Abstract
Background:
Patients with primary refractory metastatic renal cell carcinoma (mRCC) have a dismal prognosis and poor response to subsequent treatments. While there are several approved second-line therapies, it remains critical to choose the most effective treatment regimen.
Methods and patients:
We identified 7 patients with clear cell mRCC that had primary resistance to vascular endothelial growth factor (VEGF)-targeted tyrosine kinase inhibitors (TKIs) or immune checkpoint inhibitor (ICI) combination therapy. Patients were treated with lenvatinib (a multitargeted TKI) plus everolimus (a mammalian target of rapamycin inhibitor). Among these 7 patients, 2 had prior TKIs, 3 had prior ICIs, and 2 had prior TKI and ICI therapy. We collected the patients’ clinical characteristics, molecular profiles, treatment durations, and toxicity outcomes.
Results:
The median time to progression on prior therapies was 1.5 months. Lenvatinib plus everolimus was used either as a second-line (n=4) or third-line (n=3) therapy. As best responses, 3 patients had partial responses and 3 achieved stable disease. Patients were followed for ≥17 months; progression-free survival ranged from 3–15 months and overall survival ranged from 4–17 months.
Conclusions:
These 7 cases provide real-world data for the use of lenvatinib plus everolimus in patients with mRCC with primary resistance to first-line VEGF-targeted TKIs or ICI combination therapy.
Keywords: primary refractory, tyrosine kinase inhibitor, second-line therapy, mTOR inhibitor, immune checkpoint inhibitor
Introduction
The treatment landscape for metastatic renal cell carcinoma (mRCC) is constantly changing with the continuous approval of new first-line and second-line therapies.1-4 Preferred first-line treatments for clear cell mRCC include vascular endothelial growth factor (VEGF)-targeted tyrosine kinase inhibitors (TKIs; eg, sunitinib, pazopanib, or cabozantinib), and the immune checkpoint inhibitor (ICI) combination therapies (eg, ipilimumab plus nivolumab and axitinib plus pembrolizumab).5 However, a significant proportion (approximately 20%) of patients have tumors that progress during first-line treatments and require subsequent therapies.6-9 Several regimens have been approved in the second-line setting with the following preferred treatment options: cabozantinib, nivolumab, lenvatinib plus everolimus, and axitinib.4 However, given the changing treatment paradigm of metastatic RCC favoring immunotherapy over targeted therapy, the approval of second-line therapies based on clinical trials using different control arms and in different first-line settings, and the diverse patient population in terms of risk group and histology, it has become increasingly challenging for clinicians to decide on the optimal subsequent line of treatment.
Patients refractory to first-line mRCC therapy with either targeted therapy or immunotherapy are of special interest as they have a dismal prognosis due to poor response to subsequent therapy with VEGF-targeted TKIs or mammalian target of rapamycin (mTOR) inhibitors.6-9 Therefore, it is imperative to carefully select the second-line treatment. Circumventing the mechanisms underlying resistance to VEGF-targeted TKIs using angiogenic escape through the upregulation of fibroblast growth factor (FGF)-associated signaling has been shown.10 Therefore, simultaneous inhibition of VEGF and FGF pathways using a multitargeted TKI is a rational approach and has been gaining substantial interest and evidence.10,11 Lenvatinib is a potent multitargeted TKI that inhibits VEGF receptors 1–3, FGF receptors 14, platelet-derived growth factor receptor-β, RET, and KIT.12 In addition to the VEGF and FGF pathways, the mTOR pathway also has been implicated in the development of RCC.13 In the United States, the regimen combining lenvatinib and everolimus (an mTOR inhibitor) has been approved as a second-line treatment in patients with mRCC that failed targeted therapy with a prior antiangiogenic TKI.14,15
Patients whose tumors are refractory to first-line therapy with the combination of the ICIs ipilimumab plus nivolumab have yet to be studied. However, as more patients are receiving the ICI combination as first-line therapy, and as there is no guidance on the second-line therapies, it is of great importance to understand patient outcomes with subsequent therapy to guide optimal clinical decisions.
In this 7-patient case series, we report the characteristics and the outcomes of patients with clear cell mRCC whose tumors were refractory to first-line therapy with TKI or ICIs and who were subsequently treated with lenvatinib plus everolimus.
Material and Methods
We identified patients who were refractory to first-line therapy (VEGF-targeted TKI and/or ICI combination therapy) and who were subsequently treated with lenvatinib plus everolimus. All patients were treated by a single provider (J.H.) at Barnes Jewish Hospital/Washington University School of Medicine. They provided informed consent for the study of their tumors and publication of their associated clinical data (Washington University HRPO #201411135).
Clinical characteristics, treatment exposures, toxicities, and outcomes were collected from the electronic medical records. The choice of treatment and response assessment were performed at the discretion of the treating provider.
Tumor and germline DNA for most of the patients were analyzed using massive parallel sequencing with the Tempus ∣ xO Onco-seq panel (TEMPUS, Chicago, IL). The Tempus ∣ xO Onco-seq panel consists of 1714 cancer-related genes and detects clinically relevant genomic alterations (genomic variants as well as copy number variations). Tumor specimens were also analyzed for programmed death ligand-1 (PD-L1) expression via immunohistochemistry
Results
Patient characteristics
Patients who were primarily refractory to first-line therapy with either VEGF-targeted TKIs or ICIs were identified from clinical records (n=7; Supplemental Table 1). The median age of the patients at diagnosis was 57 years (range: 39–63 years old). All 7 patients were men and had clear cell histology (3 [43%] had sarcomatoid differentiation, including 2 with additional rhabdoid differentiation). Most patients had undergone prior nephrectomy (6/7; 86%); 4 patients (57%) were assigned as at “intermediate risk” using the International Metastatic renal cell carcinoma Database Consortium (IMDC) criteria and 3 (43%) were “poor risk.” The majority of these patients (6/7; 86%) had pulmonary nodules, 3 (43%) had brain lesions, 4 (57%) had bone metastases, and 1 (14%) had liver lesions (Supplemental Table 1).
Of the 7 patients included, 6 had their samples analyzed for genomic analyses and 5 for PD-L1 expression (Supplemental Table 2). Most patients tested (5/6; 83%) had genomic variants, 4 (67%) had loss of function in VHL, 2 (33%) had loss of function in PBRM1, and 1 (17%) had loss of function in PTEN. Of the 5 patients analyzed for PD-L1 expression, 1 (20%) stained positive by immunohistochemistry.
Treatment exposure
With respect to prior treatment exposure, 2 patients had prior VEGF-targeted TKI therapy (sunitinib, pazopanib, or cabozantinib), 3 had prior ICI therapy with ipilimumab plus nivolumab combination as first-line therapy, and 2 patients had prior VEGF-targeted TKI and ICI therapy (Table 1). The median time to progression on prior TKI or ICI therapy was 1.5 months (range: 0.8–3 months). Patients received the combination of lenvatinib plus everolimus as either second-line (n=4; 57%) or third-line (n=3; 43%) therapy. Of note, 2 patients changed treatment regimens to lenvatinib plus everolimus due to toxicity with prior therapies rather than disease progression.
Table 1.
Treatment exposure
| P# | Prior treatment (line) |
Time on prior therapy (months) |
Line of LEN + EVE treatment |
Discontinued LEN + EVE treatment |
Reason for discontinuation |
Time on therapy (months) |
Follow-up (months) |
|---|---|---|---|---|---|---|---|
| Prior TKI | |||||||
| 1 | Sunitinib (1) | 0.8 | 2nd | Yes | PD | 15c | 17 |
| 3 | Pazopaniba (1) | 1.5 | Start new | ||||
| Cabozantinibb (2) | 0.5 | 3rd | Yes | anticancer regimen |
7d | 9 | |
| Prior ICI | |||||||
| 4 | Ipilimumab + nivolumab (1) | 1 | 2nd | Yes | AE | 8 | 9 |
| 5 | Ipilimumab + nivolumab (1) | 2 | 2nd | No | NA | 6+ | 11+ |
| 7 | Ipilimumab + nivolumabb (1) | 1 | 2nd | Yes | AE | 7 | 9+ |
| Prior TKI and ICI | |||||||
| 2 | Sunitinib (1) | 2 | |||||
| Nivolumab + lenvatinib (2) | 3 | 3rd | Yes | PD | 8 | 11 | |
| 6 | Cabozantinibb (1) | 1.5 | |||||
| Ipilimumab + nivolumab (2) | 1.5 | 3rd | Yes | PD | 3 | 4 | |
Patient had mixed response.
Discontinued because of toxicity rather than disease progression.
Treatment was discontinued for 5 days due to toxicity, then resumed due to progression of brain metastasis.
Treatment was discontinued for 5 weeks because of the approval of nivolumab plus ipilimumab combination in mRCC but was later resumed due to progression of skin lesions on the ICI combination.
AE, adverse event; EVE, everolimus; ICI, immune checkpoint inhibitor; LEN, lenvatinib; NA, not applicable: P, patient; PD, progressive disease; TKI, tyrosine kinase inhibitor.
The patients were followed for up to 17 months after initiation of lenvatinib plus everolimus combination therapy (range: 4–17 months). At the time of analysis (October 29, 2019), the 7 patients had received the combination treatment for a median of 7 months (Figure 1). Currently, 1 patient remains on the combination therapy and had stable disease at last follow-up. The reasons for discontinuation of treatment in these 6 patients were disease progression in 3 patients (50%), treatment-emergent adverse events for 2 patients (33%), and approval of, and switch to, a new treatment regimen for mRCC in 1 patient (16.7%).
Figure 1.
Patients with mRCC that was primarily refractory to first-line therapy were identified (n=7) and treated with the combination of lenvatinib and everolimus. Their time on the combination therapy (blue bars) and their efficacy outcomes are shown here.
mRCC, metastatic renal cell carcinoma.
The combination treatment was discontinued for a brief period for 2 patients (5 days for Patient 1 and 5 weeks for Patient 3) and then resumed when these 2 patients’ tumors began to rapidly progress while off the regimen. Patient 1 experienced fatigue and weight loss, which prompted discontinuation of the lenvatinib plus everolimus regimen in preparation for ICI combination (ipilimumab plus nivolumab) therapy. Two days after discontinuation of lenvatinib plus everolimus, the patient presented with a headache and a subsequent magnetic resonance imaging scan showed edema. Five days later, the patient resumed lenvatinib plus everolimus treatment. The patient received lenvatinib plus everolimus for a total of 15 months and had an overall survival (OS) duration of 17 months (Figure 1).
Patient 3 had a history of prior treatment with VEGF-targeted TKIs; pazopanib (after 1.5 months of treatment) was discontinued because of disease progression and worsening liver lesions. The patient was then treated with cabozantinib (for 0.5 months) before treatment discontinuation due to new skin lesions. The patient then started therapy with lenvatinib plus everolimus as a third-line treatment regimen on which the patient experienced a best response of stable disease. However, due to fatigue and the hope for an objective treatment response, treatment was discontinued and was replaced with the recently approved ICI combination treatment for mRCC: ipilimumab plus nivolumab. At the first scan after initiation of this ICI combination therapy, there was visible tumor shrinkage, but the patient had also developed seizure-like episodes, which were responsive to high-dose steroids. ICI therapy was therefore discontinued, and the patient resumed treatment with lenvatinib plus everolimus. The patient continued to receive lenvatinib plus everolimus for a cumulative total of 7 months, with a reported best response of stable disease and an OS of 9 months (Figure 1).
Treatment outcomes
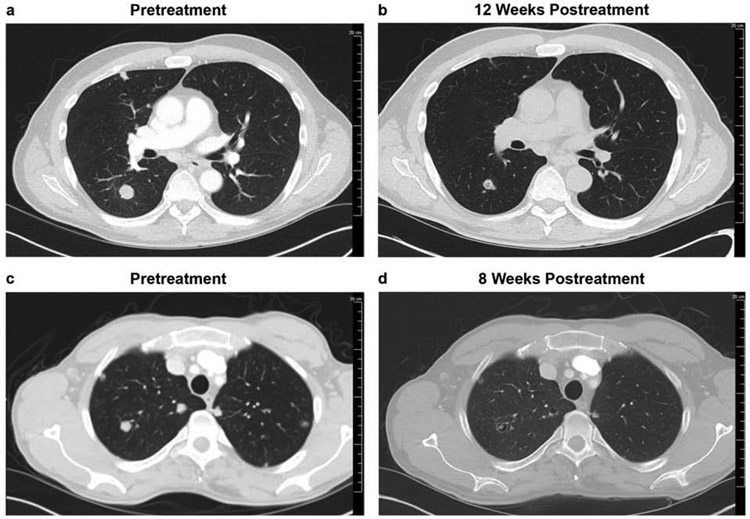
Of particular interest is the outcome of 1 patient (Patient 1) who had mRCC that was refractory to prior TKI therapy (sunitinib) and who experienced rapid disease progression after discontinuation of treatment with lenvatinib plus everolimus. Patient 1 discontinued lenvatinib plus everolimus combination therapy due to toxicity but had marked disease progression noted 5 days later, and then had an impressive rapid response to treatment once the combination regimen was resumed (Supplemental Figure 1). This patient had a best overall response of partial response (Figure 2b) and an OS of 17 months.
Figure 2.
Representative images from 2 patients with primary refractory mRCC (to prior TKI [Patient 1; images a and b] or ICI therapy [Patient 5; images c and d]) who had a partial response after lenvatinib plus everolimus treatment. Images shown are of the pretreatment scans (a, c), with tumors clearly visible, and the posttreatment scan (b, d), where tumor size is much reduced.
ICI, immune checkpoint inhibitor; mRCC, metastatic renal cell carcinoma; TKI, tyrosine kinase inhibitors.
Also, of interest is the outcome of a patient refractory to prior ICI combination therapy (ipilimumab plus nivolumab) who then had a partial response to treatment with lenvatinib plus everolimus, and whose treatment is still ongoing. This patient (Patient 5) presented with a brain metastasis, started lenvatinib plus everolimus therapy, and achieved a partial response after about 8 weeks (Figure 2d). Upon follow-up (approximately 2 months later) of a known brain lesion that had been irradiated, a brain scan showed a small asymptomatic brain lesion. The patient was treated via gamma-knife radiosurgery. Patient 5 has continued treatment with lenvatinib plus everolimus and remains in stable condition with an ongoing OS of over 11 months.
Of the 7 patients treated with lenvatinib plus everolimus in this case study, 3 (43%) patients had a partial response as best response, 3 (43%) had stable disease as best response, and 1 (14%) patient had progressive disease. At the time of this case study analysis (October 29, 2019), all patients had experienced disease progression and 5 (71%) had died (Table 2). The progression-free survival ranged from 3–15 months and the OS ranged from 4–17 months. Of note, the OS has not been reached for 2 patients because their overall survival is currently ongoing (more than 11 and 9 months, respectively).
Table 2.
Treatment outcomes
| Most recent prior therapy |
Patient number |
Best response |
Disease progression |
PFSa (months) |
Deathb | OSa (months) |
Follow-upa (months) |
|---|---|---|---|---|---|---|---|
| TKIc | 1 | PR | Yes | 15 | Yes | 17 | 17 |
| 2 | SD | Yes | 6 | Yes | 11 | 11 | |
| 3 | SD | Yes | 6 | Yes | 9 | 9 | |
| ICI | 4 | PR | Yes | 9 | Yes | 9 | 9 |
| 5 | PR | Yes | 3 | No | 11+ | 11+ | |
| 6 | PD | Yes | 3 | Yes | 4 | 4 | |
| 7 | SD | Yes | 9 | No | 9+ | 9+ |
A conversion factor of 30.4375 was used to convert number of days into months; patients who were still alive at last follow-up (October 29, 2019) are indicated with a plus for OS as survival is ongoing at the time of manuscript preparation.
Patient’s survival status as of last follow-up (October 29, 2019).
Patient 2 received lenvatinib plus nivolumab (TKI + ICI) as last prior therapy.
ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression-free survival; PD, progressive disease; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor.
Discussion
The 7 cases presented here provide real-world data on the combination of lenvatinib plus everolimus in patients with metastatic clear cell RCC whose disease appeared intrinsically refractory to front-line TKIs or ICI combination therapy. Patients with primary refractory disease are a rare and difficult-to-treat population with a poor prognosis due to poor response to subsequent therapies.6,7 Therefore, our observation that 6 of 7 primary refractory patients treated with lenvatinib plus everolimus experienced a progression-free survival range of 3–15 months is encouraging.
In clinical practice, lenvatinib plus everolimus has demonstrated a manageable tolerability profile. Treatment-emergent adverse events (TEAEs) have been consistent with class effects typical of VEGF-targeted TKIs and mTOR inhibitors, with no additive toxicity observed.14,16 The most frequent TEAEs are diarrhea, decreased appetite, and fatigue.
Based on phase 1 and phase 2 trials in mRCC, this regimen has been proposed as a preferred second-line treatment in patients with intrinsic refractory disease and those who have early disease progression.13 Lenvatinib blocks both VEGF- and FGF-driven angiogenesis, KIT-dependent angiogenesis, RET-fusion/RET-mutant tumorigenesis, and VEGF-3-associated lymphangiogenesis.12,17-20 Preclinical studies attributed the synergistic antitumor activity of lenvatinib plus everolimus to potent enhancement of antiangiogenesis by simultaneous targeting of VEGF/FGF receptor and the downstream mTOR pathway.21 Thus, our patients’ responses to lenvatinib plus everolimus may be explained by increased expression of FGF-pathway-related genes; however, additional molecular studies would be needed to confirm this.
Patients with mRCC who do not respond to early-line treatments typically have rapid disease progression. Since there is limited time for additional therapies, it is critical to prioritize the most effective therapies. With the approval of multiple subsequent lines of treatment based on clinical trials of different comparative arms, patient characteristics, and treatment settings, it has been challenging to choose the next line of treatment in mRCC patients. We believe that our case studies demonstrate that the combination of lenvatinib and everolimus can be considered a second-line therapy option for patients who are primarily refractory to VEGF-targeted TKI and/or ICIs and warrants further investigation.
Conclusions
Our study demonstrates real-world evidence of lenvatinib plus everolimus in patients with primary refractory disease. These 7 patients received a VEGF-targeted TKI (sunitinib, cabozantinib, pazopanib) or ICI combination (ipilimumab plus nivolumab) as first-line treatment, making our data applicable to the current treatment era. As responses to subsequent lines of therapy in the primary refractory patient population are rare, the use of lenvatinib plus everolimus in patients with intrinsically resistant clear cell mRCC should be studied further in this challenging group of patients with dismal prognosis.
Supplementary Material
Highlights:
Case series of 7 patients with primary refractory metastatic RCC
Patients were refractory to tyrosine kinase inhibitors (TKIs) and/or immunotherapy
Patients were then treated with a TKI and mTOR inhibitor (lenvatinib + everolimus)
Most patients (6/7) had a stable or partial response to lenvatinib + everolimus
Median overall survival was promising and ranged from 4–17 months
Clinical Practice Points:
Patients with clear cell metastatic renal cell carcinoma (RCC) that is primarily refractory to first-line treatment have a dismal prognosis due to poor response to subsequent therapies. Moreover, there is limited guidance on second-line therapy for patients with RCC after progression on, or following, the recently approved immune checkpoint inhibitor (ICI) combination therapy (ipilimumab + nivolumab). In this case series, we discuss 7 patients with primary refractory disease to either a tyrosine kinase inhibitor (TKI) or ICI combination therapy. These patients had clear cell metastatic RCC that had progressed following first-line vascular endothelial growth factor-targeted TKIs (n=4) or ICI combination therapy (n=3). All 7 patients were subsequently treated with lenvatinib (a multitargeted TKI) plus everolimus (a mammalian target of rapamycin inhibitor) as either second-line or third-line therapy. Among the 3 patients who had failed first-line TKIs, all experienced a clinical benefit of either partial response (PR; n=1) or stable disease (SD; n=2) in response to treatment with lenvatinib plus everolimus. Among the 4 patients who were primarily refractory to first-line ICI combination therapy, a clinical benefit was observed in 3 patients (PR, n=2; SD, n=1) and 1 patient experienced disease progression. This case series demonstrates that most patients with primary resistance to first-line TKI or ICI combination therapy benefitted from subsequent treatment with lenvatinib plus everolimus (6/7). These real-world data suggest that lenvatinib plus everolimus may improve the prognosis of patients with intrinsically resistant clear cell metastatic RCC and, therefore, should be given careful consideration as a potential treatment option.
Acknowledgments:
JH is supported by NIH R01 CA223231.
Funding: This work was supported by NIH R01 CA223231.
Medical writing support was provided by Tarah M. Connolly, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, with funding provided by Eisai Inc., Woodcliff Lake, NJ, USA.
Role of the funding source: Eisai Inc., funded medical writing assistance for this manuscript and reviewed the final draft.
Footnotes
Conflicts of Interest: JH has received grants and consultant fees from Novartis and Eisai, and consultant fees from OncLive. LH, RB, and VHL have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonasch E NCCN Guidelines updates: management of metastatic kidney cancer. J Natl Compr Canc Netw. 2019;17:587–589. [DOI] [PubMed] [Google Scholar]
- 3.Wei EY, Hsieh JJ. A river model to map convergent cancer evolution and guide therapy in RCC. Nat Rev Urol. 2015;12:706–712. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:706–720. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Kidney Cancer. Version 2.2020. https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf; Accessed 8 November 2019.
- 6.Heng DY, Mackenzie MJ, Vaishampayan UN, et al. Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol. 2012;23:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch J, Seidel C, Weikert S, et al. Intrinsic resistance to tyrosine kinase inhibitors is associated with poor clinical outcome in metastatic renal cell carcinoma. BMC Cancer. 2011;11:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porta C, Sabbatini R, Procopio G, Paglino C, Galligioni E, Ortega C. Primary resistance to tyrosine kinase inhibitors in patients with advanced renal cell carcinoma: state-of-the-science. Expert Rev Anticancer Ther. 2012;12:1571–1577. [DOI] [PubMed] [Google Scholar]
- 9.Seidel C, Busch J, Weikert S, et al. Progression free survival of first line vascular endothelial growth factor-targeted therapy is an important prognostic parameter in patients with metastatic renal cell carcinoma. Eur J Cancer. 2012;48:1023–1030. [DOI] [PubMed] [Google Scholar]
- 10.Sonpavde G, Willey CD, Sudarshan S. Fibroblast growth factor receptors as therapeutic targets in clear-cell renal cell carcinoma. Expert Opin Investig Drugs. 2014;23:305–315. [DOI] [PubMed] [Google Scholar]
- 11.Malouf GG, Flippot R, Khayat D. Therapeutic strategies for patients with metastatic renal cell carcinoma in whom first-line vascular endothelial growth factor receptor-directed therapies fail. J Oncol Pract. 2016;12:412–420. [DOI] [PubMed] [Google Scholar]
- 12.Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664–671. [DOI] [PubMed] [Google Scholar]
- 13.Leonetti A, Leonardi F, Bersanelli M, Buti S. Clinical use of lenvatinib in combination with everolimus for the treatment of advanced renal cell carcinoma. Ther Clin Risk Manag. 2017;13:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 15.Lenvima (lenvatinib) [prescribing information]: Woodcliff Lake, NJ: Eisai Inc.; 2019. [Google Scholar]
- 16.Molina AM, Hutson TE, Larkin J, et al. A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC). Cancer Chemother Pharmacol. 2014;73:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–5465. [DOI] [PubMed] [Google Scholar]
- 18.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97–103. [DOI] [PubMed] [Google Scholar]
- 21.Matsuki M, Adachi Y, Ozawa Y, et al. Targeting of tumor growth and angiogenesis underlies the enhanced antitumor activity of lenvatinib in combination with everolimus. Cancer Sci. 2017;108:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.