib at 20 mg/m2 for days 1 and 2 of cycle 1 only, and then increased the carfilzomib dosing to 56 mg/m2. In contrast, S1304 dosed carfilzomib at 20 mg/m2 for all of cycle 1 because that was the approved dose and schedule at the time of study initiation, and did not increase the dose to 56 mg/m2 until cycle 2.
Current and future clinical use of carfilzomib is moving towards once-weekly dosing with the recent FDA approval at 70 mg/m2 in combination with dexamethasone as well as ongoing clinical trials with additional agents.13,22 Yet, this dose and schedule is not approved in any other combination and prospective data are necessary to achieve optimal utilization of carfilzomib in order to derive maximal clinical benefit. There is data for significant heterogeneity in carfilzomib dosing and schedule leading to possibly suboptimal clinical benefit in the real-world setting.23 In this light, S1304 provides valuable data informing our clinical decisions and practice. This study shows that in a twice-weekly and fixed duration schedule in combination with dexamethasone, 56 mg/m2 dosing of carfilzomib does not provide statistically significant clinical benefit as compared to the 27 mg/m2 dose. However, treatment to progression in earlier patient populations with high-dose carfilzomib using different schedules should still be considered as part of the standard of care.
Translational Relevance.
Mutual comparison of carfilzomib doses in the same treatment schedule has not been reported previPously. The randomized trial S1304 does not show a benefit of fixed-duration twice-weekly 56 mg/m2 dosing of carfilzomib over the 27 mg/m2 dose. Certain adverse events other than cardiopulmonary were noted to be increased with the higher dose carfilzomib. The dosing and regimen of carfilzomib for treatment of patients with multiple myeloma, including weekly administration, continues to evolve.
Acknowledgments
Funding
NIH/NCI CA180888, CA180819, CA180820, CA180821, CA180830, CA189821, CA189971, CA180826, CA189830, CA180858, CA189858, CA189872, CA189829, CA180846, CA139519, CA189822, CA180798, CA189808, CA189952, legacy grants CA46282, CA13612; and in part by Onyx Pharmaceuticals, Inc. (AMGEN subsidiary)
Conflict of Interest:
S.A. has received institutional research support from Pharmacyclics, Inc., Cellectar Biosciences, Janssen, Genentech and Amgen, and is a consultant for Takeda, Amgen, Janssen and Celgene; S.L. is a consultant for Janssen, Bayer, Abbvie, Proclara, Takeda, is Chief Scientific Advisor with share options for Caelum Biosciences and receives research funding from Karyopharm and Sanofi; M.H.A. has been on the speaker’s bureau for Amgen, Takeda and Celgene; P.M.V. has served on Advisory Boards for Adaptive Biotechnologies, Bristol Meyers Squibb, Celgene, Janssen, Oncopeptides, Takeda and TeneoBio, is a consultant for Celgene, Incyte, Novartis and Oncopeptides, and has received research funding from Amgen, Celgene, Janssen, GSK Biologicals, and Takeda; A.D.C. has received research support from Bristol Myers Squibb and Novartis, and has consulted for Array Biopharma, Celgene, GlaxoSmithKline, Bristol Myers Squibb, Seattle Genetics, Janssen, Takeda, Oncopeptides, and Kite Pharma; S.H. has an immediate family member employed by Amgen Pharmaceuticals; K.K. has received honoraria from Bayer, Celgene, Janssen Pharmaceuticals, Pharmacyclics, Seattle Genetics and Novartis, and is a consultant for Verastem Inc., Agios, Astra Zeneca, Denovo Biopharma, Genentech and Jazz Pharmaceuticals; R.Z.O. has served on Advisory Boards for Amgen, Inc., Celgene Corporation, GSK Biologicals, Ionis Pharmaceuticals, Inc., Juno Therapeutics, Kite Pharma, Legend Biotech, Molecular Partners, Servier, and Takeda Pharmaceuticals, and received research funding from Amgen, Inc., Takeda Pharmaceuticals, and BioTheryX.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.www.seer.cancer.gov. Surveillance, Epidemiology, and End Results; 2018.
- 3.Ailawadhi S, Frank RD, Sharma M, et al. Trends in multiple myeloma presentation, management, cost of care, and outcomes in the Medicare population: A comprehensive look at racial disparities. Cancer. 2018;124(8):1710–1721. [DOI] [PubMed] [Google Scholar]
- 4.Scalzulli E, Grammatico S, Vozella F, Petrucci MT. Proteasome inhibitors for the treatment of multiple myeloma. Expert Opin Pharmacother. 2018;19(4):375–386. [DOI] [PubMed] [Google Scholar]
- 5.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14(7):417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8(6):508–513. [DOI] [PubMed] [Google Scholar]
- 7.Gozzetti A, Papini G, Candi V, Brambilla CZ, Sirianni S, Bocchia M. Second Generation Proteasome Inhibitors in Multiple Myeloma. Anticancer Agents Med Chem. 2017;17(7):920–926. [DOI] [PubMed] [Google Scholar]
- 8.Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36(4):561–584. [DOI] [PubMed] [Google Scholar]
- 9.Herndon TM, Deisseroth A, Kaminskas E, et al. U.s. Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clin Cancer Res. 2013;19(17):4559–4563. [DOI] [PubMed] [Google Scholar]
- 10.Berenson JR, Cartmell A, Bessudo A, et al. CHAMPION-1: a phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood. 2016;127(26):3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. [DOI] [PubMed] [Google Scholar]
- 12.Muchtar E, Gertz MA, Magen H. A practical review on carfilzomib in multiple myeloma. Eur J Haematol. 2016;96(6):564–577. [DOI] [PubMed] [Google Scholar]
- 13.Moreau P, Mateos MV, Berenson JR, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19(7):953–964. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(10):1327–1337. [DOI] [PubMed] [Google Scholar]
- 15.Carfilzomib Prescribing Information; 2018.
- 16.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64(6):1227–1234. [DOI] [PubMed] [Google Scholar]
- 18.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. [DOI] [PubMed] [Google Scholar]
- 19.Carfilzomib for multiple myeloma. Aust Prescr. 2018;41(2):56–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziogas DC, Terpos E, Kastritis E, Dimopoulos MA. An overview of the role of carfilzomib in the treatment of multiple myeloma. Expert Opin Pharmacother. 2017;18(17):1883–1897. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowiak AJ. Evolution of carfilzomib dose and schedule in patients with multiple myeloma: a historical overview. Cancer Treat Rev. 2014;40(6):781–790. [DOI] [PubMed] [Google Scholar]
- 22.Lonial S, San-Miguel J, Martinez-Lopez J, et al. Daratumumab in Combination with Carfilzomib and Dexamethasone in Patients (pts) with Relapsed Multiple Myeloma (MMY1001): An Open-Label, Phase 1b Study. Blood. 2017;130:1869 (Abstract).29051153 [Google Scholar]
- 23.Medhekar R, Hines D, Panjabi S, Welliver T, Wang X, Wade RL. Could Patients with Multiple Myeloma (MM) Derive Additional Benefit from Their Treatments? Real-World Evidence for Carfilzomib Dosing Intensity on Survival and Treatment Progression. Blood. 2018;132:836 (Abstract). [Google Scholar]