Abstract
Background:
We describe enrollment and accrual challenges in the “Promoting Maternal and Infant Survival Everywhere” (PROMISE) trial conducted in resource-limited countries, as well as the challenges in transitioning participants from the antepartum to the postpartum components of the study.
Methods:
PROMISE was a large multi-national randomized controlled trial of the safety and efficacy of interventions to reduce perinatal transmission of HIV-1 (HIV) during pregnancy and breastfeeding, and of interventions to preserve maternal health after cessation of perinatal transmission risk. The PROMISE study included two protocols for HIV-infected pregnant women in resource-limited countries who intended to either breastfeed or formula-feed their infants and did not meet country criteria for antiretroviral treatment. The PROMISE breastfeeding protocol (1077BF) used a sequential randomization design with up to three randomizations (Antepartum, Postpartum and Maternal Health). The PROMISE formula-feeding protocol (1077FF) had two randomizations (Antepartum and Maternal Health). Women presenting to the clinic during early or active labor or in the immediate postpartum period were registered as Late Presenters and screened to determine if eligible to participate in the Postpartum randomization.
Results:
The study was conducted at 14 sites in 7 countries and opened to enrollment in April 2011. A total of 3,259 pregnant women intending to breastfeed, and an additional 284 pregnant women intending to formula-feed were randomized in the Antepartum component. A total of 204 Late Presenters were registered during labor or after delivery. Enrollment was high among breastfeeding women (representing 96% of the target of 3,400 women) but was lower than expected among women intending to formula-feed (28% of 1,000 expected) and late-presenting women (8% of 2,500 expected). The successful overall enrollment and final primary study analyses results were attributed to substantial preparation before the study opened, collaboration among all stakeholders, close study monitoring during implementation and the flexibility to change and streamline the protocol.
Conclusions:
Experiences from the PROMISE study illustrate the challenges of enrolling in longer-term studies in the setting of rapidly evolving prevention and treatment standards priorities. The lessons learned will help the community, site investigators and study coordinators in the design and implementation of future clinical trials.
Keywords: HIV-1, AIDS, randomized clinical trial (RCT), antiretroviral therapy (ART), prevention of perinatal transmission, enrollment, resource-limited settings, PROMISE study
Introduction
A randomized clinical trial is the gold standard for efficacy and safety comparisons of therapeutic interventions. The number of participants is determined in advance so that the scientific objectives can be answered with high statistical power. Slower than expected or inadequate enrollment can severely compromise feasibility and can lead to an underpowered study.
The “Promoting Infant and Maternal Survival Everywhere” (PROMISE) study was a multi-national randomized controlled strategy trial in resource-limited settings, with three randomization components designed to answer key research questions about the relative efficacy and safety of interventions to prevent perinatal transmission of HIV during pregnancy and breastfeeding and preserve maternal health after cessation of perinatal transmission risk.1–3 The PROMISE study enrolled a total of 3,747 HIV-infected women in Africa and India who were pregnant or had recently given birth, had relatively high CD4 cell counts (≥350 cells/mm3) and had not met country-specific criteria for initiating antiretroviral therapy for their own care, along with their infants.
This paper describes the enrollment of mothers and their infants in the PROMISE trial, their transitions between randomization components of the study, the challenges the study team faced during study implementation and lessons learned.
Methods
The PROMISE trial was designed and conducted by the International Maternal Pediatric Adolescents AIDS Clinical Trials (IMPAACT) Network, a National Institute of Allergy and Infectious Diseases (NIAID)-sponsored clinical trials network. The PROMISE study comprised three sequential randomizations comparing the relative safety and efficacy of interventions to prevent perinatal transmission during pregnancy, labor and delivery and during breastfeeding, and to assess the relative risk versus benefit of continuing antiretroviral therapy to improve maternal health after the period of perinatal transmission risk ended (Figure 1). Two PROMISE protocols were developed that considered the variations in the standard of care for prevention of perinatal transmission during pregnancy and delivery and the recommended mode of infant feeding for HIV-infected mothers depending on safe water supply and availability, or lack thereof, of breast milk substitutes as part of a country’s standard of care.4 The Breastfeeding protocol (1077BF) with all three PROMISE randomizations (Antepartum, Postpartum and Maternal Health) was designed to enroll women who intended to breastfeed their infants. The Formula-Feeding protocol (1077FF) with two randomizations (Antepartum and Maternal Health) was designed for women who intended to formula-feed their infants. Formula-feeding mother-infant pairs were not eligible for the Postpartum component because they were no longer at risk for perinatal transmission. If however women changed their mind and wanted to breastfeed, they were given the chance to be randomized to the Postpartum component.
Figure 1.
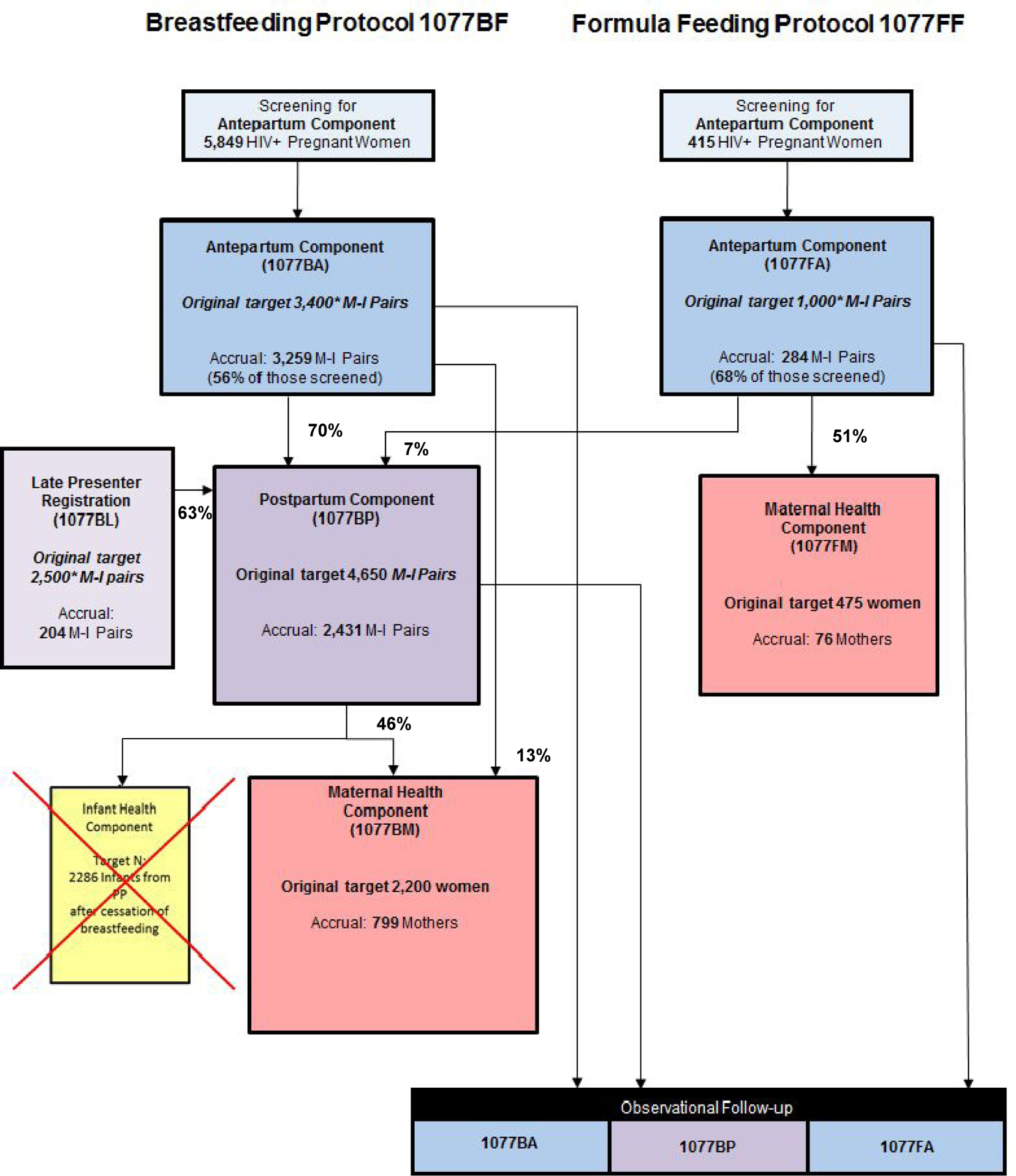
PROMISE target and actual accrual and transition rates
1077BF: PROMISE Breastfeeding protocol; enrolled women who intended to breastfeed their infants
1077FF: PROMISE Formula-feeding protocol; enrolled women who intended to formula-feed their infants
Component: one of the randomizations in the 1077BF or 1077FF protocols
* Target initial enrollment in PROMISE (in italics).
Originally, the 1077BF protocol also had a fourth randomization for HIV-uninfected infants who ceased breastfeeding prior to age 12 months, to assess the efficacy and safety of continued cotrimoxazole prophylaxis versus placebo for the prevention of infant mortality and morbidity following breastfeeding cessation. This Infant Health component however, did not open to enrollment due to a change in WHO guidelines to recommend breastfeeding of HIV-exposed infants beyond age 12 months.
It is important to note that the overall number of unique mother-infant pairs in PROMISE is much less than the sum of the component sample sizes. This is because 1077BF had only two points of entry (Antepartum and Late Presenters) and 1077FF had only one point of entry (Antepartum); the remaining PROMISE components would only enroll women and/or infants who participated in one of these initial PROMISE components. Transition and non-enrollment rates were calculated among the eligible MI pairs per component and protocol.
There were two routes of entry into the PROMISE study: through the Antepartum component,1 and through registration to the Late Presenters component (Table 1). Additional details on the PROMISE study and its design are provided elsewhere.1–3
Table 1.
Target number of mother-infant pairs, women or infants to be enrolled in each PROMISE component and protocol version
| PROMISE Component | 1077BF | 1077FF |
|---|---|---|
| Antepartum Randomization | 3,400 pairsb | 1,000 pairsb |
| Late Presenters Registration | 2,500 pairsa | 0 |
| Postpartum Randomization | ||
| From Antepartum Componentb | 3,100 pairs | 0 |
| From Late Presenters Registration | 1,550 pairs | 0 |
| Maternal Health Randomization | ||
| After deliveryb | 100 womenc | 475 women |
| After BF perinatal transmission risk ceasesb | 2,100 women | 0 |
Initial enrollment in PROMISE (in italics). It was projected that a total of 2,500 late presenting mother-infant pairs would need to be registered to the Late Presenters registration in order to identify 1,550 late presenting mother-infant pairs eligible for the Postpartum randomization.
For 1077BF and 1077FF, the numbers shown are only the numbers of pairs, women or infants who were projected to meet eligibility criteria and agree to be randomized in that component. In addition, all women and infants who participated in a previous PROMISE randomization but were not eligible for or did not agree to be randomized in a subsequent randomization continued to be followed on-study as a comparison group.
Projected number of women in the Antepartum triple antiretroviral therapy arm ineligible for the Postpartum randomization due to infant ineligibility or stillbirth but still eligible for the Maternal Health randomization.
During study design, the PROMISE team queried IMPAACT site investigators regarding the numbers of potentially eligible breastfeeding and formula-feeding women who delivered at their sites per year and the estimated numbers were included in the study protocol to justify accrual projections. The study team worked with the sites to develop detailed accrual plans with monthly projections and then distributed a report each month showing projected and actual accrual for each site.
Initially, the team assessed the protocol registration compared with targets quarterly to ensure that an adequate number of sites had registered to complete the protocol. Once one-half of eligible IMPAACT sites had registered, the team assessed accrual compared with targets on a quarterly basis. Accrual to PROMISE was monitored by the study team and by IMPAACT leadership in accordance with standard operating procedures. In addition, the team assessed feasibility quarterly.
The study was monitored by an NIAID-sponsored Data Safety and Monitoring Board (DSMB). Interim analyses focusing on safety, study logistics, and the accuracy of sample size assumptions were reviewed at least annually starting within 12 months after the first woman was randomized. Interim efficacy analyses were performed annually (or otherwise recommended by the DSMB) once at least 25% of the information on the primary efficacy outcome measure was available.
Results
Site activation and screening
PROMISE was conducted at 14 sites in 7 countries: India, Malawi, South Africa, Tanzania, Uganda, Zambia and Zimbabwe. The sites were in peri-urban or urban settings, but the recruitment areas for some sites extended to more rural areas. All 14 sites participated in 1077BF, with three South African sites and one India site also participating in 1077FF. Sites were activated for enrollment between March and July 2011, except for three sites in India (for 1077FF), Tanzania and Zambia (for 1077BF) which were activated between February and September 2012. Across sites, the median (min, max) time from protocol registration to study activation was 69 (1, 223) days and from study activation to the first randomization was 39 (14, 63) days.
A total of 6,264 pregnant women were screened for the Antepartum Component, of whom 2,721 (43.44%) did not enroll (Figure 1). The major reason for screening failure was a CD4 result at screening lower than 350 cells/mm3, the eligibility threshold for randomization in the study (72% of the reasons among those not enrolled) (Table 2).
Table 2.
Reasons of screening failure in the Antepartum component of PROMISE
| Screening failure reasona | Totalb |
|---|---|
| CD4 < 350 cells/mm3 | 1,960 (72%) |
| Otherc | 428 (16%) |
| Participant did not return to the clinic following consent | 148 (5%) |
| Requiring antiretroviral therapy for WHO Stage III or IV disease | 61 (2%) |
| Hemoglobin < 7.5 g/dL | 54 (2%) |
| Participant not willing to participate (reason provided) | 52 (2%) |
| History of antiretroviral therapy during current pregnancy | 30 (1%) |
| Active serious illness or medical condition | 23 (1%) |
| Participant not willing to participate (no reason provided) | 20 (1%) |
| Test result not available in protocol timeframe | 12 (0.4%) |
| Fetal death/condition incompatible with life | 6 (0.2%) |
| ALT > 2.5xULN | 5 (0.2%) |
| Platelets < 50,000 cells/mm3 | 4 (0.1%) |
| Fetus with serious congenital malformation | 3 (0.1%) |
| History of active TB or receipt of TB drugs within 30 days prior to study entry | 2 (<0.1%) |
| History of documented heart or cardiac defect | 2 (<0.1%) |
| Creatinine clearance < 60 mL/min | 1 (<0.1%) |
| Drug/alcohol use or dependence | 1 (<0.1%) |
| Total (2,812) | 2,812 |
More than one reason may be indicated for each participant who failed to enroll
Percentages calculated among the total number of those not enrolled
For the 1077BF protocol some of the most common “Other” reasons include but not are not limited to: the patient decided to formula-feed; participant was HIV negative; participant did not return to the clinic after consent (and relocated or is lost to follow-up); participant withdrew consent; discordant HIV results; indeterminate western-blot results; site is not screening/enrolling due to updated national guidelines; participant was not pregnant (confirmed by urine test and ultrasound scan); participant gave birth or had abortion or miscarried before enrollment; undetectable HIV-1 RNA; real time HIV-1 RNA < 5,000 copies/mL; no CD4 result or CD4 < 350 cells/mm3; participant was under age; negative perinatal transmission; low hemoglobin; Hepatitis B co-infection; previous pregnancy on triple antiretroviral therapy for her own health; participants requires antiretroviral therapy for her own health; participant was at high risk for pre-eclampsia; participant was willing to participate but her husband told her to decline participation to avoid status disclosure; social problems etc.
For the 1077FF protocol “Other” reasons include but not are not limited to: the patient decided to breastfeed (and enrolled to 1077BF); HIV test results inconclusive; participant was HIV negative; did not return to the clinic after consent (and relocated or is lost to follow-up); participant withdrew consent; site is not screening/enrolling due to updated national guidelines; participant gave birth before enrollment; administrative error (duplicate screening number; premature randomization)
Enrollment
The enrollment target for PROMISE was 6,900 MI pairs; 4,400 during pregnancy (3,400 intending to breastfeed and 1,000 intending to formula-feed), and approximately 2,500 presenting during labor or within 5 days after delivery (Table 1; Figure 1). The study was successful in enrolling 3,259 pregnant women who intended to breastfeed in the Antepartum component, 96% of the target sample size of 3,400 breastfeeding women. In addition, 284 women intending to formula-feed were enrolled (28% of the target of 1,000), bringing the total number of women enrolled in the Antepartum component to 3,543 representing 81% of the 4,400 anticipated. A total of 204 late presenters were registered to the Late Presenters component, 8% of the 2,500 anticipated (Figure 1). Malawi and South Africa were the highest enrolling countries.
The challenge of enrolling formula-feeding mothers
The low accrual of formula-feeding mother-infant pairs was because enrollment of women who intended to formula-feed primarily occurred in countries that updated their national guidelines during study implementation to recommend breastfeeding for all infants born to HIV-infected women and phased out providing free formula.
For, example, when PROMISE was initially developed, Botswana and Thailand were anticipated to enroll in the formula-feeding version of PROMISE, as the countries recommended formula-feeding for mothers with HIV and provided zidovudine antepartum as standard of care for the prevention of mother-to-child transmission. Both countries’ standards of care shifted to provide triple antiretroviral regimens. Similarly, the protocol team originally anticipated that sites in South Africa and India would contribute a substantial number of participants to the formula-feeding version of the study, as their country guidelines recommended formula-feeding for mothers with HIV. These countries also provided substantial support to mothers who formula-fed, including free or reduced costs formula and improved access to clean water. During enrollment into PROMISE, both countries’ standards of care shifted to recommend breastfeeding (consistent with a shift in WHO recommendations) and reduced support to mothers who chose to formula-feed. Combining this with standard information and counseling on infant feeding methods addressing the advantages and disadvantages of feeding options resulted in formula-feeding not being a viable option for many HIV-infected women. Thus, sites in these countries contributed more enrollments to the breastfeeding version of the PROMISE study but fewer than anticipated enrollments to the formula-feeding version.
The challenge of recruiting Late Presenters
There is a tendency for women to present later during pregnancy in resource-limited international settings, often in the late second trimester, with only half of pregnant women attending the recommended four antenatal visits prior to delivery and 10% presenting during labor/delivery.5 Before PROMISE started, late presenters were seen very frequently at some study sites and were expected to be at high risk of perinatal transmission. However, during the implementation of PROMISE, country-wide efforts were made to encourage women to present to antenatal care clinics earlier and costs around delivery were reduced or removed. Therefore, the numbers of HIV-infected women presenting in labor without having received antiretroviral therapy during pregnancy decreased sharply during the PROMISE trial.6 Moreover, the logistics of identifying and enrolling women at the time of or soon after delivery are much more challenging than the logistics of enrolling pregnant women in antenatal care. To successfully register late presenters in PROMISE, study staff needed to be physically present in labor and delivery wards 24/7 and this was not operationally feasible for most sites. Furthermore, team discussions highlighted that the identification, screening and enrollment of late presenters was costlier than identification of candidates for the Antepartum component, creating a disincentive at sites to accrue Late Presenters.
To address slow enrollment into 1077FF and the Late Presenters Registration, the study Chair, IMPAACT Leadership and the PROMISE team held regular teleconference calls with each of the top enrolling sites to encourage them to meet enrollment goals. Additionally, IMPAACT Leadership provided increased funding for each new enrollment and substantially increased funding for the intensive antepartum period and the first six months post-delivery.
The challenge of rapid evolution of HIV treatment guidelines
When the PROMISE trial opened in 2011, ZDV during pregnancy with intrapartum sdNVP to prevent perinatal transmission and a 1–2 week “tail” of two nucleosides to prevent Nevirapine resistance was standard of care (WHO 2010 “Option A”) for HIV-infected women with CD4 count > 350 cells/mm3 in the countries where PROMISE was conducted.3 Then, in April 2012, the WHO released a programmatic update on the “Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants”, in which it urged countries to consider the advantages of Option B (in which all pregnant and lactating women with HIV are offered antiretroviral therapy beginning in the antenatal period and continuing throughout the duration of breastfeeding) and Option B+ (life-long antiretroviral therapy regardless of a woman’s CD4 cell count).7
Despite the WHO updates, the Ministries of Health in most of the countries where PROMISE was being conducted, concluded that the study should continue given their current country guidelines, and that there was continued equipoise for the PROMISE research based on lack of clinical trial safety and efficacy data to back up the WHO 2012 recommendations. An independent Ethics panel in May 2012 also reviewed the PROMISE protocol design in light of evolving WHO guidelines and advised NIAID, the study sponsor, that PROMISE could be conducted ethically in countries that have chosen Option B+ since equipoise remained with respect to the relative safety and efficacy of the various antiretroviral regimens being used in PROMISE. Additionally, the Ethics panel concluded that PROMISE posed important unanswered and highly relevant scientific questions and was extremely likely to produce both relative safety and efficacy findings that would have value for informing clinical, policy or program decisions then or in the future.
Other challenges
Soon after enrollment in PROMISE began, it became apparent that the study sites faced other challenges including the complexity of the protocol, related site training, need for multiple consents with each randomization of enrolled participants, and unexpectedly high costs, especially for the first year of follow-up. Additionally, while the number of screening and enrollment visits was increasing with the ramp up of accrual, clinics were becoming crowded with a high volume of mothers and infants returning for follow-up visits. Based on the sites’ responses to an implementation survey and IMPAACT Leadership’s feedback, the study was modified in 2012 to streamline its implementation with a goal to not compromise the ability of the study to meet its overall objectives, while reducing costs and staff effort. To alleviate time-consuming procedural burdens and operational complexity, the overall frequency of study visits, the administration of questionnaires and the collection, testing and storage of laboratory specimens were decreased. The procedural time frames were extended, case report forms were modified, and the data collection schedule was adapted accordingly.
Anticipated enrollment increases after implementation of the protocol amendment were not observed immediately due to several intervening factors. First, the launch of the new version of any protocol is generally delayed due to time required for Institutional Review Board (IRB) and other regulatory approval, staff training and re-consenting of participants to the updated protocol versions. Furthermore, several PROMISE sites included caps on their enrollment in the consent forms based on conservative projections and had to halt enrollment pending IRB approval required to increase the cap. Finally, the India clinical research site had to halt enrollment due to a new law that placed requirements for participant compensation in research studies that were not acceptable to the United States sponsor. Overall, time from finalization of the amended PROMISE protocol version to the date the sites started operations was long, with a median (min, max) across sites of 159 (59, 339) days. Of note, distribution of the amended 1077FF protocol occurred when South Africa was beginning to implement their revised infant feeding policy.
Closure to enrollment
In June 2014, accrual to the Antepartum component was nearing completion. Enrollment challenges however persisted for women intending to formula-feed or presenting late in pregnancy. Having concluded that it would not be possible to achieve the planned sample sizes in a reasonable timeframe other than for women intending to breastfeed, NIAID, the study sponsor, decided to close accrual for women intending to formula-feed and Late Presenters on July 18, 2014. Since randomizations to the subsequent components were conditional on the Antepartum and Late Presenters enrollments, NIAID also decided to close subsequent randomization into Postpartum and Maternal Health when enrollment of women intending to breastfeed was achieved, or on October 1, 2014, whichever came first.
Subsequent randomizations
Maintaining high transition rates from study entry components into the subsequent components was a major focus throughout study follow-up and monitored at interim data reviews. Data summaries were created to identify obstacles to enrollment that were likely to remain fixed (based on eligibility criteria) versus obstacles that could be overcome with improvement of day-to-day operations.
One issue that emerged was that the infant HIV test results were not available quickly enough after birth at a number of sites, leaving many women ineligible for the Antepartum to Postpartum transition (8% of non-enrollment reasons) (Supplemental Table 1). The PROMISE team determined that a slight extension of the window for enrollment into the Postpartum component from 7–12 to 6–14 days to allow return of newborn HIV test results to the site would not adversely affect the interpretation of study results. Accordingly, the timeframe for assessing infant HIV status and other infant laboratory values for determining eligibility for the Postpartum component was extended to within 14 days of birth. Entry timeframes for transition to the Maternal Health component were also widened from within 29–42 to 29–84 days after complete cessation of breastfeeding, and maximum allowed interruption of antiretroviral therapy prior to entry into Maternal Health from Postpartum was also widened from 7 to 14 days. Additionally, the IMPAACT Central Laboratory started monitoring the performance of local laboratories on the infant HIV test turnaround, and developed corrective and preventative action plans, including: revising existing Standard Operating Procedures and creating new ones to cover gaps; retraining staff and improving training logs; revising backup plans to include when to notify sites regarding delays in testing and re-routing samples to a backup lab; replacing staff who supervised the HIV assay. The Central Laboratory also conducted laboratory-specific refresher training and continued to monitor laboratories very closely.
The corresponding changes in the protocol resulted in decreases in non-enrollment percentages in the subsequent components of the study. For example, with the amended protocol version, the overall non-enrollment percentage from the breastfeeding version of Antepartum to Postpartum dropped to 21% versus 27% under the previous protocol version; the availability of HIV test results became a less frequent reason for non-enrollment; and the non-enrollment percentage from Antepartum to Maternal Health decreased to 30% versus 49% under the previous protocol version (data not shown).
Transition patterns to subsequent components
By the time of study closure in October 2014, 70% (2,282/3,259) of women randomized to the breastfeeding version of Antepartum had transitioned to the Postpartum component, which was lower than the 90% rate assumed in the sample size calculations (Figure 1). Of note, a sizable percentage of non-enrollment reasons were related to protocol inclusion/exclusion criteria regarding health issues of the mother or the infant. For example, among the group of women who could not transition to Postpartum, 7% required antiretroviral therapy for their own health, meeting country clinical treatment criteria, and 3% had a CD4 count below 350 cells/mm3. Additionally, 8% of the women had decided not to breastfeed and therefore were ineligible for the breastfeeding component of the study. Moreover, 9% of the MI pairs who did not transition were ineligible for a subsequent randomization to Postpartum because of infant death, 7% because of infant birth weight below the 2 kg pre-defined eligibility criterion, 3% because of HIV perinatal transmission, and 1% because of an infant life-threatening illness (Supplemental Table 1).
The number of Antepartum women intending to breastfeed who were randomized to Maternal Health directly soon after delivery was higher than expected (242 compared to the 100 expected (Table 1)). The higher than expected direct randomizations to Maternal Health resulted from lower than expected eligibility for randomization to Postpartum in part due to protocol implementation factors discussed above.
The transition rate from Postpartum to Maternal Health was 46% (557 of 1,220 eligible women) versus the assumed 90% (Figure 1). Importantly, 15% of the women who did not transition to Maternal Health were unwilling to participate within the per protocol time for randomization (after 18 months postpartum or cessation of breastfeeding whichever came first). Many women left the site catchment area after their baby was born to live with family, especially in the immediate postpartum period. Additionally, 7% of the women required antiretroviral therapy for their own health and 6% had a CD4 count below 350 cells/mm3 (Supplemental Table 1).
The final transition rate from the formula-feeding version of Antepartum to the formula-feeding version of Maternal Health was 51% (76 of 149 eligible women) versus 95% who were projected to meet eligibility criteria and would agree to be randomized in that component (Figure 1). Twenty-one percent of the women however who did not enroll to Maternal Health were unwilling to participate in the Maternal Health randomization after the antepartum period and 13% of the women did not return to the clinic after the end of the Antepartum component mainly because women left the site catchment area in the period immediately postpartum as discussed above (Supplemental Table 1). Finally, although the number of Late Presenters was low, the rate of late presenting women transitioning to Postpartum was 63% (128 of 204 eligible women) similar to the anticipated 62% as per the protocol assumptions (Figure 1).
Discussion
The PROMISE study aimed to answer several key global public health questions related to the prevention of perinatal transmission of HIV and the health of infected mothers and their infants. PROMISE successfully enrolled 96% of the target population intending to breastfeed and both the antepartum, postpartum and maternal health components were successful in producing statistically and clinically important efficacy and safety results.1–3,6,7
The major challenges that the study team faced were the rapidly evolving standards of care and WHO HIV related treatment implementation priorities; challenges due to protocol requirements and changes to country infrastructure. Table 3 summarizes challenges, lessons learned and recommendations for future clinical trials.
Table 3.
Challenges, lessons learned and recommendations
| Challenges | Examples from the PROMISE study | Recommendations for future trials |
|---|---|---|
| Inclusion/exclusion criteria |
|
|
|
Operational challenges
(Note that operations for the PROMISE study were handled centrally however the team faced multiple issues during the conduct of the trial) |
|
|
| Unforeseeable obstacles | Challenge in recruiting formula-feeding mothers and late presenters |
|
| Changes in WHO guidelines and recommendations |
|
The PROMISE team tried to design the study to account for different feeding methods and antiretroviral treatment strategies by having multiple protocols accounting for different WHO and country-specific guidelines. During the conduct of PROMISE, the WHO implemented major changes to its treatment guidelines. Notably, the HPTN 052 trial provided evidence for the effectiveness of antiretroviral therapy in preventing transmission to uninfected adults, leading some researchers and experts to believe that WHO Option A of ZDV only during pregnancy should be abandoned, even though HPTN 052 did not provide randomized data on the safety of antiretroviral therapy in pregnancy.8 Challenges continued in 2013–2014 as WHO Option B+ of lifetime antiretroviral therapy was being considered and became available in some countries through the Global Fund and The United States President’s Emergency Plan for AIDS Relief. Furthermore, in 2015, WHO strategies to harmonize guidelines for adult treatment and for the prevention of perinatal transmission were developed to simplify implementation to focus on use of antiretroviral therapy for both treatment and secondary prevention. Specifically, the immediate initiation and lifetime administration of antiretroviral therapy at the time of diagnosis (“Test and Treat” strategy) for all HIV-infected, pregnant women, began to be actively promoted and gained support (antiretroviral therapy for everyone with HIV as soon as they are diagnosed) after the START trial results became available in July 2015.9 The changes in guidelines proved a major source of uncertainty during the conduct of the PROMISE trial and may have acted as a countering force during the time the PROMISE team worked to complete enrollment. Convening an Independent Ethics panel and coordinated outreach to Ministries of Health can help deal with guideline changes during the conduct of a study. In the case of PROMISE, both bodies confirmed that PROMISE posed important and highly relevant scientific questions and ultimately the results of the study did succeed to inform clinical, policy and program decisions.
There was a substantial learning curve associated with the study changes, but sites quickly adapted to new, streamlined procedures. They also quickly realized the importance of excellent communication around the time of delivery. The study team revised the protocol to allow more flexibility around the delivery visit and the time of breastfeeding cessation to allow for smoother transitions between components.
Some of our key recommendations for future trials were implemented in the PROMISE study and are already being used in other pregnancy studies within the IMPAACT Network. For example, more recent studies allow more flexibility around critical timepoints, early site involvement in protocol development is facilitated and encouraged, visits are aligned between mothers and infants, and procedures are streamlined to avoid errors and reduce complexity.
Our experiences suggest that substantial preparation before the study opens is key to the success of a clinical trial. Attention to study design, data management, and data analysis are important factors of the study’s success. For example, the PROMISE protocol had assumed high transition rates for the subsequent components of PROMISE, which proved difficult to achieve amidst the ever-changing scientific landscape of the HIV research field. Protocol sample size calculations were however conservative assuming 90% power, therefore all study components were successful in producing clinically important efficacy results and contributed important information on the significantly increased risk of adverse pregnancy outcomes with use of maternal antiretroviral therapy compared to other proven regimens with less fetal antiretroviral exposure.6,7 Supplemental Table 2 outlines the primary findings of the PROMISE study.
When conducting a multicenter trial, the ongoing commitment, the willingness to compromise when differences of opinion among collaborators arise, collaboration and dedication are essential for the success of the study. The site investigators, the Operations Center, the Statistical and Data Management Center, NIAID and the community all contributed to achieve the target accrual of the study. Close monitoring during the study by all stakeholders, assessment of barriers/challenges by surveying the sites, and the willingness to revise/streamline the protocol proved invaluable to the success of the study.
In conclusion, despite all the challenges, the breastfeeding version of the Antepartum Component of PROMISE reached 96% of the target sample size with satisfactory power to address the primary antepartum and postpartum objectives and provide important efficacy and safety clinical trial data to further inform the WHO recommendations and ministry of health policy.7 It is the authors’ hope that the lessons learned related to length of time for finalization of protocol development, rapidly evolving prevention and treatment guidelines and shifting research priorities will help the WHO, the ministries of health, the communities, the site investigators and study coordinators in the design and implementation of future clinical trials.
Supplementary Material
Acknowledgements
The PROMISE team gratefully acknowledges the contributions of the mothers and their infants who participated in the study; as well as the PROMISE study staff at the clinical research sites, the IMPAACT Central lab at UNC-Chapel Hill; the PROMISE Operations Center staff at FHI 360; the statistical staff at the Center for Biostatistics in AIDS Research, Harvard School of Public Health; and the data management staff at Frontier Science & Technology Research Foundation, Amherst, NY.
Funding
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. Study products were provided free of charge by AbbVie, Gilead Sciences, Boehringer Ingelheim, and ViiV/GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The PROMISE study is registered at clinicaltrials.gov:
NCT01061151 “Evaluating strategies to reduce mother-to-child transmission of HIV infection in resource-limited countries (PROMISE)”
References
- 1.Fowler MG, Qin M, Fiscus SA, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn PM, Taha TE, Cababasay M, et al. Prevention of HIV-1 transmission through breastfeeding: efficacy and safety of maternal antiretroviral therapy versus infant nevirapine prophylaxis for duration of breastfeeding in HIV-1-infected women with high CD4 cell count (IMPAACT PROMISE): a randomized, open-label, clinical trial. J Acquir Immune Defic Syndr 2018; 77: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman RM, Angelidou KN, Brummel SS, et al. Maternal health outcomes among HIV-infected breastfeeding women with high CD4 counts: results of a treatment strategy trial. HIV Clinical Trials 2018; 19: 209–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommendations for a public health approach, 2010. version, http://apps.who.int/iris/bitstream/10665/75236/1/9789241599818_eng.pdf (2010, accessed 30 January 2020). [PubMed]
- 5.WHO and UNICEF. Antenatal care in developing countries. Promises, achievements and missed opportunities. An analysis of trends, levels and differentials, 1990–2001, https://www.unicef.org/media/files/antenatal.pdf (Accessed 30 January 2020).
- 6.PEPFAR. Prevention of mother-to-child transmission of HIV: expert panel report and recommendations to the U.S. Congress and U.S. Global AIDS Coordinator, 2010, https://www.pepfar.gov/documents/organization/135465.pdf
- 7.World Health Organization. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Programmatic update, http://www.who.int/hiv/pub/mtct/programmatic_update2012/en/ (2012, accessed 30 January 2020). [PubMed]
- 8.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis 2014; 14: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The INSIGHT START Study Group, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


